In 2020, the coronavirus disease (COVID-19) pandemic led to a surge in submissions to respiratory and critical care journals, but the quality of manuscripts submitted to the American Thoracic Society family of journals on chronic obstructive pulmonary disease (COPD) did not diminish. In this yearly update, we summarize the progress made in 2020 to the understanding of the pathogenesis and clinical aspects of COPD and discuss how this knowledge could improve patient care.
Diagnosis
Diagnostic techniques that avoid aerosol generation have become highly relevant. Ruparel and colleagues (1) analyzed data from the Lung Screen Update Trial. Of the 986 participants, 560 (57%) met criteria for airflow obstruction and 67% did not have a prior diagnosis of COPD. In the undiagnosed participants, 68% had evidence of emphysema on computed tomography (CT) scans, suggesting that lung cancer screening may be a useful opportunity for the detection of COPD (1, 2).
Diagnostic improvements will likely accrue from better application of existing methods. In an analysis of 15,308 prebronchodilator spirometric assessments with same-day lung volume assessments, Ioachimescu and colleagues (3) computed the area under the expiratory flow-volume curve and found by using neural network models that the square root of the area under the expiratory flow-volume curve in combination with traditional spirometric measurements successfully distinguished among obstructive, restrictive, mixed, and normal patterns with a less than 9% rate of misclassification. This machine learning approach may reduce the need for lung volume testing to differentiate these lung function patterns.
Environmental Factors
The known risk factors for COPD do not fully explain the variance in its prevalence, and several studies in 2020 advanced our understanding of risk factors for COPD. In a secondary analysis of the MOSES (Multicenter Ozone Study of Older Subjects) trial, Rich and colleagues (4) examined whether personal exposures to indoor or outdoor air pollution in the prior 72 hours potentiated the response to ozone exposure. They found that prior medium and high exposure to ambient nitrogen dioxide (NO2) and carbon monoxide (CO) as well as indoor exposure to NO2 were associated with a lower FEV1 and FVC.
Alternative Exposures That Drive COPD Pathophysiology
The classic risk factor for the development of COPD is cigarette smoke (CS) exposure, which also has an impact on the long, noncoding transcriptome, which is implicated in airway inflammation regulation (5); however, biomass smoke exposure (6) and electronic nicotine delivery systems may contribute (7).
Little is known about the mechanisms underlying responses to biomass smoke, especially in a real-world setting. Kc and colleagues (8) examined indoor air pollutant exposures during cooking in 103 households in four villages in rural Nepal. They compared the use of traditional cook stoves, improved cook stoves, and liquefied petroleum gas, showing that use of improved cook stoves and liquefied petroleum gas resulted in substantially lower exposures of 51% and 80%, respectively, to particulate matter ⩽2.5 μm in aerodynamic diameter and of 72% and 86%, respectively, to CO. Human lung tissues were stimulated with different biomass extracts and showed release of proinflammatory cytokines, demonstrating that Nepalese biomass smoke samples have proinflammatory effects in human lung tissue (8).
Although cigarette smoker numbers are falling in the United States, cigar and cigarillo smoking are increasingly popular among students (9). The differences between these two tobacco products have not been well studied. Abdelwahab and colleagues (10) used epithelial air–liquid interface (ALI) cultures and in vivo mouse models to compare the effects of cigarillo smoke and CS. CS and cigarillo smoke similarly affected the apoptosis and transepithelial electrical resistance of ALI cultures, and the proteomic profile of both ALI culture media and BAL fluid (BALF) from exposed mice showed that cigarillo smoke exposure exerted effects similar to those of CS exposure.
There are many unknowns about the safety of electronic cigarettes (11). Epithelial cells treated with electronic nicotine delivery system aerosols led to substantial cell death and reduced transepithelial electrical resistance, with the addition of nicotine aggravating cell death further (12). Macrophages treated with electronic nicotine delivery system aerosols had reduced phagocytic capacity and underwent pyroptosis (12). These data suggest that these products are not safe, and large-scale studies are required (13).
Diet may have a modulatory effect on inflammation in COPD. Lemoine and colleagues (14) found that higher total omega-3 fatty acid intake was associated with better respiratory quality of life and a lower risk of severe exacerbation in the prior 3 months (odds ratio [OR], 0.08; 95% confidence interval [CI], 0.01–0.73). In contrast, increased omega-6 fatty acid intake was associated with worse respiratory quality of life and dyspnea, a lower FEV1% predicted, and a higher risk of having any exacerbations (OR, 1.3; 95% CI, 1.03–1.64) or severe exacerbations (OR, 1.48; 95% CI, 1.05–2.09) (14).
Residence in rural areas in the United States may be associated with a differential risk for COPD compared with urban residence. Raju and colleagues (15) evaluated 8,500 adults from the 2007–2012 National Health and Nutrition Examination Survey and found that rural areas had a higher prevalence of COPD than urban areas (12.0% vs. 5.9%) and that those living in rural areas had higher odds of reporting respiratory symptoms than urban dwellers.
Lung Function
Several studies examining lung function decline shed light on a number of early- and midlife exposures that confer a risk for accelerated disease progression. Bui and colleagues (16) examined lung function change in 857 participants who were between 45 and 53 years old in the Tasmanian Longitudinal Health Study. They found that adult exposures, including cigarette smoking, asthma, occupational exposures, and living <200 m from traffic, were associated with accelerated FEV1 decline. They also reported that a number of early-life and genetic factors modified the effects of these adult-life exposures. The effect of personal smoking on FEV1 decline was heightened by maternal smoking, and the effects of smoking and occupational exposures on FEV1 decline were accentuated by the presence of a lower childhood FEV1 and the presence of the GSTM1-null allele (16). Controlling for early- and adult-life confounders, including childhood infections, lung function, personal and parental smoking, weight, social class, and hormone replacement therapy, the results from the Tasmanian Longitudinal Health Study cohort also indicate that early menopause (age <45 yr) was associated with a lower postbronchodilator FEV1 (−168 ml; 95% CI, −273 to −63 ml) and FVC (−186 ml; 95% CI, −302 to −70 ml) than menopause at a later age (17, 18).
The clinical significance of the discordance that exists in individuals classified as having lung function lower than the lower limit of normal (LLN) but who have a FEV1/FVC ratio greater than 0.7 remains controversial. A study of 24,000 people (19) found that hospitalization and death were more common in this discordant group. The recent data reported by Neder and colleagues (20) from a general population sample included 715 tobacco-exposed people, 323 of whom had normal values as determined by the LLN and fixed definitions, 134 of whom had discordant values, and 258 in whom both metrics were abnormal. As expected, the individuals with discordance were older, more likely to be male, and reported more cardiovascular comorbidity than the healthy, concordant group, indicating that an abnormal fixed ratio identifies people at risk of both physiologic and clinical problems.
Progression and the Detection of Early COPD
Lung function trajectories remain central to understanding the development of COPD, a diagnosis indicated by a faster FEV1 decline from a normal peak value or a normal decline from a lower peak value (21). By using longitudinal data from 1,170 individuals enrolled during the 1970s and 1980s in the Copenhagen City Heart study, Marott and colleagues (22) explored trajectories by which COPD might develop and how this influences exacerbation risk and mortality. They classified 144 individuals who had developed COPD by 2003 according to whether their preceding trajectory was one of decline from normal or one of having a low initial FEV1 and found no impact on the subsequent severe exacerbation risk. However, they did link rapid decline from a normal FEV1 to higher subsequent nonmalignant respiratory mortality and all-cause mortality relative to those individuals with an initially lower FEV1. Their findings show that how an individual develops COPD has prognostic implications.
Identifying COPD at an early stage remains a prized goal in COPD research. The Journal has published a definition of early COPD, but its impact remained unclear (23). Within the Copenhagen General Population Study, Çolak and colleagues (24) identified 8,064 individuals younger than 50 years with a 10–pack-year smoking history. Of these, 15% met the early COPD criteria (23) because their FEV1/FVC ratio was less than the LLN (24). They were already more symptomatic and showed a heightened risk of acute respiratory hospitalizations and death during 14.4 years of follow-up (24). These results highlight a need to identify early interventions (25).
Young and colleagues (26) used computational modeling to analyze biomarkers derived from CT imaging of the COPDGene (COPD Genetic Epidemiology) and ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) cohorts. They propose two patterns of disease progression (see Figure 1 in Reference 26): small airway and emphysema changes followed by large airway changes versus the reverse pattern. Serial imaging to monitor progression is problematic in COPD, so the authors used cross-sectional image data, and by automatically assigning individuals to their most probable subtype and stage, they developed an analytical approach that might aid our understanding of how COPD heterogeneity develops (27).
Figure 1.
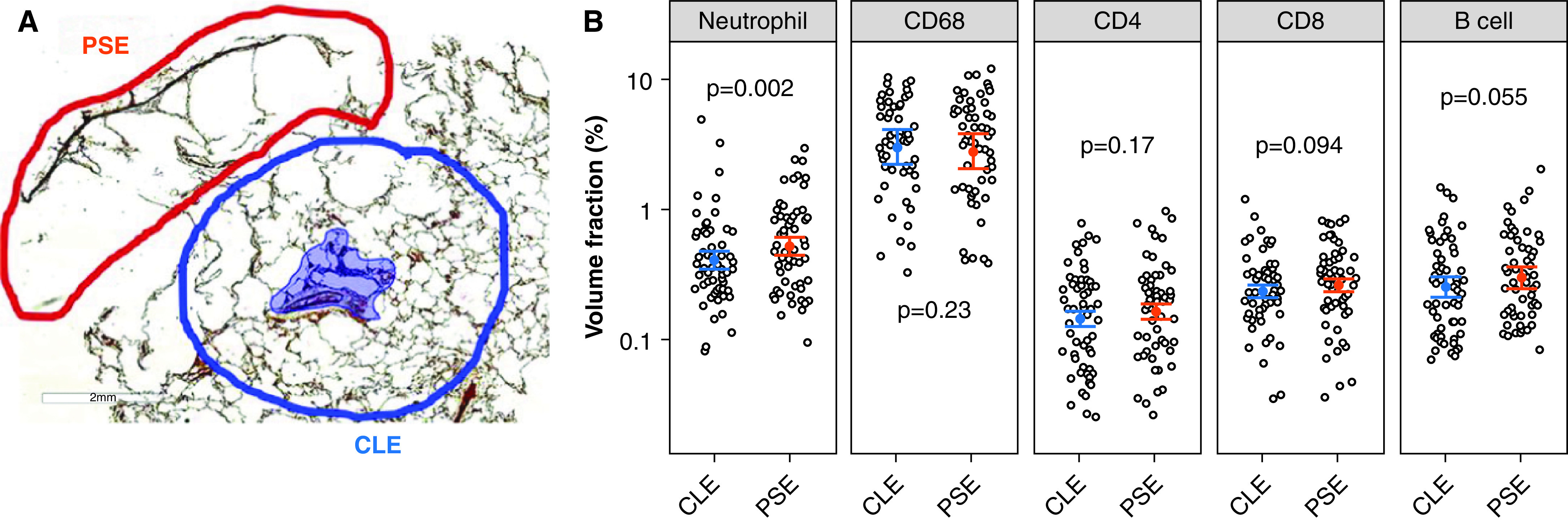
Infiltration of immune cells in centrilobular emphysema (CLE) and paraseptal emphysema (PSE) regions. (A) The regions of PSE (encircled with red line) and CLE (encircled with blue line) were manually traced on each of 60 digital images of histologic sections. Areas of airways and blood vessels were traced and excluded from the analysis (shaded blue area). The volume fractions of lung parenchyma occupied by immune-cell subtypes were calculated for PSE and CLE. (B) Data were expressed as least square means ± SEs on the basis of the linear mixed-effects model that accounted for multiple regions per core and multiple cores per subject. Reprinted by permission from Reference 60.
Genetic Studies
There are large differences in individual susceptibility to developing COPD, as only a quarter of tobacco smokers develop fixed airflow obstruction (28), whereas 25–40% of patients with COPD have never smoked (6).
Alpha-1 antitrypsin deficiency (AATD) is caused by mutations in the SERPINA1 gene and can lead to emphysema. Matamala and colleagues (29) described two novel in cis variants of the SERPINA1 gene that increase cellular retention and decrease secretion of alpha-1 antitrypsin into the bloodstream. These findings would not normally be identified by using conventional testing and suggest that complete sequencing of the SERPINA1 gene should be recommended for accurate diagnosis (30).
In their general population study, de Vries and colleagues (31) investigated novel rare genetic variants associated with airflow obstruction. They discovered seven rare variants present in the SERPINA1, CMYA5, OPA3, SUZ12P1, LRP5, KIF27, and TMC4 genes that were more frequent in individuals with airflow limitation.
Another genetic study in 2020 was that by Ortega and colleagues (32), who examined the effects of rare SERPINA1 variants on lung function and emphysema. They used the well-characterized SPIROMICS (Subpopulations and Intermediate Outcomes Measures in COPD Study) cohort and selected 2,168 subjects with a substantial 20–pack-year smoking history. Although the risk of lung disease in ZZ homozygotes in AATD is clearly established, this study showed the importance of deep gene sequencing in combination with measuring alpha-1 antitrypsin concentrations, as those individuals with additional, rare, non-Z SERPINA1 variants of the Z allele had poorer lung function.
These studies are pertinent, given that AATD remains the most common monogenic risk factor for COPD but is not wholly understood. For example, the significance of the SZ genotype is debated, primarily because of the fact that no previous comparison of individuals with the SZ genotype with a normal-risk population existed until Molloy and colleagues (33) addressed this in a comparison of an SZ cohort with normal-risk control subjects (genotypes MM and MS). They scrutinized the utility of using alpha-1 antitrypsin concentrations within the SZ genotype to determine risk and concluded that unlike in the ZZ genotype, the minimal smokers and never-smokers had lung function similar to that of control subjects and thus that the SZ genotype is not an independent risk factor for developing COPD. This has implications for better targeting in alpha-1 antitrypsin replacement therapy (34).
Mechanisms of Disease
Development of new diagnostics and treatments for COPD requires understanding the underlying mechanisms driving disease pathophysiology. In the past year, these have been examined by using both in vivo and in vitro models and human primary cells and tissues, with some notable advances being highlighted.
Understanding the Emphysematous Phenotype
Recently, Henkel and colleagues (35) examined the role of a glycoprotein, FSTL-1 (follistatin-like 1) in emphysema. They examined FSTL-1–partial-knockout mice who developed spontaneous emphysema with altered immunoregulatory gene expression mediated by reduced macrophage Nr4a1/Nur77, an inhibitor of NF-κB signaling (35, 36). This may be relevant in COPD, as SNPs in the FSTL-1 locus associate with lung function decline. Although promising, further work is needed to elucidate FSTL-1/Nr4a1/NF-κB signaling in human emphysema (37).
The protease–antiprotease balance may regulate the development of emphysema, and Uemasu and colleagues (38) investigated a murine model in which knockdown of the transcription factor C/EBPa (CCAAT/enhancer binding protein) increased protease activity and reduced expression of anti–serine proteases in CS-exposed mice. C/EBPa expression was also reduced in emphysematous human lung samples. However, more work is needed to translate this to disease (39).
Chronic CS exposure causes DNA damage and apoptosis in lung epithelial cells. Alveolar type 2 (AT2) cells act as alveolar stem cells, and the effect of CS on these was investigated by Tsutsumi and colleagues (40). Histologic assessment showed expected CS-induced emphysematous changes but also increased numbers of AT2 cells. Microarray analysis showed changes in circadian clock genes as well as antiapoptotic and proproliferation transcription factors (40). However, this was a short smoke exposure model, and whether increased AT2 cells leads to stem-cell exhaustion and/or AT2 senescence in chronic disease remains unclear.
Endothelial injury may also induce emphysema, but the mechanisms involved are unknown. Adini and colleagues (41) examined the role of PR1P (prominin-1–derived peptide), a novel synthetic peptide that increases VEGF (vascular endothelial growth factor) binding to endothelial cells. By using a computer simulation, together with in vitro and in vivo studies, they found that PR1P upregulated endogenous VEGF receptor 2 signaling (41), reducing endothelial cell apoptosis in vitro and in vivo (41). The endothelium was also examined by Pasupneti and colleagues (42), who showed that human emphysematous lungs exhibit decreased expression of HIF-2α (hypoxia-inducible factor 2α). Furthermore, endothelium-specific HIF-2α overexpression protected against the development of emphysema in a CS exposure mouse model, suggesting that decreased HIF-2α may contribute to emphysema (42). However, the mechanism of this process remains unclear, and further understanding of the global consequences of HIF-2α loss in the lung is required (43).
Antioxidants protect the lung against oxidative stress (44). Dabo and colleagues (45) examined how GPX-1 (glutathione peroxidase 1) is lost in COPD and showed that the kinase c-Src decreases GPX-1 mRNA, reducing expression of this regulator of antioxidant proteins. They also showed increased c-Src activity in COPD epithelial cells (45), further supporting that targeting c-Src pathways could elevate antioxidant capacity.
Iron dysregulation has been linked to COPD, with both iron overload and anemia being associated with disease (46). Sato and colleagues (47) fed mice an iron-deficient diet and then exposed them to CS and showed increased immune-cell responses and more severe emphysema. They suggest that iron supplementation may prevent disease progression. However, the role of iron in COPD remains controversial (48).
Elastin fibers are cleaved by proteases in the lung and act as chemoattractants (49). Mehraban and colleagues (50) examined a Syrian hamster novel elastase–LPS model that produced emphysema with prominent fragmentation of elastin fibers. Naive mice were treated with elastin fibers and showed increased immune-cell infiltrates in BALF. Leukocytes were highly chemotactic for elastin peptides in vitro (50). Targeting fragmented elastin fibers, possibly by removal or by inhibiting their activity, could reduce pulmonary leukocyte infiltration (49, 51).
Identification of Novel Immune-Cell Characteristics in COPD
Multiparameter immunophenotypic characterization of immune cells in BALF from patients with COPD is challenging because of autofluorescence and cell diversity. To overcome this, Vasudevan and colleagues (52) used time of flight mass cytometry to immunophenotype myeloid cells in COPD. By using an optimized experimental procedure, the authors identified COPD macrophages with reduced expression of PDL1 (programmed death ligand 1) and PDL2, as well as AXL, a receptor important in efferocytosis (53). This powerful technique may further the identification of novel cellular subsets in COPD lung and possible therapeutic targets.
COPD macrophages exhibit lower phagocytic and efferocytic activity (54), which may be associated with altered metabolic activity. O’Beirne and colleagues (55) examined this by looking at the mitochondria-related transcriptomic profile of alveolar macrophages from nonsmokers, smokers, and patients with COPD (55, 56). In COPD, dysregulated pathways associated with mitochondrial biogenesis and fission as well as pyruvate metabolism were seen. Mitochondrial function was stunted in both smoker and COPD macrophages; however, the former could compensate by increasing glycolytic capacity. However, this was lost in COPD macrophages (55), suggestive of an intrinsic mitochondrial defect in COPD alveolar macrophages.
Tertiary lymphoid organs (TLOs) are organized accumulations of T and B cells that resemble secondary lymphoid organs that are located at sites of chronic inflammation, including in patients with severe COPD (57). Naessens and colleagues (58) used single-cell RNA sequencing on HLA-DR1 cells from human lungs to understand the mechanisms in COPD. They identified lung EBI2+ (Epstein-Barr virus–induced gene 2–positive) OX-40L–expressing type 2 c-dendritic cells that induced IL-21+ follicular T-helper–like cells, suggesting involvement in TLO formation (58). To fully understand the role of dendritic cells and TLOs in COPD, the phenotype and origin of the type 2 c-dendritic cells in COPD are needed (59).
Imaging
Multi–detector row CT has been increasingly used to identify paraseptal and centrilobular emphysema. Although significant loss of the terminal bronchioles and the activation of the immune response is involved in the pathogenesis of centrilobular emphysema, Tanabe and colleagues (60) showed that the terminal bronchioles are relatively preserved, whereas neutrophil infiltration is increased, in paraseptal emphysema–dominant regions compared with centrilobular emphysema–dominant regions (Figure 1 [reprint of Figure 6 in Reference 60]). These findings suggest that distinct destructive processes are involved in the pathogenesis of paraseptal emphysema and might explain why clinical manifestations are less severe in paraseptal emphysema than in centrilobular emphysema.
The CT airway count is a visualization and quantification of the medium-sized airways and is a radiologic biomarker associated with FEV1 decline (61). It suggests the pathologic process responsible for terminal bronchiole reduction or remodeling may extend into the larger airways so that it can be detected using CT. This study from Kirby and colleagues (61) demonstrates an association between the CT airway count and both the number of terminal bronchioles and the distortion or remodeling in those structures that remain. There have also been developments in poly(ethylene glycol)–based hydrogel platforms for the study of encapsulation of precision-cut lung slices (62).
A key pathologic feature of COPD is lung hyperinflation because it results in impaired diaphragm function and dyspnea. Ultrasound was used to measure the thickening fraction of the diaphragm during tidal breathing and maximum volitional effort in 100 subjects (80 patients with COPD and 20 healthy control subjects). Patients had an increased diaphragm workload (tidal thickening fraction; P = 0.002), impaired diaphragm function (as assessed by using the Müller maneuver; P < 0.01), and reduced force reserve compared with healthy subjects (63). These findings worsened in line with Global Initiative for Chronic Obstructive Lung Disease stage severity and were correlated with dyspnea, airflow obstruction, and inspiratory capacity, indicating that ultrasound assessment of the diaphragm provides important functional information.
Exacerbations
The management of acute exacerbations of COPD (AECOPD) has not changed radically in several decades, but there have been many improvements in overall care and systems. By analyzing 31,991 admissions for AECOPD from 2005 to 2017 in Australia and New Zealand, Berenyi and colleagues (64) found that the admission rate per population of 1 million increased annually by 4.5% (95% CI, 3.7–5.3%) but that the proportion of ICU admissions with AECOPD for each 10,000 admissions decreased at an annual rate of 2.0% (95% CI, 0.8–3.2%), indicating an overall increase in hospital practices that mainly benefited other conditions. They also found an annual reduction in the odds of death during hospitalization (0.94; 95% CI, 0.93–0.95), a finding not seen with asthma (64, 65).
Insurance coverage can significantly impact exacerbation rates. In a study of 2,137 participants with COPD in the 2011–2017 National Health Interview Survey, Gaffney and colleagues (66) compared those who were enrolled in a high-deductible health plan with those enrolled in a traditional health plan. The authors found that delayed care, medication nonadherence due to high cost, and financial strain were more commonly reported in those with a high-deductible health plan. This group had more out-of-pocket healthcare expenses as well as a higher frequency of emergency room visits or hospitalizations (66, 67).
The impact of financial penalties to reduce COPD readmissions continues to be evaluated. To examine the effects of the financial penalties for non-COPD conditions such as congestive heart failure, myocardial infarction, and pneumonia on COPD admissions, Myers and colleagues (68) evaluated 805,764 hospitalizations for COPD and examined 30-day readmissions after an index COPD hospitalization. They found that compared with the preimplementation phase (2006–2012), the postimplementation phase (2013–2015) of penalties for non-COPD conditions was associated with a decrease in both all-cause readmissions (level change, −0.93%; 95% CI, −1.44 to −0.43) and COPD-related readmissions (level decrease, −0.52%; 95% CI, −0.93 to −0.12). Thus, the changes for other diseases as well as preemptive measures taken for COPD may have explained the reduced readmission results (68, 69).
Predicting exacerbations, especially severe exacerbations requiring hospitalization, may provide an opportunity for intervention. Tavakoli and colleagues (70) examined 6 months of data from 222,219 patients with COPD in British Columbia’s administrative health databases (1997–2016). They aimed to predict hospitalization in the next 2 months. Compared with using the area under the receiver operating characteristic curve for a model that solely relied on exacerbation history (0.68; 95% CI, 0.67–0.70), using gradient-boosting algorithms resulted in a well-calibrated model with an area under the receiver operating characteristic curve of 0.82 (95% CI, 0.80–0.83) (70, 71).
COPD exacerbations are prone to nonrecovery (72). Ritchie and colleagues (73) provide the first data on the effectiveness of antibiotic retreatment for these prolonged events. In a multicenter, double-blind, randomized, placebo-controlled trial, they examined retreatment with ciprofloxacin at Day 14 in patients with persistent symptoms or a raised C-reactive protein concentration (73). Patients derived no additional benefit from ciprofloxacin compared with placebo. This suggests that nonrecovered exacerbations, which may lead to hospital readmission, are not driven by an ongoing active bacterial infection and that nonrecovery may need to be targeted by using antiinflammatory therapies.
Comorbidities
Evidence of impact of comorbidities on patient outcomes in COPD continues to emerge.
Cardiac comorbidities
In a study of 95,987 heart failure–naive patients with COPD in the Clinical Practice Research Datalink, Axson and colleagues (74) found that the incidence rate of heart failure was steady between 2006 and 2016 (1.18 per 100 person-years; 95% CI, 1.09–1.27). Patients with comorbid incident heart failure and COPD had a greater than threefold 1-year mortality rate and had a twofold increase in 5- and 10-year mortality rates. These mortality rates did not improve over the course of the 10 years between 2006 and 2016 (74, 75).
Patients with COPD are at increased risk of developing cardiovascular disease (CVD), and this heightened risk can be assessed by using the arterial pulse wave velocity (aPWV). In 1,788 patients enrolled in the SUMMIT (Study to Understand Mortality and Morbidity in COPD) study with moderate, airflow-limited COPD and either overt CVD or CVD risk factors (76), the impact of inhaled COPD therapy on all-cause mortality and cardiovascular morbidity was assessed. Mortality was greatest in the highest aPWV quartile at baseline, but no treatment affected aPWV or all-cause mortality. This analysis implies that improved management of COPD appears unlikely to reduce the associated heightened cardiovascular risk.
Frailty
This quality is associated with significant barriers to completion of pulmonary rehabilitation. In a qualitative study of 19 individuals with COPD and frailty using semistructured interviews, Brighton and colleagues (77) found several common themes associated with noncompletion of rehabilitation. These included “striving to adapt to multidimensional loss, tensions of balancing support with independence, pulmonary rehabilitation as a challenge worth facing, and overcoming unpredictable disruptions to participation.” The use of flexibility in rehabilitation programs to accommodate patient experiences, needs, and preferences may help improve attendance (77).
Sleep-disordered breathing
Redolfi and colleagues (78) hypothesized that respiratory accessory muscle use, which is quantified as neck inspiratory muscle (NIM) activity and is present during sleep among patients with a recent severe COPD exacerbation, could predict recurrent exacerbations requiring hospitalization. Their data confirm the 6-month risk of rehospitalization for severe COPD exacerbations recorded in 29 recently hospitalized subjects (30% in the nonintermittent NIM activity group compared with 89% in the permanent NIM activity group). Importantly, as Orr and Owens (79) highlight in their editorial, these findings might represent a group that would benefit from nocturnal noninvasive ventilation with respect to NIM activity, as the activation of accessory muscles presumably serves to compensate for inadequate ventilation relative to respiratory drive.
Obstructive sleep apnea is prevalent in COPD (80), and it is believed that thickened airway walls visualized by using multi–detector row CT are due to intermittent hypoxemia and airway inflammation associated with increased COPD exacerbations and a decreased quality of life. Koch and colleagues (81) demonstrated that those with COPD–obstructive sleep apnea overlap have increased thickness of airways compared with those who have COPD alone.
Mechanistic studies into COPD comorbidities
Skeletal muscle dysfunction is a comorbidity of COPD driven by an imbalance between protein synthesis and degradation (82). High retention of CO2 (or hypercapnia) is seen in patients with COPD and is associated with worsening outcomes. Korponay and colleagues (83) showed that CO2-retaining individuals had repressed ribosomal biogenesis in quadricep muscle biopsy specimens compared with control subjects. Mechanistic work in vivo and in vitro suggests that muscle wasting in patients with hypercapnia may occur via AMPKa2-dependent depressed ribosomal biogenesis and protein synthesis.
Chan and colleagues (84) also examined muscle wasting by using CS exposure together with a BaCl2 muscle injury model in vivo. Exposure to CS increased muscle loss, decreased limb muscle contractility with an overall decline in muscle function in the injured muscle, and increased macrophage infiltration and the decline in muscle regeneration. However, whether these muscle abnormalities are specific to patients with COPD or would occur in healthy individuals exposed to chronic hypoxia remains unclear (85).
Metabolic syndrome is a comorbidity of COPD, and dysregulated lipid function is associated with both diseases. To understand this link, the role of sphingomyelins (SGMs) in COPD was assessed (86). SGMS2 (SMG synthase 2) gene expression was reduced in COPD epithelial cells, whereas CS-exposed SGMS2-knockout mice had increased collagen deposition and increased inflammatory-cell infiltrates. Although this provides a link between dysregulated lipid homeostasis and COPD, more work is needed to fully elucidate the mechanism (87).
Pharmacologic Treatment
Inhaled Therapies
In the IMPACT (Informing the Pathway of COPD Treatment) trial, fluticasone furoate (FF)/umeclidinium (UMEC)/vilanterol (VI) significantly reduced the rate of moderate or severe exacerbations compared with FF/VI or UMEC/VI in a large-scale study of a patient population enriched for symptomatic COPD and a history of exacerbations (88). In the first of two important post hoc analyses, the IMPACT investigators expanded and completed their analysis of mortality. In 10,313 enrolled subjects, there were statistically significantly fewer deaths at 12 months in the triple-combination group (FF/UMEC/VI) compared with the UMEC/VI group (adjusted rate ratio, 0.83%; relative rate ratio, 28%; P = 0.042) but not when comparing the FF/VI group with the UMEC/VI group (adjusted rate ratio, 0.55%; relative rate ratio, 18%; P = 0.190) (89). In the second post hoc analysis, the authors investigated the potential effect of prior therapy, in particular inhaled corticosteroid (ICS) withdrawal, on the overall trial results (90). Figure 2 (reprint of Figure 3 from Reference 90) shows that FF/UMEC/VI therapy resulted in a 35% reduction in severe exacerbation rates as compared with UMEC/VI therapy for both those without prior ICS use (P = 0.018) and those with prior ICS use (P < 0.001). A numerical but not statistically significant reduction in moderate or severe exacerbations was also seen among those without prior ICS use. Furthermore, when the first 30 days of data were removed to account for steroid withdrawal, the benefit of triple therapy on exacerbation reduction was maintained. These data suggest that the benefits of FF/UMEC/VI combination therapy do not appear to be related to abrupt ICS withdrawal.
Figure 2.
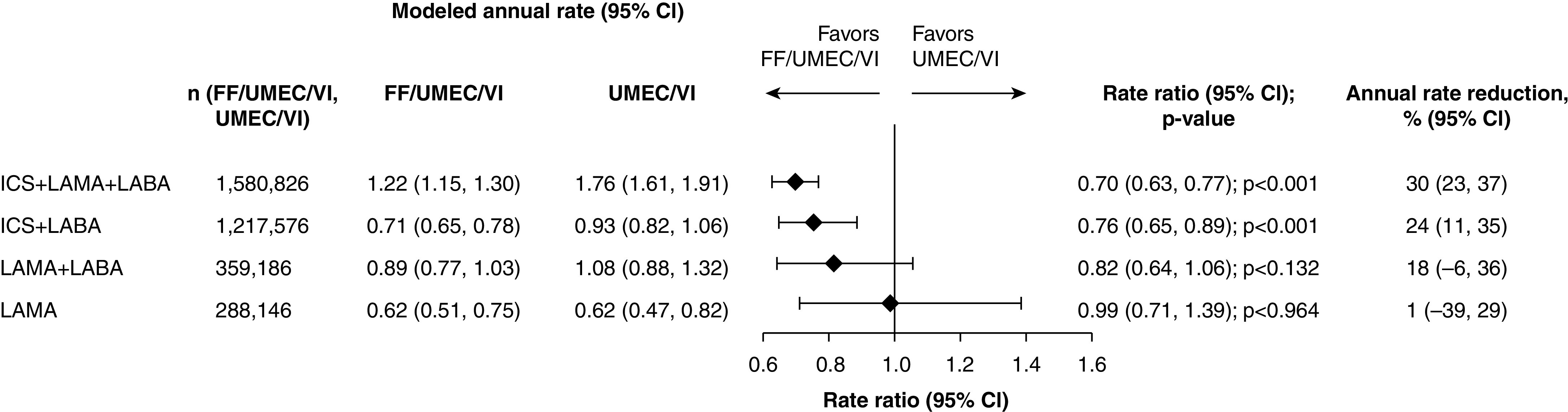
Forest plot of on-treatment moderate or severe chronic obstructive pulmonary disease (COPD) exacerbation rates by prior COPD medication class: fluticasone furoate (FF)/umeclidinium (UMEC)/vilanterol (VI) versus UMEC/VI. Throughout, n represents the number of patients receiving FF/UMEC/VI and UMEC/VI, excluding those with missing covariates (inhaled corticosteroid [ICS] + long-acting muscarinic antagonist [LAMA] + long-acting β2-agonist [LABA]: FF/UMEC/VI, n = 1; ICS + LABA: FF/UMEC/VI, n = 3; FF/VI, n = 1; LAMA + LABA: FF/UMEC/VI, n = 2; UMEC/VI, n = 1). CI = confidence interval. Reprinted by permission from Reference 90.
Studies show that ICSs convey a survival benefit and reduce the exacerbation rate (91, 92). However, the use of ICSs has to be balanced against the risk of pneumonia. Martinez-Garcia and colleagues (93) followed 201 patients with COPD for 84 months and found that a blood eosinophil count <100/μL combined with the presence of chronic bronchial infection is associated with a greater risk of pneumonia with ICS use (hazard ratio, 2.93; 95% CI, 1.30–6.22).
Lowering medication overuse can decrease harm as well as costs. Spece and colleagues (94) examined the rate of low-value use of ICS in a cohort of veterans with COPD and a low risk of exacerbation. Patient complexity was defined by using the care assessment needs score, and health system complexity was defined by using the hospitalization volume and care complexity designations. In 8,497 patients with COPD and without an indication for ICS treatment, they found that patient complexity defined by using the care assessment needs score was associated with new dispensation of ICSs (hazard ratio, 1.17 per 10-unit change; 95% CI, 1.13–1.21) and that this was not modified by the health system complexity (94).
Opioids
Although opioids are frequently prescribed to palliate dyspnea, the balance between the benefit and risk is not clear. In a retrospective evaluation of Ontario health administrative data, Vozoris and colleagues (95) found that of 169,517 older adults with COPD receiving a new opioid, 4,861 (2.9%) experienced an adverse pulmonary event, defined as an emergency room visit, hospitalization, or death due to pneumonia or COPD within 30 days. Several factors, including patient characteristics (age ⩾85 yr, long-term care residence, hospitalization for COPD in prior year), comorbidities (sleep disorder, dementia, congestive heart failure, and other lung disease), and use of other psychoactive medications (benzodiazepines, serotonergic antidepressants, and opioid-only agents), were associated with an increased risk of an adverse pulmonary event (95).
Nonpharmacologic Intervention
Bronchoscopy
Bronchoscopic lung volume reduction (BLVR) is a new therapy targeting lobar deflation to improve lung function. A post hoc analysis of 190 participants in a randomized clinical trial of BLVR with endobronchial valves showed clinically meaningful improvements in dyspnea and COPD-related quality of life with BLVR, which correlated with the degree of improvement in air trapping (96).
Valipour and colleagues (97) investigated another novel nonpharmacologic intervention termed “bronchial rheoplasty.” The procedure uses nonthermal pulsed electrical fields with the intention of ablating the abnormal, mucus-producing cells of the airway epithelium, thereby allowing normal, healthy epithelial regeneration to occur. This was the first study to evaluate the technical feasibility, safety, and initial outcomes of this therapy in patients with COPD and chronic bronchitis. Encouragingly, reductions in goblet-cell hyperplasia and changes in the patient-reported quality of life at the 12-month follow-up were demonstrated. The median (interquartile range) changes from baseline in COPD Assessment Test and St. George’s Respiratory Questionnaire scores were −8.0 (−14.0 to 1.0) points and −14.7 (−27.8 to −2.0) points, respectively.
Pulmonary Rehabilitation
Pulmonary rehabilitation is a useful intervention. However, patient uptake is low; therefore, the effects of a video intervention in addition to the standard-of-care discharge procedures on pulmonary rehabilitation uptake were assessed in a randomized controlled trial of 196 patients (98). The study found that video intervention did not lead to a significant improvement in pulmonary rehabilitation uptake.
Future Perspective
Despite the current pandemic, COPD research has continued and flourished. Patient contact for research has been restricted, but this will lead to better use of existing databases and stored samples. Familiarity with videoconferencing will accelerate collaboration. This difficult year will lead to great advances.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202102-0253UP on April 12, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Ruparel M, Quaife SL, Dickson JL, Horst C, Tisi S, Hall H, et al. Prevalence, symptom burden, and underdiagnosis of chronic obstructive pulmonary disease in a lung cancer screening cohort. Ann Am Thorac Soc. 2020;17:869–878. doi: 10.1513/AnnalsATS.201911-857OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Silvestri GA, Young RP. Strange bedfellows: the interaction between COPD and lung cancer in the context of lung cancer screening. Ann Am Thorac Soc. 2020;17:810–812. doi: 10.1513/AnnalsATS.202005-433ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ioachimescu OC, Stoller JK. An alternative spirometric measurement: area under the expiratory flow–volume curve. Ann Am Thorac Soc. 2020;17:582–588. doi: 10.1513/AnnalsATS.201908-613OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rich DQ, Thurston SW, Balmes JR, Bromberg PA, Arjomandi M, Hazucha MJ, et al. Do ambient ozone or other pollutants modify effects of controlled ozone exposure on pulmonary function? Ann Am Thorac Soc. 2020;17:563–572. doi: 10.1513/AnnalsATS.201908-597OC. [DOI] [PubMed] [Google Scholar]
- 5. Devadoss D, Long C, Langley RJ, Manevski M, Nair M, Campos MA, et al. Long noncoding transcriptome in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2019;61:678–688. doi: 10.1165/rcmb.2019-0184TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 7. Callahan-Lyon P. Electronic cigarettes: human health effects. Tob Control. 2014;23:ii36–ii40. doi: 10.1136/tobaccocontrol-2013-051470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kc B, Mahapatra PS, Thakker D, Henry AP, Billington CK, Sayers I, et al. Proinflammatory effects in ex vivo human lung tissue of respirable smoke extracts from indoor cooking in Nepal. Ann Am Thorac Soc. 2020;17:688–698. doi: 10.1513/AnnalsATS.201911-827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jamal A, Gentzke A, Hu SS, Cullen KA, Apelberg BJ, Homa DM, et al. Tobacco use among middle and high school students: United States, 2011-2016. MMWR Morb Mortal Wkly Rep. 2017;66:597–603. doi: 10.15585/mmwr.mm6623a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abdelwahab SH, Reidel B, Martin JR, Ghosh A, Keating JE, Haridass P, et al. Cigarillos compromise the mucosal barrier and protein expression in airway epithelia. Am J Respir Cell Mol Biol. 2020;63:767–779. doi: 10.1165/rcmb.2019-0085OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsai M, Mallampalli RK. E-cigarette or vaping product use-associated lung injury: opportunities and challenges. Am J Respir Cell Mol Biol. 2020;62:397–398. doi: 10.1165/rcmb.2019-0422LE. [DOI] [PubMed] [Google Scholar]
- 12. Serpa GL, Renton ND, Lee N, Crane MJ, Jamieson AM. Electronic nicotine delivery system aerosol-induced cell death and dysfunction in macrophages and lung epithelial cells. Am J Respir Cell Mol Biol. 2020;63:306–316. doi: 10.1165/rcmb.2019-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knapp S. Vaping: cell damage at the receiving ends. Am J Respir Cell Mol Biol. 2020;63:271–272. doi: 10.1165/rcmb.2020-0244ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lemoine C, Brigham E, Woo H, Koch A, Hanson C, Romero K, et al. Relationship between omega-3 and omega-6 fatty acid intake and chronic obstructive pulmonary disease morbidity. Ann Am Thorac Soc. 2020;17:378–381. doi: 10.1513/AnnalsATS.201910-740RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raju S, Brigham EP, Paulin LM, Putcha N, Balasubramanian A, Hansel NN, et al. The burden of rural chronic obstructive pulmonary disease: analyses from the National Health and Nutrition Examination Survey. Am J Respir Crit Care Med. 2020;201:488–491. doi: 10.1164/rccm.201906-1128LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bui DS, Perret JL, Walters EH, Abramson MJ, Burgess JA, Bui MQ, et al. Lifetime risk factors for pre- and post-bronchodilator lung function decline: a population-based study. Ann Am Thorac Soc. 2020;17:302–312. doi: 10.1513/AnnalsATS.201904-329OC. [DOI] [PubMed] [Google Scholar]
- 17. Campbell B, Bui DS, Simpson JA, Lodge CJ, Lowe AJ, Bowatte G, et al. Early age at natural menopause is related to lower post-bronchodilator lung function: a longitudinal population-based study. Ann Am Thorac Soc. 2020;17:429–437. doi: 10.1513/AnnalsATS.201902-180OC. [DOI] [PubMed] [Google Scholar]
- 18. Chotirmall SH. When epidemiology meets physiology: early menopause and associated respiratory risk. Ann Am Thorac Soc. 2020;17:419–420. doi: 10.1513/AnnalsATS.202002-090ED. [DOI] [PubMed] [Google Scholar]
- 19. Bhatt SP, Balte PP, Schwartz JE, Cassano PA, Couper D, Jacobs DR, Jr, et al. Discriminative accuracy of FEV1:FVC thresholds for COPD-related hospitalization and mortality. JAMA. 2019;321:2438–2447. doi: 10.1001/jama.2019.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neder JA, Milne KM, Berton DC, de-Torres JP, Jensen D, Tan WC, et al. CRRN (Canadian Respiratory Research Network); CanCOLD (Canadian Cohort of Obstructive Lung Disease) Collaborative Research Group. Exercise tolerance according to the definition of airflow obstruction in smokers [letter] Am J Respir Crit Care Med. 2020;202:760–762. doi: 10.1164/rccm.202002-0298LE. [DOI] [PubMed] [Google Scholar]
- 21. Zhang WZ. The origins of chronic obstructive pulmonary disease: sometimes the journey matters more than the destination. Am J Respir Crit Care Med. 2020;202:159–161. doi: 10.1164/rccm.202004-0959ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marott JL, Ingebrigtsen TS, Çolak Y, Vestbo J, Lange P. Lung function trajectories leading to chronic obstructive pulmonary disease as predictors of exacerbations and mortality. Am J Respir Crit Care Med. 2020;202:210–218. doi: 10.1164/rccm.201911-2115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez FJ, Han MK, Allinson JP, Barr RG, Boucher RC, Calverley PMA, et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197:1540–1551. doi: 10.1164/rccm.201710-2028PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Çolak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prevalence, characteristics, and prognosis of early chronic obstructive pulmonary disease: the Copenhagen General Population Study. Am J Respir Crit Care Med. 2020;201:671–680. doi: 10.1164/rccm.201908-1644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guerra S, Martinez FD. The complex beginnings of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201:641–642. doi: 10.1164/rccm.201912-2363ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Young AL, Bragman FJS, Rangelov B, Han M, Galban CJ, Lynch DA, et al. COPDGene Investigators. Disease progression modeling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201:294–302. doi: 10.1164/rccm.201908-1600OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Agusti A, Faner R. When Harry met Sally, or when machine learning met chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201:263–265. doi: 10.1164/rccm.201911-2123ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Løkke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61:935–939. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matamala N, Gomez-Mariano G, Perez JA, Baladrón B, Torres-Durán M, Michel FJ, et al. New cis-acting variants in PI*S background produce null phenotypes causing alpha-1 antitrypsin deficiency. Am J Respir Cell Mol Biol. 2020;63:444–451. doi: 10.1165/rcmb.2020-0021OC. [DOI] [PubMed] [Google Scholar]
- 30. Chapman KR. Bench to bedside and back: the evolving story of alpha-1 antitrypsin deficiency. Am J Respir Cell Mol Biol. 2020;63:403–404. doi: 10.1165/rcmb.2020-0243ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Vries M, van der Plaat DA, Nedeljkovic I, van der Velde KJ, Amin N, van Duijn CM, et al. Novel rare genetic variants associated with airflow obstruction in the general population. Am J Respir Crit Care Med. 2020;201:485–488. doi: 10.1164/rccm.201909-1868LE. [DOI] [PubMed] [Google Scholar]
- 32. Ortega VE, Li X, O’Neal WK, Lackey L, Ampleford E, Hawkins GA, et al. NHLBI Subpopulations and Intermediate Outcomes Measures in COPD Study (SPIROMICS). The effects of rare SERPINA1 variants on lung function and emphysema in SPIROMICS. Am J Respir Crit Care Med. 2020;201:540–554. doi: 10.1164/rccm.201904-0769OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Molloy K, Hersh CP, Morris VB, Carroll TP, O’Connor CA, Lasky-Su JA, et al. Clarification of the risk of chronic obstructive pulmonary disease in α1-antitrypsin deficiency PiMZ heterozygotes. Am J Respir Crit Care Med. 2014;189:419–427. doi: 10.1164/rccm.201311-1984OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stockley RA. Alpha-1 antitrypsin deficiency: the learning goes on. Am J Respir Crit Care Med. 2020;202:6–7. doi: 10.1164/rccm.202004-0922ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henkel M, Partyka J, Gregory AD, Forno E, Cho MH, Eddens T, et al. FSTL-1 attenuation causes spontaneous smoke-resistant pulmonary emphysema. Am J Respir Crit Care Med. 2020;201:934–945. doi: 10.1164/rccm.201905-0973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lau MC, Ng KY, Wong TL, Tong M, Lee TK, Ming XY, et al. FSTL1 promotes metastasis and chemoresistance in esophageal squamous cell carcinoma through NFκB-BMP signaling cross-talk. Cancer Res. 2017;77:5886–5899. doi: 10.1158/0008-5472.CAN-17-1411. [DOI] [PubMed] [Google Scholar]
- 37. Vlahos R. FSTL-1: a new player in the prevention of emphysema. Am J Respir Crit Care Med. 2020;201:886–888. doi: 10.1164/rccm.201912-2402ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uemasu K, Tanabe N, Tanimura K, Hasegawa K, Mizutani T, Hamakawa Y, et al. Serine protease imbalance in the small airways and development of centrilobular emphysema in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2020;63:67–78. doi: 10.1165/rcmb.2019-0377OC. [DOI] [PubMed] [Google Scholar]
- 39. Long ME, Manicone AM. Loss of C/EBPα in chronic cigarette smoke exposure: a SAD day for chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2020;63:9–10. doi: 10.1165/rcmb.2020-0069ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tsutsumi A, Ozaki M, Chubachi S, Irie H, Sato M, Kameyama N, et al. Exposure to cigarette smoke enhances the stemness of alveolar type 2 cells. Am J Respir Cell Mol Biol. 2020;63:293–305. doi: 10.1165/rcmb.2019-0188OC. [DOI] [PubMed] [Google Scholar]
- 41. Adini A, Wu H, Dao DT, Ko VH, Yu LJ, Pan A, et al. PR1P stabilizes VEGF and upregulates its signaling to reduce elastase-induced murine emphysema. Am J Respir Cell Mol Biol. 2020;63:452–463. doi: 10.1165/rcmb.2019-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pasupneti S, Tian W, Tu AB, Dahms P, Granucci E, Gandjeva A, et al. Endothelial HIF-2α as a key endogenous mediator preventing emphysema. Am J Respir Crit Care Med. 2020;202:983–995. doi: 10.1164/rccm.202001-0078OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hodson E, Ratcliffe P. Endothelial oxygen sensing in alveolar maintenance. Am J Respir Crit Care Med. 2020;202:917–919. doi: 10.1164/rccm.202006-2149ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Geraghty P, Hardigan AA, Wallace AM, Mirochnitchenko O, Thankachen J, Arellanos L, et al. The glutathione peroxidase 1-protein tyrosine phosphatase 1B-protein phosphatase 2A axis: a key determinant of airway inflammation and alveolar destruction. Am J Respir Cell Mol Biol. 2013;49:721–730. doi: 10.1165/rcmb.2013-0026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dabo AJ, Ezegbunam W, Wyman AE, Moon J, Railwah C, Lora A, et al. Targeting c-Src reverses accelerated GPX-1 mRNA decay in chronic obstructive pulmonary disease airway epithelial cells. Am J Respir Cell Mol Biol. 2020;62:598–607. doi: 10.1165/rcmb.2019-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cloonan SM, Mumby S, Adcock IM, Choi AMK, Chung KF, Quinlan GJ. The “iron”-y of iron overload and iron deficiency in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196:1103–1112. doi: 10.1164/rccm.201702-0311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sato K, Inoue S, Igarashi A, Tokairin Y, Yamauchi K, Kimura T, et al. Effect of iron deficiency on a murine model of smoke-induced emphysema. Am J Respir Cell Mol Biol. 2020;62:588–597. doi: 10.1165/rcmb.2018-0239OC. [DOI] [PubMed] [Google Scholar]
- 48. Zhang WZ, Cloonan SM. To “Fe”ed or not to “Fe”ed: iron depletion exacerbates emphysema development in murine smoke model. Am J Respir Cell Mol Biol. 2020;62:541–542. doi: 10.1165/rcmb.2019-0376ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wells JM, Gaggar A, Blalock JE. MMP generated matrikines. Matrix Biol. 2015;44-46:122–129. doi: 10.1016/j.matbio.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mehraban S, Gu G, Ma S, Liu X, Turino G, Cantor J. The proinflammatory activity of structurally altered elastic fibers. Am J Respir Cell Mol Biol. 2020;63:699–706. doi: 10.1165/rcmb.2020-0064OC. [DOI] [PubMed] [Google Scholar]
- 51. Bonvini SJ. Cause or effect? Stretching to understand the inflammatory role of elastin fiber breakdown in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2020;63:558–559. doi: 10.1165/rcmb.2020-0348ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vasudevan S, Vásquez JJ, Chen W, Aguilar-Rodriguez B, Niemi EC, Zeng S, et al. Lower PDL1, PDL2, and AXL expression on lung myeloid cells suggests inflammatory bias in smoking and chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2020;63:780–793. doi: 10.1165/rcmb.2020-0085OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Elliott MR, Koster KM, Murphy PS. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J Immunol. 2017;198:1387–1394. doi: 10.4049/jimmunol.1601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Belchamber KBR, Donnelly LE. Targeting defective pulmonary innate immunity: a new therapeutic option? Pharmacol Ther. 2020;209:107500. doi: 10.1016/j.pharmthera.2020.107500. [DOI] [PubMed] [Google Scholar]
- 55. O’Beirne SL, Kikkers SA, Oromendia C, Salit J, Rostmai MR, Ballman KV, et al. Alveolar macrophage immunometabolism and lung function impairment in smoking and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201:735–739. doi: 10.1164/rccm.201908-1683LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Belchamber KBR, Singh R, Batista CM, Whyte MK, Dockrell DH, Kilty I, et al. COPD-MAP Consortium. Defective bacterial phagocytosis is associated with dysfunctional mitochondria in COPD macrophages. Eur Respir J. 2019;54:1802244. doi: 10.1183/13993003.02244-2018. [DOI] [PubMed] [Google Scholar]
- 57. Brusselle GG, Demoor T, Bracke KR, Brandsma CA, Timens W. Lymphoid follicles in (very) severe COPD: beneficial or harmful? Eur Respir J. 2009;34:219–230. doi: 10.1183/09031936.00150208. [DOI] [PubMed] [Google Scholar]
- 58. Naessens T, Morias Y, Hamrud E, Gehrmann U, Budida R, Mattsson J, et al. Human lung conventional dendritic cells orchestrate lymphoid neogenesis during chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;202:535–548. doi: 10.1164/rccm.201906-1123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Freeman CM, Curtis JL. It’s complicated: lung dendritic cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;202:479–481. doi: 10.1164/rccm.202004-0899ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tanabe N, Vasilescu DM, Hague CJ, Ikezoe K, Murphy DT, Kirby M, et al. Pathological comparisons of paraseptal and centrilobular emphysema in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;202:803–811. doi: 10.1164/rccm.201912-2327OC. [DOI] [PubMed] [Google Scholar]
- 61. Kirby M, Tanabe N, Vasilescu DM, Cooper JD, McDonough JE, Verleden SE, et al. Computed tomography total airway count is associated with the number of micro-computed tomography terminal bronchioles. Am J Respir Crit Care Med. 2020;201:613–615. doi: 10.1164/rccm.201910-1948LE. [DOI] [PubMed] [Google Scholar]
- 62. Bailey KE, Pino C, Lennon ML, Lyons A, Jacot JG, Lammers SR, et al. Embedding of precision-cut lung slices in engineered hydrogel biomaterials supports extended ex vivo culture. Am J Respir Cell Mol Biol. 2020;62:14–22. doi: 10.1165/rcmb.2019-0232MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rittayamai N, Chuaychoo B, Tscheikuna J, Dres M, Goligher EC, Brochard L. Ultrasound evaluation of diaphragm force reserve in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2020;17:1222–1230. doi: 10.1513/AnnalsATS.202002-129OC. [DOI] [PubMed] [Google Scholar]
- 64. Berenyi F, Steinfort DP, Abdelhamid YA, Bailey MJ, Pilcher DV, Bellomo R, et al. Characteristics and outcomes of critically ill patients with acute exacerbation of chronic obstructive pulmonary disease in Australia and New Zealand. Ann Am Thorac Soc. 2020;17:736–745. doi: 10.1513/AnnalsATS.201911-821OC. [DOI] [PubMed] [Google Scholar]
- 65. Blagev DP. Better with time: reductions in mortality in patients with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2020;17:684–685. doi: 10.1513/AnnalsATS.202002-178ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gaffney A, White A, Hawks L, Himmelstein D, Woolhandler S, Christiani DC, et al. High-deductible health plans and healthcare access, use, and financial strain in those with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2020;17:49–56. doi: 10.1513/AnnalsATS.201905-400OC. [DOI] [PubMed] [Google Scholar]
- 67. Press VG, Gerald JK. High-deductible health plans make the chronically ill pay more for less. Ann Am Thorac Soc. 2020;17:30–31. doi: 10.1513/AnnalsATS.201910-808ED. [DOI] [PubMed] [Google Scholar]
- 68. Myers LC, Faridi MK, Hasegawa K, Hanania NA, Camargo CA., Jr The hospital readmissions reduction program and readmissions for chronic obstructive pulmonary disease, 2006–2015. Ann Am Thorac Soc. 2020;17:450–456. doi: 10.1513/AnnalsATS.201909-672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rinne ST, Press VG. Moving the bar on chronic obstructive pulmonary disease readmissions before and after the hospital readmission reduction program: myth or reality? Ann Am Thorac Soc. 2020;17:423–425. doi: 10.1513/AnnalsATS.202001-010ED. [DOI] [PubMed] [Google Scholar]
- 70. Tavakoli H, Chen W, Sin DD, FitzGerald JM, Sadatsafavi M. Predicting severe chronic obstructive pulmonary disease exacerbations: developing a population surveillance approach with administrative data. Ann Am Thorac Soc. 2020;17:1069–1076. doi: 10.1513/AnnalsATS.202001-070OC. [DOI] [PubMed] [Google Scholar]
- 71. Jiang L, Gershon AS. Using health administrative data to predict chronic obstructive pulmonary disease exacerbations. Ann Am Thorac Soc. 2020;17:1056–1057. doi: 10.1513/AnnalsATS.202006-704ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Donaldson GC, Law M, Kowlessar B, Singh R, Brill SE, Allinson JP, et al. Impact of prolonged exacerbation recovery in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:943–950. doi: 10.1164/rccm.201412-2269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ritchie AI, Brill SE, Vlies BH, Finney LJ, Allinson JP, Alves-Moreira L, et al. Targeted retreatment of incompletely recovered chronic obstructive pulmonary disease exacerbations with ciprofloxacin: a double-blind, randomized, placebo-controlled, multicenter phase III trial. Am J Respir Crit Care Med. 2020;202:549–557. doi: 10.1164/rccm.201910-2058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Axson EL, Sundaram V, Bloom CI, Bottle A, Cowie MR, Quint JK. Temporal trends in the incidence of heart failure among patients with chronic obstructive pulmonary disease and its association with mortality. Ann Am Thorac Soc. 2020;17:939–948. doi: 10.1513/AnnalsATS.201911-820OC. [DOI] [PubMed] [Google Scholar]
- 75. Stringer WW. Are we treating heart failure in patients with chronic obstructive pulmonary disease appropriately? Ann Am Thorac Soc. 2020;17:932–934. doi: 10.1513/AnnalsATS.202004-395ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Crim C, Anderson JA, Calverley PMA, Celli BR, Cowans NJ, Martinez FJ, et al. Pulse wave velocity in chronic obstructive pulmonary disease and the impact of inhaled therapy (SUMMIT): a randomized double-blind clinical trial. Am J Respir Crit Care Med. 2020;201:1307–1310. doi: 10.1164/rccm.201908-1639LE. [DOI] [PubMed] [Google Scholar]
- 77. Brighton LJ, Bristowe K, Bayly J, Ogden M, Farquhar M, Evans CJ, et al. Experiences of pulmonary rehabilitation in people living with chronic obstructive pulmonary disease and frailty: a qualitative interview study. Ann Am Thorac Soc. 2020;17:1213–1221. doi: 10.1513/AnnalsATS.201910-800OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Redolfi S, Grassion L, Rivals I, Chavez M, Wattiez N, Arnulf I, et al. Abnormal activity of neck inspiratory muscles during sleep as a prognostic indicator in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201:414–422. doi: 10.1164/rccm.201907-1312OC. [DOI] [PubMed] [Google Scholar]
- 79. Orr JE, Owens RL. Chronic obstructive pulmonary disease and breathing during sleep: a strain in the neck. Am J Respir Crit Care Med. 2020;201:395–396. doi: 10.1164/rccm.201911-2174ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sanders MH, Newman AB, Haggerty CL, Redline S, Lebowitz M, Samet J, et al. Sleep Heart Health Study. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167:7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 81. Koch AL, Brown RH, Woo H, Brooker AC, Paulin LM, Schneider H, et al. Obstructive sleep apnea and airway dimensions in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2020;17:116–118. doi: 10.1513/AnnalsATS.201903-220RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kryvenko V, Vadász I. Antianabolic effects of hypercapnia: no country for strong men. Am J Respir Cell Mol Biol. 2020;62:8–9. doi: 10.1165/rcmb.2019-0225ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Korponay TC, Balnis J, Vincent CE, Singer DV, Chopra A, Adam AP, et al. High CO2 downregulates skeletal muscle protein anabolism via AMP-activated protein kinase α2–mediated depressed ribosomal biogenesis. Am J Respir Cell Mol Biol. 2020;62:74–86. doi: 10.1165/rcmb.2019-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chan SMH, Cerni C, Passey S, Seow HJ, Bernardo I, van der Poel C, et al. Cigarette smoking exacerbates skeletal muscle injury without compromising its regenerative capacity. Am J Respir Cell Mol Biol. 2020;62:217–230. doi: 10.1165/rcmb.2019-0106OC. [DOI] [PubMed] [Google Scholar]
- 85. Iepsen UW, Pedersen BK. Development of limb muscle dysfunction in chronic obstructive pulmonary disease: smoking, inflammation, or simply disuse? Am J Respir Cell Mol Biol. 2020;62:134–135. doi: 10.1165/rcmb.2019-0319ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gupta G, Baumlin N, Poon J, Ahmed B, Chiang YP, Railwah C, et al. Airway resistance caused by sphingomyelin synthase 2 insufficiency in response to cigarette smoke. Am J Respir Cell Mol Biol. 2020;62:342–353. doi: 10.1165/rcmb.2019-0133OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vlahos R. Lipids in chronic obstructive pulmonary disease: a target for future therapy? Am J Respir Cell Mol Biol. 2020;62:273–274. doi: 10.1165/rcmb.2019-0338ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, et al. IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–1680. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 89. Lipson DA, Crim C, Criner GJ, Day NC, Dransfield MT, Halpin DMG, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201:1508–1516. doi: 10.1164/rccm.201911-2207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Han MK, Criner GJ, Dransfield MT, Halpin DMG, Jones CE, Kilbride S, et al. The effect of inhaled corticosteroid withdrawal and baseline inhaled treatment on exacerbations in the impact study: a randomized, double-blind, multicenter clinical trial. Am J Respir Crit Care Med. 2020;202:1237–1243. doi: 10.1164/rccm.201912-2478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP. UPLIFT Study Investigators. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:948–955. doi: 10.1164/rccm.200906-0876OC. [DOI] [PubMed] [Google Scholar]
- 92. Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 93. Martinez-Garcia MA, Faner R, Oscullo G, de la Rosa D, Soler-Cataluña J-J, Ballester M, et al. Inhaled steroids, circulating eosinophils, chronic airway infection, and pneumonia risk in chronic obstructive pulmonary disease: a network analysis. Am J Respir Crit Care Med. 2020;201:1078–1085. doi: 10.1164/rccm.201908-1550OC. [DOI] [PubMed] [Google Scholar]
- 94. Spece LJ, Donovan LM, Griffith MF, Keller T, Feemster LC, Smith NL, et al. Initiating low-value inhaled corticosteroids in an inception cohort with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2020;17:589–595. doi: 10.1513/AnnalsATS.201911-854OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vozoris NT, Pequeno P, Li P, Austin PC, O’Donnell DE, Gershon AS. Predictors of opioid-related adverse pulmonary events among older adults with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2020;17:965–973. doi: 10.1513/AnnalsATS.201910-760OC. [DOI] [PubMed] [Google Scholar]
- 96. Dransfield MT, Garner JL, Bhatt SP, Slebos D-J, Klooster K, Sciurba FC, et al. LIBERATE Study Group. Effect of Zephyr endobronchial valves on dyspnea, activity levels and quality of life at one year: results from a randomized clinical trial. Ann Am Thorac Soc. 2020;17:829–838. doi: 10.1513/AnnalsATS.201909-666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Valipour A, Fernandez-Bussy S, Ing AJ, Steinfort DP, Snell GI, Williamson JP, et al. Bronchial rheoplasty for treatment of chronic bronchitis: twelve-month results from a multicenter clinical trial. Am J Respir Crit Care Med. 2020;202:681–689. doi: 10.1164/rccm.201908-1546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Barker RE, Jones SE, Banya W, Fleming S, Kon SSC, Clarke SF, et al. The effects of a video intervention on posthospitalization pulmonary rehabilitation uptake: a randomized controlled trial. Am J Respir Crit Care Med. 2020;201:1517–1524. doi: 10.1164/rccm.201909-1878OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


