ABSTRACT.
Primary neuritic leprosy is a form of leprosy clinically limited to the peripheral nerves without obvious skin lesions. Diagnosing leprosy in the absence of typical dermatological features is challenging and often causes a delay in diagnosis. We describe a case of primary neuritic leprosy with atypical features and the roles that histological confirmation using nerve biopsy of an unenlarged nerve and newer techniques, such as polymerase chain reaction and high-resolution ultrasonography, play in improving the diagnosis.
INTRODUCTION
Hansen’s disease (HD; or leprosy) is a chronic granulomatous infection caused by two Mycobacterium species: Mycobacterium leprae and Mycobacterium lepromatosis. The disease is frequently manifested by skin lesions that are often hypo-esthetic; enlarged peripheral nerves; and, in the lepromatous form, the presence of acid-fast bacilli (AFB) in slit-skin smears. Its clinical forms are defined by the host immune response and bacillary load, resulting in a wide clinical spectrum.1 Although HD is rare in the US, as many as 2 million people are permanently disabled as a result of HD worldwide.2 At the end of 2019, there were 202,185 new leprosy cases reported to WHO. Of these, 71.3% were from southeast Asia.3
Hansen’s disease clinically limited to the peripheral nerves without skin involvement is classified as primary neuritic leprosy (PNL).4 Diagnosing leprosy in the absence of typical dermatological features is challenging and frequently causes a delay in diagnosis. It often requires histological confirmation, which is achievable only using nerve biopsy, but newer techniques such as polymerase chain reaction (PCR)5 and high-resolution ultrasonography6 have improved the diagnosis. We report an unusual case of PNL manifesting as bilateral involvement of multiple non-enlarged nerve trunks with skin biopsy revealing lepromatous leprosy in normal appearing skin. This case took 2 years to diagnose and highlights several diagnostic and management issues.
CASE REPORT
A 50-year-old, insulin-dependent diabetic man from India presented with 6 years of tingling and numbness and occasional shooting pain in his feet that had progressed to involve his hands despite gabapentin and vitamin B12. Electromyogram (EMG) showed bilateral patchy, asymmetric, and sensory predominant axonal polyneuropathy of both upper and lower extremities that was superimposed on demyelinating ulnar neuropathy at the elbows bilaterally. His pattern of involvement raised consideration of autoimmune disease and paraneoplastic syndrome, which were ruled out. He reported no exposure to leprosy. His symptoms worsened, with proximal extension prompting a referral to dermatology, where no suspicious skin lesions for leprosy were noted.
He was followed in neurology clinic. His examination was significant for progressive bilateral patchy sensory loss without significant motor changes. Repeat EMG performed a year later showed worsening of both sensory and motor conduction abnormalities involving his left median and ulnar nerves across the forearm. This raised the possibility of chronic inflammatory demyelinating polyneuropathy. His cervical spine magnetic resonance imaging (MRI) and examination of his cerebrospinal fluid were both normal. He was started on intravenous immunoglobulin, which worsened his symptoms and was stopped after two courses of treatment.
The patient subsequently developed bilateral, symmetrically swollen wrists and ankles with sausage digits. A left ankle aspiration was negative for bacterial, fungal, and mycobacterial cultures. He developed right ulnar nerve tenderness in his ulnar groove. A left sural nerve biopsy showed severely decreased density of myelinated fibers in a diffuse pattern and more empty nerve strands. Many AFB within noncaseating granulomas were detected, and PCR for M. leprae was positive. A skin biopsy from normal-appearing skin on his left hand showed foamy macrophages and noncaseating granulomas (Figure 1). The skin biopsy also demonstrated superficial perivascular and perineural lymphohistiocytic inflammatory reaction and Fite stain positive bacilli in foamy macrophages, consistent with lepromatous leprosy (Figure 2). Many AFB were also seen on Ziehl-Neelsen smear (Figure 3). Skin biopsy of his left ear lobe was Fite stain negative. His Quantiferon gold assay, hepatitis serologies, and HIV antibody were negative.
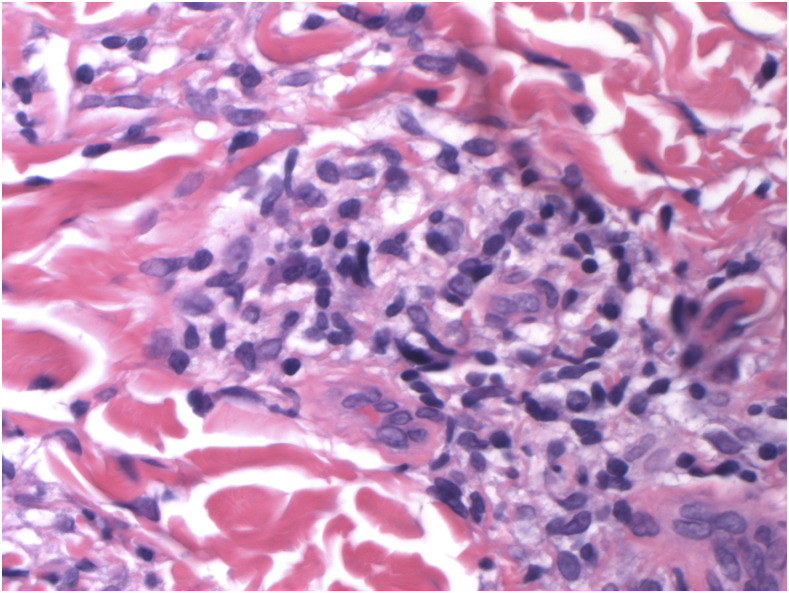
Figure 1.
Foamy macrophages and noncaseating granulomas in skin biopsy of lepromatous leprosy. H&E (hematoxylin and eosin) stain. 600x magnification. This figure appears in color at www.ajtmh.org.
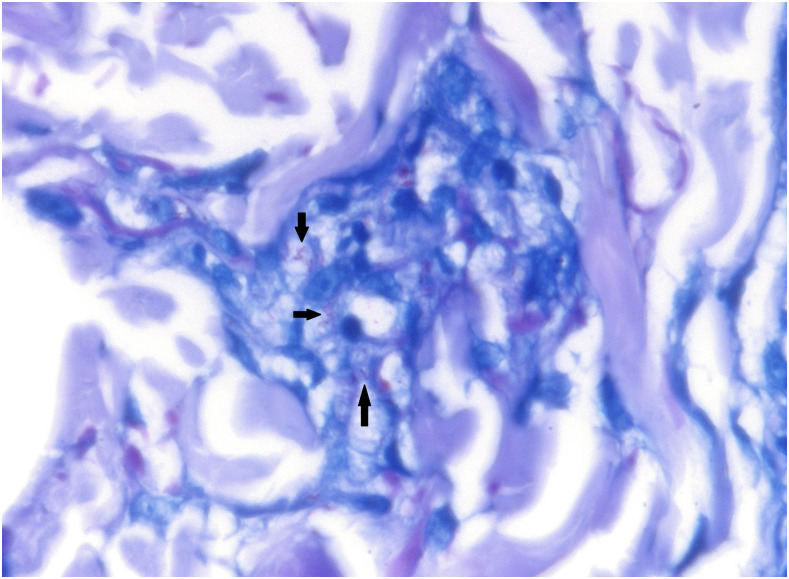
Figure 2.
Several Fite stain–positive bacilli (black arrows) within foamy macrophages, seen in a skin biopsy. Fite stain. 600x magnification. This figure appears in color at www.ajtmh.org.
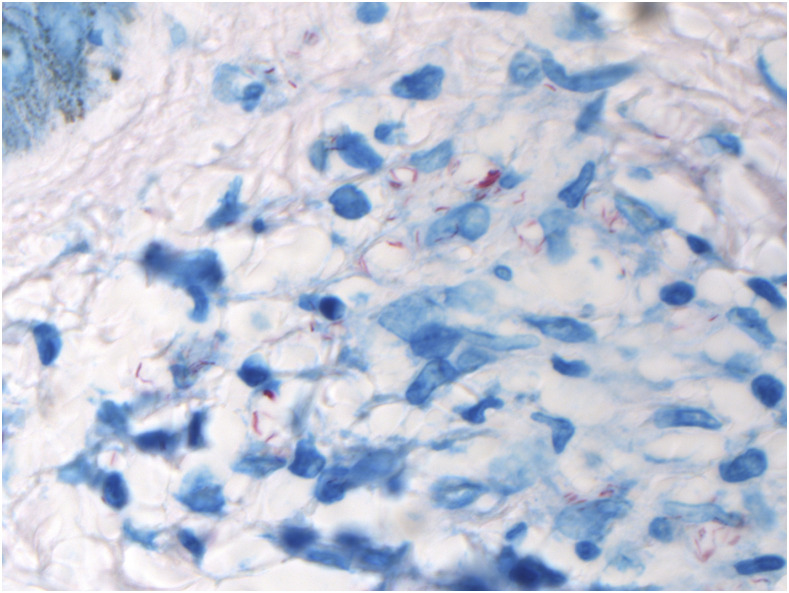
Figure 3.
Ziehl-Neelsen stain, positive on skin biopsy. Ziehl-Neelsen stain. 600x magnification. This figure appears in color at www.ajtmh.org.
The patient was started on rifampin, clofazimine, and ofloxacin (he was G6PD deficient) and prednisone for his HD and polyneuropathies. His ofloxacin was switched to minocycline due to ongoing tendonitis. Persistent neurological symptoms required prolonged steroid use for 1 year. This was replaced by methotrexate as a steroid-sparing agent. A repeat EMG performed 6 months into treatment showed mild improvement of sensory nerve conduction of his left ulnar nerve. He developed mild erythema nodosum leprosum on his face and neck area, which resolved with ongoing steroids and increased clofazimine. He completed 2 years of multidrug therapy (MDT) but remained on methotrexate for a third year for his improving neuropathy. His joint symptoms resolved after 2 months.
DISCUSSION
The WHO classification of leprosy is divided into paucibacillary disease if there are fewer than five skin lesions and multibacillary disease, if there are five or more skin lesions (or any number of skin lesions with neuritis)7 (Table 1).
Table 1.
Leprosy classification: Ridley Jopling and WHO
| Ridley Jopling Classification (1966) | WHO Classification (2017) |
|---|---|
| Tuberculoid leprosy (TT) | Paucibacillary Leprosy |
| Borderline tuberculoid (BT) | Up to five skin lesions |
| Slit skin smears negative on all sites | |
| Borderline borderline (BB) | Multibacillary leprosy |
| Borderline lepromatous (BL) | Six or more skin lesions (or any nerve involvement with fewer lesions) |
| Lepromatous leprosy (LL) | Pure neuritis |
| Slit skin smears positive |
Of patients with leprosy, 4–8% (up to 18% in some Indian series) may present with PNL4 characterized by evidence of nerve deficit or thickening, with or without tenderness in the absence of skin involvement. Our patient had involvement of 11 nerve trunks without any skin lesions, and a blind skin biopsy revealed high bacillary load and foamy macrophages consistent with lepromatous leprosy. His nerve biopsy also showed presence of AFB. These clinical findings along with biopsy results, are consistent with PNL.
Mycobacterium leprae infects Schwann cells and axons, causing a subacute demyelination involving cutaneous nerves and larger peripheral nerve trunks. The organism preferentially invades nonmyelin-producing Schwann cells (attaching via phenolic glycolipid 1, an M. leprae–specific antigen) where the organisms multiply in large numbers. After attachment of the organism, myelin-producing Schwann cells also undergo significant loss of myelination by an immune-dependent mechanism. The Schwann cells are also altered by infection, producing metabolic and functional changes that trigger the immune system in recruiting cytotoxic cells, T lymphocytes, and macrophages. The end result is unrestrained multiplication of the organism within Schwann cells, destruction of myelin, inflammatory changes leading to axonal injury, and secondary destruction of the nerve architecture. The discovery that demyelination can be induced in vivo by the bacterial cell-wall antigen alone may explain the ongoing neurologic damage that can occur in leprosy after treatment. Nerve growth factor is a neurotrophin produced by multiple cell types, including neurons and Schwann cells. The role of nerve growth factor in leprosy is not fully understood but may be involved in the nerve damage and ensuing neuropathic pain.8
The diagnosis of PNL can often be difficult and delayed, resulting in the presence of disability at the time of presentation.9 Jardim et al.10 assessed 67 patients with PNL and found the most common presentation was mono-neuritis multiplex, occurring in 61% of the patients, followed by mononeuropathy in 33% and polyneuropathy in only 6%. Sensory impairment occurred in 89% of all cases and motor dysfunction in 81%.10 Axonal neuropathy as was present in our patient was the predominant electrophysiological finding. Acid-fast bacilli were only found in 16%, and PCR for M. leprae was positive in 47% of the nerve biopsy samples. In a prospective study of 317 new cases of PNL in Brazil5, the combined evaluation of all diagnostic tools, including PCR of the skin, EMG, symptoms and neural thickening, and ELISA anti-pepsinogen 1, demonstrated a highly variable presentation with 8.6% negative in all tests. This study reinforces the need for nerve biopsy in many cases.
Any nerve may be involved in PNL. The dorsal ulnar cutaneous, the medial/lateral antebrachial, or the superficial radial or sural nerves are most suitable for biopsy. A nerve biopsy may be limited by sampling error because most PNL patients fall into the tuberculoid or borderline tuberculoid portions of the spectrum with low bacillary load.11 Nerve ultrasounds may be able to reduce the sampling error because they can identify and direct the biopsy to abnormal nerves that may be subclinical.6 High-resolution ultrasonography is an inexpensive tool that can be used to obtain information that is static (size of the nerve, echo texture, nerve abscess) and dynamic (Doppler studies of neural blood supply). It can be used on multiple nerves and can detect focal nerve thickening (not picked up clinically), and sequential studies can be used to follow increased vascularity in leprosy reactional states. Studies have shown close correlation between abnormalities on ultrasound and nerve conduction studies in leprosy. In contrast, MRI allows a more detailed but expensive evaluation of peripheral nerves, limiting its use in leprosy, where its use is not usually necessary. Leprotic peripheral nerve involvement ranges from nerve thickening on T1- and T2-weighted images, with preserved fascicular architecture to disruption of this architecture and formation of micro-abscesses.12 Magnetic resonance imaging has also allowed detection of plexopathies and lesions in the spinal cord and brain. This was not used in our patient because of the multiple nerves involved and the clinical decision that his diagnosis required a nerve biopsy.
Fine needle aspiration of affected nerves for cytology, Fite staining, and PCR for M. leprae DNA in nerves have all been helpful.5 The histopathology of PNL reveals a spectrum ranging from tuberculoid to borderline lepromatous type. Polar lepromatous leprosy in PNL as manifested in our patient is very rarely described.13 In lepromatous leprosy, there is extensive nerve fiber loss with a concomitant increase in endoneurial collagen, and numerous bacilli are seen in foamy macrophages.
Our patient illustrates the difficulty in making a diagnosis of PNL when there are atypical features present, despite a high index of suspicion, given his country of origin. His neurological symptoms had predated his diabetes, which was not severe and was well controlled. In addition, the presence of motor nerve deficits on EMG and the involvement of many large nerve trunks were felt by his neurologist to be atypical for diabetes. Given the diffuse nature of his neurological involvement, it was surmised that his clinical picture would be most consistent with lepromatous leprosy, which rarely presents as PNL. In addition, he had no skin lesions or any evidence of palpable nerve trunks based on the examination by two experienced clinicians. In retrospect, it would have been helpful for him to have had an earlier nerve biopsy in addition to a blind skin biopsy for PCR, although both may have been negative. Biopsies of apparently normal skin from the areas of sensory change in cases of PNL have revealed microscopic evidence of nerve involvement in deep dermal tissue.14 There are many reports of patients with PNL developing visible skin lesions during follow-up of months and years (with and without MDT),15 including progression to classical borderline tuberculoid leprosy in typical patients with mononeuropathy. These findings suggest that, in leprosy, a neuritic phase precedes the development of visible cutaneous lesions.
Multidrug therapy alone is aimed at treating the infection but is insufficient in preventing the nerve damage. Unlike WHO guidelines,7 in India (where PNL is most common), when one nerve trunk is involved, it is considered paucibacillary, and when more than one nerve trunk is involved, it is considered multibacillary for therapeutic purposes. Prednisone remains the drug of choice for neuritis due to its ability to reduce nerve edema, act as an immunosuppressant, and decrease postinflammatory scar formation, which are all important for improving nerve function. A prospective longitudinal trial in Brazil16 studied 24 PNL patients who were treated with MDT (dapsone, clofazimine, and rifampin) and prednisone (1 mg/kg) tapered over the 6-month treatment period. Most of the clinical parameters showed significant improvement; EMG evidence of nerve conduction block was reduced in 42% of the patients. contributing to the prevention of further neurological damage. Cyclosporine, azathioprine, and methotrexate have all been tried as steroid sparing agents in leprous neuritis. Physical therapy and education about self-care may be required in those who have residual permanent nerve function impairment. Mutations associated with resistance to rifampin, ofloxacin, or dapsone have been identified in 7.8% specimens of PNL17 but have yet to be found to be clinically significant.
In conclusion, PNL often presents with disability and poses a challenge in diagnosis. There should be a low threshold for nerve biopsy of affected nerve trunks (even if not enlarged) and blind skin biopsy of normal appearing skin in the distribution of the affected nerves for histology, Fite stain, and PCR. This will expedite diagnosis and treatment that will decrease further neurological dysfunction.
References
- 1.Jopling WH , 1956. Borderline (dimorphous) leprosy maintaining a polyneuritic form for eight years: a case report. Trans R Soc Trop Med Hyg 50: 478–480. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of High-Consequence Pathogens and Pathology (DHCPP) , 2017. Hansen’s Disease (Leprosy). Available at: www.cdc.gov/leprosy/transmission. Accessed February 10, 2017.
- 3.WHO , 2020. Global leprosy (Hansen disease) update, 2019: time to step-up prevention initiatives. Weekly Epidemiol Record 95: 417–440. [Google Scholar]
- 4.Wilder-Smith E , 2002. Diagnosis of pure neuritic leprosy. Neurol J Southeast Asia 7: 61–63. [Google Scholar]
- 5.Santos D Mendonça MR Antunes DE Sabino E Pereira RC Goulart LR Goulart I , 2017. Revisiting primary neural leprosy: clinical, serological, molecular, and neurophysiological aspects. PLoS Negl Trop Dis 11: e0006086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain S Visser LH Yerasu MR Raju R Meena AK Lokesh B Suneetha S , 2013. Use of high-resolution ultrasonography as an additional tool in the diagnosis of primary neuritic leprosy: a case report. Lepr Rev 84: 161–165. [PubMed] [Google Scholar]
- 7.WHO , 2017. Guidelines for the Diagnosis, Treatment and Prevention of Leprosy. New Delhi, India: WHO, Regional Office for South-East Asia. [Google Scholar]
- 8.Ebenezer GJ Polydefkis M Scollard DM , 2016. Mechanisms of nerve injury in leprosy, Scollard DM, Gillis TP, eds. International Textbook of Leprosy. Available at: https://www.internationaltextbookofleprosy.org/chapter/mechanisms-nerve-injury-leprosy. Accessed March 3, 2021.
- 9.Kolleri JJ Sasidharanpillai S Vadakkayil B Chathoth AT , 2019. A 10-year retrospective descriptive study on pure neuritic leprosy from a tertiary referral centre. Indian Dermatol Online J 10: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jardim MR et al. 2003. Criteria for diagnosis of pure neural leprosy. J Neurol 250: 806–809. Accessed March 3, 2021. [DOI] [PubMed] [Google Scholar]
- 11.Vijayan J Wilder-Smith EP , 2016. Neurological manifestations of leprosy. Scollard DM, Gillis TP, eds. International Textbook of Leprosy. Available at: https://www.internationaltextbookofleprosy.org/chapter/neurological-manifestations-leprosy.
- 12.Jabeen S et al. 2020. Neuroimaging in leprosy: the nerves and beyond. Radiol Infect Dis 7: 12–21. [Google Scholar]
- 13.Shetty VP Rambhia KD Khopkar US , 2017. Descriptive pathology of nerves in leprosy. Scollard DM, Gillis TP, eds. International Textbook of Leprosy. Available at https://www.internationaltextbookofleprosy.org/chapter/descriptive-pathology-nerves-leprosy. Accessed March 3, 2021.
- 14.Suneetha S Arunthathi S Chandi S Kurian N Chacko CJ , 1998. Histological studies in primary neuritic leprosy: changes in the apparently normal skin. Lepr Rev 69: 351–357. [DOI] [PubMed] [Google Scholar]
- 15.Suneetha S Sigamoni A Kurian N Chacko CJG , 2005. The development of cutaneous lesions during follow‐up of patients with primary neuritic leprosy. Int J Dermatol 44: 224–229. [DOI] [PubMed] [Google Scholar]
- 16.Mahajan NP et al. 2020. Evidence for Mycobacterium leprae drug resistance in a large cohort of leprous neuropathy patients from India. Am J Trop Med Hyg 102: 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jardim MR Illarramendi X Nascimento OJ Nery JA Sales AM Sampaio EP Sarno EN , 2007. Pure neural leprosy: steroids prevent neuropathy progression. Arq Neuropsiquiatr 65: 969–973. [DOI] [PubMed] [Google Scholar]