Abstract
Rationale: Acute respiratory distress syndrome (ARDS) is a heterogeneous syndrome with a mortality of up to 40%. Precision medicine approaches targeting patients on the basis of their molecular phenotypes of ARDS might help to identify effective pharmacotherapies. The inflammasome–caspase-1 pathway contributes to the development of ARDS via IL-1β and IL-18 production. Recent studies indicate that tetracycline can be used to treat inflammatory diseases mediated by IL-1β and IL-18, although the molecular mechanism by which tetracycline inhibits inflammasome–caspase-1 signaling remains unknown.
Objectives: To identify patients with ARDS characterized by IL-1β and IL-18 expression and investigate the ability of tetracycline to inhibit inflammasome–caspase-1 signaling in ARDS.
Methods: IL-1β and IL-18 concentrations were quantified in BAL fluid from patients with ARDS. Tetracycline’s effects on lung injury and inflammation were assessed in two mouse models of direct (pulmonary) acute lung injury, and its effects on IL-1β and IL-18 production were assessed by alveolar leukocytes from patients with direct ARDS ex vivo. Murine macrophages were used to further characterize the effect of tetracycline on the inflammasome–caspase-1 pathway.
Measurements and Main Results: BAL fluid concentrations of IL-1β and IL-18 are significantly higher in patients with direct ARDS than those with indirect (nonpulmonary) ARDS. In experimental acute lung injury, tetracycline significantly diminished lung injury and pulmonary inflammation by selectively inhibiting caspase-1–dependent IL-1β and IL-18 production, leading to improved survival. Tetracycline also reduced the production of IL-1β and IL-18 by alveolar leukocytes from patients with direct ARDS.
Conclusions: Tetracycline may be effective in the treatment of direct ARDS in patients with elevated caspase-1 activity.
Clinical Trial registered with www.clinicaltrials.gov (NCT04079426).
Keywords: inflammasomes, immunomodulation, antibacterial agents, influenza, precision medicine
At a Glance Commentary
Scientific Knowledge on the Subject
Acute respiratory distress syndrome (ARDS) is a heterogeneous syndrome with various underlying causes that is triggered by activation of the inflammasome–caspase-1 pathway and subsequent IL-1β and IL-18 release. Tetracycline can be used to treat inflammatory diseases mediated by IL-1β and IL-18, but the ability of tetracycline to inhibit inflammasome–caspase-1 signaling in ARDS remains unknown.
What This Study Adds to the Field
This study suggests that inflammasome–caspase-1 activation is much more common in patients with direct (pulmonary) than those with indirect (nonpulmonary) ARDS. Applying a precision medicine approach, we used two murine models of direct ARDS to demonstrate that tetracycline blocks the inflammasome–caspase-1 pathway via selective inhibition of caspase-1, thereby reducing lung injury and mortality. As a step toward validating the clinical relevance of our findings, we show that tetracycline reduced the production of IL-1β and IL-18 in alveolar immune cells obtained from patients with direct ARDS ex vivo.
Acute respiratory distress syndrome (ARDS) continues to have a mortality rate approaching 40% because of the absence of effective pharmacotherapy (1, 2). ARDS is a heterogeneous syndrome with various underlying illnesses that may cause either direct (e.g., pneumonia and aspiration) or indirect (e.g., nonpulmonary sepsis and pancreatitis) injury to the lungs (3, 4). Initiation of the host response to infection and cellular injury drives epithelial (direct ARDS) or the endothelial (indirect ARDS) damage and subsequent loss of the alveolar–capillary barrier function that leads to an influx of protein-rich edema fluid (5). Precision medicine approaches targeting patients on the basis of their molecular phenotype of ARDS might help to identify effective pharmacotherapies.
ARDS is characterized by inflammation of the lungs arising from activation of the innate immune system (5). Increasing evidence exists that the overproduction of IL-1β and IL-18 plays a key role in the development of ARDS (6–10). Moreover, elevated IL-1β and IL-18 concentrations are associated with increased mortality (11–13). Thus, targeting the pathway responsible for the production of these cytokines may be of therapeutic benefit to patients with ARDS (6, 14, 15).
The production of IL-1β and IL-18 is regulated via the inflammasome–caspase-1 pathway. Inflammasomes are multiprotein complexes consisting of a sensor, the adapter protein ASC (apoptosis-associated speck-like protein containing a CARD domain) and caspase-1 (16). Sensors include the NLR (nucleotide-binding oligomerization domain–like receptor) family, NLRP3 (pyrin domain–containing 3), and pyrin and HIN domain–containing protein family members AIM2 (absent in melanoma) (16). Caspase-1 activation requires two signals. Signal 1 is characterized by activation of pattern recognition receptors such as the TLRs (Toll-like receptors) by pathogen-associated molecular patterns including LPS. This initiates NF-κƙB (nuclear factor κ-light-chain-enhancer of activated B cells) regulated transcription of inflammasome components including pro–caspase-1, pro–IL-1β, and pro–IL-18. Signal 2 is provided by a wide range of stimuli, including ATP, viral RNA, and pore-forming toxins, and it activates NLRP3 or AIM2, leading to inflammasome assembly via the oligomerization of ASC. This results in the activation of caspase-1, which in turn activates pro–IL-1β and pro–IL-18 via proteolytic cleavage into the active cytokines IL-1β and IL-18 (16).
Tetracycline and its derivates have been evaluated in experimental and clinical studies of inflammatory diseases, in which they are reported to be both safe and have immunomodulatory activity (17–21). Recent evidence suggests that the antiinflammatory effects of tetracycline are mediated by the inhibition of IL-1β and L-18 production. However, the exact mechanism by which tetracycline blocks the inflammasome–caspase-1 pathway remains unclear (18, 22–25).
We hypothesized that tetracycline might improve outcomes in the subset of ARDS in which inflammation is mediated by inflammasome–caspase-1 activation (4, 26). Initial studies established that inflammasome–caspase-1–dependent disease was much more common in patients with direct ARDS. This led us to investigate the effect of tetracycline on inflammasome–caspase-1 activation in distinct murine models and on alveolar immune cells obtained from patients with direct ARDS. Finally, we revealed the underlying mechanism by which tetracycline inhibits inflammasome–caspase-1 signaling, thereby blocking IL-1β and IL-18 production. Some of the results of these studies have been previously reported in the form of an abstract (27).
Methods
Mouse Experiments
The study was approved by the authority for animal care (animal protocols: AZ 81–02.04.2018.A110). Wild-type (WT) C57BL/6J mice (Charles River Laboratories) and caspase-1–knockout (KO) mice (caspase-1ko/ko mice; kind gift of E.L.) were challenged with either LPS O111:B4 (Sigma-Aldrich), mouse-adapted A/Puerto Rico 8 strain (PR8, H1N1) Influenza A virus, or PBS (Thermo Fisher Scientific) by intratracheal instillation.
These animals were then treated by intraperitoneal injection with tetracycline (Sigma-Aldrich) or PBS for 10 days. Mice were killed and considered dead if weight loss after challenge exceeded 25% of baseline weight. BAL fluid (BALF) was collected as described previously (28). IL-1β, IL-6, and TNF-α concentrations were analyzed with LEGENDplex (BioLegend), IL-18 and albumin were analyzed by ELISA (R&D Systems and Bethyl), and protein was analyzed by bicinchoninic acid protein assay kit (Thermo Fisher Scientific). FACS Canto II (BD Bioscience) (FACSDiva software 6.1.2), FlowJo software 10.6.1 (TreeStar), and antibodies against CD16/CD32 (2.4G2) (BD Bioscience), CD45 (30-E11, eFluor450), Ly-6C (HK1.4, PE), F4/80 (BM8, PE-Cy7) (Thermo Fisher Scientific), and Ly-6G (148, APC) (BioLegend) were used for neutrophil characterization. Two blinded investigators evaluated hematoxylin and eosin–stained (Sigma-Aldrich) histological samples using an established lung injury score (29) (see online supplement).
In Vitro Analyses
Bone marrow–derived macrophages (BMDM) from C57BL/6J mice were differentiated in vitro as described previously (30), activated with LPS, nigericin, or Poly (dA:dT) (both Invivogen), and coincubated with tetracycline, VX765, or ZVAD-FMK (both Invivogen). IL-1β and TNFα were analyzed by ELISA (R&D Systems), and LDH was analyzed by CytoTox 96 assay (Promega). Immunoblotting was performed as previously described (30). Antibodies against full-length and the p20 fragment of caspase-1 (mAb Casper-1; Adipogen Life Sciences), ASC (anti-Asc, pAb [AL177]; Adipogen Life Sciences) and horseradish-peroxidase–conjugated anti-rabbit and anti-mouse IgG (both Cell Signaling Technology) were used. Complementary DNA was synthesized, and real-time PCR was performed as previously described (31). Expression levels (normalized to 18 s) were calculated using the 2−ΔΔCt method. All reagents were from Applied Biosystems. Activity of tetracycline coincubated recombinant caspase-1 or -8 was detected using Caspase-Glo Assay (Promega) (see online supplement).
ASC Speck Assay
Mouse 19.5 reporter cells expressing ASC-mCerulean (32) were activated as described for BMDM. Staining was performed with Draq5 (Thermo Fisher Scientific). Speck formation was analyzed using a Zeiss Observer.Z1 fluorescence microscope (Zeiss) (see online supplement).
Human BALF Analysis and Ex Vivo Cultures
ARDS was defined according to the Berlin Definition (33). The study was registered at clinicaltrials.gov (NCT04079426). BALF was obtained from patients of two ARDS centers (University Hospital Bonn and Hannover Medical School) with approval by the local institutional research ethics boards. To obtain alveolar leukocytes, BALF was collected after informed consent within 24 hours of ARDS onset. A total of 1 × 106 cells/well in 24-well plates were coincubated with tetracycline, COL-3 (Hycultec), VX765, or ZVAD-FMK as described above. IL-1β and IL-18 concentrations in BALF were analyzed by Luminex assay (R&D Systems). IL-1β and IL-18 concentrations in supernatant of ex vivo assays and historic control of ventilated patients without infection who received elective abdominal surgery were determined by ELSIA (R&D Systems) (34).
Statistics
Statistical analysis was performed using GraphPad Prism 8 Software. Variables were compared by Mann-Whitney U test. Kaplan-Meier survival curves were evaluated with log-rank test. Values of P < 0.05 were considered significant. All data are expressed as mean ± SEM unless otherwise noted.
Results
IL-1β and IL-18 Expression in BALF Differs in Patients with Direct Versus Indirect ARDS
To investigate the activation of the inflammasome–caspase-1 pathway in the pathophysiologically distinct subtypes direct and indirect ARDS, we measured the concentrations of IL-1β and IL-18 in BALF samples of patients (n = 61) from two ARDS centers. The clinical characteristics of patients are listed in Table 1. Of the patients with direct ARDS, 23 had viral (95% influenza and 5% herpes-simplex virus), 20 had bacterial, and five had aspiration pneumonia, whereas 13 patients had indirect ARDS caused by nonpulmonary sepsis (n = 7) or pancreatitis (n = 6).
Table 1.
Patient Characteristics with Direct and Indirect ARDS
| Direct ARDS (n = 48) | Indirect ARDS (n = 13) | P Value | |
|---|---|---|---|
| Age, yr | 55.9 ± 14.1 | 48.5 ± 11.3 | 0.05 |
| Sex, M, % | 70.8 | 69.2 | >0.99 |
| BMI | 29.7 ± 5.6 | 32.0 ± 7.5 | 0.37 |
| Leukocytes, G/L | 12.1 ± 8.9 | 17.9 ± 10.7 | <0.05 |
| Lactate, mmol/L | 2.9 ± 3.0 | 3.0 ± 3.2 | 0.91 |
| SOFA score | 14 ± 3 | 12 ± 3 | 0.10 |
| APACHE II score | 22 ± 5 | 20 ± 5 | 0.66 |
| Death in ICU, % | 35.4 | 30.8 | 0.75 |
| Immunosuppression | 29.2 | 46.2 | 0.32 |
| Exposure to immunomodulatory antibiotics, % | 43.8 | 76.9 | 0.53 |
| Steroid exposure, % | 39.6 | 30.8 | 0.71 |
| PaO2/FiO2 ratio | 100 ± 53 | 122 ± 55 | 0.15 |
| PEEP | 17 ± 4 | 15 ± 4 | 0.18 |
| Vt/kg predicted body weight | 4.8 ± 2.5 | 5.8 ± 2.4 | 0.19 |
| Driving pressure | 11 ± 4 | 10 ± 3 | 0.46 |
| Position of ventilation in the first 24 h, % prone | 70.8 | 15.4 | <0.05 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II ARDS = acute respiratory distress syndrome; BMI = body mass index; PEEP = positive end-expiratory pressure; SOFA = sepsis-related organ failure assessment.
P value represents direct versus indirect; Mann-Whitney Test.
Data are presented as mean ± SD.
Immunosuppression is defined as any malignancy, solid organ transplant, HIV, or cirrhosis, diabetes, and immunosuppressive drugs. Macrolides and fluoroquinolines are considered as immunomodulatory antibiotics.
Patients with direct ARDS displayed significantly higher IL-1β and IL-18 concentrations than individuals with the indirect subtype (P = 0.0226 and P = 0.0003, respectively). In patients with direct ARDS, the concentration of IL-1β was significantly higher in bacterial compared with viral pneumonia (Figure 1A; P = 0.0042). In contrast, patients with both virus- and bacteria-driven direct ARDS possessed significant higher concentrations of IL-18 than individuals with indirect ARDS (Figure 1B; P = 0.0010 and P = 0.0018, respectively). These data suggest that the inflammasome–caspase-1 pathway is active in direct but not in indirect ARDS and that inhibition of this pathway may provide a useful approach to the treatment of individuals with direct ARDS.
Figure 1.
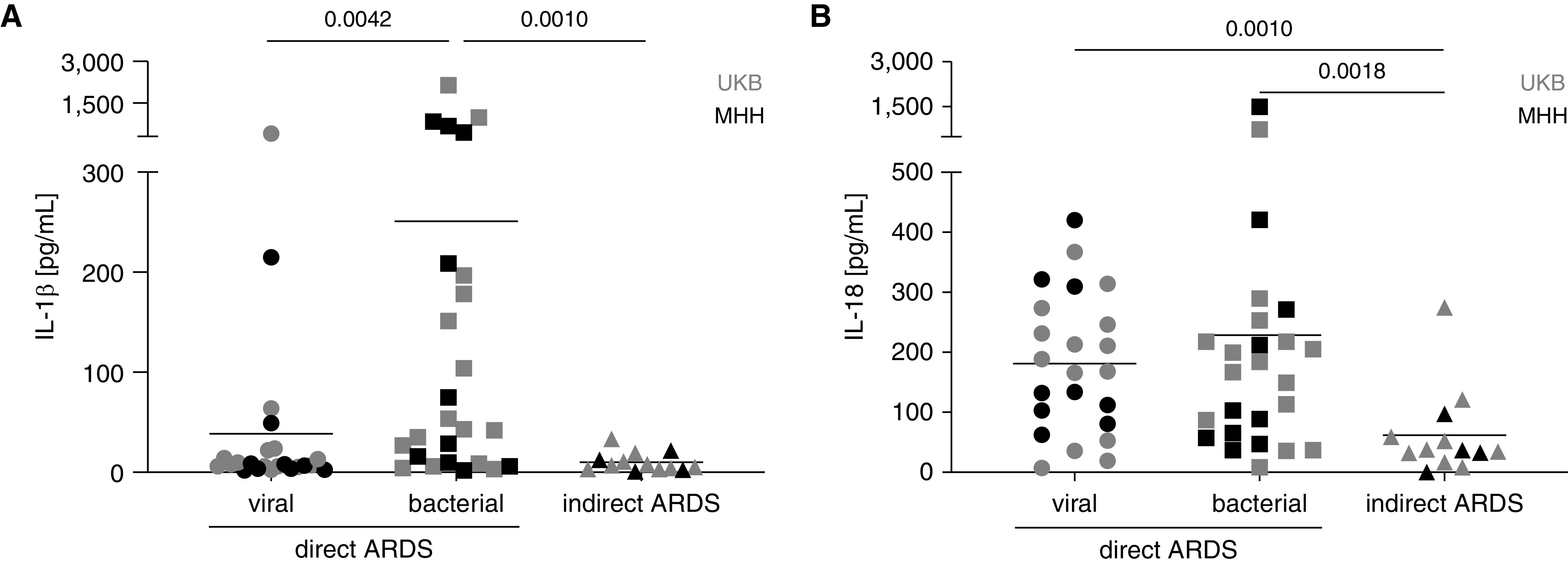
Concentrations of IL-1β and IL-18 in BAL fluid (BALF) from patients with direct and indirect acute respiratory distress syndrome (ARDS). BALF of patients with direct (n = 23 viral and n = 25 bacterial origin) or indirect (n = 13) ARDS from UKB and MHH was obtained and analyzed via bead-based multiplex assay for (A) IL-1β and (B) IL-18. Mean, Mann-Whitney Test. MHH = Hannover Medical School; UKB = University Hospital Bonn.
Tetracycline Reduces Acute Lung Injury and Pulmonary Production of IL-1β and IL-18 in Distinct Murine Models
To examine the possibility that inhibition of inflammasome–caspase-1 pathway could ameliorate disease, we tested the effects of tetracycline in a murine model of direct acute lung injury (ALI). Because bacterial ARDS expressed a strong IL-1β and IL-18 immune response (Figure 1), C57Bl/6J mice were challenged with LPS intratracheally and injected daily with tetracycline or PBS for 10 days (Figure 2A). Tetracycline treatment significantly improved survival, rising from 57% in the PBS group to 92% in the tetracycline group (Figure 2B; P = 0.0001). Lung injury was evaluated by monitoring the accumulation of protein, albumin, and neutrophils in BALF. LPS challenge led to the accumulation of all three markers over a 4-day period. This effect was significantly reduced by tetracycline treatment (Figures 2C–2E; P ≤ 0.0175). Consistent with this improvement, tetracycline reduced injury-related histopathological changes in the lung (Figure 2F; P = 0.0286). We next investigated the effect of tetracycline on the proinflammatory cytokine response in the lungs 8 and 24 hours after LPS delivery. Results show that tetracycline significantly inhibited inflammasome–caspase-1–dependent production of IL-1β and IL-18 (Figures 2G and 2H; P ≤ 0.0207), whereas non–inflammasome–caspase-1–dependent cytokines, such as TNF-α and IL-6, were not affected (see Figures E1A and E1B in the online supplement). To confirm the relevance of our findings on pulmonary inflammation and lung injury in a clinically relevant model of virus-induced ALI, mice were challenged intratracheally with influenza strain A/PR8 and treated with tetracycline (Figure 3A). Levels of protein and albumin, as well as numbers of neutrophils, were elevated in BALF of infected mice compared with naive control mice at Day 2 (Figures 3B–3D). Consistent with results in LPS-induced ALI (Figures 2C–2E), tetracycline significantly reduced these three markers of lung injury (Figures 3B–3D; P ≤ 0.0288). To further explore the effect of tetracycline on inflammasome–caspase-1–dependent cytokines in this model, we investigated the release of IL-1β and IL-18 after influenza A virus challenge by ELISA. Treating infected mice with tetracycline significantly reduced the production of both IL-1β and IL-18 (Figures 3E and 3F; P = 0.0288 and P = 0.0011, respectively). Of note, the inhibition of inflammasome–caspase-1– dependent cytokines by tetracycline had no influence on viral clearance (Figure E2). This series of findings demonstrate that tetracycline can reduce severity of direct ALI and suppresses pulmonary inflammation via a selective inhibition of the inflammasome–caspase-1 pathway.
Figure 2.
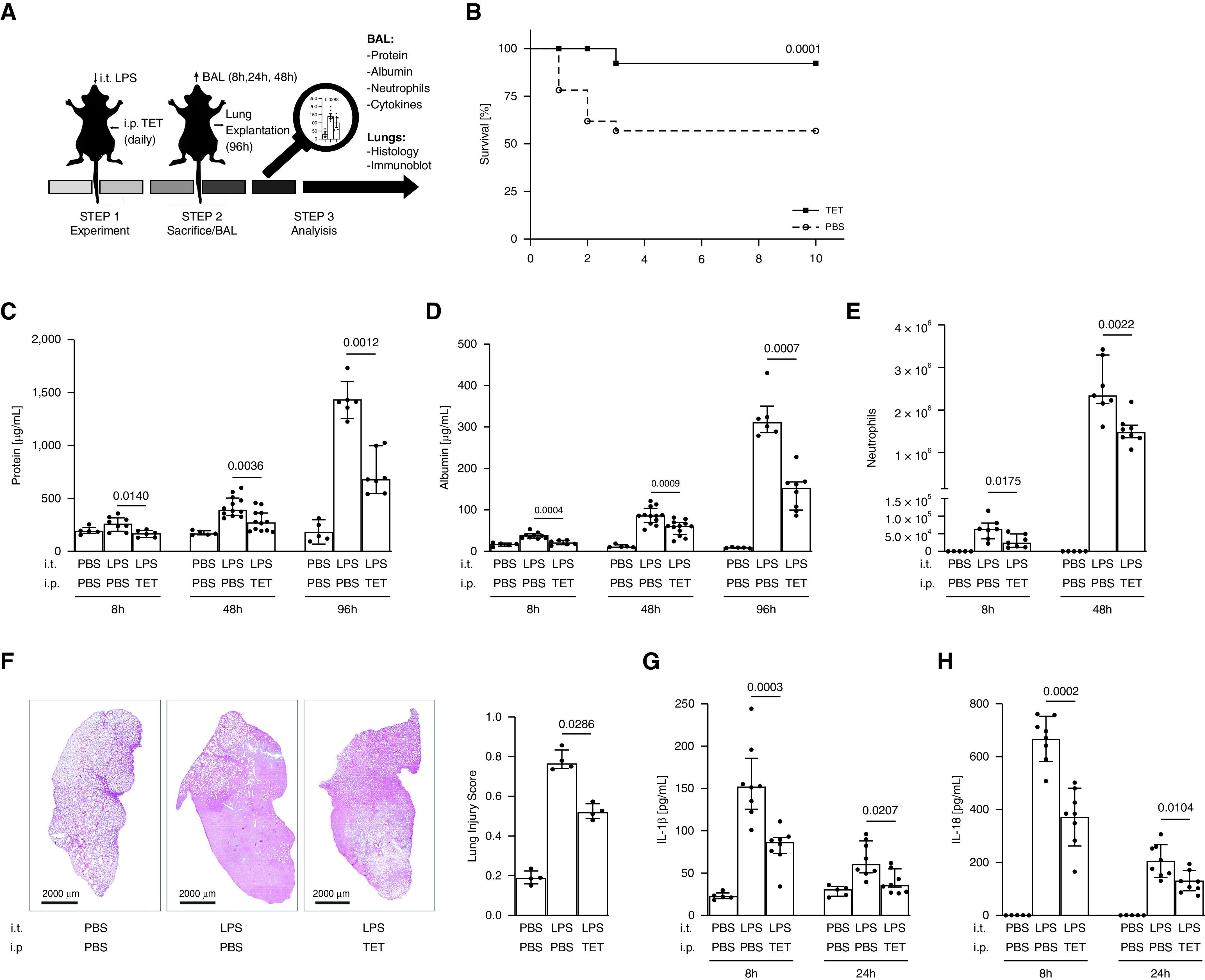
Tetracycline (TET) reduces lung injury and mortality and inhibits the production of IL-1β and IL-18 in mice with LPS-induced acute lung injury. C57BL/6J mice were challenged intratracheally with LPS (8.75 μg/g body weight) on Day 0 and treated daily with TET (75 μg/g body weight) or PBS intraperitoneally for 10 days. (A) Schematic procedure of the experiments. (B) Mice were evaluated for changes in survival (n ≥ 11 per group). The accumulation of (C) total protein, (D) albumin, and (E) neutrophils (n ≥ 5 per group) in BAL fluid (BALF) was determined by bicinchoninic acid assay (BCA), ELISA, and flow cytometry at the indicated time points. Lungs were removed at 96 hours and stained with hematoxylin and eosin. (F) Representative histologic sections are shown (magnification, 20×), and lung injury score was determined by examining 15 sections/lung/animal (n = 4 per group, magnification ×100). BALF was further analyzed for (G) IL-1β and (H) IL-18 (n ≥ 5 per group) using Luminex Assay and ELISA. Median with interquartile range of three or more independent experiments; Mann-Whitney test and log-rank test, respectively.
Figure 3.

Tetracycline (TET) reduces lung injury and inhibits IL-1β and IL-18 production in mice with influenza virus–induced acute lung injury. (A) C57BL/6J mice were challenged intratracheally with IAV (2,500 pfu/mouse) on Day 0 and treated daily with TET (75 μg/g body weight) or PBS intraperitoneally for 2 days. (B) Total protein, (C) albumin, (D) neutrophils, (E) IL-1β, and (F) IL-18 (n = 10 per group) were quantified in BAL fluid as described in Figure 2. Median with interquartile range of three independent experiments; Mann-Whitney test. IAV = influenza A virus.
Tetracycline Inhibits Caspase-1 Activation
To further explore the inhibitory effect of tetracycline on the inflammasome–caspase-1 pathway, we primed murine BMDM with LPS and then stimulated them with nigericin (NLRP3 activator) or Poly (dA:dT) (AIM2 activator) +/− tetracycline. Tetracycline dose dependently inhibited IL-1β secretion in response to both nigericin and Poly (dA:dtT) (Figures 4A and 4B; P < 0.05). Similar effects on IL-1β production were obtained when primary human macrophages were stimulated with nigericin in the presence of tetracycline (Figure E3). Consistent with earlier in vivo data (Figure E1), LPS-induced (caspase-1–independent) TNF-α production was not affected by the addition of tetracycline (Figure 4C). In addition to IL-1β and IL-18 maturation, inflammasome–caspase-1 activation causes a rapid, proinflammatory form of cell death called pyroptosis (16). Tetracycline significantly inhibited pyroptotic cell death, which is indicated by reduced LDH release (Figure 4D; P < 0.05). The expression of genes upstream of the inflammasome–caspase-1 pathway was examined to determine whether tetracycline might be targeting TLR–NF-ƙκB signaling (signal 1). Results show that tetracycline had no effect on NF-ƙκB–dependent mRNA concentrations of NLRP3, AIM2, ASC, pro–caspase-1, or pro–IL-1β after LPS stimulation (Figures E4A–E4E), suggesting that signal 1 was not affected by tetracycline.
Figure 4.

Tetracycline (TET) inhibits IL-1β production in vitro via selective inhibition of caspase-1 (Casp-1). Murine bone marrow–derived macrophages (BMDM) or immortalized murine BMDM overexpressing a fluorescently tagged ASC (apoptosis-associated speck-like protein containing a CARD domain)-mCerulean fusion protein were stimulated with either LPS (30 ng/ml) alone or in combination with nigericin (10 mM) or Poly (dA:dT) (0.5 μg/ml) and then treated with TET. (A and B) IL-1β, (C) TNF-α, and (D) LDH concentrations were measured in supernatants with ELISA or LDH assay. ASC speck formation was measured by fluorescence microscopy. (E) Six different images were taken of each condition at a magnification of ×40. (F) Immunoblots of lysates (Lys.) and supernatants (Sup.) of wild-type BMDM. Representative blots from four independent experiments. (G) Active recombinant Casp-1 was incubated with its substrate and TET. Reduction of recombinant Casp-1 activity was measured in relation to control without TET. Median with interquartile range of three or more independent experiments. *P < 0.05; Mann-Whitney test.
ASC oligomerization, which is termed “ASC specking,” is critical to NLRP3 and AIM2 inflammasome assembly (signal 2). To determine whether tetracycline might be affecting this process, the murine 19.5 reporter cell line of BMDM overexpressing fluorescently tagged ASC-mCerulean fusion protein (19.5 reporter cells) were stimulated with LPS and nigericin in the presence of tetracycline. Tetracycline had no effect on ASC speck formation as assessed by fluorescence microscopy (Figure 4E).
The effect of tetracycline on downstream caspase-1 activity was evaluated in primary BMDM. Immunoblot analyses showed that tetracycline reduced cleavage and activation of the p45 caspase-1 precursor into its p20 subunit in a dose-dependent fashion. No effect on ASC protein expression (control) was observed (Figure 4F). To investigate the specificity of tetracycline activity, its effect on recombinant caspase-1 was compared with caspase-8 (control). Tetracycline inhibited the activity of recombinant caspase-1 in a dose-dependent manner, whereas it had no effect on caspase-8 (Figures 4G and E5). Overall, these data suggest that tetracycline blocks NLRP3 and AIM2 inflammasome signaling by selectively inhibiting caspase-1 activation.
Amelioration of ALI by Tetracycline is Mediated Via Inhibition of Caspase-1
To determine whether tetracycline influenced caspase-1 in vivo, C57Bl/6 (WT) mice were challenged with either LPS or influenza strain A/PR8 and then treated with tetracycline. Lung homogenates prepared from these animals were evaluated by immunoblotting. Results show that tetracycline reduced caspase-1 activation in both models when compared with the PBS-treated control mice (Figures 5A and 5B). The effect of tetracycline on LPS-induced ALI was also examined in caspase-1–deficient mice (Caspase-1ko/ko). Caspase-1ko/ko animals showed significantly less weight loss than WT animals and were protected from death (Figure 5C). Although tetracycline treatment reduced both the magnitude and duration of weight loss in WT mice, no effect was observed on disease progression in the KO mice (Figure 5C). LPS administration induced the accumulation of albumin, neutrophils, IL-1β, and TNF-α in the BALF of Caspase-1ko/ko mice but at concentrations much lower than those observed in normal animals. Moreover, tetracycline treatment did not alter these parameters (Figures 5D–5F). Together, these data suggest that tetracycline reduces LPS-induced ALI via the inhibition of caspase-1.
Figure 5.
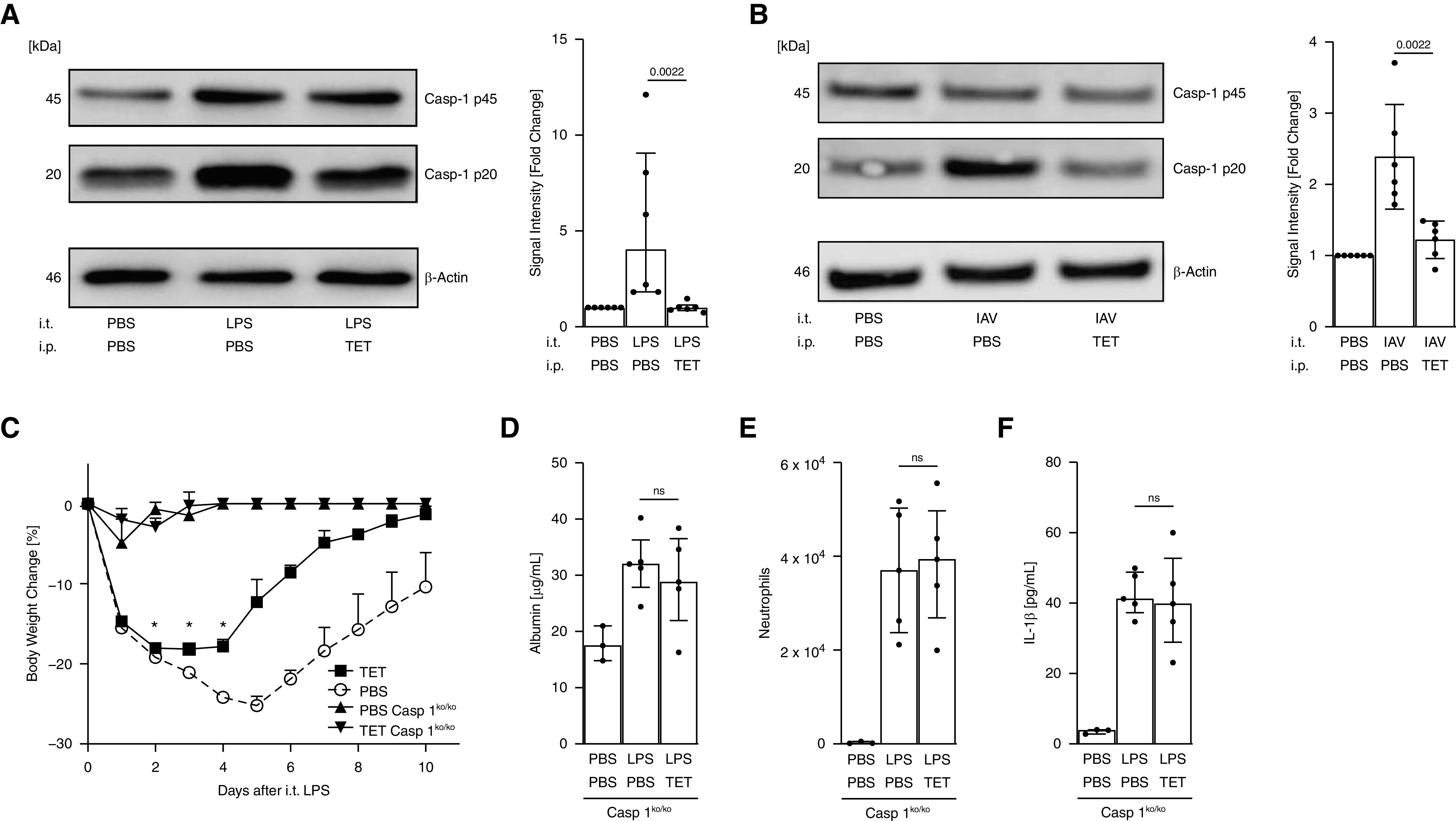
Protection against LPS- and influenza virus–induced acute respiratory distress syndrome by tetracycline (TET) is mediated via inhibition of caspase-1 (Casp-1). Wild-type (WT) C57BL/6J and Caspase-1–knockout (KO) mice were challenged with LPS or IAV as indicated and treated with TET as described in Figures 2 and 3. Lungs from WT mice were homogenized, and pro–Caspase-1 (p45) and a subunit of activated Caspase-1 (p20) were measured via immunoblot. (A and B) Representative blot of three independent experiments and quantification of signal intensity is shown. (C) Mice were evaluated daily for loss of body weight (n = 5 per group). BAL fluid of Caspase-1ko/ko mice was obtained 8 hours after LPS instillation and monitored for (D) albumin, (E) neutrophils, and (F) IL-1β via bicinchoninic acid assay, flow cytometry, and ELISA, respectively (n ≥ 3 per group). Median with interquartile range. *P < 0.05; Mann-Whitney Test. Bonferroni correction for repeated statistical tests (body weight change) and adjustment of significance level to *P < 0.005. IAV = influenza A virus; ns = not significant.
Tetracycline Reduces IL-1β and IL-18 Production by Alveolar Leukocytes Obtained from Patients with Direct ARDS
As described above, high concentrations of IL-1β and IL-18 characterize the BALF of patients with direct ARDS (Figure 1). To investigate the effect of tetracycline on both IL-1β and IL-18 production, alveolar leukocytes were isolated from patients within 24 hours of the onset of direct ARDS (see Table E2 for further patient characteristics). As previously reported, the leukocytes were primarily composed of neutrophils and macrophages (Figure 6A) (35, 36). These cells were cultured in the presence of tetracycline, and IL-1β and IL-18 concentration in the supernatant was monitored by ELISA after 16 hours.
Figure 6.
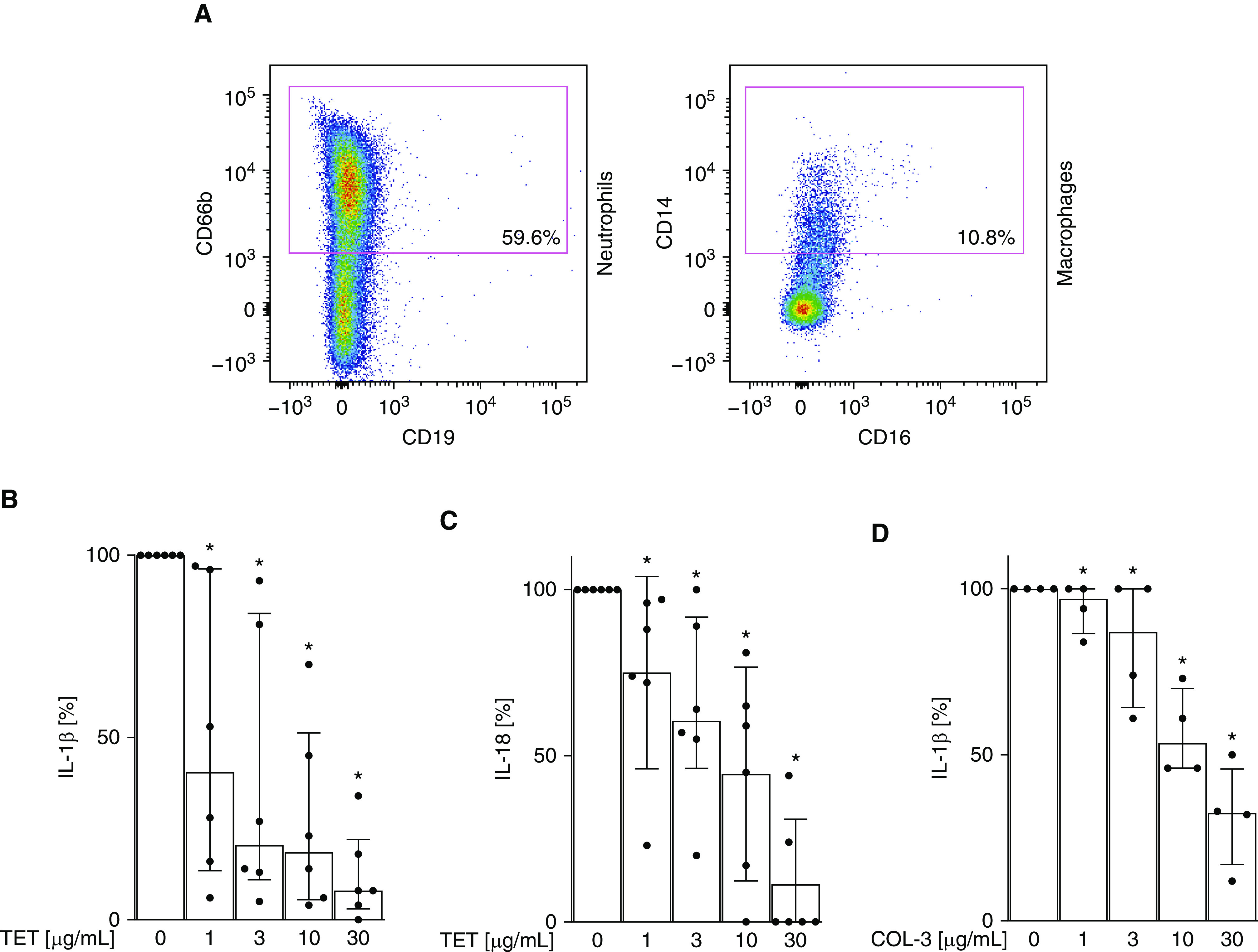
IL-1β expression by alveolar leukocytes from patients with direct acute respiratory distress syndrome (ARDS) is inhibited by tetracycline (TET). (A) BAL fluid from patients with direct ARDS was collected as described in Figure 1 and analyzed for neutrophils and macrophages via flow cytometry. Leukocytes were cultured and coincubated with increasing doses of (B and C) TET and (D) COL-3 (1, 3, 10, and 30 μg/ml) for 16 hours, and indicated cytokine production was quantified by ELISA. *P < 0.05 (vs. TET 0 μg/ml). Median with interquartile range of six patients; Mann-Whitney test.
When cultured ex vivo in the absence of further stimulation, these cells continued to produce IL-1β (308 ± 109 pg/ml) and IL-18 (34 ± 10 pg/ml), indicating that the inflammasome–capsase-1 pathway remained activated during culture. Tetracycline inhibited this production of both IL-1β and IL-18 in a dose-dependent manner (Figures 6B and 6C). Of note, the tetracycline derivate COL-3, which is antiinflammatory but manifests no antibacterial activity (37), also significantly suppressed IL-1β production (Figure 6D). These data suggest that tetracycline might be beneficial for the treatment of inflammation in patients with direct ARDS by inhibiting caspase-1–dependent IL-1β production in the lungs.
Discussion
In the present study, patients with direct ARDS possessed significant higher concentrations of inflammasome–caspase-1–dependent IL-1β and IL-18 in their lungs than individuals with indirect lung injury (Figure 1). Applying a precision medicine approach, we used two murine models of direct ALI to show that tetracycline significantly reduced pulmonary IL-1β and IL-18 concentrations, thereby improving lung injury and disease progression (Figures 2 and 3). The antiinflammatory effects of tetracycline were facilitated by blocking the inflammasome–caspase-1 pathway via direct inhibition of caspase-1 (Figures 4 and 5). As a step toward validating the clinical relevance of these findings, we show that tetracycline reduced the production of IL-1β and IL-18 in alveolar immune cells obtained from patients with direct ARDS ex vivo (Figure 6).
There is increasing evidence that ARDS is a heterogeneous syndrome (4, 38). By identifying molecular features that distinguish between ARDS subtypes, promising treatments for specific patient groups might be more easily identified (4, 39, 40). The current study is the first to document differential expression of IL-1β and IL-18 in the lungs of patients with direct versus indirect ARDS. Consistent with these findings, recent articles highlight the critical role of IL-1β and IL-18 in the direct form of human ARDS (6, 8). Even though evidence exists that NLRP3-deficient mice possess reduced amounts of injury-related proteins in the lung during abdominal sepsis (41), most studies show a benefit of inhibiting the inflammasome–caspase-1 pathway in murine models of direct ALI (6, 10, 42–44). Current findings suggest that patients with direct ARDS 1) are characterized by high concentrations of IL-1β and IL-18 and thus 2) might be more responsive to therapy targeting the inflammasome–caspase-1 pathway.
Most patients develop direct ARDS after exposure to pathogen-associated molecular patterns (such as LPS or RNA) released during bacterial and viral pneumonia (5). On the basis of our finding that IL-1β and IL-18 concentrations were increased in this subtype, the widely accepted LPS- and influenza-induced murine models of direct ALI were used to evaluate the effect of tetracycline on inflammasome–caspase-1–dependent cytokine production (10, 42, 44, 45). Consistent with our human data, both LPS and viral challenge resulted in the accumulation of IL-1β and IL-18 in the BALF of mice. Tetracycline treatment significantly reduced IL-1β and IL-18 concentrations and lung injury, thereby improving outcome. The tetracycline derivate COL-3 was previously evaluated in a model of indirect ALI (in which abdominal sepsis preceded lung disease) (46, 47). In those studies, COL-3 reduced plasma cytokine concentrations and improved lung histology. In contrast, COL-3 had no significant effect on outcome. These findings suggest that tetracycline (and its derivates) are selectively effective in direct ARDS, consistent with their ability to target caspase-1 activity (46, 47).
Caspase-1 activation is a major contributor in the development of ARDS (6, 10). A plethora of data, including current results, support a pivotal role for caspase-1 signaling in the development of ALI (6, 10, 42, 48). Current studies demonstrate that tetracycline 1) selectively inhibits the activation of caspase-1 and thus 2) has no effect on lung injury in caspase-1–deficient mice. This supports the conclusion that the inhibition of caspase-1 underlies the beneficial effects of tetracycline in the ALI models used in this study. Other groups examining LPS-, bleomycin-, or ventilation-induced pulmonary injury/inflammation similarly documented benefit from depleting inflammasome–caspase-1 signaling (6, 10, 42, 48). We found that tetracycline directly inhibits caspase-1, having no effect on upstream elements of that pathway in terms of the gene expression of NLRP3 or AIM2 or ASC speck formation. In contrast, previous studies in murine models of diabetic nephropathy and Huntington’s disease showed that the tetracycline derivate minocycline improved outcome by reducing gene expression of NLRP3 or caspase-1 (18, 49). Those studies investigated NLRP3 and caspase-1 expression after weeks of minocycline treatment, making it difficult to determine whether treatment had a direct or indirect effect (e.g., feedback loops via IL-1R–NF-ƙκB signaling) (50). Our finding that tetracycline directly blocks caspase-1 activity is supported by a model of diabetic retinopathy in which both tetracycline and minocycline inhibited caspase-1 activity within 96 hours of administration, although the effects of tetracycline on direct inflammasome activation and assembly were not investigated (25). Current studies further clarified the effect of tetracycline on caspase-1 by using recombinant caspase-1 to exclude tetracycline’s interference with the intersection between ASC and caspase-1. In summary, our data provide strong evidence that tetracycline selectively inhibits caspase-1 activation, thereby limiting pulmonary inflammation and lung injury.
To support the clinical relevance of the murine studies, we demonstrated that tetracycline also inhibited both IL-1β and IL-18 secretion by alveolar leukocytes obtained from patients with direct ARDS. The observation that leukocytes isolated from the BALF of patients with direct ARDS secreted inflammasome–caspase-1–dependent cytokines without any additional stimulus was in line with the finding of Jacobs and colleagues (8). In that work, alveolar macrophages from patients with ARDS were documented to spontaneously release more IL-1β than those from healthy control subjects. Because tetracycline possess antibacterial properties and some of the patients from whom alveolar leukocytes were isolated had bacterial pneumonia, the conclusion that tetracycline was acting as a direct immunomodulator was established by substituting COL-3, a tetracycline derivate with antiinflammatory, but not antibacterial, effects (46, 47).
In summary, this work demonstrates for the first time that 1) patients with direct ARDS have significantly higher IL-1β and IL-18 concentrations in their lungs than individuals with indirect ARDS, 2) tetracycline may be effective in the treatment of direct ARDS, and 3) tetracycline blocks the inflammasome–caspase-1 pathway by selective inhibition of caspase-1 (Figure 7). Tetracycline efficacy was supported by evidence that it inhibited inflammatory cytokine production by alveolar immune cells from patients with direct ARDS. In two relevant murine models, tetracycline reduced IL-1β and IL-18 concentrations, lung injury, and outcome by blocking inflammasome–caspase-1 pathway. Tetracycline thus warrants clinical evaluation as an immunomodulatory agent given its ability to inhibit caspase-1 and thereby interfere with the deleterious cascade of alveolar hyperinflammation in patients with direct ARDS.
Figure 7.
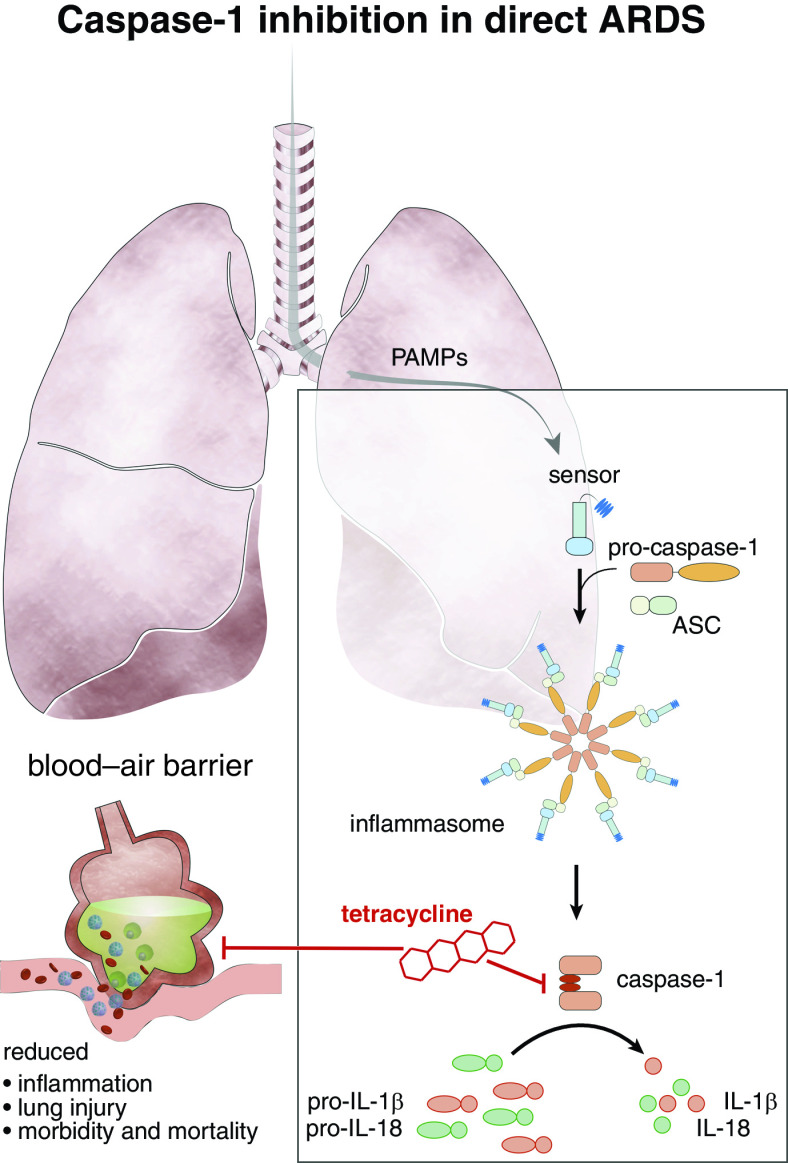
Proposed mechanism of acute respiratory distress syndrome (ARDS) treatment by tetracycline. Pathogen-associated molecular patterns (PAMPs) released during bacterial pneumonia activate pattern recognition receptors, stimulating assembly of an inflammasome complex with ASC (apoptosis-associated speck-like protein containing a CARD domain) oligomerization and caspase-1 activation. Pro–IL-1β/pro–IL-18 are cleaved by caspase-1 and secreted as active cytokines. These cause pulmonary inflammation and lung injury resulting in ARDS. Tetracycline selectively inhibits caspase-1 activation and subsequent IL-1β and IL-18 production. This reduces pulmonary inflammation, morbidity, and mortality.
Footnotes
Supported by grants and fellowships from the University of Bonn and the B. Braun Foundation.
Author Contributions: K.P., F.S., and C.B. conceived and designed the study. K.P., M.F., S.S., C.F., H.W., B.M.K., B.S., and C.B. acquired data. K.P., S.F., C.P., S.D., T.W., E.L., D.K., C.W., F.S., and C.B. analyzed and interpreted the data. K.P., F.S., and C.B. drafted the manuscript. All authors made significant contributions to the final manuscript and approve its submission.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202005-1916OC on March 24, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Meyer NJ, Calfee CS. Novel translational approaches to the search for precision therapies for acute respiratory distress syndrome. Lancet Respir Med. 2017;5:512–523. doi: 10.1016/S2213-2600(17)30187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3. Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147:1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25:12–20. doi: 10.1097/MCC.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Englert JA, Bobba C, Baron RM. Integrating molecular pathogenesis and clinical translation in sepsis-induced acute respiratory distress syndrome. JCI Insight. 2019;4:e124061. doi: 10.1172/jci.insight.124061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pugin J, Ricou B, Steinberg KP, Suter PM, Martin TR. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Respir Crit Care Med. 1996;153:1850–1856. doi: 10.1164/ajrccm.153.6.8665045. [DOI] [PubMed] [Google Scholar]
- 8. Jacobs RF, Tabor DR, Burks AW, Campbell GD. Elevated interleukin-1 release by human alveolar macrophages during the adult respiratory distress syndrome. Am Rev Respir Dis. 1989;140:1686–1692. doi: 10.1164/ajrccm/140.6.1686. [DOI] [PubMed] [Google Scholar]
- 9. Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015;194:855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV, et al. Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol. 2014;192:5974–5983. doi: 10.4049/jimmunol.1400368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS: persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 12. Makabe H, Kojika M, Takahashi G, Matsumoto N, Shibata S, Suzuki Y, et al. Interleukin-18 levels reflect the long-term prognosis of acute lung injury and acute respiratory distress syndrome. J Anesth. 2012;26:658–663. doi: 10.1007/s00540-012-1409-3. [DOI] [PubMed] [Google Scholar]
- 13. Rogers AJ, Guan J, Trtchounian A, Hunninghake GM, Kaimal R, Desai M, et al. Association of elevated plasma interleukin-18 level with increased mortality in a clinical trial of statin treatment for acute respiratory distress syndrome. Crit Care Med. 2019;47:1089–1096. doi: 10.1097/CCM.0000000000003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sinha P, Calfee CS. Inflammasomes assemble: cytoplasmic guardians or fallen heroes? Crit Care Med. 2019;47:1161–1163. doi: 10.1097/CCM.0000000000003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Nardo D, De Nardo CM, Latz E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am J Pathol. 2014;184:42–54. doi: 10.1016/j.ajpath.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Metz LM, Li DKB, Traboulsee AL, Duquette P, Eliasziw M, Cerchiaro G, et al. Minocycline in MS Study Team. Trial of minocycline in a clinically isolated syndrome of multiple sclerosis. N Engl J Med. 2017;376:2122–2133. doi: 10.1056/NEJMoa1608889. [DOI] [PubMed] [Google Scholar]
- 18. Shahzad K, Bock F, Al-Dabet MM, Gadi I, Nazir S, Wang H, et al. Stabilization of endogenous Nrf2 by minocycline protects against Nlrp3-inflammasome induced diabetic nephropathy. Sci Rep. 2016;6:34228. doi: 10.1038/srep34228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krakauer T, Buckley M. Doxycycline is anti-inflammatory and inhibits staphylococcal exotoxin-induced cytokines and chemokines. Antimicrob Agents Chemother. 2003;47:3630–3633. doi: 10.1128/AAC.47.11.3630-3633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bode C, Diedrich B, Muenster S, Hentschel V, Weisheit C, Rommelsheim K, et al. Antibiotics regulate the immune response in both presence and absence of lipopolysaccharide through modulation of Toll-like receptors, cytokine production and phagocytosis in vitro. Int Immunopharmacol. 2014;18:27–34. doi: 10.1016/j.intimp.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 21. Garrido-Mesa J, Algieri F, Rodríguez-Nogales A, Vezza T, Utrilla MP, Garcia F, et al. Immunomodulatory tetracyclines ameliorate DNBS-colitis: impact on microRNA expression and microbiota composition. Biochem Pharmacol. 2018;155:524–536. doi: 10.1016/j.bcp.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 22. Lu Y, Xiao G, Luo W. Minocycline suppresses NLRP3 inflammasome activation in experimental ischemic stroke. Neuroimmunomodulation. 2016;23:230–238. doi: 10.1159/000452172. [DOI] [PubMed] [Google Scholar]
- 23. Chen W, Zhao M, Zhao S, Lu Q, Ni L, Zou C, et al. Activation of the TXNIP/NLRP3 inflammasome pathway contributes to inflammation in diabetic retinopathy: a novel inhibitory effect of minocycline. Inflamm Res. 2017;66:157–166. doi: 10.1007/s00011-016-1002-6. [DOI] [PubMed] [Google Scholar]
- 24. Garcez ML, Mina F, Bellettini-Santos T, da Luz AP, Schiavo GL, Macieski JMC, et al. The involvement of NLRP3 on the effects of minocycline in an AD-like pathology induced by β-amyloid oligomers administered to mice. Mol Neurobiol. 2019;56:2606–2617. doi: 10.1007/s12035-018-1211-9. [DOI] [PubMed] [Google Scholar]
- 25. Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56:224–230. doi: 10.2337/db06-0427. [DOI] [PubMed] [Google Scholar]
- 26. Hendrickson CM, Calfee CS. A new frontier in ARDS trials: phenotyping before randomisation. Lancet Respir Med. 2019;7:830–831. doi: 10.1016/S2213-2600(19)30175-4. [DOI] [PubMed] [Google Scholar]
- 27. Bode C, Peukert K, Schewe J-C, Putensen C, Latz E, Steinhagen F. Tetracycline alleviates acute lung injury by inhibition of NLRP3 inflammasome. Eur Respir J. 2019;54:PA2175. [Google Scholar]
- 28. Bode C, Kinjo T, Alvord WG, Klinman DM. Suppressive oligodeoxynucleotides reduce lung cancer susceptibility in mice with silicosis. Carcinogenesis. 2014;35:1078–1083. doi: 10.1093/carcin/bgu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nardo CMD, Latz E.The inflammasome: methods and protocols. Totowa, NJ: Humana Press; 2013. [Google Scholar]
- 31. Bode C, Fox M, Tewary P, Steinhagen A, Ellerkmann RK, Klinman D, et al. Human plasmacytoid dentritic cells elicit a type I interferon response by sensing DNA via the cGAS-STING signaling pathway. Eur J Immunol. 2016;46:1615–1621. doi: 10.1002/eji.201546113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Primiano MJ, Lefker BA, Bowman MR, Bree AG, Hubeau C, Bonin PD, et al. Efficacy and pharmacology of the NLRP3 inflammasome inhibitor CP-456,773 (CRID3) in murine models of dermal and pulmonary inflammation. J Immunol. 2016;197:2421–2433. doi: 10.4049/jimmunol.1600035. [DOI] [PubMed] [Google Scholar]
- 33. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 34. Wrigge H, Uhlig U, Zinserling J, Behrends-Callsen E, Ottersbach G, Fischer M, et al. The effects of different ventilatory settings on pulmonary and systemic inflammatory responses during major surgery. Anesth Analg. 2004;98:775–781. doi: 10.1213/01.ane.0000100663.11852.bf. [DOI] [PubMed] [Google Scholar]
- 35. Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306:L709–L725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barr LC, Brittan M, Morris AC, McAuley DF, McCormack C, Fletcher AM, et al. A randomized controlled trial of peripheral blood mononuclear cell depletion in experimental human lung inflammation. Am J Respir Crit Care Med. 2013;188:449–455. doi: 10.1164/rccm.201212-2334OC. [DOI] [PubMed] [Google Scholar]
- 37. Islam MM, Franco CD, Courtman DW, Bendeck MP. A nonantibiotic chemically modified tetracycline (CMT-3) inhibits intimal thickening. Am J Pathol. 2003;163:1557–1566. doi: 10.1016/S0002-9440(10)63512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reilly JP, Calfee CS, Christie JD. Acute respiratory distress syndrome phenotypes. Semin Respir Crit Care Med. 2019;40:19–30. doi: 10.1055/s-0039-1684049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bos LDJ, Scicluna BP, Ong DSY, Cremer O, van der Poll T, Schultz MJ. Understanding heterogeneity in biologic phenotypes of acute respiratory distress syndrome by leukocyte expression profiles. Am J Respir Crit Care Med. 2019;200:42–50. doi: 10.1164/rccm.201809-1808OC. [DOI] [PubMed] [Google Scholar]
- 40. Luo L, Shaver CM, Zhao Z, Koyama T, Calfee CS, Bastarache JA, et al. Clinical predictors of hospital mortality differ between direct and indirect ARDS. Chest. 2017;151:755–763. doi: 10.1016/j.chest.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee S, Nakahira K, Dalli J, Siempos II, Norris PC, Colas RA, et al. NLRP3 inflammasome deficiency protects against microbial sepsis via increased lipoxin B4 synthesis. Am J Respir Crit Care Med. 2017;196:713–726. doi: 10.1164/rccm.201604-0892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jones HD, Crother TR, Gonzalez-Villalobos RA, Jupelli M, Chen S, Dagvadorj J, et al. The NLRP3 inflammasome is required for the development of hypoxemia in LPS/mechanical ventilation acute lung injury. Am J Respir Cell Mol Biol. 2014;50:270–280. doi: 10.1165/rcmb.2013-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dong L, Zhu Y-H, Liu D-X, Li J, Zhao P-C, Zhong Y-P, et al. Intranasal application of budesonide attenuates lipopolysaccharide-induced acute lung injury by suppressing nucleotide-binding oligomerization domain-like receptor family, pyrin domain-containing 3 inflammasome activation in mice. J Immunol Res. 2019;2019:7264383. doi: 10.1155/2019/7264383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu T, Zhou Y, Li P, Duan J-X, Liu Y-P, Sun G-Y, et al. Blocking triggering receptor expressed on myeloid cells-1 attenuates lipopolysaccharide-induced acute lung injury via inhibiting NLRP3 inflammasome activation. Sci Rep. 2016;6:39473. doi: 10.1038/srep39473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kurundkar D, Kurundkar AR, Bone NB, Becker EJ, Jr, Liu W, Chacko B, et al. SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight. 2019;4:e120722. doi: 10.1172/jci.insight.120722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roy SK, Kubiak BD, Albert SP, Vieau CJ, Gatto L, Golub L, et al. Chemically modified tetracycline 3 prevents acute respiratory distress syndrome in a porcine model of sepsis + ischemia/reperfusion-induced lung injury. Shock. 2012;37:424–432. doi: 10.1097/SHK.0b013e318245f2f9. [DOI] [PubMed] [Google Scholar]
- 47. Steinberg J, Halter J, Schiller H, Gatto L, Carney D, Lee H-M, et al. Chemically modified tetracycline prevents the development of septic shock and acute respiratory distress syndrome in a clinically applicable porcine model. Shock. 2005;24:348–356. doi: 10.1097/01.shk.0000180619.06317.2c. [DOI] [PubMed] [Google Scholar]
- 48. Hoshino T, Okamoto M, Sakazaki Y, Kato S, Young HA, Aizawa H. Role of proinflammatory cytokines IL-18 and IL-1beta in bleomycin-induced lung injury in humans and mice. Am J Respir Cell Mol Biol. 2009;41:661–670. doi: 10.1165/rcmb.2008-0182OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 50. Bogdanov AA, Jr, Gordeeva LV, Torchilin VP, Margolis LB. Lectin-bearing liposomes: differential binding to normal and to transformed mouse fibroblasts. Exp Cell Res. 1989;181:362–374. doi: 10.1016/0014-4827(89)90094-3. [DOI] [PubMed] [Google Scholar]