Abstract
Cer1p/Lhs1p/Ssi1p is a novel Hsp70-related protein that is important for the translocation of a subset of proteins into the yeast Saccharomyces cerevisiae endoplasmic reticulum. Cer1p has very limited amino acid identity to the hsp70 chaperone family in the N-terminal ATPase domain but lacks homology to the highly conserved hsp70 peptide binding domain. The role of Cer1p in protein folding and translocation was assessed. Deletion of CER1 slowed the folding of reduced pro-carboxypeptidase Y (pro-CPY) approximately twofold in yeast. In wild-type yeast under reducing conditions, pro-CPY can be found in a complex with Cer1p, while partially purified Cer1p is able to bind directly to peptides. Together, this suggests that Cer1p has a chaperoning activity required for proper refolding of denatured pro-CPY which is mediated by direct interaction with the unfolded polypeptide. Cer1p peptide binding and oligomerization could be disrupted by addition of ATP, confirming that Cer1p possesses a functional ATP binding site, much like Kar2p and other members of the hsp70 family. Interestingly, replacing the signal sequence of a CER1-dependent protein with that of a CER1-independent protein did not relieve the requirement of CER1 for import. This result suggests that an interaction with the mature portion of the protein also is important for the translocation role of Cer1p. The CER1 RNA levels increase at lower temperatures. In addition, the effects of deletion on folding and translocation are more severe at lower temperatures. Therefore, these results suggest that Cer1p provides an additional chaperoning activity in processes known to require Kar2p. However, there appears to be a greater requirement for Cer1p chaperone activity at lower temperatures.
In the first stages of the secretory pathway, proteins translocate into and fold in the endoplasmic reticulum (ER). Translocation in the yeast Saccharomyces cerevisiae depends on the activity of the luminal protein Kar2p, while folding is also believed to require Kar2p. The Kar2p folding activity in vivo was demonstrated by a study in which mutations in kar2 cause defects in the refolding of a denatured protein in the yeast ER (38). Since Kar2p is a member of the highly conserved hsp70 family, it is believed to chaperone protein folding in a manner similar to that used by other family members, although no study of this mechanism has been published.
The mechanism of hsp70-dependent protein folding has been best studied by using the bacterial hsp70, DnaK. DnaK, like other hsp70s, possesses the ability to bind short hydrophobic peptides and hydrolyze ATP (11, 33). The peptide binding is regulated by the state of the bound nucleotide. In the ATP form, the peptide exchanges rapidly (37); in the ADP state, the peptide exchanges more slowly (29). The nucleotide state and hence the peptide binding properties are also regulated by accessory proteins, or cochaperones. For DnaK, the two cochaperones are known as DnaJ and GrpE. DnaJ stimulates the ATP hydrolysis of DnaK, whereas GrpE stimulates nucleotide exchange (23). Both these cochaperones are required for rapid hydrolysis of ATP and for the chaperoning activity of DnaK. Kar2p physically interacts with the DnaJ homolog Sec63p, a transmembrane component of the ER translocon. This interaction is required for proper import of proteins into the ER. A second DnaJ homolog in the ER, the luminal Scj1p, is the most likely Kar2p cochaperone candidate for protein folding in the ER (3). Unlike Sec63p, Scj1p possesses all the domains thought necessary to function as a DnaJ-like chaperone in protein folding (42). Although no physical associations have been demonstrated between these proteins, genetic experiments do suggest interactions between SCJ1 and KAR2 (36).
Protein folding in the ER requires other chaperones, including protein disulfide isomerase and calnexin, but does not utilize classes of chaperones found in other compartments in the cell. Both the yeast cytosol and mitochondria contain chaperonins, the hsp60 class of molecular chaperones. This class can facilitate folding through a pathway independent of but cooperative with the hsp70 system (22). Surprisingly, the ER lacks any molecular chaperones of the chaperonin class. Moreover, the yeast ER lacks an hsp90, a class shown to interact with folding proteins in the mammalian ER (26).
In addition to folding, Kar2p plays a primary role in facilitating translocation across the ER membrane. Experiments with intact cells and import studies with reconstituted microsomes have confirmed the importance of Kar2p in translocation (5, 30, 40). Different models have been proposed to explain how Kar2p acts during translocation. However, in all models, the polypeptide binding properties of hsp70s would suggest that the Kar2p translocation mechanism works through direct interaction with the translocating chain (34).
In yeast, translocation can be directed by two independent routes: cotranslational and posttranslational. Kar2p is required to facilitate translocation in both of these pathways (4, 40). The two protein translocation routes are reflected by the existence of distinct translocation complexes. Two of the complexes that have been identified in yeast are trimeric and are thought to be involved solely in cotranslational translocation (10, 30). A third complex, composed of seven polypeptides (Sec61p, Sbh1p, Sss1p, Sec62p, Sec63p, Sec71p, and Sec72p), seems to import proteins independently of SRP and is capable of driving posttranslational translocation in vitro (30). Kar2p physically interacts with Sec63p, a protein with homology to DnaJ (6, 7). Furthermore, point mutations in Sec63p that block translocation also prevent association with Kar2p (7).
Recently, a luminal protein (Cer1p, Lhs1p, or Ssi1p) which possesses weak amino acid sequence identity to the hsp70 family was identified (1, 8, 15). Deletion of the CER1 gene led to defective ER import of certain secretory proteins. Analysis of well-characterized secretory proteins suggested that only proteins with the ability to be translocated posttranslationally in vitro were affected in cer1Δ strains (15, 27). These secretory proteins also seem to be the same ones affected by sec63 mutations (17), suggesting that Cer1p may be restricted to the heptameric translocation complex. Whereas the association of Kar2p with the complex has been clearly demonstrated, no such evidence has been presented for such an association between the complex and Cer1p. Genetic interactions have been observed between CER1 and KAR2, indicating possible functional similarities in the two proteins (1, 15). Since Kar2p may be acting at multiple steps in translocation (34), it is unclear at which steps, if any, both Cer1p and Kar2p act.
The limited sequence identity and common translocation defects suggest that Cer1p and Kar2p function by similar mechanisms. On the other hand, the lack of identity in the known peptide binding domain of Kar2p brings into question the functional relatedness of these two proteins. For example, whereas Kar2p is over 50% identical to the known peptide binding region of DnaK, Cer1p displays no significant identity to any hsp70 in this region. Since the chaperoning function and the translocation function of Kar2p would require direct binding to a polypeptide, any similar function in Cer1p would also seem to require such peptide binding abilities. Therefore, we examined whether Cer1p, like Kar2p, aids in protein folding in the yeast ER and compared the chaperone-related biochemical properties of hsp70s with those of Cer1p.
MATERIALS AND METHODS
Yeast strains.
We used the following strains in this study: SEY6210 (MATα leu2-3 ura3-52 his3Δ200 trp1Δ901 lys2-801), LCY22 (MATα leu2-3 ura3-52 his3Δ200 trp1Δ901 lys2-801 pep4), GHY2 (MATα leu2-3 ura3-52 his3Δ200 trp1Δ901 lys2-801 pep4 cer1::URA3), MS958 (MATa leu2-3,112 ura3-52 ade2-101 kar2-191), JHRY20-1 (MATα prc1::HIS3 his3Δ200 leu2-3 lys2-801 ura3-52 pep4), JHRY20-2 (MATα prc1::HIS3 his3Δ200 leu2-3 lys2-801 ura3-52), GHY14 (MATα prc1::HIS3 his3Δ200 leu2-3 lys2-801 ura3-52 pep4 cer1::URA3), and GHY15 (MATα prc1::HIS3 his3Δ200 leu2-3 ura3-52 cer1::URA3).
CPY folding assay.
Strain LCY22 was grown in synthetic minimal medium (SD) supplemented with methionine (31a) at 25°C to an optical density at 600 nm (OD600) of 0.3. Then 5.0 OD unit of cells was harvested, pelleted, and resuspended in 0.5 ml of fresh SD-methionine medium, and dithiothreitol DTT was added to the cells at a final concentration of 5 mM. The cells were incubated at 25°C for 10 min and then labeled with 5 mCi of [35S]methionine for 15 min. To terminate labeling and begin the chase, the cells were pelleted and resuspended in SD-methionine medium plus 1 mM cycloheximide and incubated at 25°C. These reactions were terminated by removing 50 μl of cells from the cultures at the indicated times and adding 50 μl of ice-cold phosphate-buffered saline–20 mM sodium azide. After all the samples had been removed, the cells were pelleted and resuspended in 150 μl of spheroplast buffer (1.4 M sorbitol, 50 mM Tris [pH 7.4], 10 mM NaN3, 0.3% β-mercaptoethanol, 10 mg of lyticase per ml) and incubated at 30°C for 30 min. A cocktail of protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per μg, 10 μg of pepstatin per ml) and sodium dodecyl sulfate (SDS) to a final concentration of 1% was added, and the mixture was boiled for 5 min. Then 1× IP buffer (100 mM Tris [pH 8], 0.1% Triton X-100, 2 mM EDTA) was added to increase the volume to 1 ml and 50 μl of IgG Sorb (The Enzyme Center; 2 mg of immunoglobulin G per ml) was added prior to incubation on ice for 15 min to preclear the extracts. IgG Sorb was pelleted, and the supernatants were used for immunoprecipitations with anti-CPY antiserum (generously provided by Tom Stevens). Samples were immunoprecipitated overnight at 4°C. Then 50 μl of IgG Sorb was added, and the mixture was incubated at 4°C for 1 h. Antibody complexes were pelleted and washed with 1× IP buffer–0.1% SDS, resuspended in 40 μl of 2× SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer, and boiled to disrupt antibody complexes, and 20 μl was analyzed by SDS-PAGE and autoradiography.
To investigate whether carboxypeptidase (CPY) could be posttranslationally translocated, the CPY folding experiment was modified. By using the MS958 strain, cells were labeled at the nonpermissive temperature (37°C) for 15 min and then shifted to the permissive temperature (25°C) for the chase period. Immunoprecipitation of CPY was carried out as described above at the indicated times. The control for this experiment was done identically, except that MS958 was labeled at the permissive temperature.
Plasmid construction.
The PRC1 (CPY)-SUC2 (invertase) fusion gene was constructed by generating PCR fragments from plasmids containing either full-length CPY or invertase genes, generously provided by Nils Johnsson (UTA1U and UTA22) (20). The portion of PRC1 encoding pro-CPY was PCR amplified from UTA22 with 5′ HindIII and PstI sites and a 3′ SacI site engineered into the primers and subsequently cloned into pRS315 by using the HindIII and SacI sites in the polylinker. The fragment of SUC2 encoding the invertase presequence was amplified from UTA1U by using a 5′ primer with a XhoI site and a 3′ primer with a PstI site engineered. This amplified DNA fragment also contained the CUP1 promoter upstream from the presequence. The PCR-amplified invertase signal sequence DNA fragment was digested with XhoI and PstI and ligated into pRS315 that contained pro-CPY DNA with no signal sequence, cut with the same enzymes. The resulting plasmid (pGH7) contained a chimeric gene to express the pro-CPY fused to the invertase signal sequence under the control of the copper-inducible CUP1 promoter. This plasmid was transformed into strains JHRY20-1 and JHRY20-2, and the ability of the fusion protein to be translocated into the ER was assayed by determining the steady-state levels of the fusion protein. Cells were grown to a concentration of 0.5 OD unit/ml, and Cu2+ was added to a final concentration of 1 mM. Aliquots of cells were harvested in log phase, and total cellular protein was isolated as previously described (9). Proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Western blot analysis was preformed with anti-invertase antiserum (generously provided by T. Stevens). The primary antibody was detected with secondary antibodies conjugated to horseradish peroxides and visualized with chemiluminescent reagents (Pierce Chemical Co.).
Protein purification and antibody production.
A bacterial expression vector containing the CER1 gene was constructed by amplifying the CER1 gene from the pGH2 plasmid. The amplified fragment containing the SalI-BamHI restriction sites was ligated into the expression vector pQE-41 (Qiagen) to make plasmid pQE-41–Cer1. Expression from this vector adds a 6× His affinity tag to the amino terminus of the protein. Escherichia coli JM109 harboring the pQE-41–Cer1 expression plasmid was grown at 37°C in 4 liters of Luria-Bertani broth with 50 μg of ampicillin per ml. Expression was induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside when the cell culture reached an OD600 of 0.3/ml. The cells were harvested by centrifugation at 3,000 × g for 20 min at 4°C and frozen at −20°C. The cell pellets were resuspended with 120 ml of buffer A (50 mM Tris [pH 7.5], 200 mM NaCl) and broken in a French pressure cell. The inclusion bodies containing 6× His-Cer1p were washed once with buffer A containing 0.1% Triton X-100 and then resuspended in buffer B (8 M urea, 1% Triton X-100, 3 mM β-mercaptoethanol, 100 mM Sodium phosphate [pH 8.0]). This material was cleared and loaded onto a 5-ml Ni-nitrilotriacetic acid agarose column (Qiagen). The column was washed with buffer B (pH 6.3) and eluted with buffer B (pH 4.5). The eluate was exchanged into buffer C (8 M urea, 50 mM Tris [pH 7.5]) by gel filtration. The sample was then loaded on a 3-ml Fast Flow Q column and eluted with a 40-ml gradient of 0 to 0.5 M NaCl in buffer C. The fractions eluting between 150 and 200 mM NaCl (10 ml) were pooled and concentrated in a Centriprep 10 apparatus (Amicon) to a final volume of 1.5 ml and a concentration of 1.5 mg/ml as measured by the Bradford assay. The purified material was injected into New Zealand White rabbits for antibody production. The antibody serum diluted 1:1,000 reacts against a single band corresponding to a protein of 100 kDa on a Western blot from total cellular protein of bacterial cells expressing Cer1p. There is no cross-reactivity in bacterial cells not expressing Cer1p.
Native coimmunoprecipitations.
Yeast strain LCY22 was grown to an OD600 of 0.5, at which time dithiothreitol (DTT) was added to a final concentration of 5 mM, and the cells were allowed to grow to a final concentration of 1.0 OD600 unit before being harvested. The cells were disrupted with glass beads and a bead beater in Break buffer (50 mM Tris [pH 7.5], 50 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml, 10 μg of pepstatin per ml). Cell extract was centrifuged at 3,000 × g for 5 min to pellet unbroken cells. The supernatant was centrifuged at 20,000 × g for 20 min to pellet crude membranes including nuclei, mitochondria, and the ER. The pellet was resuspended in Break buffer–1% Triton X-100 to solubilize membranes. After 30 min on ice, this fraction was centrifuged at 20,000 × g for 20 min to pellet unbroken membranes and large protein aggregates. The supernatant was used for all experiments. The solubilized membrane extract was precleared by the addition of IgG Sorb, incubation for 15 min on ice, and pelleting at top speed in a microcentrifuge for 5 min. Polyclonal antiserum (against Kar2p, Cer1p, or CPY) was added to the supernatant, the mixture was incubated for 1 h at 4°C, IgG Sorb was added, and the mixture was incubated for a further 1 h. Antibody complexes were pelleted and washed three times with phosphate-buffered saline. Pellets were resuspended in SDS-PAGE loading buffer to disrupt complexes and analyzed by immunoblotting with using anti-CPY antiserum.
Peptide binding assay.
Cer1p was partially purified from strain LCY22 transformed with plasmid pGH2, which overexpresses Cer1p. Crude microsomes were isolated and solubilized as described for native immunoprecipitations. The peptide SO81 (VKKRCSMWIIPTDDEA) was coupled to Affi-gel 10 resin (12 mg of peptide/ml of resin) for 4 h at 4°C in 100 mM HEPES (pH 7.5). The coupling reaction was stopped by the addition of 50 mM Tris (pH 7.4). The no-peptide resin was treated identically but with water used instead of peptide. Binding of peptide to the resin was confirmed by a ninhydrin assay. A 0.2-ml volume of resin was loaded in each column. Then 100 μl of extract was pelleted for 5 min at 4°C at top speed in a microcentrifuge, and an equal volume of peptide buffer (50 mM Tris [pH 7.5], 150 mM potassium acetate, 5 mM MgCl2, 1% Triton X-100) was added with or without 2 mM ATP. The extract was loaded onto the column 50 μl at a time and then washed with two 200-μl volumes of peptide buffer. Proteins bound to the column were eluted with 400 μl of peptide buffer–8 M urea. Samples were analyzed by immunoblotting with anti-Cer1p antiserum.
Sucrose gradients.
The same extract that was used in the peptide binding assay was used for sucrose gradient analysis. The SO81 peptide was added to the extract at a final concentration of 40 μM and incubated at 22°C for 20 min before being loaded onto the gradient. ATP (final concentration, 1 mM) and MgCl2 (final concentration, 4 mM) were added to the extract, which was immediately loaded onto the gradient. The total volume loaded was 100 μl: 90 μl of extract and 10 μl of ATP, peptide, or buffer in control samples. All gradients consisted of 10 to 35% sucrose, 50 mM Tris (pH 7.5), 50 mM KCl, 1 mM EDTA, 1 mM EGTA, and 1% Triton X-100. SO81 peptide and ATP were added to the gradients at the same concentration present in the samples. All gradients were run in a Beckman swinging-bucket 55K rotor for 6 h at 4°C at 55,000 rpm. Fractions were analyzed by immunoblotting with anti-Cer1p antiserum.
Northern blot analysis.
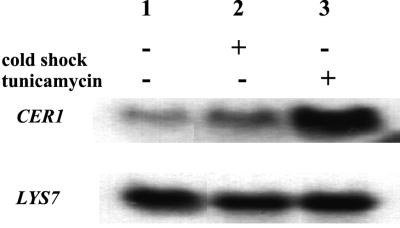
RNA was isolated from strain SEY6210. Cells were grown at 30°C to an OD600 of 0.75 and then divided among four separate containers. The heat shock culture was then grown at 37°C for 90 min, while the cold shock culture was grown at 18°C. Tunicamycin was added to a concentration of 1 μg/ml, and the cells were incubated for 90 min at 30°C, while the control culture remained at 30°C. RNA isolation, Northern blotting, and probing were done as previously described (25), except that formaldehyde was omitted from the 1% morpholinepropanesulfonic acid (MOPS) gels as suggested (24). RNA was normalized by measuring the OD260, and 10 μg of RNA was loaded in each lane of the gel. The blot was probed with a 32P-labeled CER1 mRNA riboprobe made with the Promega in vitro transcription kit. The same blot was stripped and reprobed with 32P-labeled LYS7 mRNA riboprobe to confirm that equal amounts of RNA were loaded in each lane (19).
RESULTS
Cer1p is required for proper refolding of CPY.
We had previously demonstrated that Cer1p is required for the proper import of a subset of secreted proteins into the ER (15). Kar2p, the ER hsp70 in yeast, had also been shown to play a role in translocation, possibly at multiple stages in the process (16, 34). In addition to translocation, Kar2p facilitates protein folding in the ER (38). Because of the functional similarity between Kar2p and Cer1p and weak sequence relatedness, we explored a possible protein-folding role for Cer1p.
A previous study of the chaperone function of Kar2p used the maturation of the vacuolar protease CPY as an indicator of proper folding in the ER (38). As CPY travels through the secretory pathway, it is subjected to proteolytic cleavage and carbohydrate modification (39). Three distinctly migrating species can be observed on polyacrylamide gels as a result of these modifications. The ER p1CPY form contains the core N-linked glycosylation and migrates with a molecular mass of approximately 67 kDa. In the Golgi further carbohydrate processing results in the 69-kDa p2CPY form. Finally, the mature (mCPY) 61-kDa form is obtained upon proteolytic removal of the pro region in the vacuole. Faulty import function or folding activity can be observed as an accumulation of one or more of these species.
In the Kar2p studies, the thiol reducing agent DTT was used to block disulfide formation of p1CPY. Inhibition of protein folding as a result of inhibition of disulfide formation blocked transport and hence p1CPY-to-p2CPY conversion. After removal of DTT from the medium, disulfide bonds form and CPY is transported out of the ER and therefore processed as normal. The ability to monitor the folding of CPY by observing its form conversion has made it possible to ascertain the role that Kar2p plays in the folding process. A staged assay was designed to circumvent the translocation defect of kar2 mutants (38). By maintaining temperature-sensitive kar2 yeast strains at a permissive temperature in the presence of DTT, CPY was translocated into the ER. A pool of reduced p1CPY accumulated in the ER under these conditions. Then by switching to nonpermissive conditions and removing DTT, the role of Kar2p in this process could be determined by monitoring the maturation of CPY. Several alleles of kar2 were found to strongly inhibit CPY folding in this assay (38). It should be noted that CPY maturation in this assay could be more representative of refolding in the ER. CPY folding under native conditions could occur cotranslocationally; however, the CPY-folding pathway has not been defined. Regardless, the fact that Kar2p is required for this process is extremely suggestive of its ability to function as a chaperone by binding unfolded portions of proteins and promoting refolding, as has been shown for other hsp70s.
We used a similar strategy to determine the role of Cer1p in CPY folding in the ER. Since no rapid-onset mutations of CER1 were available, we modified the CPY-folding assay outlined above. Although the CER1 deletion strain (cer1Δ) is partially defective for the translocation of prepro-CPY (ppCPY), enough ppCPY is imported during the pulse to observe through a chase period. We could therefore compare the processing of the CPY in wild-type and cer1Δ strains after removal of DTT. Unfortunately, the untranslocated ppCPY has the same electrophoretic mobility as mature CPY, thereby obscuring the p1CPY-to-mCPY conversion typically used to measure folding. By using a pep4 strain, which eliminates the p2CPY-to-mCPY conversion, we could observe the folding as p1CPY-to-p2CPY conversion and monitor the levels of untranslocated ppCPY during the chase period. However, if substantial amounts of untranslocated ppCPY were converted to p1CPY during the chase, it would not be possible to easily exact a rate for p1CPY-to-p2CPY conversion from the p1CPY-to-p2CPY ratios.
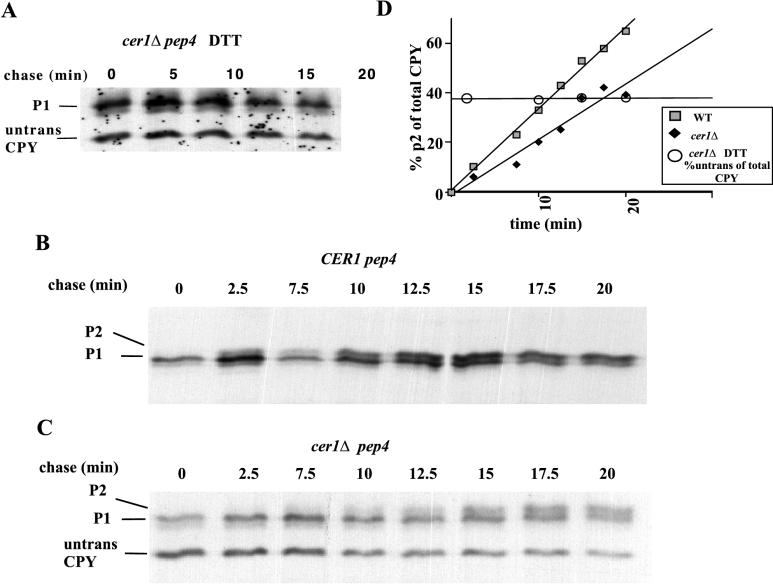
To determine if a significant amount of ppCPY is converted to p1CPY in these experiments, a cer1Δ pep4 strain was grown for 15 min in the presence of radiolabeled methionine and DTT. At this time, the radiolabeled amino acids were replaced with nonradioactive ones but DTT was included for the 20-min chase to prevent CPY from exiting the ER, resulting in the accumulation of reduced p1CPY (Fig. 1A). We found that the levels of ppCPY (38% of total) and p1CPY remained constant for the duration of the chase in the presence of DTT (Fig. 1D). Therefore, the conversion of p1CPY to p2CPY could accurately reflect the folding rate of CPY in cer1Δ pep4 yeast under these conditions, since ppCPY was not being converted to p1CPY during the chase.
FIG. 1.
Cer1p affects the folding of CPY. (A) Pulse-chase analysis of CPY translocation into the ER in the presence of DTT. CPY was immunoprecipitated at the indicated time after washing out of [35S]methionine and addition of cycloheximide. p1CPY (P1) is the ER glycosylated form of CPY, while the lower band represents untranslocated cytosolic CPY. (B) Pulse-chase analysis of CPY folding in the wild-type control strain. CPY was immunoprecipitated at the indicated time after washing out of [35S]methionine and DTT and addition of cycloheximide. p2CPY (P2) is the Golgi glycosylated form as well as the vacuolar form. (C) Pulse-chase experiment with a cer1Δ strain. (D) quantitation of the folding rates of CPY in panels A to C. The x axis shows the chase time in minutes. The y axis shows the percentage of p2CPY with respect to total CPY, which is applicable to panels B and C. It also represents the percentage of untranslocated CPY with respect to total CPY, which is applicable to panel A. WT, wild type.
A defect in CPY folding can be seen in a cer1Δ strain. Figure 1B shows the folding of CPY in a CER1 pep4 strain, while Fig. 1C shows the folding in a cer1Δ pep4 strain. In both experiments, cells were labeled in the presence of 5 mM DTT for 15 min. Both radiolabeled amino acids and DTT were removed at the beginning of the chase period. In the wild-type strain, 50% of the labeled CPY was found in the folded p2 form in 10 to 12 min. By contrast, more than 20 min was required for conversion of a similar amount of CPY in the cer1Δ strain (Fig. 1D). The loss of CER1 function slows the folding of CPY (∼twofold), although the effect is not as severe as is seen in some kar2 alleles (38). Since the cer1Δ strain may have adapted to the loss of CER1 function over time, the folding effects may be diminished compared to those seen in the rapid-onset kar2 mutant strains.
It is interesting that the cer1Δ folding defect follows the same temperature sensitivity trend that we observed with the translocation defect. When the experiments described in Fig. 1 were performed at 30 instead of 25°C, the folding defect was not as severe (data not shown). The greatest translocation defect and growth defects were also observed at lower temperatures (15). Taken together, the evidence supports a role for Cer1p as a chaperone functioning at lower temperatures.
Cer1p is required for the translocation of CPY.
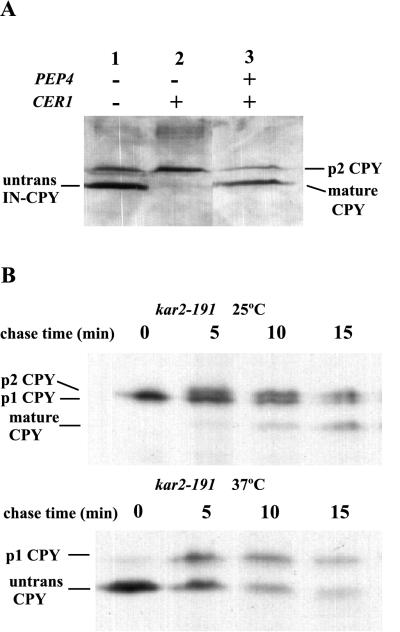
In an attempt to clarify the role of Cer1p in protein folding, we constructed an invertase-CPY (IN-CPY) fusion protein, which contained pro-CPY with the signal sequence from preinvertase. The goal was to create a form of CPY that depended on Cer1p for folding but was imported independently of Cer1p. If the signal sequence alone determined whether a translocating polypeptide required Cer1p, swapping the signal sequence on ppCPY (CER1 dependent) with the signal sequence from preinvertase (CER1 independent) should eliminate the accumulation of untranslocated CPY in these folding experiments.
To determine if the IN-CPY fusion protein could be imported independently of Cer1p activity, cells that lack chromosomal CPY (prc1Δ) were transformed with the plasmid containing the gene encoding the chimeric IN-CPY protein. As described above, carrying out these experiments in a pep4 background allowed us to distinguish between untranslocated and mature CPY. A CER1 pep4 strain efficiently translocated the fusion protein (Fig. 2A, lane 2), as indicated by the accumulation of p2CPY under steady-state conditions. In a wild-type strain the fusion protein was translocated and efficiently processed, since mature CPY is the predominant form of CPY under steady-state conditions (lane 3). However, in a cer1Δ pep4 strain, untranslocated IN-CPY accumulated under steady state conditions (lane 1), to a level similar to that seen for CPY with its own signal sequence (15). Therefore, the signal sequence is not sufficient to determine the dependence of translocation on Cer1p. The dependence of a translocating protein on Cer1p could be less a function of the signal sequence and more a reflection of the characteristics of the mature portion of the protein.
FIG. 2.
(A) Western blot analysis of the translocation IN-CPY in wild-type or cer1Δ cells. In pep4 strains, the lower band is ppCPY, while in a PEP4 strain, it is mature CPY. (B) CPY can be posttranslationally translocated in vivo. Pulse-chase analysis of CPY folding done as described in the legend to Fig. 1 was carried out. (Top) The kar2-191 strain was kept at 25°C for the entire experiment. (Bottom) The labeling was done at 37°C, and the chase was done at 25°C. The lower band represents ppCPY and mature CPY because the kar2-191 strain is PEP4.
CPY is believed to enter the ER posttranslationally based upon in vitro translocation assays (18) and the presence of a weak signal sequence (27). However, it is not clear whether changes to the CPY signal sequence could switch its translocation from posttranslational to cotranslational. In fact, the posttranslational translocation of CPY in vivo has never been demonstrated. Surprisingly, in vivo posttranslational import competence has been demonstrated only for a single protein, ppαf, which requires Cer1p activity (32). We therefore explored whether ppCPY was also competent for posttranslational import in vivo.
To determine if ppCPY is posttranslationally translocated, the CPY-folding assay was slightly modified. A kar2-191 strain was labeled in the presence of DTT at the permissive temperature (25°C). A pool of unfolded p1CPY accumulated in the ER, as seen in Fig. 2B (top panel, zero time point). After removal of DTT, the labeled p1CPY folded and was converted to p2CPY and then to mature CPY. When the cells were labeled at the nonpermissive temperature (37°C), untranslocated CPY accumulated in the cytosol. Removal of DTT in the presence of cycloheximide and shifting cells back to the permissive temperature allowed for CPY to be imported into the ER, as indicated by the conversion of untranslocated CPY to p1CPY (Fig. 2B, lower panel). This experiment demonstrates that ppCPY is competent for posttranslational translocation in vivo.
Cer1p is in a complex with pro-CPY.
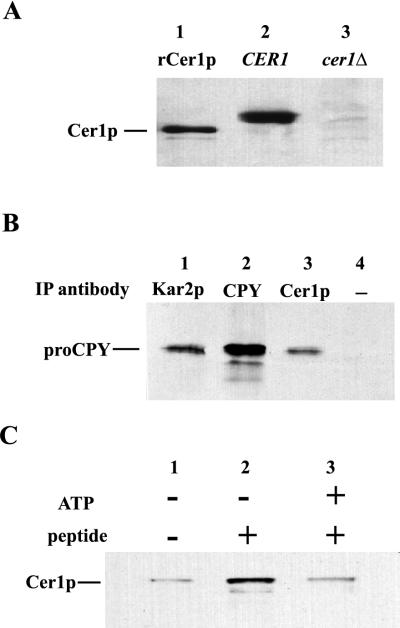
Although Cer1p appears to be involved in the process of folding denatured proCPY, it is possible that its effect is indirect. To clarify the role of Cer1p in folding, native immunoprecipitations were performed to determine whether Cer1p and CPY exist in a complex. All native primary immunoprecipitations were performed with crude microsomal extract isolated from a pep4 CER1 yeast strain. Cells were treated with 5 mM DTT for 30 min to generate a pool of unfolded CPY (reduced p1CPY or pro-CPY) in the ER. Following detergent disruption of the microsomal membranes, the extracts were immunoprecipitated with antiserum against Cer1p, CPY, or Kar2p. The resulting immunoprecipitated complexes were then analyzed by immunoblotting with the anti-CPY antiserum. Similar immunoprecipitations were carried out with microsomes that had not undergone DTT treatment. The anti-Cer1p antiserum was shown to be monospecific for Cer1p in both immunoblotting (Fig. 3A) and immunoprecipitations (results not shown), since both the full-length Cer1p from crude yeast extracts and an unglycosylated, recombinantly expressed form of Cer1p (rCer1p) were specifically recognized by the antibodies.
FIG. 3.
(A) Anti-Cer1p antiserum specifically recognizes Cer1p. Lanes: 1, 2 ng of recombinant Cer1p; 2 and 3, total-cell extract from either wild-type or cer1Δ yeast. Samples were Western blotted and probed with anti-Cer1p antiserum. (B) Cer1p is in a complex with CPY. The antibody used for the primary immunoprecipitations (IP) done in crude microsomal extract from the wild-type strain are indicated above each lane. Each immunoprecipitation was Western blotted and probed with anti-CPY antiserum. proCPY represents a mixture of p1CPY and p2CPY. (C) Cer1p binds peptide. Crude microsomal extract from a CER1-overexpressing strain was passed over Affi-Gel 10 beads alone or with the SO81 peptide covalently attached. The eluted fractions were tested for the presence of Cer1p by Western blotting and probing with anti-Cer1p antiserum. In the lane 3, ATP was added to the microsomal extract before it was loaded on the column.
Simons et al. had previously shown that Kar2p coimmunoprecipitated with pro-CPY when cells were treated with DTT (38) and hence proposed a direct role of Kar2p in protein folding. We could replicate their results, since immunoprecipitations of Kar2p also precipitates proCPY, as shown in Fig. 3B (lane 1). Interestingly, we observed a similar complex formation between Cer1p and proCPY under identical conditions (lane 3). Since the immunoprecipitations with the anti-Cer1p and anti-Kar2p antiserum were nearly quantitative (data not shown), the figure indicates the relative contributions of Cer1p and Kar2p in these complexes. A significant fraction of unfolded CPY appears to interact with Cer1p under reducing conditions in the ER. Controls indicate that no CPY precipitated without primary antibody in the reactions (lane 4). Therefore, these precipitates were not simply the result of antibody-independent cosedimentation of large aggregates of CPY. Moreover, no complex formation was observed when immunoprecipitations were performed with microsomal extracts from cells that did not receive DTT treatment (data not shown). The p1CPY accumulation and hence complex formation was dependent on DTT addition. We should note that the shorter gels used in these experiments did not resolve p1CPY from p2CPY. Therefore, although it is assumed that the accumulated p1CPY coimmunoprecipitates with Cer1p, precipitation of p2CPY cannot be ruled out. This result also illustrates the specificity of Cer1p antibody, since no coimmunoprecipitation was observed in the absence of DTT. Together, these experiments support the conclusion that Cer1p plays a direct role in the folding of denatured pro-CPY.
Cer1p peptide binding activity.
The experiments outlined above, coupled with previous work on the involvement of Cer1p in protein translocation, suggest that Cer1p has similar biological activities to Kar2p. For authentic hsp70 members, proposed mechanisms of action were derived, in part, through identification of biochemical activities, such as ATP and peptide binding. Central to both folding and translocation models involving hsp70 function is the ATP-dependent binding to unfolded polypeptides (11). The low sequence homology between Cer1p and hsp70s led us to question whether Cer1p shares the biochemical activities of hsp70s, as suggested by the similar biological activities.
Biochemical activities have been probed for a variety of hsp70 members, including BiP, the mammalian homolog of Kar2p. BiP, like Kar2p, is thought to function as a chaperone in the ER. BiP, and presumably Kar2p, binds to short hydrophobic peptides. Binding and release of the peptides is regulated by the ATP hydrolysis cycle (12). This regulated binding of peptides suggests that BiP interacts with unfolded segments of polypeptides in an ATP-dependent fashion.
Early experiments with recombinant Cer1p expressed in bacteria failed to demonstrate ATP binding or hydrolytic activities. These results were surprising considering the reasonable degree of homology between the N-terminal region of Cer1p and the ATPase domain of the hsp70 family. However, the propensity of the purified recombinant Cer1p to form aggregates led us to question whether Cer1p assumed a native conformation in bacteria. Unlike the ER, the E. coli cytosol does not allow the formation of disulfide bonds or N-linked glycosylation. Either or both of these modifications could be required to obtain active Cer1p. We therefore attempted to ascertain the biochemical activities of Cer1p expressed in yeast. Since Cer1p is expressed at relatively low levels in yeast, making purification and detection difficult, we overexpressed Cer1p in yeast to allow crude biochemical characterization from microsomal extracts.
To screen Cer1p for peptide binding activity, detergent-disrupted microsomes from yeast strains overexpressing Cer1p were passed over peptide-agarose resin. This approach was used initially to demonstrate that BiP, but few other proteins, displayed this peptide binding activity (12). Further experiments showed that purified BiP bound to soluble peptides in a similar way. The peptide S081 used in this experiment can bind and stimulate the ATPase activity of BiP and DnaK (12a). As can be seen in Fig. 3C, Cer1p bound to the peptide resin much more strongly than to resin lacking the coupled peptide. As with BiP binding, ATP lowered the peptide-Cer1p affinity (Fig. 3C, lane 3). The most straightforward conclusion from these experiments is that like BiP, Cer1p binds to peptides and is regulated by ATP. These results, taken together with the pulse-chase and immunoprecipitation experiments in particular, support a direct interaction between Cer1p and an unfolded protein substrate. Furthermore, these results suggest that the role that Cer1p plays in translocation is through direct interaction with a translocating chain.
Cer1p self-association is regulated by peptide and ATP.
A common feature of hsp70s is the ability to self-associate to form dimers and higher-order polymers. It has been demonstrated that the addition of peptide substrate causes dissociation of hsp70 dimers and oligomers (14). ATP can also drive this dissociation. Again, while many hsp70 self-association experiments have been performed with purified protein in vitro (2), it is possible to probe the association state of hsp70s by using crude extracts obtained from microsomes (13).
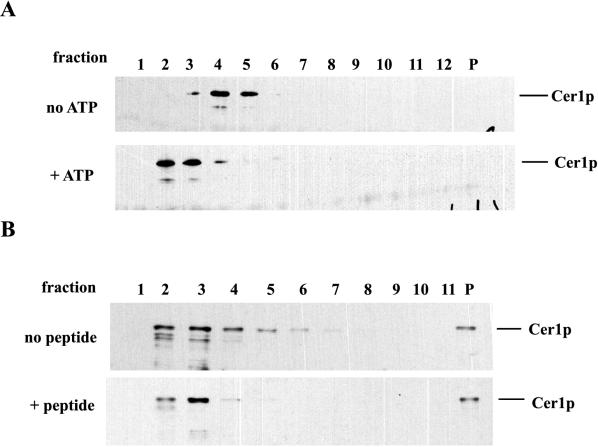
The association state of Cer1p was measured by velocity sedimentation through sucrose gradients. In microsomal extracts from a wild-type yeast strain, Cer1p sedimented as a slowly migrating species near the top of the gradient, consistent with its existing solely as a monomer (data not shown). In contrast, Cer1p sedimented more rapidly on gradients, as expected for a dimer, when extracts were prepared from a Cer1p-overproducing strain (Fig. 4A, top panel). This shift to a larger species when Cer1p is overexpressed is probably self-association and not the association of Cer1p with other ER proteins, since the ratio of oligomer to monomer would not be expected to increase at higher concentrations (see Discussion). However, other heterotypic interactions cannot be ruled out in this crude system. Previous studies have demonstrated that hsp70s can form homooligomers in a concentration-dependent manner, both in crude extracts and in a pure state (2, 13, 14). Therefore, it is likely that Cer1p overexpression also leads to the formation of homodimers. We then tested whether ratios of the two different forms of Cer1p could be influenced by ATP or peptide addition, as was found for hsp70s.
FIG. 4.
Peptide and ATP affect the self-association of Cer1p. (A) The top panel is the Western blot analysis of the fractions from a 10 to 35% sucrose gradient loaded with crude microsomal extract from a CER1-overexpressing strain. Fraction 1 represents the top of the gradient, while P is the pellet fraction. In the lower panel, ATP was added to a final concentration of 1 mM in the gradient and in the extract and then immediately loaded onto the gradient at 4°C. (B) The same gradients and analysis were used to determine the effect of peptide SO81, which was absent in the top gradient and present in the bottom gradient. In both gradients the extract was incubated at 22°C with or without peptide for 20 min before it was loaded on the column.
The top panel of Fig. 4A illustrates the position of oligomeric Cer1p from microsomal extracts of a Cer1p overexpression strain on a 10 to 35% sucrose gradient. The same extracts were incubated briefly with 1 mM ATP and then sedimented through a sucrose gradient also containing ATP. The lower panel of Fig. 4A shows that ATP addition shifted the distribution of Cer1p almost exclusively to a slowly sedimenting form. This provides further evidence that Cer1p has a functional ATPase that behaves like the Hsp70 ATPase. Cer1p oligomers could also be dissociated by the addition of peptide substrate. Since peptide-induced dissociation of hsp70s is relatively slow, extracts were incubated with and without peptide for 20 min at room temperature before being loaded sucrose gradients. Although the long incubations resulted in some degradation, partially obscuring the separation between the different forms, Cer1p sedimented as a combination of slowly and more rapidly sedimenting forms in the absence of peptide (Fig. 4B, top panel). When peptide was included in the incubations, more Cer1p migrated as a slowly sedimenting form (Fig. 4B, lower panel). These results are similar to those seen with hsp70 family members (2, 14) and suggest that Cer1p behaves like an hsp70 family member in peptide and ATP binding and self-association.
Regulation of CER1 mRNA levels.
the biological and biochemical evidence presented here indicates that Cer1p and Kar2p possess almost identical activities and functions. Why, then, does the yeast ER lumen possess two such chaperones instead of one? One possibility is that the two chaperones are utilized under different cellular conditions. For example, the cellular defects seen in cer1Δ strains are more severe at lower temperatures. To further investigate the unique role of Cer1p, we have analyzed the accumulation of CER1 mRNA.
It was previously reported that CER1 mRNA accumulates when cells are treated with the drug tunicamycin, which prevents glycosylation in the ER and results in the accumulation of misfolded proteins in the ER (8). This response is common to other ER chaperones and is dependent upon a promoter element known as the unfolded-protein response element (21). It was also shown that unlike KAR2, CER1 mRNA levels do not increase after heat shock (1). We have attempted to determine if CER1 mRNA levels accumulate under other stress conditions. Northern blot analysis of CER1 mRNA levels revealed that CER1 mRNA accumulated after cells received a cold shock treatment (Fig. 5, top panel). Cold shock produced a 2-fold increase in CER1 mRNA levels, which is not as dramatic as the 10-fold induction due to tunicamycin treatment. To ensure that equal amounts of RNA were loaded, the same blot was reprobed with the LYS7 mRNA (Fig. 5, bottom panel). Since deletion of cer1 produces yeast with a cold-sensitive phenotype, the accumulation of CER1 mRNA by cold shock suggests that Cer1p plays a unique role as a chaperone functioning at lower temperatures. This idea is further supported by the increased severity of the translocation (15) and folding defects (this study) of cer1Δ strains at lower temperatures. It is possible that the chaperone activity of Cer1p is most relevant at lower temperatures, when Kar2p might be less effective.
FIG. 5.
Northern blot analysis of CER1 mRNA levels. The top panel shows analysis of the CER1 mRNA under basal and stress conditions. The blot was probed with a 32P-labeled riboprobe corresponding to the noncoding strand of CER1. The bottom panel shows the same blot stripped and reprobed with the noncoding strand of LYS7.
DISCUSSION
The results presented here support a role for Cer1p as a chaperone in the folding of denatured proteins that accumulate in the ER under stress conditions. We found that folding of denatured proCPY in the ER is slowed in yeast cells lacking Cer1p. Moreover, the results of immunoprecipitations and peptide binding experiments suggest that this activity is mediated through direct interaction with the unfolded polypeptide. Cer1p could also play a role in the de novo folding of pro-CPY, although it has been previously suggested, based upon pulse-chase analysis of CPY maturation, that the loss of Cer1p did not affect the processing of CPY molecules that had been successfully imported into the ER (1). However, it is possible that these experiments would not have revealed a defect in CPY folding since they were performed at the permissive temperature for the cer1 deletion strain. Additionally, CPY folding might not be the rate-limiting factor of CPY maturation.
The CPY maturation assay used in our studies monitored the folding of an unfolded pool of pro-CPY, which accumulated in the ER in the presence of reducing agent. Upon removal of the reducing agent, pro-CPY could then fold. By increasing the pool of unfolded protein in the ER, the folding-specific steps in protein maturation could be more easily revealed. Using this assay, Simons et al. (38) were able to demonstrate that Kar2p was required for reduced pro-CPY folding in vivo. The staging of pro-CPY folding in the ER with DTT increased the sensitivity of the refolding steps, thus allowing the identification of the cer1Δ folding defect.
There may, however, be some concern about whether authentic pro-CPY folding is represented in either study. The protein folding that is observed initiates from the full-length reduced proCPY. It is possible that folding and/or disulfide formation initiates cotranslocationally in vivo and therefore follows a different folding pathway. The pro-CPY folding observed in both studies could be more akin to the refolding that may occur during recovery from stress. The resolution of this question will require further experiments to characterize the CPY-folding pathway in vivo.
The folding defects we observed in cer1Δ yeast, although similar, were not as severe as previously observed for some kar2 alleles (38). However, the kar2 mutations used were rapid onset (temperature sensitive). The cer1 deletion strain used in our study may have adapted to the loss of Cer1p, possibly by overexpressing other chaperones, such as Kar2p. In fact, other studies have found that increased levels of KAR2 mRNA accumulate in cer1Δ strains (8). Like the cer1Δ-dependent translocation defect, we found that the cer1Δ folding defect was more severe at low temperatures. In addition, CER1 mRNA accumulated after cold shock treatment. Together, these results suggest that Cer1p acts as a molecular chaperone in the ER, which is particularly important at lower temperatures.
Despite the lack of sequence similarity between hsp70s, such as Kar2p, and Cer1p, our results strongly indicate that there are biochemical similarities between them. We have demonstrated that Cer1p, like hsp70s, has the capacity to bind short peptides. The binding to peptide is lower in the presence of millimolar ATP concentrations. Furthermore, Cer1p oligomerization can be disrupted by the addition of ATP or peptide. These effects of ATP and peptide on Cer1p are reminiscent of ATP- and peptide-induced disruption of self-association of members of the hsp70 family. It should be noted that although these experiments were not done with purified Cer1p, it is likely that higher-molecular-weight Cer1p species are predominantly homooligomeric Cer1p. In any hetero-bimolecular binding reaction, such as A + B → AB, increasing the concentration of one species (such as A) will lead to increasing amounts of the complex at subsaturation conditions. However, this cannot increase the ratio of the complex (AB) to the free species (free A). Since the overexpression of Cer1p leads to an increase in the ratio of fast- to slow-migrating species (representing the complex-to-free-species ratio), the thermodynamics strongly argue that the fast-migrating species is due to Cer1p self-association. Furthermore, the relatively narrow and slowly sedimenting peak, coupled to the ATP-dependent dissociation, argues that this self-association is specific, possibly dimerization, and is not a nonspecific aggregation of Cer1p. Our biochemical experiments also provide the first evidence that Cer1p contains a functional ATPase. Although the sequence similarity between hsp70 and Cer1p suggested that there was a functional ATPase domain in Cer1p, the peptide binding property of Cer1p was not apparent from such comparisons.
The conclusion that Cer1p functions as a chaperone, possibly by the same mechanism as Kar2p, might be considered surprising due to the low degree of sequence identity between these proteins. Cer1p sequence identity to hsp70s is restricted to the N-terminal ATPase domain of hsp70. Even within this region, the similarity is significantly lower than is found in true hsp70s. However, much of the similarity between Cer1p and hsp70s is clustered in regions known to be in contact with nucleotide based upon the three-dimensional structure of the hsp70 ATPase. Beyond the N-terminal domains of these proteins, Cer1p has no significant similarity to hsp70s. In contrast, true hsp70s are about 50% identical to each other in the C-terminal half of the protein, which contains the peptide binding domains (corresponding to residues 386 to 545 in DnaK). The chaperoning and folding roles of hsp70s and presumably Kar2p require direct binding to unfolded polypeptides through this peptide binding domain (41). Whereas the peptide binding domain of Cer1p could have the same fold as the hsp70 peptide binding domain, it is also possible that Cer1p possesses a peptide binding domain with a completely novel structure.
How does Cer1p aid in both folding and translocation in the ER? If Cer1p acts at the same stage as Kar2p in both these processes, similar cochaperones might be expected to regulate Cer1p activity. The Kar2p translocation activity is mediated through interaction with the membrane proteins Sec63p (6) and possibly Sec61p (16), while the chaperoning activity could be mediated through interaction with Scj1p (36). Both Scj1p and Sec63p have a J domain, a region that has sequence identity to the N-terminal domain of DnaJ. Moreover, mutations in the J domain abolish interactions with Kar2p (7). Previously, we observed that overexpression of Scj1p suppressed the translocation defect of a cer1Δ strain (15). This genetic interaction could suggest a direct Cer1p-Scj1p interaction. Additionally, we have preliminary data showing that Cer1p in crude extracts binds to a fusion protein containing the J domain of Sec63p (data not shown). This result not only suggests that Cer1p could interact with Sec63p during translocation but also supports the idea that Cer1p could directly interact with Scj1p. Previous experiments have demonstrated that the J domains of Sec63p and Scj1p are interchangeable with respect to the ability to interact with Kar2p (36). Therefore, the ability of Cer1p to interact with a J domain suggests a regulatory mechanism for both translocation and folding in the ER.
Our experiments also attempted to clarify the role of Cer1p during translocation of proteins into the ER. A large collection of evidence supports the existence of two distinct import pathways in yeast: cotranslational and posttranslational translocation. It appears that soluble proteins which require Cer1p activity for proper import also require Sec63p (8, 15). Sec63p is a component of the heptameric complex believed to be required for posttranslational translocation (30). The observation that Cer1p- and Sec63p-dependent proteins can be imported independent of a functional signal recognition particle (SRP) further supports a posttranslational import pathway for these proteins (17). Several of these proteins have the ability to be posttranslationally imported into microsomes in vitro. In contrast, integral membrane proteins which do not require Cer1p or Sec63p activity for insertion into the ER membrane are assumed to do so cotranslationally due to a strict requirement for an SRP (17). It is believed that the strength of the signal sequence is a major factor in determining the pathway of import.
We attempted to determine if replacing the native signal sequence of a Cer1p-dependent protein, CPY, with that of a Cer1p-independent protein, invertase, could relieve the dependence of CPY on Cer1p activity for import. Studies done by Johnsson and Varshavsky demonstrated that the invertase signal sequence can direct SRP-dependent cotranslational translocation to a greater extent than the CPY signal sequence can (20). However, these studies did not reveal the nature of invertase translocation under native conditions. The majority of the invertase could be posttranslationally imported, but only cotranslational translocation could be detected in that assay. The ability of invertase to be imported posttranslationally is supported by results which have shown that invertase translocation is dependent on the function of Sec62p, a component of the heptameric posttranslational translocation complex (17). Also, despite the presence of its hydrophobic signal sequence, invertase import is largely unaffected by deletion of SRP (17). Our results indicated that the invertase signal sequence did not change the dependence of CPY on Cer1p activity. We do not know whether the IN-CPY fusion protein is imported differently from native CPY. However, this result could reflect the nature of all soluble proteins to be imported posttranslationally. The degree to which a protein can be posttranslationally imported may reflect its ability to remain in an unfolded or in a translocation-competent state following translation. In fact, the two proteins found to be capable of posttranslational translocation in vivo, ppαf (32) and ppCPY (this study), are most probably unable to completely fold in the cytosol. Mature α-factor is an unfolded peptide that is proteolytically processed in the Golgi from its polyprotein precursor. It is therefore unlikely that prepro-α-factor folds before it is processed into peptides. ppCPY is also not believed to obtain its final folded state until its pro region is cleaved in the vacuole. Therefore, it is possible that the degree of unfoldedness of a protein, and not the signal sequence, defines the need for the Cer1p chaperone during import.
In a previously published study by Saris et al. (35), the authors proposed that the main function of Cer1p (Lhs1p) is to allow cells to recover from extreme heat shock stress. This study also claimed to rule out normal translocation and folding roles for Cer1p. Several of our results are inconsistent with their conclusions. We observed translocation defects for several proteins and noticeable folding defects for CPY in cer1Δ strains. It is likely that these defects would become more severe with rapid-onset mutant cer1 alleles. Some of the results of the Saris et al. study do not even seem to support their own conclusions. They concluded from similar CPY pulse-chase refolding experiments with DTT that no translocation or folding defects are evident in cer1Δ strains (Fig. 9C) (35). However, a translocation defect is clearly evident in their cer1Δ strain pulse-chase 0-min time point. As we have mentioned above, untranslocated ppCPY migrates on polyacrylamide nearly the same as mature CPY. Since at 0 min of the chase more of this species is observed in the cer1Δ strain than in the wild-type strain, one would have to conclude either that processing is faster (more mature CPY) or that translocation is partially blocked (more untranslocated CPY) in the cer1Δ strain. Our evidence with pep4 strains supports the latter possibility. In addition, the long times chosen in their chase were unlikely to reveal folding defects. The shortest intermediate time point in their study was 45 min. However, in wild-type yeast, CPY is virtually completely processed from p1CPY to mCPY in 20 min. The shorter times used in our study allowed the defect to be observed. However, the long times used points in the Saris et al. (35) study did indicate that CPY does not accumulate and remain in the ER of a cer1 deletion strain for extended periods. Either the protein eventually folds in the ER and is transported to the Golgi in the absence of Cer1p or it is degraded in the ER. The lack of CER1 mRNA accumulation or functional importance seen at higher temperatures, coupled with the increased dependency and CER1 mRNA accumulation at lower temperatures, argues for a role of Cer1p at lower, not heat shock, temperatures.
Taken together, our results suggest a model in which Cer1p and Kar2p have overlapping functions in the ER. The KAR2 induction at elevated temperatures would suggest an increased need for Kar2p under these conditions (28, 31). In fact, it is possible that the temperature-sensitive phenotype of the kar2 mutants used in these previous studies was the result of this increased dependence on Kar2p at elevated temperatures and was not due to thermal destabilization of the mutant protein. Kar2p also is important at normal temperature, but its function at lower temperatures has not been published. The typical tetrad analysis used to demonstrate the importance of a gene is performed at room temperature or above. An analysis at lower temperature would be required to determine the importance of KAR2 under these conditions. Cer1p apparently shares the functions of Kar2p at normal temperatures. At lower temperatures, the role of Cer1p becomes more prominent and cannot be replaced by the normal cellular levels of Kar2p.
ACKNOWLEDGMENTS
This research was sponsored in part by grant R01 GM57978-01 from the NIH (G.C.F. and T.G.H.).
We thank N. Johnsson, M. Rose, and T. Stevens for providing strains, antibodies, and plasmids. We also thank R. Piper, S. Givan, L. Graham, N. Bryant, and T. Stevens for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Baxter B K, James P, Evans T, Craig E A. SSI1 encodes a novel Hsp70 of the Saccharomyces cerevisiae endoplasmic reticulum. Mol Cell Biol. 1996;16:6444–6456. doi: 10.1128/mcb.16.11.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benaroudj N, Triniolles F, Ladjimi M M. Effect of nucleotides, peptides, and unfolded proteins on the self-association of the molecular chaperone HSC70. J Biol Chem. 1996;271:18471–18476. doi: 10.1074/jbc.271.31.18471. [DOI] [PubMed] [Google Scholar]
- 3.Blumberg H, Silver P A. A homologue of the bacterial heat-shock gene DnaJ that alters protein sorting in yeast. Nature. 1991;349:627–629. doi: 10.1038/349627a0. [DOI] [PubMed] [Google Scholar]
- 4.Brodsky J L, Goeckeler J, Schekman R. BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc Natl Acad Sci USA. 1995;92:9643–9646. doi: 10.1073/pnas.92.21.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodsky J L, Hanamoto S, Feldheim D, Schekman R. Reconstitution of protein translocation from solubilized yeast membranes reveals topologically distinct roles for BiP and hsc70. J Cell Biol. 1993;120:85–102. doi: 10.1083/jcb.120.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodsky J L, Schekman R. A Sec63p-BiP complex from yeast is required for protein translocation in a reconstituted proteoliposome. J Cell Biol. 1993;123:1355–1363. doi: 10.1083/jcb.123.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corsi A K, Scheckman R. The lumenal domain of Sec63p stimulates the ATPase activity of BiP and mediates BiP recruitment to the translocon in Saccharomyces cerevisiae. J Cell Biol. 1997;137:1483–1493. doi: 10.1083/jcb.137.7.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craven R A, Egerton M, Stirling C J. A novel Hsp70 of the yeast ER lumen is required for the efficient translocation of a number of protein precursors. EMBO J. 1996;15:2640–2650. [PMC free article] [PubMed] [Google Scholar]
- 9.Davis N G, Horecka J L, Sprague G F J. cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J Cell Biol. 1993;122:53–65. doi: 10.1083/jcb.122.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finke K, Plath K, Panzner S, Prehn S, Rapoport T A, Hartmann E, Sommer T. A second trimeric complex containing homologs of the Sec61p complex functions in protein transport across the ER membrane of S. cerevisiae. EMBO J. 1996;15:1482–1494. [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn G C, Chappell T G, Rothman J E. Peptide binding and release by proteins implicated as catalysts of protein folding. Science. 1989;245:385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- 12.Flynn G C, Pohl J, Flocco M T, Rothman J E. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991;353:726–730. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- 12a.Flynn, G. C. Unpublished results.
- 13.Freiden P J, Gaut J R, Hendershot L M. Interconversion of three differentially modified and assembled forms of BiP. EMBO J. 1992;11:63–70. doi: 10.1002/j.1460-2075.1992.tb05028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao B, Eisenberg E, Greene L. Effect of constitutive 70kDa heat shock protein polymerization on its interaction with protein substrate. J Biol Chem. 1996;271:16792–16797. doi: 10.1074/jbc.271.28.16792. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton T G, Flynn G C. Cer1p, a novel Hsp70 related protein for posttranslational ER translocation in yeast. J Biol Chem. 1996;271:30610–30613. doi: 10.1074/jbc.271.48.30610. [DOI] [PubMed] [Google Scholar]
- 16.Hamman B D, Hendershot L M, Johnson A E. BiP maintains the permeability barrier of the ER by sealing the lumenal end of the translocon pore before and early in translocation. Cell. 1998;92:747–758. doi: 10.1016/s0092-8674(00)81403-8. [DOI] [PubMed] [Google Scholar]
- 17.Hann B C, Walter P. The signal recognition particle in S. cerevisiae. Cell. 1991;67:131–144. doi: 10.1016/0092-8674(91)90577-l. [DOI] [PubMed] [Google Scholar]
- 18.Hansen W, Walter P. Prepro-carboxypeptidase Y and a truncated form of pre-invertase, but not the full-length pre-invertase, can be posttranslationally translocated across microsomal vesicle membranes from Saccharomyces cerevisiae. J Cell Biol. 1988;106:1075–1081. doi: 10.1083/jcb.106.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horecka J, Kinsey P T, Sprague G F J. Cloning and characterization of the Saccharomyces cerevisiae LYS7 gene: evidence for function outside of lysine biosysnthesis. Gene. 1995;162:87–92. doi: 10.1016/0378-1119(95)00325-z. [DOI] [PubMed] [Google Scholar]
- 20.Johnsson N, Varshavsky A. Ubiquitin-assisted dissection of protein transport across membranes. EMBO J. 1994;13:2686–2698. doi: 10.1002/j.1460-2075.1994.tb06559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohno K, Normington K, Sambrook J, Gething M-J, Mori K. The promoter region of the yeast KAR2 (BiP) gene contains a regulatory domain that responds to the presence of unfolded proteins in the endoplasmic reticulum. Mol Cell Biol. 1993;13:877–890. doi: 10.1128/mcb.13.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langer T, Lu C, Echols H, Flanagan J, Hayer M K, Hartl F U. Successive action of DnaK, DnaJ, and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992;356:683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- 23.Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci USA. 1991;88:2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y C, Chou Y C. Formaldehyde in formaldehyde/agarose gel may be eliminated without affecting the electrophoretic separation of RNA molecules. BioTechniques. 1990;9:558–560. [PubMed] [Google Scholar]
- 25.McKinney J D, Chang F, Heintz N, Cross F R. Negative regulation of FAR1 at the start of the yeast cell cycle. Genes Dev. 1993;7:833–843. doi: 10.1101/gad.7.5.833. [DOI] [PubMed] [Google Scholar]
- 26.Melnick J, Dul J L, Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370:373–375. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- 27.Ng D T W, Brown J D, Walter P. Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J Cell Biol. 1996;134:269–278. doi: 10.1083/jcb.134.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Normington K, Kohno K, Kozutsumi Y, Gething M J, Sambrook J. S. cerevisiae encodes and essential protein homologous is sequence and function to mammalian BiP. Cell. 1989;57:1223–1236. doi: 10.1016/0092-8674(89)90059-7. [DOI] [PubMed] [Google Scholar]
- 29.Palleros D R, Reid K L, Shi L, Welch W J, Fink A L. ATP-induced protein-hsp70 complex dissociation requires K+ but not ATP hydrolysis. Nature. 1993;365:664–666. doi: 10.1038/365664a0. [DOI] [PubMed] [Google Scholar]
- 30.Panzner S, Dreier L, Hartmann E, Kostka S, Rapoport T A. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell. 1995;81:561–570. doi: 10.1016/0092-8674(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 31.Rose M D, Misra L M, Vogel J P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989;57:1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- 31a.Rose M D, Winston F, Hieter P. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 32.Rothblatt J A, Deshaies R J, Sanders S L, Daum G, Schekman R. Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J Cell Biol. 1989;109:2641–2652. doi: 10.1083/jcb.109.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 1997;16:1501–1507. doi: 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders S L, Whitfield K M, Vogel J P, Rose M D, Schekman R W. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992;69:353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- 35.Saris N, Holkeri H, Craven R A, Stirling C J, Makarow M. The Hsp70 homologue Lhs1p is involved in a novel function of the yeast endoplasmic reticulum, refolding and stabilization of heat-denatured protein aggregates. J Cell Biol. 1997;137:813–824. doi: 10.1083/jcb.137.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlenstedt G, Harris S, Risse B, Lill R, Silver P A. A yeast DnaJ homologue, Scj1p, can function in the endoplasmic reticulum with BiP/Kar2p via a conserved domain that specifies interactions with Hsp70s. J Cell Biol. 1995;129:979–988. doi: 10.1083/jcb.129.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmid D, Baici A, Gehrig H, Christen P. Kinetics of molecular chaperone action. Science. 1994;263:971–973. doi: 10.1126/science.8310296. [DOI] [PubMed] [Google Scholar]
- 38.Simons J F, Ferro-Novick S, Rose M D, Helenius A. BiP/Kar2p serves as a molecular chaperone during carboxypeptidase Y folding in yeast. J Cell Biol. 1995;130:41–49. doi: 10.1083/jcb.130.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens T H, Esmon B, Schekman R. Early stages in the yeast secretory pathway are required for the transport of carboxypeptidase Y to the vacuole. Cell. 1982;30:439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- 40.Vogel J P, Misra L M, Rose M D. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990;110:1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang T F, Chang J-H, Wang C. Identification of the peptide binding domain of hsc70. J Biol Chem. 1993;268:26049–26051. [PubMed] [Google Scholar]
- 42.Way J C, Silver P A. Eukaryotic DnaJ homologs and the specificity of Hsp70 activity. Cell. 1993;74:5–6. doi: 10.1016/0092-8674(93)90287-z. [DOI] [PubMed] [Google Scholar]