Abstract
Backgrounds
Corona virus disease 19 (Covid-19) affects especially the respiratory tract, and induces lung injury which may progress to the acute respiratory distress syndrome (ARDS). Various treatment options were tried all over the world, corticosteroids had showed beneficial effects.
The Objective of this study, is to compare the safety and efficiency of two corticosteroids: dexamethasone and prednisolone in the treatment of Covid-19 infection.
Methods
This retrospective and comparative study included 513 patients diagnosed with Covid-19 infection and were admitted to intensive care unit of our university hospital center of MOHAMMED VI Oujda from March 1, 2020, to December 31st, 2020.
Results
In this study, 513 cases were included, 230 patients were received methylprednisolone, and 283 were treated with dexamethasone. The median age in methylprednisolone group was 64 years, and 63 years in the second group treated with dexamethasone. Patients treated with dexamethasone had more critically lesions compared to patients treated with methylprednisolone (67.6%), these patients had a good evolution with a significant reduction of oxygen supplementation, lower use of invasive ventilation and a significant improvement in biological parameters. The difference in outcome between the two groups in terms of mortality was significantly reduced in the second group.
Conclusion
Both steroids are efficient in the management of mild, moderate and severe Covid-19 pneumonia with a clear superiority of dexamethasone especially in severe forms.
Keywords: COVID-19, Dexamethasone, Methylprednisolone, Safety, Efficacy
Highlights
-
-
The development of an efficient treatment in Covid-19 infection was a challenge, especially the corticosteroids.
-
-
The aim of this paper is to compare between two most used corticosteroids in the treatment of Covid 19 diseases: Dexamethasone and methylprednisolone
-
-
A significant reduction of oxygen requirement was noted in the second group of patients treated with dexamethasone.
-
-
The difference in outcome between the two groups in terms of mortality was significantly reduced in the second group with a good evolution.
1. Introduction
In the first phase of ARDS, Lung injury initiates at the epithelial-interstitial-endothelial level, with exudation of macrophages and neutrophils [1], the result is a reduction of alveolar surfactant and therefore a decrease in gas exchanges. The second phase is characterized by an uncontrolled viral replication, and then a worsening of the hyper inflammatory state [2]. Based on this pathophysiology, the use of steroids in the management of Covid-19 diseases (especially dexamethasone and methylprednisolone) was encouraged, and there are many studies that improve its beneficial effects [3].
The aim of this paper is to compare the efficacy and safety of the use of dexamethasone and methylprednisolone in Covid-19 patients after 7 days, to follow the clinical and biological evolution of the patients as well as the evolution of oxygen supplementation, complications and mortality.
2. Patients and methods
This is a retrospective and comparative study conducted in the intensive care unit (ICU) of our university hospital center of Mohammed VI in Oujda, including 513 cases, diagnosed with mild, moderate or severe Covid-19 disease, confirmed by RT swab test (PCR) positive or Chest CT Scan typically for Covid-19 From march 1, 2020, to December 30, 2020. The collect information was made retrospectively on data from patient's medical records and a questionnaire.
In this study, we defined 2 groups:
-
-
First Group: patients treated with intravenous (1 mg/kg) or oral methylprednisolone
-
-
Second Group: patients treated with intravenous dexamethasone 6 mg/kg per day.
2.1. Cases included
-
•
Patients with PCR positive or Chest CT typical for Covid-19
-
•
Patients above 18 years of age
-
•
Patients who have stayed more than 7 days, either at the ICU or hospitalized initially in ICU and transferred to another department.
2.2. Cases excluded
-
•
Patients previously treated with corticosteroids for any reason
-
•
Immunodeficiency disorders
-
•
Lack of willingness to participate in this study
2.3. Study design
Patients baseline characteristics were noted in the time of admission (day zero) such as medical histories, clinical symptoms, the initial clinical examination (temperature, respiratory rate, oxygen saturation and type of oxygen supplementation).
Biological parameters (CRP, White blood cell, serum procalcitonin level ….) were done for all patients and were also noted.
Chest Computed tomography was done for all of our patients at the admission.
All patients included were treated with corticosteroids either by methylprednisolone 1 mg/kg (First Group) or dexamethasone 6 mg/kg (Second group).
Seven days after given corticosteroids, we noted the improvement in clinical (oxygen supplementation, duration of hospitalization, mortality …) and biological parameters in the both groups, consequently, we made a comparison between this two groups to know which one is more efficient and more superior in the treatment of Covid-19 according to our experience.
2.4. Statistical analysis
Statistical analysis was performed using the SPSS software version 21.0. Qualitative variables were expressed as counts and percentage and quantitative variables as mean ± standard deviation (SD) or as median (interquartile range (IQR)). Then a univariate analysis was performed to compare between two groups of patients receiving corticosteroids. The comparison of values was performed by the 2-tailled Student t-test or the Mann-Whitney U test, as appropriate for quantitative variables and by CHI-2 test or Fisher exact test for qualitative variables.
This study was approved by the ethics committee for biomedical research of Oujda (ECBRO) of the faculty of medicine and pharmacy of Oujda. Access to patient data was authorized by the Mohammed VI university hospital and approved by the head of the department, taking into account the retrospective and comparative design of this study. Informed consent was obtained from participants. Data anonymity was respected in accordance with national and international guidelines.
This case series has been reported in line with the PROCESS Guideline [4].
Research registry 6573.
3. Results
Among the 513 patients included in this present study and whom were admitted in the ICU of the UHC Mohammed VI of Oujda, 230 patients were treated with methylprednisolone in the first group (44.8%) and 283 cases were received dexamethasone in the second group (55.2%).
The median age of patients receiving methylprednisolone (1st group) was 64 [52–73] and 63 [55–75] years in the patients receiving dexamethasone (2nd group), which explains that the 2 groups of our patients were almost the same age. Patients clinical characteristics of our cases at the admission were represented in Table 1.
Table 1.
Basic characteristics.
| Groups | Group 1 |
Group 2 |
||
|---|---|---|---|---|
| N | % | N | % | |
| Gender | ||||
| Male | 162 | 70.4 | 183 | 67.3 |
| Female | 68 | 29.6 | 100 | 35.3 |
| Medical histories | ||||
| Hypertension | 74 | 32.2 | 103 | 36.4 |
| Diabetes | 75 | 32.6 | 95 | 33.6 |
| Smoking | 19 | 8.3 | 19 | 6.7 |
| Heart diseases | 32 | 13.9 | 39 | 13.8 |
| Renal failure | 15 | 6.5 | 17 | 6.0 |
| Symptoms | ||||
| Fever | 169 | 73.5 | 219 | 77.4 |
| Dyspnea | 170 | 73.9 | 206 | 72.8 |
| Cough | 169 | 73.5 | 217 | 76.7 |
| fatigue | 152 | 66.1 | 194 | 68.6 |
All patients required oxygen supplementation, the oxygen was given by nasal cannula, face mask, high flow nasal cannula (HFNC), non-invasive ventilation (NIV) and mechanical ventilation (MV).
Types of oxygen supplementation in the two groups on admission of our patients were represented in Table 2.
Table 2.
Types of oxygen supplementation in the different groups at the admission.
| Types of oxygen supplementation |
Group 1 |
Group 2 |
||
|---|---|---|---|---|
| N | % | N | % | |
| <15L (cannula or face mask) | 170 | 73.9 | 196 | 69.5 |
| HFNC | 42 | 18.2 | 52 | 18.3 |
| VNI | 18 | 7.8 | 30 | 10.6 |
| VM | 0 | 0.0 | 3 | 1.0 |
The initial laboratory data were as presented in Table 3.
Table 3.
Initial clinical blood count in the both groups.
| Group 1 | Group 2 | P-Value | |
|---|---|---|---|
| C-ReactiveProtein (mg/l) | 188 [94–255] | 182 [88–237] | 0.98 |
| White blood count | 10 600 [7320–14895] | 10 440 [7140–14965] | 0.93 |
| Procalcitonin level | 0.38 [0.15–1.30] | 0.40 [0.17–1.30] | 0.75 |
| Lymphocytes | 840 [540–1370] | 840 [535–1260] | 0.92 |
Chest CT scan was done for 97.82% of patients in the first group and in 93.99% in the second group, and according to the degree of pulmonary lesions, 4 groups were defined:
✓10–25%: patients with mild Covid-19
✓25–50%: patients with moderate Covid-19
✓50–75%: patients with severe Covid-19
✓75–100%: patients with critical Covid-19
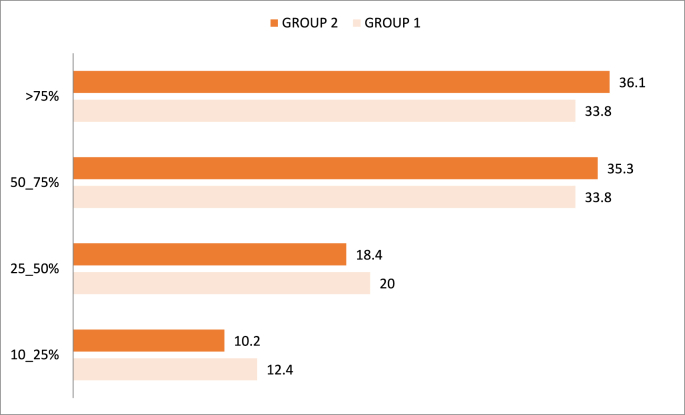
In our study, the second Group patients had more severe and critical radiological lesions with a percentage of 71.4% compared to the first group (67.6%).
Percentages of pulmonary involvement at the first CT after symptom onset are presented in Fig. 1.
Fig. 1.
Percentage of pulmonary lesions in the two groups.
Seven days later, clinical examination and biological parameters were noted in both groups.
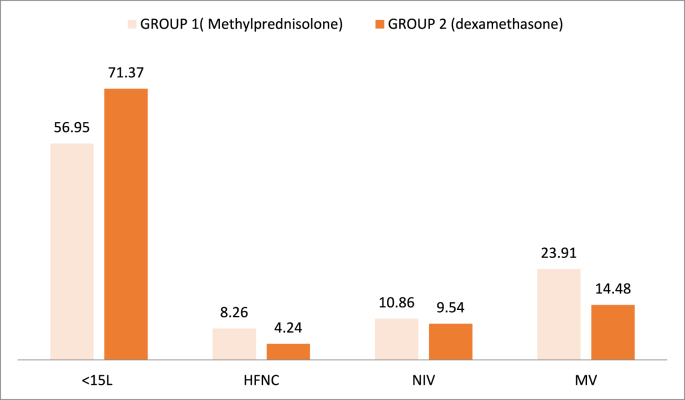
We observed initially a significant reduction of oxygen requirement in patients treated with dexamethasone for seven days versus methylprednisolone group. 71.37% of patients treated with dexamethasone required low oxygen supplementation through an oxygen cannula or face mask <15 L/min with p value of 0.05. The need of non-invasive ventilation and mechanical ventilation were also reduced in the same group (P-value 0.06) (Fig. 2).
Fig. 2.
Evolution of oxygen supplementation seven days after given steroids.
An important increase on arterial pressure of oxygen (PaO2) in both groups were noted (8.4% in the first group versus 11.6% in the second group). We noted in Table 4 that the median of the PaO2 values were higher on the 7th day in the dexamethasone group.
Table 4.
Evolution of partial pressure of oxygen after seven days.
| GROUP 1 | GROUP 2 | |
|---|---|---|
| Days 0 | 59 [49–77] | 60 [50–79] |
| Days 7 | 64 [54–77] | 67 [55–88] |
| P-value | 0.00 | 0.00 |
Biological data in the seven days showed a significant reduction of mean CRP and white blood cells in the second group (reduction of 36.81% in the first group and 45.0% in the second group in the level of CRP/reduction of 9.4% in the group 1 versus 19.5% in the group 2 in the level of WBC) (Table 5).
Table 5.
Evolution of CRP level and white blood cell seven days after given steroids.
| GROUP 1 | GROUP 2 | |
|---|---|---|
| CRP | ||
| Days 0 | 188 [94–255] | 182 [88–237] |
| Days 7 | 120 [56–187] | 100 [44–131] |
| P-value | 0.00 | 0.00 |
| WBC | ||
| Days 0 | 10 600 [7320–14 895] | 10 440 [7140–14 965] |
| Days 7 | 9 600 [6400–11 505] | 8 400 [5000–11 500] |
| P-value | 0.01 | 0.003 |
The chest scan CT were done after 7 days only in 59 of patients, and showed a worsening of lung injury in 26 patients treated with methylprednisolone (92.9%) versus 33 patients treated with dexamethasone (89.2%).
We observed more complications such as supper added infection and septic shock in the first group treated with methylprednisolone than in the second group treated with dexamethasone (Table 6).
Table 6.
Evolution of complications in the both groups seven days after given steroids.
| methylprednisolone |
Dexamethasone |
P value |
|||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Supper added infection | 59 | 25.6 | 44 | 15.5 | 0.61 |
| Septic shock | 59 | 25.6 | 65 | 22.9 | 0.59 |
| Hyperglycemia | 34 | 14.7 | 69 | 24.3 | 1.09 |
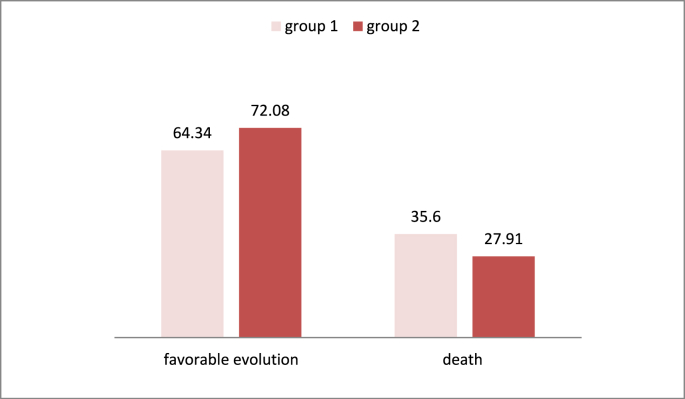
In our study, 72.08% of patients treated with dexamethasone had a favorable evolution as compared as 64.34% in the first group (P-Value 0.04), we noted also a reduction in mortality in the same group (Fig. 3).
Fig. 3.
Favorable evolution and mortality in the different groups.
4. Discussion
Over the last decade, corticosteroids were used for its anti-inflammatory properties and immune suppression for the treatment of many disorders, such as asthma, systemic lupus erythematosus, rheumatoid, exacerbation of COPD [5], and septic shock [6].
They were also indicated for the treatment of acute respiratory distress syndrome (non Covid-19 ARDS). Many studies showed the beneficial effects of the use of corticosteroids [7,8].
Since the emergence of the Covid-19, the development of an efficient treatment was a challenge. Therefore, many treatment options were tried such as Tocilizumab, plasma therapy, antibiotics, antiviral, and corticosteroids [9].
Hence, several studies were conducted to improve the impact of steroids in the treatment of Covid-19 infection. Dexamethasone and methylprednisolone were the most corticosteroids studied.
SYEDA and all [10] had compared between the both steroids in the management of moderate and severe Covid-19, and had noted that oxygen requirement was reduced from 12.5 L/mn to 10.3L/mn in patient received dexamethasone. As reported in our study, 71.37% of patients in the second group had less than 15L/mn after given dexamethasone for 7 days, as compared as 56.95% in the first group.
Our results found a significant reduction of CRP level and white blood cells count in both groups, and especially in patients treated with dexamethasone. Which joins the results of Syeda and all study [10].
In the same study of Syeda and all [10], none of the patients collected had developed super added bacterial infection, this may be explained by the short duration of the treatment (5 days) and the number of patients (35 cases treated with dexamethasone), however, in our study there was no significant difference between the two groups in terms of super added infection and septic shock. Hyperglycemia, another side effect of steroids [11], was also observed in our study especially in diabetic patients and it was effectively managed by insulin.
The oxford recovery trial [12], concluded that Dexamethasone decrease the mortality of the patients [13,14]. Nicolas Martin in his study, found that 2104 patients received dexamethasone against 4321 patients in the control group receiving usual care [15]; however, the 28-day evaluation of these 2 groups of patients found a significant reduction in mortality of 35% in patients on invasive mechanical ventilation while 20% in patients on oxygen supplementation [12]. These results are similar to those of our study, where 72.08% of patients treated with dexamethasone had a favorable evolution as compared as 64.34% in the first group (P-Value 0.04), we noted also a reduction in mortality in the same group.
The secondary outcome of recovery study showed shorter duration of hospitalization with dexamethasone [16], in our study we observed a shorter duration of hospitalization (7.21 days) in methylprednisolone group versus 8.29 days in dexamethasone group. Another study conducted by Keivan Ranjbar and all [17], found the same results as our study.
Our study has several limitations. Certain data were not available and were missing during the collection of files such as biological tests, CT scan, treatments and outcomes because this study was retrospective. Therefore, it is important to lead a more complete and thorough investigation in the future in a prospective sense.
Our results found that both drugs are efficient in the management of mild, moderate and severe Covid-19 with a clear superiority of dexamethasone especially in severe forms. However, more studies are needed in order to improve the superiority of the Dexamethasone therapy in Covid-19 patients.
5. Conclusions
The result of our study showed that both drugs used for the treatment of our patients with Covid-19 pneumonia (methylprednisolone and dexamethasone) are efficient, and they have demonstrated a good clinical and biological improvement whatever the degree of pulmonary lesion. However, patients treated with dexamethasone had a good evolution with a significant reduction of oxygen supplementation, lower use of invasive ventilation and with a significant reduction in mortality.
Ethical approval
This is a retrospective and comparative case series was approved by the ethics committee for biomedical research of Oujda (ECBRO) of the faculty of medicine and pharmacy of Oujda. Data were anonymously registered in our database. Access to data was approved by the head of the department.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
EL MEZZIOUI Sanae: Study concept, Data collection; data analysis; writing review & editing.
EL AIDOUNI Ghizlane: Study concept, Data collection; data analysis; writing review & editing.
MERBOUH Manal: Data collection.
EL KAOUINI Abderrahim: Contributor.
AFTISS Fatem-zahra: Contributor.
BERRICHI Samia: Contributor.
BERRAJAA Sara: Contributor.
BKIYAR Houssam: supervision and data validation.
ABDA Naima: Study concept, Data collection; data analysis, editing and supervision and data validation.
HOUSNI Brahim: supervision and data validation.
Consent
Obtained.
Registration of research studies
Research registry 6573.
Guarantor
EL MEZZIOUI Sanae.
EL AIDOUNI Ghizlane.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors state that they have no conflicts of interest for this case series.
Acknowledgments
We would like to thank the medical and nursing teams of Mohammed VI University Hospital for their significant involvement in the management of the patients included in our study. Particular thanks to the director of Mohammed VI University Hospital Prof. Abdelkarim Daoudi for his successful management of this outbreak.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102858.
Contributor Information
Sanae El mezzeoui, Email: sanae.elmezzeoui@gmail.com.
Ghizlane El aidouni, Email: elaidounighizlane@gmail.com.
Manal Merbouh, Email: manal.mrb@gmail.com.
Abderrahim El Kaouini, Email: abderrahimfmpo19@gmail.com.
Fatima Zahra Aftiss, Email: fatimaaf-13@hotmail.com.
Samia berrichi, Email: sammia9@gmail.com.
Sara Berrajaa, Email: berrajaasara@gmail.com.
Houssam Bkiyer, Email: 7b.houssam@gmail.com.
Naima Abda, Email: abda.naima@yahoo.com.
Brahim Housni, Email: brahimhousni@yahoo.fr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Guan W., Ni Z., Wu H., Liang W. Clinical characteristics of corona virus disease 2019 in China. N Engl. J. Med. March. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [Google Scholar]
- 2.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Song S., Ma Z., Mo P., Zhang Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isidori A., Arnaldi G., Boscaro M., Falorni A., Giordano C., Giordano R., Pivonello R., Pofi R., Hasenmajer V., Venneri M.A., Sbardella E., Simeoli C., Scaroni C., Lenzi A. COVID-19 infection and glucocorticoids: update from the Italian Society of Endocrinology Expert Opinion on steroid replacement in adrenal insufficiency. J. Endocrinol. Invest. 2020;43(8):1141–1147. doi: 10.1007/s40618-020-01266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agha R.A., Sohrabi C., Mathew G., Franchi T., Kerwan A., O'Neill N for the Process Group The PROCESS 2020 guideline: updating consensus preferred reporting of CasE series in surgery (PROCESS) guidelines. Int. J. Surg. 2020;84:231–235. doi: 10.1016/j.ijsu.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Bethesda Corticosteroids. 2012. https://www.ncbi.nlm.nih.gov/books/NBK548400/2020
- 6.Marik P.E. Steroids for sepsis: yes, no or maybe. J. Thorac. Dis. 2018;10(Suppl 9) doi: 10.21037/jtd.2018.04.35. S1070–S1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villar J., Ferrando C., Martinez D. On behalf of Dexamethasone in ARDS network. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomized controlled trial. Lancet Respir. Med. 2020;8:267e76. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 8.Gu Meduri, Annane D., Chrousos G.P., Marik P.E., Sinclair S.E. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136:1631e43. doi: 10.1378/chest.08-2408. [DOI] [PubMed] [Google Scholar]
- 9.Wang J. Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study. J. Chem. Inf. Model. 2020;60(6):3277–3286. doi: 10.1021/acs.jcim.0c00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syeda Arzinda Fatima Comparison of efficacy of dexamethasone and methylprednisolone in moderate to severe covid 19 disease. 2020. [DOI] [PMC free article] [PubMed]
- 11.Tamez-P'erez H.E., Quintanilla-Flores D.L., Rodríguez-Guti'errez R., Gonz'alez- Gonz'alez J.G., Tamez-Peña A.L. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J. Diabetes. 2015;6:1073. doi: 10.4239/wjd.v6.i8.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson E. RECOVERY trial: the UK covid-19 study resetting expectations for clinical trials. BMJ. 2020:m1626. doi: 10.1136/bmj.m1626. [DOI] [PubMed] [Google Scholar]
- 13.Ledford Heidi. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. juin 2020;16 doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 14.Morin Hervé. Coronavirus : un corticoïde réduit d’un tiers la mortalité chez les patients les plus atteints par le Covid-19. Le Monde.fr. 16 juin 2020 [Google Scholar]
- 15.Nicolas Martin, « La dexaméthasone, un remède miracle… encore ? », sur France Culture (consulté le 19 juin 2020).
- 16.Horby P, Lim WS, Emberson J, Mafham M, Bell J, Landary MJ, et al. Effect of dexamethasone in hospitalized patients with COVID-19 e preliminary report. medRxiv June 22,2020. Doi:10.1101/2020.06.22.20137273.
- 17.Ranjbar Keivan, Moghadami Mohsen, Mirahmadizadeh Alireza, Javad Fallahi Mohammad, Khaloo Vahid, Shahriarirad Reza, Erfani Amirhossein, Khodamoradi Zohre, Hasan Gholampoor Saadi Mohammad. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect. Diseases. 2021;21:337. doi: 10.1186/s12879-021-06045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.