ABSTRACT
The development of safe and effective vaccines has been an overriding priority for controlling the 2019-coronavirus disease (COVID-19) pandemic. From the onset, COVID-19 has caused high mortality and economic losses and yet has also offered an opportunity to advance novel therapeutics such as DNA and mRNA vaccines. Although it is hoped that the swift acceptance of such vaccines will prevent loss of life, rejuvenate economies and restore normal life, there could also be significant pitfalls. This perspective provides an overview of future directions and challenges in advancing promising vaccine platforms to widespread therapeutic use.
KEYWORDS: Vaccine, SARS-CoV-2, COVID-19, mRNA, DNA
Introduction
Since the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus in December 2019, it has spread throughout most countries in the world, claimed many lives, and wreaked havoc on social and economic structures. Subsequently, the world has seen a large number of vaccine development projects against this deadly pathogen. Plasmid DNA and mRNA vaccines have been touted as potential alternatives to standard vaccines because developing a gene-build coding for the antigen rather than inactivating or attenuating the pathogen is much simpler and quicker, and obviates the hazards of dealing with live pathogens.1–3 DNA vaccination has recently become the fastest-growing vaccine technology field due its many advantages: high stability, ease of construction and ease of delivery. However, other than for COVID-19, no DNA vaccine has yet to be commercially licensed or even successfully passed phase III clinical trials.3,4 Moreover, plasmid DNA has the possibility of integration into the host genome, and in many cases DNA vaccination has been hampered by poor efficacy as well as auto-immunity risk.3,5 In contrast, mRNAs represent a non-infectious, non-integrating platform that is degraded by normal cellular processes and whose in vivo half-life can be controlled by using various modifications and distribution methods.6–8 RNA vaccines are not completely error free – there are issues with mRNA instability, high innate immunogenicity, and inefficient in vivo-delivery. Despite the fact that a substantial number of mRNA/DNA vaccine candidates are in preclinical and human clinical trials, no licensed vaccine has been seen for human use since 1990, when active protein was detected in a mouse model by mRNA inoculation; mostly due to safety concerns.1,4 Nevertheless, in 11 January 2021, the first mRNA vaccine against SARS-CoV-2 was approved within just a year after COVID-19 vaccine development projects were initiated. As of 25 May 2021, at least 14 different nucleic acid-based vaccine (in 3 platforms) for SARS-CoV-2 have been issued by the U.S. Food and Drug Administration (FDA) and World Health Organization (WHO).9 To ensure global immunization against SARS-CoV-2, the COVID-19 Vaccine Global Access (COVAX) initiative has been formed.10 The primary target of COVAX is to procure and fairly distribute 2 billion doses of COVID-19 vaccines across almost 200 countries, including 92 middle- and lower-income countries that cannot fully afford to pay for COVID-19 vaccines, so that they get equal access as higher-income, self-financing countries do by the end of 2021.11,12 In this report, the possible effects of the early approval of mRNA/DNA vaccines use against SARS-CoV-2 are summarized.
Significance of the early approval of corona vaccines
The use of face masks, physical separation or social distancing, the testing of exposed or symptomatic individuals, touch tracking, and isolation have proven unsuccessful in preventing the dissemination of SARS-Cov-2. Therefore, several vaccines have been licensed to minimize COVID-19-related morbidity and mortality, despite the possible dangers associated with newly-approved vaccines. Early approval of COVID-19 vaccines might play a great role in controlling the COVID-19 pandemic.
The vaccine will reduce loss of life and will help to recover economic loss
Since the outbreak of COVID-19, the mortality rate has been estimated to be around 2.1%, with nearly 3.5 million people dead by 25 May 2021.13 Severe/fatal cases of COVID-19 are associated with immune hyperactivation and excessive cytokine release, leading to multiple organ failure.14–16 It has been estimated that the vaccines against infectious diseases have saved at least 23 million lives over the ten years from 2011 to 2020.17 However, identifying, quantifying, and balancing the possible safety risks against possible advantages is an important consideration in approving any vaccine. Despite there being no prior experience of using mRNA/DNA vaccines for human use, the contagious nature and mortality of COVID-19 means vaccines have been approved without long-term clinical follow-up. The efficacy of DNA and mRNA vaccines in producing neutralizing antibodies has already been demonstrated.4,18,19 Further, several phase-1 mRNA and DNA vaccine clinical studies have provided satisfactory safety and efficacy results, having elicited sufficient neutralizing titer to protect from infectious pathogens. For example, DNA vaccines against West Nile virus can induce neutralizing antibodies in different age groups providing safety and immunogenicity against Ebolavirus, Marburgvirus, Zika virus and HPV infection.20–24 Additionally, mRNA vaccines against Zika virus, respiratory syncytial virus (RSV), influenza virus, rabies virus, Ebolavirus, H1N1 influenza etc., have already shown satisfactory results in animals.25–28 Moreover, preclinical and clinical profiles of mRNA vaccines for H10N8, H7N9 influenza virus, rabies virus, and HIV-1 virus can induce protective immunogenicity with acceptable tolerability results.26,29–31 In the case of COVID-19 vaccines, currently approved mRNA and viral vector vaccines have a vaccine efficacy ranging from around 70% to more than 90%.32–37 It is hoped that the approved vaccines against SARS-CoV-2 will control COVID-19, as justified by results of their clinical trials.32,34 Moreover, the vaccination program should promote the herd immunity needed to get back to normal human activities, which will in turn help the global economy to recover.
Cheap, easy, and reproducible
The most important way to control and avoid pandemics is to create prophylactic or preventive vaccines against infectious diseases. Orthodox vaccination methods have often struggled to deliver successful vaccines against complicated viruses like HIV-1, herpes simplex virus, and RSV. Furthermore, commercial vaccine development generally takes many years. Since both mRNA and DNA vaccines are designed and developed independent of cellular processes, they are easily adaptable, relatively inexpensive, and their scalable manufacturing process offers the flexibility to encode virtually every protein as an antigen in a very short period of time. One of the greatest advantages of the new mRNA-based technology is the ease with which nucleotide sequences can be tweaked for revised formulation to combat emerging immune-escape mutants. Recently, a complete mutation map for the SARS-CoV-2 RBD has been developed that might enable rational design of antibody therapeutics using this new technology.38 In addition, they can be manufactured in the same manufacturing plant using the same manufacturing process. Such vaccines are consistent from batch to batch and reproducible, much as with in vitro-chemical reactions. Thus, with relatively little financial expenditure, innovative vaccines could be produced in a very short period, which is of considerable value for pandemic scenarios with infectious diseases like COVID-19 in addition to of developing cancer vaccines against patient-specific cancer-associated or mutated antigens.39,40
Suitable for both infectious and non-infectious disease
One of the greatest advantages of mRNA/DNA vaccines is that the protein is engineered and the vaccine is produced without using the infectious pathogen, and hence no pathogen cultivation or purification steps are required. Once the nucleotide sequences are known, no matter how contagious they are, vaccines can be produced. Moreover, it is equally amenable to non-infectious disease like cancer where antigen purification steps are so difficult. There are many organisms comprising multiple serotypes/genotypes, and in conventional methods, multiple epitope-based vaccine formulation is very difficult, but nucleic acid-based vaccines technology could resolve this problem. While the handling of highly contagious pathogens like SARS, MERS, and Ebola require biosafety level 4 safety cabinets with highly equipped laboratory settings, mRNA/DNA vaccines might be produced in a comparatively low-resource setting. Moreover, using several laboratories around the world should decrease reliance on specialist laboratories, increase production capacity, cut transport and production time and costs, and eventually shorten delays in distribution to the public.
Drawbacks of nucleic acid-based vaccines
The approval of nucleic acid-based vaccines will not only reduce mortality by regulating COVID-19, and reintroduce patients to daily life, but also support research into other diseases. However, there are several serious problems that must be resolved in exposing people to novel nucleic acid vaccines before they can be deemed a potential therapeutic for long-term use.
First mRNA vaccine without long term safety data
Successful vaccine development needs a long time, usually several years. Until recently, the Mumps vaccine was the fastest vaccine – it was approved in 1960 after approximately four years of initiation. On 11 December 2020, the U.S. Food and Drug Administration issued the first emergency use authorization (EUA) for the prevention of COVID-19 which allows Pfizer-BioNTech’s mRNA-based COVID-19 vaccine ‘BNT162b2ʹ to be distributed in the U.S.41 This was not only the first mRNA vaccine approved for human use but also the fastest formulated vaccine whose development was initiated just 11 months back on 10 January 2020.34 One week later, the FDA issued an EUA for the another mRNA vaccine, named ‘mRNA-1273ʹ, also known as the ‘Moderna COVID-19 Vaccine.’33 Other than these, the first DNA vector-based ‘ChAdOx1 nCoV-19 vaccine’ (the AstraZeneca/Oxford COVID-19 vaccine), was approved by the WHO on 15 February 2021.42 Subsequently, another vector-based vaccine from Janssen/Johnson & Johnson was approved on 12 March 2021.
At present, at least 3 different platforms, i.e. mRNA, DNA, and DNA vector-based vaccines comprising 14 candidates are in use worldwide.9 All these vaccine development projects were launched shortly after the SARS-CoV-2 genetic sequence was determined in January 2020.43–46 It is obviously not possible to get long-term safety data given this short time. In addition, ethical and practical barriers prevented following placebo recipients for a long time without offering active immunization and thus made it impossible to generate randomized control trial data.34 The ‘BNT162b2ʹ trail included only 2 months of follow-up of the vaccinated individuals after the second dose of the vaccine for half the trial participants, and up to 14 weeks’ maximum follow-up for a smaller subset. The median follow-up of the Moderna vaccine was 56 days for 62% of participants after the second dose.37 In contrast, the average safety follow-up was 3–4 months for the ChAdOx1 nCoV-19 vaccine produced by Oxford–AstraZeneca. In all cases, vaccinated and non-vaccinated groups developed transient adverse reactions though these were resolved within a couple of days after onset in most of the cases. However, due to early approval more comprehensive information on the vaccine effect and the duration of protection remain to be determined.34
Late side effects and duration of protection are unknown
Vaccines are generally safe, because they are approved after long clinical and pre-clinical trials. Despite precautions, and an extremely low incidence of serious systemic adverse events, numerous reports have highlighted the occurrence of untoward neurological, articular, and autoimmune effects after single or combined multi-vaccine procedures. For instance, vaccine-associated paralytic poliomyelitis has been associated with oral poliovirus vaccine.47 In the case of the Yellow Fever (YF) vaccination program, vaccine-associated severe neurotropic diseases such as post-vaccinal encephalitis, acute disseminated encephalomyelitis, and Guillain-Barré syndrome (GBS) have been reported.48 Although the side-effects are merely associated and causality has never been established, effects including GBS, multiple sclerosis, autism, arthritis, rheumatoid arthritis, systemic lupus erythematosus, and diabetes mellitus, among others, have been reported for other vaccines such as HBV, typhoid/paratyphoid, anthrax, tetanus, MMR, BCG, smallpox, DPT, influenza, pertussis, and polio vaccines.49 It is supposed that DNA vaccines bear a key risk of genomic integration. Again, this probability is thought to be extremely low and issues about integration are currently theoretical as DNA vaccines are still not licensed for humans. However, if hundreds of millions of effective doses are to be administered to healthy recipients, even a very rare event might become a potentially serious safety problem, particularly in view of vaccination fatigue in many countries.50
An mRNA vaccine has no risk of genomic integration, but there are several limitations. The length of antigen expression can last for several months in the case of both DNA and RNA vaccines. In general, mRNA/DNA vaccines serve as a constant long-term antigen production factory, which may not equate with successful immune responses and may even damage the expected immune effect and contribute to T cell exhaustion.51–53 However, the current mRNA vaccines might be the safer and cleaner than conventional vaccine technologies since mRNA is translated then quickly degrades, leaving nothing behind except vaccine-induced neutralizing antibodies. In contrast, in next-generation mRNA vaccines, the self-amplifying mRNA encodes not only the antigen but also the viral replication machinery leading to high levels of antigen expression, which may provide longer stimulation, hence a long-lasting duration of protection.4,6
Another prominent issue might be that certain nucleic acid-based vaccines elicit strong type I interferon responses that have been related to inflammation potentially leading to the development of autoimmune disease.54–56 Epitope spreading and bystander activation, as well as autoinflammatory dysregulation in genetically prone individuals, can also lead to acute and chronic autoimmunity throughout and after COVID-19 vaccination.57,58 Additionally, involvement of extracellular naked RNA or DNA results from nucleic acid-based vaccination, which has been shown to increase the permeability of closely-packed endothelial cells and thus may lead to edema.59 In vivo and in vitro evidence has shown that blood coagulation factors, particularly factors XII and XI, strongly bind to extracellular naked RNAs and activate the proteases involved in the blood coagulation contact process pathway.59 Different forms of eukaryotic and prokaryotic RNAs serve as promoters of blood coagulation and pathological thrombus formation.59 During the phase III trials of the AstraZeneca and JNJ vaccines, there were early warning signals whereby serious adverse events following immunization (AEFI), such as multiple sclerosis and transverse myelitis, were reported in like Germany, Austria, USA, and India.60–63 As of March 2, 2021, more than 51 million dosages of the COVID-19 vaccines from different platforms were administered in the United States and 9,442 adverse reactions had been reported.64 Common side effects were dizziness, headache, pain, muscle spasms, myalgia, and paresthesia, while in rare cases, tremors, diplopia, tinnitus, dysphonia, seizures, and reactivation of herpes zoster have been detected. In a few cases, serious events like stroke (17 cases), GBS (32 cases), facial palsy (190 cases), transverse myelitis (9 cases), and acute disseminated encephalomyelitis (6 cases) were observed.64 Since the evidence is based on passive surveillance, it is subject to reporting bias and might contain errors. Furthermore, due to the high number of patients being vaccinated, certain cases of neurological conditions might arise by chance alone because of background incidence of neurological disorders among the population.
Autoimmune thrombosis associated with the AstraZeneca vaccine mimics heparin-induced thrombocytopenia in different regions such as the United Kingdom, European Union, and Scandinavian countries. The rare cases, cerebral sinus vein thrombosis (CSVT) and thrombocytopenia were reported in patients who received the AstraZeneca COVID-19 vaccine (AZD1222).60,65 At least 9 patients presented with thrombosis between 4 and 16 days after vaccination. Seven of them had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had both CVT and splanchnic vein thrombosis; four of them died. While the vaccine-related effects were clinically similar to heparin-induced thrombocytopenia, the serological profile was different.60 Vaccine-induced immune thrombotic thrombocytopenia (VIITT) and was anxiety-related adverse events such as syncope (fainting) have been reported for the JNJ vector-based vaccine. Syncope was detected for 8.2 per 100,000 doses; nearly 164 times higher than for influenza vaccination, where the reporting rate of syncope was 0.05 episodes per 100,000 doses in July 2019 to June 2020.61 In contrast, the incidence of VIITT was predicted to be nearly 1 case per 500,000 doses for AstraZeneca vaccine.65 Overall, VIITT rate for the AstraZeneca/COVISHIELD vaccines have been estimated from 1 case per 26,000 to 1 case per 127,000 however, it greatly varies from one country to another.60,65–68 Due to safety concerns, vaccine trials were temporarily paused in some countries for both the JNJ and AstraZeneca vaccines. However, the effects were ultimately deemed to be unrelated to the vaccine, as the rate of cerebral venous sinus thrombosis in the general population was estimated at 0.22 to 1.57 cases per 100,000 per year.60 The benefits of the COVID-19 vaccines were deemed outweigh the risks.69 Therefore, protection will require ongoing assessment as multiple mRNA/DNA modalities and delivery mechanisms are used for the first time in humans and evaluated in wider populations of patients.
There is no authorized vaccine for human corona viruses, and vaccines against common cold viruses are short-lived and less effective
The Coronaviridae family consists of four genera: the alpha, beta, gamma, and delta coronaviruses, and which possess a large (31 kb) single-stranded positive-sense RNA genome. The highly pathogenic SARS-CoV, SARS-CoV-2, and MERS-CoV are all betacoronaviruses. Four other major human coronaviruses (HCoV) are HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1, which are responsible for nearly 15% of human common colds.70 Unfortunately, there are as yet no approved non-Covid coronavirus vaccines for human use; only some ongoing projects.71 Experimental data show that vaccines against coronaviruses are less effective and less cross-protective than vaccines against many other human viruses, with antibody titers greatly decreasing one year after initial infection, and many are able to re-infect and shed viruses.72,73 The estimated reinfection rates for HCoV-229E and HCoV-OC43 have been estimated to be 30% and 80%, respectively.74–76 Accordingly, complete protection from a mRNA/DNA vaccine has yet to be realized.
Similar to coronaviruses, others respiratory viruses such as RSV, influenza viruses, and rhinoviruses impair antibody-mediated protection and defective B-Cell memory due to the short life of the neutralizing antibody and/or presence of numerous serotypes.77–80 A recent study suggested that a relatively small number of mutations can mediate potent escape of pseudoviruses representing 10 globally-circulating SARS-CoV-2 strains from BNT162b2 or mRNA-1273 vaccine.81 A number of antibody-resistant SARS-CoV-2 variants have already been reported in the UK and South Africa.82 A novel SARS-Cov-2 variant CAL.20 C (B.1.427/B.1.429) was originally detected in California and is currently spreading throughout the US and 29 additional countries, has been reported to have escaped from a monoclonal antibody panel.83
Another important concern of a COVID-19 vaccine is whether it will generate a sufficient immune response in elderly people. Research demonstrates that elderly people are impaired in generating high-affinity antibodies, have defects in somatic hypermutation and isotype switching, their naive T cells decrease significantly, and have reduced TCR repertoires and poorly responding CD8+ CD28- T cells.84–89 The most severe risk group for SARS-Cov-2 is the elderly.90–92 While the overall case fatality rate (CFR) has been estimated to be around 4%, the rate is rapidly increasing in the age group of ≥60 years, reaching 16.9% and 24.4% in the 70–79 years and ≥80 years age groups, respectively.91,92 More than 85% of the deceased are 65 years or older.93 Recently, it has been reported that the elderly elicited a strong and persistent antibody response, similar to those younger aged, after a second dose of Pfizer or Oxford-AstraZeneca COVID-19 vaccines in UK and USA.94–96 However, continuous long-term surveillance is required in spite of initial impressive clinical data because it is unknown how long a new vaccine’s immune responses will last.
Nucleotide-mediated ectopic immune stimulation
Type I interferon production is one of the most important, first line, innate, immune defenses against any virus, and essential for the initiation of adaptive immunity. While innate immune responses to DNA viruses are thought to be initiated by pattern-recognition receptors (PRRs), the precise mechanism is unknown.97 In contrast, immune-sensing mechanisms for RNA viruses are very well studied. It is proposed that both DNA and RNA viruses produce certain RNA species during their replication cycle that are recognized by retinoic acid-inducible gene I (RIG-I).98–100 RIG-I has been shown to sense viral RNAs derived from a panel of virus families.98,101 Moreover, previously it was thought that viral replication is required to initiate RIG-I sensing, but surprisingly, viral and other nucleotide fragments are enough for an RIG-I-mediated immune response (Figure 1).102–104 Moreover, nucleotide uptake from dietary sources can also elicit immune responses.105 Among extracellular nucleotides, ATP is the most abundant and is commonly considered a classical danger signal and can act as an immune response initiator or terminator. In addition, signal transduction induced by the association of exogenous nucleotides/nucleosides and their receptors can modulate the expression of a range of genes, some of which can specifically influence the levels of cytokines.106 As previously discussed, in some platforms, advanced technologies such as mass production, self-expression, rapid degradation, etc., have eliminated the risks associated with nucleic acid-based vaccines. However, COVID-19 vaccines administered on various platforms should be monitored for prolonged periods of time.
Figure 1.
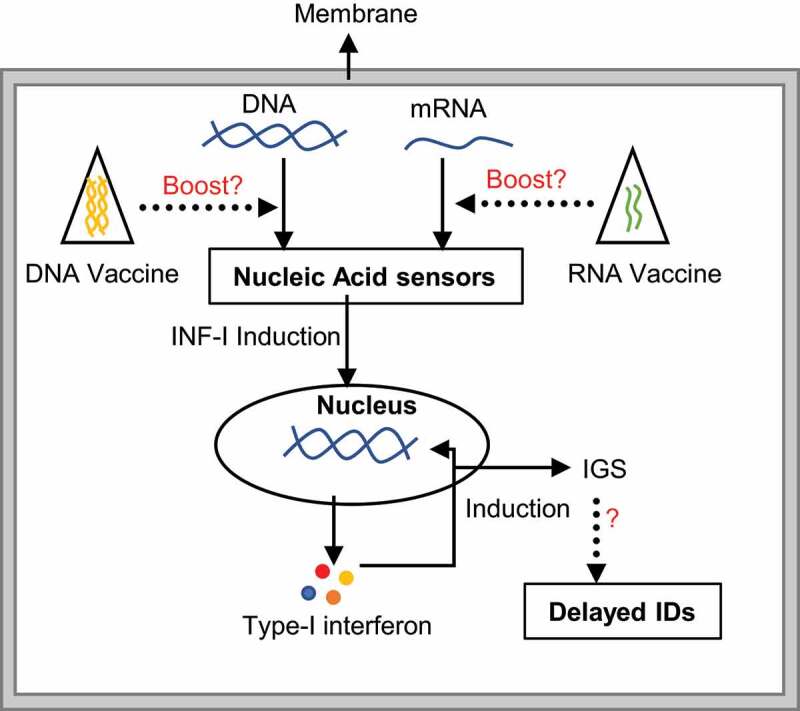
The mechanism of nucleic acid-mediated immune stimulation. The presence of naked mRNA/DNA from any source e.g. infectious organisms, diet or cellular DNA fragments, are sensed by a protein complex. Successive downstream signaling induces type-I interferon, which further stimulates the production of interferon stimulation genes (IGS). Abnormal expression of IGS might link to different immunological disorders (IDs). While second-generation mRNA vaccinations should be safer and may not strongly associated with this mechanism, long-term exposure to any nucleic acid in the form of vaccination may activate the nucleic acid-mediated immune sensing pathway, raising the risk of IDs
The risk of vaccine-associated enhancement
Vaccine-associated enhanced disease (VAED) is very rare but tends to cause serious adverse infection outcomes relative to infection without previous vaccination. VAED is also known as antibody-dependent enhancement (ADE), a disease in which secondary infection is directly facilitated by pathogen-specific antibodies produced by vaccination or primary infection (Figure 2). VAED has been observed for several vaccines such as formalin-inactivated whole-virus vaccines against respiratory syncytial virus (RSV) and measles virus vaccines, and has been reported in rare cases of secondary dengue infection.107–109 In the context of COVID-19 vaccine production, one of the possible risks posed is whether the immune responses elicited by the vaccine may boost the acquisition of SARS-CoV-2 or make the disease worse during reinfection or when infection occurs after vaccination. Although this phenomenon has not been observed yet in any of the approved vaccine trials including animal studies in non-human primates however, it is too early to settle this question since neither the principles of immunity nor the preclinical trials present a justification claiming the safety of COVID-19 vaccines at this time. Long term follow-up should be provided by post-licensing surveillance to detect adverse events, including the potential for increased severity of COVID-19 illness.
Figure 2.
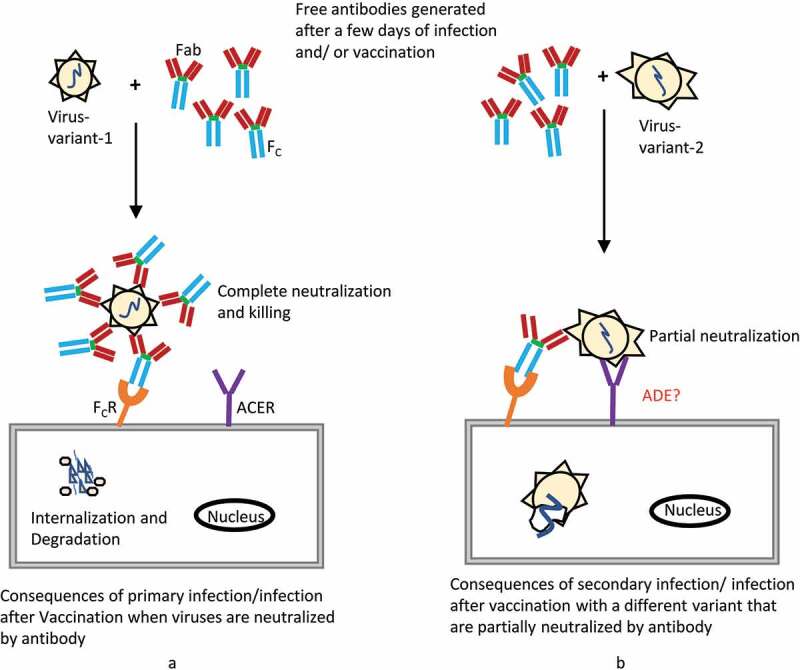
Schematic representation of antibody action and antibody dependent enhancement (ADE) to viral infection. (a) Antibodies generated after a few days of viral infection or successful vaccination, which in turn neutralize the viruses, enabling protection from further infection. (b) Secondary infection or infection after vaccination with a different viral strain causes failure of complete neutralization by the previously existing antibody. However, due to sharing some antigen similarities, partial neutralization may accelerate viral internalization by antibody-binding Fc receptors. Although nucleic-acid-based COVID-19 vaccines have been successfully approved and have shown no ADE complications in trials, including non-human primate animal studies, vaccinated individuals should be monitored across time
Future perspective
Since 1990, when a successful protein was produced from in vitro-transcribed mRNA-injected animals, researchers have been trying to produce mRNA-based vaccines. To date, several plasmid-based vaccines have already been approved for animals such as a melanoma cancer vaccine for dogs, a West Nile virus vaccine for horses, and an infectious hematopoietic necrosis virus vaccine for fish.39,110,111 A large number of preclinical vaccine development projects have been published recently, and several have entered into human clinical trials.4 Although the key ability of nucleic acid-based vaccines to cause cellular T- and B-cell responses in humans has been demonstrated in a number of clinical trials, the production of DNA vaccines for humans has so far not been equally successful. These included a number of HIV vaccines, for Zika virus, influenza virus, rabies virus, and a number of vaccines against different cancers (melanoma, breast cancer, lung cancer, prostate cancer, ovarian cancer, multiple solid tumors, etc.).4 Unlike therapeutic drugs, vaccines are injected into the healthy individuals and thus safety issues are paramount; none of them have received approval. COVID-19 however, has changed the mind-set and a year after the emergence of the SARS-CoV-2 virus, at least 14 different nucleic acid-based vaccines (3 platforms) have been approved.9 The approval of several mRNA or vector-based COVID-19 vaccines has had a great impact as it has opened a new era in vaccinology. A billion doses of the Oxford-AstraZeneca vaccine have been ordered already by many countries throughout the world, including the EU, US, China, India, Japan, UK, Brazil, Indonesia, Bangladesh, Australia, Egypt, Argentina, and Canada.112 These pioneering vaccines can obviously be considered as trials for overall ‘nucleic acid-based therapeutics.’ Thus, a large trial outcome of nucleic acid-based vaccine, irrespective of gender, ethnicity, and socioeconomic status, will be generated by the COVID-19 vaccination program worldwide. The medical world is no doubt looking forward to seeing the success of these vaccines, as approval of nucleic acid-based vaccines for other diseases will mostly depend on them.
Conclusion
The development of COVID-19 vaccines exemplify the possibilities when key sectors of society, such as the general public, government, scientists, regulators, and industry, collaborate toward a shared objective. The production of COVID-19 vaccines that are safe, reliable, inexpensive, and deployable is vital to ending the pandemic, and restoring normalcy. However, given the low efficacy of previous vaccines against the common cold/influenza viruses and the durability of immune responses, and the questions about new vaccines, the celebrations surrounding early promising results of the COVID-19 vaccines are premature. Longitudinal studies will be required to assess the reliability of the defensive adaptive immune responses following natural infection or vaccination.
Acknowledgments
The authors are thankful to Mahmuda Khatun for her co-operation.
Funding Statement
The authors have no funding to report.
Disclosure of potential conflicts of interest
The authors declare no competing interests regarding this article.
Author contributions
M.A.R conceptualized and designed the study; M.A.R and M.S.I. wrote, reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
References
- 1.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL.. Direct gene transfer into mouse muscle in vivo. Science (80-). 1990;247(4949):1465–68. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 2.Liu MA.A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines. 2019;7(2):37. doi: 10.3390/vaccines7020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leitner WW, Ying H, Restifo NP. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine. 1999;18(9–10):765–77. doi: 10.1016/S0264-410X(99)00271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines-a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–79. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols WW, Ledwith BJ, Manam SV, Troilo PJ. Potential DNA vaccine integration into host cell genome. Ann N Y Acad Sci. 1995;772:30–39. doi: 10.1111/j.1749-6632.1995.tb44729.x. [DOI] [PubMed] [Google Scholar]
- 6.Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16(11):1833–40. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kauffman KJ, Webber MJ, Anderson DG. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J Control Release. 2016;240:227–34. doi: 10.1016/j.jconrel.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Guan S, Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24(3):133–43. doi: 10.1038/gt.2017.5. [DOI] [PubMed] [Google Scholar]
- 9.#Vaccineswork. [accessed 2021May25]. https://www.gavi.org/vaccineswork?gclid=Cj0KCQjwna2FBhDPARIsACAEc_Xs53mjrz5yZ4vp5vpsxMqzGQhoWQ0jVVFlviYNGFfXMz8VkFpQ-74aAm-KEALw_wcB
- 10.WHO . COVAX. 2020. [cited 2021 Mar 2]. https://www.who.int/initiatives/act-accelerator/covax
- 11.WHO . COVID-19 vaccine doses shipped by the COVAX Facility head to Ghana, marking beginning of global rollout. 2021. [cited 2021 Mar 2]. https://www.who.int/news/item/24-02-2021-covid-19-vaccine-doses-shipped-by-the-covax-facility-head-to-ghana-marking-beginning-of-global-rollout
- 12.Winsor MWhat is COVAX? How a global initiative is helping get COVID-19 vaccines to poorer countries. [accessed 2021Mar2]. https://abcnews.go.com/Health/covax-global-initiative-helping-covid-19-vaccines-poorer/story?id=76106981
- 13.COVID live update: 167,986,676 cases and 3,487,062 deaths from the coronavirus – worldometer. 2021. [cited 2021 May 25]. https://www.worldometers.info/coronavirus/
- 14.Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, Jiang B. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395(10228):e52. doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elezkurtaj S, Greuel S, Ihlow J, Michaelis EG, Bischoff P, Kunze CA, Sinn BV, Gerhold M, Hauptmann K, Ingold-Heppner B, et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep. 2021;11(1):4263. doi: 10.1038/s41598-021-82862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renu K, Prasanna PL, Valsala Gopalakrishnan A. Coronaviruses pathogenesis, comorbidities and multi-organ damage – a review. Life Sci. 2020;255:117839. doi: 10.1016/j.lfs.2020.117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee LA, Franzel L, Atwell J, Datta SD, Friberg IK, Goldie SJ, Reef SE, Schwalbe N, Simons E, Strebel PM, et al. The estimated mortality impact of vaccinations forecast to be administered during 2011-2020 in 73 countries supported by the gavi alliance. Vaccine. 2013;31:B61–72. doi: 10.1016/j.vaccine.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann G. Nucleic acid immunity. In: Alt FW, editor. Advances in immunology. New York, NY: Academic Press; 2017. p. 121–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, Cu Y, Beard CW, Brito LA, Krucker T, et al. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci USA. 2012;109(36):14604–09. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin JE, Pierson TC, Hubka S, Rucker S, Gordon IJ, Enama ME, Andrews CA, Xu Q, Davis BS, Nason M, et al. A west Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196(12):1732–40. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ledgerwood JE, Pierson TC, Hubka SA, Desai N, Rucker S, Gordon IJ, Enama ME, Nelson S, Nason M, Gu W, et al. A west Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis. 2011;203(10):1396–404. doi: 10.1093/infdis/jir054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarwar UN, Costner P, Enama ME, Berkowitz N, Hu Z, Hendel CS, Sitar S, Plummer S, Mulangu S, Bailer RT, et al. Safety and immunogenicity of DNA vaccines encoding ebolavirus and marburgvirus wild-type glycoproteins in a phase I clinical trial. J Infect Dis. 2015;211(4):549–57. doi: 10.1093/infdis/jiu511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaudinski MR, Houser KV, Morabito KM, Hu Z, Yamshchikov G, Rothwell RS, Berkowitz N, Mendoza F, Saunders JG, Novik L, et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet. 2018;391(10120):552–62. doi: 10.1016/S0140-6736(17)33105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim TJ, Jin H-T, Hur S-Y, Yang HG, Seo YB, Hong SR, Lee C-W, Kim S, Woo J-W, Park KS, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun. 2014;5(1):1–14. doi: 10.1038/ncomms6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543(7644):248–51. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Hassett KJ, Laska ME, Smith M, Almarsson Ö, Thompson J, et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther. 2017;25(6):1316–27. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnee M, Vogel AB, Voss D, Petsch B, Baumhof P, Kramps T, Stitz L. An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Negl Trop Dis. 2016;10(6):10. doi: 10.1371/journal.pntd.0004746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chahal JS, Khan OF, Cooper CL, McPartlan JS, Tsosie JK, Tilley LD, Sidik SM, Lourido S, Langer R, Bavari S, et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc Natl Acad Sci USA. 2016;113(29):E4133–42. doi: 10.1073/pnas.1600299113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, Gottardo R, Bica MA, Garofano A, Koch SD, et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. The Lancet. 2017;390(10101):1511–20. doi: 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson JM, Routy J-P, Welles S, DeBenedette M, Tcherepanova I, Angel JB, Asmuth DM, Stein DK, Baril J-G, McKellar M, et al. Dendritic cell immunotherapy for HIV-1 infection using autologous HIV-1 RNA: a randomized, double-blind, placebo-controlled clinical trial. J Acquir Immune Defic Syndr. 2016;72(1):31–38. doi: 10.1097/QAI.0000000000000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandhi RT, Kwon DS, Macklin EA, Shopis JR, McLean AP, McBrine N, Flynn T, Peter L, Sbrolla A, Kaufmann DE, et al. Immunization of HIV-1-infected persons with autologous dendritic cells transfected with mRNA encoding HIV-1 Gag and Nef: results of a randomized, placebo-controlled clinical trial. J Acquir Immune Defic Syndr. 2016;71(3):246–53. doi: 10.1097/QAI.0000000000000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knoll MD, Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.FDA . Moderna COVID-19 vaccine. 2021. [cited 2021 Feb 23]. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
- 34.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–50. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaccines and related biological products advisory committee December 17, 2020 meeting briefing document - FDA. 2020;
- 37.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greaney AJ, Starr TN, Gilchuk P, Zost SJ, Binshtein E, Loes AN, Hilton SK, Huddleston J, Eguia R, Crawford KHD, et al. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2021;29(1):44–57.e9. doi: 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreiter S, Castle JC, Türeci Ö, Sahin U. Targeting the tumor mutanome for personalized vaccination therapy. Oncoimmunology. 2012;1(5):768–69. doi: 10.4161/onci.19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petsch B, Schnee M, Vogel AB, Lange E, Hoffmann B, Voss D, Schlake T, Thess A, Kallen KJ, Stitz L, et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol. 2012;30:1210–16. doi: 10.1038/nbt.2436. [DOI] [PubMed] [Google Scholar]
- 41.FDA . Pfizer-BioNTech COVID-19 Vaccine. 2021. [cited 2021 Feb 19]. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
- 42.WHO . WHO lists two additional COVID-19 vaccines for emergency use and COVAX roll-out. 2021. [cited 2021 Mar 3]. https://www.who.int/news/item/15-02-2021-who-lists-two-additional-covid-19-vaccines-for-emergency-use-and-covax-roll-out
- 43.Corbett K, Edwards D, Leist S, Abiona O, Boyoglu-Barnum S, Gillespie R, Himansu S, Schäfer A, Ziwawo C, DiPiazza A, et al. SARS-CoV-2 mRNA vaccine development enabled by prototype pathogen preparedness. bioRxiv Prepr Serv Biol. 2020;2020.06.11.145920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science (80-). 2020;367(6483):1260–63. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham J, Port J, Avanzato V, Bushmaker T, Flaxman A, Ulaszewska M, et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv Prepr Serv Biol. 2020;2020.05.13.093195. [cited 2021 Mar 1]. 10.1101/2020.05.13.093195 [DOI]
- 46.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–78. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Platt LR, Estivariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis. 2014;210(suppl 1):S380–9. doi: 10.1093/infdis/jiu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaves M, Riccio P, Patrucco L, Rojas J, Cristiano E. Longitudinal myelitis associated with yellow fever vaccination. J Neurovirol. 2009;15(4):348–50. doi: 10.1080/13550280903062805. [DOI] [PubMed] [Google Scholar]
- 49.Shoenfeld Y, Aharon-Maor A, Sherer Y. Vaccination as an additional player in the mosaic of autoimmunity. Clin Exp Rheumatol. 2000;18:181–84. [PubMed] [Google Scholar]
- 50.Fleeton MN, Chen M, Berglund P, Rhodes G, Parker SE, Murphy M, Atkins GJ, Liljeström P. Self‐replicative RNA vaccines elicit protection against influenza a virus, respiratory syncytial virus, and a tickborne encephalitis virus. J Infect Dis. 2001;183(9):1395–98. doi: 10.1086/319857. [DOI] [PubMed] [Google Scholar]
- 51.Han S, Asoyan A, Rabenstein H, Nakano N, Obst R. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc Natl Acad Sci USA. 2010;107(47):20453–58. doi: 10.1073/pnas.1008437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19(4):408–15. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Wherry EJ, Blattman JN, Murali-Krishna K, Van Der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edwards DK, Jasny E, Yoon H, Horscroft N, Schanen B, Geter T, Fotin-Mleczek M, Petsch B, Wittman V. Adjuvant effects of a sequence-engineered mRNA vaccine: translational profiling demonstrates similar human and murine innate response. J Transl Med. 2017;15(1):1. doi: 10.1186/s12967-016-1111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (α/β) in immunity and autoimmunity. Annu Rev Immunol. 2005;23(1):307–36. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 56.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu Y-J, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J Exp Med. 2005;202(1):135–43. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caso F, Costa L, Ruscitti P, Navarini L, Del Puente A, Giacomelli R, Scarpa R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5):102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White SMCould COVID-19 mRNA vaccines cause autoimmune diseases? 2020. [cited 2021 Mar 1]. https://www.bmj.com/content/371/bmj.m4347/rr-6
- 59.Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, Song Y, Tzima E, Kennerknecht E, Niepmann M, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci USA. 2007;104(15):6388–93. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hause AM, Gee J, Johnson T, Jazwa A, Marquez P, Miller E, Su J, Shimabukuro TT, Shay DK. Anxiety-related adverse event clusters after Janssen COVID-19 vaccination — five U.S. mass vaccination sites, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):685–88. doi: 10.15585/mmwr.mm7018e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, Streiff MB, Rao AK, Wheeler AP, Beavers SF, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448. 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh Malhotra H, Gupta P, Prabhu V, Garg RK, Dandu H, Agarwal V. COVID-19 vaccination-associated myelitis. QJM An Int J Med. 2021;2021:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goss AL, Samudralwar RD, Das RR, Nath A. ANA investigates: neurological complications of COVID-19 vaccines. Ann Neurol. 2021;89(5):856–57. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menaka P, Schull M, Razak F, Grill A, Ivers N, Maltsev A, Miller KJ, Schwartz B, Stall NM, Steiner R, et al. Vaccine-induced prothrombotic immune thrombocytopenia (VIPIT) following AstraZeneca COVID-19 vaccination: interim guidance for healthcare professionals in emergency department and inpatient settings. 2021.
- 66.Pottegård A, Lund LC, Ø K, Dahl J, Andersen M, Hallas J, Lidegaard Ø, Tapia G, Gulseth HL, Ruiz PL-D, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coronavirus vaccine - weekly summary of Yellow Card reporting - GOV.UK. 2021. [cited 2021 May 12]. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting
- 68.COVID-19 vaccine weekly safety report - 06-05-2021 | therapeutic Goods Administration (TGA). 2021. [cited 2021 May 12]. https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-06-05-2021
- 69.COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets | European medicines agency. 2021. [cited 2021 Apr 5]. https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots
- 70.Gorse GJ, Patel GB, Vitale JN, O’Connor TZ. Prevalence of antibodies to four human coronaviruses is lower in nasal secretions than in serum. Clin Vaccine Immunol. 2010;17(12):1875–80. doi: 10.1128/CVI.00278-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sariol A, Perlman S. Lessons for COVID-19 immunity from other coronavirus infections. Immunity. 2020;53(2):248–63. doi: 10.1016/j.immuni.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bradburne AF, Bynoe ML, Tyrrell DAJ. Effects of a “New” human respiratory virus in volunteers. Br Med J. 1967;3(5568):767–69. doi: 10.1136/bmj.3.5568.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Callow KA, Parry HF, Sergeant M, Tyrrell DAJ. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105(2):435–46. doi: 10.1017/S0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hendley JO, Fishburne HB, Gwaltney JM. Coronavirus infections in working adults. Eight-year study with 229 E and OC 43. Am Rev Respir Dis. 1972;105(5):805–11. doi: 10.1164/arrd.1972.105.5.805. [DOI] [PubMed] [Google Scholar]
- 75.Monto AS, Lim SK. The Tecumseh study of respiratory illness. VI. Frequency of and relationship between outbreaks of coronavirus infection. J Infect Dis. 1974;129(3):271–76. doi: 10.1093/infdis/129.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt OW, Allan ID, Cooney MK, Foy HM, Fox JP. Rises in titers of antibody to human corona viruses oc43 and 229e in Seattle families during 1975-1979. Am J Epidemiol. 1986;123(5):862–68. doi: 10.1093/oxfordjournals.aje.a114315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Habibi MS, Jozwik A, Makris S, Dunning J, Paras A, DeVincenzo JP, De Haan CAM, Wrammert J, Openshaw PJM, Chiu C. Impaired antibody-mediated protection and defective iga b-cell memory in experimental infection of adults with respiratory syncytial virus. Am J Respir Crit Care Med. 2015;191(9):1040–49. doi: 10.1164/rccm.201412-2256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu WM, Van Der Zeijst BAM, Boog CJP, Soethout EC. Aging and impaired immunity to influenza viruses: implications for vaccine development. Hum Vaccin. 2011;7(sup1):94–98. doi: 10.4161/hv.7.0.14568. [DOI] [PubMed] [Google Scholar]
- 79.Gr M. Developing a vaccine for human rhinoviruses. J Vaccines Immun. 2014;2(3):16–20. doi: 10.14312/2053-1273.2014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glanville N, Johnston SL. Challenges in developing a cross-serotype rhinovirus vaccine. Curr Opin Virol. 2015;11:83–88. doi: 10.1016/j.coviro.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 81.Garcia-Beltran WF, Lam EC, St. Denis K, Nitido AD, Garcia ZH, Hauser BM, Feldman J, Pavlovic MN, Gregory DJ, Poznansky MC, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184(9):2372–2383.e9. 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, Wang M, Yu J, Zhang B, Kwong PD, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021; 593:1–9. [DOI] [PubMed] [Google Scholar]
- 83.McCallum M, Bassi J, De Marco A, Chen A, Walls AC, Di Iulio J, Alejandra Tortorici M, Navarro M-J, Silacci-Fregni C, Agostini M, et al. SARS-CoV-2 immune evasion by variant B.1.427/B.1.429. bioRxiv. 2021;2021.03.31.437925. doi: 10.1101/2021.03.31.437925 [DOI]
- 84.Frasca D, Riley RL, Blomberg BB. Humoral immune response and B-cell functions including immunoglobulin class switch are downregulated in aged mice and humans. Semin Immunol. 2005;17(5):378–84. doi: 10.1016/j.smim.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 85.Han S, Yang K, Ozen Z, Peng W, Marinova E, Kelsoe G, Zheng B. Enhanced differentiation of splenic plasma cells but diminished long-lived high-affinity bone marrow plasma cells in aged mice. J Immunol. 2003;170(3):1267–73. doi: 10.4049/jimmunol.170.3.1267. [DOI] [PubMed] [Google Scholar]
- 86.Naylor K, Li G, Vallejo AN, Lee -W-W, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174(11):7446–52. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 87.Cicin-Sain L, Smyk-Paerson S, Currier N, Byrd L, Koudelka C, Robinson T, Swarbrick G, Tackitt S, Legasse A, Fischer M, et al. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol. 2010;184(12):6739–45. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205(3):711–23. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford RL, Kronenberg M, Cohen D, Schächter F. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29(6):601–09. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 90.Ahrenfeldt LJ, Otavova M, Christensen K, Lindahl-Jacobsen R. Sex and age differences in COVID-19 mortality in Europe. Wien Klin Wochenschr. 2020;133:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kang SJ, Jung SI. Age-related morbidity and mortality among patients with COVID-19. Infect Chemother. 2020;52(2):154–64. doi: 10.3947/ic.2020.52.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Undurraga EA, Chowell G, Mizumoto K. COVID-19 case fatality risk by age and gender in a high testing setting in Latin America: chile, March–August 2020. Infect Dis Poverty. 2021;10(1):11. doi: 10.1186/s40249-020-00785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yanez ND, Weiss NS, Romand J-A, Treggiari MM. COVID-19 mortality risk for older men and women. BMC Public Health. 2020;20(1):1742. doi: 10.1186/s12889-020-09826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bernal JL, Andrews N, Gower C, Stowe J, Robertson C, Tessier E, Simmons R, Cottrell S, Roberts R, O’Doherty M, et al. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. medRxiv. 2021;2021.03.01.21252652. 10.1101/2021.03.01.21252652 [DOI]
- 96.Hyams C, Marlow R, Maseko Z, King J, Ward L, Fox K, Heath R, Turner A, Friedrich Z, Morrison L, et al. Assessing the effectiveness of BNT162b2 and ChAdOx1nCoV-19 COVID-19 vaccination in prevention of hospitalisations in elderly and frail adults: a single centre test negative case-control study. SSRN Electron J. 2021. doi: 10.2139/ssrn.3796835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nie Y, Wang -Y-Y. Innate immune responses to DNA viruses. Protein Cell. 2013;4(1):1–7. doi: 10.1007/s13238-012-2122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–05. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 99.Schlee M, Roth A, Hornung V, Hagmann CA, Wimmenauer V, Barchet W, Coch C, Janke M, Mihailovic A, Wardle G, et al. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31(1):25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu G, Zhou Y, Tsai B. Cytoplasm and beyond: dynamic innate immune sensing of influenza a virus by RIG-I. J Virol. 2019;93(8):2299–317. doi: 10.1128/JVI.02299-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schlee M. Master sensors of pathogenic RNA – RIG-I like receptors. Immunobiology. 2013;218(11):1322–35. doi: 10.1016/j.imbio.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davis WG, Bowzard JB, Sharma SD, Wiens ME, Ranjan P, Gangappa S, Stuchlik O, Pohl J, Donis RO, Katz JM, et al. The 3′ untranslated regions of influenza genomic sequences are 5′PPP-independent ligands for RIG-i. PLoS One. 2012;7(3):e32661. doi: 10.1371/journal.pone.0032661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bowzard JB, Ranjan P, Sambhara S. RIG-I goes beyond naked recognition. Cell Host Microbe. 2013;13(3):247–49. doi: 10.1016/j.chom.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann -K-K, Schlee M, et al. 5’-triphosphate RNA is the ligand for RIG-I. Science (80-). 2006;314(5801):994–97. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 105.Gorini S, Gatta L, Pontecorvo L, Vitiello L, la Sala A. Regulation of innate immunity by extracellular nucleotides. Am J Blood Res. 2013;3:14–28. [PMC free article] [PubMed] [Google Scholar]
- 106.Gil A. Modulation of the immune response mediated by dietary nucleotides. Eur J Clin Nutr. 2002;56(S3):S1–4. doi: 10.1038/sj.ejcn.1601475. [DOI] [PubMed] [Google Scholar]
- 107.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization: a field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89(4):435–48. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 108.Smatti MK, Al Thani AA, Yassine HM. Viral-induced enhanced disease illness. Front Microbiol. 2018;9:2991. doi: 10.3389/fmicb.2018.02991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martin DB. Atypical measles in adolescents and young adults. Ann Intern Med. 1979;90(6):877–81. doi: 10.7326/0003-4819-90-6-877. [DOI] [PubMed] [Google Scholar]
- 110.Davis BS, Chang GJJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, Bowen R, Bunning ML. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75(9):4040–47. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garver KA, LaPatra SE, Kurath G. Efficacy of an infectious hematopoietic necrosis (IHN) virus DNA vaccine in chinook Oncorhynchus tshawytscha and sockeye O. nerka salmon. Dis Aquat Organ. 2005;64:13–22. doi: 10.3354/dao064013. [DOI] [PubMed] [Google Scholar]
- 112.The global race to vaccinate – foreign policy. 2021. [cited 2021 Apr 6]. https://foreignpolicy.com/2021/03/29/covid-19-vaccine-diplomacy-global-pandemic-response/


