Abstract
The signaling routes linking G-protein-coupled receptors to mitogen-activated protein kinase (MAPK) may involve tyrosine kinases, phosphoinositide 3-kinase γ (PI3Kγ), and protein kinase C (PKC). To characterize the mitogenic pathway of bradykinin (BK), COS-7 cells were transiently cotransfected with the human bradykinin B2 receptor and hemagglutinin-tagged MAPK. We demonstrate that BK-induced activation of MAPK is mediated via the α subunits of a Gq/11 protein. Both activation of Raf-1 and activation of MAPK in response to BK were blocked by inhibitors of PKC as well as of the epidermal growth factor (EGF) receptor. Furthermore, in PKC-depleted COS-7 cells, the effect of BK on MAPK was clearly reduced. Inhibition of PI3-Kγ or Src kinase failed to diminish MAPK activation by BK. BK-induced translocation and overexpression of PKC isoforms as well as coexpression of inactive or constitutively active mutants of different PKC isozymes provided evidence for a role of the diacylglycerol-sensitive PKCs α and ɛ in BK signaling toward MAPK. In addition to PKC activation, BK also induced tyrosine phosphorylation of EGF receptor (transactivation) in COS-7 cells. Inhibition of PKC did not alter BK-induced transactivation, and blockade of EGF receptor did not affect BK-stimulated phosphatidylinositol turnover or BK-induced PKC translocation, suggesting that PKC acts neither upstream nor downstream of the EGF receptor. Comparison of the kinetics of PKC activation and EGF receptor transactivation in response to BK also suggests simultaneous rather than consecutive signaling. We conclude that in COS-7 cells, BK activates MAPK via a permanent dual signaling pathway involving the independent activation of the PKC isoforms α and ɛ and transactivation of the EGF receptor. The two branches of this pathway may converge at the level of the Ras-Raf complex.
The extracellular signal-regulated kinases ERK1 and ERK2 belong to the mitogen-activated protein kinase (MAPK) family and may be regulated by both receptor tyrosine kinases (RTKs) and G-protein-coupled receptors (GPCRs). Their activation via RTKs is well defined and includes the consecutive stimulation of the adaptor protein Grb2, the Ras-guanine nucleotide exchange factor Sos, the small G protein Ras, and a cascade of protein kinases consisting of Raf, MEK, and MAPK. Finally, activated MAPK stimulates nuclear transcription, thereby regulating cell proliferation and other cellular functions.
The mechanism of GPCR-induced stimulation of MAPK activity appears to be heterogeneous and more complex (14, 41). Thus, MAPK activation via Gi-coupled receptors, such as the α2A adrenergic receptor (17) or the M2 muscarinic receptor (29) has been reported to be mediated by Gβγ subunits involving phosphoinositide 3-kinase γ (PI3Kγ) and Ras. Downstream mediators of Gβγ might be cytosolic tyrosine kinases of the Src family and the adaptor protein Shc (43, 31). In contrast, receptors coupled to G proteins of the pertussis toxin (PTX)-insensitive Gq/11 family such as the M1 muscarinic receptor or the α1 adrenergic receptor activate MAPK via a protein kinase C (PKC)-dependent pathway which does not involve Gβγ and Ras (18). Once activated, PKC stimulates MAPK independently of Ras via Raf-1 (2). Gs-coupled receptors such as the β-adrenergic receptor were found to exert an opposite effect on MAPK, involving a Gβγ-mediated activation and a cyclic AMP-mediated inhibition (5). Cyclic AMP activates protein kinase A and phosphorylates Raf-1, resulting in a decreased Raf-1 kinase activity (15).
More recently, a GPCR-induced tyrosine phosphorylation (transactivation) of the epidermal growth factor (EGF) receptor (EGFR) (7, 8) or platelet-derived growth factor receptor (19) has been demonstrated. The mechanism of RTK transactivation is poorly understood. Thus, for Gi-coupled receptors, transactivation of the EGFR via βγ complexes and Src was proposed (31). In contrast, in stably transfected human 293 cells, EGFR transactivation in response to Gq/11-coupled M1 muscarinic receptor stimulation was found to be mediated in a PKC-dependent pathway (40). In Rat-1 or COS-7 cells, EGFR transactivation by several agonists of GPCRs without any effect on PKC activity was observed (7, 8). Finally, in GN4 rat liver epithelial cells, EGFR transactivation by angiotensin II was shown to be normally suppressed by PKC and to occur only when PKC activation is prevented (26). In these cells, angiotensin II activates MAPK via a latent dual signaling pathway.
Here we demonstrate that in COS-7 cells, stimulation of the human bradykinin B2 (BK) receptor (BKR) leads to the activation of the PKC pathway as well as to tyrosine phosphorylation of the EGFR. Both pathways are independently activated by BK. The inhibition of either of these pathways results in loss of the ability of BK to stimulate MAPK activity. To our knowledge, this represents a novel mechanism of MAPK activation by a GPCR via permanent dual signaling involving both the PKC pathway and EGFR transactivation.
MATERIALS AND METHODS
Cell culture, transfections, and preparation of cell lysates.
COS-7 cells (American Type Culture Collection) were routinely grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and antibiotics. Subconfluent cells were transfected with pcDNA3 (Invitrogen) expressing hemagglutinin (HA)-tagged MAPK p42 (pcDNA-HA-MAPK), pcDNA3-BKR, and additional cDNAs as indicated by the DEAE-dextran technique. The total amount of plasmid DNA was adjusted to 3 to 4 μg per plate with vector pcDNA3. Human kidney BKR cDNA was kindly provided by H. Appelhans, Max Planck Institute for Biophysics (Frankfurt/Main, Germany). pcDNA3-HA-MAPK (4) and pCDNA3-CD8-βARK, encoding the adrenergic receptor kinase fused to the transmembrane protein CD8 (5), were generously provided by J. S. Gutkind (National Institutes of Health, Bethesda, Md.). The cDNAs encoding for PKC isoforms α, βI, ɛ, and ζ were cloned into a cytomegalovirus promoter-driven expression vector. Kinase-inactive mutants of these PKC isoforms were generated by mutation of the conserved lysine residue within the ATP binding domain (22). Constitutively active mutants were generated by mutation of the conserved alanine residue (A-to-E mutation) within the pseudosubstrate domain of PKC (9). Two days after transfection, COS-7 cells were exposed to serum-free medium overnight and then left untreated or stimulated with the various agents as indicated, washed in cold phosphate-buffered saline (PBS), and lysed at 4°C in a buffer containing 20 mM HEPES (pH 7.5), 10 mM EGTA, 40 mM β-glycerophosphate, 1% Nonidet P-40, 2.5 mM MgCl2, 1 mM dithiothreitol (DTT), 2 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 20 μg of aprotinin per ml, and 20 μg of leupeptin per ml. The lysate was centrifuged at 14,000 × g for 20 min at 4°C. Equivalent expression levels of cDNA constructs were verified by Western blotting with the appropriate antibodies.
MAPK assay.
For MAPK assay, after centrifugation, proteins from clarified supernatants were immunoprecipitated with anti-HA monoclonal antibody (MAb) 12CA5 (Babco, Berkeley, Calif.) for 1 hour at 4°C, and immunocomplexes were recovered with Gamma-bind G-Sepharose (Pharmacia, Uppsala, Sweden). Bound proteins were washed three times with PBS supplemented with 1% Nonidet P-40 and 2 mM sodium vanadate, once with 0.5 M LiCl in 100 mM Tris-HCl (pH 7.5), and once with kinase reaction buffer (12.5 mM morpholinepropanesulfonic acid [pH 7.5], 12.5 mM β-glycerophosphate, 7.5 mM MgCl2, 0.5 mM EGTA, 0.5 mM sodium fluoride, 0.5 mM sodium vanadate). Reactions were performed in 30 μl of kinase buffer containing 1 μCi of [γ-32P]ATP (NEN Life Science Products, Boston, Mass.), 20 μM unlabeled ATP, 3.3 μM DTT, and 1.5 mg of myelin basic protein (MBP) at 30°C for 20 min. Reactions were terminated by addition of 5 volumes of Laemmli buffer. Samples were boiled, and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12.5% gels). Phosphorylated MBP was visualized by autoradiography and quantified with a phosphorimager. For some experiments, cell lysates were analyzed by Western blotting using an antibody specifically recognizing activated MAPK (Promega, Madison, Wis.).
Detection of PKC isoforms and PKC translocation experiments.
For the detection of endogenous and overexpressed PKC isoforms in COS-7 cells, whole-cell lysates were subjected to SDS-PAGE on 7.5% gels and transferred to Hybond polyvinylidene difluoride (PVDF) membranes (Amersham, Braunschweig, Germany). For PKC translocation experiments, COS-7 cells were transfected with BKR cDNA only and stimulated with BK (100 nM) for the times indicated. Then, cells were washed two times with 50 mM HEPES buffer (pH 7.4), suspended in 50 mM HEPES (pH 7.4), containing phenylmethylsulfonyl fluoride (0.1 mM), pepstatin (1 μg/ml), and leupeptin (2 μg/ml), homogenized, and centrifuged at 100,000 × g for 20 min at 4°C. Pellets were subjected to SDS-PAGE on 7.5% gels and blotted onto Hybond PVDF membranes. For both types of experiments, the PVDF strips were blocked in 1% bovine serum albumin–1% nonfat dried milk powder overnight, and the various PKC isoforms were detected using polyclonal antibodies against human PKC isoforms α, βI, βII, γ, δ, ɛ, and ζ (Santa Cruz Biotechnology, Santa Cruz, Calif.). For Western blot analysis, after incubation the PVDF strips were washed twice with Tris-buffered saline (pH 7.6) containing 0.05% (vol/vol) Tween 20, treated for 45 min with goat anti-rabbit immunoglobulin G conjugated to horseradish peroxidase (Santa Cruz), and washed again four times. Secondary antibodies were detected by using an enhanced chemiluminescence Western blotting detection system (Amersham) by exposure to Biomax films.
Assay of Raf-1 kinase activity.
The activity of Raf-1 kinase was assayed by measuring the phosphorylation of syntide-2 (Santa Cruz), a peptide substrate for Raf-1. In brief, immunoprecipitates were prepared by adding to the lysates a polyclonal anti-Raf-1 antibody (Santa Cruz) and protein A-Sepharose. After 2 h of incubation, the protein A-Sepharose was spun down and washed three times with PBS containing 1% Triton X-100 and 2 mM sodium vanadate, one time with 100 mM Tris-HCl (pH 7.4) containing 0.5 M LiCl, and one time with kinase buffer (25 mM Tris-HCl [pH 7.4], 10 mM MgCl2, 0.5 mM EGTA, 1 mM DTT, 2 mM sodium vanadate). The immunoprecipitates were incubated with the substrate (10 μg of syntide-2), 2 μCi of [γ-32P]ATP, and 40 μM ATP in kinase buffer for 30 min at 25°C. The reaction was terminated by heating for 2 min at 95°C, and the phosphorylation of syntide-2 was analyzed by SDS-PAGE on a 16% gel. The gel was dried and exposed for autoradiography. Alternatively, phosphorylated syntide-2 was collected by using Whatman P81 phosphocellulose paper. The paper was washed three times in 0.5% phosphoric acid and one time with acetone, dried, and measured by Cerenkov counting.
Detection of EGFR tyrosine phosphorylation.
Lysates from treated and untreated COS-7 cells were immunoprecipitated as described for the MAPK assay. Immunoprecipitation was performed with 1 μl of anti-EGFR MAb 425 (kindly provided by A. Luckenbach, E. Merck AG, Darmstadt, Germany). Immunoprecipitates were subjected to SDS-PAGE on 7.5% gels and blotted onto PVDF membranes. Tyrosine phosphorylation of EGFR was detected with antiphosphotyrosine MAb 4G10 (Upstate Biotechnology, Lake Placid, N.Y.). For reblotting, a polyclonal anti-EGFR antibody (Santa Cruz) was used.
Phosphatidylinositol turnover.
COS-7 cells (5 × 105 cells per well) in 24-well plates were prelabeled with 4 μCi of myo-[3H]inositol (NEN Life Science Products, Boston, Mass.) per ml for 24 h. Two hours prior to stimulation, the cells were incubated in serum-free medium containing 20 mM HEPES (pH 7.4). The cells were stimulated with BK or EGF in the presence of LiCl for the times indicated. For termination, the medium was replaced by 10 ml of 10% trichloroacetic acid. After 10 min, the extracts were collected and the trichloroacetic acid was removed by washing four times with 2 volumes of water-saturated diethyl ether. After neutralization by adding Tris base, the samples were diluted in 4 ml of distilled water. The inositol phosphate fractions containing IP1, IP2, and IP3 were obtained by elution five times with 2 ml of 1.0 M ammonium formate–0.1 M formic acid from AG 1 × 8 columns (200/400 mesh, formate form; Bio-Rad, Richmond, Calif.). Radioactivity of the inositol phosphate-containing fractions was determined by liquid scintillation counting in a Flo-Scint IV scintillator (Packard Bioscience B.V., Groningen, The Netherlands).
RESULTS
BK activates MAPK in COS-7 cells via α subunits of a PTX-insensitive G protein.
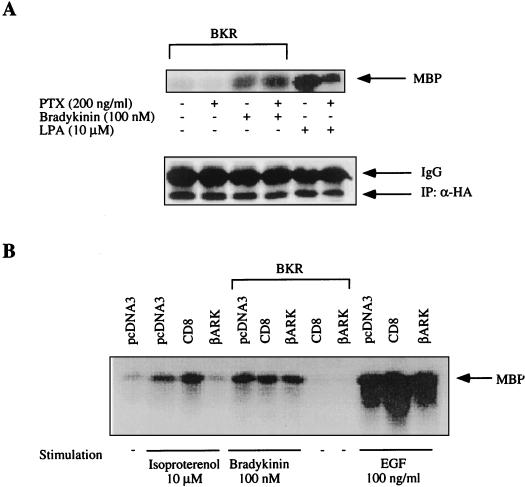
Binding studies with [3H]BK revealed an expression of human BKR in transiently transfected COS-7 cells, with ca. 2 × 105 sites per cell and a Kd of approximately 1 nM (not shown). Control cells not transfected with the BKR cDNA showed no appreciable specific binding of [3H]BK. Stimulation of the expressed receptors by BK resulted in an up to 2.5-fold concentration-dependent increase in inositol phosphate formation (not shown). BK effectively induced activation of MAPK in transfected COS-7 cells, reaching a plateau phase at BK concentrations above 100 nM, with a 50% effective concentration of ca. 3 nM (not shown), close to the Kd of BK binding. This BK-induced activation of MAPK was insensitive to treatment with PTX (Fig. 1A). For a positive control, PTX in the concentration used clearly inhibited MAPK activation by lysophasphatidic acid (LPA). The LPA receptor, which is endogenously expressed in COS-7 cells, couples to both Gi and Gq/11 proteins (11, 24). Therefore, the effect of PTX on LPA-induced MAPK activation remained incomplete. In addition, we coexpressed a chimeric construct (CD8-βARK-C) that is expected to block βγ-dependent pathways by sequestering free βγ complexes (4, 5). As shown in Fig. 1B, coexpression of CD8-βARK-C nearly abolished MAPK activation in response to isoproterenol (positive control) (5), whereas MAPK activation in response to BK was not affected. CD8 itself had no demonstrable effect on MAPK activity, and CD8-βARK-C did not influence EGF-dependent activation of MAPK (negative control).
FIG. 1.
Activation of MAPK by BK is insensitive to PTX or βγ-scavenging proteins. (A) COS-7 cells were cotransfected with 6 μg of BKR DNA and 0.5 μg of HA-MAPK DNA. After 2 days, cells were preincubated with PTX (200 ng/ml) for 24 h. The effects of PTX treatment on basal and BK-stimulated MAPK activity were determined following a 5-min exposure to BK (100 nM). For control, COS-7 cells were stimulated with 10 μM LPA (5 min). MAPK activity was assessed as phosphorylation by MBP by immunoprecipitated p42HA-MAPK. The amount of immunoprecipitated HA-MAPK was determined by Western blot analysis with HA-specific MAb 12CA5. (IP: α-HA). (B) BKR and HA-MAPK DNAs were cotransfected into COS-7 cells together with plasmids containing the CD8-βARK chimera or the pcDNA vector. Cells were then serum starved and stimulated with isoproterenol (10 μM), BK (100 nM), or EGF (100 ng/ml) for 5 min. MAPK activity was determined in immunoprecipitates with MBP as the substrate, subjected to SDS-PAGE, and autoradiographed. The results shown are representative of at least three experiments. In all relevant figures, IgG denotes immunoglobulin G.
BK-induced MAPK activation in COS-7 cells depends on PKC and EGFR but is independent of PI3K or Src family kinases.
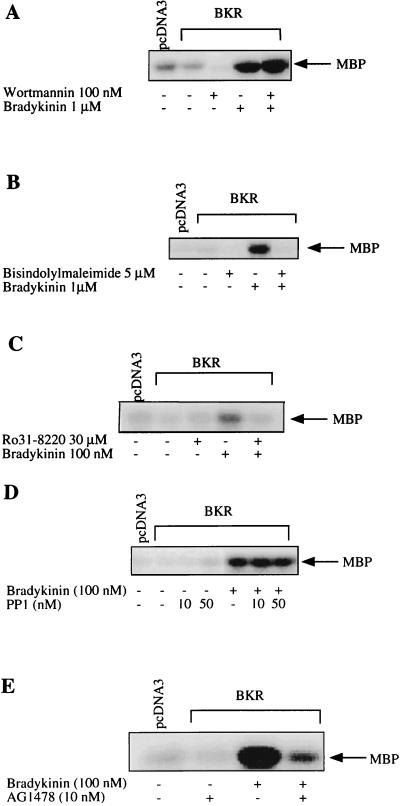
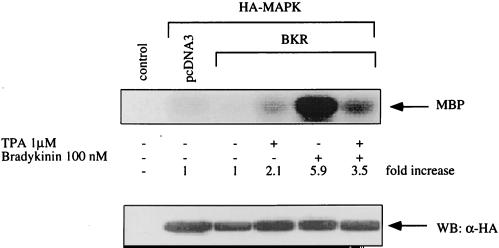
To identify the signaling route downstream of the Gq protein, we determined the effect of BK on MAPK activity in the presence of several inhibitors (Fig. 2). Neither wortmannin, a specific inhibitor of PI3K (44), nor PP-1, protein phosphatase 1 which specifically inhibits Src family tyrosine kinases (16), significantly affected the BK-induced activation of MAPK (Fig. 2A and D). These findings exclude an involvement of PI3K as well as Src kinases in BK signaling toward MAPK. In contrast, AG1478, a specific inhibitor of EGFR tyrosine kinase (35), clearly reduced the effect of BK on MAPK, suggesting an essential role of EGFR in MAPK activation by BK (Fig. 2E). In addition, PKC appears to be involved in the BK-induced MAPK activation, as suggested by two lines of evidence. First, two different inhibitors of PKC, bisindolylmaleimide (39) and Ro 31-8220 (42), prevent the MAPK activation in response to BK (Fig. 2B and C); second, depletion of PKC by long-term treatment of COS-7 cells with the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) significantly diminished the stimulatory effect of BK on MAPK (Fig. 3).
FIG. 2.
Effects of various inhibitors on BK-induced activation of MAPK. COS-7 cells, cotransfected with BKR and HA-MAPK DNAs as described in the text, were serum starved and preincubated with (A) the PI3K inhibitor wortmannin (100 nM), the PKC inhibitor bisindolylmaleimide (5 μM) (B) or Ro 31-8220 (30 μM) (C), the Src inhibitor protein phosphatase (PP1; 10 and 50 nM) (D), or the EGFR tyrosine kinase inhibitor AG1478 (10 nM) (E). R.31-8220 was kindly provided by D. Bradshaw (Roche, Welwyn Garden, United Kingdom). After preincubation for 30 min (A to C), 15 min (D), or 10 min (E), cells were stimulated with BK (100 nM to 1 μM) for 5 min. MAPK activity was assayed in lysates using the MBP phosphorylation assay as described in the text. Shown are blots representative of three independent experiments.
FIG. 3.
BK-induced activation of MAPK is decreased in PKC-depleted cells. COS-7 cells transfected with BKR and HA-MAPK were stimulated with TPA (1 μM) for 24 h to induce down-regulation of PKC and exposed to BK (10 nM) for 5 min, then lysis buffer was added, and MAPK activity was determined. The expression level of HA-MAPK was controlled by Western blotting with HA-specific MAb 12CA5 (WB: α-HA). Results are representative of three experiments.
BK stimulates MAPK activity via the PKC isoforms α and ɛ.
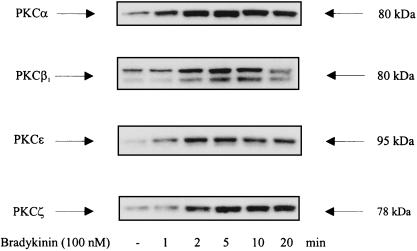
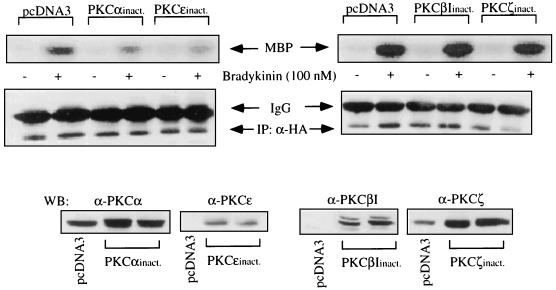
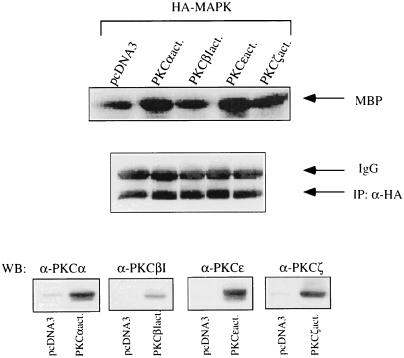
Western blotting of whole-cell extracts revealed that COS-7 cells express endogenously PKC isoforms α, βI, βII, ɛ, and ζ, whereas PKC isoforms δ and γ were not detectable in significant amounts (not shown). Treatment with BK of COS-7 cells expressing the BKR induced a rapid and transient translocation of PKC isoforms α, βI, ɛ, and ζ (Fig. 4), as detected by Western blotting of COS-7 cell membranes. Thus, these BK-sensitive PKC isoforms represent candidates for mediating the BK effect to MAPK. We next examined whether the effect of BK on MAPK activity may be mediated by a single PKC or by all isoforms translocated by BK. Overexpression of PKCα significantly increased BK-induced MAPK activation to ca. 120% of the control level. Overexpression of PKC isoform βI or ɛ failed to increase the BK effect (not shown). Cotransfection of PKCζ always led to a drastic decrease in the expression of HA-MAPK. Therefore, a putative influence of PKCζ on the effect of BK could not be analyzed in this way. In another approach, inactive mutants of the respective PKC isoforms were coexpressed together with BKR DNA and HA-MAPK. In the presence of the inactive PKC isoforms α and ɛ, we observed a significant reduction in BK-induced stimulation of MAPK, to 68 and 61%, respectively, of the control level (Fig. 5). In contrast, cotransfection of the inactive PKC isoform βI or ζ had no influence on the effect of BK. Finally, cotransfection of COS-7 cells with HA-MAPK and constitutively active mutants of PKCɛ or PKCα led to stimulation of MAPK activity (up to 150% of control values), whereas the active forms of PKCβI or PKCζ had no significant effect (Fig. 6). Thus, these experiments provided clear evidence of an essential involvement of PKCα and PKCɛ in mediating the effect of BK on MAPK.
FIG. 4.
BK induces translocation of the endogenous PKC isoforms α, βI, ɛ, and ζ. COS-7 cells were transfected with 6 μg of BKR DNA. After 2 days, serum-starved cells were stimulated with 100 nM BK for increasing times as indicated. Then a membrane fraction was prepared very quickly. Membranes were lysed, subjected to SDS-PAGE, and blotted onto PVDF membranes. Immunoblots obtained with antisera against the different PKC isoforms (1 μg/ml; Santa Cruz) are shown. Western blots shown are representative of at least two experiments.
FIG. 5.
Expression of inactive mutants of PKC isoforms in COS-7 cells. cDNA (1.5 μg) of inactive (inact.) mutants of PKC isoforms α, βI, ɛ, and ζ were cotransfected with BKR and HA-MAPK DNAs as described in Materials and Methods. After stimulation with BK (100 nM, 5 min), COS-7 cells were lysed, HA-MAPK was immunoprecipitated, and MAPK activity was assayed with MBP as the substrate. For control, the amount of immunoprecipitated HA-MAPK was determined by immunoblotting with a MAb against HA (IP:α-HA). Expression levels of the cotransfected inactive PKC isoforms were analyzed by Western blotting (WB) with antibodies against the respective isoforms (Santa Cruz). For comparison, the basal levels of the corresponding endogenous PKC isoforms are shown (pcDNA3). Results are representative of three experiments.
FIG. 6.
Effects of constitutively active mutants of the PKC isoforms on MAPK activity. In COS-7 cells, cDNA of active PKC isoform α or βI (2 μg) or ɛ or ζ (1 μg) was cotransfected with HA-MAPK DNA (0.5 μg). After 2 days, HA-MAPK was immunoprecipitated and assayed for activity as described in the text. For control, the expression levels of HA-MAPK and of the active PKC mutants determined by Western blotting are shown. Results are representative of three separate experiments. Notation is as for Fig. 5.
BK also stimulates MAPK via tyrosine phosphorylation (transactivation) of the EGFR.
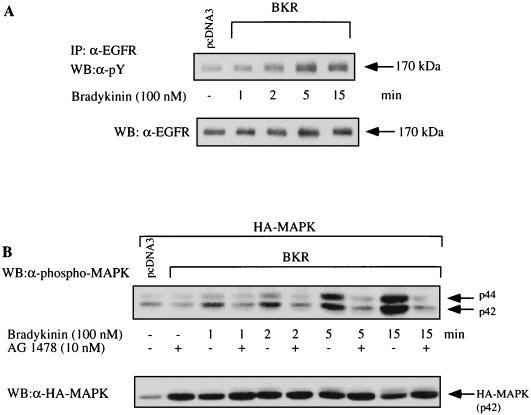
As shown in Fig. 7A, BK is capable of inducing tyrosine phosphorylation of the EGFR in COS-7 cells. For comparison, the stimulation of MAPK in response to BK was found to be dependent on time and the presence of the EGFR tyrosine kinase inhibitor AG1478 (Fig. 7B). AG1478 completely blocks the effect of BK on MAPK activity. Furthermore, there is a close correlation between the kinetics of EGFR transactivation and the increase in MAPK activity in response to BK, both reaching a maximal level after about 5 min. These kinetics also closely correspond to the time-dependent BK-induced PKC activation induced by BK (Fig. 4). Thus, both the transactivation pathway and the PKC pathway respond rapidly and with kinetics similar to those for BK. In contrast, EGF appears to activate the PKC pathway in COS-7 cells with a very slow kinetic (≥15 min [not shown]).
FIG. 7.
Time course of BK-induced tyrosine phosphorylation of the EGFR and EGFR-dependent activation of MAPK. (A) COS-7 cells expressing the BKR were serum starved for 12 h and then stimulated with BK (100 nM) for increasing times as indicated. Immunoprecipitates (IP) of the endogenous EGFR (anti-EGFR MAb; E. Merck AG, Darmstadt, Germany) were resolved by SDS-PAGE (7.5% gel) transferred to PVDF membranes, and Western blotted (WB) with either antiphosphotyrosine (α-pY) MAb 4G10 (top panel) or anti-EGFR antibody (polyclonal; Santa Cruz) (bottom panel). The results shown are representative of four separate experiments. (B) COS-7 cells were cotransfected with BKR and HA-MAPK, serum starved for 12 h, and stimulated with 100 nM BK in the absence or presence of AG1478 (10 nM, 10-min preincubation) for the times indicated. Shown is a representative Western blot with a polyclonal anti-phospho-MAPK antibody (Promega), representing two independent experiments.
BK-induced EGFR transactivation does not involve PKC, and activation of PKC by BK is independent of transactivation.
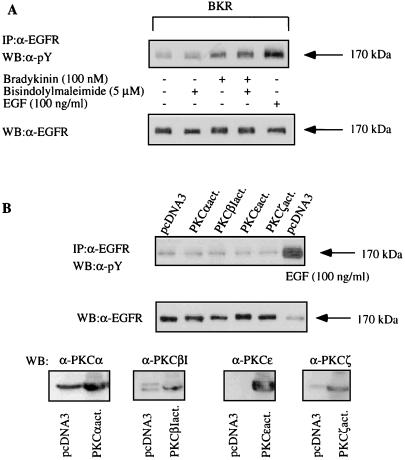
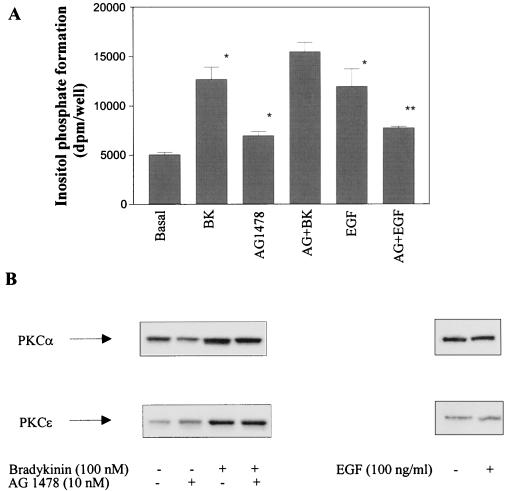
Next we examined whether activation of PKC could be involved in EGFR transactivation by BK or, vice versa, whether transactivation of EGFR and subsequent stimulation of PLCγ could mediate the BK-induced activation of PKC. We found that the presence of the PKC inhibitor bisindolylmaleimide had no effect on the tyrosine phosphorylation of the EGFR in response to BK (Fig. 8A), and the coexpression of constitutively active PKC mutants did not simulated the effect of BK on the EGFR (Fig. 8B). Furthermore, the specific EGFR tyrosine kinase inhibitor AG1478 had no effect on the BK-induced increase in inositol phosphate formation, excluding a participation of PLCγ in the effect of BK on phosphatidylinositol turnover (Fig. 9A). For a positive control, the EGF-induced stimulation of inositol phosphate formation is sensitive to AG1478. Furthermore, in the concentration that is sufficient to inhibit BK-induced activation of MAPK as well as EGF-induced stimulation of inositol phosphate production in COS-7 cells AG1487 did not affect translocation of the relevant PKC isoforms α and ɛ in response to BK (Fig. 9B). In contrast to BK, stimulation of COS-7 cells with EGF for 5 min failed to induce PKC translocation. It may be concluded that PKC is located neither upstream nor downstream of EGFR with respect to BK-induced transactivation.
FIG. 8.
BK-induced tyrosine phosphorylation of the EGFR is independent of PKC (notation is as in Fig. 7). (A) COS-7 cells transfected with pcDNA BKR were serum starved for 12 h, preincubated with the PKC inhibitor bisindolylmaleimide (5 μM) for 30 min, and stimulated with 100 nM BK for 5 min or 100 ng of EGF per ml (for control). The EGFR immunoprecipitates were subjected to SDS-PAGE, blotted, and analyzed by Western blotting with antiphosphotyrosine antibody (top). After stripping, the immunoprecipitates were quantified by using an anti-EGFR antibody (bottom). Results are representative of three independent experiments. (B) COS-7 cells were transfected with 2 μg of cDNA of constitutively active mutants of the four BK-sensitive PKC isoforms. After 2 days, cells were lysed, and the endogenous EGFR was immunoprecipitated and selected by using anti-phosphotyrosine antibody. For control, the EGFR was induced with 100 μg of EGF per ml. Also shown are the control blots with anti-EGFR antibody (middle panel) and with anti-PKC antibodies to detect the expression of the various PKC isoforms (bottom panel). The results are representative of four experiments.
FIG. 9.
Both BK-induced inositol phosphate formation and PKC translocation are independent of EGFR transactivation. (A) COS-7 cells were transfected with BKR DNA, prelabeled with [myo-3H]inositol for 24 h, subjected to serum-starved medium, preincubated for 10 min with 10 nM AG1478, and then stimulated with BK (100 nM, 5 min) or EGF (100 ng/ml, 7 min) as indicated. Inositol phosphate formation was determined in quadruplicate as described in Materials and Methods. ∗, inositol phosphate levels are significantly enhanced in the presence of BK, AG1478, and EGF compared with the control; ∗∗, EGF-induced inositol phosphate formation is significantly inhibited by AG1478 (Student’s t test; P < 0.05). (B) COS-7 cells expressing the BKR were stimulated with BK or EGF as indicated in absence or presence of AG1478 (10 nM, 10-min preincubation). PKC translocation was determined as described in Materials and Methods. Shown are Western blots for PKCs isoforms α and ɛ representative of two separate experiments.
Convergence of the PKC- and EGFR-mediated pathways on Raf-1.
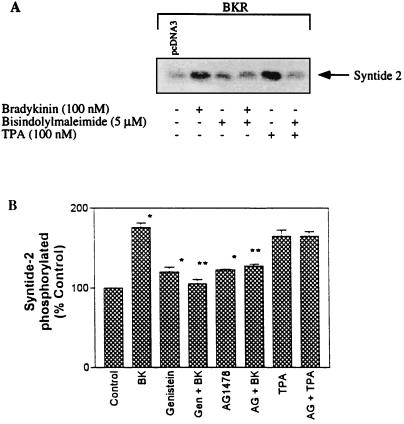
Raf-1 kinase represents the first element of the MAPK cascade and is activated via the EGFR in a Ras-dependent manner. In addition, Raf-1 may be directly activated by most of the PKC isoforms, including α and ɛ (2, 37). In COS-7 cells, on the one hand, we found a stimulation of Raf-1 activity by BK as well as by the phorbol ester TPA that is prevented in both cases by the PKC inhibitor bisindolylmaleimide (Fig. 10A). In Fig. 10, we present the results of a novel experimental approach to demonstrate the phosphorylation of the peptide substrate synthide-2 by SDS-PAGE on a 16% gel and autoradiography. Comparable results were obtained from identical experiments using the P81 Whatman phosphocellulose paper assay (not shown). The BK-induced activation of Raf-1 kinase was also abolished by tyrosine kinase inhibitors such as genistein (1) or by AG1478 (Fig. 10B). The effect of TPA, for comparison, was not inhibited by AG1478 (Fig. 1B). These findings suggest that in response to BK, Raf-1 kinase is independently activated via both the PKC pathway and the EGFR pathway.
FIG. 10.
Effects of PKC and tyrosine kinase inhibitors on activation of Raf-1 by BK. COS-7 cells transfected with the BKR were subjected to serum-starved medium and preincubated with bisindolylmaleimide (5 μM, 30 min), genistein (50 μM, 2 h), or AG1478 (10 nM, 10 min) followed by stimulation with BK (100 nM, 5 min) or TPA (100 nM, 3 min) as indicated. Raf-1 was immunoprecipitated with anti-Raf-1 polyclonal antibodies (Santa Cruz), and the immunocomplex was incubated with the substrate peptide syntide-2 and [γ-32P]ATP. Shown are results obtained with different experimental approaches to determine Raf-1 activation. (A) Autoradiogram of phosphorylated syntide-2. (B) Measurement of radioactivity (in quadruplicate) incorporated into syntide-2, using Whatman P81 phosphocellulose paper. Results are means ± standard errors of the means of three different experiments. ∗, significantly different compared with the control; ∗∗, significantly different compared with the effect of BK (Student’s t test; P < 0.05). Gen, genistein; AG, AG1478.
DISCUSSION
The signaling routes connecting GPCRs to the Ras/MAPK pathway have been shown to involve tyrosine kinases, PI3Ks, and/or PKCs (14). Recently, some interest has been focused on ligand-independent activation (transactivation) of RTKs, such as the EGFR, as a key event of MAPK activation by Gi- as well as Gq/11-coupled receptors (7, 8). However, for Gq/11-coupled receptors activating PKC, there are contradictory reports concerning the role of PKC in EGFR transactivation and, subsequently, MAPK activation. These include PKC-mediated (40), PKC-prevented (26), and PKC-independent (7, 8) EGFR transactivation. In this study, we define a novel possibility for the link of a Gq/11-coupled receptor to MAPK. In COS-7 cells transiently transfected with human BKR, stimulation of MAPK by BK was found to be dependent on the simultaneous and independent activation of both PKC and EGFR.
COS-7 cells express endogenously Gq/11, Gi, and Gs proteins (12). The BKR mainly couples to Gq/11 proteins (36) but is, in certain cells, also capable of activating Gi or Gs proteins (27, 28). In COS-7 cells, the BK-induced activation of MAPK was affected neither by PTX nor by coexpression of the βγ-complex-scavenging CD8-βARK chimera. Additionally, we found that BK stimulates phosphatidylinositol hydrolysis in COS-7 cells. It may be concluded, therefore, that the effect of BK on MAPK is mediated via the α subunits of Gq/11 protein.
There are several lines of evidence suggesting a role of PI3Kγ downstream from GPCRs to MAPK (29). PI3Kγ may be stimulated by Gβγ complexes from Gi proteins (29, 25) but also by Gqα (34). Another central player in both Gi- and Gq/11-coupled receptor-mediated MAPK activation downstream of Ca2+-calmodulin and PYK2 and upstream of Shc and Ras might be a cytosolic tyrosine kinase of the Src family (10, 13). The inability of specific inhibitors of PI3K as well as Src kinases to affect the BK-induced MAPK activation in COS-7 cells excludes a role of these kinases in that pathway. In addition, we cotransfected COS-7 cells with PI3Kγ cDNA and determined the effect of BK on MAPK in absence and presence of wortmannin. Furthermore, the ability of BK to stimulate Src activity in vitro was investigated. In both assays, we found no indication for the involvement of PI3Kγ or Src in BK signaling (not shown). In contrast, using two chemically different PKC inhibitors and by means of down-regulation of PKC activity, we found that in COS-7 cells the PKC pathway is essentially involved in MAPK activation by BK. To study which PKC isoforms may be included, we used different experimental approaches such as coexpression of wild-type, inactive mutants or constitutively active mutants of various PKC isoforms. In COS-7 cells, among the PKC isoforms α, βI, ɛ, and ζ that are sensitive to BK, only the diacylglycerol-regulated PKC isoforms α and ɛ could be identified to play a critical role in BK-induced activation of MAPK. This finding is not completely consistent with the results of Schönwasser et al. (37), demonstrating the activation of MAPK by constitutively active mutants of six PKC isotypes (α, βI, δ, ɛ, η, and ζ) in COS-7 cells. This discrepancy might be explained by the use of different PKC mutants in the two studies.
Tyrosine phosphorylation of EGFR has been recognized as a key event in signaling of LPA receptor and other GPCRs (6–8, 19). Therefore, experiments were carried out to assess an involvement of EGFR in MAPK activation by BK. Our results show that in COS-7 cells, (i) AG1478, a specific inhibitor of EGFR tyrosine kinase, potently inhibits BK-induced activation of MAPK and (ii) BK is capable of inducing EGFR tyrosine phosphorylation. The effects of BK on both the PKC pathway and the EGFR display a fast and similar kinetic indicating rather a simultaneous than a consecutive activation. Indeed, inhibition of PKC did not affect EGFR tyrosine phosphorylation by BK. In addition, expression of constitutively active PKC mutants failed to induce a tyrosine phosphorylation of EGFR. Thus, it may be concluded that PKC does not mediate the BK-induced EGFR transactivation in COS-7 cells. EGFR transactivation, vice versa, does not mediate the effect of BK on phosphatidylinositol metabolism that subsequently leads to PKC activation. This is confirmed by the inability of AG1478 to affect the BK-induced increase in inositol phosphate formation or to inhibit the BK-induced translocation of PKCs isoforms α and ɛ. Together, these findings suggest that in COS-7 cells the PKC pathway and the EGFR pathway are independently activated by BK. It is well known that PKC phosphorylates the EGFR on threonine residue 654 (21), resulting in a decreased receptor affinity toward EGF and/or in ligand-independent internalization of the EGFR (33). In A431 cells, for example, BK has been shown to stimulate both the PKC system and threonine phosphorylation of the EGFR (20). We have not yet determined whether BK phosphorylates Thr-654 in COS-7 cells. Nevertheless, it might be speculated that BK activates MAPK via a PKC-dependent pathway. BK-activated PKC desensitizes the EGFR toward EGF by threonine phosphorylation; simultaneously, BK transactivates the EGFR by tyrosine phosphorylation in a PKC-independent manner. In that way, EGFR might be efficiently recruited into the BK signaling.
In contrast with our findings in COS-7 cells, BK-induced EGFR transactivation in HaCaT human keratinocytes (3) or m1 muscarinic acetylcholine receptor-induced EGFR transactivation in human 293 cells (40) are PKC-dependent processes. In partial accordance with our results, in GN4 rat liver epithelial cells angiotensin II was found to activate MAPK via a dominant PKC pathway and a latent EGFR transactivation pathway that is suppressed PKC action (26). Another pathway is described for the LPA receptor in COS-7 cells, where a Src-mediated EGFR tyrosine phosphorylation was found to lead to MAPK activation (31). However, these controverse data suggest again that heterogeneity in MAPK activation may exist between receptors and cell types.
Whereas in BK signaling there is a divergence downstream of Gq/11, the two branches of the dual pathway might converge at the level of Raf kinase. Raf-1 serves as a central intermediate in connecting upstream RTKs and Ras as well as PKCs with the downstream kinases MEK and MAPK. Activation by Ras can occur without phosphorylation and may be due to lipid-protein interactions at the membrane (30, 38). Other results suggest a role for phosphorylation in activation of Raf-1, e.g., tyrosine phosphorylation by Src (38) or serine phosphorylation by PKCα (23). Recently, the PKC isoforms α and ɛ were used as activators of Raf-1 in vivo. Overexpression of active PKCɛ stimulated Raf kinase activity in COS-7 cells, and dominant negative mutants of both PKCɛ and PKCα inhibited activation of Raf-1 in COS-7 cells (2). In addition, constitutively active mutants of PKCα as well as PKCɛ overcame the inhibitory effects of other PKC isotypes, indicating that PKCα and PKCɛ function as redundant activators of Raf-1 (2). Our data suggest that BK as a physiological activator of PKCα and PKCɛ also activates Raf-1 in a PKC-dependent manner. In addition, we observed an equipotent activation of Raf-1 by BK in dependency on EGFR transactivation. Raf-1 thus appears to be a plausible candidate which integrates the bifurcating BK signaling upstream of MAPK. Very recently, the requirement of Ras for activation of Raf-1 by PKC was demonstrated (32). It may be assumed, therefore, that the convergence in BK signaling occurs at the level of Ras-GTP-Raf complexes.
In summary, our results suggest that in the expression model COS-7 the human BKR controls MAPK activity via a dual signaling pathway involving the independent activation of the PKC isoforms α and ɛ as well as EGFR transactivation. MAPK stimulation by BK needs signals from both pathways which are integrated at the level of the Ras-Raf complex. However, the GPCR-induced activation of an enzyme that is chiefly regulating cell growth via a two-part system makes some physiological sense.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft.
We thank Lone Hansen and Lene Nilsen (Hagedorn Research Institute) for generating the PKC mutants and Carmen Mertens (Institute of Biochemistry and Biophysics) for excellent technical assistance.
REFERENCES
- 1.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5995. [PubMed] [Google Scholar]
- 2.Cai H, Smola U, Wixler V, Eisenmann-Trappe I, Diaz-Meco M, Moscat J, Rapp U, Cooper G M. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997;17:732–741. doi: 10.1128/mcb.17.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coutant K D, Corvaia N, Ryder N S. Bradykinin induces tyrosine phosphorylation of epidermal growth factor-receptor and focal adhesion proteins in human keratinocytes. Biochem Biophys Res Commun. 1995;210:774–780. doi: 10.1006/bbrc.1995.1726. [DOI] [PubMed] [Google Scholar]
- 4.Crespo P, Xu N, Simonds W F, Gutkind J S. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 5.Crespo P, Cachero T G, Xu N, Gutkind J S. Dual effect of beta-adrenergic receptors on mitogen-activated protein kinase. Evidence for a beta gamma-dependent activation and a G alpha s-cAMP-mediated inhibition. J Biol Chem. 1995;270:25259–25265. doi: 10.1074/jbc.270.42.25259. [DOI] [PubMed] [Google Scholar]
- 6.Cunnick J M, Dorsey J F, Standley T, Turkson J, Kraker A J, Fry D W, Jove R, Wu J. Role of tyrosine kinase activity of epidermal growth factor receptor in the lysophosphatidic acid-stimulated mitogen-activated protein kinase pathway. J Biol Chem. 1998;273:14468–14475. doi: 10.1074/jbc.273.23.14468. [DOI] [PubMed] [Google Scholar]
- 7.Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 1997;16:7032–7044. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daub H, Weiss F U, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 9.Decock J B J, Gillespiebrown J, Parker P J, Sugden P H, Fuller S J. Classical, novel and atypical isoforms of PKC stimulate ANF- and TRE/AP-1-regulated-promoter activity in ventricular cardiomyocytes. FEBS Lett. 1994;356:275–278. doi: 10.1016/0014-5793(94)01283-0. [DOI] [PubMed] [Google Scholar]
- 10.Della Rocca G J, van Biesen T, Daaka Y, Luttrell D K, Luttrell L M, Lefkowitz R J. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2, and Src kinase. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 11.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 12.Federman A D, Conklin B R, Schrader K A, Reed R R, Bourne H R. Hormonal stimulation of adenylyl cyclase through Gi-protein beta gamma subunits. Nature. 1992;356:159–161. doi: 10.1038/356159a0. [DOI] [PubMed] [Google Scholar]
- 13.Felsch J S, Cachero T G, Peralta E G. Activation of protein tyrosine kinase PYK2 by the m1 muscarinic acetylcholine receptor. Proc Natl Acad Sci USA. 1998;95:5051–5056. doi: 10.1073/pnas.95.9.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutkind J S. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 15.Häfner S, Adler H S, Mischak H, Janosch P, Heidecker G, Wolfman A, Pippig S, Lohse M, Ueffing M, Kolch W. Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol. 1994;14:6696–6703. doi: 10.1128/mcb.14.10.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 17.Hawes B E, Luttrell L M, van Biesen T, Lefkowitz R J. Phosphatidylinositol 3-kinase is an early intermediate in the G beta gamma-mediated mitogen-activated protein kinase signaling pathway. J Biol Chem. 1996;271:12133–12136. doi: 10.1074/jbc.271.21.12133. [DOI] [PubMed] [Google Scholar]
- 18.Hawes B E, van Biesen T, Koch W J, Luttrell L M, Lefkowitz R J. Distinct pathways of Gi- and Gq-mediated mitogen-activated protein kinase activation. J Biol Chem. 1995;270:17148–17153. doi: 10.1074/jbc.270.29.17148. [DOI] [PubMed] [Google Scholar]
- 19.Herrlich A, Daub H, Knebel A, Ullrich A, Schultz G, Guderman T. Ligand-independent activation of platelet-derived growth factor receptor is a necessary intermediate in lysophosphatidic-acid stimulated mitogenic activity in L cells. Proc Natl Acad Sci USA. 1998;95:8985–8990. doi: 10.1073/pnas.95.15.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosoi K, Kurihara K, Ueha T. Bradykinin-stimulated transient modulation of epidermal growth factor receptors in A-431 human epidermoid carcinoma cells. J Cell Physiol. 1993;157:1–12. doi: 10.1002/jcp.1041570102. [DOI] [PubMed] [Google Scholar]
- 21.Hunter T, Ling N, Cooper J A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984;311:480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- 22.Jaken S. Protein kinase C isozymes and substrates. Curr Opin Cell Biol. 1996;8:168–173. doi: 10.1016/s0955-0674(96)80062-7. [DOI] [PubMed] [Google Scholar]
- 23.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp U. Protein kinase C alpha activates Raf-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 24.Kranenburg O, Verlaan I, Hordijk P L, Moolenaar W H. Gi-mediated activation of the Ras/MAP kinase pathway involves a 100 kDa tyrosine-phosphorylated Grb2 SH3 binding protein, but not Src nor Shc. EMBO J. 1997;16:3097–3105. doi: 10.1093/emboj/16.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leopoldt D, Hanck T, Exner T, Maier U, Wetzker R, Nürnberg B. Gbetagamma stimulates phosphoinositide 3-kinase-gamma by direct interaction with two domains of the catalytic p110 subunit. J Biol Chem. 1998;273:7024–7029. doi: 10.1074/jbc.273.12.7024. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Lee J W, Graves L M, Earp H S. Angiotensin II stimulates ERK via two pathways in epithelial cells: protein kinase C suppresses a G-protein coupled receptor-EGF receptor transactivation pathway. EMBO J. 1998;17:2574–2583. doi: 10.1093/emboj/17.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liebmann C, Graness A, Ludwig B, Adomeit A, Boehmer A, Boehmer F D, Nürnberg B, Wetzker R. Dual bradykinin B2 receptor signalling in A431 human epidermoid carcinoma cells: activation of protein kinase C is counteracted by a Gs-mediated stimulation of the cyclic AMP pathway. Biochem J. 1996;313:109–118. doi: 10.1042/bj3130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liebmann C, Offermanns S, Spicher K, Hinsch K D, Schnittler M, Morgat J L, Reissmann S, Schultz G, Rosenthal W. A high-affinity bradykinin receptor in membranes from rat myometrium is coupled to pertussis toxin-sensitive G-proteins of the Gi family. Biochem Biophys Res Commun. 1990;167:910–917. doi: 10.1016/0006-291x(90)90610-y. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Illasaca M, Crespo P, Pellici P G, Gutkind J S, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 30.Luo Z, Diaz B, Marshall M S, Avruch J. An intact Raf zinc finger is required for optimal binding to processed Ras and for ras-dependent Raf activation in situ. Mol Cell Biol. 1997;17:46–53. doi: 10.1128/mcb.17.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luttrell L M, Della Rocca G J, van Biesen T, Luttrell D K, Lefkowitz R J. Gbetagamma subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. A scaffold for G protein-coupled receptor-mediated Ras activation. J Biol Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- 32.Marais R, Light Y, Mason C, Paterson H, Olson M F, Marshall C J. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–112. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- 33.McCune B K, Earp H S. The epidermal growth factor receptor tyrosine kinase in liver epithelial cells. The effect of ligand-dependent changes in cellular location. J Biol Chem. 1989;264:15501–15507. [PubMed] [Google Scholar]
- 34.Murga C, Laguinge L, Wetzker R, Cuadrado A, Gutkind J S. Activation of Akt/protein kinase B by G protein-coupled receptors. A role for alpha and beta gamma subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinaseγ. J Biol Chem. 1998;273:19080–19085. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- 35.Osherov N, Levitzki A. Epidermal-growth-factor-dependent activation of the src-family kinases. Eur J Biochem. 1994;225:1047–1053. doi: 10.1111/j.1432-1033.1994.1047b.x. [DOI] [PubMed] [Google Scholar]
- 36.Ransom R W, Goodman C B, Young G S. Bradykinin stimulation of phosphoinositide hydrolysis in guinea-pig ileum longitudinal muscle. Br J Pharmacol. 1992;105:919–924. doi: 10.1111/j.1476-5381.1992.tb09078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schönwasser D C, Marais R M, Marshall C J, Parker P J. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stokoe D, McCormick F. Activation of c-Raf-1 by Ras and Src through different mechanisms: activation in vivo and in vitro. EMBO J. 1997;16:2384–2396. doi: 10.1093/emboj/16.9.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Koriolle F, Duhamel L, Charon D, Kirilovski J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 40.Tsai W, Morielli A D, Peralta E G. The m1 muscarinic acetylcholine receptor transactivates the EGF receptor to modulate ion channel activity. EMBO J. 1997;16:4597–4605. doi: 10.1093/emboj/16.15.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Biesen T, Luttrell L M, Hawes B E, Lefkowitz R J. Mitogenic signaling via G protein-coupled receptors. Endocrine Rev. 1996;17:698–714. doi: 10.1210/edrv-17-6-698. [DOI] [PubMed] [Google Scholar]
- 42.van Dijk M C M, Muriana F J G, van der Hoeven P C J, de Widt J, Schaap D, Moolenaar W H, van Blitterswijk W J. Diacylglycerol generated by exogenous phospholipase C activates the mitogen-activated protein kinase pathway independent of Ras- and phorbol ester-sensitive protein kinase C: dependence on protein kinase C-zeta. Biochem J. 1997;323:693–699. doi: 10.1042/bj3230693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan Y, Kurosaki T, Huang X Y. Tyrosine kinases in activation of the MAP kinase cascade by G-protein-coupled receptors. Nature. 1996;380:541–544. doi: 10.1038/380541a0. [DOI] [PubMed] [Google Scholar]
- 44.Wymann M P, Bulgarelli-Leva G, Zvelebil M J, Pirola L, Vanhaesebroeck B, Waterfield M D, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]