Abstract
Purpose:
To measure financial toxicity and explore its association with quality of life (QOL) in an emerging population of survivors: advanced melanoma patients treated with immunotherapy.
Design:
Cross-sectional survey and medical record review.
Sample:
106 survivors (39% response). Median time since start of immunotherapy was 36.4 months (range: 14.2, 133.9).
Methods:
The Comprehensive Score for Financial Toxicity measured financial toxicity, and the EORTC-QLQ30 assessed QOL and functioning across five domains. Data were collected online, by phone, or in clinic.
Findings:
Younger patients (<65 years) reported higher financial toxicity (p<.001) than older patients. Controlling for age, financial toxicity was correlated with QOL (p<.001), financial difficulties (p<.001), and EORTC-QLQ30 functioning subscales.
Conclusions:
Given the demonstrated association between financial toxicity and QOL, our study highlights the importance of addressing financial toxicity, particularly among patients receiving high-cost treatments.
Implications for Psychosocial Providers:
Providers should educate patients and their caregivers about cost-management techniques, link them with available resources, and provide psychosocial counseling to alleviate related distress.
Keywords: financial toxicity, quality of life, melanoma, immunotherapy
Introduction
Immune checkpoint inhibitors (CI) have changed approaches to the treatment of advanced (unresectable stage III or IV) melanoma and have led, for some patients, to prolonged survival.1 These immunotherapies, which include ipilimumab, the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitor, and nivolumab and pembrolizumab, programmed cell death 1 (PD-1) inhibitors, allow for targeted treatment that typically yields fewer side effects than standard chemotherapeutic regimens. Such advances in treatment, however, come at high financial costs—with most estimates of yearly CI price tags exceeding 100,000 USD.2, 3
Across all cancer types, higher costs of drugs have led to increases in expense-sharing between insurers and patients, resulting in rising out-of-pocket expenses for patients (higher deductibles, co-pays, etc.).2, 4 When combined with the potential loss of income due to an inability to work, these higher expenses have resulted in a growing incidence of financial toxicity in patients and their caregivers.4 Financial toxicity refers to the financial distress that results from cancer treatment.5, 6 Emerging frameworks have characterized financial toxicity as having both objective and subjective components: the objective experience is the measurable financial impact of treatment (e.g., out-of-pocket copays and medications; lost wages, etc.), while the subjective experience refers to the psychosocial impact of the resulting negative financial outcomes [e.g., lower quality of life (QOL), mental health issues, etc.].4, 5 Although previous literature describes the negative impact of financial toxicity on QOL among other cancer types, this association is poorly described among advanced melanoma patients and survivors treated with CI, who represent an emergent population of cancer survivors.2,7, 8 This analysis reports financial toxicity in a sample of these patients and survivors and explores the association between financial toxicity and QOL. The data presented here are part of a larger study to describe QOL and immune-related adverse events in advanced melanoma survivors treated with CI.9
Methods
Data collection and recruitment
This was an exploratory cross-sectional survey of patients diagnosed with advanced (unresectable stage III or IV) melanoma treated with a CI beginning at least 12 months prior to study enrollment. The Memorial Sloan Kettering Cancer Center Institutional Review Board approved the study. Patients were identified via an institutional database of patients treated with CI (either on or off protocol), and they were eligible for participation if they had been diagnosed over age 18, read and spoke English, and had not received other systemic therapy after CI initiation. We excluded patients who experienced symptomatic progression. Interested patients provided informed consent and completed study measures online via REDCap (Research Electronic Data Capture), over the phone with a member of the research team, or in person at a clinic visit.10
Measures
We assessed financial toxicity using the Comprehensive Score for Financial Toxicity (COST), a patient-reported outcome measure of financial distress in cancer patients.11 Respondents ranked agreement (0=“Not at all,” 4=“Very much”) with 11 items relating to both objective and subjective aspects of financial toxicity. We created a summed composite score, with lower scores indicating greater financial toxicity.
Quality of life was measured using the EORTC-QLQ30, a widely-used tool to assesses global QOL, symptom burden, financial difficulty, and social, emotional, physical, role, and cognitive functioning.12 For global QOL, respondents selected a response from 1=“Bad” to 7=“Excellent”; for the functioning subscales and level of financial difficulty, respondents ranked agreement on a 4-point scale (1=“Not at all,” 4=“Very much”). For analysis, responses were reversed as appropriate and scaled 0–100 so that higher scores represented positive outcomes; we do not report symptom burden in this analysis.
Statistical Analyses
We used descriptive statistics to characterize the data. Independent sample t-tests and chi-square tests were used to evaluate associations between demographic (age, gender, race, language) and clinical variables [treatment type, length, or status (on-treatment vs. off)] and COST and EORTC-QLQ30 subscale scores. To assess the relationship between financial toxicity and QOL, we calculated Pearson correlation coefficients and partial correlations that controlled for age. We computed Cronbach’s alpha to determine the internal consistency of the COST measure and EORTC-QLQ30 subscales. Analysis was conducted using IBM SPSS Statistics 25, and figures were produced using the Likert package in R, version 3.6.1.13
Findings
Sample demographics
One hundred six patients and survivors participated (39% response rate). Most participants were white (94%), and 43% of respondents were female. Mean age at survey completion was 63.0 years [standard deviation (sd)=12.54]; mean age at CI therapy start was 59.1 years (sd=12.36). Median time since start of first CI regimen was 36.4 months [interquartile range (IQR): 28.1, 55.0], while median length of CI therapy was 7.3 months (IQR: 2.1–24.3); 15 respondents were undergoing treatment when they completed the survey. For patients off treatment, median time since CI therapy completion was 27.1 months (IQR: 16.7–40.4 months). (Table 1)
Table 1.
Demographics, clinical information, and scale responses
| Mean | sd | |
|
| ||
| Age at survey | 63.0 | 12.54 |
| Age at diagnosis | 59.1 | 12.36 |
|
| ||
| % | ||
|
| ||
| Gender | ||
| Male | 57 | |
| Female | 43 | |
| Race | ||
| White | 94 | |
| Asian | 1 | |
| Unknown | 5 | |
| Primary language | ||
| English | 99 | |
| Other | 1 | |
| Stage at diagnosis | ||
| I | 11 | |
| II | 21 | |
| III | 16 | |
| IV | 52 | |
| Initial immunotherapy regimen | ||
| Ipilimumab+nivolumab | 57 | |
| Ipilimumab | 28 | |
| Pembrolizumab | 12 | |
| Nivolumab | 3 | |
| Durvalumab | 2 | |
| Prior Treatment | ||
| Chemotherapy | 22 | |
| Radiation | 16 | |
| 2nd immunotherapy regimen | 38 | |
| 3rd immunotherapy regimen | 5 | |
|
| ||
| Mean | sd | |
|
| ||
| EORTC-QLQ30 Subscales | ||
| Global QOL | 84.0 | 19.51 |
| Social | 89.2 | 19.80 |
| Emotional | 84.6 | 16.74 |
| Physical | 90.7 | 16.45 |
| Role | 89.3 | 20.98 |
| Cognitive | 87.1 | 16.47 |
| Financial difficulties | 88.4 | 23.92 |
| COST | 30.0 | 9.46 |
Financial toxicity and financial difficulty
Respondent scores on the COST ranged from 0–44 (possible range: 0–44); mean score was 30.0 (sd=9.46). Lower scores indicated worse outcomes. Internal consistency was very good (α = 0.88). In the EORTC measure, 23% of respondents reported some financial difficulties.
Scores on the COST and report of financial difficulties did not vary significantly by gender, race, or language or by treatment type, length, or status. However, younger patients (<65 years; n=56) reported higher financial toxicity [t(103)=3.3, p<.001] and more financial difficulties [t(104)=2.6, p=.01] than older patients. At the item-level, younger patients reported the lowest mean scores for having enough money and assets for retirement and future costs of treatment; satisfaction with current financial situation; and feeling no choice about healthcare spending: Figure 1 highlights the disparities in responses to the COST by age (<65 years vs. 65+ years).
Figure 1.
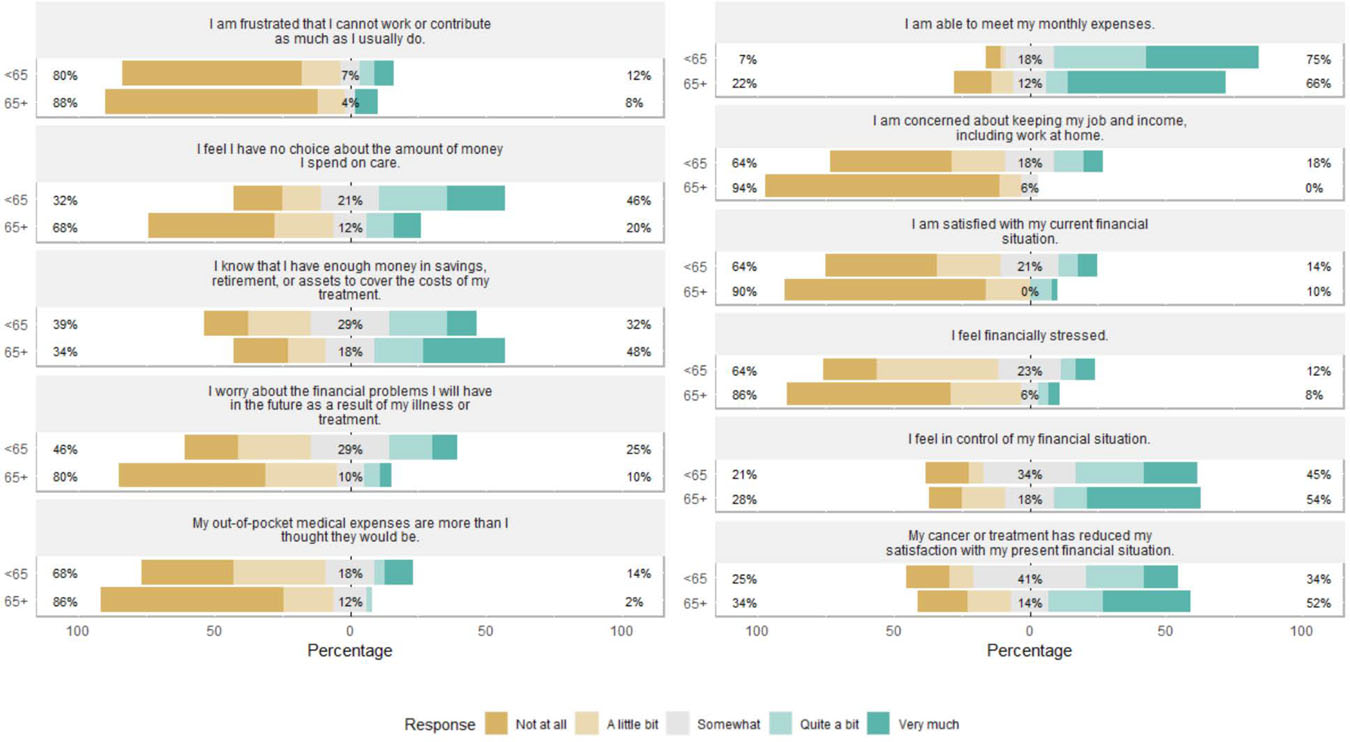
Item-level Comprehensive Score for Financial Toxicity response, by age category
Beige-side percent: combined “Not at all” and “A little bit” responses;
Teal-side percent: combined “Quite a bit” and “Very much” response
Quality of life
Participants reported high levels of global QOL and functioning: mean global QOL score was 84.0 (sd=19.51), and functioning subscale means ranged from 84.6–90.7. (Table 1). Subscale internal consistency ranged from 0.68–0.92. Global QOL and functioning subscales did not vary significantly by demographic or clinical variables.
Financial toxicity and quality of life
Financial toxicity was correlated with global QOL (r=.44, p<.001), functioning subscales [social (r=.55, p<.001), emotional (r=.45, p<.001), physical (r=.33, p=.001), role (r=.52, p<.001), cognitive (r=.22, p=.02)], and report of financial difficulties (r=.70, p<.001). When controlling for age, financial toxicity maintained a significant correlation with financial difficulties (r=.62, p<.001), global QOL (r=.11, p=.03), and the social (r=.34, p<.001), emotional (r=.22, p=.03), role (r=.26, p=.01) functioning subscales.
Discussion
Financial toxicity in this sample of patients with advanced melanoma was lower than that reported elsewhere in the literature.11 However, financial toxicity was inversely associated with QOL in all measured domains, which supports earlier findings in samples of cancer survivors with varying diagnoses.6 Our findings are also consistent with previous work indicating that younger age is a risk factor for both objective and subjective financial toxicity: younger patients and survivors in our survey reported higher financial toxicity than older patients, and partial correlations between financial toxicity and QOL were significant when controlling for age.8
Representing an emerging population of cancer survivors, one-half of our respondents had survived at least 36 months since initiating CI therapy, and median time since completion was over two years. To our knowledge, this study is the first to evaluate, at the individual level, financial toxicity in survivors of advanced melanoma treated with CI, as the extant cost-related literature in melanoma patients focuses on cost-effectiveness comparisons between therapies. Although our findings were on par with analyses of the association between QOL and financial toxicity in general cancer survivor populations, future research should continue to monitor this relationship in advanced melanoma survivors, exploring the interaction with potential immune-related toxicities and adverse events that may follow immune system activation.
This paper provides insight into the association between financial toxicity and QOL in a population of cancer survivors whose psychosocial experience after treatment is poorly described. Future research is needed to explore financial toxicity in larger, broader, and more diverse samples of melanoma patients, with attention paid to relevant demographic covariates as well as out-of-pocket expenses and insurance coverage. Future research should also follow these survivors over time to explore the impact of financial toxicity on ongoing survivorship care.
Limitations
The homogenous demographic makeup of our respondents limits our findings’ generalizability and implications for practice, and the cross-sectional nature of our survey precludes conclusions about causality. Due to the small sample size, we did not assess differences by CI type. As this analysis was part of a larger study addressing symptom burden and quality of life, we did not collect data on income, education, or employment status, variables typically predictive of financial toxicity. Our previous work with patients and survivors at our institution suggests respondents have higher socioeconomic status than the general population, and thus the generalizability of our findings may be impacted.
Implications for psychosocial oncology practice
Our study highlights the importance of addressing financial toxicity, particularly among patients receiving high-cost treatments, as there was a consistent association with components of QOL. Our study also supports previous work identifying age as a potential risk factor for financial toxicity.4,7 In practice, healthcare providers must be aware of this risk and initiate financial toxicity screenings within their practice as appropriate. Our findings, however, speak only to influences on individual-level experiences of financial toxicity, and providers should take care to acknowledge the complex contexts and interplay of systems that give rise to both financial toxicity itself and the individual-level experience of it.
At the individual-level, oncology social workers, psychologists, and nurses are well-positioned within the healthcare team to educate patients and their caregivers about cost-management techniques, link them with available financial resources, and provide psychological counseling to alleviate related objective and subjective financial distress. In addition, efforts on the part of psychosocial oncology providers to increase the financial capability of patients and their caregivers are vital to the long-term well-being of this emergent group of cancer survivors.
Acknowledgments
Funding: This manuscript was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Disclosure: The authors have no conflicts of interest.
Data availability:
De-identified data that support the findings of this study are available on request from the corresponding author, BT. The data are not publicly available due to the privacy of research participants.
References
- 1.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. The Lancet Oncology. November2018;19(11):1480–1492. doi: 10.1016/s1470-2045(18)30700-9 [DOI] [PubMed] [Google Scholar]
- 2.Tran G, Zafar SY. Financial toxicity and implications for cancer care in the era of molecular and immune therapies. Annals of translational medicine. May2018;6(9):166. doi: 10.21037/atm.2018.03.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma V, Sprave T, Haque W, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. Journal for immunotherapy of cancer. November232018;6(1):128. doi: 10.1186/s40425-018-0442-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon LG, Merollini KM, Lowe A, Chan RJ. A Systematic Review of Financial Toxicity Among Cancer Survivors: We Can’t Pay the Co-Pay. The patient. October312016;doi: 10.1007/s40271-016-0204-x [DOI] [PubMed] [Google Scholar]
- 5.Witte J, Mehlis K, Surmann B, et al. Methods for measuring financial toxicity after cancer diagnosis and treatment: a systematic review and its implications. Annals of oncology : official journal of the European Society for Medical Oncology. July2019;30(7):1061–1070. doi: 10.1093/annonc/mdz140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zafar SY, Abernethy AP. Financial toxicity, Part I: a new name for a growing problem. Research Support, Non-U S Gov’t. Oncology. 2013;27(2):80–1. [PMC free article] [PubMed] [Google Scholar]
- 7.Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. September2014;10(5):332–8. doi: 10.1200/jop.2013.001322 [DOI] [PubMed] [Google Scholar]
- 8.Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. Journal of oncology practice. March2015;11(2):145–50. doi: 10.1200/jop.2014.001542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamoor M, Postow MA, Lavery JA, et al. Quality of life in long-term survivors of advanced melanoma treated with checkpoint inhibitors. J Immunother Cancer. March2020;8(1)doi: 10.1136/jitc-2019-000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. April2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Souza JA, Yap BJ, Wroblewski K, et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST). Cancer. February12017;123(3):476–484. doi: 10.1002/cncr.30369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aaronson NKAS, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A,, Flechtner HFS, de Haes JCJM, Kaasa S, Klee MC, Osoba D, Razavi D,, Rofe PBSS, Sneeuw KCA, Sullivan M, Takeda F. The European Organisation for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute. 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 13.likert: Analysis and Visualization Likert Items. Version R package version 1.3.5 2016. https://CRAN.R-project.org/package=likert
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified data that support the findings of this study are available on request from the corresponding author, BT. The data are not publicly available due to the privacy of research participants.


