Abstract
Of the thousands of per- and polyfluoroalkyl substances (PFAS) in the environment, few have been investigated in detail. In this study, we analyzed 36 legacy and emerging PFAS in multiple seabird tissues collected from individuals from Massachusetts Bay, Narragansett Bay and the Cape Fear River Estuary. PFOS was the dominant compound across multiple tissues, while long-chain perfluorinated carboxylic acids (PFCAs) dominated in brain (mean = 44% of total concentrations). Emerging perfluoroalkyl ether acids (PFEAs)—Nafion byproduct-2 and PFO5DoDA – were detected in greater than 90% of tissues in birds obtained from a nesting region downstream from a major fluorochemical production site. Compound ratios, relative body burden calculations, and electrostatic surface potential calculations were used to describe partitioning behavior of PFEAs in different tissues. Novel PFEAs preferentially partition into blood compared to liver, and were documented in brain for the first time. PFO5DoDA showed a reduced preference for brain compared to PFCAs and Nafion BP2. These results suggest future monitoring efforts and toxicological studies should focus on novel PFAS and long-chain PFCAs in multiple tissues beyond liver and blood, while exploring the unique binding mechanisms driving uptake of multi-ether PFEAs.
Keywords: PFOS, PFAS, seabirds, bioaccumulation, partitioning
Graphical Abstract
Introduction
Per- and polyfluoroalkyl substances (PFAS) are manmade chemicals associated with adverse effects in biota; global biomonitoring studies document the pervasive accumulation of these compounds in wildlife1–4. The majority of these studies measured PFAS in select matrices only, such as serum, liver, feather, or egg5–7. These studies provide snapshots of PFAS levels in single tissue compartments but cannot assess total exposure across multiple tissues and organs.
Some research has recognized this shortcoming by measuring PFAS within multiple tissues of wild organisms, including tissues such as brain, lungs, heart, muscle, kidney, gonads, and adipose tissue (fat) 8–14. Such work demonstrates highly unique partitioning behavior of PFAS. Verreault et al. (2005) examined plasma, liver, egg, and brain from glaucous gull (Larus hyperboreus) adults and eggs from the Norwegian Arctic and determined perfluorooctanesulfonic acid (PFOS) concentrations were greatest in plasma, followed by approximately equal concentrations in liver and egg9. Investigations in herring gull (Larus argentatus) female adults and eggs from the Great Lakes (US) and common guillemot (Uria aalgae) adults, chicks, and eggs from the Baltic Sea subsequently found adult and chick liver to contain higher concentrations of PFOS compared to plasma or muscle10,15. Results from other taxa also suggest blood, liver, and kidney contain the highest concentrations of multiple perfluoroalkyl acids (PFAAs), with some indication of preferential uptake of long-chain perfluoroalkyl carboxylic acids (PFCAs, CnF2n+1COOH, n ≥ 7) in the brains of marine mammals8,14,16. This is in stark contrast to the behavior of hydrophobic organic chemicals like polychlorinated biphenyls (PCBs), which prefer fatty tissue storage.
Tissue-specific measurements also help constrain drivers of PFAS uptake and internal distribution. Empirical data from tissue-specific studies, modeling efforts, and data from controlled laboratory studies indicate interactions with specific proteins like albumin and liver fatty acid binding protein (L-FABP) determine the partitioning of PFAS in liver, kidney, and blood matrices17,18. Non-specific phospholipid-mediated pathways have also been hypothesized to drive PFAA uptake in brain and liver18–20. These mechanisms are poorly described empirically and mechanistically across different taxa.
Notably, little or no information exists evaluating tissue-specific behavior of novel and replacement PFAS 11,12, such as per- and polyfluoroalkyl ether acids (PFEAs) 21. We hence analyzed legacy and novel PFAS in 8 different tissues in seabirds from the U.S. Atlantic Coast to conduct the first study examining the tissue-specific distribution of select PFEAs. The aims of this study were to (i) derive the tissue distributions of 36 legacy and emerging PFAS in birds from three habitats and (ii) evaluate partitioning behavior of PFEAs, relative to PFAAs in different tissues. We hypothesized that novel ether-containing PFAS would be detected less frequently and at lower concentrations in all tissue matrices compared to legacy PFCA and PFSA homologs.
Materials and Methods
Sample Collection
Deceased immature seabirds including Royal Terns, Sandwich Terns, Laughing Gulls, and Brown Pelicans were collected from the Cape Fear River Estuary (CFRE) in southeastern North Carolina (n = 12), downstream from a major fluoropolymer production facility. Herring Gulls (n = 4) were obtained from urbanized Narragansett Bay and Great Shearwaters (n = 6) were obtained from offshore Massachusetts Bay. All birds were freshly or recently deceased, with little or no apparent decomposition. These species generally rely on small forage fish and marine invertebrates for food. All individuals were less than six months old, aged 1 – 5 months. The total sample set (n = 20 individuals), while seeemingly small, is larger than the number of individuals used in prior tissue-specific work (e.g. 522, 810, 5–1015, or 413 individuals). More details about the appropriateness of our unique sample set, and particulars about each seabird species, individual, and location are provided in the Supplementary Information (SI) and in previous work (Tables S1, S2, Fig. S1)9,15. Tissues or samples collected included heart, brain, kidney, lungs, adipose fat, liver, whole blood, and pectoral muscle. 11/160 tissue samples (livers from CFRE individuals) were also reported in previous work from our group, but over 93% of the sample set is investigated here for the first time23.
PFAS analysis via HRMS
A total of 36 PFAS were assessed in 160 seabird tissue samples from 20 immature seabird individuals via targeted and suspect screening (Table S4). PFAS were extracted and analyzed following a previously described HRMS workflow23–26 using a Thermo Orbitrap Fusion mass spectrometer (ThermoFisher Scientific, Waltham, MA). Further details about sample preparation, analysis, quantification, and quality control are available in the SI.
Data treatment, statistical analysis, and structural comparison
Data manipulation and statistical analyses were performed in R version 3.6.1 (R Core Team, 2020)27. Concentrations were converted to a wet weight, per gram basis for ease of comparability. Reporting limits (RLs) were determined by comparison to process blank values plus three times the standard deviation; in the absence of blank concentrations, the lowest calibration curve point was used. Responses below RLs were considered zero for summation purposes. Summary statistics, compound ratios, tissue-blood ratios, and relative body burdens were calculated for compounds found in greater than 50% of samples in at least one tissue as described in the SI; non-detects were substituted with zeros to avoid artificial inflation of derived statistics and ratios given the small sample size per tissue and habitat. As a result, ratio calculations are conservative and may underestimate true values. Compound ratios in multiple tissues of CFRE birds (n = 12, 96 tissues) were compared using ANOVA with post hoc application of Tukey’s test with Bonferroni correction for multiple testing. Additionally, TURBO-MOLE software included in the COSMOconf software suite was used to evaluate differences in electrostatic surface potential between the lowest energy conformers of PFOS, PFOA, Nafion BP2 and PFO5DoDA. These visualizations portray a molecule’s probable surface charge as a three-dimensional surface, providing insight about likely molecular interactions 28.
Results and Discussion
Detection of PFAS by tissue and region
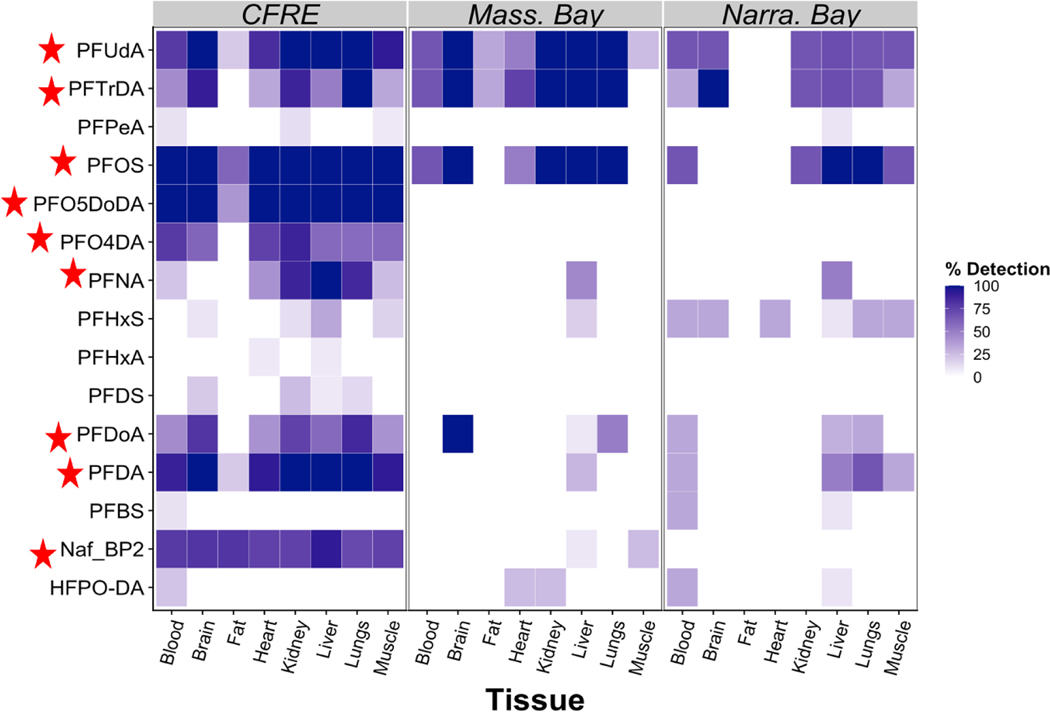
17 of 36 analytes were detected in at least one seabird tissue (Fig. 1, Table S3). The largest number of analytes and the highest detection frequencies across each tissue type were observed in individuals from the Cape Fear sample set (Fig. 1).
Figure 1.
Detection frequencies by habitat and tissue type, including 15 compounds that were found at least two samples. Nine PFAS (Nafion BP2, PFO5DoDA, PFO4DA, PFOS, PFNA, PFDA, PFUdA, PFDoA, and PFTrDA) were found in ≥ 50% of at least two tissues from at least one habitat, marked with a red star in the figure. See SI for compound abbreviations.
PFOS and PFCA concentrations across multiple tissues
PFOS was detected at the highest mean concentration in most tissues when it was detected, and dominated sum totals in most tissues (Fig. S4, Tables S5, S7). Considering birds from all habitats, the highest mean concentrations of PFOS were observed in liver, kidney, lungs, and blood across all habitats (Fig. S2, Tables S5). High PFOS abundances in these relative to other tissues have been reported in wildlife from regions unimpacted by PFOS point sources 9,10,15. PFOS was found in all CFRE tissues examined, and at the highest concentrations (up to 480 ng/g in blood) (Tables S5). High levels of PFOS have also been observed in fish and human serum samples from the wider Cape Fear region 26,29,30.
CFRE birds displayed the largest number of PFCA detections across multiple tissues (Fig. 1). PFUdA was the most abundant PFCA in liver, with a mean concentration of 10, 10, and 5 ng/g in CFRE, Massachusetts Bay, and Narragansett Bay livers, respectively (Fig. S2, Tables S5). PFTrDA was the most abundant PFCA detected in the lungs and brain of Massachusetts Bay individuals, at a mean concentration of 19 ng/g in both tissues, higher than or similar to concentrations observed in other tissue-specific research involving seabirds9,10,15.
PFEA concentrations across multiple tissues of CFRE birds
PFEAs were detected in multiple tissues in birds from two habitats but were by far most abundant in CFRE birds. PFEAs made up the largest single proportion of total PFAS in fat (54% in CFRE birds), dominated by Nafion BP2, but concentrations were lower in fat compared to other tissues (Fig. S4, Tables S5). The highest PFEA levels were found in brain of CFRE individuals, with a maximum of 360 ng/g Nafion BP2 (Tables S5). Mean concentrations of Nafion BP2 in blood from CFRE individuals (mean = 21 ng/ml) were approximately 70 times higher than Nafion BP2 levels observed in striped bass from the Cape Fear River (mean = 0.3 ng/ml)26, and about 7 times higher than Nafion BP2 concentrations observed in human serum from Wilmington, NC (median = 2.7 ng/ml), a community where upstream industrial PFAS discharges in the Cape Fear River are known to impact finished drinking water31. Mean PFO5DoDA concentrations in blood of CFRE chicks (mean = 27 ng/ml) were about 90 times higher than in human serum from Wilmington residents (median = 0.3 ng/ml). PFEA concentrations in CFRE bird blood are also approximately two orders of magnitude higher than concentrations observed in humans surrounding a fluoropolymer production site in China (Nafion BP2 median = 0.097 ng/ml, PFO5DoDA median = 0.987 ng/ml), which were found to be associated with altered glucose, cholesterol, and other enzymes and proteins in human serum32. The higher observed concentrations in birds may be related to dose as a function of body weight and maternal offloading, with the smaller birds evidencing higher tissue concentrations when subjected to the same air/water dose.
Relative body burdens by tissue and body weight
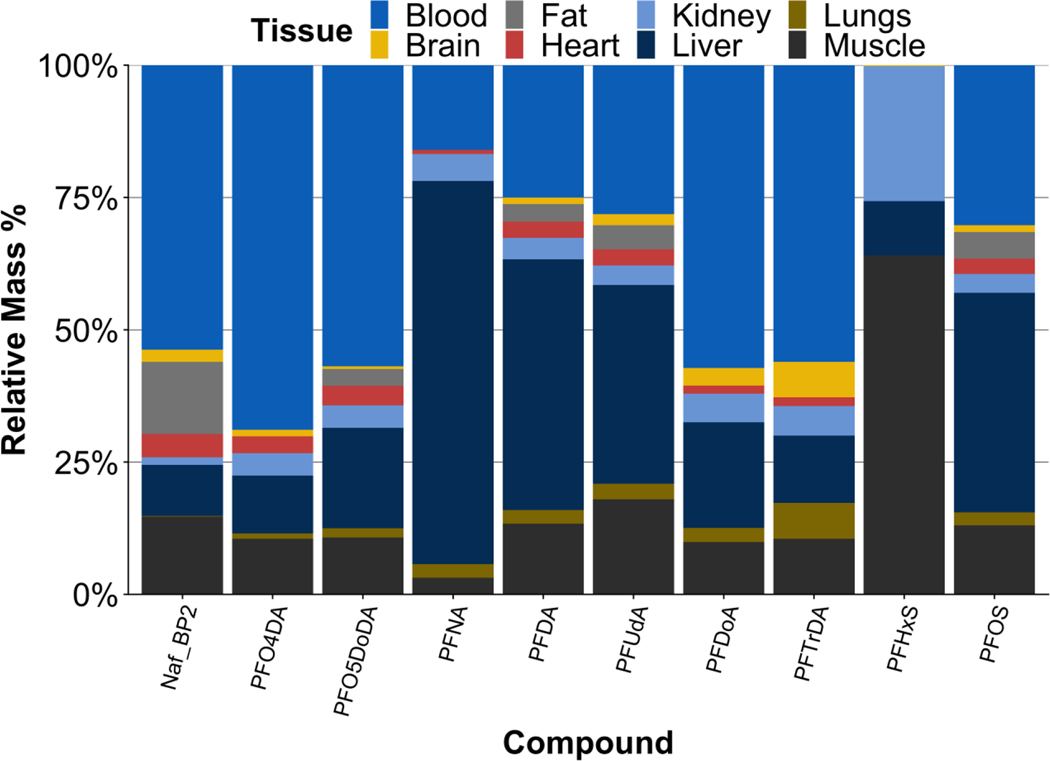
We derived relative body burdens (RBBs) as the product of PFAS concentrations, organ weights, and total body weight (Eq. S1). The highest RBB of PFOS was found in liver, followed by blood and muscle (Fig. 2, Fig. S3).
Figure 2.
Relative body burdens (RBBs) of each PFAS detected in more than 50% of samples in at least one tissue of CFRE individuals, stratified by tissue compartment. RBB data from other habitats is limited by lower detection frequencies, and is presented in Fig. S3.
In muscle, the high RBB of PFOS is likely driven by the large mass fraction of this component (~15 – 20% of body weight) whereas in blood (~10%) and liver (4 – 6%) the large RBB also results from the increased PFOS affinity for these tissues via specific uptake mechanisms including albumin, L-FABP, and membrane phospholipids17,33,34. RBBs also suggest a chain-length dependence of PFCA accumulation in liver and brain (Fig. 2). C9 PFNA had the highest relative mass in liver, with decreasing relative mass with increasing chain length (Fig. 2). C12 PFDoDA and C13 PFTrDA showed significantly decreased relative masses in liver and increased mass in brain compared to C9 – C11 PFCAs. These trends were likely driven by relative binding affinity of PFCAs with specific proteins in brain and blood-brain membrane transport pathways 14,17,34. The largest RBBs of Nafion BP2, PFO4DA, PFO5DoDA were found in blood of CFRE individuals, driven by the large mass fraction of this compartment coupled with relatively high concentrations of PFEAs in whole blood (Fig. 2).
Legacy and novel PFAS in brain
10 compounds were detected in at least one brain sample and 8 detected in at least 50% of brain samples, demonstrating that multiple PFAS can migrate across the blood-brain barrier. PFOS displayed the highest mean concentration in brain, and PFCAs made up the largest fraction observed in brain (44% of total concentration across all birds), with PFUdA found at mean concentrations of 13, 8, and 3 ng/g in brains from CFRE, Massachusetts Bay, and Narragansett Bay individuals, respectively. The dominance of PFCAs was particularly notable in brains from Massachusetts Bay and Narragansett Bay individuals in which [PFOS] was lower, with PFCAs constituting a mean of 57% of total concentrations observed in brains (n = 6) (Tables S5). Pairwise concentration comparison suggests PFTrDA is preferentially taken up into the brain compared to structurally analogous C9 – C12 PFCAs (Table S6). Similar results for long-chain PFCAs in marine mammals brain14 have been hypothesized to be driven by phospholipid-mediated uptake.
Nafion byproduct-2, PFO4DA, and PFO5DoDA were all detected in brain tissue of individuals from the CFRE, with detection frequencies of 80%, 60%, and 100%, respectively. Migration across the blood-brain barrier (BBB) is highly restricted for ionic species like PFAAs and PFEAs, and is thought to occur via active transport pathways.35 Empirical observations in marine mammals and birds demonstrate the presence of long-chain PFCAs, PFOS, and PFDS in brain tissue, suggesting BBB transport of PFCAs with C >9 or PFSAs with C ≥ 8 is possible, whether by OATP1A2 or another unspecified transport pathway related to or mediated by phospholipid interactions9,13,14.
Our results indicate that ether-based PFAS chemistries readily migrate across the highly selective blood-brain barrier in vertebrate wild animals subject to environmentally relevant PFEA exposures. Data from polar bears16, frogs36, and mice37 suggest some legacy PFAS (PFOS, PFCAs) are associated with alterations of neurochemical signaling and proteins critical for brain development, with uncertain long-term implications. Modeling suggests ether-based PFAS have similar or higher toxic potency compared to PFAAs38. Further research is required to examine PFEA impacts in the brain, given their proven ability to migrate into this tissue compartment.
Decreased preference of PFO5DoDA for brain
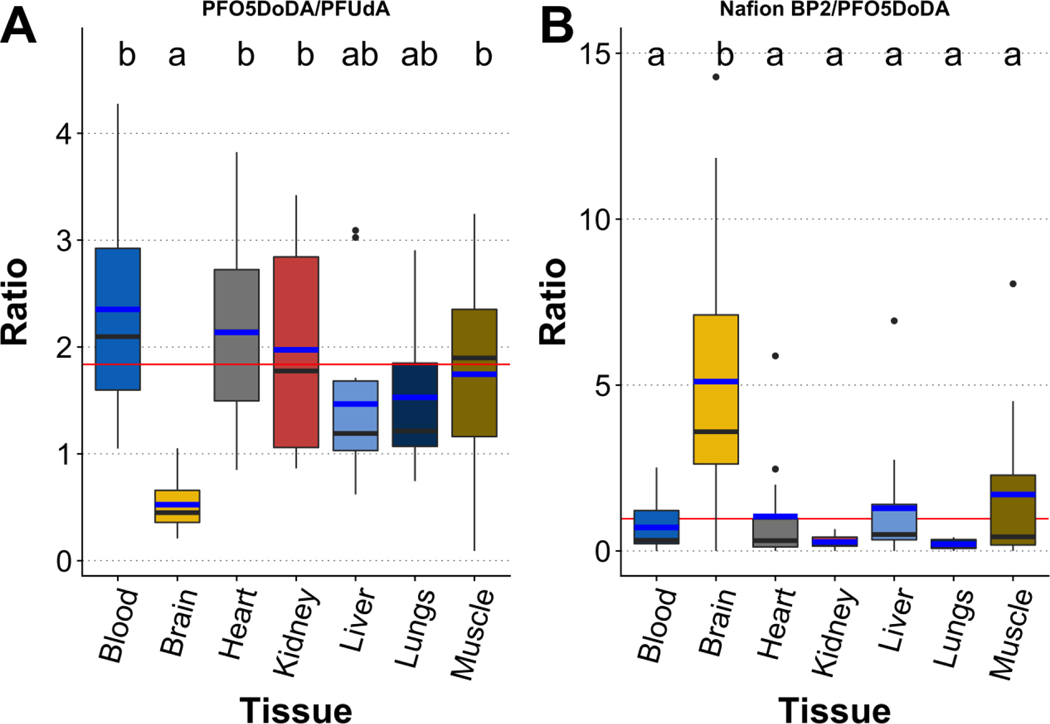
Concentration ratios facilitate the evaluation of compound partitioning between different tissues, as compounds subject to similar exposure and distribution pathways across multiple tissues likely share similar ratios between different tissues and vice versa. Using concentration ratios, we note that PFO5DoDA, a PFOA substitute with a backbone incorporating multiple ether linkages, partitions differently into brain than in other tissues compared to PFUdA, PFTrDA, PFOS, and Nafion BP2 (Fig. 3, Table S6).
Figure 3.
Ratios of PFAS concentrations in seven tissues from all CFRE individuals, with A) presenting concentrations of PFO5DoDA divided by PFUdA and B) presenting concentrations of Nafion BP2 divided by concentrations of PFO5DoDA. Fat was excluded due to the very low detection frequency of PFO5DoDA and PFUdA in fat. The blue line within each box plot indicates the arithmetic mean while the black line indicates the median. The box hinges represent the first and third quartiles, and the whiskers indicate 1.5 times the interquartile range. Outliers are indicated by black dots. The red line indicates the mean of each concentration ratio including all tissues except brain. Common letters above each boxplot indicate tissues that share statistically indistinguishable concentration ratios as determined using Tukey’s post-hoc test.
This suggests a combination of structural features, such as the inclusion of multiple ether linkages (>2) that increase backbone flexibility, the molecular size of PFO5DoDA (11-member backbone), and/or the -COOH head group, reduce transport across the BBB compared to other examined PFAAs and the PFESA Nafion BP2. We consider the ether-linkages, molecular size, and/or increased backbone flexibility of PFO5DoDA to be the most important features for its accumulation in brain, given that the -COOH functional group is also present in long-chain PFCAs that preferentially partition to brain. The ether-based chemicals distribute across most other tissues in a similar manner as long-chain PFAA analogues, with a preference for blood and kidney apparent in some comparisons (Table S6).
Relative sorption strength of PFEAs to proteins
The high PFAS concentrations observed in blood suggests albumin may offer increased binding opportunity for PFEAs via its multiple binding sites with distinct binding affinities that favor a range of PFAS chain lengths and functional groups28,39. We used quantum chemical calculations performed via COSMOconf/TURBOMOLE to explore this hypothesis; these calculations allowed the comparison of electrostatic surface potential between the lowest energy PFEA conformations and their respective PFAA homologues (Table S9). Nafion BP2 possessed increased probabilities of positive surface charge density compared to PFOS, and reduced probabilities of neutral surface charge. Given the sensitivity of binding cavity interactions to charge, we hypothesize that these aberrations in charge potential between PFEAs and PFAAs may result in a reduced ability for Nafion BP2 to engage in hydrophobic interactions in the L-FABP active site. This could help explain the reduced presence of Nafion BP2 in liver compared to blood. However, PFO5DoDA possessed an increased probability of neutral surface charge compared to PFOA, but was found at higher concentrations in blood compared to liver. Steric effects may drive differences in binding of PFO5DoDA to L-FABP compared to C8 - C10 PFAAs, which were found at higher concentrations in liver compared to blood in CFRE individuals. Sheng et al. (2018) found that several ether- and chlorine-containing emerging PFAS were structurally distorted during the binding process in orientations distinct from the “head-out” binding observed for PFAAs; they also interacted with a different suite of amino acid residues40.
Based on calculated and observational results observed across this study, we suggest multi-ether PFEAs engage with the L-FABP active site in a way distinct from the structural deformation and binding interactions observed between L-FABP and long-chain PFAAs. These differences may reduce the favorability of interaction in the primary L-FABP cavity, leading to reduced liver concentrations compared to blood (Figs. S3, S4). Alternatively, phospholipid binding is an additionally important mechanism driving PFAS accumulation in liver, in tandem with L-FABP interactions18. The multi-ether PFAS, with altered electron densities or conformational behavior compared to PFAAs, may be less likely to engage with uptake pathways that favor phospholipid-like species with a distinctly hydrophobic tail and a hydrophilic head group.
Implications
This study is the first to document the internal distribution behavior of PFEAs in any vertebrate organism, and details the presence of PFAAs and PFEAs in multiple tissues and organs of young seabirds. Multi-ether PFEAs were generally most abundant in blood rather than liver; we suggest compounds containing multiple ether linkages may find more flexible binding sites within serum albumin compared to the primary active site of L-FABP. Little research to date has examined the binding behavior of multi-ether PFEAs, which is needed considering their burgeoning environmental relevance and continued incorporation in industrial processes. Overall, we note that continued reliance on ether-based PFAS may not translate to reduced tissue accumulation of PFAS as our work indicates ether-based chemicals are readily transported to multiple body compartments such as the brain, with unknown biological consequences. Our results also reinforce the utility of birds to better understand environmental distributions and internal partitioning of PFAS.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Northeast Fisheries Observer Program, Gina Shield, Johanna Pedersen, Michael Moore, Lindsay Addison, Matthew McIver, the Wildlife Clinic of Rhode Island, and Kevin Powers for assistance obtaining and necropsying bird samples used in this studyWe acknowledge the help of Detlef Knappe (NCSU) in making this study feasible, Michaela Cashman, Diane Nacci, and Steve Rego (all US EPA) for critical feedback prior to peer review. The TOC art was created with BioRender.com, with the tern photo provided courtesy of Lindsay Addison. We thank Andrea Ebert and Kai Uwe Goss (Both UFZ Leipzig) for assistance with COSMOconf analysis and interpretation. Although EPA employees contributed to this article, the research presented was not funded by EPA and was conceived, designed, and implemented by URI. EPA’s role was limited to advising PFAS analysis and therefore not subject to EPA’s quality system requirements. Consequently, the views, interpretations, and conclusions expressed in the article are solely those of the authors and do not necessarily reflect or represent EPA’s views or policies.
Funding Sources
A. Robuck acknowledges support from the National Oceanic and Atmospheric Administration Dr. Nancy Foster Scholarship program, the Robert and Patricia Switzer Foundation, the STEEP Superfund Research Program (NIEHS Award Number P42ES027706), and the Oak Ridge Institute for Science and Education (ORISE) program.
ABBREVIATIONS
- PFOS
Perfluorooctanesulfonic acid
- PFAAs
Perfluoroalkyl acids
- PFEAs
Per- and polyfluoroalkyl ether acids
Footnotes
ASSOCIATED CONTENT
Supporting Information.
The following files are available free of charge.
Additional summary data, analyte list (Excel workbook)
Additional text describing methods, quality assurance, and providing additional figures in support of the text (PDF)
REFERENCES
- (1).Giesy JP; Kannan K. Global Distribution of Perfluorooctane Sulfonate in Wildlife Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol 2001, 35 (March), 1339–1342. 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- (2).Gebbink WA; Letcher RJ; Hebert CE; Chip Weseloh DVV Twenty Years of Temporal Change in Perfluoroalkyl Sulfonate and Carboxylate Contaminants in Herring Gull Eggs from the Laurentian Great Lakes. J. Environ. Monit 2011, 13 (12), 3365–3372. 10.1039/c1em10663e. [DOI] [PubMed] [Google Scholar]
- (3).Braune BM; Letcher RJ Perfluorinated Sulfonate and Carboxylate Compounds in Eggs of Seabirds Breeding in the Canadian Arctic: Temporal Trends (1975–2011) and Interspecies Comparison. Environ. Sci. Technol 2013, 47 (1), 616–624. 10.1021/es303733d. [DOI] [PubMed] [Google Scholar]
- (4).Ahrens L. Polyfluoroalkyl Compounds in the Aquatic Environment: A Review of Their Occurrence and Fate. J. Environ. Monit 2011, 13 (1), 20–31. 10.1039/c0em00373e. [DOI] [PubMed] [Google Scholar]
- (5).Jahnke A; Berger U. Trace Analysis of Per- and Polyfluorinated Alkyl Substances in Various Matrices-How Do Current Methods Perform? J. Chromatogr. A 2009, 1216 (3), 410–421. 10.1016/j.chroma.2008.08.098. [DOI] [PubMed] [Google Scholar]
- (6).Nakayama SF; Yoshikane M; Onoda Y; Nishihama Y; Iwai-Shimada M; Takagi M; Kobayashi Y; Isobe T. Worldwide Trends in Tracing Poly- and Perfluoroalkyl Substances (PFAS) in the Environment. TrAC - Trends Anal. Chem 2019, No. xxxx. 10.1016/j.trac.2019.02.011. [DOI] [Google Scholar]
- (7).Valsecchi S; Rusconi M; Polesello S. Determination of Perfluorinated Compounds in Aquatic Organisms: A Review. Anal. Bioanal. Chem 2013, 405 (1), 143–157. 10.1007/s00216-012-6492-7. [DOI] [PubMed] [Google Scholar]
- (8).Greaves AK; Letcher RJ; Sonne C; Dietz R; Born EW Tissue-Specific Concentrations and Patterns of Perfluoroalkyl Carboxylates and Sulfonates in East Greenland Polar Bears. Environ. Sci. Technol 2012, 46 (21), 11575–11583. 10.1021/es303400f. [DOI] [PubMed] [Google Scholar]
- (9).Verreault J; Houde M; Gabrielsen GW; Berger U; Haukås M; Letcher RJ; Muir DCG Perfluorinated Alkyl Substances in Plasma, Liver, Brain, and Eggs of Glaucous Gulls (Larus Hyperboreus) from the Norwegian Arctic. Environ. Sci. Technol 2005, 39 (19), 7439–7445. 10.1021/es051097y. [DOI] [PubMed] [Google Scholar]
- (10).Gebbink WA; Letcher RJ Comparative Tissue and Body Compartment Accumulation and Maternal Transfer to Eggs of Perfluoroalkyl Sulfonates and Carboxylates in Great Lakes Herring Gulls. Environ. Pollut 2012, 162, 40–47. 10.1016/j.envpol.2011.10.011. [DOI] [PubMed] [Google Scholar]
- (11).Shi Y; Vestergren R; Nost TH; Zhou Z; Cai Y. Probing the Differential Tissue Distribution and Bioaccumulation Behavior of Per- and Polyfluoroalkyl Substances of Varying Chain-Lengths, Isomeric Structures and Functional Groups in Crucian Carp. Environ. Sci. Technol 2018, 52 (8), 4592–4600. 10.1021/acs.est.7b06128. [DOI] [PubMed] [Google Scholar]
- (12).Shi Y; Vestergren R; Zhou Z; Song X; Xu L; Liang Y; Cai Y. Tissue Distribution and Whole Body Burden of the Chlorinated Polyfluoroalkyl Ether Sulfonic Acid F-53B in Crucian Carp (Carassius Carassius): Evidence for a Highly Bioaccumulative Contaminant of Emerging Concern. Environ. Sci. Technol 2015, 49 (24), 14156–14165. 10.1021/acs.est.5b04299. [DOI] [PubMed] [Google Scholar]
- (13).Ahrens L; Siebert U; Ebinghaus R. Total Body Burden and Tissue Distribution of Polyfluorinated Compounds in Harbor Seals (Phoca Vitulina) from the German Bight. Mar. Pollut. Bull 2009, 58 (4), 520–525. 10.1016/j.marpolbul.2008.11.030. [DOI] [PubMed] [Google Scholar]
- (14).Dassuncao C; Pickard H; Pfohl M; Tokranov AK; Li M; Mikkelsen B; Slitt A; Sunderland EM Phospholipid Levels Predict the Tissue Distribution of Poly- and Perfluoroalkyl Substances in a Marine Mammal. Environ. Sci. Technol. Lett 2019, 6 (3), 119–125. 10.1021/acs.estlett.9b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Holmström KE; Berger U. Tissue Distribution of Perfluorinated Surfactants in Common Guillemot (Uria Aalge) from the Baltic Sea. Environ. Sci. Technol 2008, 42 (16), 5879–5884. 10.1021/es800529h. [DOI] [PubMed] [Google Scholar]
- (16).Eggers Pedersen K; Basu N; Letcher R; Greaves AK; Sonne C; Dietz R; Styrishave B. Brain Region-Specific Perfluoroalkylated Sulfonate (PFSA) and Carboxylic Acid (PFCA) Accumulation and Neurochemical Biomarker Responses in East Greenland Polar Bears (Ursus Maritimus). Environ. Res 2015, 138, 22–31. 10.1016/j.envres.2015.01.015. [DOI] [PubMed] [Google Scholar]
- (17).Ng CA; Hungerbühler K. Bioconcentration of Perfluorinated Alkyl Acids: How Important Is Specific Binding? Environ. Sci. Technol 2013, 47 (13), 7214–7223. 10.1021/es400981a. [DOI] [PubMed] [Google Scholar]
- (18).Ng CA; Hungerbühler K. Bioaccumulation of Perfluorinated Alkyl Acids: Observations and Models. Environ. Sci. Technol 2014, 48 (9), 4637–4648. 10.1021/es404008g. [DOI] [PubMed] [Google Scholar]
- (19).Armitage JM; Arnot JA; Wania F. Potential Role of Phospholipids in Determining the Internal Tissue Distribution of Perfluoroalkyl Acids in Biota. Environ. Sci. Technol 2012, 46 (22), 12285–12286. 10.1021/es304430r. [DOI] [PubMed] [Google Scholar]
- (20).Armitage JM; Arnot JA; Wania F; Mackay D. Development and Evaluation of a Mechanistic Bioconcentration Model for Ionogenic Organic Chemicals in Fish. Environ. Toxicol. Chem 2013, 32 (1), 115–128. 10.1002/etc.2020. [DOI] [PubMed] [Google Scholar]
- (21).Zhao HX; Jiang JQ; Wang YL; Xie Q; Qu BC Phototransformation of 2,4,6-Tribromophenol in Aqueous Solution: Kinetics and Photolysis Products. J. Environ. Sci. Heal. Part A 2017, 52 (1), 45–54. 10.1080/10934529.2016.1229926. [DOI] [PubMed] [Google Scholar]
- (22).Zhang B; He Y; Yang G; Chen B; Yao Y; Sun H; Kannan K; Zhang T. Legacy and Emerging Poly- and Perfluoroalkyl Substances in Finless Porpoises from East China Sea: Temporal Trends and Tissue-Specific Accumulation. 2021. 10.1021/acs.est.1c00062. [DOI] [PubMed] [Google Scholar]
- (23).Robuck A; Cantwell MG; McCord JP; Addison LM; Pfohl M; Strynar MJ; McKinney RA; Katz DR; Wiley DN; Lohmann R. Legacy and Novel Per- and Polyfluoroalkyl Substances (PFAS) in Juvenile Seabirds from the US Atlantic Coast. Environ. Sci. Technol 2020. 10.1021/acs.est.0c01951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Mccord J; Strynar M. Identifying Per- and Polyfluorinated Chemical Species with a Combined Targeted and Non-Targeted-Screening High-Resolution Mass Spectrometry Workflow. J. Vis. Exp 2019, 146 (e59142), 1–15. 10.3791/59142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Strynar M; Dagnino S; McMahen R; Liang S; Lindstrom A; Andersen E; McMillan L; Thurman M; Ferrer I; Ball C. Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS). Environ. Sci. Technol 2015, 49 (19), 11622–11630. 10.1021/acs.est.5b01215. [DOI] [PubMed] [Google Scholar]
- (26).Guillette TC; McCord J; Guillette M; Polera ME; Rachels KT; Morgeson C; Kotlarz N; Knappe DRU; Reading BJ; Strynar M; Belcher SM Per- and Polyfluoroalkyl Substances Exposure in Cape Fear River Striped Bass (Morone Saxatilis) Is Associated with Biomarkers of Altered Immune and Liver Function Research. Environ. Int 2019, 2 (1), 105358. 10.1016/j.envint.2019.105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria: 2020. [Google Scholar]
- (28).Allendorf F; Berger U; Goss K-UU; Ulrich N. Partition Coefficients of Four Perfluoroalkyl Acid Alternatives between Bovine Serum Albumin (BSA) and Water in Comparison to Ten Classical Perfluoroalkyl Acids. Environ. Sci. Process. Impacts 2019, 21 (11), 1852–1863. 10.1039/c9em00290a. [DOI] [PubMed] [Google Scholar]
- (29).Kotlarz N; McCord J; Collier D; Lea C; Strynar M; Lindstron A; Wilkie A; Islam J; Matney K; Tarte P; Polera M; Burdette K; DeWitt J; May K; Smart R; Knappe D; Hoppin JA Measurement of Novel, Drinking Water-Associated PFAS in Blood from Adults and Children in Wilmington, North Carolina. Env. Heal. Perspect 2020, 128 (July), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Nakayama S; Strynar MJ; Helfant L; Egeghy P; Ye X; Lindstrom AB Perfluorinated Compounds in the Cape Fear Drainage Basin in North Carolina. Environ. Sci. Technol 2007, 41, 5271–5276. 10.1021/es070792y. [DOI] [PubMed] [Google Scholar]
- (31).Hopkins ZR; Sun M; DeWitt JC; Knappe DRU Recently Detected Drinking Water Contaminants : GenX and Other Per- and Polyfluoroalkyl Ether Acids. J. AWWA 2018, 110 (7), 13–28. 10.1002/awwa.1073. [DOI] [Google Scholar]
- (32).Yao J; Pan Y; Sheng N; Su Z; Guo Y; Wang J; Dai J. Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs): Occurrence and Association with Serum Biochemical Parameters in Residents Living Near a Fluorochemical Plant in China. Environ. Sci. Technol 2020. 10.1021/acs.est.0c02888. [DOI] [PubMed] [Google Scholar]
- (33).Jones PD; Hu WY; De C; Newsted JL; Giesy JP Binding of Perfluorinated Fatty Acids to Serum Proteins. Environ. Toxicol. Chem 2003, 22, 2639–2649. [DOI] [PubMed] [Google Scholar]
- (34).Zhang L; Ren XM; Guo LH Structure-Based Investigation on the Interaction of Perfluorinated Compounds with Human Liver Fatty Acid Binding Protein. Environ. Sci. Technol 2013, 47 (19), 11293–11301. 10.1021/es4026722. [DOI] [PubMed] [Google Scholar]
- (35).Barar J; Rafi MA; Pourseif MM; Omidi Y. Blood-Brain Barrier Transport Machineries and Targeted Therapy of Brain Diseases. BioImpacts 2016, 6 (4), 225–248. 10.15171/bi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Foguth RM; Hoskins TD; Clark GC; Nelson M; Flynn RW; de Perre C; Hoverman JT; Lee LS; Sepúlveda MS; Cannon JR Single and Mixture Per- and Polyfluoroalkyl Substances Accumulate in Developing Northern Leopard Frog Brains and Produce Complex Neurotransmission Alterations. Neurotoxicol. Teratol 2020, 81 (June), 106907. 10.1016/j.ntt.2020.106907. [DOI] [PubMed] [Google Scholar]
- (37).Johansson N; Eriksson P; Viberg H. Neonatal Exposure to PFOS and PFOA in Mice Results in Changes in Proteins Which Are Important for Neuronal Growth and Synaptogenesis in the Developing Brain. Toxicol. Sci 2009, 108 (2), 412–418. 10.1093/toxsci/kfp029. [DOI] [PubMed] [Google Scholar]
- (38).Gomis MI; Vestergren R; Borg D; Cousins IT Comparing the Toxic Potency in Vivo of Long-Chain Perfluoroalkyl Acids and Fluorinated Alternatives. Environ. Int 2018, 113 (November 2017), 1–9. 10.1016/j.envint.2018.01.011. [DOI] [PubMed] [Google Scholar]
- (39).Chen YM; Guo LM Fluorescence Study on Site-Specific Binding of Perfluoroalkyl Acids to Human Serum Albumin. Arch. Toxicol 2009, 83, 255–261. 10.1007/s00204-008-0359-x. [DOI] [PubMed] [Google Scholar]
- (40).Sheng N; Cui R; Wang J; Guo Y; Wang J; Dai J. Cytotoxicity of Novel Fluorinated Alternatives to Long-Chain Perfluoroalkyl Substances to Human Liver Cell Line and Their Binding Capacity to Human Liver Fatty Acid Binding Protein. Arch. Toxicol 2018, 92 (1), 359–369. 10.1007/s00204-017-2055-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.