Abstract
Background
Ethnodentistry is the use of indigenous oral cleansing agents such as plant parts by local folks not only to maintain oral hygiene but also to treat oral infections. Mostly, ethnodentistry is inspired by traditions and belief systems of local communities. Musa paradisiaca is extensively cultivated and used in many cultures for its nutritional and medicinal values. In Ghana, the fruit stalk of Musa paradisiaca is used as an oral cleansing agent to maintain oral hygiene; yet this folk claim remains to be ascertained scientifically.
Objective
The study assessed the antibacterial and antifungal effects of three extract fractions (aqueous, ethanol, and ethyl acetate fractions) of Musa paradisiaca fruit stalk against Lactobacillus acidophilus, Aggregatibacter actinomycetemcomitans, and Candida albicans, common oral pathogens implicated in dental caries and periodontitis.
Materials and Methods
Aqueous, ethanol, and ethyl acetate fractions of Musa paradisiaca fruit stalk were prepared by cold maceration and qualitatively screened for their phytochemical composition. Antimicrobial effects of the three extract fractions were assessed by using serial broth dilutions at increasing concentrations (62.5, 125, 250, 500, and 1000 µg/ml) and compared to standard antimicrobial agents (erythromycin, doxycycline, and fluconazole). Subsequently, the absorbances of the microbial suspensions treated with increasing concentrations of the extract fractions were measured at 450 nm, and the cell densities were determined.
Results
Except for the aqueous extract, which was less effective in decreasing microbial growth, the ethyl acetate and the ethanol extract fractions demonstrated antimicrobial efficacies comparable to those of the standard drugs. All three extract fractions demonstrated concentration-dependent growth inhibitory effects on the tested oral pathogens although not as effective as the standard drugs used.
Conclusion
Musa paradisiaca fruit stalk has demonstrated antimicrobial effects against Lactobacillus acidophilus, Aggregatibacter actinomycetemcomitans, and Candida albicans, common oral pathogens implicated in dental caries and periodontitis, and this finding confirms in part folk use of Musa paradisiaca fruit stalk as a traditional dental care agent. Thus, the fruit stalk of Musa paradisiaca could be explored for use as a cheap and readily available dental care agent for people entrapped in the poverty line.
1. Introduction
Despite advancements in dentistry, oral diseases (dental caries, periodontitis, bleeding gums, toothache, oral sores, bad breath, tooth sensitivity, tooth loss, and oral cancer) remain a major health problem worldwide [1, 2]. Over 2.3 billion people are reported suffering from caries of permanent teeth and more than 530 million children are suffering from caries of primary teeth [3]. Evidence abounds linking risk of colorectal cancer, gum bleeding, toothache, preterm birth among pregnant mothers, chronic kidney disease, myocardial infarction, and stroke to oral diseases [4–9]. It is reported that most forms of periodontal diseases such as plaque, dental caries, halitosis, gingivitis, periodontitis, and toothache are caused by a complex and elusive activity of over 600 polymicrobial species inhabiting the oral cavity [10]. Common among these oral pathobionts are Gram-positive and -negative bacteria such as Veillonella species, Atopobium species, Prevotella species, Streptococcus mutans, Lactobacillus species, Enterococcus faecalis and some nonmutant streptococci, which are associated with caries formation and progression [10–12]. Also, common Gram-negative bacteria, such as Aggregatibacter actinomycetemcomitans, have been associated with aggressive periodontitis [13], while commensal yeasts, such as Candida albicans, are also implicated in oral candidiasis [14], requiring holistic treatments.
Treatment of oral diseases involves the use of various agents having demonstrable antimicrobial, antioxidant, anti-inflammatory, antifungal, and analgesic effects. Risk of urticaria, taste masking, increased calculus formation, stained teeth and mucous membranes, oral mucosa desquamation, and parotid swelling are associated with long-term use of some conventional oral care agents, such as chlorhexidine [11, 15, 16]. Given the incidence of oral disease, increased microbial resistance, adverse effects associated with some conventional oral care agents currently used in oral care, and the high cost of these conventional oral care agents, it has become necessary to prospect for alternatives treatments which are relatively cheaper, readily available, effective, and safe.
Use of plants and plant-derived products to improve human health has been in existence for centuries even before the dawn of modern medicine. Most of the curative effects of medicinal plants have been determined over hundreds of years through centuries of uneventful use [17, 18]. The World Health Organization (WHO) has reported that in developing countries, 65–80% of populations depend on traditional therapies, mostly plant-based therapies [17]. Thus, the WHO has recommended that countries should formulate national policies and regulations to integrate plant-based therapies into their healthcare systems [19].
Interestingly, use of plant-based therapies to improve general health is quite common in Ghana [20]. For instance, Musa paradisiaca fruit stalk is commonly used in rural Ghana to maintain oral hygiene; however, this folk claim remains to be ascertained scientifically. Ecologically, M. paradisiaca (family, Musaceae) is distributed in the tropical and subtropical regions of the world. Traditionally, Musa paradisiaca is used for treatment of diarrhea, dysentery, intestinal lesions in ulcerative colitis, diabetes, in sprue, uremia, nephritis, gout, hypertension, and cardiac disease [21, 22]. In view of the ethnobotanical importance of M. paradisiaca, many studies have investigated some of its traditional uses. For example, the peels of the fruit have demonstrated antifungal activity against C. albicans [23]. Extracts of unripe fruit peels and leaves of Musa paradisiaca have demonstrated antimicrobial activity against Pseudomonas species, Staphylococcus aureus, Escherichia coli, and Proteus mirabilis [23, 24]. Also, extracts from various parts of M. paradisiaca have demonstrated antidiarrheal, hypoglycemic, antioxidant, antihypertensive, wound healing, antiallergic, antimalarial, leishmanicidal, and anti-snake venom activities [21, 25, 26]. The observed pharmacological properties were attributed to organic and inorganic components of M. paradisiaca including vitamins, lutein, carotene, potassium, and magnesium [23, 27] as well as phytochemicals such as flavonoids, serotonin, tryptophan, indole, tannins, and triterpenes [21, 22]. The present study assessed the antimicrobial effect of M. paradisiaca fruit stalk extracts against three common oral pathogens implicated in dental caries and periodontitis.
2. Materials and Methods
2.1. Chemicals and Reagents
The materials used in the study included Sabouraud Dextrose agar (SDA), peptone water, blood agar, doxycycline (Actavis, Barnstaple, UK), erythromycin (Concordia International, Capital House, UK), and fluconazole (FDC International, Fareham Harts, UK). Also, ethyl acetate, ethanol, sodium hydroxide (VWR Prolab Chemicals, France), hydrogen peroxide, Wagner's reagent, Mayer's reagent, and ferric chloride chemicals used were of analytical grade. Equipment such as a gas bath oscillator, dry-air oven, aerobic incubator, and other apparatus such as Petri dishes, beakers, and pipettes were also used.
2.2. Plant Collection, Identification, and Authentication
Fresh M. paradisiaca fruit stalks (red arrows in Figure 1) were collected from Abura market in the Cape Coast Metropolis, Ghana. Identification and authentication were done by Mr. Francis Otoo, the curator at the Herbarium Unit, School of Biological Sciences, University of Cape Coast. The green outer coverings of the fruit stalk were removed with a clean sharp knife and discarded. The remains were then chopped into small pieces and shade dried for two weeks. The dried fruit stalk remains were pulverized manually by pounding with mortar and pestle. The powdered samples were packed into transparent plastic sample bags, labeled, and stored at room temperature.
Figure 1.

Areal part of Musa paradisiaca (plantain) showing fruit stalk (red arrow) and fruits.
2.3. Preparation of Musa paradisiaca Fruit Stalk Extracts
Three flat-bottomed flasks were each filled with 20 g of the powdered sample. A 200 ml each of distilled water, ethanol, and ethyl acetate were added to each of the flasks and appropriately labeled. The flasks were corked with clean cotton wool and aluminum foil and allowed to stand with intermittent shaking on a mechanical shaker for 72 hours. After shaking, all four samples were vacuum filtered through filter paper (Whatman No. 54) separately with a Buckner funnel and a suction pump. The filtrates were each collected into separate conical flasks and labeled. The four samples were then concentrated using a rotary evaporator and the concentrates transferred into weighed and labeled crucibles for further evaporation on a water bath. All four samples were then placed into a desiccator and allowed to dry. The resultant fractions were weighed and labeled according to the solvent used to extract them as aqueous (AEMP), ethanol (EEMP), and ethyl acetate fractions (EAMP). Five different concentrations (62.5, 125, 250, 500, and 1000 µg/ml) were prepared from each of the extracts by serial dilution.
2.4. Preliminary Phytochemical Screening
The phytochemicals screened for included alkaloids, flavonoids, tannins, terpenoids, saponins, and phenols as follows.
2.4.1. Test for Alkaloids
Presence of alkaloids was determined as previously described [28]. Briefly, 0.4 g of the dried extracts was dissolved in 5 ml of 1% HCl and the mixture warmed and filtered. 1 ml of filtrate was treated separately with (a) a few drops of potassium mercuric iodide (Mayer's reagent) and (b) potassium bismuth (Dragendorff's reagent). Turbidity or precipitation with either of these reagents was taken as evidence for the existence of alkaloids.
2.4.2. Test for Flavonoids
The presence of flavonoids was assessed by using a previously described method. Briefly, 0.5 g of each extract fraction was suspended in 1 ml of distilled water stirred and filtered to obtain a filtrate. A 0.5 ml of dilute ammonia solution was added to 0.5 ml of the filtrate followed by the addition of few drops of concentrated sulphuric acid. Appearance of yellow coloration confirmed the presence of flavonoids.
2.4.3. Test for Tannins
Tannins were detected using a modified version of the Ferric chloride test as described previously [28]. Briefly, 2 drops of 1% aqueous ferric chloride reagent were added to 0.5 ml of crude extract and observed for the formation of blue-black or green coloration, which indicated the presence of tannins.
2.4.4. Test for Phenolic Compounds
Phenolic compounds were detected using a modified version of the Folin–Ciocalteu procedure [29]. Briefly, 200 μl of crude extract was added to 2 ml of 3% aqueous sodium carbonate, followed by the addition of 200 μl Folin–Ciocalteu reagent. The mixture was allowed to stand for 30 minutes at room temperature. The formation of blue/gray colour indicated the presence of phenolic groups.
2.4.5. Test for Triterpenoids
Triterpenoids were detected using a modified version of the Salkowski test [30]. Briefly, 1 ml of extract was slowly added to 400 µl chloroform, followed by the careful addition of 400 µl concentrated sulphuric acid. Formation of a red/brown/purple colour at the interface indicated the presence of triterpenoids.
2.4.6. Test for Cardiac Glycosides
Cardiac glycosides were detected using a modified version of the Keller–Kiliani test [29]. 500 μl of extract was added to 500 μl glacial acetic acid. A few drops of 1% aqueous iron chloride and concentrated sulphuric acid were then carefully added. The presence of a red/brown ring of the interface indicated deoxy sugar characteristic of cardiac glycosides.
2.4.7. Test for Anthraquinones
Anthraquinones were detected using modified versions of the Kumar and Ajaiyeoba tests [28, 29]. The modified Kumar test involved the addition of a few drops of concentrated sulphuric acid to 500 μl pure extract, followed by the careful addition of 500 μl of ammonia. A rose-pink colour indicated the presence of free anthraquinones. For the Ajaiyeoba test, 450 μl of crude extract was added to 50 μl concentrated HCl and allowed to stand at room temperature for several minutes. 500 μl of chloroform was then carefully added. The formation of a rose-pink colour indicated the presence of free hydroxyl anthraquinones.
2.5. Ethical Clearance
The study was approved (UCCIRB/CHAS/2016/29) by the University of Cape Coast Institutional Review Board (UCCIRB) and the management of University of Cape Coast Dental Clinic. Also, patients' consent was sought for by means of a verbal or written agreement after the aim of the study was clearly explained to the patients.
2.6. Acquisition, Culturing, and Identification of Test Organisms
This was a laboratory experimental study to determine the antibacterial and antifungal activities of aqueous, ethanol, and ethyl acetate extracts of Musa paradisiaca fruit stalk against three common oral pathogens. The test bacteria were acquired from the Dental Clinic of University of Cape Coast. The research was carried out in the laboratories at the Department of Medical Laboratory Science, University of Cape Coast and Cape Coast Teaching Hospital. The plant extracts were screened for phytochemicals present and then assayed for the antibacterial activity using serial broth dilution. Test fungal strains, Candida albicans, that were used in the study are clinical isolates and were provided by the Microbiology Laboratory of Cape Coast Teaching hospital. These species were cultured on Sabouraud Dextrose agar (SDA) plates and incubated at room temperature (28 ± 3°C) for 3 days. Candida species were subcultured unto SDA supplemented with chloramphenicol and incubated at 37°C for 72 hours to obtain pure isolates. Identification of Candida albicans was done using germ tube test. Bacterial strains, Lactobacillus acidophilus and Aggregatibacter actinomycetemcomitans, were obtained by taking dental swabs from the patients who visited the University of Cape Coast Dental Clinic. Using sterile cotton swab sticks, swabs were taken from deep cavities in the teeth and also from plaques on the teeth. The swabs were placed in Bijou bottles containing sterile peptone water and incubated overnight at 35°C. Bacterial colonies were subcultured on blood agar and incubated at 35°C for 24 hours. After incubation, colony appearance, biochemical tests as previously performed [31], and Gram staining to confirm the presence of Lactobacillus acidophilus and Aggregatibacter actinomycetemcomitans were performed. Table 1 shows a summary of results of biochemical tests that were conducted to confirm or otherwise the identity of the test organisms.
Table 1.
Results of biochemical tests to confirm Lactobacillus acidophilus and Aggregatibacter actinomycetemcomitans.
| Test | Reaction |
|---|---|
| Lactobacillus acidophilus | |
| Gram reaction | + |
| 2% NaCl tolerance | + |
| 2% bile tolerance | + |
| Glucose fermentation | + |
| Galactose | + |
| Sucrose | + |
| Lactose | + |
| Mannose | + |
| Catalase | − |
| Motility | − |
| Indole | − |
| TSI | − |
| Citrate | − |
|
| |
| Aggregatibacter actinomycetemcomitans | |
| Gram reaction | − |
| Glucose fermentation | + |
| Motility | − |
| Fructose | + |
| Maltose | + |
| Indole | − |
| Catalase | + |
| Oxidase | − |
(+) indicates positive reaction; (−) indicates negative reaction.
2.7. Serial Broth Dilution
Each fraction of the extract was dissolved in sterile distilled water to form stock concentrations of 10380 µg/ml, 10230 µg/ml, and 10300 µg/ml for ethanol, aqueous, and ethyl acetate fractions, respectively. From these stock solutions, working concentrations of 1000, 500, 250, 125, and 62.5 µg/ml were prepared and used for the tests for antimicrobial activity. Fluconazole (FDC International, Fareham Harts, UK), doxycycline (Actavis, Barnstaple, UK), and erythromycin (Concordia International, Capital House, UK) were used as positive control drugs for Candida albicans, Aggregatibacter actinomycetemcomitans, and Lactobacillus acidophilus, respectively, with the same concentration as the fractions. A 0.5 McFarland standard was used to standardize each of the three freshly cultured isolates to a density of 1.5 × 108 CFU/ml in sterile peptone water. 0.5 ml of this inoculum was added to sterile test tubes with the label of the fraction and the concentration. 1 ml of each concentration of the fractions was added to the corresponding test tubes containing the inoculum. The same was done for the drug control with the drug replacing the fractions instead. Turbidity standard for the bacteria was prepared by adding 0.5 ml of the inoculum to 1 ml of sterile peptone water whereas sterility standard was prepared by using sterile peptone without any additions. Bacterial and fungal suspensions were incubated at 35°C and 25°C, respectively, for 24 hours. Absorbance of the content of each of the test tubes was determined at a wavelength of 450 nm. The density of each suspension after incubation was determined by proportion as it was compared to the density and absorbance of the 0.5 McFarland. Each experiment was repeated at least three times.
2.8. Statistical Analysis
Data generated from the antibacterial effect of the three plant extracts of Musa paradisiaca on the 2 test bacteria and 1 fungi specie were entered and organized in Microsoft Excel. These data were then exported to IBM SPSS version 24.0 (SPSS Inc., Chicago, IL, USA) to compute descriptive statistics of the mean and standard deviations. Data were also analyzed with GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA) to perform one-way analysis of variance (ANOVA) using Tukey's multiple comparisons to compare between different antibacterial activities of different extract concentrations against each test bacteria versus controls. Two-way ANOVA was used to determine if there were significant differences in the antibacterial activities between the aqueous, ethanol, and ethyl acetate extracts at varying concentrations against the test bacteria. P values ≤ 0.05 were considered statistically significant in all analyses.
3. Results
3.1. Yield of Extracts and Phytochemical Screening
The aqueous extract yielded the highest fraction of crude extract of M. paradisiaca stalk. Upon phytochemical screening of M. paradisiaca fruit stalk extracts (MPFSEs), it was observed that phenols were present in all the three extracts fractions (Table 2). Triterpenoids and cardiac glycosides were detected in the ethyl acetate fraction. All fractions except the aqueous fractions showed presence of alkaloids (Table 2).
Table 2.
Percentage yield and qualitative phytochemical profile of the three extract fractions of Musa paradisiaca fruit stalk.
| Solvents for extraction | |||
|---|---|---|---|
| Aqueous | Ethanol | Ethyl acetate | |
| Yield∗N (%) | 1.82 (9.10) | 0.53 (2.65) | 0.24 (1.20) |
| Phytochemicals | |||
| Phenols | + | + | + |
| Flavonoids | − | − | − |
| Anthraquinones | − | − | − |
| Tannins | − | − | − |
| Triterpenoids | + | − | + |
| Alkaloids | − | − | + |
| Cardiac glycosides | − | + | + |
+ = present; − = absent; and ∗N/Y × 100, where N = mass of the extract and Y = mass of dry powdered fruit stalk (20 g).
3.2. Effect of Extracts on Mean Cell Density (CFU/mL) of L. acidophilus
Erythromycin demonstrated concentration-dependent decrease in mean cell density of L. acidophilus. Although the extracts fractions demonstrated inhibitory effects on mean cell density of L. acidophilus, this was not comparable to that of erythromycin (Figure 2). Among the three extract fractions, the ethyl acetate fraction demonstrated significant inhibitory effects on cell density, and this inhibitory effect was concentration dependent.
Figure 2.

Effect of extracts on microbial cell density of Lactobacillus acidophilus. Each bar is the mean cell density ± SD, n = 3.
3.3. Effect of Extracts on Mean Cell Density (CFU m/L) of A. actinomycetemcomitans
Both doxycycline, aqueous and ethanol fractions did not demonstrate significant inhibitory effects on mean cell density of A. actinomycetemcomitans. The ethyl acetate fraction demonstrated concentration-dependent inhibitory effect on mean cell density (Figure 3).
Figure 3.

Effect of extracts on microbial cell density of A. actinomycetemcomitans. Each bar is the mean cell density ± SD, n = 3.
3.4. Effect of Extracts on Mean Cell Density (CFU m/L) of Candida albicans
Aqueous fractions of the extract had no inhibitory effect on mean cell density of C. albicans compared to that of fluconazole. Ethyl acetate and ethanol fractions of the extract demonstrated concentration-dependent inhibitory effects on the mean cell density of C. albicans comparable to that of fluconazole (Figure 4). At the highest equipotent concentrations, the ethyl acetate fraction demonstrated more inhibitory potency than fluconazole (Figure 4).
Figure 4.
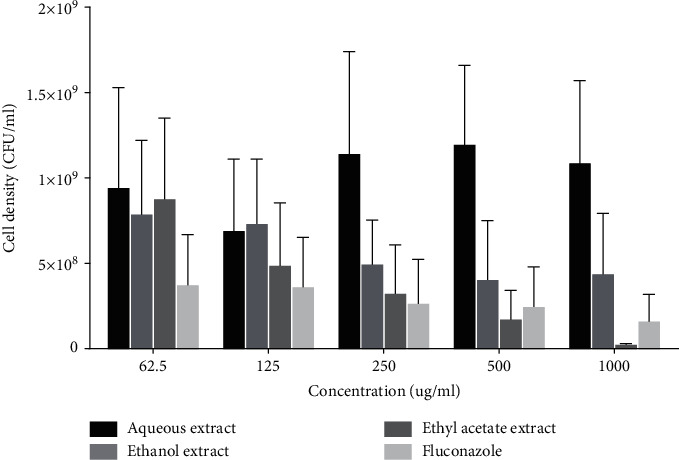
Effect of extracts on microbial cell density of C. albicans. Each bar is the mean cell density ± SD, n = 3.
3.5. Effect of Extracts on Growth of Test Organisms
All the three fractions (aqueous, ethanol, and ethyl acetate) demonstrated some degree of growth inhibition against the three test organisms (L. acidophilus, A. actinomycetemcomitans, and C. albicans). Among the fractions, ethyl acetate fraction was the most potent against all the test organisms (Figure 5).
Figure 5.

Inhibitory effects of extract fractions of M. paradisiaca fruit stalk on the of growth of (a) Lactobacillus acidophilus, (b) Aggregatibacter actinomycetemcomitans, and (c) Candida albicans.
4. Discussion
This study has demonstrated that extracts from M. paradisiaca fruit stalk have antimicrobial effects against common oral pathogens (Lactobacillus acidophilus, Aggregatibacter actinomycetemcomitans, and Candida albicans), mostly implicated in oral infections. For instance, these pathobionts have long been linked to the initiation and progression of dental caries, oral thrush, and periodontitis [12, 14, 32]. Antimicrobial property is considered as one of the key hallmarks of oral care agents, in view of the involvement of pathogenic bacteria and fungal species in oral infections. The fruit stalk of Musa paradisiaca is used in rural Ghana to maintain oral hygiene. Specifically, a pulverized preparation of the fruit stalk of Musa paradisiaca with or without charcoal is used to clean the teeth and also to maintain oral hygiene. Use of M. paradisiaca fruit stalk by rural folks has been handed down many generations, and this age-old practice has been uneventful. At a time, that use of conventional oral cleansing agents has not only received a backlash for claimed adverse effects but also has been seen as expensive yet ineffective; it is refreshing to learn of readily available alternative or complementary oral care therapies. However, such alternative oral care therapies need to be verified scientifically to substantiate these claims.
In this study, three (aqueous, ethanol, and ethyl acetate) extract fractions of M. paradisiaca produced concentration-dependent inhibition of growth of the test oral pathogens; in particular, the ethyl acetate extract fraction demonstrated significant antimicrobial activity compared to the other extract fractions. Except for the aqueous extract, which did not demonstrate significant decrease in cell density of the test pathogens, the ethyl acetate and ethanol fractions demonstrated significant antimicrobial activity. The ethyl acetate extract demonstrated concentration-dependent broad-spectrum decrease in cell densities of the three test pathogens. In the case of the ethanol extract, antifungal activity against Candida albicans was observed. The current observed antimicrobial activity of M. paradisiaca fruit stalk extracts against oral pathogens implicated in oral infections complements that of other studies which showed that extracts from fruits, florets, root, leaves, and peels of M. paradisiaca possess antimicrobial properties [23, 27, 33, 34]. The current observation together with that of previous studies which demonstrated that M. paradisiaca has antimicrobial activity against A. actinomycetemcomitans [35] and C. albicans [23] clearly shows the possible prospects of M. paradisiaca as an alternative or complementary oral therapy that can be exploited for use by indigenous people.
The bioactivity of medicinal plants has always been linked to their phytochemical composition and functional group enrichment [36]. Antioxidant, antiproliferative, chemopreventive, and antimicrobial properties demonstrated by synergistic interactions of naturally occurring phytocompounds including alkaloids, phenolic compounds, tannins, saponins, and terpenoids have been reported in oral health [16, 37]. The present study showed that Musa paradisiaca fruit stalk contains alkaloids, terpenoids, cardiac glycosides, and phenols, which corroborates earlier reports on Musa paradisiaca [21, 23] and these phytocompounds possibly may account for the observed antimicrobial activity in the current study.
In increasing order, the antimicrobial activity of the three extract fractions of M. paradisiaca fruit stalk was in the order aqueous < ethanol < ethyl acetate, and this order of antimicrobial activity reflected their phytochemical composition and probably solvent system dependent. It was not surprising to observe that ethyl acetate fraction demonstrated significant antimicrobial activity as it extracted most of the phytocompounds, including alkaloids and cardiac glycosides, which were absent in the aqueous fraction (Table 2). Cardiac glycosides from other medicinal plants have demonstrated antifungal properties [38]. Thus, it is also possible that the antifungal activity demonstrated by the ethyl acetate and ethanol extract fractions of M. paradisiaca fruit stalk could be due to their cardiac glycoside components. Surprisingly, alkaloids and cardiac glycosides were absent in the aqueous extract fraction of M. paradisiaca fruit stalk in the current study. However, in a study involving fruit peel of M. sapientum, it was shown that the aqueous fraction demonstrated significant antibacterial effect compared to that of absolute ethanol fraction and this was linked to its alkaloidal components [39] and this observation stands at variance with the current observation where the aqueous fraction of M. paradisiaca fruit stalk demonstrated the least antimicrobial activity. In summary, the observed antimicrobial activity of the fractions (aqueous, ethanol, and ethyl acetate) directly relates to their ability to extract diverse compounds from the fruit stalk; however, disparity between the current results and that of other species in the genus Musa could be due to the differences in both species and the part of plant investigated.
5. Conclusion
M. paradisiaca fruit stalk extracts have demonstrated antimicrobial effects against Lactobacillus acidophilus, Aggregatibacter actinomycetemcomitans, and Candida albicans, and this observation perhaps adds credence to its folk use in maintaining oral hygiene. The observed antimicrobial activity is attributable to the phytochemical composition of M. paradisiaca fruit stalk; therefore, future studies should focus on bio-assay guided extraction, identification, and purification of specific antimicrobial phytocompounds with specific antimicrobial activity against oral pathogens.
Acknowledgments
The authors thank Emmanuel Birikorang and Jonathan Ntow (Department of Laboratory Technology, University of Cape Coast) and Francis Kobina Arthur (Microbiology Laboratory, Cape Coast Teaching Hospital, Cape Coast).
Data Availability
All data used to support the findings of this study are available within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Alex Boye conceived, designed, and wrote and critically reviewed the final manuscript for intellectual content. Ernest Owusu-Boadi, Gabriel Mensah, and Emmanuel Ayamba Ayimbissa performed experiments. Mainprice Akuoko Essuman analyzed the results and retrieved literature information.
References
- 1.Jin L., Lamster I., Greenspan J., Pitts N., Scully C., Warnakulasuriya S. Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Diseases. 2016;22(7):609–619. doi: 10.1111/odi.12428. [DOI] [PubMed] [Google Scholar]
- 2.Peres M. A., Macpherson L. M. D., Weyant R. J., et al. Oral diseases: a global public health challenge. The Lancet. 2019;394(10194):249–260. doi: 10.1016/s0140-6736(19)31146-8. [DOI] [PubMed] [Google Scholar]
- 3.James S. L., Abate D., Abate K. H., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. The Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flemer B., Warren R. D., Barrett M. P., et al. The oral microbiota in colorectal cancer is distinctive and predictive. Gut. 2018;67(8):1454–1463. doi: 10.1136/gutjnl-2017-314814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han Y. W. Oral health and adverse pregnancy outcomes - what’s next? Journal of Dental Research. 2011;90(3):289–293. doi: 10.1177/0022034510381905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oyetola E. O., Owotade F. J., Agbelusi G. A., Fatusi O. A., Sanusi A. A. Oral findings in chronic kidney disease: implications for management in developing countries. BMC Oral Health. 2015;15(1):24–28. doi: 10.1186/s12903-015-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han Y. W., Fardini Y., Chen C., et al. Term stillbirth caused by oral fusobacterium nucleatum. Obstetrics & Gynecology. 2010;115(2):442–445. doi: 10.1097/aog.0b013e3181cb9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich T., Webb I., Stenhouse L., et al. Evidence summary: the relationship between oral and cardiovascular disease. British Dental Journal. 2017;222(5):381–385. doi: 10.1038/sj.bdj.2017.224. [DOI] [PubMed] [Google Scholar]
- 9.Hamza S. A., Asif S., Khurshid Z., Zafar M. S., Bokhari S. A. H. Emerging role of epigenetics in explaining relationship of periodontitis and cardiovascular diseases. Diseases. 2021;9(3):p. 48. doi: 10.3390/diseases9030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simón-Soro A., Mira A. Solving the etiology of dental caries. Trends in Microbiology. 2015;23(2):76–82. doi: 10.1016/j.tim.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Shafiei Z., Shuhairi N. N., Md Fazly Shah Yap N., Harry Sibungkil C. A., Latip J. Antibacterial activity of myristica fragrans against oral pathogens. Evidence-Based Complementary and Alternative Medicine: ECAM. 2012;2012 doi: 10.1155/2012/825362.825362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakshmi T., Roy A., Merlin A. R. S. Antibacterial activity of achyranthes aspera extract against oral pathogens–an in vitro study. Plant Cell Biotechnology and Molecular Biology. 2020;21(25):37–40. [Google Scholar]
- 13.Fine D. H., Patil A. G., Velusamy S. K. Aggregatibacter actinomycetemcomitans (aa) under the radar: myths and misunderstandings of aa and its role in aggressive periodontitis. Frontiers in Immunology. 2019;10:p. 728. doi: 10.3389/fimmu.2019.00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hebecker B., Naglik J. R., Hube B., Jacobsen I. D. Pathogenicity mechanisms and host response during oral candida albicans infections. Expert Review of Anti-infective Therapy. 2014;12(7):867–879. doi: 10.1586/14787210.2014.916210. [DOI] [PubMed] [Google Scholar]
- 15.Varghese J., Ramenzoni L., Shenoy P., et al. In vitro evaluation of substantivity, staining potential, and biofilm reduction of guava leaf extract mouth rinse in combination with its anti-inflammatory effect on human gingival epithelial keratinocytes. Materials. 2019;12(23):p. 3903. doi: 10.3390/ma12233903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palombo E. A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evidence-Based Complementary and Alternative Medicine. 2011;2011 doi: 10.1093/ecam/nep067.680354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzari A. L., Prieto J. M. Herbal medicines in Brazil: pharmacokinetic profile and potential herb-drug interactions. Frontiers in Pharmacology. 2014;5:p. 162. doi: 10.3389/fphar.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fridlender M., Kapulnik Y., Koltai H. Plant derived substances with anti-cancer activity: from folklore to practice. Frontiers of Plant Science. 2015;6:p. 799. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baptista A., Gonçalves R. V., Bressan J., Pelúzio M. D. C. G. Antioxidant and antimicrobial activities of crude extracts and fractions of cashew (anacardium occidentale l.), cajui (anacardium microcarpum), and pequi (caryocar brasiliense c.): a systematic review. Oxidative Medicine and Cellular Longevity. 2018;2018 doi: 10.1155/2018/3753562.3753562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oppong Bekoe E., Agyare C., Boakye Y. D., et al. Ethnomedicinal survey and mutagenic studies of plants used in accra metropolis, Ghana. Journal of Ethnopharmacology. 2020;248 doi: 10.1016/j.jep.2019.112309.112309 [DOI] [PubMed] [Google Scholar]
- 21.Mondal A., Banerjee S., Bose S., et al. Cancer preventive and therapeutic potential of banana and its bioactive constituents: a systematic, comprehensive, and mechanistic review. Frontiers in Oncology. 2021;11:2214. doi: 10.3389/fonc.2021.697143.697143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira A., Maraschin M. Banana (musa spp) from peel to pulp: ethnopharmacology, source of bioactive compounds and its relevance for human health. Journal of Ethnopharmacology. 2015;160:149–163. doi: 10.1016/j.jep.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Behiry S. I., Okla M. K., Alamri S. A., et al. Antifungal and antibacterial activities of wood treated with musa paradisiaca L. Peel extract: HPLC analysis of phenolic and flavonoid contents. Processes. 2019;7(4):p. 215. doi: 10.3390/pr7040215. [DOI] [Google Scholar]
- 24.Ahmad I., Beg A. Z. Antimicrobial and phytochemical studies on 45 indian medicinal plants against multi-drug resistant human pathogens. Journal of Ethnopharmacology. 2001;74(2):113–123. doi: 10.1016/s0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- 25.Yakubu M. T., Nurudeen Q. O., Salimon S. S., et al. Antidiarrhoeal activity of musa paradisiaca sap in wistar rats. Evidence-Based Complementary and Alternative Medicine: ECAM. 2015;2015 doi: 10.1155/2015/683726.683726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Accioly M. P., Bevilaqua C. M., Rondon F. C., et al. Leishmanicidal activity in vitro of musa paradisiaca l. and spondias mombin l. Fractions. Veterinary Parasitology. 2012;187(1-2):79–84. doi: 10.1016/j.vetpar.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Mostafa H. S. Banana plant as a source of valuable antimicrobial compounds and its current applications in the food sector. Journal of Food Science. 2021;98 doi: 10.1111/1750-3841.15854. [DOI] [PubMed] [Google Scholar]
- 28.Saeed N., Khan M. R., Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts torilis leptophylla l. BMC Complementary and Alternative Medicine. 2012;12(1):221–312. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harborne J. B., Williams C. A. Anthocyanins and other flavonoids (January 1998 to December 2000) Natural Product Reports. 2001;18(3):310–333. doi: 10.1039/b006257j. [DOI] [PubMed] [Google Scholar]
- 30.Oyedeji A., Ekundayo O., Olawore O. N., Adeniyi B. A., Koenig W. A. Antimicrobial activity of the essential oils of five eucalyptus species growing in Nigeria. Fitoterapia. 1999;70(5):526–528. doi: 10.1016/s0367-326x(99)00083-0. [DOI] [Google Scholar]
- 31.Nørskov-Lauritsen N. Classification, identification, and clinical significance of haemophilus and aggregatibacter species with host specificity for humans. Clinical Microbiology Reviews. 2014;27(2):214–240. doi: 10.1128/CMR.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fine D. H., Kaplan J. B., Kachlany S. C., Schreiner H. C. How we got attached to actinobacillus actinomycetemcomitans: a model for infectious diseases. Periodontology 2000. 2006;42(1):114–157. doi: 10.1111/j.1600-0757.2006.00189.x. [DOI] [PubMed] [Google Scholar]
- 33.Fahim M., Ibrahim M., Zahiruddin S., et al. TLC‐bioautography identification and GC‐MS analysis of antimicrobial and antioxidant active compounds in Musa × paradisiaca L. fruit pulp essential oil. Phytochemical Analysis. 2019;30(3):332–345. doi: 10.1002/pca.2816. [DOI] [PubMed] [Google Scholar]
- 34.Ariffin M. M., Khong H. Y., Nyokat N., Liew G. M., Hamzah A. S., Boonpisuttinant K. In vitro antibacterial, antioxidant, and cytotoxicity evaluations of musa paradisiaca cv. Sekaki florets from sarawak, Malaysia. MPC Journal of Applied Pharmaceutical Science. 2021;11(5):91–99. [Google Scholar]
- 35.Kapadia S., Pudakalkatti P., Shivanaikar S. Detection of antimicrobial activity of banana peel (musa paradisiaca l.) on porphyromonas gingivalis and aggregatibacter actinomycetemcomitans: an in vitro study. Contemporary Clinical Dentistry. 2015;6(4):p. 496. doi: 10.4103/0976-237x.169864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acheampong D. O., Baffour I. K., Addo J. K., Essuman M. A., Boye A. Zanthoxylum zanthoxyloides alkaloidal extract improves ccl4-induced hepatocellular carcinoma-like phenotypes in rats. Evidence-Based Complementary and Alternative Medicine. 2021;2021 doi: 10.1155/2021/3804379.3804379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karygianni L., Al-Ahmad A., Argyropoulou A., Hellwig E., Anderson A. C., Skaltsounis A. L. Natural antimicrobials and oral microorganisms: a systematic review on herbal interventions for the eradication of multispecies oral biofilms. Frontiers in Microbiology. 2016;6:1529. doi: 10.3389/fmicb.2015.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Javed S., Shoaib A., Mahmood Z., Mushtaq S., Iftikhar S. Analysis of phytochemical constituents of eucalyptus citriodora l. Responsible for antifungal activity against post-harvest fungi. Natural Product Research. 2012;26(18):1732–1736. doi: 10.1080/14786419.2011.607451. [DOI] [PubMed] [Google Scholar]
- 39.Siddique S., Nawaz S., Muhammad F., Akhtar B., Aslam B. Phytochemical screening and in-vitro evaluation of pharmacological activities of peels of musa sapientum and carica papaya fruit. Natural Product Research. 2018;32(11):1333–1336. doi: 10.1080/14786419.2017.1342089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support the findings of this study are available within the article.


