Abstract
Synthetic bioconjugation at cysteine (Cys) residues in peptides and proteins has emerged as a powerful tool in chemistry. Soft nucleophilicity of the sulfur in Cys renders an exquisite chemoselectivity with which various functional groups can be placed onto this residue under benign conditions. While a variety of reactions have been successful at producing Cys-based bioconjugates, the majority of these feature sulfur-carbon bonds. We report Cys-borylation, wherein a benchtop stable Pt(II)-based organometallic reagent can be used to transfer a boron-rich cluster onto a sulfur moiety in unprotected peptides forging a boron-sulfur bond. Cys-borylation proceeds at room temperature and tolerates a variety of functional groups present in complex polypeptides. Further, the bioconjugation strategy can be applied to a model protein modification of Cys-containing DARPin (designed ankyrin repeat protein). The resultant bioconjugates show no additional toxicity compared to their Cys aryl-based congeners. Finally, we demonstrate how the developed Cys-borylation can enhance the proteolytic stability of the resultant peptide bioconjugates while maintaining the binding affinity to a protein target.
Graphical Abstract
INTRODUCTION
Synthetic bioconjugation has emerged as a powerful tool towards understanding and altering biomolecular interactions. Mimicking the post-translational modifications ubiquitous to natural biological systems, a variety of C-S, C-O, C-N and C-C bond forming reactions have been previously reported.1,2 These synthetic tools have been used to install handles on biomolecules for applications ranging from imaging3,4 to enhancement of therapeutic efficacy.5,6
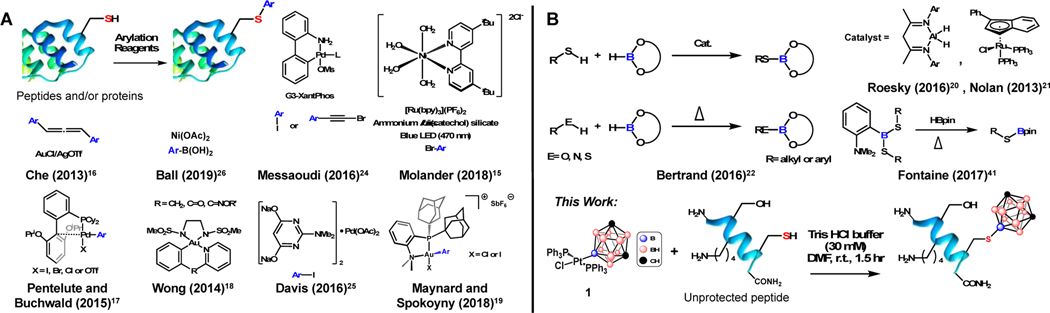
Cysteine (Cys) residues in proteins and peptides represent a historically attractive site for synthetic bioconjugation development owing to its soft nucleophilicity and low natural abundance which contributes to its capacity to undergo site-directed mutagenesis and subsequent selective modification.7 These chemoselective Cys conjugations can be achieved through both metal-free and recently developed metal-mediated routes (Figure 1A). Conjugate addition to Michael acceptors (e. g., maleimides and vinyl sulfones1,2) as well as SN2 reactions with alkyl electrophilic centers1,2 represent the most common metal-free transformations; however, a number of noteworthy other alkylation and arylation strategies have been reported.1,7, 10–16 Recently, transition metal-mediated C-S bond forming reactions have attracted considerable attention due to the rapid kinetics, mild reaction conditions and high functional group tolerance often associated with these metal-based transformations (Figure 1A).1,17 For example, Buchwald, Pentelute and co-workers demonstrated a unique approach towards chemoselective Cys modification via arylation using well-defined and benchtop-stable Pd-based organometallic reagents.17 The reaction conditions were amenable to covalent linkage of fluorescent and affinity tags, drug molecules and handles for further conjugation. This concept was also demonstrated using a stochiometric Au(I/III) platform18,19 as well as several notable catalytic strategies.15,24
Figure 1.
A: Summary of selective C-S bond forming reactions for bioconjugation of unprotected peptides and proteins. Metal-mediated strategies result in thiol arylation with the transferred group highlighted in blue. B: Summary of selective S-B bond forming reactions.
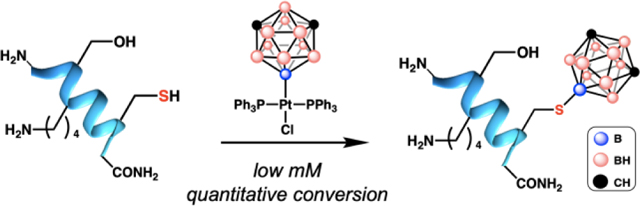
While a large breadth of chemoselective C-S bond forming bioconjugations have been reported, analogous transformations resulting in the formation of boron-sulfur bonds (B-S) are conspicuously absent. While substrates containing free thiols have been borylated through metal-mediated and metal-free routes (Figure 1B), these methods generally lack selectivity for thiols over other competing nucleophilic centers which is a key prerequisite for successful bioconjugation reactions. These include thiol borylation reactions using pinacol, 9-BBN or catechol borane and either aluminum-20 or ruthenium-based21 catalysts, where alcohols and amines can also be competent substrates for borylation under similar catalytic conditions (Figure 1B). Similar selectivity issues arise in uncatalyzed borylation of thiols as evidenced by the work of Bertrand and co-workers.22 In their recent elegant work, Fontaine et al.23 used frustrated Lewis pairs to improve the selectivity of the sulfur borylation of aromatic and aliphatic thiol substrates; however, the use of relatively high temperatures and nonpolar solvents preclude the application of this chemistry towards Cys containing biomolecules. analogous borylation reactions might further expand the biomolecular toolbox. As there are no existing methods competent towards peptide and protein borylation with tricoordinate boron substrates, we hypothesized that these limitations could be overcome by using a different source of boron-based fragment. Icosahedral boranes represent a promising platform to probe this hypothesis given their three-dimensional delocalized aromaticity27–33 analogous to the carbon-based aromatic molecules previously employed for bioconjugation. Here, we show that a Pt(II) complex34 supported by a boron-bound carboranyl cluster (1) is capable of chemoselective Cys borylation across multiple unprotected peptide substrates to generate the first B-S bond linkages post-synthetically, which was a previously inaccessible modality in bioconjugation. Importantly, the borylated peptides were stable towards excess base, acid and external thiol and did not display any appreciable toxicity up to 50 μM in cell culture. In addition to providing a new chemical connectivity, the developed Cys borylation offers new opportunities in areas of multivalent binding and tuning of ligand-receptor interactions in biomolecular targeting.
RESULTS/DISCUSSION
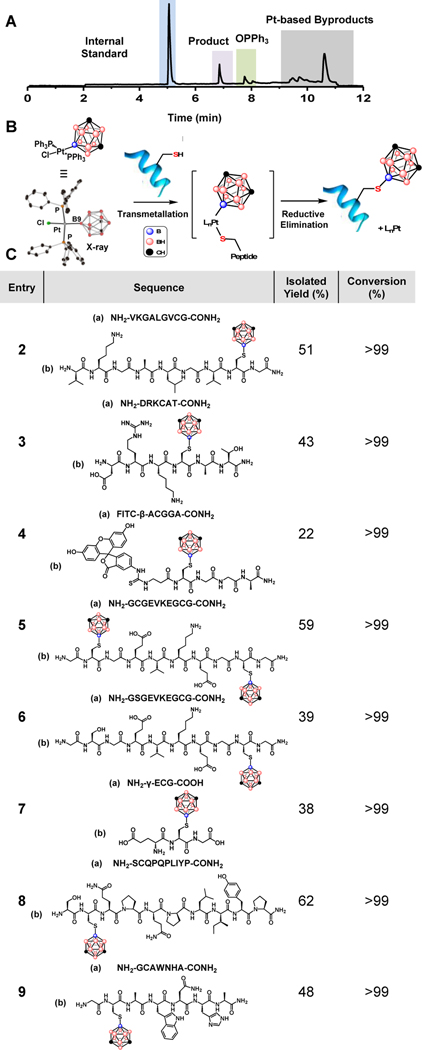
To test whether one can use boron-cluster supported organometallic reagents for successful Cys borylation, we subjected model peptide H2N-VKGALGVCG-CONH2 (2a) with 1 under various conditions. Nearly quantitative conversion to a peptide containing a mass consistent with a Cys-borylated peptide was observed within 1.5 h when 2a (5 mM) was treated with 1.2 equivalents of 1 at 25 °C in the presence of Tris•HCl buffer (30 mM) in dimethylformamide (DMF) as assessed by LC-MS analysis of the crude reaction mixture (Figure 2A). In order to verify that the reaction conditions were selective for Cys over other nucleophiles within the model peptide substrate, tandem MS/MS of Cys-borylated peptide 2b was conducted. Fragmentation patterns support exclusive Cys borylation (Figure S19). Analogous to arylation bioconjugation involving Pd-based reagents17, we propose this chemoselectivity arises from the transmetallation of the soft nucleophilic Cys sulfur residue that has a high propensity towards binding a Pt(II) metal center (Figure 2B).35,36 A subsequent reductive elimination process affords Cys-borylated peptide and Pt-based byproducts (Figure 1B). Importantly, this process proceeds efficiently despite the carboranyl group being significantly more sterically encumbering than the previously demonstrated aryl-based species27 ultimately highlighting the advantages of the organometallic approach used.
Figure 2.
A: Representative LC trace collected after 1 (1.2 equiv) and H2N-VKGALGVCG-CONH2 (5 mM) were allowed to react for 1.5 h at 25 °C in the presence of Tris•HCl buffer (30 mM) in dimethylformamide (DMF). Internal standard was produced through alkylation of H2N-VKGALGVCG-CONH2 (see SI section I). B: Proposed reaction scheme between 1 and cysteine-containing peptide C: Peptide substrate scope with isolated yields (%) and conversion (%).
To ensure generality of this method, we then applied the reaction conditions used to generate 2b towards other peptide sequences (Figure 2C). Across all peptide substrates tested (entries 2–9), we observed nearly quantitative conversion towards the corresponding borylated product with isolated yields ranging from 22–59% after HPLC purification (Figure 2C). Furthermore, the borylation reaction conditions were tolerant to the common labeling dye, fluorescein isothiocyanate (FITC) attached to the N-terminus of the peptide chain despite the presence of a thiourea linkage which could also serve as a chelating ligand towards the metal center (entry 4). Even though carboranyl ligands are sterically bulky, diborylation of a peptide containing two Cys residues is possible; nearly quantitative conversion of 5a to 5b was observed under the optimized reaction conditions (entry 5). Importantly, only monoborylation was observed when 6a, which contains identical residues as 5a except one Cys is mutated to a serine residue, was subjected to identical treatment (Figures S12 and S13). Overall, the borylation selectivity towards Cys thiols in unprotected peptides using 1 as a transfer reagent mirrors the selectivity of both Pd(II) and Au(III) organometallic Cys arylation reagents.17,19
After assuring Cys-borylation was successful across multiple thiol containing peptides, we decided to perform an additional robustness screen for additional reagents that are often used in bioconjugation reactions on peptides. Diluting the DMF reaction mixture with water did not diminish conversion up to 25% water when model peptide H2N-VKGALGVCG-CONH2 (2a) was treated with 1. Reaction mixtures exceeding 25% water resulted in a significant reduction in conversion, likely due to the poor solubility of 1 in water (Figure S17). Further, the bioconjugation was compatible with a common denaturing agent guanidine•HCl (3 M, Figure S18A), suggesting the organometallic complex 1 is stable under highly ionic conditions on the timescale of the reaction. Further, addition of Tris(2-carboxyethyl)phosphine hydrochloride (TCEP HCl), a common reducing agent for disulfide moieties in biomolecules, does not significantly alter the conversion efficiency of the reaction (Figure S18B) indicating excess phosphine does not shut down the reactivity of 1 on the timescale of the reaction.
In order to probe the local environment of the carboranyl cluster on the purified Cys-borylated peptides using 11B NMR spectroscopy, we decided to apply the borylation reaction to a peptide substrate with a low molecular weight to increase the signal to noise outcome of this experiment. Commercially available L-glutathione (7a) was thus chosen for these studies. Successfully Cys-borylated glutathione (7b) was subjected to a routine HPLC purification followed by 11B NMR spectroscopic analysis. A distinct singlet resonance was observed at 2 ppm along with multiple broad doublets in an integral ratio of 1:9 consistent with the splitting pattern of a B(9)-substituted, intact m-carboranyl cluster (Figure S21A). Importantly, the 11B{1H} NMR spectrum of the same sample indicates that the doublets observed stem from the 11B-1H coupling and the resonance at 2 ppm is consistent with a 11B atom that is not bound to a 1H nucleus (Figure S21B). Based on these observations and previously reported NMR spectroscopy data collected on B9 substituted carborane thiols and thioether species34, the resonance at 2 ppm was assigned to the 11B(9)-S nucleus on the intact m-carborane cluster attached to the peptide. Finally, IR spectroscopy was used to further corroborate structural elements of 7b. A diagnostic stretching band ascribed to the B-H bond vibrations on the cluster (2532–2681 cm−1) was observed in samples of 7b and was absent in 7a when both samples were analyzed as powders by ATR IR spectroscopy (Figure S22). Additional evidence that Cys thiol conjugation had proceeded is provided by the disappearance of a free S-H stretch (2454–2545 cm−1) in the spectrum of 7b compared to the spectrum of 7a (Figure S22). The spectroscopic techniques outlined above unequivocally confirm successful B-S bond formation using 1 and unprotected peptides containing Cys residues.
We then extended the bioconjugation to a more complex substrate, DARPin (designed ankyrin repeat protein), which has been previously shown to successfully undergo Cys arylation with various organometallic reagents.17,19 After treatment of DARPin with excess 1 for 6.5 hr, a deconvoluted mass consistent with complete DARPin borylation was observed by LCMS (Figure S23). The capacity to fully conjugate a protein target further highlights the potential of this Pt-mediated route to transfer carboranyl clusters directly to large biomacromolecules at micromolar concentrations.
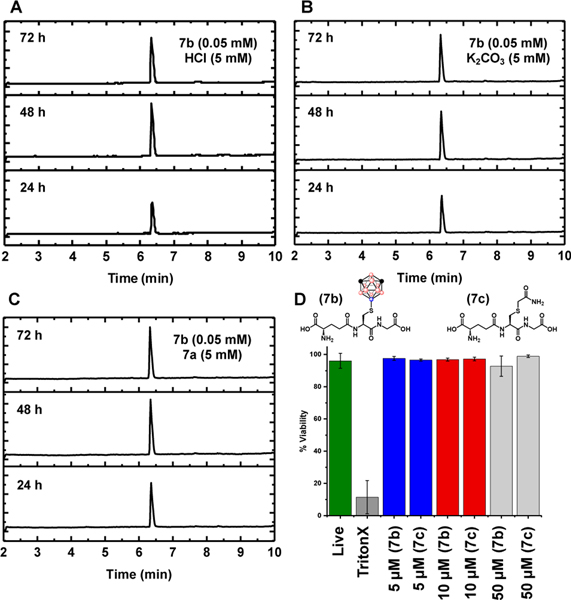
Next, we evaluated the stability of the B-S bond using 7b as a model Cys-borylated peptide. Specifically, we assessed the fidelity of 7b towards an acidic environment with a pH of 2.3 as this value falls within the normal pH range for stomach acid (pH= 1–3).37 Sample of 7b (0.05 mM) was incubated in the presence of hydrochloric acid (5 mM) at 25 °C and 37 °C for 72 hours (Figure 3A). No fragmentation was observed by LC-MS analysis of the mixture, indicating the S-B bond within 7b stays intact at pH 2.3 under the timeframe of the experiment. Further, stability towards highly acidic environments suggests Cys-boryl conjugates could be compatible with harsh reagents used normally to cleave synthesized peptides from solid supports.2i To probe this hypothesis, we treated a model peptide bound to Rink amide resin with excess 1 under buffered conditions for 1.5 hr (SI Figure S24). Resin was then isolated and peptide was globally cleaved using a standard solution of trifluoroactetic acid, water and triisopropyl silane (90%, 5%, 5% v/v) (SI Section VIII). Full conversion of Cys-borylated peptide was observed by LC-MS (SI Figure S24) indicating the bioconjugation could be also successfully applied to peptides appended to the solid supports. After assessment of stability towards acid, we probed the resistance of 7b towards basic conditions. While intracellular pH remains close to neutral, published reports suggest alkaline microenvironments could be important in cellular signaling.38 To rigorously determine the tolerance of Cys-borylated peptides towards base, we incubated 7b at pH 11, a significantly more alkaline environment than would be anticipated for human cells. Sample of 7b (0.05 mM) was incubated in the presence of potassium carbonate (5 mM) at 25 °C and 37 °C for 72 hours. No fragmentation was observed by LC-MS analysis of the mixture, indicating the stability of the S-B bond within 7b (Figure 3B). Finally, we evaluated the stability of 7b towards external thiol source. Glutathione (7a) was selected as it represents the most abundant non-protein source of thiol in eukaryotic cells with intracellular concentrations ranging from 1–10 mM.39 Sample of 7b (0.05 mM) was incubated in the presence of 7a (5 mM) at 25 °C and 37 °C for 72 hours. No fragmentation was observed by LC-MS analysis of the mixture, indicating the S-B bond within 7b does not undergo thiol exchange chemistry in the presence of large excess of external thiol (Figure 3C). Importantly, this stability towards external thiol is in contrast to classical Cys-based bioconjugation tools that employ Michael acceptors which often undergo retro-Michael addition when subjected to the presence of other thiol substrates.1 Altogether, the stability of 7b demonstrated herein suggests the S-B bond within Cys-borylated peptides can be bioorthogonal towards harsh biological milieu.
Figure 3.
LC-MS traces of 7b incubated with 100 excess A: hydrochloric acid B: potassium carbonate and C: 7a after 24 hrs at r.t. followed by 48 and 72 hrs at 37 °C (See SI Section VII for full details). D: The % cell viability assessed after four-hour incubation of 7b and 7c with Chinese Hamster Ovarian (CHO) cells at 5 μM, 10 μM and 50 μM concentration of analyte (See SI Section IX for full details).
After confirming the stability of these constructs, we decided to evaluate the toxicity of Cys-borylated peptides towards Chinese Hamster Ovarian (CHO) cells using 7b as a model peptide. As Pt(II) complexes have well described toxicity40, we first assessed the efficiency of the purification method towards removing all metal-based byproducts. ICP-AES analysis of 7b purified by reversed-phase HPLC indicated >99.9% platinum content had been removed (See SI Section II, Figure S1). Four-hour incubation of pure, isolated 7b with CHO cells resulted in no appreciable toxicity up to 50 μM (Figure 3D). As a control sample, acetamide-glutathione (7c) was also incubated under the same conditions and exhibited no appreciable toxicity up to 50 μM (Figure 3D). The similar cell viability profiles for Cys-boryl glutathione and Cys-acetamide glutathione suggest no inherent cytotoxicity is added through forging a B-S bond by appending a boron cluster to unprotected peptides.
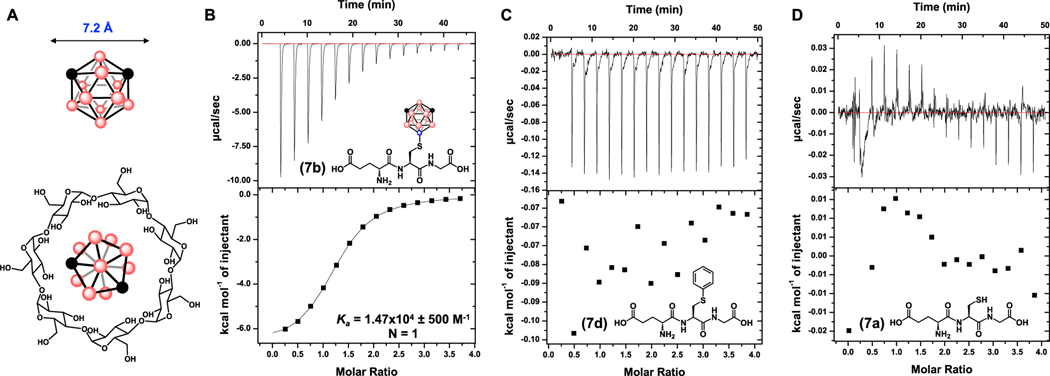
Few post translational modifications are competent towards the chemoselective transfer of bulky substrates containing large cone angles directly at amino acid sites. A rare example was demonstrated independently by both Park41 and Davis42 who prepared tert-Leu residues via dehydroalanine; however, the bioconjugates formed are racemates. The present strategy offers the first organometallic-based route towards unprotected peptide modification with a highly bulky moiety that does not alter the local stereochemistry, affording unique opportunities to assess the role steric encumbering at amino acid sites has upon downstream peptidic properties. Specifically, we probed the capacity of Cys-boryl peptides to participate in receptor binding by titrating 7b as a model peptide to β-cyclodextrin, a sugar macrocycle which is widely used in catalysis, analytical separation and solubilization.43 Isothermal titration calorimetry (ITC) experiment was carried out where peptide 7b (10 mM) was titrated to β-cyclodextrin (0.25 mM) in an aqueous buffered solution at pH 3.4 revealing a binding stoichiometry (N = 1) and an association constant (Ka = 1.47 × 104 ± 500 M−1) (Figure 4A and 4B). The approximated binding stoichiometry of N= 1 is consistent with a 1:1 7b⊂β-cyclodextrin inclusion complex promoted by the chaotropic properties of the cluster cage inserting into the macrocycle cavity.44,45 The observed Ka is an order of magnitude higher than was observed between unmodified m-carborane and β-cyclodextrin when measured by a displacement binding technique46, which could be attributed to the enhanced solubility of 7b over unmodified m-carborane in aqueous media. Cys-modified S-phenyl glutathione (7d, Figure 4C) and 7a (Figure 4D) were used as controls and did not exhibit any appreciable binding affinity to β-cyclodextrin by ITC under the same measurement conditions. These controls highlight the receptor specificity and the capacity of borylation to engender a unique binding profile between 7b and a macrocyclic sugar.
Figure 4.
A: Representation of the inclusion complex formed between m-carborane and β-cyclodextrin.53,54 B: ITC binding plot for carboranylated glutathione and β-cyclodextrin to yield an association constant (Ka = 1.47 × 104 ± 500 M−1) and binding stoichiometry (N = 1). C: ITC binding plot for phenyl-glutathione and β-cyclodextrin. D: ITC binding plot for unmodified glutathione and β-cyclodextrin.
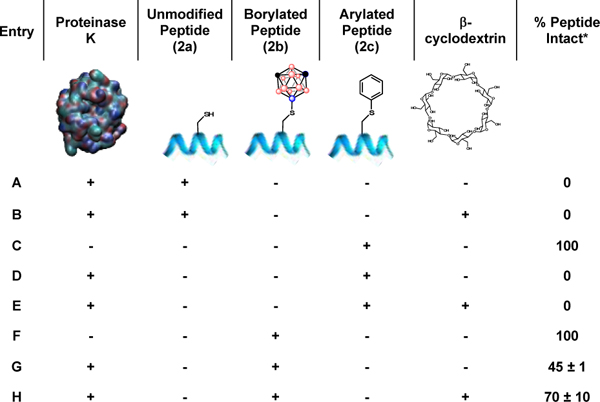
Finally, we evaluated the stability of Cys-boryl peptides towards a broadly acting serine protease, Proteinase K. Specifically, Cys-boryl peptide 2b was chosen as a model substrate owing to the many aliphatic amino acid residues in the sequence which are cleavable by Proteinase K.46 We envisaged 2b might resist the activity of Proteinase K to a higher degree than 2a owing to the steric hindrance of the carboranyl group positioned proximally to the peptide backbone thereby blocking access to cleavable residues. To test this hypothesis, 2b was incubated with Proteinase K for 5 min at 60 °C in the presence of Tris•HCl buffer (50 mM, pH=8.2) and CaCl2 (5 mM). After treatment, 45 ± 1% 2b remained intact as assessed by integration of the peak compared to integration of the internal standard (Table 1, Entry G). In contrast, no intact peptide remained when 2a was subjected to the same reaction conditions (Table 1, Entry A), indicating Cys-borylation is competent towards inhibiting the action of Proteinase K within this timeframe. We then subjected Cys-aryl peptide 2c to Proteinase K treatment for 5 min at 60 °C in the presence of buffer (50 mM) and CaCl2 (5 mM) (Table 1, Entry D). No intact peptide was observed under these conditions, suggesting inhibition of proteolytic degradation is unique to Cys-boryl conjugates compared to aryl-based congeners.
Table 1.
The reaction schemes and the observed corresponding degradation of 2a, 2band 2c with Proteinase K under various conditions.
|
To extend the steric hindrance surrounding the Cys moiety, we next subjected the 2b⊂β-cyclodextrin inclusion complex to Proteinase K treatment (see SI for further details on the characterization of 2b⊂β-cyclodextrin). The complex 2b⊂β-cyclodextrin was heated for 5 min at 60 °C in the presence of buffered Proteinase K (Tris•HCl, 50 mM, pH=8.2) and CaCl2 (5 mM). After this treatment, 70 ± 10% intact 2b remained (Table 1, Entry H). Importantly, incubation of 2a and 2c with β-cyclodextrin prior to treatment with the same Proteinase K reaction conditions resulted in full degradation (Table 1, Entries B and E), indicating the presence of β-cyclodextrin alone is insufficient towards inhibiting degradation. Overall, these experiments suggest that Cys-borylation can render peptides more proteolytically stable.
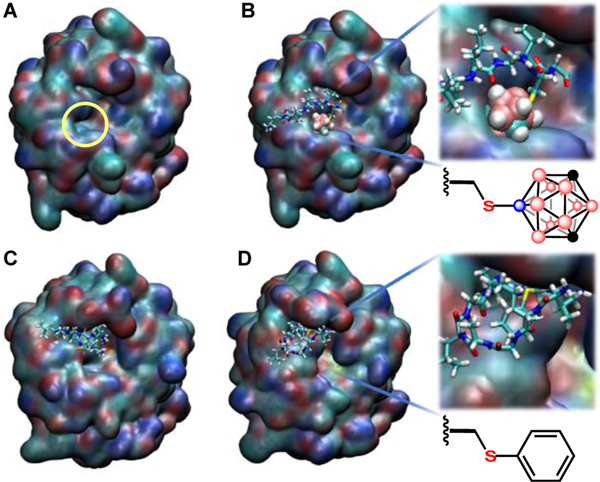
Molecular dynamics (MD) simulations were used to elucidate possible nature of the stability enhancement towards proteolysis. MD simulations of 2a, 2b and 2c with Proteinase K demonstrated stable binding of all peptides to the reported substrate recognition site47 by two segments, Gly100 to Tyr104 and Ser132 to Gly136 (Movies 1-3). A second binding pocket (Figure 5A) formed by Gly134-Gly135-Gly-136 and Gly160-Asn161-Asn162 was identified for the unique capacity to bind the carborane of 2b as a result of B-H···H-N dihydrogen48 and C-H···O or C-H···N hydrogen bonding interactions27 (Figures 5B, S40-41). This binding might prevent the peptide from approaching the Asp39-His69-Ser224 catalytic triad, since 2b was on average 2.12 Å further away from the catalytic triad than 2a over the entire simulation timeframe (Figures 5C, S43). Importantly, 2c did not exhibit stable binding to the pocket formed by Gly134-Gly135-Gly-136 and Gly160-Asn161-Asn162 (Movie 3, Figures 5D, S42-43) indicating the proteolytic protection is unique to Cys-borylation and is likely a result of placing a sterically encumbering functional group like carborane in close proximity to the polypeptide chain.
Figure 5.
Molecular dynamics simulations of A: the secondary binding pocket identified as an important docking site B: 2b binding with Proteinase K, C: 2a binding with Proteinase K and D: 2c binding with Proteinase K at 120 ns of equilibration. Proteinase K is represented using QuickSurf representation in VMD.
While Cys-borylation can render peptides more resistant towards proteolytic degradation, we wondered whether one can design hybrid agents with preserved binding capabilities to the protein target. To probe protein binding, we designed a model peptide (8a) containing a Ile-Tyr-Pro sequence which was previously identified as active towards inhibiting the activity of angiotensin-converting enzyme (ACE)49, an important protein class implicated in the regulation of blood pressure and, recently, SARS-CoV-2 infection.50 Specifically, at 5 μM unmodified 8a and Cys-borylated 8b were found to inhibit the activity of ACE by 24 ± 5% and 17 ± 4%, respectively (See SI Section XII). Increasing the concentration of 8a and 8b to 50 μM enhanced the inhibition of ACE activity by 54 ± 7% and 52 ± 1%, respectively (See SI Section XII). The observed similarity in rates of inhibition between 8a and 8b suggests that Cys-borylation could maintain binding efficacy of the therapeutically relevant peptides while at the same time enhancing their proteolytic stability.
CONCLUSION
In conclusion, an organometallic strategy for borylating Cys residues within unprotected peptide sequences and a model protein has been demonstrated. This work significantly expands our fundamental ability to construct new bioconjugates via a Cys residue by introducing a new room-temperature boron-sulfur forming pathway in unprotected peptides. Importantly, Cys-borylation can engender stable hybrid peptides featuring unique recognition and binding properties towards a macrocyclic sugar as well as render the resulting peptides more proteolytically stable while maintaining their binding affinity towards a protein target. This work further demonstrates the growing importance of organometallic chemistry in the field of bioconjugation,51–60 where a designer post-translational synthetic modification can forge a chemoselective delivery of a sterically encumbering, abiotic functional group (e.g., carborane) directly to a native amino acid residue in a complex biomolecule.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health (NIH) Maximizing Investigators Research Award (MIRA, R35GM124746). A. M. S. is a Research Corporation for Science Advancement (RCSA) Cottrell Scholar and a Dreyfus Foundation Camille Dreyfus Teacher Scholar. Authors thank Mr. Nicholas Bernier for assistance with ICP-AE experiments.
Footnotes
Supporting Information
Data supporting the results and conclusions are available within the paper and the Supplementary Information (SI).
Experimental procedures and characterization for all new compounds (PDF)
Notes
The authors declare no competing financial interests.
REFERENCES
- (1).Hermanson GT Bioconjugate Techniques, 3rd ed.; Academic Press: San Diego, CA, 2013. [Google Scholar]
- (2).(a) Recent representative reviews on general methods: Stephanopoulos N & Francis MB Choosing an Effective Protein Bioconjugation Strategy. Nature Chem. Bio 2011, 7, 876–884. [DOI] [PubMed] [Google Scholar]; (b) McKay CS & Finn MG Click Chemistry in Complex Mixtures: Biorthogonal Bioconjugation. Chem. Biol 2014, 21, 1075–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Boutureira O & Bernardes GJL Advances in Chemical Protein Modification. Chem. Rev 2015, 115, 2174–2195. [DOI] [PubMed] [Google Scholar]; (d) Albaba B & Metzler-Nolte N Organometallic-Peptide Bioconjugates: Synthetic Strategies and Medicinal Applications. Chem. Rev 2016, 116, 11797–11839. [DOI] [PubMed] [Google Scholar]; (e) Hu Q-Y; Berti F; Adamo R Towards the Next Generation of Biomedicines by Site-Selective Conjugation. Chem. Soc. Rev 2016, 45, 1691–1719. [DOI] [PubMed] [Google Scholar]; (f) Vinogradova EV Organometallic Chemical Biology: An Organometallic Approach to Bioconjugation. Pure & Appl. Chem 2017, 89, 1619–1640. [Google Scholar]; (g) Jbara M; Maity SK; Brik A Palladium in the Chemical Synthesis and Modification of Proteins. Angew. Chem., Int. Ed 2017, 56, 10644–10655. [DOI] [PubMed] [Google Scholar]; (h) Ohata J; Martin SC; Ball ZT Metal-Mediated Functionalization of Natural Peptides and Proteins: Panning for Bioconjugation Gold. Angew. Chem., Int. Ed 2019, 58, 6176–6199. [DOI] [PubMed] [Google Scholar]; (i) Conibear AC; Watson EE; Payne RJ; Becker CFW Native Chemical Ligation in Protein Synthesis and Semi-Synthesis. Chem. Soc. Rev 2019, 47, 9046–9068. [DOI] [PubMed] [Google Scholar]; (j) Isenegger PG & Davis BG Concepts of catalysis in site-selective protein modifications. J. Am. Chem. Soc 2020, 141, 8005–8013. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Latocheski E; Dal Forno GM; Ferreira TM; Oliveira BL; Bernardes GJL; Domingos JB Mechanistic insights into transition metal-mediated bioorthogonal uncaging reactions Chem. Soc. Rev 2020, 49, 7710–7729. [DOI] [PubMed] [Google Scholar]; (l) Szijj PA; Kostadinova KA; Spears RJ; Chudasama V Tyrosine Bioconjugation - An Emergent Alternative. Org. Biomol. Chem 2020, 18, 9018–9028. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Patra M & Gasser G Organometallic Compounds: An Opportunity for Chemical Biology? ChemBioChem 2021, 13, 1232–1252. [DOI] [PubMed] [Google Scholar]
- (3).Lavis LD Teaching Old Dyes New Tricks: Biological Probes Built from Fluoresceins and Rhodamines. Annu. Rev. Biochem 2017, 86, 825–43. [DOI] [PubMed] [Google Scholar]
- (4).Li Y Commonly Used Tag Combinations for Tandem Affinity Purification. Biotechnol. Appl. Biochem 2010, 55, 73–83. [DOI] [PubMed] [Google Scholar]
- (5).Liu H; Bolleddula J; Nichols A; Tang L; Zhao Z; Prakash C Metabolism of Bioconjugate Therapeutics: Why, When and How? Drug Metab. Rev 2020, 52, 66–124. [DOI] [PubMed] [Google Scholar]
- (6).Acar H; Ting JM; Srivastava S; LaBelle JL; Tirrell MV Molecular Engineering Solutions for Therapeutic Peptide Delivery. Chem. Soc. Rev 2017, 46, 6553–6569. [DOI] [PubMed] [Google Scholar]
- (7).Chalker JM; Bernardes GJL; Lin YA; Davis BG Chemical Modification of Proteins at Cysteine: Opportunities in Chemistry and Biology. Chem. Asian J 2009, 4, 630–640. [DOI] [PubMed] [Google Scholar]
- (8).Masri MS & Friedman M Protein Reactions with Methyl and Ethyl Vinyl Sulfones. J. Protein Chem 1988, 7, 49–54. [DOI] [PubMed] [Google Scholar]
- (9).Morales-Sanfrutos J; Lopez-Jaramillo J; Ortega-Muñoz M; Megia-Fernandez A; Perez-Balderas F; Hernandez-Mateo F; Santoyo-Gonzales F Vinyl Sulfone: a Versatile Function for Simple Bioconjugation and Immobilization. Org. Biomol. Chem 2010, 8, 667–675. [DOI] [PubMed] [Google Scholar]
- (10).Spokoyny AM; Zou Y; Ling JJ; Yu H; Lin Y-S; Pentelute BL A Perfluoroaryl-Cysteine SNAr Chemistry Approach to Unprotected Peptide Stapling. J. Am. Chem. Soc 2013, 135, 5946–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Kalhor-Monfared S; Jafari MR; Patterson JT; Kitov PI; Dwyer JJ; Nuss JM; Derda R Rapid Biocompatible Macrocyclization of Peptides with Decafluorodiphenylsulfone. Chem. Sci 2016, 7, 3785–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Valkevich EM; Guenette RG; Sanchez NA; Chen Y-C; Ge Y; Strieter ER Forging Isopeptide Bonds Using Thiol-Ene Chemistry: Site-Specific Coupling of Ubiquitin Molecules for Studying the Activity of Isopeptidases. J. Am. Chem. Soc 2012, 134, 6916–6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Conte ML; Staderini S; Marra A; Sanchez-Navarro M; Davis BG; Dondoni A Multi-molecule Reaction of Serum Albumin Can Occur Through Thiol-yne Coupling. Chem. Commun 2011, 47, 11086–11088. [DOI] [PubMed] [Google Scholar]
- (14).Bottecchia C; Rubens M; Gunnoo SB; Hessel V; Madder A; Noël T Visible-Light-Mediated Selective Arylation of Cysteine in Batch and Flow. Angew. Chem., Int. Ed 2017, 56, 12702–12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Vara BA; Li X; Berritt S; Walters CR; Petersson EJ; Molander GA Scalable Thioarylation of Unprotected Peptides and Biomolecules Under Ni/Photoredox Catalysis. Chem. Sci 2018, 9, 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chan AO-Y; Tsai JL-L; Lo VK-Y; Li L-G; Wong M-K; Che C-M Gold-mediated Selective Cysteine Modification of Peptides Using Allenes. Chem. Commun 2013, 49, 1428–1430. [DOI] [PubMed] [Google Scholar]
- (17).Vinogradova EV; Zhang C; Spokoyny AM; Pentelute BL; Buchwald SL Organometallic Palladium Reagents for Cysteine Bioconjugation. Nature 2015, 526, 687–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Kung KK-Y; Ko H-M; Cui J-F; Chong H-C; Leung Y-C; Wong M-K Cyclometalated Gold(III) Complexes for Chemoselective Cysteine Modification via Ligand Controlled C-S Bond-forming Reductive Elimination. Chem. Commun 2014, 50, 11899–11902. [DOI] [PubMed] [Google Scholar]
- (19).Messina MS; Stauber JM; Waddington MA; Rheingold AL; Maynard HD; Spokoyny AM Organometallic Gold(III) Reagents for Cysteine Arylation. J. Am. Chem. Soc 2018, 140, 7065–7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Yang Z; Zhong M; Ma X; Nijesh K; De S; Parameswaran P; Roesky HW An Aluminum Dihydride Working as a Catalyst in Hydroboration and Dehydrocoupling. J. Am. Chem. Soc 2016, 138, 2548–2551. [DOI] [PubMed] [Google Scholar]
- (21).Fernández-Salas JA; Manzini S; Nolan SP Efficient Ruthenium-Catalysed S-S, S-Si and S-B Bond Forming Reactions. Chem. Commun 2013, 49, 5829–5831. [DOI] [PubMed] [Google Scholar]
- (22).Romero EA; Peltier JL; Jazzar R; Bertrand G Catalyst-free Dehydrocoupling of Amines, Alcohols, and Thiols with Pinacol Borane and 9-Borabicyclononane (9-BBN). Chem. Commun 2016, 52, 10563–10565. [DOI] [PubMed] [Google Scholar]
- (23).Rochette É; Boutin H; Fontaine F-G Frustrated Lewis Pair Catalyzed S-H Bond Borylation. Organometallics 2017, 36, 2870–2876. [Google Scholar]
- (24).Al-Shuaeeb RAA; Kolodych S; Koniev O; Delacroix S; Erb S; Nicoläy S; Cintrat J-C; Brion J; Cianférani S; Alami M; Wagner A; Messaoudi S Palladium-Catalyzed Chemoselective and Biocompatible Functionalization of Cysteine-Containing Molecules at Room Temperature. Chem. Eur. J 2016, 22, 11365–11370. [DOI] [PubMed] [Google Scholar]
- (25).Willwacher J; Raj R; Mohammed S; Davis BG Selective Metal-Site-Guided Arylation of Proteins. J. Am. Chem. Soc 2016, 138, 8678–8681. [DOI] [PubMed] [Google Scholar]
- (26).Hanaya K; Ohata J; Miller MK; Mangubat-Medina AE; Swierczynski MJ; Yang DC; Rosenthal RM; Popp BV; Ball ZT Rapid Nickel(II)-Promoted Cysteine S-Arylation with Arylboronic Acids. Chem. Commun 2019, 55, 2841–2844. [DOI] [PubMed] [Google Scholar]
- (27).Scholz M & Hey-Hawkins E Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies. Chem. Rev 2011, 111, 7035–7062. [DOI] [PubMed] [Google Scholar]
- (28).Leitao EM; Jurca T; Manners I Catalysis in Service of Main Group Chemistry Offers a Versatile Approach to p-Block Molecules and Materials. Nature Chem. 2013, 5, 817–829. [DOI] [PubMed] [Google Scholar]
- (29).Keener M; Hunt C; Carroll TG; Kampel V; Dobrovetsky R; Hayton TW; Ménard G Redox-Switchable Carboranes for Uranium Capture and Release. Nature 2020, 577, 652–655. [DOI] [PubMed] [Google Scholar]
- (30).Fisher SP; Tomich AW; Lovera SO; Kleinsasser JF; Guo J; Asay MJ; Nelson HM; Lavallo V Nonclassical Applications of closo-Carborane Anions: From Main Group Chemistry and Catalysis to Energy Storage. Chem. Rev 2019, 119, 8262–8290. [DOI] [PubMed] [Google Scholar]
- (31).Quan Y & Xie Z Controlled Functionalization of o-Carborane via Transition Metal Catalyzed B-H Activation. Chem. Rev 2019, 48, 3660–3673. [DOI] [PubMed] [Google Scholar]
- (32).Lin F; Yu J-L; Shen Y; Zhang S-Q; Spingler B; Liu J; Hong X; Duttwyler S Palladium-Catalyzed Selective Five-Fold Cascade Arylation of the 12-Vertex Monocarborane Anion by B-H Activation. J. Am. Chem. Soc 2018, 140, 13798–13807. [DOI] [PubMed] [Google Scholar]
- (33).Yin Y; Ochi N; Craven TW; Baker D; Takigawa N; Suga H De Novo Carborane-Containing Macrocyclic Peptides Targeting Human Epidermal Growth Factor Receptor. J. Am. Chem. Soc 2019, 141, 19193–19197. [DOI] [PubMed] [Google Scholar]
- (34).Saleh LMA; Dziedzic RM; Khan SI; Spokoyny AM Forging Unsupported Metal-Boryl Bonds with Icosahedral Carboranes. Chem. Eur. J 2016, 22, 8466–8470. [DOI] [PubMed] [Google Scholar]
- (35).Pearson RG The Principle of Maximum Hardness. Acc. Chem. Res 1993, 26, 250–255. [Google Scholar]
- (36).Dedon PC & Borch RF Characterization of the Reactions of Platinum Antitumor Agents with Biologic and Nonbiologic Sulfur-Containing Nucleophiles. Biochem. Pharmacol 1987, 36, 1955–1964. [DOI] [PubMed] [Google Scholar]
- (37).Machen TE & Paradiso AM Regulation of Intracellular pH in the Stomach. Ann. Rev. Physiol 1987, 49,19–33. [DOI] [PubMed] [Google Scholar]
- (38).Ro H-A & Carson JH pH Microdomains in Oligodendrocytes. J. Biol. Chem 2004, 279, 37115–37123. [DOI] [PubMed] [Google Scholar]
- (39).Forman HJ; Zhang H; Rinna A Glutathione: Overview of its Protective Roles, Measurement, and Biosynthesis. Mol. Aspects Med 2009, 30, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Hartmann JT & Lipp H Toxicity of Platinum Compounds. Expert Opin. Pharmacol 2003, 6, 889–901. [DOI] [PubMed] [Google Scholar]
- (41).Yang A; Ha S; Ahn J; Kim R; Kim S; Lee Y; Kim J; Söll D; Lee H-Y; Park H-S A Chemical Biology Route to Site-Specific Authentic Protein Modifications. Science 2016, 354, 623–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Wright TH; Bower BJ; Chalker JM; Bernardes GJL; Wiewiora R; Ng W-L; Raj R; Faulkner S; Vallée MRJ; Phanumartwiwath A; Coleman OD; Thézénas M-L; Khan M; Galan SRG; Lercher L; Schombs MW; Gerstberger S; Palm-Espling ME; Baldwin AJ; Kessler BM; Claridge TDW; Mohammed S; Davis BG Posttranslational Mutagenesis: A Chemical Strategy for Exploring Protein Side-Chain Diversity. Science 2016, 354, aag1465-1–aag1465–11. [DOI] [PubMed] [Google Scholar]
- (43).Singh M; Sharma R; Banerjess UC Biotechnological Applications of Cyclodextrins. Biotechnol. Adv 2002, 20, 341–359. [DOI] [PubMed] [Google Scholar]
- (44).Assaf KI & Nau WM The Chaotropic Effect as an Assembly Motif in Chemistry. Angew. Chem., Int. Ed 2018, 57, 13968–13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Sadrerafi K; Moore EE; Lee MW Association Constant of β-cyclodextrin with Carboranes, Adamantane, and Their Derivatives Using Displacement Binding Technique. J. Incl. Phenom. Macrocycl. Chem 2015, 83, 159–166. [Google Scholar]
- (46).Ebeling W; Hennrich N; Klockow M; Metz H; Orth HD; Lang H Proteinase K From Tritirachium Album Limber. Eur. J. Biochem 1974, 47, 91–97. [DOI] [PubMed] [Google Scholar]
- (47).Wolfs WM; Bajorath J; Muller A; Raghunathan S; Singh TP; Hinrichs W; Saenger W Inhibition of Proteinase K by Methoxysuccinyl-Ala-Ala-Pro-Ala-Chloromethyl Ketone. J. Biol. Chem 1991, 266, 17695–17699. [DOI] [PubMed] [Google Scholar]
- (48).Fanfrlík J; Lepšík M; Horinek D; Havlas Z; Hobza P Interaction of Carboranes with Biomolecules: Formation of Dihydrogen Bonds. ChemPhysChem 2006, 7, 1100–1105. [DOI] [PubMed] [Google Scholar]
- (49).Kohmura M; Nio N; Kubo K; Minoshima Y; Munekata E; Ariyoshi Y Inhibition of Angiotensin-Coverting Enzyme by Synthetic Peptides of Human β-Casein. Agric. Biol. Chem 1989, 53, 2107–2114. [Google Scholar]
- (50).Ghafouri-Farda S; Noroozib R; Omrania MD; Branickib W; Pośpiechb E; Sayada A; Pyrcb K; Łabajb PP; Vafaeec R; Taherid M; Sanak M Angiotensin Converting Enzyme: A review on Expression Profile and its Association with Human Disorders with Special Focus on SARS-CoV-2 Infection. Vascul. Pharmacol 2020, 130, 106680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Antos JM; McFarland JM.; Iavarone AT; Francis MB Chemoselective Tryptophan Labeling with Rhodium Carbenois at Mild pH. J. Am. Chem. Soc 2009, 131, 6301–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Schischko A; Ren H; Kaplaneris N; Ackermann L Bioorthogonal Diversification of Peptides through Selective Ruthenium(II) Catalyed C-H Activation. Angew. Chem., Int. Ed 2017, 129, 1598–1602. [DOI] [PubMed] [Google Scholar]
- (53).Bauer M; Wang W; Lorion MM; Dong C; Ackermann L Internal Peptide Late-Stage Diversification: Peptide-Isosteric Triazoles for Primary and Secondary C(sp3)-H Activation. Angew. Chem., Int. Ed 2018, 57, 203–207. [DOI] [PubMed] [Google Scholar]
- (54).Lee HG; Lautrette G; Pentelute BL; Buchwald SL Palladium-Mediated Arylation of Lysine in Unprotected Peptides. Angew. Chem., Int. Ed 2017, 56, 3177–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Kubota K; Dai P; Pentelute BL; Buchwald SL Palladium Oxidative Addition Complexes for Peptide and Protein Cross-Linking. J. Am. Chem. Soc 2018, 140, 3128–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Stenton BJ; Oliveira BL; Matos MJ; Sinatra L; Bernardes GJ L. A Thioether-Directed Palladium-Cleavable Linker for Targeted Biorthogonal Drug Decaging. Chem. Sci 2018, 9, 4184–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Zhao W; Lee HG; Buchwald SL; Hooker JM Direct 11CN-Labeling of Unprotected Peptides via Palladium-Mediated Sequential Cross-Coupling Reactions. J. Am. Chem. Soc 2017, 139, 7152–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Schlatzer T; Kriegesmann J; Schroder H; Trobe M; Lembacher-Fadum C; Santner S; Kravchuk AV; Becker CFW; Breinbauer R Labeling and Natural Post-Translational Modification of Peptides and Proteins via Chemoselective Pd-Catalyzed Prenylation of Cysteine. J. Am. Chem. Soc 2019, 141, 14931–14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Stauber JM; Qian EA; Han Y; Rheingold AL; Kral P; Fujita D; Spokoyny AM An Organometallic Strategy for Assembling Atomically Precise Hybrid Nanomaterials. J. Am. Chem. Soc 2020, 142, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Dhanjee HH; Saebi A; Buslov I; Loftis AR; Buchwald SL; Pentelute BL Protein-Protein Cross-Coupling via Palladium-Protein Oxidative Addition from Cysteine Residues. J. Am. Chem. Soc 2020, 142, 9124–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.