Abstract
Qianghuo Shengshi decoction (QHSSD) is a classical Chinese medicine formula, which is used in clinical practice for the treatment of rheumatoid arthritis (RA) in China. However, the pharmacological mechanism of QHSSD on RA has remained unclear by now. We collected and screened active compounds and its potential targets by the pharmacology platform of Chinese herbal medicines. In addition, the therapeutic targets of RA were obtained and selected from databases. Network construction analyzed that 128 active compounds may act on 87 candidate targets and identified a total of 18 hub targets. GO annotation and KEGG enrichment investigated that the action mechanism underlying the treatment of RA by QHSSD might be involved in cell proliferation, angiogenesis, anti-inflammation, and antioxidation. Finally, molecular docking verification showed that TP53, VEGFA, TNF, EGFR, and NOS3 may be related to the RA treatment and molecular dynamics simulation showed the stability of protein-ligand interactions. In this work, QHSSD might exert therapeutic effect through a multicomponent, multitarget, and multipathway in RA from a holistic aspect, which provides basis for its mechanism of action and subsequent experiments.
1. Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease with the following symptoms: tender, warm, swollen, and stiff joints in the morning and as RA progresses, resulting in bone erosion, joint deformity, and physical disabilities [1–4]. RA affects approximately 1% of the worldwide population and mostly occurs in middle-aged women [5]. The drug treatment of RA mainly focuses on nonsteroidal anti-inflammatory drugs (NSAIDs), steroids, disease-modifying antirheumatic drugs (DMARDs), and glucocorticoids. However, these drugs can only delay the progression of RA, which may also increase the risk of infection. Long-term use of these drugs will cause drug dependence and a cascade of side effects, such as digestive tract irritation reaction, rashes, liver toxicity, and hair loss [2, 6, 7].
There is a rising trend of the application of traditional Chinese medicine in the treatment of RA, and the RA patients are more inclined to choose complementary medicine as a medical treatment [8–10]. Traditional Chinese medicine (TCM) has a long history of clinical use in China, used as complementary and alternative medicines in treatment [11]. Qianghuo Shengshi decoction (QHSSD) is a classic clinical prescription from internal and external causes (Nei Wai Shang Bian Huo Lun). Accordingly, QHSSD can dispel wind and dampness and sweat and alleviate pain, consisting of seven herbs: Notopterygii rhizoma et radix (Qianghuo, QH), Angelicae pubescentis radix (Duhuo, DH), Saposhnikoviae radix (Fangfeng FF), Ligustici rhizoma et radix (Gaoben, GB), Chuanxiong rhizoma (Chuangxiong, CX), Viticis fructus (Manjingzi, MJZ), and Glycyrrhizae radix et rhizoma (Gancao, GC). QHSSD has been used to treat influenza, allergic purpura, and inflammatory diseases, such as RA [12–15]. Unlike the single-target agent with a clear mechanism, however, the mechanism of QHSSD underlying the treatment of RA still remains a blur.
Network pharmacology is a novel approach to understand drug's pharmacological mechanism in the network perspective and to find out the interaction between the drug and the body on the basis of biological networks [16]. There is a growing number of researches that applied the network pharmacology to elucidate the mechanism between TCM/herbs and disease treatment, involving multicompound and multitarget ones [17–24]. We therefore tried to explore the mechanism of action of QHSSD on RA by network pharmacology and to further predict the interaction modes between QHSSD and the predicted targets by molecular docking and molecular dynamics. The flowchart of our study is presented in Figure 1.
Figure 1.
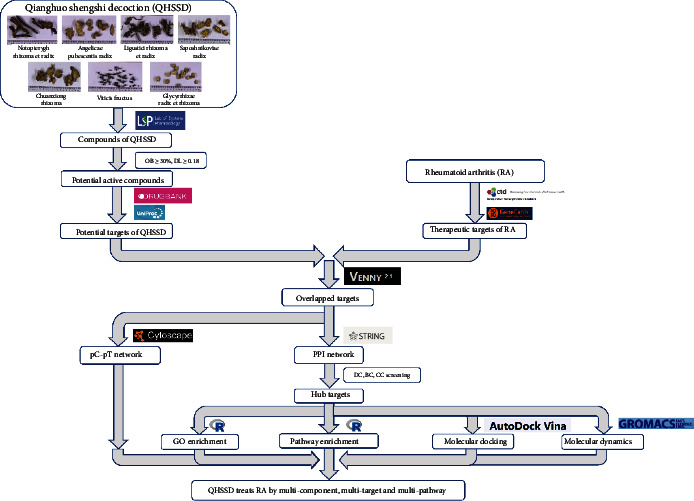
Flowchart of network pharmacology of QHSSD in the treatment of RA.
2. Materials and Methods
2.1. Collecting and Screening of Active Components of QHSSD
We collected the chemical compounds of QHSSD from the traditional Chinese medicine systems pharmacology database and analysis platform (TCMSP) (https://tcmspw.com/tcmsp.php), a common tool for network pharmacology research of TCM [25]. Oral bioavailability (OB) and druglikeness (DL) were set as the main parameters to screen the active components according to the absorption, distribution, metabolism, and excretion (ADME) process. We set OB ≥ 30% and DL ≥ 0.18 as the screening threshold values to select the candidate components through literature search [23, 26, 27].
2.2. Potential Targets of QHSSD
Considering that the target screening criteria differ between different databases (including TCMSP, PubChem, and Swiss Target Prediction databases), therefore, only the TCMSP database was applied in this work. We predicted relevant targets (DrugBank, https://go.drugbank.com/) of active components in QHSSD on the TCMSP and then transformed the target name to a standard gene name on Uniport (https://www.uniprot.org/) database and removed the duplications.
2.3. Identification of Associated Targets of RA
RA-related genes were collected from the CTD (http://ctdbase.org/) database [28] and GeneCards (https://www.genecards.org/) database [29], with the keyword “rheumatoid arthritis.” The genes from the above databases merged and removed the duplications.
2.4. Compound-Target Network Construction and Analysis
Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/) [30] was used to find out the overlapping targets between compound targets and disease targets. To explore the relationship between compounds and disease more reasonably, Cytoscape 3.7.2 (https://cytoscape.org/) [31] with a visualized tool and all node degrees of networks calculated was used to construct a compound-potential target network.
2.5. Protein-Protein Interaction (PPI) Network Construction and Analysis
The Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) (https://string-db.org/) was used to predict the interaction between proteins [32]. According to the previous steps, drug-associated genes and disease targets were analyzed on STRING to create a protein-protein interaction network (PPI network) by importing overlapping targets. Cytoscape was then used to construct a core PPI network.
2.6. Gene Ontology (GO) Enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis
We imported the hub targets on the Bioconductor clusterProfiler org.Hs.eg.db and DOSE packages [33, 34] of R 4.0.2 (https://cran.r-project.org/src/base/R-4/) software to create the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment and construct Gene Ontology (GO) biological function enrichment analysis with p < 0.05 and q < 0.05 as the thresholds.
2.7. Compound-Target Molecular Docking
According to previous studies [19, 20], compounds with a higher degree value and potential targets were evaluated by molecular docking. The 3D structure format file of the active compounds screened from the previous step and the hub targets were downloaded from the PubChem (https://pubchem.ncbi.nlm.nih.gov/) database and the RCSB PDB (https://www.rcsb.org/), respectively. The active compounds are ligands and the targets are receptors. AutoDockTools 1.5.6 software was used to remove the water molecules, isolate proteins, add nonpolar hydrogen, and calculate Gasteiger charges for the structure. Both ligands and receptors were kept as PDBQT format docked through AutoDock Vina [35]. The Grid Box size and position of macromolecule were predicted by POCASA 1.1 (http://g6altair.sci.hokudai.ac.jp/g6/service/pocasa/). Then, the protein-ligand interaction was visualized with PyMOL 2.3.0 software.
2.8. Molecular Dynamics Simulations
Molecular dynamics (MD) simulations were used to evaluate the structural characteristics of the protein-ligand system [36]. In this work, the GROMACS Program package [37] and CHARMM36 force field [38] were used to simulate the molecular dynamics of the complexes in the TIP3P water model. Ions (Na+ or Cl−) were added to neutralize charges. The energy of systems was minimized through a 4000-step steepest descent method and a 2000-step conjugate gradient method to eliminate high-energy collisions between molecules. Then, the systems were heated from 0 K to 300 K within 100 ps and simulated as an NPT ensemble with normal temperature (300 K) and normal pressure (101 kPa) for 100 ps; unconstrained MD simulation was carried out for 10 ns after the system reached dynamic equilibrium. Periodic boundary conditions and particle mesh Ewald (PME) methods were used to deal with long-range electrostatic interactions. The short-range Coulomb interaction was 0.9 nm, and the SHAKE algorithm was used to restrict the bond to the hydrogen atom. The temperature and pressure of the system were controlled by V-rescale and Parrinello-Rahman. The stability of systems was evaluated using the root-mean-square deviation (RMSD) of the aligned protein-ligand coordinate set calculated against the initial frame.
3. Results
3.1. Compound-Target Network and Analysis
We collected 153 compounds and 152 putative targets of QHSSD (Table S1 and Figure S1) on the screening criteria from the TCMSP. Among these compounds, we excluded 25 compounds without RA-related targets and 128 active compounds were obtained in this work. 1846 targets were selected from 29368 genes related to RA by the screening threshold of inference score ≥ 24.46 on the CTD database. Meanwhile, 306 genes were obtained from 4465 targets with a relevance score ≥ 19.94 on GeneCards. Targets from the above database merged and removed the overlapping parts, and at last, we received a total of 1992 RA-related targets. Based on the Venn diagram, we obtained 87 overlapping targets from 152 putative targets of QHSSD and 1992 RA-related targets. Consequently, 87 targets (Figure 2 and Table 1) were considered as potential targets for the treatment. To further investigate the interaction between the compounds and targets, we constructed a compound-target network (Figure 3) with 215 nodes and 1239 edges. The network results manifested that the average degree value of the compounds was 9.68; we received 66 active compounds. Five compounds were considered to have high connection with putative targets of RA: quercetin (degree = 57), kaempferol (degree = 31), luteolin (degree = 27), wogonin (degree = 21), and isorhamnetin (degree = 20) (Table 2).
Figure 2.
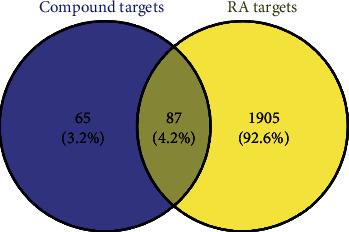
Venn diagram of overlapping targets of QHSSD and RA.
Table 1.
Information of 87 potential targets.
| No. | Target name | Gene name | Gene ID |
|---|---|---|---|
| T1 | Prostaglandin G/H synthase 2 | PTGS2 | 5743 |
| T2 | Estrogen receptor | ESR1 | 2099 |
| T3 | Heat shock protein HSP 90-beta | HSP90AB1 | 3326 |
| T4 | Androgen receptor | AR | 367 |
| T5 | Nitric oxide synthase, inducible | NOS2 | 4843 |
| T6 | Serine/threonine-protein kinase pim-1 | PIM1 | 5292 |
| T7 | Cyclin-dependent kinase 2 | CDK2 | 1017 |
| T8 | Peroxisome proliferator-activated receptor gamma | PPARG | 5468 |
| T9 | Glycogen synthase kinase-3 beta | GSK3B | 2932 |
| T10 | Prostaglandin G/H synthase 1 | PTGS1 | 5742 |
| T11 | Cyclin-A2 | CCNA2 | 890 |
| T12 | Mitogen-activated protein kinase 14 | MAPK14 | 1432 |
| T13 | Serine/threonine-protein kinase Chk1 | CHEK1 | 1111 |
| T14 | cAMP-dependent protein kinase catalytic subunit alpha | PRKACA | 5566 |
| T15 | Acetylcholinesterase | ACHE | 43 |
| T16 | DNA topoisomerase 2-alpha | TOP2A | 7153 |
| T17 | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | PIK3CG | 5294 |
| T18 | Vascular endothelial growth factor receptor 2 | KDR | 3791 |
| T19 | Nitric oxide synthase, endothelial | NOS3 | 4846 |
| T20 | Amine oxidase | MAOB | 4129 |
| T21 | Carbonic anhydrase 2 | CA2 | 760 |
| T22 | Apoptosis regulator Bcl-2 | BCL2 | 596 |
| T23 | Transcription factor AP-1 | JUN | 3725 |
| T24 | DNA topoisomerase 2-beta | TOP2B | 7155 |
| T25 | Interstitial collagenase | MMP1 | 4312 |
| T26 | Tumor necrosis factor | TNF | 7124 |
| T27 | Xanthine dehydrogenase/oxidase | XDH | 7498 |
| T28 | Glutathione S-transferase P | GSTP1 | 2950 |
| T29 | Mitogen-activated protein kinase 1 | MAPK1 | 5594 |
| T30 | Cellular tumor antigen p53 | TP53 | 7157 |
| T31 | Interleukin-6 | IL6 | 3569 |
| T32 | Glucocorticoid receptor | NR3C1 | 2908 |
| T33 | Heme oxygenase 1 | HMOX1 | 3162 |
| T34 | Insulin receptor | INSR | 3643 |
| T35 | Retinoblastoma-associated protein | RB1 | 5925 |
| T36 | Superoxide dismutase | SOD1 | 6647 |
| T37 | Serum paraoxonase/arylesterase 1 | PON1 | 5444 |
| T38 | C-C motif chemokine 2 | CCL2 | 6347 |
| T39 | Cyclin-dependent kinase 1 | CDK1 | 983 |
| T40 | Cytochrome P450 3A4 | CYP3A4 | 1576 |
| T41 | Cytochrome P450 1A2 | CYP1A2 | 1544 |
| T42 | E-selectin | SELE | 6401 |
| T43 | Vascular cell adhesion protein 1 | VCAM1 | 7412 |
| T44 | Arachidonate 5-lipoxygenase | ALOX5 | 240 |
| T45 | Aryl hydrocarbon receptor | AHR | 196 |
| T46 | Glutathione S-transferase Mu 1 | GSTM1 | 2944 |
| T47 | Glutathione S-transferase Mu 2 | GSTM2 | 2946 |
| T48 | Urokinase-type plasminogen activator | PLAU | 5328 |
| T49 | Epidermal growth factor receptor | EGFR | 1956 |
| T50 | Vascular endothelial growth factor A | VEGFA | 7422 |
| T51 | 72 kDa type IV collagenase | MMP2 | 4313 |
| T52 | Cyclin-dependent kinase 4 | CDK4 | 1019 |
| T53 | DNA topoisomerase 1 | TOP1 | 7150 |
| T54 | Interleukin-2 | IL2 | 3558 |
| T55 | Interferon gamma | IFNG | 3458 |
| T56 | Peroxisome proliferator-activated receptor delta | PPARD | 5467 |
| T57 | Mitogen-activated protein kinase 8 | MAPK8 | 5599 |
| T58 | Serine/threonine-protein phosphatase 2B catalytic subunit alpha isoform | PPP3CA | 5530 |
| T59 | Aldo-keto reductase family 1 member C3 | AKR1C3 | 8644 |
| T60 | Amine oxidase | MAOA | 4128 |
| T61 | Amyloid-beta precursor protein | APP | 351 |
| T62 | Caspase-7 | CASP7 | 840 |
| T63 | Hepatocyte growth factor receptor | MET | 4233 |
| T64 | Stromelysin-1 | MMP3 | 4314 |
| T65 | Pro-epidermal growth factor | EGF | 1950 |
| T66 | NADPH--cytochrome P450 reductase | POR | 5447 |
| T67 | Ornithine decarboxylase | ODC1 | 4953 |
| T68 | Endoplasmic reticulum chaperone BiP | HSPA5 | 3309 |
| T69 | Acetyl-CoA carboxylase 1 | ACACA | 31 |
| T70 | Tissue factor | F3 | 2152 |
| T71 | Gap junction alpha-1 protein | GJA1 | 2697 |
| T72 | Interleukin-1 beta | IL1B | 3553 |
| T73 | Tissue-type plasminogen activator | PLAT | 5327 |
| T74 | Thrombomodulin | THBD | 7056 |
| T75 | Collagen alpha-1 | COL1A1 | 1277 |
| T76 | Myeloperoxidase | MPO | 4353 |
| T77 | Collagen alpha-1 | COL3A1 | 1281 |
| T78 | Cathepsin D | CTSD | 1509 |
| T79 | Mitogen-activated protein kinase 3 | MAPK3 | 5595 |
| T80 | Fatty acid synthase | FASN | 2194 |
| T81 | Low-density lipoprotein receptor | LDLR | 3949 |
| T82 | Catalase | CAT | 847 |
| T83 | Aromatase | CYP19A1 | 1588 |
| T84 | Glutathione reductase, mitochondrial | GSR | 2936 |
| T85 | Multidrug resistance-associated protein 1 | ABCC1 | 4363 |
| T86 | Aldo-keto reductase family 1 member C1 | AKR1C1 | 1645 |
| T87 | Aspartate aminotransferase, cytoplasmic | GOT1 | 2805 |
Figure 3.
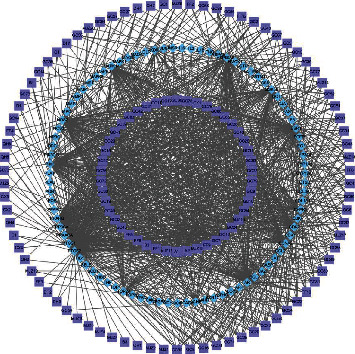
Compound-target network of QHSSD. The rectangle nodes–the compounds of herbs, the diamond nodes–the targets of compounds.
Table 2.
Information of the top five active compounds of QHSSD.
| No. | Mol ID | Chemical component | Degree | CAS | Herb |
|---|---|---|---|---|---|
| A1 | MOL000098 | Quercetin | 57 | 117-39-5 | MJZ/GC |
| A2 | MOL000422 | Kaempferol | 31 | 520-18-3 | MJZ/GC |
| MJZ18 | MOL000006 | Luteolin | 27 | 491-70-3 | MJZ |
| FF9 | MOL000173 | Wogonin | 21 | 632-85-9 | FF |
| GC7 | MOL000354 | Isorhamnetin | 20 | 480-19-3 | GC |
QHSSD: Qianghuo Shengshi decoction; FF: Fangfeng; MJZ: Manjingzi; GC: Gancao.
3.2. PPI Network and Analysis
Based on the previous results, 87 potential targets were imported in STRING with the organism set as “homo sapiens.” Those data with a confidence level higher than 0.4 constructed a complete PPI network including 87 nodes and 1062 edges. We chose three important parameters, including “degree (DC),” “betweenness centrality (BC),” and “closeness centrality (CC),” as the thresholds to screen the key genes to establish major hub nodes by Cytoscape. First, we set the medium of DC, BC, and CC as thresholds, DC ≥ 21, BC ≥ 0.003, and CC ≥ 0.555, respectively, and obtained 37 nodes and 472 edges. Then, the 37 key nodes were further filtered by the second threshold of DC ≥ 27, BC ≥ 0.006, and CC ≥ 0.800 and we got 18 nodes and 153 edges at last (Figure 4). The hub targets of QHSSD in RA therapy are as follows: IL-6, TP53, VEGFA, JUN, TNF, MAPK1, MAPK8, EGF, PTGS2, MAPK3, EGFR, ESR1, IL-1B, CAT, CCL2, MAPK14, MMP2, and NOS3.
Figure 4.
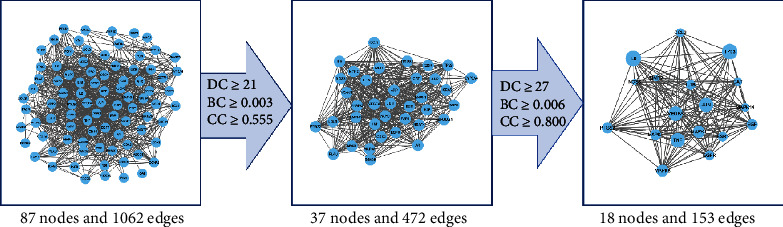
The screening process of the PPI network by Cytoscape (the larger nodes mean higher degree values).
3.3. GO and KEGG Pathway Enrichment Analysis
We performed GO and KEGG pathways to further elucidate the biological functions of 18 hub targets systematically. There were 1534 GO items (1474 terms of biological process, 40 terms of molecular function, and 20 terms of cellular component) and 142 pathways of KEGG enrichment. The top 20 GO items and KEGG pathways are displayed in Figure 5. The biological processes of targets were mainly enriched in oxidative response/stress (GO: 0034599, GO: 0006979, GO: 0000302, and GO: 0034614), inflammatory response (GO: 0032496, GO: 0031663, and GO: 0071222), and biochemical stimulation (GO: 0062197, GO: 0002237, GO: 0071216, and GO: 0071219). Additionally, the KEGG pathways were most enriched in immune- and inflammatory-associated pathways, including the MAPK signaling pathway (hsa04010), IL-17 signaling pathway (hsa04657), TNF signaling pathway (hsa04668), Toll-like receptor signaling pathway (hsa04620), and C-type lectin receptor signaling pathway (hsa04625), and involved in angiogenesis and atherosclerosis pathways: fluid shear stress and atherosclerosis (hsa05418) and relaxin signaling pathway (hsa04926). The compounds of QHSSD may act on these signaling pathways and yield a therapeutic effect.
Figure 5.
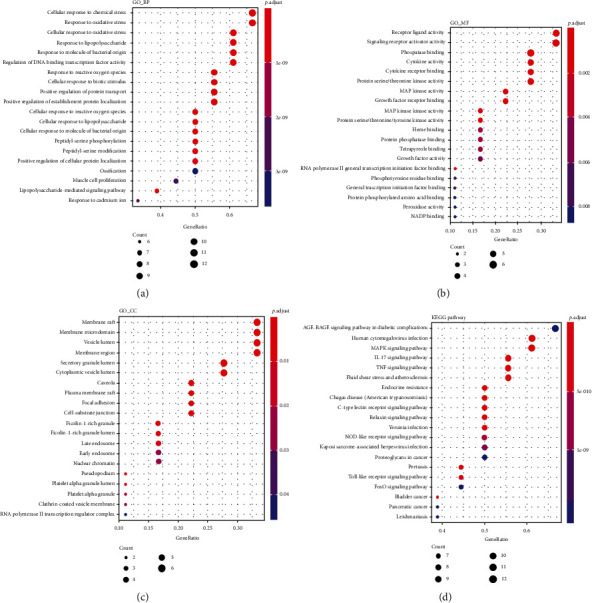
Enrichment analysis of potential targets. (a) GO biological process analysis, (b) GO molecular function, (c) GO cellular component, and (d) KEGG pathway analysis.
3.4. Molecular Docking Analysis
To further elucidate the mechanism of action, 18 hub targets with their related compounds were docked and screened by the binding affinity on AutoDock Vina based on the compound-target network. We investigated a total of 39 pairs of ligand-protein interaction (Table 3), with binding affinity < −5 kcal/mol as the screening threshold. The lower the value of the docking affinity, the stronger the binding ability between the compound and the active site of the target. Accordingly, JUN and TNF get a low binding ability with their related compounds, as well as JUN-quercetin, JUN-kaempferol, and TNF-quercetin. Therefore, 3 pairs of combinations were excluded, and at last, we retrieved 47 compound-target pairs. In addition, cognate docking was used to verify the rationality of molecular docking. In order to verify the rationality of the docking for the TP53 and quercetin system, we investigated the docking of IRAK4 (PDB ID: 2a9i) and quercetin; the binding affinity of IRAK4-quercetin was −6.6 kcal/mol. Small-molecule ligand potentially could fit into the interface port formed by the interaction of amino acid residues in protein. Overall, these complexes provide further evidence that these proteins could act as therapeutic target for the compound of QHSSD.
Table 3.
Results of 18 hub targets and related compounds of molecular docking.
| No. | Targets | PDB ID | Compound | Binding affinity (kcal/mol) |
|---|---|---|---|---|
| 1 | IL6 | 4O9H | Quercetin | −6.4 |
| Luteolin | −6.4 | |||
| Wogonin | −6.4 | |||
| 2 | TP53 | 3DCY | Quercetin | −7.2 |
| Luteolin | −7.5 | |||
| Wogonin | −7.7 | |||
| 3 | VEGFA | 5DN2 | Quercetin | −8.1 |
| Luteolin | −8.1 | |||
| 4 | JUN | 1JUN | Quercetin | −4.8 |
| Kaempferol | −4.9 | |||
| Luteolin | −5.0 | |||
| Wogonin | −5.0 | |||
| 5 | TNF | 1TNF | Quercetin | −4.9 |
| Kaempferol | −5.3 | |||
| Luteolin | −5.4 | |||
| Wogonin | −5.3 | |||
| 6 | MAPK1 | 6RSM | Quercetin | −8.3 |
| Luteolin | −8.5 | |||
| Naringenin | −8.2 | |||
| 7 | MAPK8 | 4YR8 | Kaempferol | −8.6 |
| 8 | EGF | 2KV4 | Quercetin | −6.7 |
| 9 | PTGS2 | 5F19 | Quercetin | −9.4 |
| Kaempferol | −8.9 | |||
| Luteolin | −9.8 | |||
| Wogonin | −9.0 | |||
| Isorhamnetin | −8.8 | |||
| Hesperetin | −10.2 | |||
| Glepidotin B | −9.3 | |||
| Naringenin | −9.3 | |||
| 10 | MAPK3 | 2ZOQ | Naringenin | −8.7 |
| 11 | EGFR | 4R3P | Quercetin | −7.3 |
| Luteolin | −7.5 | |||
| 12 | ESR1 | 4XI3 | Wogonin | −7.2 |
| Isorhamnetin | −7.6 | |||
| Hesperetin | −7.5 | |||
| Glepidotin B | −6.8 | |||
| Naringenin | −8.0 | |||
| 13 | IL1B | 5BVP | Quercetin | −5.7 |
| 14 | CAT | 1DGH | Naringenin | −7.9 |
| 15 | CCL2 | 4ZK9 | Quercetin | −7.7 |
| Wogonin | −6.9 | |||
| 16 | MAPK14 | 3MGY | Wogonin | −7.6 |
| Isorhamnetin | −8.0 | |||
| Hesperetin | −8.2 | |||
| 17 | MMP2 | 1EAK | Quercetin | −7.2 |
| Luteolin | −7.6 | |||
| 18 | NOS3 | 1M9Q | Quercetin | −9.4 |
| Kaempferol | −8.5 | |||
| Isorhamnetin | −8.5 | |||
| Glepidotin B | −8.4 |
3.5. Molecular Dynamics Simulations
To further research the stability of the molecular dynamics trajectory, we took a complex (TP53-quercetin) as an example to analyze the root mean square deviation (RMSD) of the TP53 backbone atoms in this complex, as shown in Figure 6. The RMSD of the TP53 backbone atom began to show an upward trend, whose trajectory was stable and reached an equilibrium state. The RMSD remained stable at about 0.2 nm after 4 ns, but in 7 ns, the trajectory fluctuated slightly. In addition, after 2 ns of the molecular dynamics simulation, the potential energy of the system also tends to be the minimum and stabilizes. The total interaction energy value was −136.2 ± 19.0 kJ/mol. The 4 ns simulated trajectory was extracted by GROMACS as the representative frame of the interaction between quercetin and TP53 (Figure 7); we could find that the amino acid ARG15 of TP53 and quercetin have hydrophobic interactions, resulting in quercetin and TP53 more closely. Moreover, quercetin and TP53 could form three hydrogen bonds (the hydrogen bond between the oxygen atom of quercetin and the hydrogen atom of amino acid residue GLY204 and the hydrogen bond between the hydrogen atom of quercetin and the nitrogen atom of amino acid residues GLU209 and GLN28) and caused the complex to bind more tightly.
Figure 6.
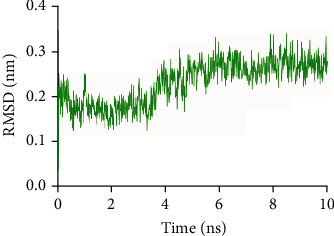
RMSD change of TP53 backbone atoms in MD simulation.
Figure 7.
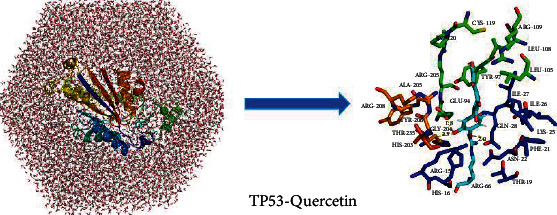
Detailed view of hydrogen bonds between TP53 and quercetin.
4. Discussion
There has been increased researches on how traditional Chinese herbal medicine or formulae work on various diseases based on network pharmacology [21, 22, 24]. In our study, we first collected the putative targets of compounds in QHSSD from the database. And then, the compound-target network was constructed, which revealed that one herb could correspond with multiple components and multiple targets. The traditional Chinese medicine integrative database (TCMID) and a Bioinformatics Analysis Tool for Molecular Mechanism of Traditional Chinese Medicine (BATMAN-TCM) database were used to predict the targets of Chinese herbal medicine [27, 28], whereas we found that the results of these databases were different because of the screening criteria. To ensure the consistency of data, we only used the TCMSP database to collect active compounds and related targets. In compound-target network analysis, the top five compounds (quercetin, kaempferol, luteolin, wogonin, and isorhamnetin) were considered to be crucial compounds in QHSSD. Surprisingly, these compounds are both flavonoids which have antioxidant and anti-inflammatory effects [39–42]. For instance, researches indicated that quercetin mediate the anti-inflammatory mechanism of reducing acute laryngitis by restoring the Th17/Treg balance and activating heme oxygenase 1 and isorhamnetin increased the levels of TNF-α, IL-1β, IL-6, IL-17A, and IL-17F as well as decreased levels of IL-35 and IL-10 in the CIA mice, resulting in an anti-inflammatory effect [43, 44]. Luteolin prevented cartilage destruction in OA rats in vivo through inhibiting IL-1β-induced phosphorylation of NF-κB [45]. In conclusion, these active compounds are crucial material basis of the potential therapeutic effect of QHSSD on RA.
Further, we selected 18 hub targets from the PPT network, as follows: IL-6, TP53, VEGFA, JUN, TNF, MAPK1, MAPK8, EGF, PTGS2, MAPK3, EGFR, ESR1, IL-1B, CAT, CCL2, MAPK14, MMP2, and NOS3. Combined with GO annotation and pathway enrichment analysis, we preliminarily considered that the treatment of RA by QHSSD may participate in biological processes of proliferation, inflammatory response, and angiogenesis. Previous studies indicated that IL-6 and TNF antagonists decrease the inflammatory response of RA patients and IL-1B stimulates inflammation and degradation of the bone and cartilage [46–49]. EGF is an important growth factor, regulating the proliferation of cells. MAPK enables the growth and proliferation of cells by binding with other protein kinases or factors [50]. In addition, the formation of new blood vessels supplied nutrients and oxygen to the augmented inflammatory cell mass, contributing to perpetuation of joint disease [51]. VEGFA, a proangiogenic molecule, promotes the angiogenic phenotype of RA and is upregulated in RA [52, 53]. These literatures are consistent with our prediction. Therefore, these hub targets are considered as crucial proteins in RA therapy.
To further verify the hypothesis of action mechanism, we performed molecular docking on the hub target binding with crucial compounds. Surprisingly, we found that quercetin, luteolin, and wogonin could both fitly bind with TP53, a transcription factor, regulating cell cycle. Moreover, quercetin and luteolin also act on VEGFA. Previous studies revealed that TP53 mutations are linked to the VEGF pathway and showed that the transcriptional target of TP53 inhibited proliferation and angiogenesis via VEGFA [54–56]. We therefore hypothesized that quercetin and luteolin positively worked on TP53 to regulate cell cycle, and meanwhile act on VEGFA to suppress vascular formation. Additionally, luteolin could simultaneously act on EGFR and TNF, decreasing cell proliferation and the level of proinflammatory cytokines. This is in line with the researches before; the result of which manifested that luteolin inhibited IL-1β-induced expression of NO, PGE2, TNF-α, and MMP-2 [45, 50, 57, 58]. On the one hand, the synergistic action of quercetin and luteolin produces the effect of inhibiting cell proliferation and angiogenesis by multiple proteins and also has an anti-inflammatory effect. On the other hand, kaempferol acting on NOS3 may increase NO levels and prevent oxidative damage [59]. From a holistic aspect, QHSSD could inhibit the cell proliferation and angiogenesis and yield anti-inflammatory and antioxidant effects through regulating TP53, VEGFA, EGFR, TNF, and NOS3.
MD is crucial to the discovery of new drug [60] and is a powerful method for studying protein-ligand interactions [61]. In contrast to experimental methods alone, MD simulations can address diseases caused by protein misfolding and virtual screening; it also identifies the stability of protein-ligand complexes and ligand binding kinetics [62]. Previous studies found that quercetin can play an important role in inflammation by acting on the TP53 target [54–56]. In our study, MD simulation was used to investigate the interactions of TP53-quercetin. This complex was simulated within 10 ns and reached an equilibrium state. We found that ligand has three hydrogen bonds with protein; a study [63] had shown that hydrogen bonding has an advantage over shapeless forces if it can form hydrogen bonds. In this study, MD simulation revealed that the structure of TP53-quercetin was very stable when the simulation temperature was 300 K. It is important to explore the application of new drug by molecular docking and MD simulations [61]; however, it is still necessary to perform in vitro experiment to verify the prediction.
5. Conclusion
To conclude, we could learn that multicompounds act on multitargets in TCM therapy. We speculated that the therapeutic effect on the treatment of RA by QHSSD may be focused on antiangiogenesis, meanwhile, anti-inflammation, especially TP53 and VEGFA targets, plays a vital role during the treatment. This study was based on the combination of network pharmacology and molecular docking to predict and identify the mechanism of QHSSD in the treatment of RA, which provides the foundation for subsequent experimental investigation and offers ideas for the multidimensional and multilevel research of TCM formulae.
Acknowledgments
Thanks are due for Warren L. DeLano, the original PyMOL author of the open-source software. Our research was supported by the “Significant News Drugs Creation” Science and Technology Major Project of China (grant number 2018ZX09721005).
Data Availability
The data used to support the findings of this study are included within the supplementary information files.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Authors' Contributions
Xiaoli Bi designed this study and provided technical support toward the study. Zhihao Zeng, Guanlin Xiao, Ruipei Yang, Huajing Huang, and Huixian Zhong collected the data. Zhihao Zeng and Jiaoting Hu analyzed the data and wrote the manuscript. Jieyi Jiang, Sumei Li, and Yangxue Li revised the manuscript.
Supplementary Materials
Table S1: information of active compounds of QHSSD. Figure S1: herb-compound-target network of QHSSD. (Supplementary Materials).
References
- 1.Li T.-p., Zhang A.-h., Miao J.-h., et al. Applications and potential mechanisms of herbal medicines for rheumatoid arthritis treatment: a systematic review. RSC Advances. 2019;9(45):26381–26392. doi: 10.1039/C9RA04737A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilsdon T. D., Hill C. L. Managing the drug treatment of rheumatoid arthritis. Australian Prescriber. 2017;40(2):51–58. doi: 10.18773/austprescr.2017.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelotti F., Parma A., Cafaro G., Capecchi R., Alunno A., Puxeddu I. One year in review 2017: pathogenesis of rheumatoid arthritis. Clinical and Experimental Rheumatology. 2017;35 [PubMed] [Google Scholar]
- 4.Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. American Journal of Managed Care. 2012;18:295–302. [PubMed] [Google Scholar]
- 5.Nemtsova M. V., Zaletaev D. V., Bure I. V., et al. Epigenetic Changes in the Pathogenesis of Rheumatoid Arthritis. Frontiers in Genetics. 2019;10 doi: 10.3389/fgene.2019.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahlenberg J. M., Fox D. A. Advances in the medical treatment of rheumatoid arthritis. Hand Clinics. 2011;27(1):11–20. doi: 10.1016/j.hcl.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conigliaro P., Triggianese P., Martino E. D., Chimenti M. S., Perricone R. Challenges in the treatment of rheumatoid arthritis. Autoimmunity Reviews. 2019;18(7):706–713. doi: 10.1016/j.autrev.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Pan H.-D., Xiao Y., Wang W.-Y., Ren R.-T., Leung E. L.-H., Liu L. Traditional Chinese Medicine as a Treatment for Rheumatoid Arthritis: From Empirical Practice to Evidence-Based Therapy. Engineering. 2019;5(5):895–906. doi: 10.1016/j.eng.2019.01.018. [DOI] [Google Scholar]
- 9.Xing Q., Fu L., Yu Z., Zhou X., Vannacci A. Efficacy and Safety of Integrated Traditional Chinese Medicine and Western Medicine on the Treatment of Rheumatoid Arthritis: A Meta-Analysis. Evidence-Based Complementary and Alternative Medicine. 2020;2020:15. doi: 10.1155/2020/4348709.4348709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H., Pan T., Liu P., Wang P., Xu S., Yamada H. Baihu Jia Guizhi Decoction Improves Rheumatoid Arthritis Inflammation by Regulating Succinate/SUCNR1 Metabolic Signaling Pathway. Evidence-Based Complementary and Alternative Medicine. 2019;2019:11. doi: 10.1155/2019/3258572.3258572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sham T.-T., Chan C.-O., Wang Y.-H., Yang J.-M., Mok D. K.-W., Chan S.-W. A review on the traditional Chinese medicinal herbs and formulae with hypolipidemic effect. BioMed Research International. 2014;2014:21. doi: 10.1155/2014/925302.925302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J.-J. Treatment of arthralgia by Qianghuo Shengshi decoction and Huangqi Guizhi Wuwu decoction (report of 300 cases) Journal of Hubei University of Science and Technology(Medical Sciences) 2014;28:p. 150. [Google Scholar]
- 13.Li M.-Z. Clinical Observation on Treatment of Rheumatoid Arthritis with Modification of Qianghuo Shengshi Decoction Combined with Traditional Chinese Medicine. Mod. Health Care. 2017. pp. 187–188.
- 14.Li Y., Liu H., Deng X., Ding G., Lin S. The clinical application status of Qianghuo Shengshi decoction. Journal of Jiangxi College of Traditional Chinese Medicine. 2017;29:113–114. [Google Scholar]
- 15.Hongxia F., Chunhao W., Lijuan P., et al. Study on the Anti-Inflammatory and Analgesic Effects of Qianghuo Shengshi Decoction. The 9th Annual Meeting of Pharmaceutical Toxicology-New Era·New Technology·New Strategy·New HealthWuhan; 2019; Hubei, China. p. p. 1. [Google Scholar]
- 16.Zhang W. Network pharmacology: a further description. Network Pharmacology; 2016. [Google Scholar]
- 17.Zhao J., Yang J., Tian S., Zhang W. A survey of web resources and tools for the study of TCM network pharmacology. Frontiers of Electrical and Electronic Engineering in China. 2019;7:17–29. [Google Scholar]
- 18.Zhang R., Zhu X., Bai H., Ning K. Network pharmacology databases for traditional Chinese medicine: review and assessment. Frontiers in Pharmacology. 2019;10 doi: 10.3389/fphar.2019.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y., Li X., Guo C., Dong J., Liao L. Mechanisms of Spica Prunellae against thyroid-associated ophthalmopathy based on network pharmacology and molecular docking. BMC Complementary Medicine and Therapies. 2020;20(1):p. 229. doi: 10.1186/s12906-020-03022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J., Liu X., Zhou W., et al. A bioinformatics investigation into molecular mechanism of Yinzhihuang granules for treating hepatitis B by network pharmacology and molecular docking verification. Scientific Reports. 2020;10 doi: 10.1038/s41598-020-68224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An H.-M., Huang D.-R., Yang H., et al. Comprehensive chemical profiling of Jia-Wei-Qi-Fu-Yin and its network pharmacology-based analysis on Alzheimer's disease. Journal of Pharmaceutical and Biomedical Analysis. 2020;189, article 113467 doi: 10.1016/j.jpba.2020.113467. [DOI] [PubMed] [Google Scholar]
- 22.Gao D., Wu S.-N., Zhang C.-E., et al. Exploration in the mechanism of rhubarb for the treatment of hyperviscosity syndrome based on network pharmacology. Journal of Ethnopharmacology. 2020;261, article 113078 doi: 10.1016/j.jep.2020.113078. [DOI] [PubMed] [Google Scholar]
- 23.Tao W., Xu X., Wang X., et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal _Radix Curcumae_ formula for application to cardiovascular disease. Journal of Ethnopharmacology. 2013;145(1):1–10. doi: 10.1016/j.jep.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y., Wu R., Cai F.-F., et al. Xiaoyaosan decoction alleviated rat liver fibrosis via the TGFβ/Smad and Akt/FoxO3 signaling pathways based on network pharmacology analysis. Journal of Ethnopharmacology. 2021;264, article 113021 doi: 10.1016/j.jep.2020.113021. [DOI] [PubMed] [Google Scholar]
- 25.Ru J., Li P., Wang J., et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics. 2014;6(1):p. 13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X., Zhang W., Huang C., et al. A novel chemometric method for the prediction of human oral bioavailability. International Journal of Molecular Ences. 2012;13(6):6964–6982. doi: 10.3390/ijms13066964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian S., Wang J., Li Y., Li D., Xu L., Hou T. The application of _in silico_ drug-likeness predictions in pharmaceutical research. Advanced Drug Delivery Reviews. 2015;86:2–10. doi: 10.1016/j.addr.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Grondin C. J., Davis A. P., Wiegers T. C., Wiegers J. A., Mattingly C. J. Accessing an expanded exposure science module at the comparative toxicogenomics database. Environmental Health Perspectives. 2018;126(1):p. 014501. doi: 10.1289/EHP2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebhan M., Chalifa-Caspi V., Prilusky J., Lancet D. GeneCards: a novel functional genomics compendium with automated data mining and query reformulation support. Bioinformatics. 1998;14(8):656–664. doi: 10.1093/bioinformatics/14.8.656. [DOI] [PubMed] [Google Scholar]
- 30.Venny J. C. O. An interactive tool for comparing lists with Venn’s diagrams. 2007, https://bioinfogp.cnb.csic.es/tools/venny/index.html.
- 31.Shannon P., Markiel A., Ozier O., et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Routledge; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damian S., Gable A. L., David L., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Research. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou J. B., Chai H. B., Zhang X. F., Guo D. Y., Shi Y. J. Reconstruction of the lncRNA-miRNA-mRNA network based on competitive endogenous RNA reveal functional lncRNAs in cerebral infarction. Scientific Reports. 2019;9(1):p. 12176. doi: 10.1038/s41598-019-48435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guangchuang Y., Li-Gen W., Guang-Rong Y., Qing-Yu H. DOSE: an R/Bioconductor package for disease ontology semantic and enrichment analysis. Bioinformatics. 2015;31:608–609. doi: 10.1093/bioinformatics/btu684. [DOI] [PubMed] [Google Scholar]
- 35.Trott O., Olson A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry. 2009;31 doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marco T., Alessia V., Maria A. A. Reverse screening on Indicaxanthin from Opuntia ficus-indica as natural chemoactive and chemopreventive agent. Journal of Theoretical Biology. 2018;455:147–160. doi: 10.1016/j.jtbi.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Kutzner C., Páll S., Fechner M., Esztermann A., De Groot B. L., Grubmüller H. More bang for your buck: improved use of GPU nodes for GROMACS 2018. Journal of Computational Chemistry. 2019;40(27):2418–2431. doi: 10.1002/jcc.26011. [DOI] [PubMed] [Google Scholar]
- 38.Huang J., Rauscher S., Nawrocki G., et al. Mackerell, CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Biophysical Journal. 2017;112:175a–176a. doi: 10.1038/nmeth.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Florez S., González-Gallego J., Culebras J. M., Tuñón M. J. Flavonoids: properties and anti-oxidizing action. Nutricion Hospitalaria. 2002;17 [PubMed] [Google Scholar]
- 40.Zhang L., Ravipati A. S., Koyyalamudi S. R., et al. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. Journal of Agricultural and Food Chemistry. 2011;59(23):12361–12367. doi: 10.1021/jf203146e. [DOI] [PubMed] [Google Scholar]
- 41.Johnson M. H., de Mejia E. G., Fan J., Lila M. A., Yousef G. G. Anthocyanins and proanthocyanidins from blueberry–blackberry fermented beverages inhibit markers of inflammation in macrophages and carbohydrate-utilizing enzymes in vitro. Molecular Nutrition & Food Research. 2013;57(7):1182–1197. doi: 10.1002/mnfr.201200678. [DOI] [PubMed] [Google Scholar]
- 42.Yi Y. S. Regulatory roles of flavonoids on inflammasome activation during inflammatory responses. Molecular Nutrition & Food Research. 2018;62, article e1800147 doi: 10.1002/mnfr.201800147. [DOI] [PubMed] [Google Scholar]
- 43.Li L., Lin S., Zhang D., Xie H., Wei Z. Quercetin restores Th17/Treg balance and activates heme oxygenase 1 mediated anti-inflammatory effect on the mechanism of reducing acute laryngitis. Hebei Medical Journal. 2020;42:2730–2734. [Google Scholar]
- 44.Huang Y., Guo L., Chitti R., et al. Wogonin ameliorate complete Freund's adjuvant induced rheumatoid arthritis via targeting NF-κB/MAPK signaling pathway. BioFactors (Oxford, England) 2020;46(2):283–291. doi: 10.1002/biof.1585. [DOI] [PubMed] [Google Scholar]
- 45.Fei J., Liang B., Jiang C., Ni H., Wang L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2019;109:1586–1592. doi: 10.1016/j.biopha.2018.09.161. [DOI] [PubMed] [Google Scholar]
- 46.Tak P. P., Kalden J. R. Advances in rheumatology: new targeted therapeutics. Arthritis Research & Therapy. 2011;13(Supplement 1):p. S5. doi: 10.1186/1478-6354-13-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smolen J. S., Landewé R. B. M., Bijlsma J. W. J., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Annals of the Rheumatic Diseases. 2020;79(6):685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 48.McInnes I. B., Schett G. The pathogenesis of rheumatoid arthritis. The New England Journal of Medicine. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 49.Mateen S., Zafar A., Moin S., Khan A. Q., Zubair S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clinica Chimica Acta; International Journal of Clinical Chemistry. 2016;455:161–171. doi: 10.1016/j.cca.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Rimawi M. F., Shetty P. B., Weiss H. L., et al. Epidermal growth factor receptor expression in breast cancer association with biologic phenotype and clinical outcomes. Cancer. 2010;116(5):1234–1242. doi: 10.1002/cncr.24816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor P. C. VEGF and imaging of vessels in rheumatoid arthritis. Arthritis Research. 2002;4(Supplement 3) doi: 10.1186/ar582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paradowska-Gorycka A., Pawlik A., Romanowska-Prochnicka K., et al. Relationship between VEGF gene polymorphisms and serum VEGF protein levels in patients with rheumatoid arthritis. PLoS One. 2016;11(8, article e0160769) doi: 10.1371/journal.pone.0160769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi J. P., Wu Y. Z., Yu N., Yu Z. W., Xie F. Y., Yuan Q. VEGF gene polymorphisms affect serum protein levels and alter disease activity and synovial lesions in rheumatoid arthritis. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research. 2016;22:316–324. doi: 10.12659/MSM.894912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li A. M., Boichard A., Kurzrock R. MutatedTP53is a marker of increasedVEGFexpression: analysis of 7,525 pan-cancer tissues. Cancer Biology & Therapy. 2020;21(1):95–100. doi: 10.1080/15384047.2019.1665956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X., Zeng K., Xu M., et al. P53-induced miR-1249 inhibits tumor growth, metastasis, and angiogenesis by targeting VEGFA and HMGA2. Cell Death & Disease. 2019;10(2):p. 131. doi: 10.1038/s41419-018-1188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheler J. J., Janku F., Naing A., et al. TP53 alterations correlate with response to VEGF/VEGFR inhibitors: implications for targeted therapeutics. Molecular Cancer Therapeutics. 2016;15(10):2475–2485. doi: 10.1158/1535-7163.MCT-16-0196. [DOI] [PubMed] [Google Scholar]
- 57.Zhang B. C., Li Z., Xu W., Xiang C. H., Ma Y. F. Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. American Journal of Translational Research. 2018;10(1):265–273. [PMC free article] [PubMed] [Google Scholar]
- 58.Anson D. M., Wilcox R. M., Huseman E. D., et al. Luteolin decreases epidermal growth factor receptor-mediated cell proliferation and induces apoptosis in glioblastoma cell lines. Basic & Clinical Pharmacology & Toxicology. 2018;123(6):678–686. doi: 10.1111/bcpt.13077. [DOI] [PubMed] [Google Scholar]
- 59.Olabiyi A. A., Carvalho F. B., Bottari N. B., et al. Tiger nut and walnut extracts modulate extracellular metabolism of ATP and adenosine through the NOS/cGMP/PKG signalling pathway in kidney slices. Phytomedicine : International Journal of Phytotherapy and Phytopharmacology. 2018;43:140–149. doi: 10.1016/j.phymed.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 60.Hollingsworth S. A., Dror R. O. Molecular dynamics simulation for all. Neuron. 2018;99(6):1129–1143. doi: 10.1016/j.neuron.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morris C. J., Corte D. D. Using molecular docking and molecular dynamics to investigate protein-ligand interactions. Modern Physics Letters B. 2021;35(8):p. 2130002. doi: 10.1142/S0217984921300027. [DOI] [Google Scholar]
- 62.Liu X., Shi D., Zhou S., Liu H., Liu H., Yao X. Molecular dynamics simulations and novel drug discovery. Expert Opin Drug Discov. 2018;13(1):23–37. doi: 10.1080/17460441.2018.1403419. [DOI] [PubMed] [Google Scholar]
- 63.Cao Z., Song Z., Liang F., An X., Yang Y. Hydrogen bonding sewing interface. RSC Advances. 2020;10(30):17438–17443. doi: 10.1039/D0RA00366B. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: information of active compounds of QHSSD. Figure S1: herb-compound-target network of QHSSD. (Supplementary Materials).
Data Availability Statement
The data used to support the findings of this study are included within the supplementary information files.


