Abstract
The Drosophila CLOCK (dCLOCK) and CYCLE (CYC) (also referred to as dBMAL1) proteins are members of the basic helix-loop-helix PAS (PER-ARNT-SIM) superfamily of transcription factors and are required for high-level expression of the circadian clock genes period (per) and timeless (tim). Several lines of evidence indicate that PER, TIM, or a PER-TIM heterodimer somehow inhibit the transcriptional activity of a putative dCLOCK-CYC complex, generating a negative-feedback loop that is a core element of the Drosophila circadian oscillator. In this report we show that PER and/or TIM inhibits the binding of a dCLOCK-CYC heterodimer to an E-box-containing DNA fragment that is present in the 5′ nontranscribed region of per and acts as a circadian enhancer element. Surprisingly, inhibition of this DNA binding activity by PER, TIM, or both is not accompanied by disruption of the association between dCLOCK and CYC. The results suggest that the interaction of PER, TIM, or both with the dCLOCK-CYC heterodimer induces a conformational change or masks protein regions in the heterodimer, leading to a reduction in DNA binding activity. Together with other findings, our results strongly suggest that daily cycles in the association of PER and TIM with the dCLOCK-CYC complex probably contribute to rhythmic expression of per and tim.
A wide variety of organisms, from cyanobacteria to humans, manifest circadian rhythms in biochemical, physiological, and behavioral phenomena (reviewed in reference 25). These daily rhythms are controlled by endogenous cell-autonomous oscillators or clocks that can be entrained (synchronized) by environmental stimuli, most notably the daily changes in light-dark cycles. Of fundamental importance in the study of circadian biology is an understanding of the molecular circuitry underlying timekeeping mechanisms. The isolation and characterization of “clock genes” in several model systems has provided significant insights into this problem. A common theme that has emerged from these studies is that transcriptional feedback loops constitute a central feature of many, if not all, timekeeping mechanisms (for a recent review, see reference 10).
In Drosophila melanogaster, the period (per) and timeless (tim) genes are essential components of the timekeeping apparatus (reviewed in references 17, 22, 46–48, 53, and 62). For example, several missence mutations in per and tim can either lengthen or shorten the free-running periods of rhythms in eclosion (emergence from pupal cases) and locomotor activity (11, 18, 31, 32, 50). Furthermore, these behavioral rhythms are abolished in the absence of functional per or tim proteins (PER and TIM, respectively) (31, 54). The RNA and protein products from per and tim undergo daily fluctuations in abundance (12, 24, 29, 40, 55, 64, 65), consistent with an important role in pacemaker function. Mutations in coding regions of per and tim have similar effects on behavioral output rhythms and cycles in both per and tim transcripts, indicating that PER and TIM proteins participate in a codependent feedback loop. Daily fluctuations in the levels of per and tim transcripts are driven mainly at the transcriptional level (1, 23, 49, 57), suggesting that PER and TIM can somehow regulate their own transcription. Because PER has a PAS (PER-ARNT-SIM) domain that can mediate protein dimerization but does not appear to bind DNA directly, consistent with the absence of a known DNA binding motif, it was originally proposed by Huang et al. that PER might inhibit transcription by forming nonfunctional heterodimers with PAS-containing transcription factors (28). The codependency for PER and TIM in maintaining this transcriptional feedback loop is at least partly based on the fact that these two proteins form a heterodimeric partnership in the cytoplasm that appears necessary for nuclear entry of both subunits (14, 29, 34, 40, 51, 61, 64). In addition to a transcriptional component, other posttranscriptional regulatory pathways which affect the metabolism of the RNA and protein products from per and tim contribute to the maintenance of a transcriptional-translational feedback loop that can be entrained by external stimuli and is the core of a circadian clock in Drosophila (see, e.g., references 4, 5, 9, 12, 29, 30, 34, 40, 43, 44, 56, 57, 59, and 64).
Recent studies have provided significant insights into our understanding of the components that are involved in the stimulation of per and tim transcription and how PER and/or TIM might function as a negative regulator of gene expression. Two basic-helix-loop-helix (bHLH) PAS transcription factors, termed dCLOCK and CYCLE (CYC) (CYC is also referred to as dBMAL1), probably function as a heterodimeric complex that binds E-box DNA elements present in 5′ regulatory regions found in either per or tim driving their expression (1, 2, 8, 20, 21, 49). For example, mutations in dClock or cyc lead to constitutively low levels of per and tim transcription and arrhythmic behaviors (1, 49). Furthermore, Darlington et al. (8) showed that ectopic expression of dClock in Drosophila tissue culture cells can drive the expression of a reporter gene flanked at the 5′ end with E-box elements present in either per or tim. The stimulatory effect of dCLOCK on E-box-mediated expression of a reporter gene in cultured Drosophila cells is inhibited when PER and TIM are present together but not when one of them is present alone (8). The role of TIM in this inhibition is not clear, because it does not contain a PAS domain (41) and might simply function to shuttle PER into the nucleus (51, 61), where it can participate in transcriptional inhibition. Nonetheless, TIM interacts with the PAS domain of PER (14, 51, 52, 61), raising the possibility that TIM also directly associates with PAS-containing transcription factors to modulate their activity. A similar scenario is also postulated for circadian clocks in mammals, whereby the mammalian homologs of PER (mPER-1 to mPER-3) and TIM block CLOCK-BMAL1/MOP3-mediated expression, generating a circadian feedback loop (15, 27, 52).
Recently, we showed that in Drosophila, PER, TIM, and the PER-TIM heterodimer interact with dCLOCK or a dCLOCK-containing complex during the night but not during most of the day (33). This is consistent with the demonstration that the transcriptional rates of per and tim increase during the day but decline during the night (57). Together, the results suggest that once PER and TIM enter the nucleus, one or more of these proteins interact with a putative dCLOCK-CYC complex, somehow inhibiting its transcriptional activity. In this study, we show that PER and TIM, either separately or together, block the ability of a dCLOCK-CYC heterodimer to specifically bind DNA in an E-box-dependent manner, suggesting a biochemical mode of action for how PER and TIM participate in transcriptional autorepression. Our findings raise the possibility that once in the nucleus, monomeric PER or TIM can directly participate in regulating dCLOCK-CYC-mediated transcriptional activity. Surprisingly, the dCLOCK-CYC complex is not disrupted by interactions with PER, TIM, or both. This result indicates that PAS-containing proteins can stably interact with a bHLH-PAS heterodimer and regulate its activity without disrupting the ability of the two bHLH-PAS subunits to associate with each other.
MATERIALS AND METHODS
Recombinant plasmids.
The construction of a full-length version of dClock was described previously (2). Briefly, we incubated total head RNA isolated from wild-type Canton-S (CS) flies with dClock-specific primers and subjected the mixture to 5′ rapid amplification of cDNA ends (5′ RACE) followed by PCR. A ∼1.3-kb 5′ RACE-PCR product that terminated 387 bp upstream of the dClock translation start site was digested with SacI and AatII (the SacI site is at position −368 relative to the start of translation, whereas the AatII site is at position 890). A dClock expressed sequence tag (EST)-containing plasmid (obtained from Genome Systems Inc. [GenBank accession no. AA698290]) was digested with SacI (present in the polylinker region of the backbone vector) and AatII. Subsequently, the digested 5′ RACE-PCR product and dClock EST plasmid were ligated to generate a contiguous full-length dClock open reading frame [referred to as SK(−)/dClock] (2). To generate a hemagglutinin (HA)-tagged version of dCLOCK (dCLOCK-HA), we digested SK(−)/dClock with SacI and HindIII (position 36 relative to the start of translation). Subsequently, two oligonucleotides containing complementary sequences were hybridized to yield a double-stranded DNA fragment that contained sequences coding for the first two amino acids of dCLOCK followed by the HA epitope and amino acids 3 to 12 of dCLOCK. The oligonucleotides used were 5′-CCTCGAGATGGACTACCCGTACGACGTCCCGGACTACGCCTCCCTGGACGAGAGCGACGACAAG GATGATACAAAA-3′ and 5′-AGCTTTTTGTATCATCCTTGTCGTCGCTCTCGTCCAGGGAGGCGTAGTCCGGGACGTCGTACGGGTAGTCCATCTCGAGGAGCT-3′ (dClock sequences are underlined). The double-stranded oligonucleotide also generated 5′ SacI and 3′ HindIII overhang restriction sites, which were used to ligate the fragment into the SacI-HindIII-digested SK(−)/dClock vector to generate SK(−)/HA-dClock. Finally, to increase the yield of the in vitro-generated transcription product, we digested SK(−)HA-dClock with SacI and KpnI [present in the pBlueScript SK(−) polylinker region] and subcloned the resulting fragment into the pGEM-7Zf(+) vector (Promega) to yield pGEM/HA-dClock. Expression is under the control of the SP6 promoter in the pGEM-based vector, which generally produces larger amounts of transcription products compared to the T3 promoter used to drive expression in the pBlueScript SK(−) vector (data not shown).
To generate a myc epitope-tagged version of CYC (CYC-myc), we subjected a full-length cyc EST plasmid (obtained from Genome System Inc. [GenBank accession number AA695336]) to PCR. The oligonucleotides used were 5′-AATTCCGCGGATGGAAGAGCAGAAGCTGATCTCCGAGGAGGACCTGA ACGTTCAGGAGTTCTGCG-3′ and 5′-GGATGCAGGACGTCGAACCAG-3′ (the cyc sequences are underlined). The resulting PCR product contains at the 5′ end (orientation relative to the sense strand) a SacII site, followed by sequences coding for the myc epitope placed in frame between amino acids 2 and 3 of CYC, and ends at the 3′ end with sequences just downstream of an AatII site present in the cyc open reading frame (position 460 relative to start of translation). Subsequently, the cyc EST-containing plasmid was digested with SacII (present in the polylinker region of the backbone vector) and AatII, and the released fragment was replaced with the PCR product to yield SK(−)/myc-cyc. Finally, SK(−)/myc-cyc was digested with SacII and ApaI (present in the polylinker region of the backbone vector), and the insert was ligated into the pGEM-5Zf(+) vector (Promega) to place expression under the SP6 promoter (the final construct is referred to as pGEM/myc-cyc). Expression plasmids to generate in vitro-translated PER (pSP65ATper [6, 28]), TIM (a pSP65 [Promega] derivative termed pSP65/tim that was constructed based on tim sequences described in references 41 and 42; the open reading frame for the full-length TIM product in this construct begins at the translation start site proposed in reference 41), mammalian ARNT (pSP64/mARNT; a derivative of pCDNAI/Neo/mARNT described in reference 39), c-Rel (36), and IκBα (36) have been described previously.
In vitro transcription and translation.
In vitro-radiolabeled translation products were produced by using appropriate circular plasmids (described above) to prime a coupled transcription-translation rabbit reticulocyte system (TNT; Promega) in the presence of l-[35S]methionine (Amersham) as specified by the manufacturer. A carboxy-terminally truncated version of TIM was generated by digesting a full-length tim cDNA with BamHI followed by recircularization (BamHI cleaves at position 3010 from the start of translation and immediately following the translation stop signal). This deleted plasmid contains the entire tim open reading frame until the nucleotides encoding amino acid 1005 followed by sequences derived from the beginning of the 3′ untranslated region of tim that add 16 heterologous amino acids to the carboxy terminus of the truncated TIM polypeptide (data not shown). The amount of protein produced in each translation was determined by subjecting an aliquot of the translation mixture to trichloroacetic acid precipitation. An incubation that did not include exogenously added RNA served as a background control for the translation reactions. To increase the amount of target protein for use in mobility shift assays (described below), transcription-translation reaction mixtures also contained nonradiolabeled methionine at a molar ratio of 50:1 (cold to hot). Where indicated, in some immunoprecipitation experiments (see below) a version of dCLOCK-HA containing trace amounts of radiolabel was synthesized by performing transcription-translation reactions with a methionine ratio of 50:1 or 400:1 (cold to hot).
DNA mobility shift assay.
Mobility shift assays were performed essentially as previously described (27). The 32P-labeled synthetic oligonucleotide probe used as a substrate in the mobility shift assay contains per sequences from −548 to −508 (numbering according to reference 20). This region includes an E-box element (CACGTG) that is part of a 69-bp fragment previously shown to function as a circadian enhancer region in Drosophila (20). To generate a wild-type E box containing double-stranded DNA fragment, the following two oligonucleotides were annealed: 5′-TTTAGTGAAAAGCCGCCGCTCACGTGGCGAACTGCGTGACTTG-3′ and 5′-TTTCAAGTCACGCAGTTCGCCACGTGAGCGGCGGCTTTTCACT-3′ (the sequence for the E box is underlined). A mutant E-box version was based on one described in reference 8 and was identical to the wild-type DNA probe except that the E-box sequence was changed from CACGTG to CAGCTG (the alteration is underlined). The 5′-TTT overhangs were used to radiolabel the annealed oligonucleotide by a Klenow fill-in reaction in the presence of [α-32P]ATP, and unincorporated nucleotides were removed on a Biospin column (Bio-Rad). For mobility shift assays with NF-κB DNA binding sites (see Fig. 3), we used a previously described synthetic oligonucleotide containing an NF-κB DNA binding site derived from the interleukin-6 enhancer (36). Aliquots of reticulocyte lysates containing the amounts of dCLOCK (or dCLOCK-HA), CYC (or CYC-myc), PER, TIM, ARNT, c-Rel, and IκBα indicated in the figure legends were mixed in different combinations. To minimize nonspecific interactions, 50 mM KCl (final) was added to the mixtures. If necessary, unprogrammed reticulocyte lysate was added to normalize volumes. The mixtures were incubated for 30 min at 30°C, 1.5 μg total of poly(dI-dC) was added, and a further incubation for 10 min was carried out. Subsequently, 0.5 × 106 cpm of 32P-labeled oligonucleotide (∼10 ng) was added and the mixture was incubated for 20 min at 30°C. In competition reactions, nonradiolabeled oligonucleotide was added at a 100-fold molar excess (∼1 μg [final amount]) compared to the radiolabeled version. In experiments that included serum, 1 μl of either an anti-dCLOCK antibody (dCGP-90) (33) or preimmune serum was added to the mixtures after completion of the incubation step with oligonucleotides, and an additional incubation of 10 min at 30°C was carried out. Mixtures were resolved by electrophoresis on nondenaturing 5% polyacrylamide gels in 0.5× TBE (45 mM Tris-borate [pH 7.5], 1 mM EDTA). Subsequently, gels were fixed in 35% methanol–10% acetic acid and dried, and putative protein-DNA complexes were visualized by autoradiography. The relative intensities of bands on gels were quantified by using a PhosphorImager and ImageQuant software (Molecular Dynamics).
FIG. 3.
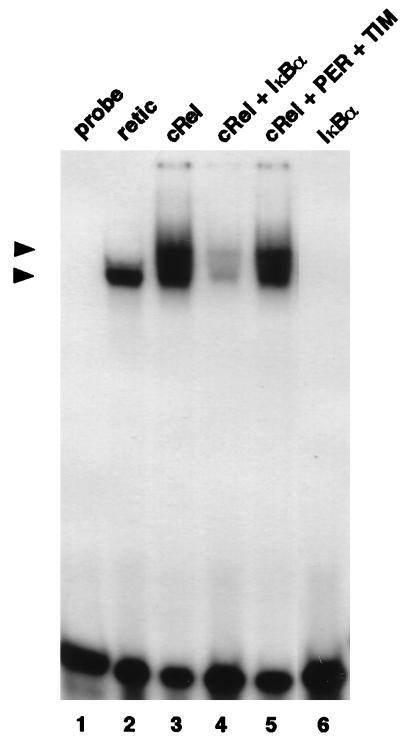
PER and TIM do not inhibit the DNA binding activity of the non-bHLH-PAS transcription factor c-Rel. Reticulocyte lysates containing 7 fmol of c-Rel (lanes 3 to 5), 14 fmol of IκBα (lane 4 and 6), or 14 fmol each of PER and TIM (lane 5) were incubated with a 32P-radiolabeled synthetic oligonucleotide containing NF-κB DNA binding sites (lanes 1 to 6), and protein-DNA complexes visualized as described in the legend to Fig. 1. The positions of a complex formed by a c-Rel homodimer (top arrowhead) and an endogenously produced complex (bottom arrowhead) that is also observed when using nonprogrammed reticulocyte lysate (lane 2) are indicated.
Immunoprecipitation.
Reticulocyte lysates containing known amounts of [35S]methionine-labeled target proteins (i.e., PER, TIM, dCLOCK-HA, and CYC-myc) were mixed in different combinations and incubated under conditions similar to those used for the mobility shift assays (e.g., 30 min at 30°C). Subsequently, 500 μl of IB solution (5 mM Tris-Cl, 10 mM HEPES [pH 7.5], 10% glycerol, 50 mM KCl, 0.05% Triton X-100, 1 mM EDTA, 1 mM dithiothreitol, 0.25 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml, 5 μg of leupeptin per ml, 1 μg of pepstatin A per ml) was added. To eliminate nonspecific interactions, 15 μl of GammaBind Plus beads (Promega) was added, the mixture was incubated with gentle rotation for 30 min at 25°C and centrifuged, and clarified supernatant was transferred to a new tube. A slurry containing 3 μl of an antibody against the HA epitope (12CA5), the myc epitope (9E10), or PER (GP73) (56) and 15 μl of GammaBind Plus beads (the beads were equilibrated in IB solution containing 1% nonfat dry milk) were added to the precleared mixtures as indicated in the figure legends and incubated with gentle rotation for 2 h at 25°C. Subsequently, the beads were collected by centrifugation, washed three times in 0.5 ml of IB for 10 min each, mixed with 10 μl of 2× sodium dodecyl sulfate (SDS) sample buffer, boiled, and centrifuged. The resulting supernatants were resolved by SDS-polyacrylamide gel electrophoresis (10% polyacrylamide), and radiolabeled peptides were visualized by autoradiography. The relative stoichiometries of the individual target proteins present in immune complexes were calculated by using a densitometer (Computing Densitometer Scan v 5.0) and ImageQuant software followed by normalizing for methionine content.
RESULTS
dCLOCK and CYC bind an E-box DNA element found in the per circadian regulatory enhancer.
Hao et al. showed that a 69-bp fragment in the 5′ nontranscribed region of per that contains an E-box DNA element (CACGTG) can confer rhythmic expression to a reporter gene with a similar phase and amplitude to wild-type per (20, 21). This element can also drive dCLOCK-mediated expression of a reporter gene in cultured Drosophila cells (8). Furthermore, mutations in dClock and cyc yield arrhythmic flies that manifest low and constitutive levels of per and tim transcripts (1, 49). These lines of evidence strongly suggest that a dCLOCK-CYC complex interacts with E-box elements found in per and tim 5′ regulatory DNA, stimulating their transcription.
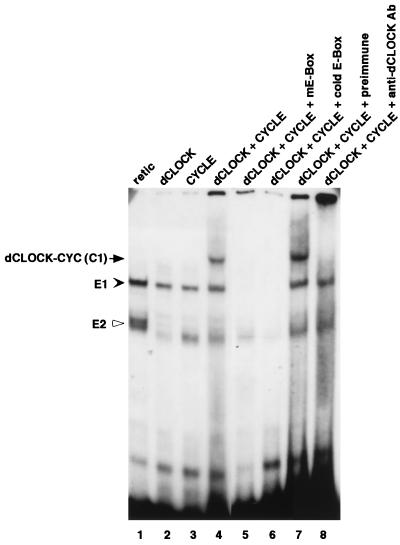
To obtain biochemical evidence that dCLOCK and CYC, when present together, can interact with the per cycling region, we performed mobility shift assays with proteins that were produced in vitro by a coupled transcription-translation rabbit reticulocyte system. The reactions were carried out in the presence of trace amounts of [35S]methionine to enable accurate quantitation of target protein levels. Reticulocyte lysates containing known amounts of in vitro-produced target proteins were mixed at the desired molar concentrations and subsequently incubated with a 40-bp 32P-radiolabeled synthetic DNA oligonucleotide that contained the E box and portions of flanking sequences found in the 69-bp per circadian enhancer fragment. Putative DNA-protein complexes were resolved by subjecting the mixture to electrophoresis on low-ionic-strength native polyacrylamide gels, and the relative intensities of appropriate bands were quantified (Fig. 1). When lysates containing dCLOCK and CYC were incubated together, a novel band was detected (lane 4) that was absent in nonprogrammed lysates (lane 1) or when either dCLOCK or CYC was produced separately (lanes 2 and 3, respectively).
FIG. 1.
dCLOCK/CYC-mediated binding to an E box present in a per circadian regulatory element. Reticulocyte lysates containing 10 fmol each of dCLOCK (lane 2), CYC (lane 3), or a mixture of the two (lanes 4 to 8) were incubated with either the wild-type pwtE oligonucleotide (lanes 1 to 4 and 6 to 8) or the pmE E-box mutant version (lane 5). Subsequently, mixtures were resolved by electrophoresis on native polyacrylamide gels followed by autoradiography. The dCLOCK/CYC specific mobility shift (C1) is indicated by the arrow (left). Also indicated are two complexes, termed E1 (solid arrowhead) and E2 (open arrowhead), that are observed when using nonprogrammed reticulocyte lysate (lane 1). Addition of a 100-fold molar excess (1 μg) of cold wild-type E-box DNA abolished detection of C1, E1, and E2 (lane 6), whereas addition of antibodies (Ab) to dCLOCK specifically inhibited C1 (lanes 7 and 8). The “free” unbound radiolabeled DNA probe is at the bottom of the gel.
Several control experiments indicate that the appearance of this novel band represents a specific interaction between dCLOCK, CYC, and the E-box sequence-containing DNA fragment (herein referred to as pwtE). First, the dCLOCK-CYC-specific band was absent when a mutant 32P-radiolabeled oligonucleotide (pmE) that differed from the “wild-type” pwtE sequence only by a 2-nucleotide inversion in the canonical E-box sequence was used (CAGCTG; alteration is underlined) (lane 5). This mutation was previously shown to abrogate dCLOCK-mediated expression of a reporter gene in cultured Drosophila cells (8). Second, an excess amount of cold pwtE oligonucleotide but not an equivalent amount of the pmE version functioned as an effective competitive inhibitor of complex formation (lane 6 and data not shown). Finally, a polyclonal antibody against dCLOCK but not preimmune sera eliminated detection of the dCLOCK/CYC specific mobility shift (compare lanes 7 and 8). In addition to the specific mobility shift detected in the presence of dCLOCK and CYC (herein referred to as dCLOCK/CYC complex 1 [C1]), two other bands were also observed in the presence of pwtE (these complexes are referred to as E-box complex 1 and 2 [E1 and E2, respectively]) but not of pmE. Also, the intensities of E1 and E2 were not reduced by the addition of antibodies against dCLOCK (lane 8). Although the nature of the complexes giving rise to E1 and E2 is not known, it is possible that they involve bHLH or bHLH-PAS proteins endogenously present in the reticulocyte system.
PER and TIM inhibit the dCLOCK/CYC specific mobility shift.
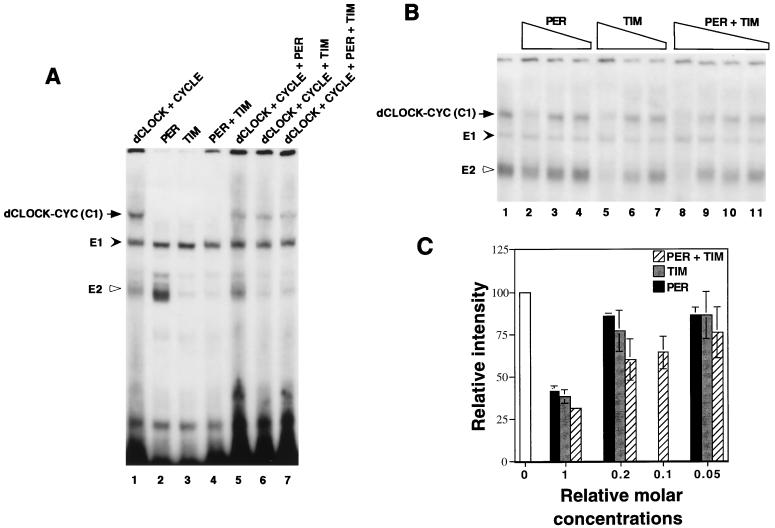
Having established conditions to measure the specific binding of a putative dCLOCK-CYC complex to an E-box element, we next sought to determine whether PER, TIM, or both can inhibit this DNA binding activity (Fig. 2A). Each protein was produced separately and subsequently incubated together in different combinations prior to the addition of the radiolabeled pwtE oligonucleotide (the final concentrations of dCLOCK and CYC were 10 fmol, whereas PER and TIM were each present at 20 fmol). PER or TIM did not form a detectable complex with pwtE (lanes 2 to 4), consistent with the lack of any apparent DNA binding motifs on either protein. However, the presence of PER, TIM, or both reduced the intensity of the dCLOCK/CYC specific mobility shift band by ∼80% (Fig. 2A, compare lanes 5 to 7 with lane 1). Similar concentration-dependent reductions in the intensity of the C1 mobility shift were observed for PER, TIM, or both (Fig. 2B and C), suggesting that TIM is also a potent inhibitor of transcription. Under our experimental conditions, strong inhibition required a ∼1:1 molar ratio of inhibitor (i.e., PER, TIM, or PER-TIM complex) to dCLOCK or CYC (Fig. 2A and B and data not shown). This suggests that inhibition is due to stoichiometric binding of PER or TIM to one or both subunits of the putative dCLOCK-CYC heterodimer (see below). An intriguing observation is that whereas the endogenously produced E1 complex is essentially unaffected by the addition of PER, TIM, or both, the intensity of the faster-migrating E2 band is severely reduced in the presence of TIM but not PER (Fig. 2A, compare lanes 2 to 4; Fig. 2B, compare lanes 2, 5, and 8). These results suggest that TIM but not PER can interact with one or more proteins participating in the formation of E2. Thus, although PER and TIM can interact with each other, they probably do not share equivalent protein binding specificities (also see Fig. 5).
FIG. 2.
PER and TIM inhibit the DNA binding activity of dCLOCK/CYC. (A) Reticulocyte lysates containing dCLOCK (10 fmol), CYC (10 fmol), PER (20 fmol), and TIM (20 fmol) in different combinations (indicated at the top) were incubated with the pwtE [32P]DNA fragment and complexes visualized as described in the legend to figure 1. E2 is inhibited by TIM but not PER (compare lanes 2 and 3). (B) Mixtures containing 10 fmol of dCLOCK and 10 fmol of CYC (lane 1) were incubated with decreasing amounts of PER (lanes 2 to 4), TIM (lanes 5 to 7), or PER and TIM (lanes 8 to 11). PER and TIM were added at a final concentration of 20 fmol (1×; lanes 2, 5, and 8), 4 fmol (0.2×; lanes 3, 6, and 9), 2 fmol (0.1×; lane 10), or 1 fmol (0.05×; lane 4, 7, and 11). (C) The relative intensities of the C1 complex in panel B were pooled with data obtained from two other independent experiments. The intensity of the C1 complex in the control reaction (e.g., panel B, lane 1) was set to 100, and all other values were normalized to this. “Relative molar concentrations” refers to the amount of PER and TIM in the mixture where 20 fmol is defined as 1×.
FIG. 5.
The dCLOCK-CYC complex is not disrupted by interactions with PER and/or TIM. (A) Expression plasmids containing open reading frames for different proteins (indicated at the top) were used to program a coupled transcription-translation system in the presence of [35S]methionine. An aliquot of each mixture containing 3 fmol of each target radiolabeled protein was resolved by SDS-polyacrylamide gel electrophoresis (10% polyacrylamide) and visualized by autoradiography. The positions of molecular weight standards (Bio-Rad) (in thousands) are shown at the left. A higher-molecular-weight band was routinely detected in translation reaction mixtures containing per plasmid (lane 3; identified by the right arrowhead). The production of two electrophoretic mobility variants of CYC-myc (lane 2) is due to the occasional utilization of an in-frame translation start site (data not shown). (B) Reticulocyte lysates containing 20 fmol each of [35S]methionine-labeled dCLOCK-HA (lanes 1 and 2), CYC-myc (lane 3), or both (lane 4) were incubated with antibodies (Ab) against myc (lane 1) or HA (lanes 2 to 4), and immune complexes were recovered by centrifugation. dCLOCK-HA(∗) refers to a transcription-translation reaction mixture containing no exogenously added cold (i.e., nonradiolabeled) methionine, whereas all other dCLOCK-HA proteins were produced with a mixture of [35S]methionine to nonradiolabeled methionine (1:50). The positions of dCLOCK-HA and CYC-myc are indicated on the right. (C and D) Three independent experiments are shown (C, lanes 1 to 4 and lanes 5 to 8, and D, lanes 1 to 10). Reticulocyte lysates containing 20 fmol of dCLOCK-HA (produced by using a mixture of [35S]methionine to nonradiolabeled methionine [1:400]) (as a control experiment dCLOCK-HA was omitted; C, lanes 1 and 2), 20 fmol of CYC-myc, and different amounts of PER and TIM were mixed, and immune complexes were recovered in the presence of either anti-HA antibodies (C, lanes 1 to 4 and 6 to 8, and D, lanes 1 to 10) or a nonspecific monoclonal antibody (C, lane 5). (C) The final amounts of PER and TIM in the mixtures were 20 fmol (lane 1), 60 fmol (lane 2), 20 fmol (lane 3), 60 fmol (lane 4), 60 fmol (lane 5), 0 fmol (lane 6), 20 fmol (lane 7), and 60 fmol (lane 8). (D) The final amounts of PER and TIM in the mixtures were 20 fmol (lanes 2, 5, and 8), 40 fmol (lanes 3, 6, and 9), and 60 fmol (lanes 4, 7, and 10). The positions of PER, TIM, and CYC-myc are indicated (note that because of the low-specific-activity radiolabeling, dCLOCK-HA is barely visible at the exposures shown). (E) Different combinations of in vitro-translated proteins (in these experiments dCLOCK-HA was produced with a mixture of [35S]methionine to nonradiolabeled methionine [1:50]) were subjected to immunoprecipitation with anti-PER (lane 1), anti-myc (lanes 2 and 3), or anti-HA (lane 4 and 5) antibodies and analyzed by SDS-polyacrylamide gel electrophoresis (10% polyacrylamide). In the experiment shown, 20 fmol of PER or TIM and 80 fmol of dCLOCK-HA or CYC-myc were used. The positions of PER, dCLOCK-HA, TIM, and CYC-myc are indicated on the right.
To rule out the possibility that PER and TIM “nonspecifically” inhibit the ability of a dCLOCK/CYC complex to bind DNA, we used the c-Rel transcription factor, which does not contain a PAS domain and can form a homodimer that specifically interacts with NFκB DNA binding sites (reviewed in reference 3). Furthermore, the DNA binding activities of the Rel/NF-κBα superfamily of transcription factors can be specifically blocked by interactions with the IκBα inhibitor. In agreement with previous results (36), incubation of a synthetic oligonucleotide containing an NF-κB DNA binding site with c-Rel produced in a rabbit reticulocyte lysate yields two specific mobility band shifts: one that is slower migrating and contains c-Rel, and one that migrates faster and is also present in unprogrammed lysates (Fig. 3, compare lanes 2 and 3). As expected, IκBα reduces the intensities of both complexes (lane 4). However, the addition of PER and TIM at relative concentrations that were effective in strongly blocking dCLOCK/CYC binding to DNA (Fig. 2) had no effect on the ability of c-Rel to interact with DNA (lane 5).
PER and TIM can inhibit the DNA binding activity of other bHLH-PAS proteins.
We next sought to determine whether PER or TIM could inhibit E-box-mediated DNA binding of other bHLH-PAS-containing proteins. As an initial test case, we used the mammalian arylhydrocarbon receptor nuclear translocator protein (ARNT). Several studies indicate that in vitro the Drosophila PER protein can interact with mammalian ARNT (28, 35, 58). This is consistent with the observation that PER can block the ability of an arylhydrocarbon receptor (AHR)-ARNT heterodimer to bind DNA in vitro and stimulate gene expression in cultured mammalian cells (35). Although ARNT was initially characterized as a bHLH-PAS protein that forms a heterodimer with AHR to mediate the induction of xenobiotic genes in response to ligand binding (e.g., to dioxin) (reviewed in reference 19), numerous lines of evidence strongly suggest that ARNT is widely expressed and interacts with a large variety of bHLH-PAS proteins (reference 26 and references therein). Furthermore, unlike many bHLH-PAS proteins, ARNT can form a stable homodimer in vitro that can interact with DNA containing a CACGTG type E-box (58, 60). In agreement with this result, a translation mixture containing ARNT and incubated with pwtE resulted in the detection of a novel mobility shift band (Fig. 4A, compare lanes 1 and 2). The ARNT-specific band is eliminated when the pmE E-box mutant is used as the DNA substrate (lane 6). Whether the ARNT-specific band is due to homodimeric formation or interactions with an unknown partner(s) in the reticulocyte lysate is not clear. In any event, PER, TIM, or both inhibit this ARNT-specific mobility shift (lanes 3 to 5). Similar to results obtained with dCLOCK/CYC-mediated DNA binding, TIM is a slightly more potent inhibitor than PER when present at equimolar concentrations (Fig. 4B). In addition to the inhibition of ARNT-mediated DNA binding, PER and TIM were effective in reducing the DNA binding activities of other bHLH-PAS proteins to various extents (data not shown). This is perhaps not surprising for Drosophila PER, because it has been shown to interact in vitro with several nonphysiologically relevant bHLH-PAS proteins (e.g., SIM, ARNT, and AHR) (28, 35, 38, 58). With respect to TIM however, our results suggest that it also has the intrinsic capacity to directly interact with a broad range of bHLH-PAS transcription factors.
FIG. 4.
PER and TIM can inhibit the DNA binding activity of ARNT. (A) Reticulocyte lysates containing equivalent molar amounts (i.e., 20 fmol) of different exogenously produced proteins (indicated at the top) were incubated with either a wild-type (lanes 1 to 5) or mutant (lane 6) E-box [32P]DNA probe and analyzed as described in the legend to Fig. 1. A novel band is visible in the presence of ARNT (lane 2; left arrow) compared to nonprogrammed lysates (lane 1). (B) The relative intensities of the C1 complex in panel A were pooled with data obtained from two other independent experiments. The intensity of the C1 complex in the control reaction (e.g., panel A, lane 2) was set to 100, and all other values were normalized to this.
Interactions between dCLOCK, CYC, PER, and TIM.
PER and TIM might inhibit dCLOCK/CYC-mediated DNA binding by acting as competitive inhibitors, whereby stable nonfunctional heterodimers are formed (e.g., PER-dCLOCK), reducing the yield of putative dCLOCK-CYC complexes. This scenario is based on the well-demonstrated ability of the PAS domain to function as a protein dimerization interface (7, 28). However, we recently showed that in vivo PER, TIM, and dCLOCK are present in the same complex, indicating that PAS-containing proteins are not limited to binary interactions (33). This raises the possibility that PER and TIM interact with a dCLOCK-CYC dimer, somehow blocking its DNA binding activity.
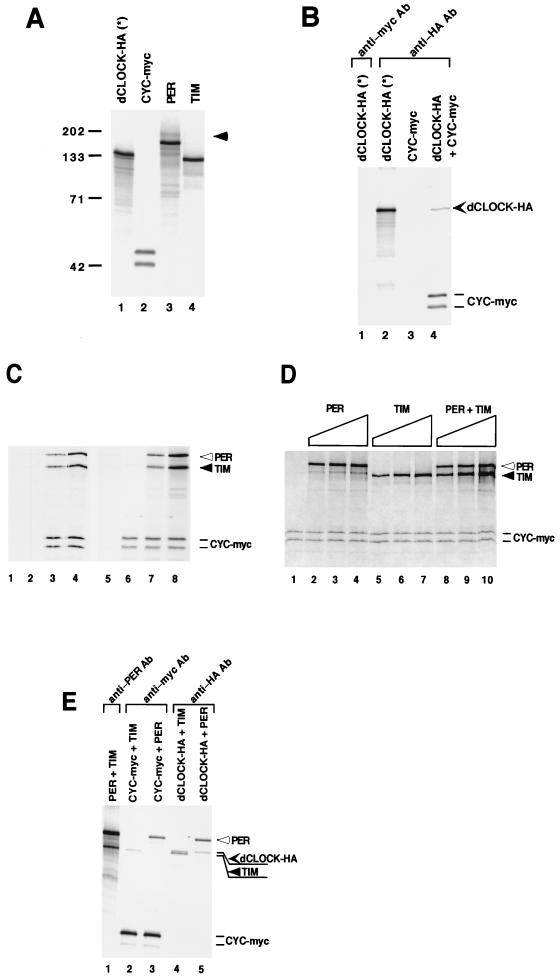
To begin to address these possibilities, we performed coimmunoprecipitation experiments with [35S]methionine-labeled proteins (Fig. 5). During the course of these studies, we noticed the appearance of a high-molecular-weight product in extracts primed with per cDNA expression vectors that migrated more slowly than full-length PER (lane 3; this upper band might be due to a fraction of PER being phosphorylated in vitro [data not shown]). Because this upper band comigrates with full-length TIM (data not shown), we used a carboxy-terminally deleted version of TIM that ends at amino acid 1005 (Fig. 5A, lane 4; see Materials and Methods), which was previously shown to interact with PER (14, 51). In addition, dCLOCK (lane 1) and CYC (lane 2) were modified at their amino-terminals with either the HA or myc tagging peptide, respectively. Two versions of CYC-myc differing in electrophoretic mobility were observed (lane 2; the faster-migrating band is probably due to translation beginning at an in frame start codon, data not shown). Finally, because dCLOCK-HA has a similar size to the deleted version of TIM (compare lanes 1 and 4), we used a dCLOCK-HA protein with a much lower specific activity in [35S]methionine (see Materials and Methods).
First, we sought to determine the ability of dCLOCK-HA and CYC-myc to interact in vitro. Equivalent molar concentrations (20 fmol each) of dCLOCK-HA and CYC-myc were incubated under conditions similar to those used for the mobility shift assays. Subsequently, immune complexes were recovered with a monoclonal anti-HA antibody and the relative stoichiometries of target proteins present in immune pellets were determined (Fig. 5B). In four independent experiments, the average stoichiometry of dCLOCK-HA and CYC-myc recovered in immune complexes was 1:0.7 to 1:0.75. Thus, approximately 70 to 75% of the dCLOCK molecules are stably bound to CYC. Similar results were obtained when anti-myc antibodies were added to purify CYC-myc (data not shown). Several control experiments, including the use of irrelevant antibodies and the omission of dCLOCK-HA, confirmed that the interaction of CYC with dCLOCK was specific (lanes 1 and 3 and data not shown).
Having established reproducible conditions that yield a significant fraction of stable dCLOCK-CYC complexes, we next determined the efficiency of CYC to interact with dCLOCK in the presence of increasing amounts of PER, TIM, or both (Fig. 5C and D). Extracts containing individual radiolabeled proteins were mixed, and immune complexes were recovered in the presence of anti-HA antibodies. Specificity was confirmed by showing that only negligible amounts of PER, TIM, or CYC were present in immune pellets when dCLOCK-HA was omitted from the mixture (Fig. 5C, lanes 1 and 2) or when nonspecific antibodies were used (lane 5). When all four proteins were mixed at equimolar concentrations (20 fmol), there was no decrease in the amount of CYC bound to dCLOCK, although approximately 20 to 40% (n = 3) of the recovered dCLOCK-HA was bound to PER and TIM (Fig. 5C, lanes 3 and 7; Fig. 5D, lane 8). This was the case even under conditions where PER and TIM were present in relative molar concentrations that were greater than those effective in producing strong inhibition of DNA binding activity (Fig. 5C, lane 4 and 8; Fig. 5D, lanes 3, 4, 6, 7, 9, and 10; compare with the results shown in Fig. 2C). For example, when dCLOCK-HA, CYC-myc, PER, and TIM were mixed at a 1:1:3:3 molar ratio (Fig. 5C, lanes 4 and 8; Fig. 5D, lane 10), ∼70% of the recovered dCLOCK was still bound to CYC, even though a significantly larger fraction of dCLOCK (∼50 to 90%) (n = 3) also interacted with PER and TIM. Similar results were also obtained when PER or TIM was added separately (Fig. 5D, lanes 2 to 7). In this case comparable molar amounts of either PER or TIM interacted with the dCLOCK-CYC complex (Fig. 5D, lanes 2 to 7), consistent with the observation that they have similar abilities to inhibit dCLOCK-CYC-mediated DNA binding activity (Fig. 1 and 2). Furthermore, under our experimental conditions, approximately 50% of the PER and TIM present in a mixture interacted to form a stable complex (Fig. 5E, lane 1). It is possible that the assembly of a PER-TIM heterodimer is the basis for the slightly better inhibition of dCLOCK-CYC binding to DNA obtained in the presence of both PER and TIM compared to either alone (Fig. 2B and C).
Roughly similar amounts of PER and TIM copurified with dCLOCK-HA (Fig. 5E, lanes 2 to 5). However, in several independent experiments (n = 3), we noted that whereas equivalent molar amounts of PER associated with dCLOCK-HA and CYC-myc (lanes 3 and 5), the levels of TIM that copurify with CYC-myc were barely above background values obtained with nonspecific antibodies (lane 2 and data not shown), indicating that under the conditions used, TIM probably does not interact specifically with CYC. At the very least, these results indicate that TIM has a higher affinity for dCLOCK than for CYC. Taken together, the results strongly suggest that PER, TIM, or both do not disrupt the dCLOCK-CYC complex but, rather, associate with this heterodimer to form stable trimeric or tetrameric structures that have an impaired ability to bind DNA.
DISCUSSION
In this study, we showed that PER, TIM, or both can inhibit dCLOCK-CYC-dependent binding to an E-box-containing circadian enhancer element. This is consistent with our previous findings showing that in adult fly heads, PER and TIM interact with dCLOCK or a dCLOCK-containing complex during times in a daily cycle when the transcription rates of per and tim are at trough values (33, 57). Because dCLOCK and CYC are required for the stimulation of per and tim transcription (1, 8, 49), our results strongly suggest that in Drosophila PER and TIM inhibit their own transcription by physically interacting with the dCLOCK-CYC complex and impairing its DNA binding activity. Likewise, mammalian TIM and PER (mPER1) were recently shown to specifically inhibit CLOCK-BMAL1-induced stimulation of the mPer-1 promoter in cultured cells (52), suggesting that a similar mode of inhibition also operates in mammals. Unexpectedly, however, our findings demonstrate that PER and TIM do not disrupt the dCLOCK-CYC heterodimer, indicating that inhibition of DNA binding activity is not due to the formation of nonfunctional heterodimers. In addition, our studies demonstrate that TIM can directly interact with bHLH-PAS proteins and that it has the same intrinsic capacity as PER to function as a repressor of transcription.
The ability of PER to inhibit transcription was formally demonstrated for the first time by using the mammalian dioxin signaling pathway, which is mediated by the ligand-generated complex of AHR and ARNT (35). The results indicated that (i) the PAS domain of PER can dimerize with both AHR and ARNT subunits in vitro and disrupt AHR-ARNT binding to DNA and (ii) ectopic expression of Drosophila PER blocks the ability of the AHR-ARNT complex to activate transcription in mammalian cells. Recent studies identified dCLOCK, CYC, or both as possible physiologically relevant targets of PER in the Drosophila circadian feedback loop (1, 2, 8, 33, 49). The findings presented in this report strongly suggest that the molecular underpinning for how PER inhibits its own transcription is based at least partly on its ability to interact stably with one or both subunits of the dCLOCK-CYC complex and abrogate its DNA binding activity.
Although per and tim RNA cycling are both abolished in a tim null mutant (tim0) (54, 55), the role(s) of TIM in autoinhibition has been more enigmatic than that of PER. Based on the apparently exclusive cytoplasmic localization of a series of PER–β-galactosidase fusion proteins in tim0 flies, it was initially suggested that the biochemical function of TIM is to transport PER to the nucleus (61). Thus, the observation that maximal inhibition of dCLOCK-mediated transcription in Drosophila tissue culture cells is observed when PER and TIM are present together could result from a blocked or reduced nuclear localization of PER in the absence of TIM (8). However, the reciprocal relationship, whereby the nuclear localization of TIM requires PER, also seems to operate (29, 40, 51), raising the formal possibility that TIM is the active negative regulator of transcription and PER plays a less direct role in enhancing the nuclear entry of TIM. Although TIM does not contain a PAS region (41), it can nonetheless interact with the PAS domain of PER (14, 51, 52), raising the possibility that it binds other PAS-containing proteins. Indeed, we show that in vitro TIM interacts strongly with dCLOCK (and presumably ARNT [Fig. 3]) but weakly or not at all with CYC (Fig. 5), suggesting that TIM has a protein interaction region that functionally operates like a PAS domain to mediate specific associations with a limited number of PAS-containing members. Likewise, not all members of the bHLH-PAS superfamily interact with each other, and some of the specificity lies in the PAS region (7). The observation that PER, TIM, or both have similar inhibitory effects on the DNA binding activity of the dCLOCK-CYC complex (Fig. 2) raises the possibility that in the nucleus either monomeric PER or TIM can directly inhibit the transcriptional activity of a dCLOCK-CYC heterodimer.
Notwithstanding this possibility, circumstantial evidence suggests that in Drosophila the PER-TIM complex is the major functional entity participating in the per-tim-based autoinhibitory loop. This contention is buttressed by two key observations made during times in a daily cycle when per and tim transcription rates begin to decline. (i) PER protein is found almost exclusively as a heterodimeric complex stably bound to TIM (33, 64). This is consistent with the observations that at these times in a daily cycle TIM is present in approximately twice the molar amount of PER and that the PER-TIM complex is in a 1:1 stoichiometric relationship (33, 64). (ii) dCLOCK or a dCLOCK-containing complex interacts mainly with the PER-TIM complex compared to “free” TIM (i.e., free of PER) (33). This observation suggests that in Drosophila free TIM contributes little or nothing to the direct inhibition of transcription. Based on the behavior of a per transcript expressed from a transgene missing 5′ regulatory elements, So and Rosbash (57) also proposed that PER or the PER-TIM heterodimer, but not free TIM, plays a more prominent role in the negative feedback of transcription. The preferential binding of the PER-TIM complex to dCLOCK in vivo might suggest that free TIM is bound to other factors, precluding its ability to interact with the dCLOCK-CYC complex. In addition, phosphorylation and/or other posttranslational modifications might alter the relative affinities of free TIM and TIM associated with PER (e.g., the PER-TIM complex) to interact with dCLOCK-CYC in vivo.
PER and/or TIM might also function in transcriptional inhibition by retaining one or more subunits of the dCLOCK-CYC heterodimer in the cytoplasm. In at least one other case, it has been shown that the nuclear entry of a bHLH-PAS protein is regulated: AHR is bound to the heat shock protein HSP90 in the cytoplasm and, in response to ligand (e.g., dioxin), translocates to the nucleus to form a transcriptionally active AHR-ARNT heterodimer (reviewed in reference 19). Thus, similar to IκB inhibitors, which abrogate the activities of the NF-κB family of transcription factors in the cytoplasm and nucleus (references 36 and 37 and references therein), PER and TIM might have dual sites of action, interacting with dCLOCK/CYC in the cytoplasm to block its nuclear entry and in the nucleus to inhibit its DNA binding activity. Future studies probing the subcellular distributions of dCLOCK and CYC as a function of time in wild-type flies and in the different circadian clock mutants should provide insights into these possibilities.
Based on the demonstrated ability of the PAS domain to promote heterotypic and homotypic dimer formation, it was originally postulated that PER might inhibit a PAS-containing transcription factor by forming nonfunctional heterodimers (28). Although not extensively studied, biochemical evidence for this type of mechanism has been obtained by using the bHLH-PAS factors hypoxia-inducible factor 1α (HIF-1α) and AHR; HIF-1α functionally interferes with the dioxin signal transduction pathway at the level of heterodimer formation by competing for recruitment of the common partner, ARNT (16). An unexpected finding from our study is that the interaction of PER, TIM, or both with a mixture of dCLOCK/CYC does not inhibit the ability of dCLOCK and CYC to interact with each other (Fig. 5). Rather, our results suggest that PER, TIM, dCLOCK, and CYC can assemble to form a stable tetrameric complex with impaired DNA binding capabilities. Although the protein regions that govern how these four components interact in the different combinations are not clear and will require extensive structure-function analysis, our results suggest that the binding of PER, TIM, or both to dCLOCK-CYC either blocks its DNA binding regions or induces conformational changes that inhibit its ability to bind DNA. A clear precedent for this type of inhibition is based on the ability of members of the IκB family of proteins to block the activity of the Rel/NF-κB family of transcription factors. Binding of IκB to Rel/NF-κB dimers can mask both the nuclear localization signal and DNA binding regions of the intact dimer blocking its transcriptional activity (see, e.g., references 36 and 37).
It will be of interest to determine whether the PAS domain can engage in trimeric and higher-order structures, in addition to its well-characterized ability to form dimers. The canonical PAS region found in bHLH-PAS proteins is ∼250 to 300 amino acids long and contains two conserved repeats of approximately 50 amino acids, designated PAS-A and PAS-B (reviewed in reference 7). Several studies indicate that whereas both PAS-A and PAS-B are required for maximal interactions between bHLH-PAS proteins, in some cases deletion of either region does not severely impair dimer formation (see, e.g., references 13 and 45). These findings could imply that the PAS-A and PAS-B repeats independently promote protein-protein interactions and that this is the basis for the ability of at least some bHLH-PAS proteins to simultaneously accommodate more than one partner. In this context, it is interesting that Zelzer et al. (63) showed that replacement of the Trachealess (Trh) PAS domain by an analogous region of Single-minded (SIM) was sufficient to convert it into a functional SIM protein. They suggested that bHLH-PAS heterodimers can associate, through their PAS regions, with additional distinct proteins to confer specificity for binding to target gene DNA. Nonetheless, it is important to point out that other regions besides the PAS domain might be involved in mediating trimeric and tetrameric interactions between PER, TIM, dCLOCK, and CYC. For example, in both the Drosophila and mammalian systems, several distinct fragments of PER that do not include the PAS domain can physically interact with different sites on TIM (51, 52). In any event, we speculate that the abilities of PER and TIM to interact with a dCLOCK-CYC heterodimer without dissociating the two transcription factors provides a mechanistic framework that can account for the observation that PER and TIM also function as transcriptional activators of dClock expression (2, 33). For example, PER, TIM, or both might selectively interact with a range of dimeric transcription factors and act as coactivators to modulate their activities.
An emerging theme in the study of circadian clocks from a wide variety of organisms is that transcriptional feedback loops encompassing both positive and negative elements play prominent roles in the oscillatory mechanisms underlying circadian clocks. Based on our findings and the overall similarities of clock mechanisms, we predict that negative factors in circadian transcriptional feedback loops will function, at least partly, by physically interacting with positive transcription factors inhibiting their DNA binding activities. Rhythmic changes in the levels or activities of negative factors would result in positively acting transcription factors with cyclical activities.
ACKNOWLEDGMENTS
We thank the following people for kindly providing plasmids: Jerry Pelletier and Peter Moffett for pSP64/mARNT, Amita Sehgal for a plasmid containing tim cDNA sequences, and Celine Gelinas for plasmids that encode c-Rel and IκBα.
This work was supported by an NIH grant to I.E.
C.L. and K.B. contributed equally to this work.
REFERENCES
- 1.Allada R, White N E, So W V, Hall J C, Rosbash M. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell. 1998;93:791–804. doi: 10.1016/s0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- 2.Bae K, Lee C, Sidote D, Chuang K, Edery I. Circadian regulation of a Drosophila homolog of the mammalian clock gene: PER and TIM function as positive regulators. Mol Cell Biol. 1998;18:6142–6151. doi: 10.1128/mcb.18.10.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin A S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Hunter-Ensor M, Schotland P, Sehgal A. Alterations of per RNA in noncoding regions affect periodicity of circadian behavioral rhythms. J Biol Rhythms. 1998;13:364–379. doi: 10.1177/074873098129000192. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, Gvakharia B, Hardin P E. Two alternatively spliced transcripts from the Drosophila period gene rescue rhythms having different molecular and behavioral characteristics. Mol Cell Biol. 1998;18:6505–6514. doi: 10.1128/mcb.18.11.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Citri Y, Colot H V, Jacquier A C, Yu Q, Hall J C, Baltimore D, Rosbash M. A family of unusually spliced biologically active transcripts encoded by a Drosophila clock gene. Nature. 1987;326:42–47. doi: 10.1038/326042a0. [DOI] [PubMed] [Google Scholar]
- 7.Crews S T. Control of cell lineage-specific development and transcription by bHLH-PAS proteins. Genes Dev. 1998;12:607–620. doi: 10.1101/gad.12.5.607. [DOI] [PubMed] [Google Scholar]
- 8.Darlington T K, Wager-Smith K, Ceriani M F, Staknis D, Gekakis N, Steeves T D L, Weitz C J, Takahashi J S, Kay S A. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- 9.Dembinska M E, Stanewsky R, Hall J C, Rosbash M. Circadian cycling of a PERIOD-beta-galactosidase fusion protein in Drosophila: evidence for cyclical degradation. J Biol Rhythms. 1997;12:157–172. doi: 10.1177/074873049701200207. [DOI] [PubMed] [Google Scholar]
- 10.Dunlap J C. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 11.Dushay M S, Konopka R J, Orr D, Greenacre M L, Kyriacou C P, Rosbash M, Hall J C. Phenotypic and genetic analysis of Clock, a new circadian rhythm mutant in Drosophila melanogaster. Genetics. 1990;125:557–578. doi: 10.1093/genetics/125.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edery I, Zwiebel L J, Dembinska M E, Rosbash M. Temporal phosphorylation of the Drosophila period protein. Proc Natl Acad Sci USA. 1994;91:2260–2264. doi: 10.1073/pnas.91.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukunaga B N, Probst M R, Reisz-Porszasz S, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor. J Biol Chem. 1995;270:29270–29278. doi: 10.1074/jbc.270.49.29270. [DOI] [PubMed] [Google Scholar]
- 14.Gekakis N, Saez L, Delahaye-Brown A M, Myers M P, Sehgal A, Young M W, Weitz C J. Isolation of timeless by PER protein interaction: defective interaction between timeless protein and long-period mutant PERL. Science. 1995;270:811–815. doi: 10.1126/science.270.5237.811. [DOI] [PubMed] [Google Scholar]
- 15.Gekakis N, Staknis D, Nguyen H B, Davis F C, Wilsbacher L D, King D P, Takahashi J S, Weitz C J. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 16.Gradin K, McGuire J, Wenger R H, Kvietikova I, Whitelaw M L, Toftgard R, Tora L, Gassmann M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall J C. Genetics of biological rhythms in drosophila. Adv Genet. 1998;38:135–184. doi: 10.1016/s0065-2660(08)60143-1. [DOI] [PubMed] [Google Scholar]
- 18.Hamblen M J, White N E, Emery P T, Kaiser K, Hall J C. Molecular and behavioral analysis of four period mutants in Drosophila melanogaster encompassing extreme short, novel long, and unorthodox arrhythmic types. Genetics. 1998;149:165–178. doi: 10.1093/genetics/149.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 20.Hao H, Allen D L, Hardin P E. A circadian enhancer mediates PER-dependent mRNA cycling in Drosophila melanogaster. Mol Cell Biol. 1997;17:3687–3693. doi: 10.1128/mcb.17.7.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao H, Glossop N R, Lyons L, Qiu J, Morrish B, Cheng Y, Helfrich-Forster C, Hardin P. The 69 bp circadian regulatory sequence (CRS) mediates per-like developmental, spatial, and circadian expression and behavioral rescue in Drosophila. J Neurosci. 1999;19:987–994. doi: 10.1523/JNEUROSCI.19-03-00987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardin P E. Activating inhibitors and inhibiting activators: a day in the life of a fly. Curr Opin Neurobiol. 1998;8:642–647. doi: 10.1016/s0959-4388(98)80093-7. [DOI] [PubMed] [Google Scholar]
- 23.Hardin P E, Hall J C, Rosbash M. Circadian oscillations in period gene mRNA levels are transcriptionally regulated. Proc Natl Acad Sci USA. 1992;89:11711–11715. doi: 10.1073/pnas.89.24.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardin P E, Hall J C, Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990;343:536–540. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- 25.Hastings J W, Rusak B, Boulos Z. Circadian rhythms: the physiology of biological timing. In: Prosser C L, editor. Neural and integrative animal physiology. New York, N.Y: Wiley-Liss, Inc.; 1991. pp. 435–546. [Google Scholar]
- 26.Hogenesch J B, Chan W K, Jackiw V H, Brown R C, Gu Y Z, Pray-Grant M, Perdew G H, Bradfield C A. Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway. J Biol Chem. 1997;272:8581–8593. doi: 10.1074/jbc.272.13.8581. [DOI] [PubMed] [Google Scholar]
- 27.Hogenesch J B, Gu Y Z, Jain S, Bradfield C A. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci USA. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Z J, Edery I, Rosbash M. PAS is a dimerization domain common to Drosophila period and several transcription factors. Nature. 1993;364:259–262. doi: 10.1038/364259a0. [DOI] [PubMed] [Google Scholar]
- 29.Hunter-Ensor M, Ousley A, Sehgal A. Regulation of the Drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell. 1996;84:677–685. doi: 10.1016/s0092-8674(00)81046-6. [DOI] [PubMed] [Google Scholar]
- 30.Kloss B, Price J L, Saez L, Blau J, Rothenfluh A, Wesley C S, Young M W. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iε. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- 31.Konopka R J, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konopka R J, Hamblen-Coyle M J, Jamison C F, Hall J C. An ultrashort clock mutation at the period locus of Drosophila melanogaster that reveals some new features of the fly’s circadian system. J Biol Rhythms. 1994;9:189–216. doi: 10.1177/074873049400900303. [DOI] [PubMed] [Google Scholar]
- 33.Lee C, Bae K, Edery I. The Drosophila CLOCK protein undergoes daily rhythms in abundance, phosphorylation, and interactions with the PER-TIM complex. Neuron. 1998;21:857–867. doi: 10.1016/s0896-6273(00)80601-7. [DOI] [PubMed] [Google Scholar]
- 34.Lee C, Parikh V, Itsukaichi T, Bae K, Edery I. Resetting the Drosophila clock by photic regulation of PER and a PER-TIM complex. Science. 1996;271:1740–1744. doi: 10.1126/science.271.5256.1740. [DOI] [PubMed] [Google Scholar]
- 35.Lindebro M C, Poellinger L, Whitelaw M L. Protein-protein interaction via PAS domains: role of the PAS domain in positive and negative regulation of the bHLH/PAS dioxin receptor-Arnt transcription factor complex. EMBO J. 1995;14:3528–3539. doi: 10.1002/j.1460-2075.1995.tb07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luque I, Gelinas C. Distinct domains of IκBα regulate c-Rel in the cytoplasm and in the nucleus. Mol Cell Biol. 1998;18:1213–1224. doi: 10.1128/mcb.18.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malek S, Huxford T, Ghosh G. IκBα functions through direct contacts with the nuclear localization signals and the DNA binding sequences of NF-κB. J Biol Chem. 1998;273:25427–25435. doi: 10.1074/jbc.273.39.25427. [DOI] [PubMed] [Google Scholar]
- 38.McGuire J, Coumailleau P, Whitelaw M L, Gustafsson J, Poellinger L. The basic helix-loop-helix/PAS factor Sim is associated with hsp90. J Biol Chem. 1996;270:31353–31357. doi: 10.1074/jbc.270.52.31353. [DOI] [PubMed] [Google Scholar]
- 39.Moffett P, Reece M, Pelletier J. The murine Sim-2 gene product inhibits transcription by active repression and functional interference. Mol Cell Biol. 1997;17:4933–4947. doi: 10.1128/mcb.17.9.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers M P, Wager-Smith K, Rothenfluh-Hilfiker A, Young M W. Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science. 1996;271:1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- 41.Myers M P, Wager-Smith K, Wesley C S, Young M W, Sehgal A. Positional cloning and sequence analysis of the Drosophila clock gene, timeless. Science. 1995;270:805–808. doi: 10.1126/science.270.5237.805. [DOI] [PubMed] [Google Scholar]
- 42.Ousley A, Zafarullah K, Chen Y, Emerson M, Hickman L, Sehgal A. Conserved regions of the timeless (tim) clock gene in Drosophila analyzed through phylogenetic and functional studies. Genetics. 1998;148:815–825. doi: 10.1093/genetics/148.2.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price J L, Blau J, Rothenfluh A, Abodeely M, Kloss B, Young M W. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- 44.Price J L, Dembinska M E, Young M W, Rosbash M. Suppression of PERIOD protein abundance and circadian cycling by the Drosophila clock mutation timeless. EMBO J. 1995;14:4044–4049. doi: 10.1002/j.1460-2075.1995.tb00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reisz-Porszasz S, Probst M R, Fukunaga B N, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor nuclear translocator protein (ARNT) Mol Cell Biol. 1994;14:6075–6086. doi: 10.1128/mcb.14.9.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reppert S M. A clockwork explosion! Neuron. 1998;21:1–4. doi: 10.1016/s0896-6273(00)80234-2. [DOI] [PubMed] [Google Scholar]
- 47.Rosato E, Piccin A, Kyriacou C P. Circadian rhythms: from behaviour to molecules. Bioessays. 1997;19:1075–1082. doi: 10.1002/bies.950191206. [DOI] [PubMed] [Google Scholar]
- 48.Rosbash M, Allada R, Dembinska M, Guo W Q, Le M, Marrus S, Qian Z, Rutila J, Yaglom J, Zeng H. A Drosophila circadian clock. Cold Spring Harbor Symp Quant Biol. 1996;61:265–278. [PubMed] [Google Scholar]
- 49.Rutila J E, Suri V, Le M, So W V, Rosbash M, Hall J C. CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell. 1998;93:805–814. doi: 10.1016/s0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- 50.Rutila J E, Zeng H, Le M, Curtin K D, Hall J C, Rosbash M. The timSL mutant of the Drosophila rhythm gene timeless manifests allele-specific interactions with period gene mutants. Neuron. 1996;17:921–929. doi: 10.1016/s0896-6273(00)80223-8. [DOI] [PubMed] [Google Scholar]
- 51.Saez L, Young M W. Regulation of nuclear entry of the Drosophila clock proteins period and timeless. Neuron. 1996;17:911–920. doi: 10.1016/s0896-6273(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 52.Sangoram A M, Saez L, Antoch M P, Gekakis N, Staknis D, Whiteley A, Fruechte E M, Vitaterna M H, Shimomura K, King D P, Young M W, Weitz C J, Takahashi J S. Mammalian circadian autoregulatory loop: a timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1-induced transcription. Neuron. 1998;21:1101–1113. doi: 10.1016/s0896-6273(00)80627-3. [DOI] [PubMed] [Google Scholar]
- 53.Sehgal A. Molecular genetic analysis of circadian rhythms in vertebrates and invertebrates. Curr Opin Neurobiol. 1995;5:824–831. doi: 10.1016/0959-4388(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 54.Sehgal A, Price J L, Man B, Young M W. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994;263:1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- 55.Sehgal A, Rothenfluh-Hilfiker A, Hunter-Ensor M, Chen Y, Myers M P, Young M W. Rhythmic expression of timeless: a basis for promoting circadian cycles in period gene autoregulation. Science. 1995;270:808–810. doi: 10.1126/science.270.5237.808. [DOI] [PubMed] [Google Scholar]
- 56.Sidote D, Majercak J, Parikh V, Edery I. Differential effects of light and heat on the Drosophila circadian clock proteins PER and TIM. Mol Cell Biol. 1998;18:2004–2013. doi: 10.1128/mcb.18.4.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.So W V, Rosbash M. Post-transcriptional regulation contributes to Drosophila clock gene mRNA cycling. EMBO J. 1997;16:7146–7155. doi: 10.1093/emboj/16.23.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sogawa K, Nakano R, Kobayashi A, Kikuchi Y, Ohe N, Matsushita N, Fujii-Kuriyama Y. Possible function of Ah receptor nuclear translocator (Arnt) homodimer in transcriptional regulation. Proc Natl Acad Sci USA. 1995;92:1936–1940. doi: 10.1073/pnas.92.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stanewsky R, Jamison C F, Plautz J D, Kay S A, Hall J C. Multiple circadian-regulated elements contribute to cycling period gene expression in Drosophila. EMBO J. 1997;16:5006–5018. doi: 10.1093/emboj/16.16.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swanson H I, Chan W K, Bradfield C A. DNA binding specificities and pairing rules of the Ah receptor, ARNT, and SIM proteins. J Biol Chem. 1995;270:26292–26302. doi: 10.1074/jbc.270.44.26292. [DOI] [PubMed] [Google Scholar]
- 61.Vosshall L B, Price J L, Sehgal A, Saez L, Young M W. Block in nuclear localization of period protein by a second clock mutation, timeless. Science. 1994;263:1606–1609. doi: 10.1126/science.8128247. [DOI] [PubMed] [Google Scholar]
- 62.Young M W. The molecular control of circadian behavioral rhythms and their entrainment in Drosophila. Annu Rev Biochem. 1998;67:135–152. doi: 10.1146/annurev.biochem.67.1.135. [DOI] [PubMed] [Google Scholar]
- 63.Zelzer E, Wappner P, Shilo B Z. The PAS domain confers target gene specificity of Drosophila bHLH/PAS proteins. Genes Dev. 1997;11:2079–2089. doi: 10.1101/gad.11.16.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeng H, Qian Z, Myers M P, Rosbash M. A light-entrainment mechanism for the Drosophila circadian clock. Nature. 1996;380:129–135. doi: 10.1038/380129a0. [DOI] [PubMed] [Google Scholar]
- 65.Zerr D M, Hall J C, Rosbash M, Siwicki K K. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci. 1990;10:2749–2762. doi: 10.1523/JNEUROSCI.10-08-02749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]