Abstract
SARS-CoV-2, a newly emerged and highly pathogenic coronavirus, is identified as the causal agent of Coronavirus Disease (2019) (COVID-19) in the late December 2019, in China. The virus has rapidly spread nationwide and spilled over to the other countries around the globe, resulting in more than 120 million infections and 2.6 million deaths until the time of this review. Unfortunately, there are still no specific drugs available against this disease, and it is very necessary to call upon more scientists to work together to stop a further spread. Hence, the recent progress in the development of drugs may help scientific community quickly understand current research status and further develop new effective drugs. Herein, we summarize the cellular entry and replication process of this virus and discuss the recent development of potential viral based drugs that target bio-macromolecules in different stages of the viral life cycle, especially S protein, 3CLPro, PLPro, RdRp and helicase.
Keywords: Coronavirus, SARS-CoV-2, Entry inhibitors, Protease inhibitors
1. Introduction
Coronaviruses (CoVs) are enveloped, positive-sense, single-stranded RNA viruses with the genome size ranging from 26 to 32 kb, belonging to the subfamily Coronavirinae of family Coronaviridae in order Nidovirales. The subfamily Coronavirinae includes four major genera, namely, Alpha-, Beta-, Delta- and Gamma-CoV. CoVs mainly cause respiratory, intestinal and neurological diseases in a broad spectrum of animals and humans. It is generally accepted that CoVs originated from wild animals such as bats, which can't be transmitted to humans, but the occasional mutation of CoVs gene can pave the way for its transmission to humans (Jones et al., 2008). A total of six different types of human CoV (HCoV) have been identified before 2019, including two Alpha-CoVs (HCoV-229E and HCoV-NL63), and four Beta-CoVs (HCoV-OC43, SARS-CoV, HCoV-HKU1 and MERS-CoV). HCoV-229E, NL63, OC43 and HKU1 are widely spread in humans and responsible for approximately one third of common cold infections, causing mild respiratory and intestinal diseases. In contrast, SARS-CoV and MERS-CoV are both highly pathogenic viruses, which could cause severe respiratory syndrome and sometimes death in humans.
SARS-CoV-2 was first reported in the late December 2019 in Wuhan, China and has rapidly spread to other countries around the world (Wang et al., 2020, Wang et al., 2020). The disease caused by the virus is called Coronavirus Disease 2019 (COVID-19). As of March 15, 2021, the outbreak has led to 119, 220, 681 total confirmed cases and 2, 642, 826 deaths, and the virus has spread to 223 countries, areas or territories. The continuing threat of this disease caused a panic globally, and governments of all countries have taken a series of actions to stop a further spread. However, there are still no approved specific drugs against CoVs at present, so the efforts are mainly focused on quarantine of infected individuals and symptomatic treatment. Therefore, there is an urgent need to call upon more scientists to work together to explore more effective therapies to tackle with the infections and avoid the threat of death immediately. Thus, it is of great importance to provide the recent progress in the development of drugs against SARS-CoV-2, which may help the scientists to understand the current situation and deficiencies in this field and quickly find out the future research direction. In this review, we summarize the cellular entry and replication of SARS-CoV-2, focus on the important drug targets, and review the recent progress in the development of potential viral based drugs.
2. Cellular entry and replication of SARS-CoV-2
The cellular entry of CoV is started by binding of the viral spike glycoprotein (S protein) to the host cell receptors, which can activate the fusion process in two ways, the endosomal pathway and the cell surface non-endosomal pathway (Chan et al., 2015). As for the endosomal pathway, proton influx into the endosome and cysteine protease (cathepsins) will trigger the membrane fusion activity in S protein and facilitate endosomal cellular entry of CoV. On the other hand, the S protein is cleaved at the S1/S2 cleavage site by host cell proteases, leading to the formation of S1 and S2 subunit to facilitate the non-endosomal cellular entry of CoV. It was reported that SARS-CoV-2 uses the same cell receptor (Angiotensin Converting Enzyme-2, ACE2) as SARS-CoV(Hoffmann et al., 2020). The high structural similarity of receptor binding domain (RBD) in S1 subunit between SARS-CoV-2 and SARS-CoV suggest that SARS-CoV-2 may enter the host cell in the similar manner with SARS-CoV(Ou et al., 2020). The RBD mediates the initial binding to ACE2 on the surface of cells, following by the insertion of the fusion peptide (FP) having characteristics of “class 1” fusion proteins, such as those of HIV, influenza virus, and Ebola virus into the cell membrane (Li et al., 2005). After that, the heptad repeats 1 and 2 (HR1 and HR2) in S2 subunit react with each other to form six-helical bundle (6-HB), leading to the fusion between the viral envelope and cell membrane (Shibo Jiang, 2020). The cellular entry process of SARS-CoV-2 is depicted in Fig. 1 (upper panel).
Fig. 1.
The cell entry and replication process of SARS-CoV-2. The combination of the spike protein (S) and the host cell receptor-ACE2 leads to fusion of the viral and cell membranes. Proton influx into the endosome and cathepsins will trigger the membrane fusion activity in S protein and facilitate endosomal cellular entry of CoV. In contrast, the S protein is cleaved to form S1 and S2 subunit to facilitate the non-endosomal cellular entry of CoV. After the viral RNA is released, ORF1a and ORF1ab are translated into Pp1a and Pp1ab, which are processed by 3CLPro and PLPro to produce NSPs. Subsequently, mRNAs that synthesized with the catalytic of RdRp are translated to produce the structural and accessory proteins, while newly synthesized genomic RNA is assembled by N protein to form helical nucleocapsid. After that, the structural proteins including S, E, and M are inserted into the ER and then move along the pathway into the ERGIC, where the interactions between the helical nucleocapsid and the structural proteins are occurred to form the assembled virion. Finally, virion is transported to the cell surface by intracellular vesicles and released across the plasma membrane by exocytosis.
After the entry process completed, the viral genome is then released into the cellular cytoplasm (Zhu et al., 2013). The ORF1a/1b of the genome is translated into replicase polyprotein (pp1a and pp1ab), which are processed by 3C-like protease (3CLPro) and papain-like protease (PLPro) to produce Non-structural proteins (NSPs), such as RNA-dependent RNA polymerase (RdRp). In the presence of RdRp, the replication of a full length anti-genome and synthesis of sub-genomic RNAs are started, which are used as templates for the synthesis of new genomic RNA and mRNAs, respectively. The mRNAs are then used to encode the structural proteins, including spike protein (S), membrane protein (M), envelope protein (E) and nucleocapsid protein (N), some (S, M, E) of which are inserted into rough endoplasmic reticulum (ER), while N protein is integrated with the newly synthesized genomic RNA to form a nucleocapsid. Finally, the nucleocapsid and those structural proteins are assembled in the ER–Golgi intermediate compartment (ERGIC) to form the new virus, which is then released from the cell by exocytosis (Locker et al., 1994). The whole replication process of SARS-CoV-2 is depicted in Fig. 1 (lower panel). Thus, inhibiting the cellular entry or any replication processes of SARS-CoV-2 could be a potential strategy to design and develop viral based drugs. Numerous antiviral agents have been identified to interrupt with the entry and/or replication of CoVs in cell culture or animal models. However, it usually takes more than ten years to develop new drugs for clinical use, so until now there are still no specific drugs approved for treating the diseases induced by CoVs (Lipsky and Sharp, 2001; Copeland, 2016). In this outbreak, the discovery of potential treatment options should be speeded up, and drug repurposing (also known as drug repositioning or reprofiling) will be a good choice, especially after the structures of key viral proteins (potential drug targets) are resolved (Yulong et al., 2020). It is a process of identifying new indications for drugs that are already approved or under investigation, through which much of the cost and time spent on new drug development would be saved. Computer aided drug design (CADD, including virtual screening, virtual library design, lead optimization and de novo design) and cell-based cytopathic effect (CPE) assay are two main useful tools for drug repurposing. The potential drugs identified by the two methods against cellular entry and replication processes of SARS-CoV-2, especially targeting S protein, 3CLPro PLPro, RdRp and helicase, are discussing in the following sections. The most potential drugs repurposed by cell-based CPE assay are summarized in Table 1.
Table 1.
Viral targeting drugs evaluated by cell based CPE assay.
| Drug candidates | Disease indications | Targets | Inhibition activity |
|---|---|---|---|
| EK1 (Xia et al., 2020) | under investigation | S protein | IC50= 2.38 μM |
| SARS-CoV-2-HR2P (Xia et al., 2020) | under investigation | S protein | IC50= 0.98 μM |
| EK1C4 (Xia et al., 2020) | under investigation | S protein | IC50 = 1.3 nM |
| ACE2-Ig (Changhai Lei, 2020) | under investigation | S protein | IC50 = 0.65 μg/ml |
| mACE2-Ig (Changhai Lei, 2020) | under investigation | S protein | IC50 = 0.48 μg/ml |
| F (ab’)2 (Pan et al., 2020) | under investigation | S protein | EC50 = 0.07 μg/ml |
| sdAbs (Chi et al., 2020) | under investigation | S protein | IC50 = 0.23∼0.50 μg/mL |
| α-ketoamide (13b) (Zhang et al., 2020) | under investigation | 3CLPro | IC50 = 0.67 μM EC50 = 4–5 μM |
| compound 11a (Dai et al., 2020) | under investigation | 3CLPro | IC50 = 0.05 μM EC50 = 0.42 μM |
| compound 11b (Dai et al., 2020) | under investigation | 3CLPro | IC50 = 0.04 μM EC50 = 0.33 μM |
| inhibitor N3 (Yang et al., 2005) | under investigation | 3CLPro | EC50 = 16.77 μM |
| Ebselen (Jin et al., 2020) | treatment for stroke | 3CLPro | EC50 = 4.67 μM |
| Nelfinavir (Xu et al., 2020) | treatment of HIV infection | 3CLPro | EC50 = 2.89 μM |
| Atazanavir (Fintelman-Rodrigues et al., 2020) | treatment of HIV infection | 3CLPro | EC50 = 2.0 μM |
| Dipyridamole (Liu et al., 2020) | platelet inhibitor | 3CLPro | IC50 = 0.53 μM EC50 = 0.1 μM |
| Baicalin (Su et al., 2020) | under investigation | 3CLPro | EC50 = 10.27 μM |
| Baicalein (Su et al., 2020) | under investigation | 3CLPro | EC50 = 1.69 μM |
| Chloroquine (Wang et al., 2020) | treat or prevent malaria | PLPro | EC50 = 1.13 μM |
| Hydroxychloroquine (Liu et al., 2020) | treat or prevent malaria | PLPro | EC50 = 4.51 μM |
| Remdesivir (Wang et al., 2020) | an investigational antiviral compound | RdRp | EC50 = 0.77 μM |
| Ribavirin (Wang et al., 2020) | Treat viral infections | RdRp | EC50 = 109.50 μM |
| Penciclovir (Wang et al., 2020) | treat cold sores | N/A | EC50 = 95.96 μM |
| Favipiravir (Wang et al., 2020) | the treatment of influenza | N/A | EC50 = 61.88 μM |
| Nafamostat (Wang et al., 2020) | treat acute pancreatitis | N/A | EC50 = 22.50 μM |
| Nitazoxanide (Wang et al., 2020) | treat diarrhea caused by Giardia or Cryptosporidium | N/A | EC50 = 2.12 μM |
| Niclosamide (Jeon et al., 2020) | treatment of worm infections | N/A | IC50=0.88 μM |
| Ciclesonide (Jeon et al., 2020) | prevent and reduce the symptoms caused by asthma | N/A | IC50=4.33 μM |
| Ribonucleoside analog β-D-N4-hydroxycytidine (Sheahan et al., 2020) | under investigation | N/A | IC50=0.3 μM |
| Cepharanthine (Fan et al., 2020) | treatment of leukopenia, snake bites, xerostomia and alopecia | N/A | EC50 = 0.98 μM |
| Auranofin (Rothan et al., 2020) | treat rheumatoid arthritis | N/A | EC50 = 1.5 μM |
| Ivermectin (Caly et al., 2020) | anti-parasitic drugs | N/A | IC50 = 2 μM |
3. Cellular entry inhibitors
The cellular entry process is mediated by the interaction between the S protein of SARS-CoV-2 and the receptor ACE2 of host cell, as shown above in Fig. 1. Therefore, many drugs focused on virus entry process either by inhibition of S1 unit mediates virus attachment or by blocking of S2 unit regulates the fusion of viral and cellular membranes are identified (Hofmann and Pöhlmann, 2004). The conventional lab based assays for drug development are limited because of the limited time and the absence of necessary experimental conditions. Fortunately, the rapid development of genomics and proteomics makes it possible to identify drug candidates against virus in a short time by CADD. To find an appropriate anti-viral drug against the SARS-CoV-2 virus. Recently (Peele et al., 2020), targeted the main protease (pdb id: 6LU7) to design anti-CoV drug. In this conceptual context, an attempt has been made to suggest an in silico computational relationship between US-FDA approved drugs, plant-derived natural drugs, and Coronavirus main protease (6LU7) protein. The evaluation of results was made based on Glide (Schrödinger) dock score. Out of 62 screened compounds, the best docking scores with the targets were found for compounds: lopinavir, amodiaquine, and theaflavin digallate (TFDG). In another study a total of 33 molecules which includes natural products, anti-virals, anti-fungals, anti-nematodes and anti-protozoals were screened (Das et al., 2020). All the studied molecules could bind to the active site of the SARS-CoV-2 protease (PDB: 6Y84), out of which rutin (a natural compound) has the highest inhibitor efficiency among the 33 molecules studied, followed by ritonavir (control drug), emetine (anti-protozoal), hesperidin (a natural compound), lopinavir (control drug) and indinavir (anti-viral drug). All the molecules, studied out here could bind near the crucial catalytic residues, HIS41 and CYS145 of the main protease, and the molecules were surrounded by other active site residues like MET49, GLY143, HIS163, HIS164, GLU166, PRO168, and GLN189. In a study the molecular docking study, the binding energy and inhibition of 6 Gingesulphonic acid from Zingiber officinalis (Sunthi) is greater than hydroxychloroquine and quinine (Singh et al., 2021). Most of the selected phytoconstituents follow the Lipinski rule of five. Target prediction of selected phytoconstituents was done on target of SARS-CoV-2, humoral immunity, and antiviral activity. Every selected phytoconstituents works on minimum one of the targets.
3.1. Targeting the S protein
Given that the S protein plays a critical in the cellular entry process, it has been taken as a pivotal drug target. Therefore, natural compounds extracted from medicine plants including crocin, digitoxigenin and β-Eudesmol that targeting the S protein were identified to has the ability to combat SARS-CoV-2 by molecular docking (Aanouz et al., 2020). Similarly, resveratrol (a natural compound belonging to stilbene) (Wahedi et al., 2020) has also been found to be potential antiviral drug against COVID-19 by disrupting the S protein, according to the molecular docking studies. RBD in S1 unit is used by the virus to attach itself to the host cell and served as a key target to fight infection. Kumar et al. employed a computational approach to screen nine natural compounds against RBD, and found that tanshinone iia and methyl tanshinonate have the highest binding affinity score, suggesting they both could be potential drugs for the treatment of COVID-19 (Kumar 2020). Aptamers, short single-stranded DNA or RNA molecules, have been designed to fight COVID-19 by targeting RBD (Yanling et al., 2020). Peptide inhibitors against RBD were designed by using classical molecular dynamics simulations based on the protease domain of ACE2 (Han and Král, 2020). Besides, zorubicin, aclarubicin, a food dye (E 155), phillyrin and chlorogenic acid were identified to be potent inhibitors of RBD by virtual screening (Jiuwang et al., 2020; Senathilake, 2020).
It is well known that HR1 and HR2 both in S2 unit can interact with each other to form 6-HB, which helps to bring the viral and cellular membranes in close proximity for viral fusion/entry process (Ahmadi et al., 2021). Therefore, they have become important target sites for inhibition against specific CoVs. A synthesized peptide OC43-HR2P, derived from the HR2 domain of HCoV-OC43, could compete with HR2 and thus inhibit the formation of 6-HB, resulting in preventing the process; furthermore, the optimized form of OC43-HR2P (EK1) exhibited significantly improved inhibitory activity against multiple HCoVs (Xia et al., 2019). Recently, it has been confirmed that HR1 and HR2 in S2 unit of SARS-CoV-2 still play key roles in mediating viral fusion/entry process like other HCoVs; and EK1, as well as SARS-CoV-2-HR2P (peptide derived from the HR2 domain of SARS-CoV-2), showed potent inhibitory activity against the virus in a dose-dependent manner with IC50 values of 2.38 and 0.98 μM, respectively (Xia et al., 2020a, Xia et al., 2020b). Therefore, both EK1 and SARS-CoV-2-HR2P hold the promise to being developed as antiviral drugs to prevent and treat COVID-19. After that, a series of lipopeptides were generated by the same group through the incorporation of cholesterol or palmitic acid to the C terminus of EK1 sequence using polyethylene glycol as spacer (Xia et al., 2020a, Xia et al., 2020b). It was found that EK1C4 (with cholesterol modified) hold the strongest activity to block the S protein-mediated membrane fusion with IC50 of 1.3 nM, indicating it has 241 fold more potent than EK1 (Xia et al., 2020a, Xia et al., 2020b).
Besides, monoclonal antibodies that produced by a single clone of cells are consisting of identical antibody molecules, and have been widely used as therapeutic agents for arthritis, cancer, bacterial and viral infections. A latest trial demonstrated that monoclonal antibodies had significant therapeutic effects and reduced the case fatality rate of Ebola virus disease (Levine, 2019), suggesting that monoclonal antibodies may also reduce deaths from other viral infections like SARS, MERS and COVID-19. Actually, some potent monoclonal antibodies targeting RBD in the S protein of SARS-CoV have been identified, including m396, CR3014 and CR3022 (ter Meulen et al., 2004; ter Meulen, van den Brink et al., 2006; Zhu et al., 2007). In consideration of the relatively high identity (73%) of RBD in SARS-CoV-2 and SARS-CoV, Tian et al. determined the binding ability of those monoclonal antibodies to RBD of SARS-CoV-2 by ELISA (Enzyme-linked immunosorbent assay) and BLI (Bio-layer interferometry) (Tian et al., 2020). It was found that the SARS-CoV-specific monoclonal antibody CR3022 had potent affinity with SARS-CoV-2, thereby having the potential to be developed as a drug candidate for the prophylactic and treatment of COVID-19. Another study also identified CR3022 as an effective neutralizing antibody for SARS-CoV-2 by using an antibody-antigen docking approach (Park et al., 2020). Similarly, Lei et al. constructed a new recombinant protein (ACE2-Ig) that consists of ACE2 and the Fc region of IgG1, and measured the affinity of it and its variety (mACE2-Ig) with RBD by using Biacore assays (Changhai Lei, 2020). The results showed that both ACE2-Ig and mACE2-Ig potently inhibited the SARS-CoV-2 protein-mediated fusion with IC50 of 0.65 and 0.48 μg/ml, respectively. Moreover, it has also been found that both fusion proteins potently neutralized SARS-CoV-2 in vitro. A similar study demonstrated that immunoglobulin fragment F (ab’)2 prepared from horse antisera inhibited SARS-CoV-2 with EC50 of 0.07 μg/ml, suggesting a highly potential therapeutic candidate for COVID-19 (Pan et al., 2020). Recently, a set of humanized single domain antibodies (sdAbs) were developed by taking RBD as a target, and the IC50 values of pseudotyped particle neutralization assay and authentic SARS-CoV-2 neutralization assay were 0.003∼0.3 and 0.23∼0.50 μg/mL, respectively (Chi et al., 2020). Evidently, the development of monoclonal antibodies is of great significance in the treatment of COVID-19. However, since hybridoma technology for development and production of monoclonal antibodies is laborious and time consuming, we still face a hard and challenging situation in fight this pandemic.
4. Replication inhibitors
The large replicase polyprotein pp1a and pp1ab are processed and cleaved by two viral proteases, the 3-chymotrypsin-like protease (3CLPro) and papain-like protease (PLPro), to produce NSPs such as RNA-dependent RNA polymerases (RdRp) and helicase, which are involved in the transcription and replication of the virus. These protease and NSPs are recognized as attractive targets to develop antiviral drugs. And actually, the most potential drugs, such as remdesivir, favipiravir, lopinavir, ritonavir, oseltamivir, chloroquine and hydroxychloroquine, have entered the clinical trials (Rosa and Santos, 2020).
4.1. 3CLPro inhibitors
3CLPro (also known as the main protease), a homodimeric cysteine protease, has the ability to cleave the polyproteins into individual polypeptides that are required for replication and transcription (Fan et al., 2004), and it thus becomes an attractive antiviral drug target. Recently, the X-ray structures of the unliganded 3CLPro of SARS-CoV-2 and its complex with an α-ketoamide inhibitor were reported, which help to identify α-ketoamide derivative (13b) as an effective inhibitor of SARS-CoV-2 with IC50 of 0.67 ± 0.18 μM (Zhang et al., 2020, Zhang et al., 2020, Zhang et al., 2020). Further studies have indicated that 13b protected the human Calu3 cells by inhibiting the virus with an EC50 of 4–5 μM and presented appropriate pharmacokinetic properties in CD-1 mice, suggesting optimized α-ketoamide inhibitors has great potential to be developed as drugs against SARS-CoV-2. Another group designed and synthesized series of compounds targeting 3CLPro, and found that compound 11a and 11b exhibited excellent inhibitory activity with IC50 of 0.05 and 0.04 μM, as well as antiviral activity in Vero E6 cells with EC50 of 0.42 and 0.33 μM, respectively (Dai et al., 2020). A Michael acceptor inhibitor N3 that was designed previously by using computer aided drug design can specifically inhibit multiple CoV 3CLPro (Yang et al., 2005). And thus it was repurposed by both molecular docking and in cell-based assay against SARS-CoV-2, the results of which showed that it fitted inside the substrate-binding pocket of 3CLPro by an irreversible manner and presented the EC50 of 16.77 μM in vitro assay (Jin et al., 2020). Based on the crystal structure of 3CLPro in complex with N3, a model for identifying lead inhibitors to target 3CLPro has been set up, by which ebselen was then identified as a potential inhibitor of 3CLPro with EC50 of 4.67 μM (Jin et al., 2020). A recent study applied a deep docking to all 1.3 billion compounds from ZINC15 library to identify top 1000 potential ligands as potential 3CLPro inhibitors (Ton et al., 2020). Besides, existed drugs that have been used for treating viral disease (HIV, hepatitis C and influenza) (Shah et al., 2020), bacterial diseases, cancers and other diseases, as well as Traditional Chinese Medicine (TCM) and natural compounds extracted from plants are all found to be the potential drugs against SARS-CoV-2, which will be discussed in the following.
At present, there are more antiviral drugs for HIV than for any other viral disease (Kanwugu and Adadi, 2021). HIV protease inhibitors and integrase inhibitors designated the suffix with –navir and –gravir, respectively, have been identified to possess the potential to combat SARS-CoV-2 by targeting 3CLPro. Nutho et al. docked ritonavir and lopinavir to 3CLPro, and found that both ritonavir and lopinavir bind well to 3CLPro, suggesting the effective therapeutic effect of Kaletra (a co-formulation of lopinavir and ritonavir) on COVID-19 may be due to the directly inhibitory effect on 3CLPro (Nutho et al., 2020). Another study found that indinavir has stronger affinity with 3CLPro than both lopinavir and ritonavir (Chang, 2020). Xu et al. docked 1903 small molecule drugs to 3CLPro and identified nelfinavir as a potential inhibitor against COVID-19 (Xu et al., 2020, Xu et al., 2020, Xu et al., 2020), and a following study by the same group demonstrated that it inhibited SARS-CoV-2 in Vero E6 cells with EC50 of 2.89 ± 0.65 μM (Xu et al., 2020, Xu et al., 2020, Xu et al., 2020). Beck et al. found atazanavir was the most potential drug among those commercially available drugs (Beck et al., 2020), and it was further confirmed to has the ability to inhibit SARS-CoV-2 in Vero cells with EC50 of 2.0 ± 0.12 μM (Fintelman-Rodrigues et al., 2020). Besides, saquinavir, tipranavir, amprenavir, fosamprenavir, darunavir, bictegravir and raltegravir were all identified to have the potential to target 3CLPro by using virtual screening technology (Calligari, 2020; Hall and Ji, 2020; Khan et al., 2020; Khan et al., 2020; Li et al., 2020; Mohammed Hakmi, 2020; Pant et al., 2020; Sang et al., 2020). Although some of these anti-HIV drugs have been used for CoVs treatment such as SARS and MERS, the effectiveness for COVID-19 still needs further clinical examination.
The anti-Hepatitis C drugs identified through the suffix –asvir, –buvir or –previr, have also been evaluated to whether have the ability to bind to 3CLPro. It was found through virtual screening study that ledipasvir and velpatasvir were particularly attractive as therapeutics to combat COVID-19 (Yu Wai Chen, 2020). Tegobuvir (Li et al., 2020) and beclabuvir (Talluri, 2020), non-nucleoside polymerase inhibitors, were both identified to have the potential to target 3CLPro by virtual high throughput screening, and they may also become the drug candidates for COVID-19 therapy. In addition, asunaprevir, ciluprevir, danoprevir, glecaprevir, simeprevir, faldaprevir, deldeprevir and paritaprevir all have been identified as lead candidates that target 3CLPro by using virtual screening technology (Khan et al., 2020; Mohammed Hakmi, 2020; Sohini and Narayanaswamy, 2020). Some drugs that used for fighting influenza have been repurposed to fight COVID-19. For example, oseltamivir, an antiviral medication that treat symptoms caused by influenza virus types A and B, were found to have high binding ability to 3CLpro by using molecular docking and molecular dynamics (MD) simulations (Muralidharan et al., 2020). Zanamivir, an antiviral drug used to treat or prevent the influenza, has been identified by virtual screening (Hall and Ji, 2020).
Anti-bacterial drugs, anti-cancer drugs and drugs for other diseases were all been repurposed. Anti-bacterial drugs including prulifloxacin (a quinolone antibiotic) (Li et al., 2020), colistin (a cyclic polypeptide antibiotic) (Liu and Wang, 2020) and eravacycline (a tetracycline antibiotic) (Wang, 2020) were identified as promising candidates that targeting 3CLPro. Anti-cancer drugs, such as carfilzomib (used to treat multiple myeloma) (Wang, 2020), poziotinib (used to treat kidney cancer) (Liu et al., 2020, Liu et al., 2020, Liu et al., 2020, Liu et al., 2020), sonidegib (used to treat basal cell carcinoma) (Hasanain Abdulhameed Odhar and Hashim, 2020) and valrubicin (used to treat bladder cancer) (Wang, 2020), were identified as potential inhibitors of 3CLPro by virtual docking screening. Dipyridamole that belongs to a class of drugs known as platelet inhibitors has been found to inhibit SARS-CoV-2 by molecular docking and in vitro evaluating assay, with the IC50 of 0.53 ± 0.01 μM in an enzymatic assay targeting 3CLPro and EC50 of 0.1 μM in Vero E6 cells (Liu et al., 2020). Moreover, a proof-of-concept trial of this study demonstrated that supplementation of dipyridamole significantly improved clinical outcomes of severely ill patients in comparison to the control patients (Liu et al., 2020). Similarly, Perampanel (an anticonvulsant), conivaptan (an inhibitor of antidiuretic hormone) and vidupiprant (a potential antiasthmatic agent) were identified by a computational protocol (Hasanain Abdulhameed Odhar and Hashim, 2020) (Liu et al., 2020).
Traditional Chinese Medicine (TCM) is an ancient form of healthcare that dates back over 2500 year, and it is seldom harmful when prescribed correctly. It has been suggested as an alternative treatment for COVID-19 (Du et al., 2020), and actually, the administration of TCM has significantly improved the symptoms of patients with COVID-19 (Huan-Tian Cui, 2020). Clinical effectiveness of Lung-toxin Dispelling Formula No. 1 (LDFN1) for control and prevention COVID-19 has been demonstrated but without knowing the inherent mechanism of its action. More than one hundred constituents of LDFN1 were identified to have a high binding affinity with 3CLpro by molecular docking, and the 3CLpro inhibition assay further showed that 22 chemical constituents were inhibitors of 3CLpro (Zhang et al., 2020, Zhang et al., 2020, Zhang et al., 2020). Several major components from TCM, including rutin, hesperidin, glycyrrhizin, saikosaponin A, puerarin, hesperidin and baicalin, have been identified as potential 3CLPro inhibitors by using molecular docking (Das et al., 2020; Yan, 2020). Notably, baicalin and baicalein presented potent antiviral activities targeting 3CLPro in vitro with EC50 of 10.27 and 1.69 μM, respectively (Su et al., 2020). Another study also found that scutellaria baicalensis extract and baicalein inhibited the replication of SARS-CoV-2 in Vero cells with EC50 of 0.74 g/ml and 17.6 μM, respectively (Liu et al., 2020). 13 compounds that exist in TCM, such as betulinic acid, coumaroyltyramine and cryptotanshinone, were also found to have potential anti-SARS-CoV-2 activity (Zhang et al., 2020).
Natural compounds extracted from natural plants or other herbal medicines are also taken into consideration. 5,7,3′,4′-Tetrahydroxy-2'-(3,3-dimethylallyl) isoflavone, myricitrin and methyl rosmarinate have been considered as potential lead compounds for drug development to combat COVID-19 (Tahir ul Qamar 2020). Some natural ingredients like δ-viniferin, myricitrin, taiwanhomoflavone A, lactucopicrin 15-oxalate, nympholide A, afzelin, biorobin, hesperidin and phyllaemblicin B were identified to have high affinity to 3CLPro (Joshi et al., 2020). As an anti-inflammatory and immunostimulant, andrographolide was discovered as a potential inhibitor of 3CLPro by using computational approaches, which also predicts it has good solubility, pharmacodynamics property and target accuracy, indicating it is a promising drug candidate against COVID-19 (Enmozhi et al., 2020). Several natural compounds such as apigenin coriandrin and curcumin showed high binding affinity to 3CLPro by using molecular docking (H S, Vishwakarma et al., 2020). Carnosol a chemical compound from Indian spices was taken as potent inhibitor against 3CLPro (Umesh et al., 2020). 8,8′-Bieckol, 6,6′-Bieckol and dieckol, all belonging to the family of phlorotannins, were identified as the most active inhibitors targeting 3CLPro, according to the results of the virtual screening of a Marine Natural Product library (Davide Gentile, 2020). Hypericin, cyanidin 3-glucoside, baicalin, glabridin and α-ketoamide-11r were considered to have high binding affinity with 3CLPro by CAAD (Islam et al., 2020).
4.2. PLPro inhibitors
PLPro, a multifunctional cysteine protease, plays a key role in processing the viral polyprotein and host cell proteins. A virtual ligand screening targeting PLPro was performed to identify a series of anti-virus drugs (valganciclovir and thymidine), anti-bacterial drugs (chloramphenicol and cefamandole), muscle relaxant drug (chlorphenesin carbamate), anti-tussive drug (levodropropizine), as well as several natural compounds (platycodin D and baicalin) may have high binding affinity to PLPro, suggesting the potential utility of these compounds in the treatment of COVID-19 (Wu et al., 2020). Natural ingredients of TCM were also been docked to PLPro, and the most promising drugs including gingerketophenol, ginkgol alcohol and ferulic acid have been identified (Ma et al., 2020). In another docking study, 16 FDA approved drugs, including chloroquine and formoterol, were found to bind PLPro with high affinity, suggesting their potential to treat COVID-19 (Rimanshee et al., 2020). Chloroquine is a cheap and well-tolerated drug using in clinical for treating malarial and autoimmune disease, and it showed antiviral activity against SARS-CoV-2 in vitro with EC50 of 1.13 μM (Wang et al., 2020). Numerous clinical trials have been conducted in China to test the efficacy and safety of chloroquine in the treatment of COVID-19, demonstrating that chloroquine phosphate could inhibit the deterioration of pneumonia, improve the imaging manifestations of lung, turn virus nucleic acid negative and shorten the course of disease without severe adverse reactions (Gao et al., 2020; Gautret et al., 2020). Moreover, hydroxychloroquine that is a less toxic derivative of chloroquine was also found to efficiently inhibit SARS-CoV-2 infection in vitro with EC50 of 4.51 μM (Liu et al., 2020), and the synergy between hydroxychloroquine and azithromycin was observed in vitro (Julien Andreania and Jean-Marc Rolaina, 2020). The drug used to treat Covid-19 is given in Table 2.
Table 2.
2D structural information and dosage of current suggested drugs in COVID-19 treatment.
| Drug Name and Structure | Dosage (for adults) | Route of administration | Course of treatment |
|---|---|---|---|
 |
200 mg (Three time daily) | Oral | ≤10 days |
 |
100 mg (Twice daily) | Oral | ≤10 days |
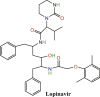 |
400 mg (Twice daily) | Oral | ≤10 days |
 |
500 mg (2–3 time daily) | Intravenous injection | ≤10 days (Combination with interferon or lopinavir/ritonavir recommended) |
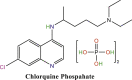 |
500 mg (Twice daily) weight ≥50 kg and for weight <50 kg, day 1–2 (twice daily); day 3–7 (once daily) | Oral | 7 days |
4.3. RdRp inhibitors
RdRp (also called RNA replicases) that catalyze the replication of RNA from an RNA template have been considered as the important druggable targets (Ganeshpurkar et al., 2019). RdRp of SARS-CoV-2 was expressed and purified recently, and it efficiently incorporated the active triphosphate form of remdesivir into RNA, which induced the termination of RNA synthesis in enzyme kinetics assay, indicating remdesivir is a direct-acting antiviral drug against this virus (Gordon et al., 2020). Moreover, the cryo-EM structure of the SARS-CoV-2 RdRp and remdesivir was reported, which sheds lights on the mechanism of viral RNA replication and provides a rational template for drug design (Gao et al., 2020; Yin et al., 2020). An in vitro study showed remdesivir inhibited SARS-CoV-2 with EC50 of 0.77 μM, indicating it is an attractive potential drug against COVID-19 (Wang et al., 2020). Remdesivir drug was first developed for Ebola and is now being tested in clinical trials for the treatment of COVID-19. At first, this drug is being tested in clinical trials of 761 patients in a double-blind, randomized, and placebo-controlled study at numerous hospitals in Wuhan. Besides, Clinical trials of this drug are also carried by the University of Nebraska Medical Center to test its safety and effectiveness. When peer-reviewed journals showed remdesivir, as a potential antiviral candidate, Gilead has begun clinical trials in China and having positive results in the case involving Chinese in vitro tests and an American patient. At this moment, a specific vaccine or drug is required for the treatment of COVID-19, which would be approved after different clinical trials. However, until the development of such compounds; Scientist is optimistic that remdesivir, an experimental drug could be possibly effective against COVID-19 because this drug has also been proved to be operative against the MERS, SARS and other bat-related coronaviruses irrespective to common cold coronavirus [10, 11].
It has been found that SARS-CoV-2 and the hepatitis C virus (HCV) use a similar viral genome replication mechanism, and thus the inhibitors against RdRp of HCV may also be used as potential drugs against SARS-CoV-2. Therefore, IDX-184, ribavirin, sofosbuvir, galidesivir and tenofovir act as RNA polymerase inhibitors are recommended to inhibit the activity of RdRp by competing with natural ribonucleotides (Elfiky, 2020; Elfiky, 2020). Notably, ribavirin inhibited SARS-CoV-2 in vitro with EC50 of 109.5 μM (Wang et al., 2020), and it has been recommended for treatment in this SARS-CoV-2 pandemic by the Novel Coronavirus Infection Pneumonia Diagnosis and Treatment Standards since the fifth version.
4.4. Helicase and 2′-O-ribose methyltransferase inhibitors
Helicase that bind and remodel RNA and RNA-protein complexes, an ATP-dependent enzyme, plays critical roles in essentially all RNA metabolism processes (Jankowsky, 2011). Given a necessary component for the replication of CoVs, it has become a potential drug target for developing antiviral drugs. According to a study of virtual screening, some approved drugs, including lymecycline, itraconazole, saquinavir, dabigatran and canrenoic acid were identified to be helicase inhibitors, suggesting the potential use in clinical for the treatment of COVID-19 (Wu et al., 2020). 2′-O-ribose methyltransferase (2′-O-MTase), a functional protein, methylates the ribose 2′-O position of the first and second nucleotide of viral mRNA, through which the viral mRNA obtain the ability to sequester itself from the host cell, thus preventing recognition and activation of the host immune response. Taken 2′-O-MTase as a drug target, dolutegravir and bictegravir were found to become potent drug candidates against COVID-19 (Khan et al., 2020).
4.5. Drugs evaluated in vitro with unspecified targets
The antiviral activities of some drugs including penciclovir, favipiravir, nafamostat and nitazoxanide against SARS-CoV-2 in vitro have been tested (Wang et al., 2020). Penciclovir (EC50 = 95.96 μM, CC50 > 400 μM, SI > 4.17) and favipiravir (EC50 = 61.88 μM, CC50 > 400 μM, SI > 6.46) exhibited the protection of cell against SARS-CoV-2 only in the high concentrations. A recent review that summarized the pharmacokinetic characteristics of favipiravir and possible drug-drug interactions may be helpful to the further study (Du and Chen, 2020). EC50, CC50 and SI (CC50/IC50) stand for the half-maximal effective concentration, the half cytotoxic concentration and selectivity index, respectively. Nafamostat (EC50 = 22.50 μM, CC50 > 100 μM, SI > 4.44) and nitazoxanide (EC50 = 2.12 μM; CC50 > 35.53 μM; SI > 16.76) both hold promise for combat this virus. Jeon et al. found a total of 24 drugs that exhibited antiviral efficacy (0.1 μM < IC50 < 10 μM) against SARS-CoV-2 in Vero cells, and niclosamide (recently reviewed by Xu et al.) (Xu et al., 2020) and ciclesonide exhibited very potent antiviral activity with IC50 of 0.28 and 4.33 μM, respectively, but without knowing the mechanism of antiviral action (Jeon et al., 2020). Ribonucleoside analog β-D-N4-hydroxycytidine (NHC, EIDD-1931) was found to inhibit SARS-CoV-2 in both Vero cells and Calu-3 cells with IC50 of 0.3 and 0.08 μM, respectively, and the antiviral activity may result from the induction of error catastrophe in the virus (Sheahan et al., 2020). Cepharanthine, a bisbenzylisoquinoline alkaloid from tubers of Stephania, inhibited a SARS-CoV-2-related CoV(a model for SARS-CoV-2 research) in Vero cells with EC50 of 0.98 μM, which suggest that it can potently inhibit SARS-CoV-2 (Fan et al., 2020). Auranofin, a gold-containing triethyl, has been found to inhibit the replication of SARS-CoV-2 in Huh7 cells with EC50 of approximately 1.5 μM and induce significant reduction in virus-induced inflammation, suggesting it could be a useful drug to limit SARS-CoV-2 infection and associated lung injury (Rothan et al., 2020). Recently, ivermectin (an FDA-approved anti-parasitic drug) has been found to inhibit the replication of SARS-CoV-2 due to its nuclear transport inhibitory activity with IC50 of appropriately 2 μM (Caly et al., 2020). Although cell-based CPE assays of drugs are integral to and essential for the FDA drug approval process and commercialization, it is far from being able to predict the functioning of a complex organism from the study of separate cells, and thereby the vitro system is not going to replace the in vivo study. Therefore, further in vivo studies or clinical trials are recommended to evaluate these antiviral drugs.
5. Conclusions and outlook
Over the past decade, the outbreaks of SARS-CoV, MERS-CoV and the newly emerging SARS-CoV-2 have demonstrated that CoVs will continue to spill over into humans, resulting in potentially disastrous consequences both now and in the future. However, no specific drug or vaccine is currently available for HCoVs to date, which highlights the urgent need for more international cooperation on developing specific drugs and vaccines against SARS-CoV-2 and other HCoVs. Herein, we summarized the new advancements of viral based drugs against SARS-CoV-2, which are potential interrupting any processes of the viral lifecycle, such as cellular entry and replication. We found that the way of identified the potential drugs were mainly based on the computer aided drug designs (CADD) and cell-based CPE assays, the combination of which can accelerate the development of drugs against SARS-CoV-2 with greater efficacy. CADD includes a number of computational methodologies, such as virtual screening, virtual library design, lead optimization and de novo design, and virtual screening is the most frequently used method in the development of drugs against SARS-CoV-2. However, due to the difference of virtual screening tools, parameters setting and receptors selection, the results of each study were quite different, thus which should be confirmed by further cell, animal or clinical studies. The cell-based CPE assays are limited due to limited experimental conditions.
The drug repurposing by using the mentioned tools is a great choice and actually it has played a key role in the fighting of COVID-19, and some of these drugs have entered clinical trials. For instance, a clinical trial carried out with dexamethasone has lowered 28-day mortality than usual care in patient's aggressive power-driven ventilation at randomization (Horby et al., 2021). Although, the current gloval vaccine drive will help to eradicate this current pandemic but there are high chance that sporadic cases of this viral infection will surface time to time – in that case a highly effective new drug or repurposed drug should be on standby to cut the infection right at the beginning. Nevertheless, there are still some obstacles ahead. First, virus isolation, culture and related research should only be conducted in a Biosafety Level 3 laboratory, which is only a few available leading to the limitation of the development of therapeutics. Second, there have been a limited number of animal models available for SARS-CoV and MERS-CoV so far, which will also be the challenge SARS-CoV-2 should face. Third, the specific drugs or vaccines targeting SARS-CoV-2 may not be effective against its new varieties due to the rapidly mutation of this virus. Thus, there is an urgent need to call upon more multi-disciplinary scientists to jointly promote the development of specific drugs against SARS-CoV-2, and this review will help them accurately and rapidly grasp the current status of the viral based drugs.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on request.
Funding
This work was supported by the Natural Science Foundation of Fujian Province (Grant No. 2019J01806), the Putian Science and Technology Bureau (Grant No. 2018SP3004) and Training Program of Innovation and Entrepreneurship for Undergraduates (Grant No. 201911498055, 201811498003 and 201811498027), and the Science and Technology Development Fund, Macau University of Science and technology, Macau SAR (FDCT (0030/2018/A1, 067/2018/A2, 033/2017/AMJ, 0007/2019/AKP, 0002/2019/APD, and 0052/2020/A)).
CRediT authorship contribution statement
Jianmin Chen: collected the information and wrote the manuscript. Fayaz Ali: collected the information and wrote the manuscript. Imran Khan: revised and supervised the manuscript. Yi Zhun Zhu: revised and supervised the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Contributor Information
Imran Khan, Email: ikhan@must.edu.mo, rustamkhan31@yahoo.com.
Yi Zhun Zhu, Email: yzzhu@must.edu.mo.
References
- Aanouz I., Belhassan A., El Khatabi K., Lakhlifi T., El Idrissi M., Bouachrine M. Moroccan Medicinal plants as inhibitors of COVID-19: computational investigations. J. Biomol. Struct. Dyn. 2020:1–12. doi: 10.1080/07391102.2020.1758790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi K., Farasat A., Rostamian M., Johari B., Madanchi H. Enfuvirtide, an HIV-1 fusion inhibitor peptide, can act as a potent SARS-CoV-2 fusion inhibitor: an in silico drug repurposing study. J. Biomol. Struct. Dyn. 2021:1–11. doi: 10.1080/07391102.2021.1871958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B.R., Shin B., Choi Y., Park S., Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (2019-nCoV), Wuhan, China through a drug-target interaction deep learning model. Comput. Struct. Biotechnol. J. 2020;18:784–790. doi: 10.1016/j.csbj.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calligari P.B.S., Ricci G., Bocedi A. Molecular investigation of SARS–CoV-2 proteins and their interactions with antiviral drugs. Viruses. 2020;12(445):1–13. doi: 10.3390/v12040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.W., Lau S.K.P., To K.K.W., Cheng V.C.C., Woo P.C.Y., Yuen K.-Y. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015;28(2):465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.T.Y., Lee K., Chen T., Hsiao Y., Chang H., Hsieh T., Su C., Wang S., Yu J., Shih S., Lin Y., Lin Y., Tu Y.E., Tung C., Chen C. 2020. Potential therapeutic agents for COVID-19 based on the analysis of protease and RNA polymerase docking. Preprints 2020. [Google Scholar]
- Changhai Lei W.F., Qian Kewen, Tian Li, Zhang Sheng, Ding Min, Hu Shi. 2020. Potent Neutralization of 2019 Novel Coronavirus by Recombinant ACE2-Ig. bioRxiv: 2020.2002.2001.929976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Liu X., Wang C., Zhang X., Ren L., Jin Q., Wang J., Yang W. Humanized single domain antibodies neutralize SARS-CoV-2 by targeting spike receptor binding domain. bioRxiv. 2020 doi: 10.1038/s41467-020-18387-8. 2020.2004.2014.042010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland R.A. The drug–target residence time model: a 10-year retrospective. Nat. Rev. Drug Discov. 2016;15(2):87. doi: 10.1038/nrd.2015.18. [DOI] [PubMed] [Google Scholar]
- Dai W., Zhang B., Jiang X.-M., Su H., Li J., Zhao Y., Xie X., Jin Z., Peng J., Liu F., Li C., Li Y., Bai F., Wang H., Chen X., Cen X., Hu S., Yang X., Wang J., Liu X., Xiao G., Jiang H., Rao Z., Zhang L.-K., Xu Y., Yang H., Liu H. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020 doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Sarmah S., Lyndem S., Singha Roy A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2020:1–11. doi: 10.1080/07391102.2020.1763201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Sarmah S., Lyndem S., Singha Roy A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2020:1–18. doi: 10.1080/07391102.2020.1763201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davide Gentile V.P., Angela Scala, Teresa Sciortino Maria, Piperno Anna, Rescifina Antonio. Putative inhibitors of SARS-CoV-2 main protease from A library of marine natural products: a virtual screening and molecular modeling study. Mar. Drugs. 2020;18(225):1–19. doi: 10.3390/md18040225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H.-Z., Hou X.-Y., Miao Y.-H., Huang B.-S., Liu D.-H. Traditional Chinese Medicine: an effective treatment for 2019 novel coronavirus pneumonia (NCP) Chin. J. Nat. Med. 2020;18(3):206–210. doi: 10.1016/S1875-5364(20)30022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y.-X., Chen X.-P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin. Pharmacol. Therapeut. 2020 doi: 10.1002/cpt.1844. n/a(n/a) [DOI] [PubMed] [Google Scholar]
- Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enmozhi S.K., Raja K., Sebastine I., Joseph J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1760136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H.H., Wang L.Q., Liu W.L., An X.P., Liu Z.D., He X.Q., Song L.H., Tong Y.G. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin Med J (Engl) 2020;133(9):1051–1056. doi: 10.1097/CM9.0000000000000797. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan K., Wei P., Feng Q., Chen S., Huang C., Ma L., Lai B., Pei J., Liu Y., Chen J., Lai L. Biosynthesis, purification, and substrate specificity of severe acute respiratory syndrome coronavirus 3C-like proteinase. J. Biol. Chem. 2004;279:1637–1642. doi: 10.1074/jbc.M310875200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fintelman-Rodrigues N., Sacramento C.Q., Lima C.R., da Silva F.S., Ferreira A.C., Mattos M., de Freitas C.S., Soares V.C., Gomes Dias S.d.S., Temerozo J.R., Miranda M., Matos A.R., Bozza F.A., Carels N., Alves C.R., Siqueira M.M., Bozza P.T., Souza T.M.L. Atazanavir inhibits SARS-CoV-2 replication and pro-inflammatory cytokine production. bioRxiv. 2020 2020.2004.2004. [Google Scholar]
- Ganeshpurkar A., Gutti G., Singh S.K. In: Viral Polymerases. Gupta S.P., editor. Academic Press; 2019. Chapter 1 - RNA-dependent RNA polymerases and their emerging roles in antiviral therapy; pp. 1–42. [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioScience Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L., Ge J., Zheng L., Zhang Y., Wang H., Zhu Y., Zhu C., Hu T., Hua T., Zhang B., Yang X., Li J., Yang H., Liu Z., Xu W., Guddat L.W., Wang Q., Lou Z., Rao Z. Structure of RNA-dependent RNA polymerase from 2019-nCoV, a major antiviral drug target. bioRxiv. 2020 2020.2003.2016.993386. [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Gotte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;13(13679):1–26. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- H S M., Vishwakarma R., Umashaanker R. Molecular docking analysis of selected natural products from plants for inhibition of SARS-CoV-2 main protease. Curr. Sci. 2020;118(7):1087–1092. [Google Scholar]
- Hall D.C., Ji H.-F. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Trav. Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Král P. Computational design of ACE2-based peptide inhibitors of SARS-CoV-2. ACS Nano. 2020;14(4):5143–5147. doi: 10.1021/acsnano.0c02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanain Abdulhameed Odhar S.W.A., Mohammed Ali Albeer Ali A., Ahmed Fadhil, Hashim A.M.R., Suhad Sami Humadi Molecular docking and dynamics simulation of FDA approved drugs with the main protease from 2019 novel coronavirus. Bioinformation. 2020;16(3):236–244. doi: 10.6026/97320630016236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Pöhlmann S. Cellular entry of the SARS coronavirus. Trends Microbiol. 2004;12(10):466–472. doi: 10.1016/j.tim.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., et al. Dexamethasone in hospitalized patients with covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan-Tian Cui Y.-T.L., Guo Li-Ying, Liu Xiang-Guo, Wang Lu-Shan, Jia Jian-Wei, Liao Jia-Bao, Miao Jing, Zhang Zhai-Yi, Wang Li, Wang Hong-Wu, Wen Wei-Bo. Traditional Chinese medicine for treatment of coronavirus disease 2019: a review. <span style='display:none'>1</span>Traditional Medicine Research. 2020;5(2):65–73. [Google Scholar]
- Islam R., Parves R., Paul A.S., Uddin N., Rahman M.S., Mamun A.A., Hossain M.N., Ali M.A., Halim M.A. A molecular modeling approach to identify effective antiviral phytochemicals against the main protease of SARS-CoV-2. J. Biomol. Struct. Dyn. 2020:1–20. doi: 10.1080/07391102.2020.1761883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky E. RNA helicases at work: binding and rearranging. Trends Biochem. Sci. 2011;36(1):19–29. doi: 10.1016/j.tibs.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S., Ko M., Lee J., Choi I., Byun S.Y., Park S., Shum D., Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. bioRxiv. 2020 doi: 10.1128/AAC.00819-20. 2020.2003.2020.999730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature. 2020 doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- Jiuwang Y., Lu W., Lidao B. Intervention of Novel Coronavirus (2019-nCoV) Based on Molecular Docking Method. 2020. Exploring the active compounds of traditional Mongolian medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R.S., Jagdale S.S., Bansode S.B., Shankar S.S., Tellis M.B., Pandya V.K., Chugh A., Giri A.P., Kulkarni M.J. Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main Protease$ J. Biomol. Struct. Dyn. 2020:1–16. doi: 10.1080/07391102.2020.1760137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien Andreania M.L.B., Duflota Isabelle, Jardota Priscilla, Rollanda Clara, Boxbergera Manon, Jacques Yaacoub Bou Khalila. Baudouin Jean-Pierre, Wurtza Nathalie, Jean-Marc Rolaina P.C., La Scolaa Bernard, Raoulta Didier. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020;145(104288):1–4. doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwugu O.N., Adadi P. HIV/SARS-CoV-2 coinfection: a global perspective. J. Med. Virol. 2021;93(2):726–732. doi: 10.1002/jmv.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R., Jha R., Amera G., Jain M., Singh E., Pathak A., Singh R., M J., Singh A. Targeting SARS-CoV-2: a systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 20-O-ribose methyltransferase. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1753577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.A., Zia K., Ashraf S., Uddin R., Ul-Haq Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1751298. [DOI] [PubMed] [Google Scholar]
- Kumar S. 2020. Drug and vaccine design against novel coronavirus (2019-nCoV) spike protein through computational approach. Preprints 2020 2020020071. [DOI] [Google Scholar]
- Levine M.M. Monoclonal antibody therapy for Ebola virus disease. N. Engl. J. Med. 2019;381(24):2365–2366. doi: 10.1056/NEJMe1915350. [DOI] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang J., Wang N., Li H., Shi Y., Guo G., Liu K., Zeng H., Zou Q. Therapeutic drugs targeting 2019-nCoV main protease by high-throughput screening. bioRxiv. 2020 2020.2001.2028.922922. [Google Scholar]
- Lipsky M.S., Sharp L.K. From idea to market: the drug approval process. J. Am. Board Fam. Pract. 2001;14(5):362–367. [PubMed] [Google Scholar]
- Liu H., Ye F., Sun Q., Liang H., Li C., Lu R., Huang B., Tan W., Lai L. em>Scutellaria baicalensis</em> extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease <em>in vitro</em>. bioRxiv. 2020 doi: 10.1080/14756366.2021.1873977. 2020.2004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery. 2020;6(1):16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zheng Q., Wang Z. Potential covalent drugs targeting the main protease of the SARS-CoV-2 coronavirus. Bioinformatics. 2020 doi: 10.1093/bioinformatics/btaa224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Li Z., Liu S., Sun J., Chen Z., Jiang M., Zhang Q., Wei Y., Wang X., Huang Y.-Y., Shi Y., Xu Y., Xian H., Bai F., Ou C., Xiong B., Lew A.M., Cui J., Fang R., Huang H., Zhao J., Hong X., Zhang Y., Zhou F., Luo H.-B. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang X.-J. Potential inhibitors for 2019-nCoV coronavirus M protease from clinically approved medicines. Journal of Genetics and Genomics. 2020;47:119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J., Ericsson M., Rottier P., Griffiths G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the golgi complex requires only one vesicular transport step. J. Cell Biol. 1994;124:55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Huo X.Q., Chen X., Zhu W.X., Yao M.C., Qiao Y.J., Zhang Y.L. [Study on screening potential traditional Chinese medicines against 2019-nCoV based on Mpro and PLP] Zhongguo Zhongyao Zazhi. 2020;45(6):1219–1224. doi: 10.19540/j.cnki.cjcmm.20200216.401. [DOI] [PubMed] [Google Scholar]
- Mohammed Hakmi E.M.B., Kandoussi Ilham, El Harti Jaouad, Azeddine Ibrahimi Repurposing of known anti-virals as potential inhibitors for SARS-CoV-2 main protease using molecular docking analysis. Biomedical Informatics. 2020;16(4):301–306. doi: 10.6026/97320630016301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan N., Sakthivel R., Velmurugan D., Gromiha M.M. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 protease against COVID-19. J. Biomol. Struct. Dyn. 2020:1–6. doi: 10.1080/07391102.2020.1752802. [DOI] [PubMed] [Google Scholar]
- Nutho B., Mahalapbutr P., Hengphasatporn K., Pattaranggoon N.C., Simanon N., Shigeta Y., Hannongbua S., Rungrotmongkol T. Why are lopinavir and ritonavir effective against the newly emerged coronavirus 2019? Atomistic insights into the inhibitory mechanisms. Biochemistry. 2020 doi: 10.1021/acs.biochem.0c00160. [DOI] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Zhou P., Fan T., Wu Y., Zhang J., Shi X., Shang W., Fang L., Jiang X., Shi J., Sun Y., Zhao S., Gong R., Chen Z., Xiao G. Immunoglobulin fragment F(ab’)2 against RBD potently neutralizes SARS-CoV-2 in vitro. bioRxiv. 2020 doi: 10.1016/j.antiviral.2020.104868. 2020.2004.2007.029884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant S., Singh M., Ravichandiran V., Murty U.S.N., Srivastava H.K. Peptide-like and small-molecule inhibitors against Covid-19. J. Biomol. Struct. Dyn. 2020:1–15. doi: 10.1080/07391102.2020.1757510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T., Lee S.-Y., Kim S., Kim M.J., Kim H.G., Jun S., Kim S.I., Kim B.T., Park E.C., Park D. Spike protein binding prediction with neutralizing antibodies of SARS-CoV-2. bioRxiv. 2020 2020.2002.2022.951178. [Google Scholar]
- Peele K.A., Durthi C.P., Srihansa T., Krupanidhi S., Ayyagari V.S., Babu D.J., Indira M., Reddy A.R., Venkateswarulu T. Molecular docking and dynamic simulations for antiviral compounds against SARS-CoV-2: a computational study. Informatics in medicine unlocked. 2020;19:100345. doi: 10.1016/j.imu.2020.100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimanshee A., Amit D., Vishal P., Mukesh K. 2020. Potential Inhibitors against Papain-like Protease of Novel Coronavirus (SARS-CoV-2) from FDA Approved Drugs. [Google Scholar]
- Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev. Panam. Salud Públic. 2020;20(44):1–7. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Stone S., Natekar J., Kumari P., Arora K., Kumar M. The FDA- approved gold drug Auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. bioRxiv. 2020 doi: 10.1016/j.virol.2020.05.002. 2020.2004.2014.041228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang P., Tian S.-H., Meng Z.-H., Yang L.-Q. Anti-HIV drug repurposing against SARS-CoV-2. RSC Adv. 2020;10(27):15775–15783. doi: 10.1039/d0ra01899f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senathilake K.S.S., Tennekoon K. 2020. Virtual screening of inhibitors against spike glycoprotein of 2019 novel corona virus: a drug repurposing approach. Preprints 2020. [Google Scholar]
- Shah B., Modi P., Sagar S.R. In silico studies on therapeutic agents for COVID-19: drug repurposing approach. Life Sci. 2020;252:117652. doi: 10.1016/j.lfs.2020.117652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., Leist S.R., Schäfer A., Dinnon K.H., Stevens L.J., Chappell J.D., Lu X., Hughes T.M., George A.S., Hill C.S., Montgomery S.A., Brown A.J., Bluemling G.R., Natchus M.G., Saindane M., Kolykhalov A.A., Painter G., Harcourt J., Tamin A., Thornburg N.J., Swanstrom R., Denison M.R., Baric R.S. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020 doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibo Jiang L.D.Z.S. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerg. Microb. Infect. 2020;9(1):275–277. doi: 10.1080/22221751.2020.1723441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Nogai L., Bohra R. Dealing with SARS COV-2 pandemic: perusing remdesivir and hydroxychloroquine as therapeutic alternative. European Journal of Molecular & Clinical Medicine. 2021;7(10):2132–2148. [Google Scholar]
- Sohini C., Narayanaswamy S. 2020. Drug Repurposing Approach Targeted against Main Protease of SARS-CoV-2 Exploiting ‘Neighbourhood Behaviour’ in 3D Protein Structural Space and 2D Chemical Space of Small Molecules. [Google Scholar]
- Su H., Yao S., Zhao W., Li M., Liu J., Shang W., Xie H., Ke C., Gao M., Yu K., Liu H., Shen J., Tang W., Zhang L., Zuo J., Jiang H., Bai F., Wu Y., Ye Y., Xu Y. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease <em>in vitro</em>. bioRxiv. 2020 2020.2004.2013. [Google Scholar]
- Tahir ul Qamar M.A., S M., Alamri M.A., Chen L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. Journal of Pharmaceutical Analysis. 2020 doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talluri S. 2020. Virtual high throughput screening based prediction of potential drugs for COVID-19. Preprints 2020. [DOI] [PubMed] [Google Scholar]
- ter Meulen J., Bakker A.B.H., van den Brink E.N., Weverling G.J., Martina B.E.E., Haagmans B.L., Kuiken T., de Kruif J., Preiser W., Spaan W., Gelderblom H.R., Goudsmit J., Osterhaus A.D.M.E. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. 2004;363(9427):2139–2141. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Meulen J., van den Brink E.N., Poon L.L.M., Marissen W.E., Leung C.S.W., Cox F., Cheung C.Y., Bakker A.Q., Bogaards J.A., van Deventer E., Preiser W., Doerr H.W., Chow V.T., de Kruif J., Peiris J.S.M., Goudsmit J. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3(7) doi: 10.1371/journal.pmed.0030237. e237-e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microb. Infect. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton A.-T., Gentile F., Hsing M., Ban F., Cherkasov A. Rapid identification of potential inhibitors of SARS-CoV-2 main protease by deep docking of 1.3Billion compounds. Molecular Informatics. 2020;39(2000028):1–8. doi: 10.1002/minf.202000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesh, Kundu D., Selvaraj C., Singh S.K., Dubey V.K. Identification of new anti-nCoV drug chemical compounds from Indian spices exploiting SARS-CoV-2 main protease as target. J. Biomol. Struct. Dyn. 2020:1–9. doi: 10.1080/07391102.2020.1763202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahedi H.M., Ahmad S., Abbasi S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. 2020:1–16. doi: 10.1080/07391102.2020.1762743. [DOI] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Fast identification of possible drug treatment of coronavirus disease -19 (COVID-19) through computational drug repurposing study. J. Chem. Inf. Model. 2020 doi: 10.1021/acs.jcim.0c00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30(4):343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Yan L., Xu W., Agrawal A.S., Algaissi A., Tseng C.-T.K., Wang Q., Du L., Tan W., Wilson I.A., Jiang S., Yang B., Lu L. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Science Advances. 2019;5(4) doi: 10.1126/sciadv.aav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y., Ying T., Liu S., Shi Z., Jiang S., Lu L. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell. Mol. Immunol. 2020 doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Shi P.-Y., Li H., Zhou J. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect. Dis. 2020 doi: 10.1021/acsinfecdis.0c00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Peng C., Shi Y., Zhu Z., Mu K., Wang X., Zhu W. Nelfinavir was predicted to be a potential inhibitor of 2019-nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation. bioRxiv. 2020 2020.2001.2027.921627. [Google Scholar]
- Xu Z., Shen J., Wu N., Xu Y., Lu X., Zhu W., Li L.-J. 2020. Nelfinavir Is Active against SARS-CoV-2 in Vero E6 Cells. [Google Scholar]
- Yan Y.S.X., Cao Y., Zhang J., Wang Y., Cheng Y. 2020. Discovery of anti-2019-nCoV agents from 38 Chinese patent drugs toward respiratory diseases via docking screening. Preprints 2020 2020020254. [Google Scholar]
- Yang H., Xie W., Xue X., Yang K., Ma J., Liang W., Zhao Q., Zhou Z., Pei D., Ziebuhr J., Hilgenfeld R., Yuen K.Y., Wong L., Gao G., Chen S., Chen Z., Ma D., Bartlam M., Rao Z. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005;3(10) doi: 10.1371/journal.pbio.0030324. e324-e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanling S., Jia S., Xinyu W., Mengjiao H., Miao S., Lin Z., Bingqian L., Haicong S., Zhi Z., Chaoyong Y. 2020. Discovery of Aptamers Targeting Receptor-Binding Domain of the SARS-CoV-2 Spike Glycoprotein. [DOI] [PubMed] [Google Scholar]
- Yin W., Mao C., Luan X., Shen D.D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M., Chang S., Xie Y.C., Tian G., Jiang H.W., Tao S.C., Shen J., Jiang Y., Jiang H., Xu Y., Zhang S., Zhang Y., Xu H.E. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;1(10) doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Wai Chen C.-P.Y., Wong Kwok-Yin. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL ) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research. 2020;9(129):1–17. doi: 10.12688/f1000research.22457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yulong S., Xinben Z., Kaijie M., Cheng P., Zhengdan Z., Xiaoyu W., Yanqing Y., Zhijian X., weiliang z. D3Targets-2019-nCoV: a webserver for predicting drug targets and for multi-target and multi-site based virtual screening against COVID-19. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.-h., Wu K.-l., Zhang X., Deng S.-q., Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. Journal of Integrative Medicine. 2020;18(2):152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020 doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.-j., Wu W.-y., Hou J.-j., Zhang L.-l., Li F.-f., Gao L., Wu X.-d., Shi J.-y., Zhang R., Long H.-l., Lei M., Wu W.-y., Guo D.-a., Chen K.-x., Hofmann L.A., Ci Z.-h. Active constituents and mechanisms of Respiratory Detox Shot, a traditional Chinese medicine prescription, for COVID-19 control and prevention: network-molecular docking-LC–MSE analysis. Journal of Integrative Medicine. 2020 doi: 10.1016/j.joim.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Liu Q., Du L., Lu L., Jiang S. Receptor-binding domain as a target for developing SARS vaccines. J. Thorac. Dis. 2013:S142–S148. doi: 10.3978/j.issn.2072-1439.2013.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Chakraborti S., He Y., Roberts A., Sheahan T., Xiao X., Hensley L.E., Prabakaran P., Rockx B., Sidorov I.A., Corti D., Vogel L., Feng Y., Kim J.-O., Wang L.-F., Baric R., Lanzavecchia A., Curtis K.M., Nabel G.J., Subbarao K., Jiang S., Dimitrov D.S. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc. Natl. Acad. Sci. U.S.A. 2007;104(29):12123–12128. doi: 10.1073/pnas.0701000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on request.



