Supplemental Digital Content is available in the text.
Keywords: epidemiology, health services research, respiratory failure, tracheostomy
Objectives:
Describe the longitudinal national epidemiology of tracheostomies performed in acute care hospitals and describe the annual rate of tracheostomy performed for patients with respiratory failure with invasive mechanical ventilation.
DESIGN:
Serial cross-sectional study.
Setting:
The 2002–2014 and 2016–2017 Healthcare Utilization Project’s National Inpatient Sample datasets.
Patients:
Discharges greater than or equal to 18 years old, excluding those with head and neck cancer or transferred from another hospital. We used diagnostic and procedure codes from the International Classification of Diseases, 9th and 10th revisions to define cases of respiratory failure, invasive mechanical ventilation, and tracheostomy.
Interventions:
None.
Measurements and Main Results:
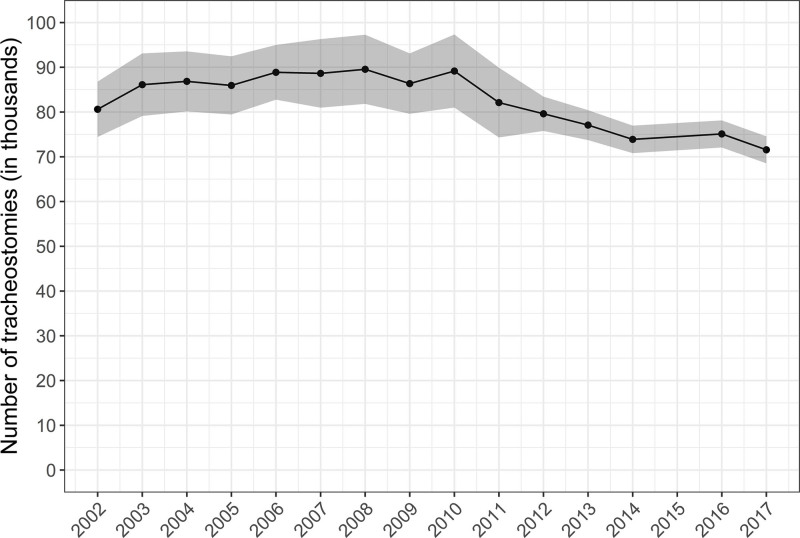
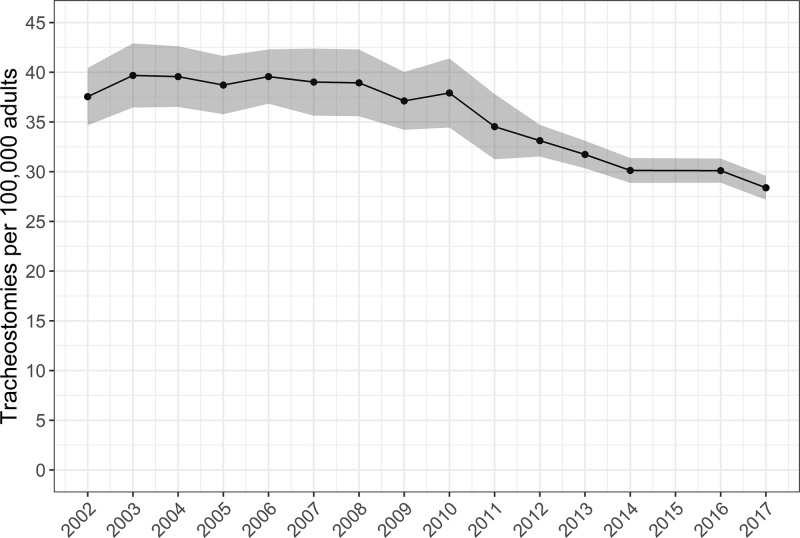
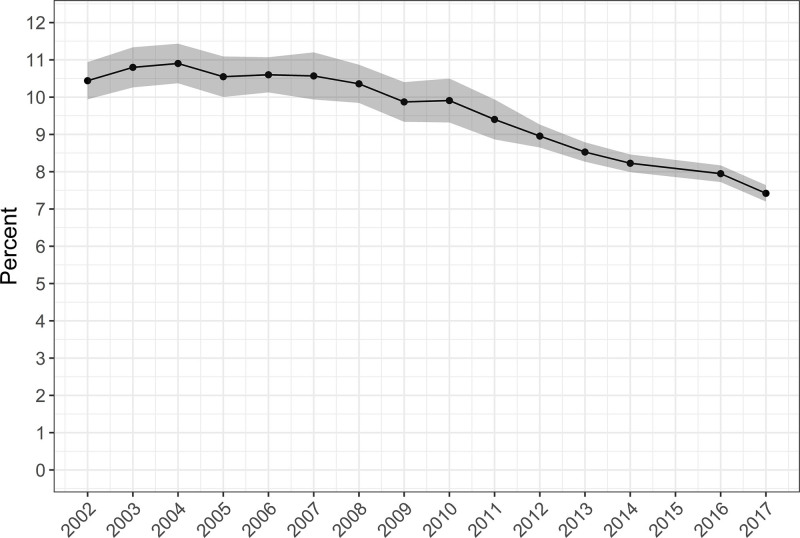
There were an estimated 80,612 tracheostomies performed in 2002, a peak of 89,545 tracheostomies in 2008, and a nadir of 58,840 tracheostomies in 2017. The annual occurrence rate was 37.5 (95% CI, 34.7–40.4) tracheostomies per 100,000 U.S. adults in 2002, with a peak of 39.7 (95% CI, 36.5–42.9) in 2003, and with a nadir of 28.4 (95% CI, 27.2–29.6) in 2017. Specifically, among the subgroup of hospital discharges with respiratory failure with invasive mechanical ventilation, an annual average of 9.6% received tracheostomy in the hospital. This changed over the study period from 10.4% in 2002, with a peak of 10.9% in 2004, and with a nadir of 7.4% in 2017. Among respiratory failure with invasive mechanical ventilation discharges with tracheostomy, the annual proportion of patients 50–59 and 60–69 years old increased, whereas patients from 70 to 79 and greater than or equal to 80 years old decreased. The mean hospital length of stay decreased, and in-hospital mortality decreased, whereas discharge to intermediate care facilities increased.
Conclusions:
Over the study period, there were decreases in the annual total case volume and adult occurrence rate of tracheostomy as well as decreases in the rate of tracheostomy among the subgroup with respiratory failure with invasive mechanical ventilation. There is some evidence of changing patterns of patient selection for in-hospital tracheostomy among those with respiratory failure with invasive mechanical ventilation with decreasing proportions of patients with advanced age.
Respiratory failure (RF) with invasive mechanical ventilation (IMV) is one of the most common diagnoses in adults admitted to the ICU, with over 90% of these patients requiring ICU services (1). Patients with RF who require prolonged IMV with an endotracheal tube may be offered tracheostomy to alleviate some of the discomfort and potential complications from prolonged endotracheal tube use such as ventilator-associated pneumonia, decreased mobility, prolonged sedation, pressure ulcers, direct damage to oropharyngeal structures, delirium, and muscle weakness (2). Tracheostomy has become one of the most common procedures done for ICU patients with prolonged RF and IMV (3). One study done by Mehta et al (4) showed that age-adjusted rates of tracheostomy among all patients with MV increased from 16.7 to 34.3 cases per 100,000 adults from 1993 to 2012.
Given the significant morbidity and mortality of tracheostomy patients as well as their costs to health systems, understanding and ongoing surveillance of the epidemiology is essential for careful patient selection, resource allocation, and healthcare workforce planning. Data on the overall occurrence rate of tracheostomy vary widely depending on patient subgroup (5–8), and our first objective in this article is to describe the annual national case volume and adult occurrence rate of tracheostomy procedures in acute care hospitals in the United States. We then narrow our focus to describe the annual rate of tracheostomies and characteristics of the subgroup of patients hospitalized with a diagnosis of RF and a procedural code for IMV.
MATERIALS AND METHODS
Study Design and Database
This is a serial cross-sectional study using the 2002–2014 and 2016–2017 Healthcare Utilization Project’s (HCUP) National Inpatient Sample (NIS). The NIS is a complex, stratified sample of administrative hospital discharge records from nonfederal, short-term hospitals from participating states and is the largest publicly available all-payer inpatient healthcare database in the United States, including more than 7 million hospital stays per year. There have been important changes in the annual sample over our study period: 1) the number of participating states increased from 35 to 48, including the District of Columbia; 2) in 2012, the sample no longer included long-term acute care (LTAC) hospitals; 3) in 2012, the sample design changed from a sample of all discharges from selected hospitals to a selected sample of discharges from all available hospitals; and 4) in 2015, diagnosis and procedure reporting changed from International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) coding to the International Classification of Diseases, 10th revision, Clinical Modification (ICD-10-CM). To facilitate national estimation in trend analyses across these years, the NIS constructed new sampling weights. Relevant to this study, these new HCUP trend weights set the weight of discharge records from LTACs to zero for the 2002–2011 years of this study. Therefore, although LTAC data are still included in the dataset 2002–2011, the discharges are not counted in our weighted analyses. The 2015 year was not included in this analysis, given that the International Classification of Diseases (ICD) coding changed during the year, preventing the use of standard methods for making annual estimates with either coding system for that year.
Study Populations and Main Measurements
From the NIS, we included all discharges with age greater than or equal to 18 years old and excluded those patients with a diagnosis of head and neck cancer or who were transferred from other hospitals. Head and neck cancer was identified using the HCUP’s Clinical Classification Software, one of the tools provided by HCUP that collapses ICD codes into smaller, manageable, clinically meaningful groups (9). In addition to HCUP-NIS, we used the annual U.S. Census Bureau data for our annual adult population denominators to calculate national, annual occurrence rate. Specifically, we used annual population estimates for those greater than or equal to 18 years old (10, 11).
There were three national, annual measurements we sought to estimate: 1) the total number of tracheostomies performed in U.S. acute care hospitals to estimate case volume for practitioners, 2) the occurrence rate of tracheostomies per 100,000 U.S. adults to estimate population burden, and 3) the proportion of those specifically with both RF and IMV (RF-IMV) that received a tracheostomy to estimate practice pattern in this clinical population. We used combinations of ICD diagnosis and procedure codes to capture these measurements. ICD-9-CM codes were used for years 2002–2014 and ICD-10-CM codes for years 2016–2017 (Tables 1–3, Supplemental Digital Content 1, http://links.lww.com/CCX/A762 for complete list codes used). First, we used a case definition that specifically captured discharges with just a procedure code for tracheostomy. We used this definition to calculate national, annual weighted case volume of tracheostomies. Second, we identified discharges with both a diagnosis code for RF and a procedure code for IMV. This served as the denominator to calculate the annual percentage of this population who received a tracheostomy. This also served to define the population with both RF-IMV and tracheostomy for further descriptive examination of population characteristics and outcomes. We used the combination of diagnosis and procedure code as it conceptually approached our study population of interest, and one published validation study provides evidence that the combination may optimize the balance between sensitivity and specificity over using either diagnosis or procedure codes alone (10).
Outcomes and Other Variables
Among the population with RF-IMV and tracheostomy, we examined the hospital outcomes of length of stay (LOS) and discharge disposition. NIS categorizes discharge disposition into seven categories: “routine;” “transfer to short-term hospital;” “transfer other: includes skilled nursing facility, intermediate care facility (ICF), another type of facility;” “home healthcare;” “against medical advice;” “died;” and “discharged alive, destination unknown,” the names of which we have abbreviated in our Results section (11). Other variables extracted to describe this population included demographic characteristics, hospital characteristics, chronic comorbidities, category of principle diagnosis, and mortality risk subclasses. For identifying chronic comorbidities in discharge records, HCUP provides software using the Elixhauser framework that uses ICD codes to identify 28 common chronic comorbidities (12). For the risk of mortality classification, HCUP provides software using the All Patient Refined Diagnosis Related Groups classification system that uses age, principle diagnosis, secondary diagnoses, and procedures to classify discharges into “minor,” “moderate,” “severe,” and “extreme” risk of mortality groups (13, 14). For describing discharges’ principle diagnosis, we first used HCUP’s Clinical Classifications Software to categorize the principle diagnosis ICD code into a shorter list of clinical categories. Since this still produces many principle diagnosis categories (285 for ICD-9 and 530 for ICD-10), we additionally recategorized these into six categories of principle diagnoses: respiratory, infection, cardiovascular, neurologic, neurotrauma, and trauma (Tables 4 and 5, Supplemental Digital Content 1, http://links.lww.com/CCX/A762 for our recategorization crosswalk).
Statistical Analysis
We used the SAS 9.4 (by SAS Institute, Cary, NC) survey family of procedures to account for the complex, multistage NIS sampling design and produce national estimates. We used the HCUP’s strata and cluster survey design variables along with HCUP’s trend weights that were especially designed to examine trends across multiple years in order to estimate annual counts of cases. We used the U.S. Census Bureau annual population estimates for persons greater than or equal to 18 years to determine tracheostomy rates per 100,000 U.S. adults (15, 16). We calculated weighted annual proportions for all categorical variables and means for continuous variables. We included weighted 95% CIs for all our estimates and defined statistical significance between annual measures as those with nonoverlapping 95% CIs.
This work was performed with publicly available, deidentified data and therefore is not considered human subjects research or requires Institutional Review Board review.
RESULTS
Annual Case Volumes and Occurrence Rate of Tracheostomy
From 2002 to 2014 and 2016 to 2017, there was an estimated weighted total of 554,346,148 hospital discharges. After excluding those less than 18 years old (n = 90,734,549) or with a diagnosis of head and neck cancer (n = 1,859,049), there were a total estimated 1,241,428 tracheostomies over the study period with an average of 84,762 tracheostomies per year. Over the study period, there were 80,612 tracheostomies performed in 2002, a peak of 89,545 tracheostomies in 2008, and a nadir of 58,840 tracheostomies in 2017 (Fig. 1) (Table 6, Supplemental Digital Content 1, http://links.lww.com/CCX/A762). National estimates of the annual occurrence rate were 37.5 (95% CI, 34.7–40.4) tracheostomies per 100,000 U.S. adults in 2002, with a peak of 39.7 (95% CI, 36.5–42.9) per 100,000 U.S. adults in 2003, and with a nadir of 28.4 (95% CI, 27.2–29.6) per 100,000 U.S. adults in 2017 (Fig. 2).
Figure 1.
Annual case volume of adult tracheostomies in acute care hospitals. Figure includes annual point estimates with shaded area representing 95% CIs of the estimates. We excluded patients with head and neck cancer. Over the study period: 1) the number of participating states in sample increased from 35 to 48, including the District of Columbia; 2) in 2012, there was a change in sample design; and 3) in 2015, diagnosis and procedure reporting changed from International Classification of Diseases (ICD), 9th revision, Clinical Modification coding to the ICD, 10th revision, Clinical Modification (8). The 2015 year was not included, given that the ICD coding changed during the year, preventing the use of standard methods for making annual estimates with either coding system for that year.
Figure 2.
Annual occurrence rate of adult tracheostomies in acute care hospitals. Figure includes annual point estimates with shaded area representing 95% CIs of the estimates. We excluded patients with head and neck cancer. Over the study period: 1) the number of participating states in sample increased from 35 to 48, including the District of Columbia; 2) in 2012, there was a change in sample design; and 3) in 2015, diagnosis and procedure reporting changed from International Classification of Diseases (ICD), 9th revision, Clinical Modification coding to the ICD, 10th revision, Clinical Modification (8). The 2015 year was not included, given that the ICD coding changed during the year, preventing the use of standard methods for making annual estimates with either coding system for that year.
Tracheostomy in RF-IMV
After our exclusions mentioned above, we identified an estimated 10,096,755 hospital discharges coded for RF-IMV. Of those with RF-IMV, an estimated 958,856 (9.4%) had an ICD code for tracheostomy with an annual average of 9.6%. This changed over the study period from 10.4% in 2002, with a peak of 10.9% in 2004, and with a nadir of 7.4% in 2017 (Fig. 3) (Table 6, Supplemental Digital Content 1, http://links.lww.com/CCX/A762).
Figure 3.
Annual rate of tracheostomy among U.S. adults hospitalized with respiratory failure with invasive mechanical ventilation. Figure includes annual point estimates with shaded area representing 95% CIs of the estimates. We excluded patients with head and neck cancer. Over the study period: 1) the number of participating states in sample increased from 35 to 48, including the District of Columbia; 2) in 2012, there was a change in sample design; and 3) in 2015, diagnosis and procedure reporting changed from International Classification of Diseases (ICD), 9th revision, Clinical Modification coding to the ICD, 10th revision, Clinical Modification (8). The 2015 year was not included, given that the ICD coding changed during the year, preventing the use of standard methods for making annual estimates with either coding system for that year. Annual U.S. Census Bureau annual population estimates for those greater than or equal to 18 yr old comprise the denominators in the calculation of annual occurrence rates (10, 11).
For readability, in this section, we compare select patient characteristics of discharges with both RF-IMV and tracheostomy between the 2 years 2002 and 2017 (Table 1). See Tables 7–10 (Supplemental Digital Content 1, http://links.lww.com/CCX/A762) for complete data on each study year. From 2002 to 2017, the proportion of female patients decreased, and there was a high proportion of patients with missing race/ethnicity (some years over 20%) that precludes informative reporting. Over the study period, the mean age of patients decreased, with an examination of age as a categorical variable revealing more specifically that the proportions of patients in the 18–29- and 50–69-year-old groups increased, the proportions in the 30–49-year-old groups remained stable, and the proportions in the greater than or equal to 70 age groups decreased. Throughout the study period, the most common expected primary payer was Medicare followed by private insurance and Medicaid. From 2002 to 2017, the proportion of patients with expected payer as Medicare decreased, the proportion of patients with Medicaid increased, and the proportions of patients with private insurance, on self-pay, no charge, and other payer remained unchanged.
TABLE 1.
Demographic Characteristics of Patients With Respiratory Failure With Invasive Mechanical Ventilation and Tracheostomy
| Characteristic | 2002 | 2006 | 2010 | 2014 | 2017 |
|---|---|---|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | |
| Age, yr | |||||
| Mean | 63.8 (63.0–64.6) | 62.2 (61.5–63.0) | 60.1 (59.5–60.8) | 60.3 (59.8–60.7) | 59.6 (59.2–60.1) |
| Categories | |||||
| 18–29 | 4.5 (3.8–5.2) | 5.3 (4.7–6.1) | 6.5 (5.8–7.3) | 6.4 (5.9–7.0) | 6.8 (6.2–7.3) |
| 30–39 | 6.0 (5.3–6.7) | 5.0 (4.4–5.5) | 6.5 (6.0–7.0) | 6.0 (5.6–6.5) | 6.9 (6.4–7.4) |
| 40–49 | 10.2 (9.5–10.8) | 11.5 (10.7–12.3) | 11.9 (11.2–12.7) | 10.3 (9.8–10.9) | 10.1 (9.6–10.7) |
| 50–59 | 14.8 (14.0–15.6) | 18.1 (17.3–18.9) | 20.1 (19.4–20.8) | 20.8 (20.1–21.5) | 21.0 (20.2–21.7) |
| 60–69 | 20.0 (19.1–20.9) | 21.5 (20.8–22.3) | 22.3 (21.5–23.0) | 24.9 (24.1–25.8) | 24.8 (24.0–25.6) |
| 70–79 | 26.8 (25.5–28.1) | 23.2 (22.2–24.2) | 20.2 (19.2–21.1) | 20.0 (19.2–20.8) | 20.8 (19.92–1.6) |
| ≥ 80 | 17.8 (16.6–19.0) | 15.4 (14.2–16.6) | 12.6 (11.7–13.4) | 11.4 (10.7–12.1) | 9.6 (9.01–0.3) |
| Female, % | 46.7 (45.4–48.0) | 45.5 (44.3–46.7) | 43.5 (42.3–44.6) | 43.4 (42.4–44.3) | 41.3 (40.4–42.3) |
| Payer | |||||
| Medicare | 57.5 (55.4–59.7) | 54.8 (52.7–56.8) | 48.9 (46.9–50.8) | 51.4 (50.2–52.6) | 49.8 (48.6–51.0) |
| Medicaid | 12.3 (11.2–13.4) | 14.3 (13.0–15.7) | 17.9 (16.3–19.4) | 18.5 (17.6–19.4) | 20.4 (19.4–21.3) |
| Private including HMO | 23.9 (22.3–25.5) | 22.9 (21.4–24.3) | 24.1 (22.7–25.5) | 22.5 (21.6–23.4) | 22.6 (21.7–23.6) |
| Self-pay | 2.9 (2.2–3.6) | 3.8 (3.1–4.4) | 4.8 (3.6–5.9) | 3.9 (3.4–4.4) | 3.6 (3.1–4.1) |
| No charge | 0.3 (0.1–0.6) | 0.5 (0.2–0.7) | 0.5 (0.2–0.9) | 0.3 (0.2–0.5) | 0.2 (0.1–0.4) |
| Other | 2.8 (2.2–3.4) | 3.8 (3.0–4.5) | 3.6 (2.9–4.4) | 3.3 (2.9–3.7) | 3.2 (2.8–3.6) |
The most common principle diagnosis category for discharges with RF-IMV and tracheostomy changed over the study period (Table 11, Supplemental Digital Content 1, http://links.lww.com/CCX/A762). In 2002, the most common principle diagnosis category was respiratory (26%), followed by infection (12%), cardiovascular (10%), neurologic (8%), neurotrauma (8%), and trauma (6%). In 2017, the most common principle diagnosis category was infection (25%), followed by respiratory (15%), neurologic (10%), neurotrauma (9%), trauma (9%), and cardiovascular (8%). Additionally, there were changes in patterns of comorbidities among RF-IMV patients who underwent tracheostomy between 2002 and 2017 (Table 2). The comorbidities that demonstrated a relative doubling of proportion among the annual RF-IMV with tracheostomy study populations were deficiency anemias, coagulopathy, depression, diabetes mellitus with complications, drug abuse, hypertension, hypothyroidism, liver disease, obesity, paralysis, peripheral vascular disease, and renal failure. The comorbidities that showed smaller relative increases in proportion were alcohol abuse, rheumatoid arthritis, fluid and electrolyte disorders, other neurologic disorders, psychoses, pulmonary circulation disorders, peptic ulcer disease, and weight loss. The comorbidities that demonstrated no change include AIDS/HIV, congestive heart failure, pulmonary disease, lymphoma, metastatic cancer, and valvular disorder. The comorbidities that demonstrated a decrease in proportion among the annual study groups were blood loss anemia, diabetes mellitus without complications, and solid tumor without metastases. For mortality risk subclasses, the majority of RF-IMV patients with tracheostomy had a predicted “extreme likelihood of dying,” followed by “major likelihood of dying.” From 2002 to 2017, the proportion with “extreme likelihood of dying” increased, whereas the proportion with a “major,” “moderate,” and “minor” likelihood of dying decreased. In terms of outcomes, from 2002 to 2017, the mean LOS per patient decreased, hospital mortality decreased by half, and mean hospital charges more than doubled. Further examining discharge disposition, the proportion of patients with routine discharge home or transfer to short-term care decreased, and the proportion discharged to intermediate care facilities increased.
TABLE 2.
Clinical Characteristics of Patients With Respiratory Failure With Invasive Mechanical Ventilation and Tracheostomy
| Characteristic | 2002 | 2006 | 2010 | 2014 | 2017 |
|---|---|---|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | |
| Elixhauser comorbidity | |||||
| HIV/AIDS | 0.4 (0.2–0.5) | 0.6 (0.4–0.7) | 0.6 (0.3–0.9) | 0.4 (0.3–0.5) | 0.6 (0.5–0.8) |
| Alcohol abuse | 6.3 (5.6–7) | 7.8 (7.1–8.5) | 8.5 (7.7–9.3) | 9.7 (9.1–10.2) | 9.1 (8.6–9.7) |
| Deficiency anemias | 13.7 (11.9–15.5) | 18.1 (16–20.1) | 26.2 (24.1–28.4) | 29.1 (28–30.3) | 28.1 (26.9–29.2) |
| Rheumatoid arthritis | 1.4 (1.1–1.6) | 1.7 (1.5–2) | 2.3 (2–2.6) | 2.5 (2.2–2.8) | 2.6 (2.3–2.8) |
| Blood loss anemia | 2.9 (2.4–3.4) | 3.1 (2.5–3.6) | 1.8 (1.4–2.1) | 1.5 (1.3–1.7) | 1.3 (1.1–1.5) |
| Congestive heart failure | 28.7 (26.8–30.7) | 31.4 (29.5–33.3) | 24 (22.5–25.5) | 27.8 (26.8–28.9) | 30.6 (29.5–31.6) |
| Pulmonary disease | 32.2 (30.2–34.2) | 32.8 (31.1–34.5) | 25.7 (24–27.5) | 28.3 (27.3–29.3) | 29.5 (28.4–30.5) |
| Coagulopathy | 11 (9.9–12) | 14.7 (13.6–15.9) | 17.8 (16.4–19.2) | 20.9 (20–21.7) | 22.5 (21.6–23.4) |
| Depression | 2.9 (2.5–3.3) | 4.5 (3.9–5.2) | 6.5 (5.8–7.1) | 8.7 (8.1–9.3) | 9.8 (9.2–10.4) |
| Diabetes without complications | 13.4 (12.2–14.7) | 16.7 (15.4–18) | 19.5 (18.1–20.9) | 22.6 (21.8–23.5) | 9.2 (8.6–9.7) |
| Diabetes with complications | 4 (3.5–4.6) | 4.6 (4–5.2) | 4.9 (4.3–5.4) | 6.4 (5.9–6.9) | 20.8 (19.9–21.7) |
| Drug abuse | 2.3 (1.9–2.7) | 3.7 (3.3–4.2) | 3.8 (3.3–4.3) | 5.6 (5.1–6) | 5.8 (5.3–6.2) |
| Hypertension | 20.3 (18.4–22.2) | 38.1 (35.8–40.4) | 44.8 (42.1–47.4) | 55.4 (54.2–56.6) | 58.8 (57.5–60.1) |
| Hypothyroidism | 3.8 (3.3–4.4) | 5.3 (4.7–6) | 7.3 (6.6–8) | 9.4 (8.8–9.9) | 8.8 (8.2–9.3) |
| Liver disease | 2.7 (2.3–3) | 3.5 (3.2–3.8) | 4.3 (3.7–4.8) | 5.1 (4.7–5.5) | 6.1 (5.7–6.6) |
| Lymphoma | 0.8 (0.6–1) | 0.9 (0.7–1) | 1.1 (0.9–1.2) | 1 (0.9–1.2) | 1 (0.8–1.2) |
| Fluid and electrolyte disorders | 43.1 (40.4–45.9) | 52.1 (50–54.2) | 62.4 (60–64.9) | 73.7 (72.6–74.8) | 74.8 (73.5–76.1) |
| Metastatic cancer | 3 (2.6–3.4) | 3 (2.7–3.4) | 3.2 (2.7–3.7) | 2.7 (2.4–3) | 2.9 (2.6–3.2) |
| Other neurologic disorders | 20.9 (19.4–22.4) | 16.8 (15.8–17.8) | 18.2 (17–19.4) | 21.8 (21–22.7) | 25 (24.2–25.9) |
| Obesity | 4.5 (3.9–5.1) | 7 (6.2–7.8) | 12.5 (11.4–13.5) | 20.7 (19.8–21.5) | 21.5 (20.7–22.4) |
| Paralysis | 6.7 (5.9–7.5) | 7.5 (6.8–8.2) | 13.6 (12.7–14.5) | 15.3 (14.6–16.1) | 20.3 (19.5–21.2) |
| Peripheral vascular disease | 3.3 (2.7–3.8) | 4 (3.5–4.5) | 6.7 (6.1–7.3) | 8.7 (8.2–9.3) | 8.6 (8.1–9.1) |
| Psychoses | 2.7 (2.3–3.1) | 3.5 (3.1–4) | 5 (4.6–5.4) | 6.4 (6–6.8) | 4.7 (4.3–5.1) |
| Pulmonary circulation disorders | 3.3 (2.7–4) | 3.5 (2.9–4) | 9.3 (8.5–10) | 10.9 (10.4–11.5) | 5.2 (4.8–5.6) |
| Renal failure | 9.8 (8.8–10.8) | 17.5 (16.3–18.7) | 16.6 (15.3–17.9) | 20.1 (19.2–21) | 21.2 (20.3–22) |
| Solid tumor without metastasis | 4.3 (3.8–4.8) | 2 (1.7–2.2) | 2.3 (2–2.5) | 2.1 (1.8–2.3) | 2.7 (2.4–3) |
| Peptic ulcer disease | 1.2 (0.9–1.5) | 0.1 (0.1–0.2) | 0.1 (0–0.1) | 0.1 (0–0.1) | 3.1 (2.8–3.5) |
| Valvular disease | 6.9 (6–7.7) | 7.7 (6.8–8.6) | 5 (4.4–5.6) | 6.8 (6.2–7.3) | 6.3 (5.8–6.8) |
| Weight loss | 19.3 (16.7–21.9) | 21.3 (19.2–23.4) | 35.5 (32.2–38.7) | 37.7 (36.3–39.2) | 33.3 (31.9–34.7) |
| Elixhauser mortality score, mean | 13.7 (13–14.4) | 14.4 (13.8–15) | 16.4 (15.7–17.1) | 18.3 (17.9–18.6) | 18.2 (17.9–18.6) |
| Risk of mortality, % | |||||
| Minor | 3.3 (2.8–3.8) | 2.8 (2.3–3.2) | 1.5 (1.3–1.8) | 1.2 (1–1.5) | 0.8 (0.7–1) |
| Moderate | 12.1 (11.1–13) | 10.8 (9.8–11.8) | 7 (6.3–7.7) | 4.6 (4.1–5) | 3.9 (3.5–4.3) |
| Major | 37.3 (35.6–39) | 34.7 (33.6–35.8) | 28.5 (27.4–29.6) | 27.5 (26.5–28.4) | 24.3 (23.4–25.2) |
| Extreme | 42.2 (39.8–44.6) | 51.7 (49.8–53.6) | 63 (61.3–64.6) | 66.7 (65.6–67.9) | 71 (69.9–72) |
| Discharge location | |||||
| Routine | 8.2 (6.9–9.5) | 7.5 (5.9–9) | 8.2 (6.2–10.2) | 6.3 (5.7–6.8) | 6.1 (5.5–6.7) |
| Transfer to short-term | 7 (6–8) | 5.8 (4.8–6.9) | 6.3 (5.1–7.6) | 6 (5.3–6.8) | 4.9 (4.4–5.4) |
| Transfer to intermediate | 52.6 (50.7–54.6) | 59.1 (56.9–61.2) | 62.7 (60–65.4) | 67.4 (66.3–68.5) | 70.2 (69.1–71.3) |
| Home healthcare | 5 (4.3–5.8) | 5.4 (4.8–5.9) | 5.9 (5–6.8) | 5.5 (5–5.9) | 5.7 (5.2–6.2) |
| Against medical advice | 0.1 (0.1–0.2) | 0.1 (0.1–0.2) | 0.1 (0.1–0.2) | 0.2 (0.1–0.3) | 0.3 (0.2–0.4) |
| Died in hospital | 25.7 (24.4–27) | 21.9 (20.7–23.1) | 16 (15–17) | 14.4 (13.8–15.1) | 12.7 (12–13.4) |
| Length of stay, mean days | 39.4 (38–40.7) | 36.7 (35.5–37.9) | 34.9 (33.2–36.6) | 33 (32.3–33.8) | 32.8 (32.1–33.5) |
| Total charges, mean dollars | 233,949 (221,692.3–246,204.9) | 275,808 (262,928.3–288,688.5) | 360,747 (337,423.2–384,070.9) | 470,727 (454,006.2–487,447.3) | 566,857 (543,750.2–589,964.8) |
Hospital Characteristics
In terms of hospital characteristics among patients with RF-IMV and tracheostomy, the South had the greatest proportion of tracheostomy patients followed by the Northeast. From 2002 to 2017, the proportion of patients in the Southern region increased, the proportion in the Northeast decreased, and proportions in the West and Midwest remained stable. The proportion of patients from urban-teaching hospitals increased, whereas those from urban nonteaching and rural hospitals decreased by more than half. The majority of patients were from private, nonprofit hospitals with this proportion remaining stable from 2002 to 2017. The proportion of patients from small hospitals increased, whereas those from large hospitals decreased (Table 9, Supplemental Digital Content 1, http://links.lww.com/CCX/A762).
DISCUSSION
In this study, we report modern annual case volume and occurrence rate of adult tracheostomies performed in acute care hospitals from 2002 to 2017. Over the study period, the overall case volume and occurrence rate for tracheostomies in adults without head and neck cancer appear to increase from 2002 till around 2008 and then maintain an annual decrease from 2010 onward. In addition to examining total adult tracheostomy procedures, we additionally examined the rate of tracheostomies specifically in patient group with RF-IMV, demonstrating that the tracheostomy rate appears stable from 2002 to 2008 with annual decreases from 2008 onward. Given that tracheostomy and the subsequent ventilator weaning for these patients are highly specialized and resource-intensive services, continued descriptive knowledge of the epidemiology for tracheostomy for RF-IMV patients is critical for workforce and healthcare resource planning.
The findings from this study must be interpreted within the context of its strengths and limitations. Our study has several strengths. We used the largest all-payer hospitalization database available, which in turn uses a complex national sampling design in order to generate nationally representative estimates of tracheostomy occurrence rate and outcomes over a 15-year period across two ICD classification systems. We used the HCUP’s trend analysis design weights and appropriate statistical procedures to produce generalizable national estimates available from this data. We used an exhaustive list of ICD-9 and ICD-10 diagnosis and procedure codes to capture an important patient population that we believe is relevant from clinical and healthcare resource utilization perspectives.
As with any study, there are important limitations to the data. These are administrative hospital data and therefore subject to any potential inaccuracies or biases in the utilization of ICD coding to identify diagnoses and conditions. Furthermore, it does not account for procedures performed in the outpatient setting. Notably, for tracheostomies performed in the ambulatory setting, providers use Common Procedural Terminology coding which may lead to an underestimation of the national tracheostomies from this dataset. Additionally, there were changes in the NIS over the study period described in our Methods section that could influence results. In 2012, the NIS sample no longer included LTAC hospitals. However, our use of HCUP’s trend weights in the analysis additionally excludes the counts of discharges from LTACs in weighted estimates making our results generalizable to procedures performed in acute care hospitals. Additionally, although the NIS sample design variables may help adjust national estimates for the underlying increase in the number of states represented in the study sample over time, our results demonstrating changes in the proportions of tracheostomies from different U.S. regions suggest that changes in the underlying representation may influence results over time. Although the change in ICD coding in 2015 precluded including this year in our annual estimates and likely effects occurrence rate, this characteristic of our study is also a strength in providing data across this important transition.
Within the context of these strengths and limitations, we believe that these data represent an important piece of the puzzle of the epidemiology of tracheostomy in the United States but also exercise caution in our speculations as to the causes of the visible trends. Our work is a comparable extension of the work done by Mehta et al (4) that examined tracheostomy epidemiology using the NIS data from 1993 to 2012. They demonstrated increases in both the national occurrence rate of tracheostomy and in the rate of IMV patients who received tracheostomy from 1993 with a visible plateau followed by annual decreases from around 2008 onward. Of note, although our two articles appear to have similar trends in their years of overlap, there are differences in the actual national estimates. For example, in 2012, Mehta et al estimated 34.3 tracheostomies per 100,000 U.S. adults, whereas we estimated 33.1 (95% CI, 31.5–34.7) (4). These differences may be attributed to different use of survey design variables in analysis, case definition, and exclusions. Specifically, the HCUP trend weights that were used to generate national, weighted estimates underwent a significant change in 2012 to account for the redesign of the NIS sampling strategy and may effect final weighted estimates. Additionally, for our occurrence rate calculations, we counted only those with a procedure code for tracheostomy, whereas to our understanding from their article, Mehta et al counted those with IMV and tracheostomy (4). Additionally, we excluded those with head and neck cancer as we thought this represented a distinct clinical population.
In interpreting the trends in our data, in general, the hypothetical reason for any changes in estimates over time may be due to changes in ICD coding utilization, changes in location of tracheostomy procedures outside of the hospital setting, changes in the underlying sample frame of the data, changes in the occurrence rates of acute conditions that precede tracheostomy, changes in the utilization of tracheostomy, or some combination of these factors. We offer our speculations on each of these components but encourage future research to better understand the patterns documented here. First, we note that, unfortunately, the dataset is not able to assess the accuracy of or changes in coding practices, and this remains an important unknown factor in our results. In regard to changes in the underlying occurrence rate of RF-IMV, our previously published work in these data has demonstrated the annual occurrence rates of RF-IMV over the same study period (17). Briefly, among U.S. adults from 2002 to 2017, the occurrence rate of discharges with RF diagnoses increased nearly two-fold, occurrence rate of discharges with procedural codes for IMV remained stable, and discharges with RF and any mechanical ventilation (including non-IMV) increased 83% (17). Although that work did not specifically describe the RF-IMV population of this study, it seems reasonable to infer that the underlying patient RF-IMV population for potential tracheostomy is stable to growing. Although speculative, such growth could potentially drive the reduced proportions of RF-IMV patients receiving tracheostomy that we observe in this study if there is not proportional growth in the procedural workforce or LTAC capacity for these patients. Regarding changing patient selection over time, it is difficult to unpack this scenario in the present analysis. Although decreasing annual proportions of advanced age groups and changing proportions of various comorbidities among RF-IMV tracheostomy discharges seem to suggest evolving selection, in the context of our prior analysis, these changes actually seem to reflect changes in the underlying RF-IMV population in general (17). Finally, another potential factor playing a role in the decreases in tracheostomies among RF-IMV discharges is greater collaboration with palliative care physicians and earlier initiation of goals of care discussion that preclude decisions to perform tracheostomy in this population (18, 19).
In regard to the reported outcomes of RF-IMV patients with tracheostomy, it is not possible in this dataset to elucidate the reasons for the declining mean LOS and hospital mortality demonstrated in the data. In general, some potential reasons for such a finding include changes in selection of RF patients for IMV, changes in selection of RF-IMV patients for tracheostomy, improvements in general critical care management, changes of the underlying discharge sample, changes in where patients eventually expire outside of hospital encounter, or some combination of any of these factors. Of note, average LOS from LTACs is greater than 30 days, and discharges to these long-term facilities have likely shortened the LOS of our population in acute care hospitals (20). Although lower LOS could be due to better implementation of multidisciplinary teams that facilitate efficient discharge out of the hospital (21, 22), it is worth mentioning that despite decreasing hospital LOS, these patients may possibly be staying longer in other facilities. The decreased in-hospital mortality among RF-IMV patients with tracheostomy follows the same rationale—either we are truly improving patient outcomes given the improving care in RF-IMV patients (23–25) or there is increased selection of patients appropriate for IMV and tracheostomy and those who receive these therapies may be increasingly dying outside of the hospital. Of note, the NIS discharge disposition category of “transfer to ICF” includes LTACs and Hospice. Within this context, it is worth observing that although hospital mortality for this study decreased from 25.7% to 12.7% (absolute difference of 13.0%), the proportion of patients discharged to an “ICF” rose from 52.6% to 70.2% (absolute difference of 17.6%).
CONCLUSIONS
Although there are challenges to the study of the epidemiology of tracheostomy, given that this is a highly specialized procedure and these patients subsequently have very specialized healthcare resource needs in LTACs, it is paramount to continue to strive toward a comprehensive understanding of the annual case volumes and patient characteristics of this population. Better understanding of outcomes of these patients after discharge can help us identify what their resource needs are in the community, recognize if any subgroup requires specialized needs, and promote better collaboration between inpatient and outpatient healthcare teams to allow smoother transition of tracheostomy patient care after hospital discharge.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Martin received research support from the National Institutes of Health’s National Center for Advancing Translational Science (UL1 TR-002378) and the Marcus Foundation and has served as a consultant for Grifols, Inc. Dr. Kempker received support from the Agency for Healthcare Quality and Research (K08HS025240) and has received consulting fees from Grifols, Inc. The remaining authors have disclosed that they do not have any conflicts of interest.
This work was performed at Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Emory University School of Medicine, Atlanta, GA.
REFERENCES
- 1.Barrett ML, Smith MW, Elixhauser A, et al. : Utilization of intensive care services, 2011: Statistical brief #185. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD, Agency for Healthcare Research and Quality (US); 2014 [PubMed] [Google Scholar]
- 2.Loss SH, de Oliveira RP, Maccari JG, et al. : The reality of patients requiring prolonged mechanical ventilation: A multicenter study. Rev Bras Ter Intensiva. 2015; 27:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung NH, Napolitano LM: Tracheostomy: Epidemiology, indications, timing, technique, and outcomes. Respir Care. 2014; 59:895–915 [DOI] [PubMed] [Google Scholar]
- 4.Mehta AB, Syeda SN, Bajpayee L, et al. : Trends in tracheostomy for mechanically ventilated patients in the United States, 1993-2012. Am J Respir Crit Care Med. 2015; 192:446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee A, Chen M, Gialdini G, et al. : Trends in tracheostomy after stroke: Analysis of the 1994 to 2013 National Inpatient Sample. Neurohospitalist. 2018; 8:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leiser Y, Barak M, Ghantous Y, et al. : Indications for elective tracheostomy in reconstructive surgery in patients with oral cancer. J Craniofac Surg. 2017; 28:e18–e22 [DOI] [PubMed] [Google Scholar]
- 7.Mehta AB, Syeda SN, Wiener RS, et al. : Epidemiological trends in invasive mechanical ventilation in the United States: A population-based study. J Crit Care. 2015; 30:1217–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathens AB, Rivara FP, Mack CD, et al. : Variations in rates of tracheostomy in the critically ill trauma patient. Crit Care Med. 2006; 34:2919–2924 [DOI] [PubMed] [Google Scholar]
- 9.Elixhauser A, Steiner C, Palmer L: Clinical Classifications Software (CCS). 2015. Available: http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed October 29, 2019
- 10.Kerlin MP, Weissman GE, Wonneberger KA, et al. : Validation of administrative definitions of invasive mechanical ventilation across 30 intensive care units. Am J Respir Crit Care Med. 2016; 194:1548–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healthcare Cost and Utilization Project (HCUP): HCUP NIS Description of Data Elements. 2020. Available at: https://www.hcup-us.ahrq.gov/db/vars/dispuniform/nisnote.jsp. Accessed March 12, 2021
- 12.Elixhauser A, Steiner C, Harris DR, et al. : Comorbidity measures for use with administrative data. Med Care. 1998; 36:8–27 [DOI] [PubMed] [Google Scholar]
- 13.Healthcare Cost and Utilization Project: Overview of Disease Severity Measures Disseminated With the Nationwide Inpatient Sample (NIS) and Kids’ Inpatient Database (KID). 2005. Available at: https://www.hcup-us.ahrq.gov/db/nation/nis/OverviewofSeveritySystems.pdf. Accessed January 16, 2020
- 14.De Marco MF, Lorenzoni L, Addari P, et al. : [Evaluation of the capacity of the APR-DRG classification system to predict hospital mortality]. Epidemiol Prev. 2002; 26:183–190 [PubMed] [Google Scholar]
- 15.US Census Bureau: Intercensal Estimates of the Resident Population by Sex and Age for the United States: April 1, 2000 to July 1, 2010. 2016. Available at: https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. 2016. Accessed January 7, 2020
- 16.US Census Bureau: Annual Estimates of the Resident Population for Selected Age Groups by Sex for the United States, States, Counties, and Puerto Rico Commonwealth and Municipios: April 1, 2010 to July 1, 2014. 2016. Available at: https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed January 7, 2020
- 17.Kempker JA, Abril MK, Chen Y, et al. : The epidemiology of respiratory failure in the United States 2002-2017: A serial cross-sectional study. Crit Care Explor. 2020; 2:e0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhulani N, Gupta A, Gao A, et al. : Palliative care and end-of-life health care utilization in elderly patients with pancreatic cancer. J Gastrointest Oncol. 2018; 9:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang J, Shen J, Kim SJ, et al. : Ten-year trends of utilization of palliative care services and life-sustaining treatments and hospital costs associated with patients with terminally ill lung cancer in the United States from 2005 to 2014. Am J Hosp Palliat Care. 2019; 36:1105–1113 [DOI] [PubMed] [Google Scholar]
- 20.Votto JJ, Scalise PJ, Barton RW, et al. : An analysis of clinical outcomes and costs of a long term acute care hospital. J Med Econ. 2011; 14:141–146 [DOI] [PubMed] [Google Scholar]
- 21.Bonvento B, Wallace S, Lynch J, et al. : Role of the multidisciplinary team in the care of the tracheostomy patient. J Multidiscip Healthc. 2017; 10:391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Mestral C, Iqbal S, Fong N, et al. : Impact of a specialized multidisciplinary tracheostomy team on tracheostomy care in critically ill patients. Can J Surg. 2011; 54:167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brower RG, Matthay MA, Morris A, et al. : Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000; 342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 24.Papazian L, Forel JM, Gacouin A, et al. ; ACURASYS Study Investigators: Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010; 363:1107–1116 [DOI] [PubMed] [Google Scholar]
- 25.Trikha A, Singh PM: The PROSEVA trial: Is it time to flip over the ARDS patient? Natl Med J India. 2013; 26:284–285 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.