ABSTRACT
The goal of achieving herd immunity to the coronavirus requires high vaccination acceptance levels on the part of the population. The objectives of this study were to: 1) Measure individuals’ willingness to pay (WTP) for a COVID-19 vaccine in Kenya; 2) evaluate the effect of vaccine characteristics (duration of protection and efficacy) and individuals’ socioeconomic variables on WTP, and 3) estimate the aggregate demand and economic value of a COVID-19 vaccine. The contingent valuation (CV) method was used as the basis for the analyses. Data for this study were obtained from a survey of 1,050 individuals in Kenya conducted from April 7 to April 15, 2020. The survey included CV questions using a double-bounded dichotomous choice format. Results reveal that most of the individuals in Kenya (at least 96%) were willing to accept a COVID-19 vaccine. Approximately 80% of individuals were willing to pay a positive amount. Conservative estimates of individuals’ mean WTP for the vaccine range from USD 49.81 to USD 68.25 (depending on vaccine characteristics). Both vaccine duration of protection and efficacy were found to influence WTP (p < .10). The perceived probability of being hospitalized, age, gender, education, location and region of residence, and household income were also found to be associated with WTP for the vaccine (p < .10). In conclusion, the COVID-19 vaccine is highly valued and accepted by the Kenyan population; however, a high percent of the population is unwilling to pay for it or is only willing to pay a low price.
KEYWORDS: COVID-19, SARS-C0V-2, vaccine acceptance, willingness to pay, vaccine attributes, Africa
1. Introduction
The global pandemic attributable to the coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),1 has disrupted people’s lives, and damaged the world economy. By April 30, 2021, more than 150 million confirmed cases, and 3.17 million deaths had been reported globally.2 It has also been estimated that the COVID-19 pandemic caused the global gross domestic product (GDP) to decrease by 4.3% during 2020.3 Kenya has suffered over 150,000 confirmed cases and over 2,500 deaths.4 Government measures to reduce the virus’s spread and domestic and international market disruptions as a consequence of the pandemic were predicted to result in a decline in Kenya’s GDP.5
The race to create a vaccine against COVID-19 began in January 2020. As of March 2021, there are 76 vaccines in clinical trials on humans and 6 vaccines approved for full use.6 Further, the ultimate goal of achieving herd immunity requires high levels of vaccination’s acceptance on the part of the population.7 Therefore, in addition to the work needed to develop a vaccine, it is very important to design plans to distribute it, including aspects related to individuals’ demand for, and acceptance of, the vaccine.7 This study’s goal is to evaluate a COVID-19 vaccine’s demand and acceptability in Kenya. The specific objectives are to: 1) Measure individuals’ willingness to pay (WTP) for a COVID-19 vaccine; 2) evaluate the effect of vaccine characteristics (duration of protection and efficacy) and individuals’ socioeconomic variables on WTP values, and 3) estimate the aggregate demand and economic value of a COVID-19 vaccine in the country. The results of this study are intended to help public and private health organizations’ efforts to plan COVID-19 vaccination programs.
This study uses the economic concept of WTP to assess individuals’ demand for, and acceptability of, a COVID-19 vaccine. WTP is a measure of the maximum amount of money individuals are willing to pay to obtain a product with specific characteristics. The WTP concept has been used to assess the demand for novel products, including potential vaccines.8–10 Moreover, in the case of vaccines, the WTP value provides an estimate of individuals’ expected indirect and direct cost of contracting the illness.11 A body of literature has evaluated individuals’ WTP for hypothetical vaccines, including for example, dengue,9,12 HIV,13 and influenza.14 Recently, several authors have evaluated individuals’ WTP for, and acceptance of, a COVID-19 vaccine in Europe,15 Asia,16–19 and America,20–23 but no studies have been reported from Africa.
2. Methods
2.1. Data and survey instrument
Data for this study were obtained from a survey of Kenyan households.24 A team of researchers from Kenya and the United States collaborated in the survey’s development. The survey was divided in three parts: (1) Individuals’ knowledge of COVID-19; perceptions of institutions’ response to the pandemic; level of preparedness for emergencies, and the pandemic’s effects; (2) a set of contingent valuation (CV) questions to determine whether households would buy a COVID-19 vaccine at stated bid prices, and (3) the respondents’ sociodemographic characteristics. A pilot survey of 100 respondents was used to evaluate survey completion (including the time required to complete the survey), identify problematic questions, and assess the bid values’ validity.
Online data collection from a national sample of 1,050 households in Kenya was conducted from April 7 to April 15, 2020 using the Qualtrics platform. The scope of the study was all Kenyan households’ heads 18 years or older. Data collection generated 963 complete observations. The survey was designed to match the distribution of household size estimated by the 2014 Kenya Demographic and Health Survey, and the household income distribution estimated by Ipsos Public Affairs in 2018 supplemented with the 2019 Economic Survey.25–27 Although Kenya is the third largest economy in Sub-Saharan Africa, it is classified as a lower middle-income country.5,28,29 Kenya’s health coverage is very deficient; for example, only 20% of its population have health insurance coverage, with even lower levels of coverage (approximately 3%) in marginalized and rural areas.30
2.2. Contingent valuation questions
First, the section with the CV questions provided respondents with a description of a hypothetical vaccine to prevent COVID-19 (see Appendix A). The vaccine’s description included two key vaccine quality characteristics (efficacy and duration of protection) and its price. Vaccine efficacy describes the reduction in the risk of infection for an individual after vaccination relative to one who has not received the vaccine.31 Vaccine duration of protection refers to the length of time a vaccine protects against a disease.32
At the time the survey was conducted, no approved vaccines against COVID-19 were available, so their efficacy and duration were still unknown; thus, in the CV questions, two levels of efficacy (70% and 98%) and two levels of duration of protection (1 and 20 years) were considered. The 70% efficacy level was based on the average efficacy of flu vaccines, while the 98% efficacy was selected based on the highest efficacy levels reported for vaccines in the literature.9,33 A 1-year duration of protection assumed the coronavirus would behave like the flu and become a seasonal disease that requires individuals to receive a vaccine every year. As some studies have reported the coronavirus mutates very slowly, a 20-year duration of protection was also considered.34,35 The combination of all levels of efficacy and duration of protection resulted in 4 vaccine profiles that were assigned randomly to the survey respondents (70% efficacy and 1 year of protection, 70% efficacy and 20 years of protection, etc.).
A double-bounded dichotomous choice format was used for the CV questions. Respondents were asked first whether they were willing to buy a COVID-19 vaccine at an initial price (BI) assigned randomly. If the respondent answered yes to the initial price, they were asked whether they were willing to buy the vaccine at a higher price (BH). If the respondent was unwilling to buy the vaccine at the initial price, they were offered the vaccine at a lower price (BL). The initial price bids used were 325, 1,300, 3,250, 6,500, and 13,000 Kenyan shillings (KES). The follow-up prices included 160 KES for respondents who did not buy the vaccine at the 325 KES initial price, and 19,500 KES for respondents who agreed to buy the vaccine at 13,000 KES.
The bid prices for the pilot test were selected based on recent literature using CV methods to evaluate the demand for hypothetical vaccines in other middle-income countries.9,21 Specifically, the bids were calculated relative to the monthly minimum wage in Kenya using proportions similar to those used in9 and;21 thus, during the pilot study, the bid prices included values approximately equal to 2.5%, 5%, 20%, 50%, 100%, 150%, and 200% of Kenya’s minimum wage for unskilled workers.36 Since several respondents were willing to buy the vaccine even at the highest bid prices (200%) during the pilot test, an additional bid price approximately equal to 250% of the monthly minimum wage was added in the final survey instrument. The exchange rate at the time of the survey was 107.52 KES = 1.00 USD; thus, in USD, the prices used in the study ranged from USD 1.49 to USD 181.36.
There are two main approaches to analyzing data from the CV dichotomous choice format.37,38 One option is to use bivariate discrete choice models (e.g., bivariate probit model). In this approach, the emphasis is modeling the dichotomous choices. The second approach models the WTP function directly. There are several advantages of modeling the WTP directly.37,38 First, the WTP function modeling approach is more transparent. For example, in most cases, the coefficient estimates of a parametric WTP model can be interpreted as regression coefficients (i.e., as the effects in the mean willingness to pay). The WTP function approach also facilitates the use of several parametric and nonparametric distribution models; however, estimation requires researchers to write specialized programs to implement the statistical procedures instead of using programs from canned statistical software. The WTP function modeling approach was used in this study.
The four possible outcomes to the two price scenarios are (1) “yes” to the first and second prices, (2) a “yes” to the first price followed by a “no” to the second price, (3) a “no” to the first price followed by a “yes” to the second price, and (4) “no” to the first and second prices. This sequence of potential discrete responses defines the following ranges for the true WTP values:
| (1) |
The parametric approach for estimation uses parametric forms for the choice probabilities corresponding to the components of equation 1.39 The probability that a respondent j answers “yes” to the first and second prices () is
| (2) |
where is a parametric statistical cumulative density function with parameters The probability that a respondent j answers “yes” to the first price and then “no” to the second price () is
| (3) |
Likewise, the probability that a respondent answers “no” to the first price and subsequently “yes” to the second price (is
| (4) |
Lastly, the probability that a respondent answers “no” to the first and second prices (is
| (5) |
.
When considering a sample of N individuals, the log-likelihood can be written as:
where indicates the individuals belonging to the th bidding process outcome, and the super scripts and n in are used to denote the responses (yes or no) to the first and second binary choice questions.
2.3. Statistical analyses
Parametric and nonparametric procedures for analyzing the WTP function were used for the statistical analyses. Nonparametric methods were used to estimate the mean WTP values for the COVID-19 vaccine and to create a demand schedule (percentage of the population willing to buy the vaccine at different prices). Parametric methods were also used to estimate the mean of the distribution of WTP values and to evaluate the effect of vaccine and respondents’ characteristics on WTP values.
The nonparametric procedure to estimate a lower bound estimate of the mean WTP values and the demand schedule are based on methods Turnbull proposed originally.40 The procedure involves estimating a nonparametric WTP distribution function first, which is then used to estimate the lower bound of the mean WTP values.39,41,42 Moreover, the WTP distribution function can be used to generate a demand schedule for the COVID-19 vaccine.39
Estimating the parameters in the parametric model requires specific distribution assumptions for the WTP distribution function, , in which is the vector of the distribution’s parameters (see Appendix A). The normal, Weibull, log-normal, exponential, log-logistic, and gamma distributions were considered.43,44 Two methods were used to select the “best” model: The Akaike information criterion (AIC), and the ratio of the maximum likelihood method.45,46
To evaluate the explanatory variables’ effect on the WTP values, components of the parameter vector can be expressed as functions of the explanatory variables. For example, the Weibull distribution is defined by two parameters (α . The explanatory variables’ (X) effect can be modeled by setting θ, in which θ is a vector of parameters. The vector of explanatory variables (X) included vaccine efficacy and duration of protection, respondents’ sociodemographic characteristics, knowledge of the disease, perceptions of the probability of infection, hospitalization, and death if one contracts the disease, and health insurance availability.9,11,20 Statistical analyses were performed using MATLAB and STATA.
3. Results
3.1. Summary statistics
The respondents’ average age was 30, and most were males (63%) and college-educated (87%: Table 1). Most respondents reported having employment (73%) and health insurance (72%), and living in urban areas (76%). The majority (39%) lived in Nairobi, the country’s capital, followed by the Rift Valley (18%) and the Central Regions (11%). The average household income was KES 17.54 thousand (USD 163) and the average household size was 4.11 members.
Table 1.
Characteristics of Survey Respondents
| Characteristic | Respondents mean (SD) | |
|---|---|---|
| Mean (SD) Age (years) | 30.05 (7.94) | |
| Mean (SD) Household size (people) | 4.11 (2.64) | |
| Income (KSh)a | ||
| Less than 3,000 | 0.13 (0.34) | |
| 3,000 to 6,000 | 0.10 (0.30) | |
| 6,001 to 8,000 | 0.06 (0.25) | |
| 8,001 to 10,000 | 0.10 (0.30) | |
| 10,001 to 17,000 | 0.16 (0.37) | |
| 17,001 to 25,000 | 0.17 (0.37) | |
| 25,001 to 40,000 | 0.13 (0.34) | |
| More than 40,000 | 0.14 (0.35) | |
| Female | 0.37 (0.48) | |
| College educated | 0.87 (0.34) | |
| Possess health insurance b | 0.72 (0.45) | |
| Employed a | 0.73 (0.44) | |
| Rural residence | 0.24 (0.43) | |
| Religious believe | ||
| Christian | 0.95 (0.23) | |
| Other | 0.05 (0.23) | |
| Region of residence | ||
| Central | 0.11 (0.32) | |
| Coast | 0.08 (0.27) | |
| Eastern | 0.07 (0.25) | |
| Nairobi | 0.39 (0.49) | |
| Nyanza | 0.08 (0.28) | |
| Rift Valley | 0.18 (0.39) | |
| Western | 0.05 (0.22) | |
*Sample size 963.
With respect to the respondents’ knowledge of COVID-19, the average knowledge score was 10.07 of 15 possible points (approximately 67%). The score was calculated using the responses to a set of 15 dichotomous (yes/no) questions related to the virus’s forms of transmission, methods to decrease its dissemination, and COVID-19 symptoms.24 The mean values for the perceived probabilities of contracting the coronavirus, requiring hospitalization, and dying if one contracts COVID-19 were approximately 28%, 45%, and 18%, respectively (Table A1).
With respect to the answers to the double-bounded dichotomous choice questions, 58% of the respondents indicated that they would buy the COVID-19 vaccine at the initial bid price, and 51% of respondents combined indicated that they would buy it at the follow-up bid prices (Table A2). In total, 80% of the survey participants indicated that they were willing to pay a positive amount to purchase the vaccine. This proportion includes the survey participants who answered yes to at least one of the two willingness to buy questions (68%), as well as those who answered yes to a third willingness to buy question (12%), asked only to individuals who responded “no” to the first two willingness to buy questions.
3.2. Willingness to pay distribution functions
The estimated nonparametric Turnbull WTP cumulative distribution functions (CDF) are step functions (Table 2), thus, they are interpreted in relation to the upper value of the bid ranges. For instance, the CDF for the vaccine with 1 year of protection and 70% efficacy showed that 18% of respondents were willing to pay USD 1.49 or less for the COVID-19 vaccine, and 16.9% were willing to pay USD 3.12 or less.
Table 2.
Turnbull willingness to pay distribution functions (n = 963)
| Turnbull CDF | ||||
|---|---|---|---|---|
| Duration (years) | 1 | 20 | 1 | 20 |
| Efficiency (%) | 70 | 70 | 98 | 98 |
| Bid Range ($) | ||||
| 0–1.49 | 0.148 | 0.166 | 0.099 | 0.139 |
| 1.49–3.12 | 0.169 | 0.237 | 0.099 | 0.139 |
| 3.12–12.09 | 0.365 | 0.330 | 0.296 | 0.294 |
| 12.09–30.23 | 0.527 | 0.429 | 0.427 | 0.389 |
| 30.23–60.45 | 0.647 | 0.608 | 0.574 | 0.509 |
| 60.45–120.90 | 0.785 | 0.695 | 0.681 | 0.651 |
| 120.90–181.35 | 0.846 | 0.749 | 0.781 | 0.798 |
| 181.35+ | 1.000 | 1.000 | 1.000 | 1.000 |
With respect to the parametric models, the Weibull distribution was selected as the distribution that fit best among the six alternatives considered based on the smallest AIC value and the ratio of maximized likelihood method.46 Finally, likelihood ratio Chi-squared tests rejected the null hypothesis that none of the explanatory variables influenced the distribution model (p < .05: Table 3).
Table 3.
Estimation results of willingness to pay models for a COVID-19 vaccine in Kenya
| |
Model 1 |
Model 2 |
Model 3 |
|||
|---|---|---|---|---|---|---|
| Variable | Coefficient | Coefficient | Coefficient | |||
| Constant | −0.516*** | (0.137) | −1.398*** | (0.501) | −2.066*** | (0.727) |
| Duration of protection | 0.218* | (0.163) | 0.012* | (0.009) | 0.258* | (0.167) |
| Efficiency of protection | 0.348** | (0.163) | 0.012** | (0.006) | 0.404*** | (0.168) |
| Contracting probability | 0.001 | (0.004) | ||||
| Hospitalized probability | 0.006** | (0.007) | ||||
| Death probability | 0.007 | (0.007) | ||||
| Age | −0.024** | (0.011) | ||||
| Household size | 0.118*** | (0.037) | ||||
| Income (USD) | 0.002** | (0.001) | ||||
| Gender (Female = 1, Male = 0) | −0.413** | (0.178) | ||||
| College education (Yes = 1, No = 0) | 0.475** | (0.243) | ||||
| Health insurance (Yes = 1, No = 0) | 0.052 | (0.192) | ||||
| Employment status (Employed = 1, Other = 0) | 0.023 | (0.058) | ||||
| Knowledge score | 0.272 | (0.221) | ||||
| Household location (Rural = 1, Urban = 0) | 0.264* | (0.204) | ||||
| Religion (Other = 1, Christian = 0) | 0.070 | (0.396) | ||||
| Region of Residence | ||||||
| Nairobi | 0.460** | (0.238) | ||||
| Rift Valley | 0.357** | (0.204) | ||||
| Sample size | 963 | 963 | 902 | |||
| Alpha parameter | 0.519 | 0.519 | 0.533 | |||
| Wald Chi2 | 6.412 | 6.412 | 58.230 | |||
| P-value (Prob>Chi2) | 0.040 | 0.040 | <0.001 | |||
| Log likelihood | −1169.990 | −1169.990 | −1071.104 | |||
The parametric model used is a Weibull distribution with parameters ɑ ˄ σ, β. Standard error in parenthesis. Dummy variables used as base are Western for region and Christian for religion. *p < 0.10 **p < 0.05 ***p < 0.01. Model 1 uses dummy explanatory variables for duration of protection and efficiency (higher levels take a value of 1). Model 2 uses the actual values of efficiency (70 and 98) and duration (1 and 20) as explanatory variables.
3.3. Mean WTP for COVID-19 vaccine
Parametric and non-parametric estimates of the mean WTP values for the vaccines are shown in Table 4. The vaccine with the lowest level of efficacy (70%) and duration of protection (1 year) had the lowest estimated mean WTP values (nonparametric lower bound WTP = USD 49.81). In contrast, the vaccine with the highest level (98%) and duration of protection (20 years) had the highest estimated values (nonparametric lower bound WTP = USD 68.25).
Table 4.
Mean willingness to pay (USD) for hypothetical COVID-19 vaccine
| Attribute | Parametric mean WTP (n = 963) |
Nonparametric lower bound mean WTP (n = 963) |
||
|---|---|---|---|---|
| 1 year protection, 70% efficacy | 102.00 | (17.36) | 49.81 | (5.20) |
| 20 years protection, 70% efficacy | 131.96 | (23.85) | 64.37 | (6.27) |
| 1 year protection, 98% efficacy | 152.83 | (29.31) | 64.90 | (5.87) |
| 20 years protection, 98% efficacy | 197.72 | (36.60) | 68.25 | (5.73) |
Standard errors in parenthesis.
Parametric mean WTP values were estimated from a model (Model 1), in which the scale parameter of the Weibull distribution was a function of dummy variables that denoted vaccines with a longer duration of protection (20 years) and a high level of efficacy (98%: Table 3). As expected, the estimated parametric mean values were larger than their corresponding nonparametric lower bound estimates. For example, the estimated mean WTP value for the vaccine with a 98% level of efficiency and 20 years duration was USD 197.06, or approximately 2.9 times the estimated nonparametric lower bound (USD 68.25).
3.4. Vaccine and respondents’ characteristics associated with WTP
Parametric models estimated to test the association between vaccine attributes and respondent characteristics on WTP values are shown in Table 3. Both vaccine duration of protection and efficacy were found to influence mean WTP (p < .10), although only efficacy was significant at the 5% level. With respect to respondents’ characteristics, the majority of characteristics considered were found to be associated with their WTP for the vaccine (p < .10), including their perceived probability of being hospitalized, age, gender, education, location and region of residence, and household income.
The estimated coefficients in Model 1 measured the difference in the mean WTP values between vaccines with different characteristics; thus, the mean WTP for a COVID-19 vaccine with 20 years of protection was estimated to be approximately 24% ((x100%) higher than that for a vaccine with 1 year of protection (p < .10). Moreover, the mean WTP for a COVID-19 vaccine with 98% efficacy was estimated to be 42% higher than that for a vaccine with 70% efficacy (p < .05). On the other hand, the estimated coefficients in Model 2 measured the effect of an additional unit of the vaccine’s attributes on the mean WTP; therefore, it was estimated that both an additional year of vaccine protection and a 1% increase in its efficacy increased the mean WTP for the vaccine by approximately 1.2%.
According to Model 3, a 1% increase in the perceived probability of being hospitalized if the disease is contracted was found to be associated with a 0.6% increase in the average WTP for the vaccine, a 1 USD increase in income with a 0.2% increase, and an additional household member with a 12.5% increase. The results also indicated that college-educated individuals were willing to pay, on average, 61% more for a COVID-19 vaccine than were those with no college education, and that individuals in rural areas were willing to pay approximately 30% more for the vaccine than those who live in urban areas, ceteris paribus. In contrast, an additional 1 year in age was found to be associated with a 2.4% decrease in the WTP for the vaccine, and female respondents’ mean WTP for the vaccine was approximately 34% lower than males’ mean WTP. The individuals’ region of residence was also found to affect the WTP for the vaccine (p < .05). Specifically, individuals who live in Nairobi and the Rift Valley Regions were estimated to be willing to pay approximately 59% and 43% more, respectively, than those who live in other regions of Kenya.
Approximately 16% of respondents (n = 158) answered “no” to both bids and to a follow-up question asking them if they would be willing to pay any amount (Figure 1). Among this group of respondents, 73% appeared to be willing to accept a vaccine, but not willing to pay any amount, including 63% who believe that the government should provide vaccines for free, and approximately 10% who indicated that the bid prices given were too high. The remaining 27% of respondents appeared to reject vaccines because of their lack of trust and confidence in them; therefore, when extrapolated to the total sample of survey participants, this indicates that 4.32% (27% of 16%) of respondents would not accept vaccines and 95.68% would.
Figure 1.
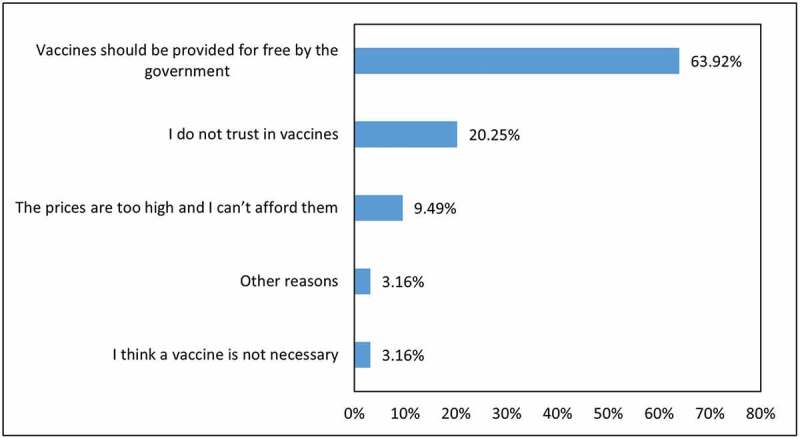
Reasons for rejection of bid scenarios for a COVID-19 vaccine (n = 158)
4. Discussion
Information about the individuals’ acceptance of, and demand for, a vaccine against SARS-CoV-2 is key in planning vaccination campaigns. In addition, the social valuation of a vaccine is important to assess the benefits of public and private investments required for its development and distribution. This study shows that most of the individuals in Kenya (at least 96%) are willing to accept a COVID-19 vaccine, and approximately 80% of individuals are willing to pay a positive amount. These acceptability estimates are higher than those from developed countries, including France47 and the United States,48 which report acceptability rates of approximately 76–80%. On the other hand, it is consistent with studies from middle-income countries (Ecuador, Chile, Malaysia, and Indonesia) that reported vaccine acceptability rates greater than 90%.15,17,20,21,49 These results reflect high levels of acceptance and trust in a potential COVID-19 vaccine in developing countries, a key factor in the success of vaccination campaigns.
Conservative estimates of the mean WTP for a vaccine in Kenya ranged from USD 49.81 to USD 68.26 (depending on the vaccine’s characteristics). Past studies that have evaluated WTP for potential vaccines for other diseases have reported a wide range of values depending upon the disease, the populations of study, and methods used (e.g.,).[50,51] However, few studies have been conducted to estimate the WTP for a COVID-19 vaccine. For example, a study in Chile reported an average WTP of USD 184.72,20 another in Ecuador an average value of at least USD 147,21 another in Romania between (23.8 and 238 USD: exchange rate 1 EUR-1.19, August 20, 2020),15 another in Indonesia, USD 57.20,16 and one in Malaysia, USD 30.66.17 These differences in values may reflect differences in the methods used, but also each country’s cultural, health, and economic conditions, as well as the status of COVID-19 at the time data were collected. Data collection for this study and three studies cited formerly were conducted between late March and early May 2020. The study’s results revealed that both the duration of protection and efficacy were important determinants of Kenyans’ WTP for the vaccine. The respondents were found to be willing to pay 25% to 30% more for a vaccine with 20 years of protection relative to one with only 1 year of protection (see Table 3), and 40%-50% more for a vaccine with a 98% efficacy relative to one with 70% of efficacy. Several previous studies have reported that the duration of protection and efficacy are among the most important attributes that influence individuals’ WTP for vaccines.21,51–53 Only the previous study on the WTP for COVID-19 in Ecuador explored the effect of the vaccine’s duration of protection and efficacy on individuals’ WTP for the vaccine; however, only the duration of protection was found to have an effect. Thus, the understanding of the meaning and importance of vaccines’ attributes may vary from country to country.
The WTP for the vaccine varied substantially across the subpopulation groups defined by region of residence, location (rural vs. urban), education (college- vs. not college-educated), the respondents’ gender, income, age, income, and perceptions of the probability of being hospitalized. This finding reflects a high degree of heterogeneity with respect to the valuation and demand for the vaccine, which might present certain challenges in the implementation of a vaccination campaign. Of notable importance is the negative association between WTP for the vaccine and age, given that the risk of severe illness from COVID-19 is well known to increase with age.54 The estimated positive association between income and WTP for the vaccine implies differences in WTP values across individuals from different income groups. For example, the difference in income between the second and seventh deciles of the sample’s income distribution (average income of approximately USD 42 and 160, respectively) is approximately USD 118,24 which would result in an approximately USD 34 USD difference in average WTP values for a vaccine with 98% efficacy and 20 years of protection (difference calculated using the parametric model and estimating mean WTP values at the explanatory variables’ specified values and using mean values for all other explanatory variables). A positive association between income and WTP for vaccines was expected, as it is reported frequently12,20,21,55 and used as a justification to subsidize vaccination for lower-income groups in the population.
The heterogeneity in the valuation of the vaccine also has implications concerning behavioral responses to the pandemic, as a higher valuation for the vaccine also reflects higher expected costs if getting the disease (e.g., because they have higher lost income if contracting the disease). Higher expected costs if infected should also make people more cautious, so they follow medical experts’ recommendations.
Three variables associated with perceived probabilities of contracting the disease, being hospitalized, and dying of the disease were included in the regression models, but only the variable that measured the perceived probability of being hospitalized was found to be associated positively with individuals’ WTP for the vaccine. This result is consistent with the previous study in Ecuador,21 and emphasizes the need for governments to provide accurate information on the dissemination of the virus and the effects if contracted, including hospitalization and mortality rates. The survey respondents’ perceived mortality rates (17.63%) at the time of the survey were nearly 10 times higher than the fatality rate reported currently (1.85%).56
4.1. Aggregate COVID-19 vaccine demand and economic value of preventing COVID-19
Figure 2 shows the aggregate demand curves for the COVID-19 vaccine in Kenya; thus, the curves show the proportion of the Kenyan population willing to pay a given price for the vaccine. At least 83% of the population is willing to pay USD 1.49 or less for the vaccine. The fraction of the population willing to pay for the vaccine declined to approximately 50% to 60% when the price was USD 30.23, and to approximately 15% to 20% when the price was USD 181.35. Thus, depending on the vaccine’s final market price, some subsidies may be necessary for individuals who cannot afford it. Moreover, as an important proportion of individuals is willing to pay for the vaccine when prices are above USD 70 (the highest estimate of the vaccine market price),57 the private sector could also play a role to hasten vaccine deployment and coverage.
Figure 2.
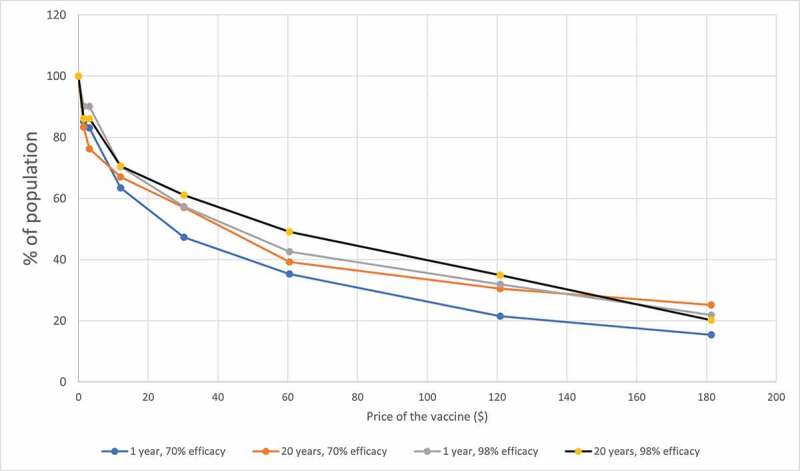
Aggregate demand curves for the COVID-19 vaccine
The total economic value of preventing COVID-19 can be calculated by multiplying the estimated mean WTP for the vaccine times the Kenyan population willing to pay a positive amount for the vaccine. For the calculations, we considered the vaccine with the longest duration (20 years) of protection and highest level of efficacy (98%), and the estimated percentage of the population willing to pay a positive amount for the vaccine (83.6%)(i.e., individuals rejecting the bid scenarios were assumed to have a WTP value of zero). Given that the estimated population in Kenya in 2019 was 52.57 million, and the estimated lower bound mean WTP for the vaccine with 98% of efficacy and 20 years duration of protection is USD 68.26, a conservative estimated aggregate value to prevent COVID-19 in Kenya is USD 2.87 billion.
The analyses have several limitations. Although the study used an established panel of online consumers throughout Kenya and the distributions of income and household size in the sample matched those in the population closely, the sample’s characteristics differed from those of the population across other important dimensions, including education, region, location of residence, and gender. Therefore, the ability to generalize the results should be interpreted with caution. Another concern is the use of hypothetical vaccine scenarios at a specific time. Preferences may change both over time and when a vaccine becomes available. However, this study provides relevant information on the acceptability of, and demand for, a potential COVID-19 vaccine.
5. Conclusions
This study contributes to the literature that has evaluated individuals’ acceptance of, and willingness to pay for, a COVID-19 vaccine, and as such, it provides information for decision makers in Kenya in their planning efforts to distribute a vaccine successfully. Although this is one of the first studies to explore the effect of a COVID-19 vaccine’s attributes on demand, only the efficacy and duration of protection attributes were considered. Future research should also explore the effect of other vaccine attributes such as the number of doses of the vaccine needed and risks of side effects.52
Supplementary Material
Appendix A.
A1. Description paragraph for contingent valuation question
Currently, there is not a vaccine that protects against the novel coronavirus, but doctors and scientists are working to develop the vaccine. Imagine that they have successfully developed the vaccine that can prevent people from getting coronavirus disease (COVID-19). We would like to know what you would do if the new coronavirus vaccine was available for sale at a convenient location like a health or medical center. This new vaccine could be given to individuals to prevent them from having the coronavirus disease in the future. It could not be used to treat someone who currently has coronavirus. The vaccine would be available for people of all ages including newborn babies. Your honest answers to the question below are important to better understand the potential demand for a new vaccine, and also to measure the economic impact of the coronavirus health emergency. The results will be disseminated to government and non-government organizations and the public.
Suppose that this vaccine has no side effects, and is safe, that is, after you were vaccinated you would have no chance to get coronavirus from the vaccine. The vaccine requires an injection. The vaccine is (70% or 98%%) effective for (1 or 20) years. In other words, of 100 people receiving the vaccine, there will be (70 or 98) of the people who have taken the vaccine that are protected for a period of (1 or 20 year) years. The other people who have been vaccinated will not be protected against coronavirus disease, even though they have taken the vaccine.
Initial question: Would you be willing to pay KES P for a coronavirus vaccine that is E% effective for D years: (P, E, and D are replaced by the values described in the methods section)
Yes
No
I don’t know/Refuse
The follow-up questions were based on the answer to this question.
Table A1.
Respondents COVID-19 perceptions, knowledge, and vaccine acceptability
| Variable | Mean | Standard deviation |
|---|---|---|
| What do you think is your probability of contracting COVID-19?a (n = 930) | 28.07 | 24.52 |
| What do you think is your probability of being hospitalize in case of contracting COVID-19? a (n = 929) | 44.80 | 35.24 |
| What do you think is the mortality rate in case of contracting COVID-19? a(n = 940) | 17.63 | 14.11 |
| Knowledge score with respect to recommendation to reduce spread, forms of transmission and symptoms of COVID-19 | 10.07 | 1.59 |
Notes. Sample size n = 963, except when noted. a Sample size as noted because of respondents skips.
Table A2.
Responses to double-bounded dichotomous questions (n = 963)
| Question | Responses | ||||||
|---|---|---|---|---|---|---|---|
| First discrete choice question | Yes | No | |||||
| Percentage of respondents | 58.36 | 41.64 | |||||
| Second discrete choice question | Yes | No | Yes | No | |||
| Percentage of respondents | 25.57 | 74.43 | 23.94 | 76.06 | |||
Funding Statement
Funding for this project was provided by the Larry Combest Endowed Chair for Agricultural Competitiveness, Texas Tech University.
Contributions
Conceptualization, methodology, CC, OS, DH, AM, MS; formal analysis investigation, resources, writing – original draft preparation CC, OS, DH; writing – review and editing CC, OS, DH, AM, MS. All authors attest they meet the ICMJE criteria for authorship.
Disclosure of potential conflicts of interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1938494
References
- 1.World Health Organization . Novel Coronavirus (2019-nCoV) Situation Report 1. 2020.
- 2.John Hopkins University . COVID-19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU); 2020 [accesed 2021 Apr 30]. https://coronavirus.jhu.edu/map.html.
- 3.The World Bank . HIGHLIGHTS from CHAPTER 1 THE GLOBAL OUTLOOK. Glob. Econ. Prospect., Washington: (DC): The World Bank; 2021. [Google Scholar]
- 4.Corona Tracker . Corona Tracker; 2020 [accesed 2021 Mar 30]. https://www.coronatracker.com/country/kenya/.
- 5.IMF . Regional economic outlook for Sub-Saharan Africa, June 2020 Update. IMF; 2020 [accesed 2020 Sep 17]. https://www.imf.org/en/Publications/REO/SSA/Issues/2020/06/29/sreo0629.
- 6.The New York Times . Coronavirus Vaccine Tracker; 2020. [accessed 2021 Dec 30]. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.
- 7.Deroo SS, Pediatrics C, George T, Sciences H, Pudalov NJ, George T, Sciences H, Sciences H, Pudalov NJ, George T, et al. Planning for a COVID-19 vaccination program. JAMA. 2020;323:2458–59. 10.1001/jama.2020.8711. [DOI] [PubMed] [Google Scholar]
- 8.Lusk JL, Hudson D.. Willingness-to-pay estimates and their relevance to agribusiness decision making. Rev Agric Econ. 2004;26:152–69. doi: 10.1111/j.1467-9353.2004.00168.x. [DOI] [Google Scholar]
- 9.Lee JS, Mogasale V, Lim JK, Carabali M, Sirivichayakul C, Anh DD, Lee K-S, Thiem VD, Limkittikul K, Tho LH, et al. A multi-country study of the household willingness-to-pay for dengue vaccines: household surveys in Vietnam, Thailand, and Colombia. PLoS Negl Trop Dis. 2015;9:1–15. doi: 10.1371/journal.pntd.0003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ughasoro MD, Esangbedo DO, Tagbo BN, Mejeha IC.. Acceptability and willingness-to-pay for a hypothetical ebola virus vaccine in Nigeria. PLoS Negl Trop Dis. 2015;9:1–15. doi: 10.1371/journal.pntd.0003838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cropper ML, Haile M, Lampietti J, Poulos C, Whittington D. The demand for a malaria vaccine: evidence from Ethiopia. J Dev Econ. 2004;75:303–18. doi: 10.1016/j.jdeveco.2003.02.006. [DOI] [Google Scholar]
- 12.Hadisoemarto PF, Castro MC. Public acceptance and willingness-to-pay for a future dengue vaccine: a community-based survey in Bandung, Indonesia. PLoS Negl Trop Dis. 2013;7:e2427. doi: 10.1371/journal.pntd.0002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNeil SA, Shinde V, Andrew M, Hatchette TF, Leblanc J, Ambrose A, Boivin G, Bowie WR, Diaz-Mitoma F, ElSherif M, et al. Interim estimates of 2013/14 influenza clinical severity and vaccine effectiveness in the prevention of. Euro Surveill. 2014;19:1–6. [DOI] [PubMed] [Google Scholar]
- 14.Hou Z, Chang J, Yue D, Fang H, Meng Q, Zhang Y. Determinants of willingness to pay for self-paid vaccines in China. Vaccine. 2014;32:4471–77. doi: 10.1016/j.vaccine.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 15.Berghea F, Berghea CE, Abobului M, Vlad VM. Willingness to Pay for a for a Potential Vaccine Against SARS-CoV-2/COVID-19 Among Adult Persons; 2020.
- 16.Harapan H, Wagner AL, Yufika A, Winardi W, Anwar S, Gan AK, Setiawan AM, Rajamoorthy Y, Sofyan H, Vo TQ, et al. Willingness-to-pay for a COVID-19 vaccine and its associated determinants in Indonesia. Hum Vaccin Immunother. 2020;16:3074–80. doi: 10.1080/21645515.2020.1819741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong LP, Alias H, Wong P-F, Lee HY, AbuBakar S. The use of the health belief model to assess predictors of intent to receive the COVID-19 vaccine and willingness to pay. Hum Vaccin Immunother. 2020;16(9):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Lyu Y, Zhang H, Jing R, Lai X, Feng H, Knoll MD, Fang H. Willingness to pay and financing preferences for COVID-19 vaccination in China. Vaccine. 2021;39:1968–76. doi: 10.1016/j.vaccine.2021.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Luo X, Ma ZF. Willingness of the general population to accept and pay for COVID-19 vaccination during the early stages of COVID-19 pandemic: a nationally representative survey in mainland China. Hum Vaccin Immunother. 2021;17(6):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García LY, Cerda AA. Contingent assessment of the COVID-19 vaccine. Vaccine. 2020;38:5424–29. doi: 10.1016/j.vaccine.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarasty O, Carpio CE, Hudson D, Guerrero-Ochoa PA, Borja I. The demand for a COVID-19 vaccine in Ecuador. Vaccine. 2020;38:8090–98. doi: 10.1016/j.vaccine.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerda AA, García LY. Willingness to pay for a COVID-19 vaccine. Appl Health Econ Health Policy. 2021;19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catma S, Reindl D. Parents’ willingness to pay for a COVID-19 vaccine for themselves and their children in the United States. Hum Vaccin Immunother. 2021;1–7. doi: 10.1080/21645515.2021.1919453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpio C, Hudson D, Sarasty O, Macharia A, Shibia M. Public knowledge, perceptions, responses and effects related to the COVID-19 health emergency: The Case of Kenya; 2020.
- 25.Ipsos Public Affairs . SPEC barometer, 1st QTR 2018 second media release. Ipsos Public Aff; 2018 [accesed 2020 Sep 14]. https://www.ipsos.com/sites/default/files/ct/news/documents/2019-08/ipsoske_spec_1st_release_presentation_pa_v1.pdf.
- 26.Kenya National Bureau of Statistics . Economic survey 2019. Kenya Natl Bur Stat; 2019 [accesed 2020 Oct 13]. https://www.knbs.or.ke/?wpdmpro=economic-survey-2019.
- 27.Kenya National Bureau of Statistics . Kenya demographic and health survey 2014. Kenya Natl Bur Stat; 2015 [accesed 2020 Sep 14]. https://dhsprogram.com/pubs/pdf/FR308/FR308.pdf.
- 28.The World Bank . Kenya overview. World Bank; 2020 [accesed 2020 Sep 17]. https://data.worldbank.org/country/kenya.
- 29.Mboya EKenya overtakes Angola as third-largest economy in Sub-Sahara Africa. Bus Dly; 2020 [accesed 2020 Sep 17]. https://theconversation.com/covid-19-exposes-weaknesses-in-kenyas-healthcare-system-and-what-can-be-done-143356.
- 30.The Conversation . COVID-19 exposes weaknesses in Kenya’s healthcare system. And what can be done. Conversat; 2020 [accesed 2020 Sep 17]. https://theconversation.com/covid-19-exposes-weaknesses-in-kenyas-healthcare-system-and-what-can-be-done-143356.
- 31.Halloran ME, Haber M, Longini IM Jr, Struchiner CJ. Direct and indirect effects in vaccine efficacy and effectiveness. Am J Epidemiol. 1991;133:323–31. doi: 10.1093/oxfordjournals.aje.a115884. [DOI] [PubMed] [Google Scholar]
- 32.Orenstein WA, Bernier RH, Dondero TJ, Hinman AR, Marks JS, Bart KJ, Sirotkin B. Field evaluation of vaccine efficacy. Bull World Health Organ. 1985;63:1055–68. [PMC free article] [PubMed] [Google Scholar]
- 33.Centers of Disease Control and Prevention . CDC seasonal flu vaccine effectiveness studies; 2020 [accesed 2020 Aug 23]. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm.
- 34.O’Donnell TOxford scientist says if coronavirus vaccine is effective it will likely be seasonal; 2020 [accesed 2020 Aug 23]. https://news.yahoo.com/oxford-scientist-says-coronavirus-vaccine-172827584.html.
- 35.Kekatos MCoronavirus mutates slower than the flu say experts - which will give scientists longer to develop an effective vaccine; 2020 [accesed 2020 Aug 23]. https://www.dailymail.co.uk/news/article-8208379/Coronavirus-mutating-slowly-giving-scientists-time-develop-vaccine.html.
- 36.AfricaPay . Minimum wages in Kenya with effect from 01-05-2018; 2018. [accessed 2021 Mar 31]. https://africapay.org/kenya/salary/minimum-wages/archive-before-2019/minimum-wages-in-kenya-with-effect-from-01-05-2018
- 37.Cameron TA, James MD. Efficient estimation methods for” closed-ended” contingent valuation surveys. Rev Econ Stat. 1987;69:269–76. doi: 10.2307/1927234. [DOI] [Google Scholar]
- 38.Haab TC, McConnell KE. Valuing environmental and natural resources: the econometrics of non-market valuation. Northampton (MA): Edward Elgar Publishing; 2002. [Google Scholar]
- 39.Carpio CE, Ramirez OA, Boonsaeng T. Potential for tradable water allocation and rights in Jordan. Land Econ. 2011;87:595–609. doi: 10.3368/le.87.4.595. [DOI] [Google Scholar]
- 40.Turnbull BW. The empirical distribution function with arbitrarily grouped, censored and truncated data. J R Stat Soc Ser B. 1976;38:290–95. doi: 10.1111/j.2517-6161.1976.tb01597.x. [DOI] [Google Scholar]
- 41.Day B. Distribution-free estimation with interval-censored contingent valuation data: troubles with Turnbull? Environ Resour Econ. 2007;37:777–95. doi: 10.1007/s10640-006-9061-8. [DOI] [Google Scholar]
- 42.Haab TC, McConnell KE. Referendum models and negative willingness to pay: alternative solutions. J Environ Econ Manage. 1997;32:251–70. doi: 10.1006/jeem.1996.0968. [DOI] [Google Scholar]
- 43.Cameron T, New A. Paradigm for valuing non-market goods using referendum data: maximum likelihood estimation by censored logistic regression. J Environ Econ Manage. 1988;15:355–79. doi: 10.1016/0095-0696(88)90008-3. [DOI] [Google Scholar]
- 44.López-Feldman A. Munich Personal RePEc Archive Introduction to contingent valuation using Stata Introduction to Contingent Valuation using Stata *; 2012.
- 45.Cameron A, Trivedi P. Microeconometrics: methods and application. New York: Cambridge University Press; 2009. [Google Scholar]
- 46.Raqab MZ, Al-Awadhi SA, Kundu D. Discriminating among weibull, log-normal, and log-logistic distributions. Commun Stat - Simul Comput. 2018;47:1397–419. doi: 10.1080/03610918.2017.1315729. [DOI] [Google Scholar]
- 47.Detoc M, Bruel S, Frappe P, Botelho-Nevers E, Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. MedRxiv 2020. 2020April23;20076513. doi: 10.1101/2020.04.23.20076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thunstrom L, Ashworth M, Finnoff D, Newbold S. Hesitancy Towards a COVID-19 Vaccine and Prospects for Herd Immunity. Available SSRN 3593098 2020.
- 49.Harapan H, Wagner AL, Yufika A, Winardi W, Anwar S, Gan AK, Setiawan AM, Rajamoorthy Y, Sofyan H, Mudatsir M. Acceptance of a COVID-19 vaccine in southeast Asia: a cross-sectional study in Indonesia. Front Public Heal. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mudatsir M, Anwar S, Fajar JK, Yufika A, Ferdian MN, Salwiyadi S, Imanda AS, Azhars R, Ilham D, Timur AU, et al. Willingness-to-pay for a hypothetical Ebola vaccine in Indonesia: a cross-sectional study in Aceh. F1000Research. 2019;8:1441. doi: 10.12688/f1000research.20144.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown DS, Johnson FR, Poulos C, Messonnier ML. Mothers’ preferences and willingness to pay for vaccinating daughters against human papillomavirus. Vaccine. 2010;28:1702–08. doi: 10.1016/j.vaccine.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 52.Arbiol J, Yabe M, Nomura H, Borja M, Gloriani N, Yoshida SI. Using discrete choice modeling to evaluate the preferences and willingness to pay for leptospirosis vaccine. Hum Vaccines Immunother. 2015;11:1046–56. doi: 10.1080/21645515.2015.1010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cameron MP, Newman PA, Roungprakhon S, Scarpa R. The marginal willingness-to-pay for attributes of a hypothetical HIV vaccine. Vaccine. 2013;31:3712–17. doi: 10.1016/j.vaccine.2013.05.089. [DOI] [PubMed] [Google Scholar]
- 54.CDC . Coronavirus disease older adults. CDC; 2020 [accesed 2020 Sep 13]. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html.
- 55.Bishai D, Brice R, Girod I, Saleh A, Ehreth J. Conjoint analysis of French and German parents’ willingness to pay for meningococcal vaccine. Pharmacoeconomics. 2007;25:143–54. doi: 10.2165/00019053-200725020-00006. [DOI] [PubMed] [Google Scholar]
- 56.WHO . The current COVID-19 situation: kenya; 2020 [accesed 2020 Aug 27]. https://www.who.int/countries/ken/.
- 57.Loftus PCovid-19 vaccine makers signal prices 2020 [accesed 2020 Aug 23]. https://www.wsj.com/articles/covid-19-vaccine-makers-signal-prices-11596648639.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


