Abstract
Background:
Episodic memory decline varies by age and underlying neuropathology. Whether ambient air pollution contributes to the heterogeneity of episodic memory decline in older populations remains unclear.
Objectives:
We estimated associations between air pollution exposures and episodic memory decline according to pollutant, exposure time window, age, and latent class subgroups defined by episodic memory trajectories.
Methods:
Participants were from the Women’s Health Initiative Memory Study–Epidemiology of Cognitive Health Outcomes. Older women (; 74–92 years of age) completed annual (2008–2018) episodic memory assessments using the telephone-based California Verbal Learning Test (CVLT). We estimated 3-y average fine particulate matter [PM with an aerodynamic diameter of ()] and nitrogen dioxide () exposures at baseline and 10 y earlier (recent and remote exposures, respectively), using regionalized national universal kriging. Separate latent class mixed models were used to estimate associations between interquartile range increases in exposures and CVLT trajectories in women and , adjusting for covariates.
Results:
Two latent classes were identified for women (), “slow-decliners” { [95% confidence interval (CI): , ] and “fast-decliners” [ (95% CI: , )]}. In the slow-decliner class, but not the fast-decliner class, exposures were associated with a greater decline in CVLT scores over time, with a stronger association for recent vs. remote exposures [ (95% CI: , ) per and (95% CI: , 0.01) per , respectively]. Among women (), the largest latent class comprised “steady-decliners” [ (95% CI: , )], whereas the second class, “cognitively resilient”, had no decline in CVLT on average. was not associated with episodic memory decline in either class. A increase in recent was associated with nonsignificant acceleration of episodic memory decline in the -y-old fast-decliner class [ (95% CI: , 0.04)], and in the cognitively resilient class [ (95% CI: , 0.03)] and steady-decliner class [ (95% CI: , 0.05)]. Associations with recent exposure in women were stronger and statistically significant when 267 women with incident probable dementia were excluded [e.g., (95% CI: , ) for the cognitively resilient class]. In contrast with changes in CVLT over time, there were no associations between exposures and CVLT scores during follow-up in any subgroup.
Discussion:
In a community-dwelling U.S. population of older women, associations between late-life exposure to ambient air pollution and episodic memory decline varied by age-related cognitive trajectories, exposure time windows, and pollutants. https://doi.org/10.1289/EHP7668
Introduction
Decline in episodic memory (e.g., the ability to remember details from daily experience, as well as the spatial and temporal context of events) is commonly associated with normal cognitive aging (Tulving 2002), but more severe changes are the hallmark symptom of Alzheimer’s disease. Given that the field of Alzheimer’s disease has shifted its focus to the preclinical stage of the disease (Dubois et al. 2016), research attention has been placed on episodic memory as one of the most sensitive cognitive domains with early decline detectable in preclinical Alzheimer’s disease (Gallagher and Koh 2011). Episodic memory performance undergoes significant changes throughout the life span, following a curvilinear shape with rapid improvement during childhood, early decline beginning around middle age, and accelerated decline in very old age () (Shing et al. 2010; Singer et al. 2003). However, there is considerable heterogeneity in episodic memory decline, with individual trajectories varying from average population trajectories in terms of both starting levels and rates of change (Olaya et al. 2017). This heterogeneity has been demonstrated in longitudinal studies in general populations that have identified from two to four distinct trajectories of episodic memory over time among older individuals (Lee et al. 2018; McFall et al. 2019; Olaya et al. 2017; Wilson et al. 2020; Zahodne et al. 2015), and there is evidence suggesting that heterogeneity in memory performance increases into very old age (Finkel and Reynolds 2014; Olaya et al. 2017). For instance, across old age, memory performance may remain relatively unchanged until very late in life, decline linearly over time, or decline rapidly with the acceleration becoming more evident in very old age (Ding et al. 2019; Small and Bäckman 2007). Studies using a data-driven approach to identify latent classes of cognitive trajectories have also shown that the influence of modifiable risk factors on cognitive change may differ across latent classes (Wu et al. 2020). Although twin studies suggest that late-life episodic memory performance is heritable, approximately 40–60% of the total variance has been attributed to environment factors (Finkel and McGue 1998; Giubilei et al. 2008; Swan et al. 1999). Previous studies have focused primarily on the social environment (Josefsson et al. 2012; McFall et al. 2019) while overlooking the influence of the physical environment.
Data has emerged over the past decade supporting the detrimental effects of air pollution exposure on brain aging (The Lancet Neurology 2018). Longitudinal studies have shown that late-life exposures to ambient air pollution, especially fine particulate matter [PM with an aerodynamic diameter of ()] and oxides of nitrogen [nitrogen oxide () and nitrogen dioxide ()], are associated with increased risk of dementia, including Alzheimer’s disease (Peters et al. 2019). However, published studies have reported mixed findings for associations between air pollution and episodic memory decline (Kulick et al. 2020a, 2020b; Oudin et al. 2017; Petkus et al. 2020; Tonne et al. 2014; Weuve et al. 2012; Wurth et al. 2018; Younan et al. 2020). These studies assumed a single common trajectory for changes in episodic memory performance over time, and only one (Kulick et al. 2020a) investigated whether associations varied by age. In addition, the majority of previous studies investigated recent exposures averaged over a few years prior to the neuropsychological assessment, and it remains unclear whether exposures that occurred earlier in time are associated with episodic memory decline in later life.
To address these knowledge gaps, we conducted a longitudinal study to examine the association between long-term exposure to ambient air pollution and late-life episodic memory assessed annually (2008–2018) in a geographically diverse sample of community-dwelling older women. The aim of our study was to investigate whether long-term exposures were associated with changes in episodic memory and whether the putative exposure effects differed by sample age ( vs. ), exposure time window, or pollutants ( vs. ).
Methods
Study Sample
We conducted a prospective cohort study on community-dwelling women enrolled in the Women’s Health Initiative Memory Study–Epidemiology of Cognitive Health Outcomes (WHIMS-ECHO). WHIMS-ECHO was an extension of the WHIMS, a Women’s Health Initiative (WHI)–Hormone Therapy (HT) trials ancillary study designed to investigate the role of postmenopausal hormone therapy on the incidence of all-cause dementia (Shumaker et al. 1998). Women enrolled in the WHI-HT trials were recruited to participate in the WHIMS ancillary study if they were at WHIMS enrollment in 1995–1998. WHIMS participants completed annual cognitive assessments in the WHI-HT trial phase (which was terminated in 2002 or 2004, depending on the WHI trial arm) and posttrial extension phase (which continued through May 2008). Starting in September 2008, WHIMS participants who were still engaged in WHI follow-up were enrolled in WHIMS-ECHO if they provided informed consent to undergo annual telephone-based assessments of their cognitive function, allowed a friend or family member to be contacted, and had adequate hearing to complete telephone interviews (Espeland et al. 2017). From WHIMS-ECHO enrollment until June 2018, participants completed annual neuropsychological assessments through centralized telephone-administered cognitive interviews conducted by trained and certified staff.
For this study, we excluded women who were classified as probable dementia cases at or before WHIMS-ECHO enrollment (), women who did not complete any California Verbal Learning Test (CVLT) assessments of episodic memory, including women who were lost to follow-up before the CVLT was added to the annual assessment (), those with missing air pollution data for one or both of the exposure time windows examined in the analysis (), and those with missing data on key covariates at WHI inception (), including education, employment status, alcohol intake, smoking, diabetes, high cholesterol, hypertension, cardiovascular disease (CVD), or neighborhood socioeconomic characteristics. The final analytic sample comprised 2,056 women, including 828 who were and 1,228 who were at WHIMS-ECHO enrollment (Figure 1A).
Figure 1.
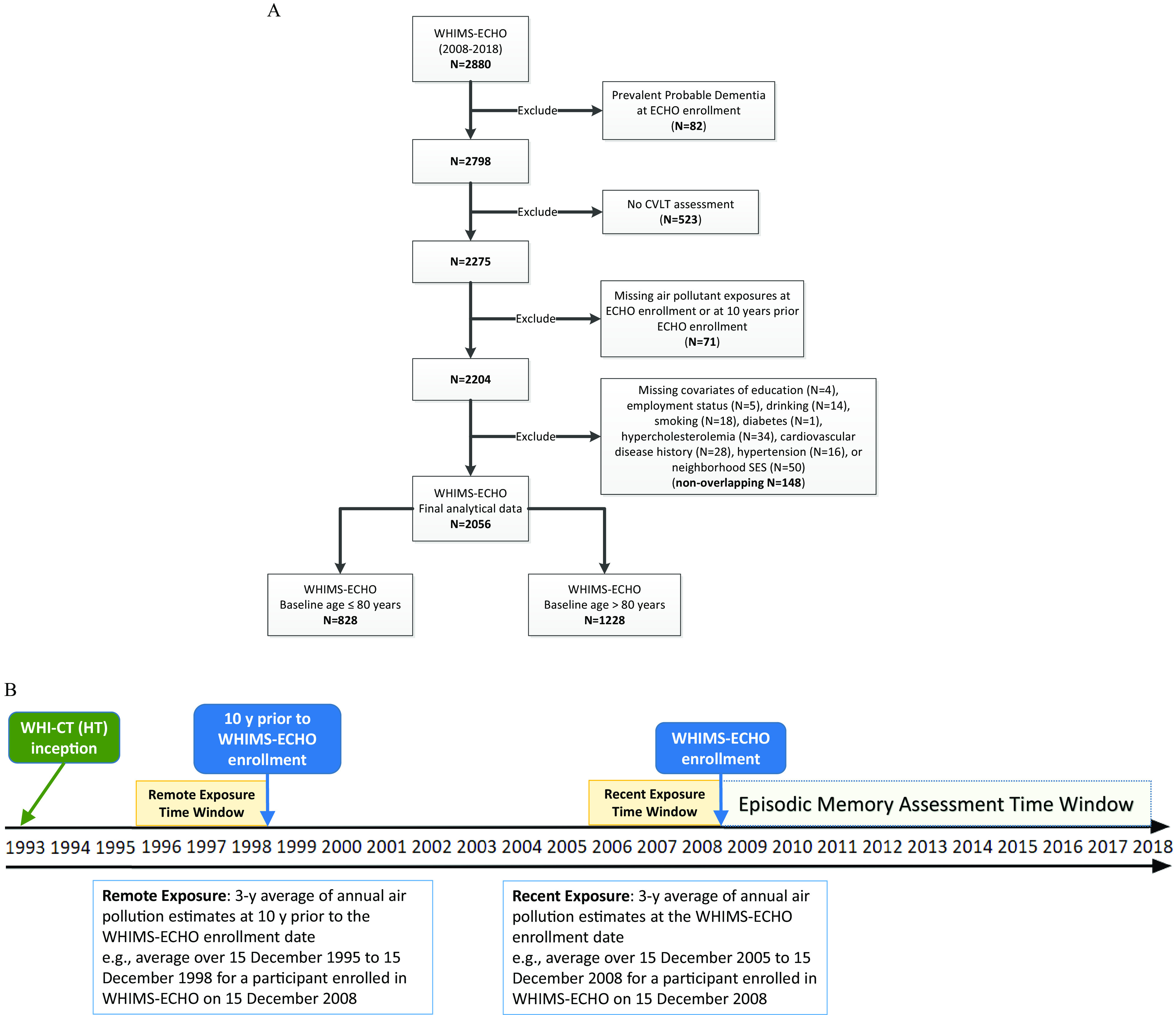
(A) Flowchart of study population and (B) illustration of the study timeline. Exposure time windows in (B) were defined based on each participant’s WHIMS-ECHO enrollment date; the example shown is used for illustrative purposes. Note: CVLT, California Verbal Learning Test; WHI-CT (HT), Women’s Health Initiative-Clinical Trial (Hormone Therapy); WHIMS-ECHO, Women’s Health Initiative Memory Study-Epidemiology of Cognitive Health Outcomes.
The institutional review board at the University of Southern California reviewed all study protocols. Written informed consent was obtained from all participants as part of the original WHIMS-ECHO study.
Measures of Episodic Memory
Episodic memory was assessed annually over the phone using a modified version of the CVLT (Delis et al. 1987). CVLT data collected through June 2018 were used in the present study. Participants were read a 16-item list of words from four semantically related categories. Each time the list was read the participant was instructed to immediately repeat back as many words as she could remember. We defined the episodic memory score as the total number of correct responses across three learning trials (0 to 48), with higher scores representing better performance. In contrast with the standard version of the CVLT that includes five learning trials, the modified version administered in WHIMS-ECHO was limited to three learning trials and did not include interference trials, short- or long-delayed free or cued recall, or word recognition.
Air Pollution Exposures
Data on participants’ residential addresses were collected at each WHI assessment and updated either during regular follow-up contacts at least semiannually or when participants alerted WHI staff about any change of address between regularly scheduled follow-ups from WHI clinical trial inception. The exact date of the change in residence was entered and used in analyses when available, otherwise the date when the change in residence was ascertained was used. The location of each residence was geocoded using standardized procedures (Whitsel et al. 2006). Annual mean concentrations of in micrograms per meter cubed and in parts per billion (a proxy measure for traffic-related air pollutants) were estimated at each participant’s address, using validated regionalized national universal kriging models with partial least squares regression of geographic covariates and U.S. Environmental Protection Agency monitoring data. More than 300 geographic covariates covering categories of population, land use, vegetative index, impervious surfaces, roadway, and proximity to features were used in the historical models for pre-1999 estimation or the national models for post-1999 estimation (Kim et al. 2017; Sampson et al. 2013). For estimation, satellite data and geographic covariates covering proximity and buffer measures were used in models (Young et al. 2016). The average cross-validation was 0.88 for and 0.85 for (Sampson et al. 2013; Young et al. 2016). We then used the annual estimates of each pollutant to calculate the “recent” 3-y average spanning the 3-y time window prior to the WHIMS-ECHO enrollment date and the “remote” 3-y average exposure, which was lagged 10 y from the WHIMS-ECHO enrollment date, accounting for residential mobility (Figure 1B). The length of stay at each residential location within the 3-y time window was used as the weight in each calculation.
Ascertainment of Probable Dementia
Incident cases of probable dementia were determined using published WHIMS-ECHO protocols (WHI Memory Study 2020). Briefly, participants underwent an annual, validated telephone interview that comprised a neuropsychological battery, including the modified Telephone Interview for Cognitive Status (TICSm) and additional neuropsychological tests. If a woman scored on the TICSm, the standardized, validated Dementia Questionnaire (DQ) was administered to a previously identified proxy (friend or family member). The results and the cognitive scoring history were then reviewed by a panel of experts in the diagnosis of dementia. A Supplemental Case Ascertainment Protocol (SCAP) was also implemented in the WHIMS-ECHO to identify cases of probable dementia in the deceased and proxy-dependent participants (Gaussoin et al. 2019). In the SCAP, the DQ data administrated to a participant-identified proxy and all prior assessments were used for adjudication of dementia. Clinical diagnoses of dementia were decided by the central adjudication committee for final confirmation.
Covariate Data
A structured questionnaire was administered to participants at WHI inception (in 1993–1998) to gather information on demographics [geographic region (Northeast, South, Midwest, or West), age], socioeconomic factors {educational attainment [ school or General Educational Diploma (GED), school but of college, or of college]; family income [, , , , , or missing/unknown (as a separate category)]; employment status (currently working, not working, or retired), and lifestyle factors (smoking status [never, past or current smoker]; alcohol intake [nondrinker, past drinker, drink/d, or drink/d]; physical activity (defined as episodes per week of moderate and strenuous recreational physical activity of min: no activity, some activity, 2–4 episodes/wk, or episodes/wk)}. We also collected information on race/ethnicity, which was reported by participants in response to “How would you describe your racial or ethnic group? If you are of mixed blood, which group do you identify with most?,” with the following options for responding: “American Indian or Alaskan Native,” “Asian or Pacific Islander (ancestry is Chinese, Indo-Chinese, Korean, Japanese, Pacific Islander, Vietnamese),” “Black or African-American (not of Hispanic origin),” “Hispanic/Latino (ancestry is Mexican, Cuban, Puerto Rican, Central American, or South American),” “White (not of Hispanic origin),” and “Other.” Although we provide descriptive information according to the original response categories, it was necessary to aggregate women who self-classified as “American Indian or Alaskan Native” or “Asian or Pacific Islander” into the “Other” category because of the small numbers in these groups. Therefore, the race/ethnicity categories used in data analyses were “Black, non-Hispanic,” “Hispanic/Latino,” “White, non-Hispanic,” and “Other.” The “other” category also included women with missing data for race/ethnicity.
Clinical characteristics were also ascertained (yes or no), including any postmenopausal hormone treatment and self-reported histories of CVD (defined as physician-diagnosed heart problems, problems with blood circulation, or blood clots), hypertension (defined as physician-diagnosed hypertension, not including high blood pressure during pregnancy), hypercholesterolemia (defined as physician-diagnosed high cholesterol requiring pills), and diabetes mellitus (defined as physician diagnosis plus oral medications or insulin therapy). Good reliability and validity of both the self-reported medical histories and the physical measures have been previously documented (Heckbert et al. 2004; Johnson-Kozlow et al. 2007; Margolis et al. 2008). Specifically, for cardiovascular events, there was substantial agreement between self-report and review by study physicians at clinical centers () (Heckbert et al. 2004). Self-reported prevalent diabetes was consistent with medication inventories in 77% and with fasting values of in 75% of women (Margolis et al. 2008). In addition, a study investigating the psychometric properties of the physical activity measure of the WHI showed that it was highly correlated with accelerometer data (, ) and the widely used 7-d Physical Activity Recall questionnaire (, ) (Johnson-Kozlow et al. 2007). In addition to the covariates collected at WHI inception, clinical covariates (hypertension; history of CVD) that were updated before the WHIMS-ECHO enrollment were also available for sensitivity analyses. History of hypertension was updated using measured blood pressures at annual in-person visits between 1993 and 2005 (elevated blood pressures were defined as systolic or diastolic ). History of CVD was updated using data on incident CVD events (coronary heart disease; myocardial infarction; coronary revascularization; coronary angioplasty; coronary artery bypass graft; atrial fibrillation; stroke) that were identified through initial self-report in annually updated medical questionnaires and subsequent medical record review by central adjudicators before the WHIMS-ECHO enrollment. Last, socioeconomic characteristics of residential neighborhood characterized at the U.S. Census tract level were calculated at both WHI inception and WHIMS-ECHO enrollment (Diez Roux et al. 2001). Briefly, six attributes covering domains of wealth/income, education, and occupation from the “U.S. Census of Population and Housing 2000 Summary File 3” (U.S. Census Bureau and ICPSR 2006), or their 5-y analogs from the American Community Survey 2005–2009 to 2013–2017 (U.S. Census Bureau 2019), were temporally matched to geocoded participant addresses (Whitsel et al. 2004, 2006). Each attribute had been aggregated at the U.S. Census tract level (i.e., the lowest geographic level historically associated with accurate and reliable assignment of Federal Information Processing System codes) (Whitsel et al. 2006). The six variables included a) log transformation of median household income; b) log transformed median value of owner-occupied housing units; c) percentage of households receiving interest, dividend, or net rent income; d) percentage of adults with a high school degree; e) percentage of adults with a college degree; and f) percentage of employed persons with a professional, managerial, or executive occupation. These variables were standardized using the corresponding population-specific mean and standard deviation and then summed to derive a -score for neighborhood socioeconomic status (SES). As computed, a higher neighborhood SES score implied a more advantageous neighborhood SES.
Statistical Analysis
Latent class mixed models.
We stratified our statistical analyses by age at WHIMS-ECHO enrollment ( vs. ) because of known heterogeneity in late-life episodic memory trajectories and the evidence of accelerated decline in very old age (Finkel and Reynolds 2014; Lee et al. 2018; McFall et al. 2019; Olaya et al. 2017; Wilson et al. 2020; Zahodne et al. 2015). Within each age group, we fitted latent class mixed models (LCMMs) (Proust-Lima et al. 2017) to identify groups of women with similar trajectories of episodic memory over time, where trajectories were characterized by a random intercept and the linear change of CVLT scores within each latent class. We determined the optimal number of latent classes using age-stratified models with follow-up time and age at WHIMS-ECHO enrollment as the only predictors, beginning with a one-class solution and sequentially increasing the number of classes until we identified the optimal set of latent classes for each age group on the basis of the Bayesian Information Criterion (the lower the better), the number of women in each class (at least 5% of the population), and interpretability of the identified trajectories, similar to our prior work (Petkus et al. 2019). Posterior probabilities were also evaluated to ensure that the average posterior probability for women assigned to a given latent class by the baseline LCMM was . To avoid convergence at a local maximum, we used a grid of 10 random initial values and retained the estimates of the random initialization with the best log-likelihood.
After determining the optimal number of latent classes within each age group, we used separate LCMMs to estimate either class-specific or global exposure effects of recent or remote exposures on linear changes in episodic memory over time, and we used similar models to estimate associations with recent or remote . The LCMM with global exposure effects assumed a common association across latent classes, allowing us to explore heterogeneity in associations between age groups, exposure time windows, and pollutants without regard to latent class. In the data-driven LCMM, the number of latent classes was held constant according to our initial analysis, but the posterior probability of each class for a given woman, and thus each woman’s specific class assignment, could vary when air pollution exposures and additional predictors or covariates were added to the model. Two sets of covariates were considered in the models. Our base model included age at WHIMS-ECHO enrollment, follow-up time, interaction of age with follow-up time, and time-varying propensity score. Our fully adjusted model contained a full set of covariates with additional covariates including geographic region, race/ethnicity, education, income, employment status, neighborhood SES, lifestyle factors (smoking, drinking, and physical activities), and clinical characteristics (hormone treatment, CVD risk factors, and CVD histories). Except for the time-varying propensity scores, all covariates in the primary fully adjusted model were classified at WHI inception. These two sets of covariates were chosen in order to evaluate if there were significant associations with minimal covariates adjusted and whether the estimated associations were robust after further adjusting for known potential confounders. In these models, the parameter of interest is the interaction of the exposure with follow-up time. The age-equivalent effect for the association between each exposure and change in CVLT scores within the same class was calculated as , where and were the parameters estimated for the interaction of the exposure with follow-up time or the interaction of age with follow-up time, respectively. Finally, in addition to estimating associations between air pollution exposures and changes in episodic memory over time, we estimated average associations with episodic memory scores modeled as repeated outcomes during follow-up using the same models, but without interaction terms for exposure with follow-up time.
The time-varying propensity score approach to adjust for selective attrition due to loss to follow-up.
To account for selective attrition during the WHIMS-ECHO follow-up, models also included time-varying propensity scores (Robins et al. 2000; Wyss et al. 2020), which were generated using a two-stage modeling approach for each woman at each year of follow-up. We calculated the probability of having the observed exposures over different follow-up intervals and included this periodically updated probability as a time-varying covariate in the LCMM to control for potential bias due to differential attrition. The procedure included four steps. First, we divided follow-up time into intervals based on time since WHIMS-ECHO enrollment (, , , , , , , and since enrollment) so that data from women with cognitive function measurements in the same time interval could be grouped together. In the second step, we constructed linear regression models with air pollution exposure as the continuous outcome and used forward selection to assess each of the 15 covariates included in the fully adjusted LCMM and to identify statistically significant predictors of exposure using a significance level of 0.05/15 to account for multiple tests. Our final model included race/ethnicity and geographic region as independent variables and air pollution () as the dependent variable for each follow-up time interval (), as shown below:
We used the parameters (, , , and ) to estimate the probability of having the observed exposure for each subject i by the normal density (Robins et al. 2000), as shown below:
This is the preliminary propensity score () for each individual with a visit in time interval . In the third step, we improved the propensity scores by adding an interaction term between preliminary propensity scores for different time intervals to the linear regression model as a surrogate for possible covariate interactions that may impact the prediction model, as shown below:
where and represent the preliminary propensity scores for the closest time intervals that an individual had a visit in before and after time interval , respectively. The updated was then calculated as follows:
In the last step, we included the updated propensity score, or the preliminary version if it was unable to be updated, in the LCMM as a time-varying covariate to control for differential attrition in all analyses. Models without adjustment for propensity scores were also conducted for comparison.
Additional analyses.
In order to compare the estimated exposure effects on episodic memory decline between recent and remote exposures, we repeated the analyses of remote exposures scaled by the interquartile range (IQR) of recent exposures. To account for potential practice effects resulting from women having previously completing the CVLT or recalling the word list administered, we included a fixed effect of “practice” in models that was represented by a time-varying indicator variable coded as “0” at the initial assessment and “1” at each subsequent assessment. To further control for possible residual confounding that may result from the temporal misspecification of covariates measured only at WHI inception, we adjusted analyses of recent exposures using covariates (hypertension; CVD history; neighborhood SES characteristics) that were updated at WHIMS-ECHO enrollment.
Two sets of sensitivity analyses were also conducted to evaluate the robustness of our findings on episodic memory declines. We excluded participants who had either a prevalent stroke or an incident stroke during the follow-up to examine whether associations with recent exposures might be explained by cerebrovascular risk. To explore whether associations with recent ambient air pollution exposure might be explained by underlying dementia risk, we repeated analyses after excluding women who were newly classified as having probable dementia during follow-up. Latent class memberships based on fully adjusted models without these exclusions were carried forward to these analyses.
All statistical analyses were performed using R (version 3.6.2; R Development Core Team) and SAS (version 9.4; SAS Institute). All statistical tests were interpreted at the 0.05 significance level.
Results
Population Characteristics, Air Pollution Exposure Distribution, and Trajectories of Episodic Memory
The entire study sample comprised predominantly non-Hispanic White women (91%) with only 5% non-Hispanic Black women, 1% Hispanic women, and American Indian or Alaskan Native and Asian or Pacific Islander. There was also 1% who selected “other” instead of one of the five specific race/ethnicity categories. Most (75%) of these women had retired at the time of WHI inception (Table 1). Half of the women had never smoked (55%) or reported no physical activity (54%). The majority of women did not have a history of hypercholesterolemia (84%), diabetes (96%), hormone treatment (55%), hypertension (66%), or CVD (86%) at WHI inception. Compared with the 824 WHIMS-ECHO participants excluded from our analyses, the 2,056 women who were included had lower average air pollution exposures (for recent and for recent and remote ) and were a year younger on average, more likely to be non-Hispanic White (91% vs. 83%), and more likely to reside in the Northeast (32% vs. 26%) and less likely to reside in the South (20% vs. 26%) (Table S1). They were also more educated (37% vs. 28% with of college), had higher incomes (10% vs. 6% had incomes of ), and resided in neighborhoods with higher average SES scores at both WHI inception and WHIMS-ECHO enrollment. These women were also less likely to self-report a history of hypercholesterolemia at WHI inception (17% vs. 21%).
Table 1.
Distribution of recent and remote 3-y average and exposures and population characteristics [ or (%)], overall and according to age at WHIMS-ECHO enrollment and cognitive function trajectory subgroups determined by a latent class mixed model with follow-up time and age at WHIMS-ECHO enrollment as the only predictors.
| Characteristics | All women () | Age () | Age () | -Valuea | Age | Age | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Slow-decliners () | Fast-decliners () | -Valueb | Cognitively resilient () | Steady-decliners () | -Valuec | |||||
| Air Pollution Exposures | ||||||||||
| Remote () | 0.11 | 0.48 | 0.54 | |||||||
| Recent () | 0.85 | 0.81 | 0.93 | |||||||
| Remote (ppb) | 0.79 | 0.97 | ||||||||
| Recent (ppb) | 0.40 | 0.66 | ||||||||
| Age at WHIMS-ECHO enrollment (y) | 0.02 | 0.001 | ||||||||
| Neighborhood SESd | ||||||||||
| At WHI inception | 0.78 | 0.93 | ||||||||
| At WHIMS-ECHO enrollment | 0.88 | 0.73 | ||||||||
| Region | 0.18 | 0.98 | ||||||||
| Northeast | 662 (32.2) | 273 (33.0) | 389 (31.7) | 200 (31.1) | 73 (39.5) | 112 (31.9) | 277 (31.6) | |||
| South | 408 (19.8) | 183 (22.1) | 225 (18.3) | 145 (22.6) | 38 (20.5) | 62 (17.7) | 163 (18.6) | |||
| Midwest | 498 (24.2) | 225 (27.2) | 273 (22.2) | 178 (27.7) | 47 (25.4) | 80 (22.8) | 193 (22.0) | |||
| West | 488 (23.7) | 147 (17.8) | 341 (27.8) | 120 (18.7) | 27 (14.6) | 97 (27.6) | 244 (27.8) | |||
| Race/ethnicity | 0.10 | 0.73 | 0.35 | |||||||
| American Indian or Alaskan Native | 4 (0.2) | 1 (0.1) | 3 (0.2) | 1 (0.2) | 0 | 2 (0.6) | 1 (0.1) | |||
| Asian or Pacific Islander | 17 (0.8) | 6 (0.7) | 11 (0.9) | 4 (0.6) | 2 (1.1) | 1 (0.3) | 10 (1.1) | |||
| Black, non-Hispanic | 107 (5.2) | 57 (6.9) | 50 (4.1) | 44 (6.8) | 13 (7.0) | 10 (2.8) | 40 (4.6) | |||
| Hispanic/Latino | 29 (1.4) | 13 (1.6) | 16 (1.3) | 12 (1.9) | 1 (0.5) | 4 (1.1) | 12 (1.4) | |||
| White, non-Hispanic | 1,875 (91.2) | 744 (89.9) | 1,131 (92.1) | 577 (89.7) | 167 (90.3) | 329 (93.7) | 802 (91.4) | |||
| Othere | 23 (1.1) | 7 (0.9) | 16 (1.3) | 5 (0.8) | 2 (1.1) | 5 (1.4) | 11 (1.3) | |||
| Missing | 1 (0.1) | 0 | 1 (0.1) | 0 | 0 | 0 | 1 (0.1) | |||
| Education | 0.58 | 0.52 | 0.16 | |||||||
| school or GED | 500 (24.3) | 196 (23.7) | 304 (24.8) | 158 (24.6) | 38 (20.5) | 74 (21.1) | 230 (26.2) | |||
| school to of college | 789 (38.4) | 329 (39.7) | 460 (37.5) | 253 (39.4) | 76 (41.1) | 136 (38.8) | 324 (36.9) | |||
| of college | 767 (37.3) | 303 (36.6) | 464 (37.8) | 232 (36.1) | 71 (38.4) | 141 (40.2) | 323 (36.8) | |||
| Employment | 0.82 | 0.13 | ||||||||
| Currently working | 321 (15.6) | 181 (21.9) | 140 (11.4) | 143 (22.2) | 38 (20.5) | 48 (13.7) | 92 (10.5) | |||
| Not working | 195 (9.5) | 81 (9.8) | 114 (9.3) | 64 (10.0) | 17 (9.2) | 26 (7.4) | 88 (10.0) | |||
| Retired | 1,540 (74.9) | 566 (68.4) | 974 (79.3) | 436 (67.8) | 130 (70.3) | 277 (78.9) | 697 (79.5) | |||
| Income () | 0.11 | 0.59 | 0.002 | |||||||
| 68 (3.3) | 31 (3.7) | 37 (3) | 23 (3.6) | 8 (4.3) | 6 (1.7) | 31 (3.5) | ||||
| 10,000–34,999 | 906 (44.1) | 350 (42.3) | 556 (45.3) | 270 (42.0) | 80 (43.2) | 158 (45.0) | 398 (45.4) | |||
| 35,000–49,999 | 450 (21.9) | 206 (24.9) | 244 (19.9) | 165 (25.7) | 41 (22.2) | 58 (16.5) | 186 (21.2) | |||
| 50,000–74,999 | 321 (15.6) | 125 (15.1) | 196 (16) | 97 (15.1) | 28 (15.1) | 75 (21.4) | 121 (13.8) | |||
| 212 (10.3) | 80 (9.7) | 132 (10.7) | 64 (10.0) | 16 (8.6) | 43 (12.3) | 89 (10.1) | ||||
| Not known | 99 (4.8) | 36 (4.3) | 63 (5.1) | 24 (3.7) | 12 (6.5) | 11 (3.1) | 52 (5.9) | |||
| Smoking status | 0.62 | 0.94 | ||||||||
| Never smoked | 1,138 (55.4) | 416 (50.2) | 722 (58.8) | 328 (51.0) | 88 (47.6) | 205 (58.4) | 517 (59.0) | |||
| Past smoker | 823 (40.0) | 359 (43.4) | 464 (37.8) | 273 (42.5) | 86 (46.5) | 133 (37.9) | 331 (37.7) | |||
| Current smoker | 95 (4.6) | 53 (6.4) | 42 (3.4) | 42 (6.5) | 11 (5.9) | 13 (3.7) | 29 (3.3) | |||
| Alcohol use | 0.66 | 0.73 | 0.57 | |||||||
| Nondrinker | 243 (11.8) | 101 (12.2) | 142 (11.6) | 76 (11.8) | 25 (13.5) | 35 (10.0) | 107 (12.2) | |||
| Past drinker | 342 (16.6) | 129 (15.6) | 213 (17.3) | 97 (15.1) | 32 (17.3) | 57 (16.2) | 156 (17.8) | |||
| drink/d | 1,204 (58.6) | 494 (59.7) | 710 (57.8) | 390 (60.7) | 104 (56.2) | 209 (59.5) | 501 (57.1) | |||
| drink/d | 267 (13) | 104 (12.6) | 163 (13.3) | 80 (12.4) | 24 (13.0) | 50 (14.2) | 113 (12.9) | |||
| Physical activityf | 0.71 | 0.56 | 0.49 | |||||||
| No activity | 1,109 (53.9) | 445 (53.7) | 664 (54.1) | 344 (53.5) | 101 (54.6) | 184 (52.4) | 480 (54.7) | |||
| Some activity | 114 (5.5) | 47 (5.7) | 67 (5.5) | 39 (6.1) | 8 (4.3) | 20 (5.7) | 47 (5.4) | |||
| 2–4 episodes/wk | 439 (21.4) | 169 (20.4) | 270 (22.0) | 135 (21.0) | 34 (18.4) | 73 (20.8) | 197 (22.5) | |||
| episodes/wk | 394 (19.2) | 167 (20.2) | 227 (18.5) | 125 (19.4) | 42 (22.7) | 74 (21.1) | 153 (17.4) | |||
| Hypercholesterolemia | 0.99 | 0.15 | 0.86 | |||||||
| No | 1,716 (83.5) | 691 (83.5) | 1,025 (83.5) | 543 (84.4) | 148 (80.0) | 294 (83.8) | 731 (83.4) | |||
| Yes | 340 (16.5) | 137 (16.5) | 203 (16.5) | 100 (15.6) | 37 (20.0) | 57 (16.2) | 146 (16.6) | |||
| Diabetes | 0.86 | 0.05 | 0.64 | |||||||
| No | 1,976 (96.1) | 795 (96.0) | 1,181 (96.2) | 622 (96.7) | 173 (93.5) | 339 (96.6) | 842 (96.0) | |||
| Yes | 80 (3.9) | 33 (4.0) | 47 (3.8) | 21 (3.3) | 12 (6.5) | 12 (3.4) | 35 (4.0) | |||
| Hormone treatment | 0.58 | 0.33 | 0.33 | |||||||
| No | 1,127 (54.8) | 460 (55.6) | 667 (54.3) | 363 (56.5) | 97 (52.4) | 183 (52.1) | 484 (55.2) | |||
| Yes | 929 (45.2) | 368 (44.4) | 561 (45.7) | 280 (43.5) | 88 (47.6) | 168 (47.9) | 393 (44.8) | |||
| Hypertension | ||||||||||
| At WHI inception | 0.37 | 0.01 | 0.14 | |||||||
| No | 1,357 (66.0) | 556 (67.1) | 801 (65.2) | 446 (69.4) | 110 (59.5) | 240 (68.4) | 561 (64.0) | |||
| Yes | 699 (34.0) | 272 (32.9) | 427 (34.8) | 197 (30.6) | 75 (40.5) | 111 (31.6) | 316 (36.0) | |||
| At WHIMS-ECHO enrollment | 0.04 | 0.24 | 0.18 | |||||||
| No | 676 (32.9) | 294 (35.5) | 382 (31.1) | 235 (36.5) | 59 (31.9) | 119 (33.9) | 263 (30.0) | |||
| Yes | 1,380 (67.1) | 534 (64.5) | 846 (68.9) | 408 (63.5) | 126 (68.1) | 232 (66.1) | 614 (70.0) | |||
| Cardiovascular disease history | 0.22 | 0.18 | 0.34 | |||||||
| At WHI inception | ||||||||||
| No | 1,759 (85.6) | 718 (86.7) | 1,041 (84.8) | 563 (87.6) | 155 (83.8) | 303 (86.3) | 738 (84.2) | |||
| Yes | 297 (14.4) | 110 (13.3) | 187 (15.2) | 80 (12.4) | 30 (16.2) | 48 (13.7) | 139 (15.8) | |||
| At WHIMS-ECHO enrollment | 0.01 | 0.28 | 0.77 | |||||||
| No | 1,583 (77.0) | 663 (80.1) | 920 (74.9) | 520 (80.9) | 143 (77.3) | 265 (75.5) | 655 (74.7) | |||
| Yes | 473 (23.0) | 165 (19.9) | 308 (25.1) | 123 (19.1) | 42 (22.7) | 86 (24.5) | 222 (25.3) | |||
| WHI hormone therapy assignment | 0.44 | 0.38 | 0.74 | |||||||
| Estrogen-alone control | 375 (18.2) | 149 (18.0) | 226 (18.4) | 110 (17.1) | 39 (21.1) | 71 (20.2) | 155 (17.7) | |||
| Estrogen-alone intervention | 362 (17.6) | 159 (19.2) | 203 (16.5) | 120 (18.7) | 39 (21.1) | 55 (15.7) | 148 (16.9) | |||
| control | 688 (33.5) | 267 (32.2) | 421 (34.3) | 209 (32.5) | 58 (31.4) | 117 (33.3) | 304 (34.7) | |||
| intervention | 631 (30.7) | 253 (30.6) | 378 (30.8) | 204 (31.7) | 49 (26.5) | 108 (30.8) | 270 (30.8) | |||
Note: All characteristics were classified at WHI inception (1993–1998) unless otherwise indicated. All -values were calculated using -test for continuous variables, Fisher’s exact test for race/ethnicity, or chi-square test for other categorical variables. GED, General Education Development; , nitrogen dioxide; , fine particulate matter (PM with an aerodynamic diameter of ); SD, standard deviation; SES, socioeconomic status; WHI, Women’s Health Initiative; WHIMS-ECHO, Women’s Health Initiative Memory Study–Epidemiology of Cognitive Health Outcomes.
-Value comparing women vs. years of age.
-Value comparing women years of age classified as slow- vs. fast-decliners.
-Value comparing women years of age classified as Cognitively resilient vs. steady-decliners.
Neighborhood SES is the sum of six standardized U.S. Census tract–level variables measuring domains of wealth/income, education, and occupation. Higher values indicate higher neighborhood SES.
“Other” race/ethnicity includes women who did not select one of the five specific race/ethnicity groups.
Moderate or strenuous physical activity for min.
Estimated 3-y average exposures at the remote (10 y before the WHIMS-ECHO enrollment) and recent (immediately before the WHIMS-ECHO enrollment) time periods were moderately correlated for (Pearson for both age groups, ) and highly correlated for (Pearson and 0.90 for women and years of age, respectively, ) (Table S2).
Overall, average and levels decreased from remote to recent exposure time windows (Table 1). Average and exposures during both time windows were lower in non-Hispanic White women than in all others, and higher in women with more education, higher income (except for recent exposure), a history of hypertension, a history of hypercholesterolemia, and no history of hormone treatment compared with other women at WHI inception (Table 2). Mean exposures during both time periods were higher in women of age compared with younger women (Table 1), and in current and past smokers compared with never smokers (Table 2), whereas mean exposures were similar by age (Table 1) and smoking status (Table 2). Average exposures were highest during both time periods in women living in the Northeast or West at WHI inception, whereas average exposures were highest in women living in the South and Midwest (Table 2). Average exposures tended to be higher and average exposures were lower in women who did not drink alcohol compared with women who were past or current alcohol consumers. Finally, average exposures at both exposure time periods were higher in the highest quartile of neighborhood SES at both WHI inception and WHIMS-ECHO enrollment. However, higher mean exposures were seen in both the lowest and highest quartiles of neighborhood SES.
Table 2.
Distribution of air pollution exposure by population characteristics in the WHIMS-ECHO cohort.
| Characteristicsa | 3-y average of ()b | 3-y average of (ppb)b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Recent exposure | Remote exposure | Recent exposure | Remote exposure | ||||||
| -Valuec | -Valuec | -Valuec | -Valuec | ||||||
| Region | |||||||||
| Northeast | 662 | ||||||||
| South | 408 | ||||||||
| Midwest | 498 | ||||||||
| West | 488 | ||||||||
| Race/ethnicity | |||||||||
| American Indian or Alaskan Native | 4 | ||||||||
| Asian or Pacific Islander | 17 | ||||||||
| Black, non-Hispanic | 107 | ||||||||
| Hispanic/Latino | 29 | ||||||||
| White, non-Hispanic | 1,875 | ||||||||
| Otherd | 23 | ||||||||
| Missing | 1 | 11.43 | 14.79 | 24.95 | 35.09 | ||||
| Education | 0.004 | ||||||||
| school or GED | 500 | ||||||||
| school to y of college | 789 | ||||||||
| y of college | 767 | ||||||||
| Employment | 0.37 | 0.75 | 0.56 | 0.54 | |||||
| Currently working | 321 | ||||||||
| Not working | 195 | ||||||||
| Retired | 1,540 | ||||||||
| Income () | 0.88 | 0.14 | 0.005 | 0.004 | |||||
| 68 | |||||||||
| 10,000–34,999 | 906 | ||||||||
| 35,000–49,999 | 450 | ||||||||
| 50,000–74,999 | 321 | ||||||||
| 212 | |||||||||
| Not known | 99 | ||||||||
| Smoking status | 0.23 | 0.40 | 0.02 | ||||||
| Never smoked | 1,138 | ||||||||
| Past smoker | 823 | ||||||||
| Current smoker | 95 | ||||||||
| Alcohol use | 0.001 | 0.09 | 0.001 | ||||||
| Nondrinker | 243 | ||||||||
| Past drinker | 342 | ||||||||
| drink/d | 1,204 | ||||||||
| drink/d | 267 | ||||||||
| Physical activitye | 0.28 | 0.61 | 0.77 | 0.39 | |||||
| No activity | 1,109 | ||||||||
| Some activity | 114 | ||||||||
| 2–4 episodes/wk | 439 | ||||||||
| episodes/wk | 394 | ||||||||
| Hypercholesterolemia | 0.03 | 0.02 | 0.002 | 0.02 | |||||
| No | 1,716 | ||||||||
| Yes | 340 | ||||||||
| Diabetes | 0.20 | 0.14 | 0.39 | 0.31 | |||||
| No | 1,976 | ||||||||
| Yes | 80 | ||||||||
| Hormone treatment | 0.02 | 0.06 | 0.19 | 0.10 | |||||
| No | 1,127 | ||||||||
| Yes | 929 | ||||||||
| Hypertension | |||||||||
| At WHI inception | 0.02 | 0.03 | 0.047 | 0.06 | |||||
| No | 1,357 | ||||||||
| Yes | 699 | ||||||||
| At WHIMS-ECHO enrollment | 0.69 | 0.44 | 0.29 | 0.21 | |||||
| No | 676 | ||||||||
| Yes | 1,380 | ||||||||
| Cardiovascular disease history | |||||||||
| At WHI inception | 0.57 | 0.68 | 0.83 | 0.82 | |||||
| No | 1,759 | ||||||||
| Yes | 297 | ||||||||
| At WHIMS-ECHO enrollment | 0.65 | 0.93 | 0.41 | 0.48 | |||||
| No | 1,583 | ||||||||
| Yes | 473 | ||||||||
| Neighborhood SESf | |||||||||
| At WHI inception | |||||||||
| 514 | |||||||||
| to | 514 | ||||||||
| to | 514 | ||||||||
| 514 | |||||||||
| At WHIMS-ECHO enrollment | |||||||||
| 515 | |||||||||
| to | 513 | ||||||||
| to | 514 | ||||||||
| 514 | |||||||||
| WHI hormone therapy assignment | 0.31 | 0.11 | 0.39 | 0.13 | |||||
| Estrogen-alone control | 375 | ||||||||
| Estrogen-alone intervention | 362 | ||||||||
| control | 688 | ||||||||
| intervention | 631 | ||||||||
Note: ANOVA, analysis of variance; GED, General Education Development; , nitrogen dioxide; , fine particulate matter (PM with an aerodynamic diameter of ); SD, standard deviation; SES, socioeconomic status; WHI, Women’s Health Initiative; WHIMS-ECHO, Women’s Health Initiative Memory Study–Epidemiology of Cognitive Health Outcomes.
All covariates were assessed at WHI inception unless otherwise noted.
Recent exposures were 3-y average exposures estimated at the WHIMS-ECHO enrollment. Remote exposures were 3-y average exposures estimated 10 y before the WHIMS-ECHO enrollment.
-Values were calculated using ANOVA -tests for mean exposures.
“Other” race/ethnicity includes women who did not select one of the five specific race/ethnicity groups.
Moderate or strenuous physical activity for min.
Neighborhood SES is the sum of six standardized U.S. Census tract–level variables measuring domains of wealth/income, education, and occupation. Higher values indicate higher neighborhood SES.
At the time of WHIMS-ECHO enrollment, the 828 women in the group (40% of the analytic sample) were 74.8–80.0 years of age (mean ), whereas the 1,228 women in the group were 80.0–92.9 years of age (mean ) (Table 1). Compared with women in the younger group, women in the group lived in neighborhoods with higher average SES scores at WHI inception and WHIMS-ECHO enrollment; were more likely to live in the West (28% vs. 18%), be never smokers (59% vs. 50%), and be retired (79% vs. 68%) at WHI inception; and were less likely to have a history of hypertension and CVD at WHIMS-ECHO enrollment (Table 1).
For both age-defined subcohorts, the LCMM with two latent classes met our criteria for the optimal number of latent classes (i.e., lower Bayesian Information Criterion (BIC) values, of each subgroup in each class) and mean probabilities of assignment to the resulting class in all classes (Table S3). When latent class membership was determined by posterior probabilities from a baseline LCMM with follow-up time and age at WHIMS-ECHO enrollment as the only predictors, the majority of women of age (, 78%) were assigned to the slow-decliner latent class, which had only modest declines in average CVLT scores over follow-up [ (95% CI: , )], whereas the remaining 22% () were assigned to the fast-decliner latent class, which experienced more rapid declines in CVLT scores over time [ (95% CI: , )] (Figure 2). Compared with the slow-decliner class, the fast-decliner class was slightly older (mean age 78.4 vs. 78.1 y) and had a higher prevalence of hypertension (41% vs. 31%) and a higher prevalence of diabetes (6.5% vs. 3.3%) at WHI inception, but there were no significant differences in air pollution exposures or other population characteristics between the two latent class groups in women (Table 1).
Figure 2.
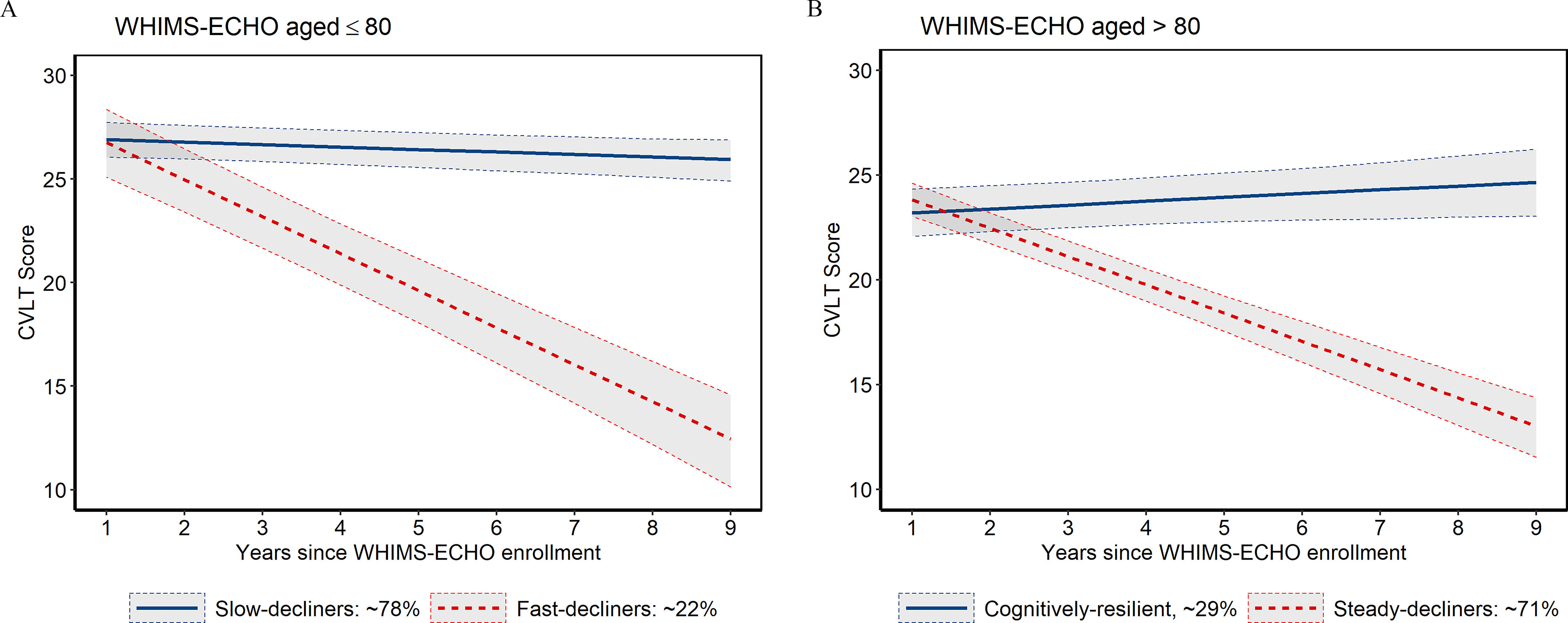
Median predicted CVLT trajectory over time by latent class in the WHIMS-ECHO cohort, stratified by age. (A) In women , the average rate of change in CVLT scores was (95% CI: , ; ) for the slow-decliner latent class (, according to the baseline LCMM) and (95% CI: , ; ) for the fast-decliner class (). (B) In women , the average rate of change in CVLT scores was 0.18/y (95% CI: , 0.36; ) for the cognitively resilient latent class (, according to the baseline LCMM) and (95% CI: , ; ) for the steady-decliner class (). Solid lines indicate the median predicted change in CVLT scores over time for women who were (A) 78 or (B) 84 years of age at WHIMS-ECHO enrollment. The predicted CVLT scores were estimated using baseline LCMMs with age at WHIMS-ECHO enrollment and follow-up time as the only predictors. Shaded regions denote 95% CI. Note: CI, confidence interval; CVLT, California Verbal Learning Test; LCMM, latent class mixed model; WHI-CT (HT), Women’s Health Initiative-Clinical Trial (Hormone Therapy); WHIMS-ECHO, Women’s Health Initiative Memory Study-Epidemiology of Cognitive Health Outcomes.
When class membership was determined based on the baseline LCMM, the majority of women of age (, 71%) were assigned to the steady-decliner latent class, which had average reductions in CVLT scores of (95% CI: , ), whereas the remaining 29% () were assigned to the cognitively resilient latent class, which showed modest improvements in average CVLT scores over time [0.18/y (95% CI: , 0.36)] (Figure 2). Compared with the cognitively resilient class, the steady-decliner class was significantly older (mean age 83.9 vs. 83.4 y) and had lower average household incomes at WHI inception (24% vs. 34% had incomes of ); otherwise there were no significant differences between the two latent classes (Table 1).
Associations between Exposure and Episodic Memory Decline
For women at WHIMS-ECHO enrollment, an IQR () increase in recent was associated with a 0.14/y faster decline in the CVLT score [ (95% CI: , )] based on a fully adjusted model (Model 2) that assumed homogeneous exposure effects across the two latent classes (Table S4). The association was stronger for the slow-decliner latent class [ (95% CI: , )] and close to null for the fast-decliner class [ (95% CI: , 0.20)] (Table 3). Using the estimated effect of a 1-y increase in age from the same model for comparison ( (95% CI: , )], the association between a increase in recent and CVLT decline in the slow-decliner latent class was equivalent to the estimated effect of a 1.9-y increase in age at WHIMS-ECHO enrollment. Global and class-specific estimates with recent exposures based on minimally adjusted models (Model 1) were similar to the fully adjusted model estimates (Table 3; Table S4). Associations for the slow- and fast-decliner classes were also similar to primary model estimates when not adjusted for propensity scores, when adjusted for practice effects, and when adjusted for hypertension, CVD history, and neighborhood SES at WHIMS-ECHO enrollment instead of WHI inception (Table S5). For the slow-decliner class, associations between recent and CVLT decline were weaker but still significant when 42 women who were newly classified with probable dementia during follow-up were excluded [ (95% CI: , )] and when 34 women with a history of stroke at WHIMS-ECHO enrollment or during follow-up were excluded [ (95% CI: , )] (Table 3). In contrast, corresponding associations for the fast-decliner class were somewhat stronger when 73 women who were newly classified with probable dementia (32% of women assigned to the fast-decliner class based on the fully adjusted LCMM) were excluded [ (95% CI: , 0.04)] and when 19 women with prevalent or incident stroke were excluded [ (95% CI: , 0.10)].
Table 3.
Summary of latent class-specific associations between air pollution exposures and episodic memory decline in the WHIMS-ECHO cohort, stratified by age and exposure time windows.
| Subgroup/Scenario | effect estimates | effect estimates | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recent exposurea | Remote exposurea | Recent exposurea | Remote exposurea | |||||||||||||
| b | 95% CI | -Value | b | 95% CI | -Value | b | 95% CI | -Value | b | 95% CI | -Value | |||||
| Slow-decliners | ||||||||||||||||
| Model 1c | 613 | , | 0.01 | 612 | , 0.01 | 0.07 | 610 | , 0.05 | 0.96 | 624 | 0.01 | , 0.09 | 0.90 | |||
| Model 2d | 597 | , | 0.01 | 596 | , 0.01 | 0.06 | 602 | , 0.18 | 0.97 | 612 | 0.003 | , 0.16 | 0.97 | |||
| Excl. dementiae | 555 | , | 0.02 | — | — | — | — | 559 | 0.01 | , 0.10 | 0.80 | — | — | — | — | |
| Excl. strokef | 563 | , | 0.02 | — | — | — | — | 568 | , 0.09 | 0.91 | — | — | — | — | ||
| Fast-decliners | ||||||||||||||||
| Model 1c | 215 | , 0.24 | 0.69 | 216 | 0.05 | , 0.32 | 0.71 | 218 | , 0.04 | 0.10 | 204 | , 0.22 | 0.67 | |||
| Model 2d | 231 | , 0.20 | 0.64 | 232 | 0.06 | , 0.32 | 0.62 | 226 | , 0.04 | 0.10 | 216 | , 0.24 | 0.76 | |||
| Excl. dementiae | 158 | , 0.04 | 0.11 | — | — | — | — | 154 | , 0.004 | 0.054 | — | — | — | — | ||
| Excl. strokef | 212 | , 0.10 | 0.33 | — | — | — | — | 207 | , 0.05 | 0.15 | — | — | — | — | ||
| Cognitively resilient | ||||||||||||||||
| Model 1c | 364 | , 0.17 | 0.74 | 368 | 0.09 | , 0.22 | 0.19 | 370 | , 0.03 | 0.12 | 369 | , 0.12 | 0.77 | |||
| Model 2d | 406 | , 0.13 | 0.71 | 432 | 0.09 | , 0.22 | 0.18 | 413 | , 0.03 | 0.12 | 418 | , 0.12 | 0.76 | |||
| Excl. dementiae | 392 | , 0.10 | 0.88 | — | — | — | — | 396 | , | 0.02 | — | — | — | — | ||
| Excl. strokef | 376 | , 0.11 | 0.85 | — | — | — | — | 378 | , | 0.03 | — | — | — | — | ||
| Steady-decliners | ||||||||||||||||
| Model 1c | 864 | , 0.13 | 0.70 | 860 | , 0.12 | 0.77 | 858 | , 0.05 | 0.17 | 859 | , 0.10 | 0.52 | ||||
| Model 2d | 822 | , 0.14 | 0.72 | 796 | , 0.13 | 0.79 | 815 | , 0.05 | 0.17 | 810 | , 0.11 | 0.52 | ||||
| Excl. dementiae | 569 | , 0.06 | 0.27 | — | — | — | — | 565 | , | 0.047 | — | — | — | — | ||
| Excl. strokef | 744 | , 0.09 | 0.62 | — | — | — | — | 742 | , 0.01 | 0.08 | — | — | — | — | ||
Note: Numbers of women within each latent class vary because women were assigned to the latent class with the highest probability according to each latent class mixed model. —, Analyses Not Performed; CI, confidence interval; CVD, cardiovascular disease; CVLT, California Verbal Learning Test; Excl., excluding; IQR, interquartile range; , nitrogen dioxide; , fine particulate matter (PM with an aerodynamic diameter of ); SES, socioeconomic status; WHIMS-ECHO, Women’s Health Initiative Memory Study–Epidemiology of Cognitive Health Outcomes.
Recent exposures were 3-y average exposures estimated at the WHIMS-ECHO enrollment. Remote exposures were 3-y average exposures estimated 10 y before the WHIMS-ECHO enrollment. Recent 3-y : . Remote 3-y : . Recent 3-y : . Remote 3-y : .
represents the average increase in CVLT score per year for each IQR increase of 3-y average exposure. A negative means higher air pollution exposure was associated with a greater decline in episodic memory.
Model 1 was adjusted for age, follow-up time, interaction of age with follow-up time, and time-varying propensity scores.
Model 2 was adjusted for age, follow-up time, geographic region, self-reported race/ethnicity (where “other” includes women who reported they were American Indian or Alaskan Native, or Asian or Pacific Islander, or women who selected “other” instead of one of the five specific race/ethnicity categories, and women with missing data for race/ethnicity), SES (education, income, employment status, and neighborhood SES), lifestyle factors (smoking, drinking and physical activities), hormone treatment, cardiovascular risk factors (hypertension, diabetes, and hypercholesterolemia), CVD histories, interaction of age with follow-up time, and time-varying propensity scores.
Excluding women with incident probable dementia during the follow-up. Latent class memberships were assigned based on Model 2.
Excluding women with either prevalent stroke or incident stroke during the follow-up. Latent class memberships were assigned based on Model 2.
For women , an IQR increase in remote exposure () was associated with a 0.11/y faster decline in CVLT [ (95% CI: , 0.01)] in the slow-decliner latent class, whereas the association was positive but close to null for the fast-decliner class [0.06/y (95% CI: , 0.32)] (Table 3). For both latent classes, associations with remote were similar when based on a minimally adjusted model (Table 3) and when scaled to an IQR increase in recent exposure (), adjusted for practice, and not adjusted for propensity scores (Table S5).
Among women , the estimated associations between CVLT decline and an IQR increase in recent were close to null for the cognitively resilient latent class [ (95% CI: , 0.13)] and for the steady-decliner class [ (95% CI: , 0.14)] based on the fully adjusted model [Model 2 (Table 3)]. These associations were also close to null when based on the minimally adjusted model, when women with incident probable dementia or stroke (prevalent or incident) were excluded (Table 3), and for all other sensitivity analyses (Table S6). Associations with an IQR increase in remote exposure were nonsignificant but positive for the cognitively resilient latent class [0.09/y (95% CI: , 0.22)] and close to null for the steady-decliner class [ (95% CI: , 0.13)], with similar estimates for both groups when minimally adjusted (Table 3) and for all sensitivity analyses (Table S6).
Associations between Exposure and Episodic Memory Decline
For women , an IQR () increase in recent was associated with a 0.21/y faster decline in CVLT scores in the fast-decliner latent class [ (95% CI: , 0.04)] and close to null for the slow-decliner class based on a fully adjusted model [Model 2 (Table 3)]. Associations with recent exposures for both latent classes were similar when based on minimally adjusted models (Model 1), and for all other sensitivity analyses (Table 3; Table S5). For the fast-decliner class, associations between recent and CVLT decline were slightly stronger when 72 women who were newly classified as having probable dementia during follow-up (32% of women assigned to the class based on the fully adjusted LCMM) were excluded [ (95% CI: , 0.004)] and much weaker when 19 women with prevalent or incident stroke were excluded [ (95% CI: , 0.05)] (Table 3).
For women , an IQR increase in recent exposure was associated with 0.10/y faster decline in CVLT score [ (95% CI: , 0.01)] based on a fully adjusted model (Model 2) that assumed homogeneous exposure effects across the two latent classes (Table S4). The associations were similar for the cognitively resilient [ (95% CI: , 0.03)] and steady-decliner latent classes [ (95% CI: , 0.05)] [Model 2 (Table 3)]. Global and class-specific associations were similar based on minimally adjusted and fully adjusted models (Table 3; Table S4). Associations estimated for the cognitively resilient and steady-decliner classes were also similar for all other sensitivity analyses (Table S6). After excluding women with incident probable dementia [17 in the cognitively resilient class, and 250 (31%) in the steady-decliner class based on the fully adjusted LCMM], estimated associations with recent exposure were stronger for the global estimate [ (95% CI: , )] and for the cognitively resilient [ (95% CI: , )] and steady-decliner classes [ (95% CI: , )] (Table 3; Table S4). After excluding women with prevalent or incident stroke among women , the association was stronger in the cognitively resilient class [ (95% CI: , )] but was similar for both the global estimate [ (95% CI: , )] and the steady-decliner class [ (95% CI: , 0.01)] (Table 3; Table S4).
For women in both age groups, the associations between remote exposure and CVLT decline were close to null in global or class-specific models regardless of whether we adjusted for minimal or a full set of covariates, propensity scores, or practice effects (Table 3; Tables S4–S6).
Associations between Air Pollution Exposures and Episodic Memory Level
The LCMM used to estimate average associations between air pollutants (recent or remote) and CVLT scores during follow-up did not indicate associations regardless of age, latent class, exposure time window, or pollutant, except for a marginally significant association between recent exposure and higher CVLT scores for the steady-decliner class [ (95% CI: , 2.02)] (Table S7).
Discussion
In this longitudinal study conducted on a geographically diverse cohort that included older women with distinctive trajectories of episodic memory, associations between late-life exposure to ambient air pollution and episodic memory decline varied by age and underlying cognitive trajectories, and possibly differed by timing of exposure and pollutant. Specifically, for recent , there was a significant association between increased exposure and greater episodic memory decline among women in the latent class characterized by slowly declining CVLT scores, whereas no associations were found among women in the latent class characterized by more rapid declines in CVLT scores or for the steady-decliner or cognitively resilient latent classes in women . For recent exposure, there was some evidence of an association with episodic memory decline among women with fast decline and among women regardless of their cognitive trajectories, but none of these associations reached statistical significance. The association with was stronger for recent 3-y average exposures (immediately before the start of WHIMS-ECHO follow-up) compared with remote 3-y average exposures that were lagged 10 y, whereas no associations were found with remote exposure. These patterns of associations were consistent between minimally adjusted LCMMs and LCMMs adjusted for socio-demographic factors (age, geographic region, race/ethnicity, education, income employment status), lifestyle (smoking, alcohol, physical activity), and clinical characteristics (diabetes, high cholesterol, hypertension, CVD, hormone therapy). After excluding women with incident probable dementia during the follow-up or women with either prevalent stroke or incident stroke during follow-up, associations with recent among women were slightly attenuated (but still statistically significant) for the slow-decliner latent class and stronger for the fast-decliner class. After excluding women with a history of stroke, associations with recent , were weaker for the fast-decliner class but stronger and statistically significant for the cognitively resilient class, whereas associations were stronger and became significant for both latent classes among women after excluding women with probable dementia. To the best of our knowledge, this is the first longitudinal study to examine the heterogeneity of air pollution exposure effects on episodic memory trajectories of older women including those .
Our study adds novel epidemiologic data to the growing literature on environmental neurosciences of brain aging, suggesting the neurotoxic effects of air pollution on cognitive decline may depend on the age-related heterogeneity in cognitive trajectories. Our results provide evidence of an adverse association between air pollution and episodic memory decline in older women who were 74–92 at the start of follow-up (Table 3). To our knowledge, only one other study has investigated whether associations between air pollution exposures and cognitive function vary by age in older adults (Kulick et al. 2020a). Based on their analysis of data from the Washington Heights Inwood Community Aging Project (WHICAP), Kulick et al. (2020a) reported stronger associations of and exposure with episodic memory decline among participants compared with those 65–74 years of age. The study population comprised a localized sample of 4,821 older men and women living in northern Manhattan who had exposure to higher average air pollution concentrations at WHICAP baseline [mean (IQR) : 13.5 ; : 33.0 (11.2) ppb when averaged over the three recruitment waves in 1990, 1992, and 2010] than our nationwide, geographically diverse cohort of older women at WHIMS-ECHO enrollment [mean (IQR) : 10.52 ; : 10.47 (6.25) ppb in 2008–2012]. In addition, prevalent cognitive problems or dementia did not affect eligibility for WHICAP, whereas women with prevalent dementia at the time of WHIMS-ECHO enrollment were excluded from our analysis. Additional studies of older adults are needed to better understand age-related heterogeneity in associations between air pollution and brain aging. Because the oldest-old (i.e., people ) are the fastest growing segment of the U.S. population (Ortman et al. 2014), it is especially important to better understand how air pollution exposure may affect brain health in this susceptible population.
Our study illustrates the advantage of using LCMMs to examine the association between ambient air pollution exposure and brain health outcomes, such as episodic memory declines, with significant heterogeneity in their longitudinal trajectories. Although well recognized in neuropsychology literature (Lee et al. 2018; McFall et al. 2019; Olaya et al. 2017; Wilson et al. 2020; Zahodne et al. 2015), the heterogeneity in episodic memory trajectories in late life has been largely ignored in air pollution epidemiologic studies on brain aging. We identified two episodic memory trajectories in each age group, and found differences in associations between air pollution exposures and CVLT declines between latent classes. Previous longitudinal studies of air pollution exposure and episodic memory decline (Table S8) reported mixed results. These included no associations in the Nurses’ Health Study (Weuve et al. 2012), Betula cohort (Oudin et al. 2017), and Northern Manhattan Study (NOMAS) (Kulick et al. 2020b) and significant associations between air pollution exposure and episodic memory decline in the Whitehall II study (Tonne et al. 2014), the WHICAP study (Kulick et al. 2020a, 2020b), and the WHIMS (Petkus et al. 2020; Younan et al. 2020) (38% and 26% overlap, respectively, between participants in these studies and the present study). However, previous studies used traditional approaches (linear mixed models, generalized estimating equations) (Kulick et al. 2020a, 2020b; Oudin et al. 2017; Tonne et al. 2014; Weuve et al. 2012) or structural equation models (latent change scores, multilevel models) (Petkus et al. 2020; Younan et al. 2020) that assumed air pollution has the same exposure effect on longitudinal trajectories of episodic memory across subjects.
Our study is likely the first to directly compare the episodic memory decline associated with ambient air pollutants across different exposure time windows in late life. Only two other longitudinal studies explored associations between declines in episodic memory and air pollution exposures during different time windows later in life. Weuve et al. (2012) evaluated associations of PM exposures 1 month to 13 y before baseline with 2-y declines in memory, whereas Tonne et al. (2014) evaluated associations of 5-y average exposure and exposure 4 y preceding the final assessment with 5-y memory decline. However, both studies assessed overlapping exposure time windows, making it difficult to assess the influence of distinct exposure periods (Tonne et al. 2014; Weuve et al. 2012). In the present study, we found that 3-y average exposures immediately before and 10-y before the start of the WHIMS-ECHO follow-up were both associated with accelerated decline in CVLT scores in the slow-decliner class among women , although the association with remote exposure was not statistically significant (). In addition, recent exposure was associated with greater episodic memory decline in the fast-decliner class and both latent class subgroups in women , but associations were not significant and memory decline did not appear to be associated with remote exposure. Recent and remote exposures were highly correlated (Pearson correlations of 0.80 for and 0.90–0.91 for ) (Table S2), and remote exposures were higher, on average, than recent exposures ( vs. for ; vs. for ) (Table 1). However, although findings provide more support for potential effects of recent and exposures on episodic memory decline in late life, they also suggest that potential effects of might have begun as early as 10 y before follow-up began.
Accumulating evidence suggests that higher levels of ambient exposure are associated with increased risk of Alzheimer’s disease and related dementias (Carey et al. 2018; Chang et al. 2014; Chen et al. 2017; Grande et al. 2020; Li et al. 2019; Oudin et al. 2016), but findings from studies of exposures and episodic memory decline have been inconsistent (Kulick et al. 2020a, 2020b; Oudin et al. 2017). In the Betula study of 1,469 adults in Northern Sweden who were 60–85 years of age at baseline, cumulative annual mean exposures were not associated with changes in episodic memory over 5 y; however, was not evaluated in the Betula study (Oudin et al. 2017). Kulick et al. (2020a, 2020b) found significant associations of and exposures 1-y before baseline with episodic memory decline over an average of 6 y of follow-up in WHICAP participants at baseline. However, associations with and were weaker and nonsignificant for changes in episodic memory measured at baseline and 5-y later among 1,093 adults of age in the NOMAS cohort, another racially and ethnically diverse northern Manhattan study population (Kulick et al. 2020b). A very important difference between these two studies was that up to six measures of episodic memory were used in the WHICAP study, whereas the NOMAS cohort only had two time points from which to analyze episodic memory change over time. Epidemiological data demonstrating an increased risk of dementia associated with late-life exposure to are corroborated by experimental data in rodent models demonstrating the neurotoxicity of on structural and functional changes in the brain (Bhatt et al. 2015; Cheng et al. 2017; Fonken et al. 2011; Ku et al. 2017; Liu et al. 2018; Wei et al. 2019; Zhang et al. 2018). Experimental evidence also supports neurotoxic effects of inhaled concentrated particles from traffic emissions (Cacciottolo et al. 2020; Costa et al. 2020), although direct evidence for neurotoxicity is less clear (Jayaraj et al. 2017).
In addition to providing some possible mechanistic insight, our study findings raise several important questions that need to be addressed to better understand the neuropathological and neurodegenerative processes underlying the episodic memory decline associated with air pollution exposure in late life. First, after excluding 53 women with a history of stroke before baseline or during follow-up, class-specific associations with episodic memory decline were attenuated for recent exposure in the slow-decliner latent class (34 excluded of 597 assigned to the class based on Model 2) and for recent exposure in the fast-decliner class (19 excluded of 226 women assigned to the class). Cerebral small vessel diseases (e.g., white matter hyperintensities, lacunar infarcts) and stroke are established risk factors for cognitive decline (Bayram et al. 2018; Lim and Alexander 2009; Rabin et al. 2018) and vascular brain damage caused by air pollution neurotoxicity has been proposed as a potential mechanism (Block and Calderón-Garcidueñas 2009) contributing to associations between exposures and cognitive decline. Recent epidemiological studies conducted in Sweden (Grande et al. 2020) and Canada (Ilango et al. 2020) suggest that associations between exposure and dementia may be partially mediated or moderated by CVD (e.g., stroke, coronary heart disease, arrhythmia, congestive heart failure), consistent with a role of vascular pathways. However, epidemiologic studies of air pollution and magnetic resonance imaging–based measurements of late-life subclinical cerebrovascular disease (Chen et al. 2015; Kulick et al. 2017; Power et al. 2018; Wilker et al. 2015, 2016) have not provided strong evidence to support this hypothesis. Results from our sensitivity analyses suggest clinical stroke in late life may have partly contributed to the association between air pollution and episodic memory decline among women at baseline. Second, after excluding 115 women with probable dementia identified after WHIMIS-ECHO enrollment, the association between recent exposure and episodic memory decline among women in the slow-decliner latent class (42 excluded of 597 assigned to the class based on Model 2) remained significant, but was noticeably weaker. Our previous findings for WHIMS participants at baseline suggested that the association between late-life exposure and accelerated decline of episodic memory was partially mediated by increased gray matter atrophy in brain areas where Alzheimer’s disease neuropathologies are thought to first emerge (Younan et al. 2020). declines in episodic memory were also associated with subsequent increases in depressive symptoms, and the indirect association was modestly diminished after women with incident dementia were excluded (Petkus et al. 2020). Taken together, these findings suggest that the association between and episodic memory decline in women assigned to the slow-decliner latent class may be partly explained by the progression of neuropathological processes underlying clinical dementia, whereas the association in women who did not develop dementia might reflect neurotoxic effects of ambient air pollution on the cognitive aging process or the early neuropathological processes underlying Alzheimer’s disease and related dementias. Alternatively, the slow-decliner latent class may include women with mixed neurodegenerative and cerebrovascular disease pathologies that might lower the threshold for cognitive impairment and dementia (Kapasi et al. 2017). However, we also found that some associations between recent exposures and episodic memory decline were stronger after we excluded women with stroke or dementia, including associations with among women in the fast-decliner class and associations with among women in both latent classes.
We recognize several limitations of our study. First, our study examined the association with regional exposures, so we could not characterize the toxic components of the mixture that may play a critical role in air pollution neurotoxicity. Second, our well-validated air pollution estimates were estimated at the participants’ addresses because we did not have personal exposure data, and may be subject to nondifferential exposure misclassification that may have attenuated the results. Third, WHIMS-ECHO did not have neuropathological biomarker data available to elucidate the underlying mechanisms for the estimated associations. Fourth, because our study sample came from a highly selective process (Figure 1A), we could not completely rule out the possibility of selection biases. However, there was little difference in the results of our sensitivity analyses with or without adjustment for propensity scores. Fifth, we were unable to examine whether there are other exposure windows (e.g., midlife) that may show stronger impact on episodic memory decline. Sixth, we were unable to adjust associations with recent exposure for remote exposure and vice versa or account for coexposure to and in two-pollutant models owing to issues of multicollinearity that could make interpretation of the parameters difficult. Seventh, latent class membership was not a fixed trait of individual women, which might vary when different covariates were included in the LCMM. Eighth, the small numbers of women assigned to the fast-decliner latent class limited our power to detect statistically significant associations for this class. Last, our findings were based on a sample of mostly non-Hispanic White, well-educated, middle-class women, which may limit the generalizability of our findings.
This study has several major strengths. First, our cohort included older women in a wide age range (74–92 y at baseline), making it possible to examine heterogeneity in episodic memory trajectory among different age ranges ( vs. ). Second, we used a data-driven LCMM approach that allowed us to investigate whether associations between air pollution exposures and episodic memory decline varied between latent classes characterized by different episodic memory trajectories. Third, we had data on annual air pollution measures estimated prior to the neuropsychological assessment in the WHIMS-ECHO cohort, which provided a unique population context to explore the exposure effects over two time windows. Fourth, the high-quality, comprehensive data of the geographically diverse WHIMS-ECHO cohort allowed us to adjust for a variety of covariates and conduct sensitivity analyses to evaluate and control for potential confounding and biases.
In summary, late-life exposure to ambient air pollution was associated with accelerated episodic memory decline in subgroups of older women with different trajectories of episodic memory. The association was most consistent for average exposure during the 3-y prior to baseline in women of age at baseline with a higher probability of a slow rate of episodic memory decline than other women in the same age group. Future studies are needed to further examine heterogeneity in associations between air pollution and episodic memory decline in older populations and substantiate our study findings.
Supplementary Material
Acknowledgments
We thank E.W. Spalt, A.J. Gassett, and L. Piepmeier for their assistance in revising this publication. This study is supported by the National Institutes of Health (NIH) National Institute on Aging (NIA) (RF1AG054068; J-C.C.), the National Institute of Environmental Health Sciences (NIEHS) (R01ES025888; J-C.C. and J.D.K.; and 5P30ES007048), and the Alzheimer’s Disease Research Center at the University of Southern California (NIA; P50AG005142 and P30AG066530). D.Y. and J-C.C. are also supported by the NIA (P01AG055367). D.Y. is also supported by a grant from the Alzheimer’s Association (AARF-19-591356). M.A.E. receives funding from the Wake Forest Alzheimer’s Disease Core Center (P30AG049638–01A1). S.M.R. is supported by the Intramural Research Program, NIA, NIH. The air pollution models were developed under a Students Tackling Advanced Research assistance agreement [RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S. Environmental Protection Agency]. The Women’s Health Initiative (WHI) program is funded by the NIH/National Heart, Lung, and Blood Institute through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. The WHI Memory Study was funded by Wyeth Pharmaceuticals, St. Davids, Pennsylvania, and Wake Forest University, Winston-Salem, North Carolina. For a list of all the investigators who have contributed to WHI science, please visit https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
References
- Bayram E, Caldwell JZK, Banks SJ. 2018. Current understanding of magnetic resonance imaging biomarkers and memory in Alzheimer’s disease. Alzheimers Dement (NY) 4:395–413, PMID: 30229130, 10.1016/j.trci.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DP, Puig KL, Gorr MW, Wold LE, Combs CK. 2015. A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer-like changes in the mouse brain. PLoS One 10(5):e0127102, PMID: 25992783, 10.1371/journal.pone.0127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Calderón-Garcidueñas L. 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32(9):506–516, PMID: 19716187, 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciottolo M, Morgan TE, Saffari AA, Shirmohammadi F, Forman HJ, Sioutas C, et al. 2020. Traffic-related air pollutants (TRAP-PM) promote neuronal amyloidogenesis through oxidative damage to lipid rafts. Free Radic Biol Med 147:242–251, PMID: 31883973, 10.1016/j.freeradbiomed.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey IM, Anderson HR, Atkinson RW, Beevers SD, Cook DG, Strachan DP, et al. 2018. Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open 8(9):e022404, PMID: 30206085, 10.1136/bmjopen-2018-022404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K-H, Chang M-Y, Muo C-H, Wu T-N, Chen C-Y, Kao C-H. 2014. Increased risk of dementia in patients exposed to nitrogen dioxide and carbon monoxide: a population-based retrospective cohort study. PLoS One 9(8):e103078, PMID: 25115939, 10.1371/journal.pone.0103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kwong JC, Copes R, Hystad P, van Donkelaar A, Tu K, et al. 2017. Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ Int 108:271–277, PMID: 28917207, 10.1016/j.envint.2017.08.020. [DOI] [PubMed] [Google Scholar]
- Chen J-C, Wang X, Wellenius GA, Serre ML, Driscoll I, Casanova R, et al. 2015. Ambient air pollution and neurotoxicity on brain structure: evidence from Women’s Health Initiative Memory Study. Ann Neurol 78(3):466–476, PMID: 26075655, 10.1002/ana.24460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Lau WKW, Fung TKH, Lau BWM, Chau BKH, Liang Y, et al. 2017. PM2.5 exposure suppresses dendritic maturation in subgranular zone in aged rats. Neurotox Res 32(1):50–57, PMID: 28275902, 10.1007/s12640-017-9710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Dao K, Chang Y-C, Coburn J, Garrick JM. 2020. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol Ther 210:107523, PMID: 32165138, 10.1016/j.pharmthera.2020.107523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. 2001. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 345(2):99–106, PMID: 11450679, 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- Ding X, Charnigo RJ, Schmitt FA, Kryscio RJ, Abner EL, Alzheimer’s Disease Neuroimaging Initiative. 2019. Evaluating trajectories of episodic memory in normal cognition and mild cognitive impairment: results from ADNI. PLoS One 14(2):e0212435, PMID: 30802256, 10.1371/journal.pone.0212435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. 2016. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement 12(3):292–323, PMID: 27012484, 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Manson JE, Goveas JS, Shumaker SA, Hayden KM, et al. 2017. Long-term effects on cognitive trajectories of postmenopausal hormone therapy in two age groups. J Gerontol A Biol Sci Med Sci 72(6):838–845, PMID: 27506836, 10.1093/gerona/glw156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, McGue M. 1998. Age differences in the nature and origin of individual differences in memory: a behavior genetic analysis. Int J Aging Hum Dev 47(3):217–239, PMID: 9879022, 10.2190/JEPX-G60A-6QK3-9DR3. [DOI] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA. 2014. Behavior Genetics of Cognition Across the Lifespan. New York, NY: Springer. [Google Scholar]
- Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S, et al. 2011. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol Psychiatry 16(10):987–995, PMID: 21727897, 10.1038/mp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. 1987. California Verbal Learning Test-Research Edition. New York: The Psychological Corporation. [Google Scholar]
- Gallagher M, Koh MT. 2011. Episodic memory on the path to Alzheimer’s disease. Curr Opin Neurobiol 21(6):929–934, PMID: 22079495, 10.1016/j.conb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaussoin SA, Espeland MA, Beavers DP, Casanova R, Garcia KR, Snively BM, et al. 2019. Dementia outcomes after addition of proxy-based assessments for deceased or proxy-dependent participants. Int J Geriatr Psychiatry 34(10):1403–1411, PMID: 31034676, 10.1002/gps.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giubilei F, Medda E, Fagnani C, Bianchi V, De Carolis A, Salvetti M, et al. 2008. Heritability of neurocognitive functioning in the elderly: evidence from an Italian twin study. Age Ageing 37(6):640–646, PMID: 18641001, 10.1093/ageing/afn132. [DOI] [PubMed] [Google Scholar]
- Grande G, Ljungman PLS, Eneroth K, Bellander T, Rizzuto D. 2020. Association between cardiovascular disease and long-term exposure to air pollution with the risk of dementia. JAMA Neurol 77(7):801–809, PMID: 32227140, 10.1001/jamaneurol.2019.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, et al. 2004. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol 160(12):1152–1158, PMID: 15583367, 10.1093/aje/kwh314. [DOI] [PubMed] [Google Scholar]
- Ilango SD, Chen H, Hystad P, van Donkelaar A, Kwong JC, Tu K, et al. 2020. The role of cardiovascular disease in the relationship between air pollution and incident dementia: a population-based cohort study. Int J Epidemiol 49(1):36–44, PMID: 31347651, 10.1093/ije/dyz154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraj RL, Rodriguez EA, Wang Y, Block ML. 2017. Outdoor ambient air pollution and neurodegenerative diseases: the neuroinflammation hypothesis. Curr Environ Health Rep 4(2):166–179, PMID: 28444645, 10.1007/s40572-017-0142-3. [DOI] [PubMed] [Google Scholar]
- Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. 2007. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav 31(2):193–202, PMID: 17269909, 10.5993/AJHB.31.2.8. [DOI] [PubMed] [Google Scholar]
- Josefsson M, de Luna X, Pudas S, Nilsson LG, Nyberg L. 2012. Genetic and lifestyle predictors of 15-year longitudinal change in episodic memory. J Am Geriatr Soc 60(12):2308–2312, PMID: 23110764, 10.1111/jgs.12000. [DOI] [PubMed] [Google Scholar]
- Kapasi A, DeCarli C, Schneider JA. 2017. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 134(2):171–186, PMID: 28488154, 10.1007/s00401-017-1717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-Y, Olives C, Sheppard L, Sampson PD, Larson TV, Keller JP, et al. 2017. Historical prediction modeling approach for estimating long-term concentrations of PM2.5 in cohort studies before the 1999 implementation of widespread monitoring. Environ Health Perspect 125(1):38–46, PMID: 27340825, 10.1289/EHP131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku T, Li B, Gao R, Zhang Y, Yan W, Ji X, et al. 2017. NF-κB-regulated microRNA-574-5p underlies synaptic and cognitive impairment in response to atmospheric PM2.5 aspiration. Part Fibre Toxicol 14(1):34, PMID: 28851397, 10.1186/s12989-017-0215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulick ER, Elkind MSV, Boehme AK, Joyce NR, Schupf N, Kaufman JD, et al. 2020a. Long-term exposure to ambient air pollution, APOE-ε4 status, and cognitive decline in a cohort of older adults in northern Manhattan. Environ Int 136:105440, PMID: 31926436, 10.1016/j.envint.2019.105440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulick ER, Wellenius GA, Boehme AK, Joyce NR, Schupf N, Kaufman JD, et al. 2020b. Long-term exposure to air pollution and trajectories of cognitive decline among older adults. Neurology 94(17):e1782–e1792, PMID: 32269113, 10.1212/WNL.0000000000009314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulick ER, Wellenius GA, Kaufman JD, DeRosa JT, Kinney PL, Cheung YK, et al. 2017. Long-term exposure to ambient air pollution and subclinical cerebrovascular disease in NOMAS (the Northern Manhattan Study). Stroke 48(7):1966–1968, PMID: 28455324, 10.1161/STROKEAHA.117.016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhou X, Gao Y, Vardarajan B, Reyes-Dumeyer D, Rajan KB, et al. 2018. Episodic memory performance in a multi-ethnic longitudinal study of 13,037 elderly. PLoS One 13(11):e0206803, PMID: 30462667, 10.1371/journal.pone.0206803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-Y, Li C-H, Martini S, Hou W-H. 2019. Association between air pollution and risk of vascular dementia: a multipollutant analysis in Taiwan. Environ Int 133(pt B):105233, PMID: 31678904, 10.1016/j.envint.2019.105233. [DOI] [PubMed] [Google Scholar]
- Lim C, Alexander MP. 2009. Stroke and episodic memory disorders. Neuropsychologia 47(14):3045–3058, PMID: 19666037, 10.1016/j.neuropsychologia.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Liu X, Qian X, Xing J, Wang J, Sun Y, Wang Q, et al. 2018. Particulate matter triggers depressive-like response associated with modulation of inflammatory cytokine homeostasis and brain-derived neurotrophic factor signaling pathway in mice. Toxicol Sci 164(1):278–288, PMID: 29688525, 10.1093/toxsci/kfy086. [DOI] [PubMed] [Google Scholar]
- Margolis KL, Qi L, Brzyski R, Bonds DE, Howard BV, Kempainen S, et al. 2008. Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials 5(3):240–247, PMID: 18559413, 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, McDermott KL, Dixon RA. 2019. Modifiable risk factors discriminate memory trajectories in non-demented aging: precision factors and targets for promoting healthier brain aging and preventing dementia. J Alzheimers Dis 70(s1):S101–S118, PMID: 30775975, 10.3233/JAD-180571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaya B, Bobak M, Haro JM, Demakakos P. 2017. Trajectories of verbal episodic memory in middle-aged and older adults: evidence from the English Longitudinal Study of Ageing. J Am Geriatr Soc 65(6):1274–1281, PMID: 2863362, 10.1111/jgs.14789. [DOI] [PubMed] [Google Scholar]
- Ortman JM, Velkoff VA, Hogan H. 2014. An aging nation: the older population in the United States: population estimates and projections. Washington, DC: U.S. Census Bureau, Economics and Statistics Administration. https://www.census.gov/prod/2014pubs/p25-1140.pdf [accessed 28 August 2021]. [Google Scholar]
- Oudin A, Forsberg B, Adolfsson AN, Lind N, Modig L, Nordin M, et al. 2016. Traffic-related air pollution and dementia incidence in northern Sweden: a longitudinal study. Environ Health Perspect 124(3):306–312, PMID: 26305859, 10.1289/ehp.1408322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin A, Forsberg B, Lind N, Nordin S, Oudin Åström D, Sundström A, et al. 2017. Is long-term exposure to air pollution associated with episodic memory? A longitudinal study from northern Sweden. Sci Rep 7(1):12789, PMID: 28986549, 10.1038/s41598-017-13048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. 2019. Air pollution and dementia: a systematic review. J Alzheimers Dis 70(s1):S145–S163, PMID: 30775976, 10.3233/JAD-180631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkus AJ, Younan D, Wang X, Serre M, Vizuete W, Resnick S, et al. 2019. Particulate air pollutants and trajectories of depressive symptoms in older women. Am J Geriatr Psychiatry 27(10):1083–1096, PMID: 31311712, 10.1016/j.jagp.2019.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkus AJ, Younan D, Widaman K, Gatz M, Manson JE, Wang X, et al. 2020. Exposure to fine particulate matter and temporal dynamics of episodic memory and depressive symptoms in older women. Environ Int 135:105196, PMID: 31881430, 10.1016/j.envint.2019.105196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power MC, Lamichhane AP, Liao D, Xu X, Jack CR, Gottesman RF, et al. 2018. The association of long-term exposure to particulate matter air pollution with brain MRI findings: the ARIC study. Environ Health Perspect 126(2):027009, PMID: 29467108, 10.1289/EHP2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proust-Lima C, Philipps V, Liquet B. 2017. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J Stat Soft 78(2):1–56, 10.18637/jss.v078.i02. [DOI] [Google Scholar]
- Rabin JS, Schultz AP, Hedden T, Viswanathan A, Marshall GA, Kilpatrick E, et al. 2018. Interactive associations of vascular risk and β-amyloid burden with cognitive decline in clinically normal elderly individuals: findings from the Harvard Aging Brain Study. JAMA Neurol 75(9):1124–1131, PMID: 29799986, 10.1001/jamaneurol.2018.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JM, Hernán MA, Brumback B. 2000. Marginal structural models and causal inference in epidemiology. Epidemiology 11(5):550–560, PMID: 10955408, 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Richards M, Szpiro AA, Bergen S, Sheppard L, Larson TV, et al. 2013. A regionalized national universal kriging model using partial least squares regression for estimating annual PM2.5 concentrations in epidemiology. Atmos Environ (1994) 75:383–392, PMID: 24015108, 10.1016/j.atmosenv.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing YL, Werkle-Bergner M, Brehmer Y, Müller V, Li S-C, Lindenberger U. 2010. Episodic memory across the lifespan: the contributions of associative and strategic components. Neurosci Biobehav Rev 34(7):1080–1091, PMID: 19896974, 10.1016/j.neubiorev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, et al. 1998. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials 19(6):604–621, PMID: 9875839, 10.1016/S0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- Singer T, Verhaeghen P, Ghisletta P, Lindenberger U, Baltes PB. 2003. The fate of cognition in very old age: six-year longitudinal findings in the Berlin Aging Study (BASE). Psychol Aging 18(2):318–331, PMID: 12825779, 10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- Small BJ, Bäckman L. 2007. Longitudinal trajectories of cognitive change in preclinical Alzheimer’s disease: a growth mixture modeling analysis. Cortex 43(7):826–834, PMID: 17941341, 10.1016/s0010-9452(08)70682-8. [DOI] [PubMed] [Google Scholar]
- Swan GE, Reed T, Jack LM, Miller BL, Markee T, Wolf PA, et al. 1999. Differential genetic influence for components of memory in aging adult twins. Arch Neurol 56(9):1127–1132, PMID: 10488814, 10.1001/archneur.56.9.1127. [DOI] [PubMed] [Google Scholar]
- The Lancet Neurology. 2018. Air pollution and brain health: an emerging issue. Lancet Neurol 17(2):103, PMID: 29413304, 10.1016/S1474-4422(17)30462-3. [DOI] [PubMed] [Google Scholar]
- Tonne C, Elbaz A, Beevers S, Singh-Manoux A. 2014. Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology 25(5):674–681, PMID: 25036434, 10.1097/EDE.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. 2002. Episodic memory: from mind to brain. Annu Rev Psychol 53:1–25, PMID: 11752477, 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2019. American community survey 5-year estimates. Social Explorer. http://www.socialexplorer.com [accessed 5 September 2021]
- U.S. Census Bureau, ICPSR (Inter-university Consortium for Political and Social Research). 2006. Census of Population and Housing, 2000 [United States]: Selected Subsets from Summary File 3 (ICPSR 13402). Ann Arbor: Inter-university Consortium for Political and Social Research. https://www.icpsr.umich.edu/web/ICPSR/studies/13402/datadocumentation [accessed 28 August 2021]. [Google Scholar]
- Wei W, Chen M, Li G, Sang N. 2019. Atmospheric PM2.5 aspiration causes tauopathy by disturbing the insulin signaling pathway. Ecotoxicol Environ Saf 169:301–305, PMID: 30458396, 10.1016/j.ecoenv.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. 2012. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med 172(3):219–227, PMID: 22332151, 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHI Memory Study (Women’s Health Initiative Memory Study). 2020. WHI Memory Study (WHIMS) investigator data release data preparation guide. https://sp.whi.org/researchers/data/_layouts/15/WopiFrame.aspx?sourcedoc=/researchers/data/whims%22documents/Data%20Preparation%20for%20WHIMS%20ECHO%20Data%20Set%20May%202020.pdp&action=default.
- Whitsel EA, Quibrera PM, Smith RL, Catellier DJ, Liao D, Henley AC, et al. 2006. Accuracy of commercial geocoding: assessment and implications. Epidemiol Perspect Innov 3(1):8, PMID: 16857050, 10.1186/1742-5573-3-8. https://epi-perspectives.biomedcentral.com/articles/10.1186/1742-5573-3-8 [accessed 5 September 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsel EA, Rose KM, Wood JL, Henley AC, Liao D, Heiss G. 2004. Accuracy and repeatability of commercial geocoding. Am J Epidemiol 160(10):1023–1029, PMID: 15522859, 10.1093/aje/kwh310. [DOI] [PubMed] [Google Scholar]
- Wilker EH, Martinez-Ramirez S, Kloog I, Schwartz J, Mostofsky E, Koutrakis P, et al. 2016. Fine particulate matter, residential proximity to major roads, and markers of small vessel disease in a memory study population. J Alzheimers Dis 53(4):1315–1323, PMID: 27372639, 10.3233/JAD-151143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker EH, Preis SR, Beiser AS, Wolf PA, Au R, Kloog I, et al. 2015. Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke 46(5):1161–1166, PMID: 25908455, 10.1161/STROKEAHA.114.008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Wang T, Yu L, Bennett DA, Boyle PA. 2020. Normative cognitive decline in old age. Ann Neurol 87(6):816–829, PMID: 32144793, 10.1002/ana.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Phyo AZZ, Al-Harbi T, Woods RL, Ryan J. 2020. Distinct cognitive trajectories in late life and associated predictors and outcomes: a systematic review. J Alzheimers Dis Rep 4(1):459–478, PMID: 33283167, 10.3233/ADR-200232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurth R, Kioumourtzoglou M-A, Tucker KL, Griffith J, Manjourides J, Suh H. 2018. Fine particle sources and cognitive function in an older Puerto Rican cohort in Greater Boston. Environ Epidemiol 2(3):e022, PMID: 30506018, 10.1097/EE9.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss R, Gagne JJ, Zhao Y, Zhou EH, Major JM, Wang SV, et al. 2020. Use of time-dependent propensity scores to adjust hazard ratio estimates in cohort studies with differential depletion of susceptibles. Epidemiology 31(1):82–89, PMID: 31569120, 10.1097/EDE.0000000000001107. [DOI] [PubMed] [Google Scholar]
- Younan D, Petkus AJ, Widaman KF, Wang X, Casanova R, Espeland MA, et al. 2020. Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain 143(1):289–302, PMID: 31746986, 10.1093/brain/awz348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MT, Bechle MJ, Sampson PD, Szpiro AA, Marshall JD, Sheppard L, et al. 2016. Satellite-based NO2 and model validation in a national prediction model based on universal kriging and land-use regression. Environ Sci Technol 50(7):3686–3694, PMID: 26927327, 10.1021/acs.est.5b05099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Wall MM, Schupf N, Mayeux R, Manly JJ, Stern Y, et al. 2015. Late-life memory trajectories in relation to incident dementia and regional brain atrophy. J Neurol 262(11):2484–2490, PMID: 26259562, 10.1007/s00415-015-7871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Li Q, Ma J, Zhao Y. 2018. PM2.5 impairs neurobehavior by oxidative stress and myelin sheaths injury of brain in the rat. Environ Pollut 242(pt A):994–1001, PMID: 30373045, 10.1016/j.envpol.2018.07.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


