Abstract
The aim of this study is to describe radiographic, ultrasonographic, and computed tomographic appearance of normal cinereous vulture’s eye and to determine normal biometric values of intraocular structures. Twenty-six eyes of thirteen healthy cinereous vultures were examined. Under general anesthesia with isoflurane, ultrasonography (US), computed tomography (CT) and skull radiography were performed. Differences between both eyes as well as between US and CT measurements were investigated and correlation of measurements between both eyes as well as correlation between CT and US measurements of the various ocular structures were calculated. Most of paired data did not show any significant differences between both eyes and the CT and US measurements, while there were significant differences (P<0.05) between CT and US measurements of depth of both vitreous and anterior chambers, and axial length of the lens in right eyes. There was also a significant difference (P<0.05) between both eyes in depth of vitreous measured by CT. All the measurements had strong correlations between both eyes and between US and CT. In conclusion, ocular imaging techniques provided useful data of biometry and morphology, showing good correlation between CT and US in cinereous vulture’s eye. Especially, when ophthalmoscopic examinations would not be available due to opaque anterior segment, imaging techniques could be essential for diagnosing and managing of the eye.
Keywords: cinereous vulture, computed tomography, ocular biometry, ocular imaging, ultrasonography
The Eurasian black or cinereous vulture (Aegypius monachus) has been categorized as “Near Threatened” in all over the world [10, 18]. It breeds in Asia and the Mediterranean region of the world but it has been found in entire region in Eurasian continent. Being listed as near threatened or endangered species, it has been raised all over the world including South Korea. It has been raise since 1973 in different wildlife rescue centers in South Korea. In every winter, cinereous vultures (Aegypius monachus) migrate from Mongolia to Korea and return to the north in next season [14]. Due to various ailments, i.e., poor nutritional conditions, poisoning, skeletal fracture and weakness, they have been presented often at the wildlife rescue centers in Korea [10, 18].
A thorough ophthalmic examination should be included in physical examination of raptors because it has been reported a high prevalence to be up to 50% of ocular disease in free living raptors [4]. Most common ailments found in raptors are retinal degeneration, corneal lesions, retinal dysplasia, cataract, glaucoma, ciliary body malformations, and traumatic uveitis [1, 3, 7]. Above mentioned ocular diseases induce weakened eyesight that causes life-threatening results sometimes.
There are rare studies about radiography of the head or more specifically about the eyes of the birds [4]. In some species, few anatomic structures of avian scleral ossicles and orbit have been reported [12, 19, 23, 24]. Radiological examination of avian eye is restricted to assessment of fractures of scleral ossicles, organomegaly, or mass defects [11, 15]. In certain study, Computed tomography (CT) and routine radiography in evaluating the heads of raptors and psittacine have been compared [13] but ophthalmological explanation is missing. In modern diagnosis, CT has been used for examination of avian head occasionally to diagnose cerebral or skeletal diseases like meningo-encephalocele, cranial malformation, hemorrhage, or otitis [9]. It is not a routine practice to use ultrasonography (US) in avian ophthalmology, however, it has been used in small animals. Few examples are available to show the possibilities of use of ultrasonography in avian ophthalmology [4].
There are few studies related the anatomy and ultrasonography of raptor’s eyes. Recently, US has become routine procedure for veterinary ophthalmology. It provides biometric measurement of eye and different structures of the eye including pecten oculi (LP), lens, anterior chamber, and vitreous chamber. It also allows assessment of the eyes with an opacity of transparent media. There is variation among anatomy of birds, so it is impossible to use same ultrasonographic and biometric measurements for every species [4].
The objectives of this study were to describe radiographic, ultrasonographic, and computer tomographic appearance of normal cinereous vulture’s eye. It was also included to determine normal values of biometry of intraocular structures and axial length for further treatment.
MATERIALS AND METHODS
Animals
Thirteen healthy cinereous vultures (mean body weight, 9.21 ± 0.84 kg; range, 8.0 to 10.8 kg) were examined. These birds were obtained from captivity of Gyeongnam Wildlife Rescue Center present in College of Veterinary Medicine, Gyeongsang National University, South Korea. Twenty-six eyes of all birds were examined in this study. All animals were young adult, suspected between 1 to 5 years of age, but gender and exact age were unknown. They were previously free living birds but could not be released because of devastating injuries not related to the eyes. They have been provided proper treatment and care by veterinarians to resolve their health issues prior to be brought for this study. This study was approved by Institutional Animal Care and Use Committee (IACUC) of College of Veterinary Medicine, Gyeongsang National University, South Korea with an approval number GNU-140307-E0017.
Restraining and Anesthesia to raptors
All raptors were restrained manually and examined in sternal recumbent position under general anesthesia with isoflurane. Mask induction was performed using 5% isoflurane (Ifran®, Hana Pharm, Co., Ltd., Korea) saturated in 100% oxygen (3 l/min). Trachea was intubated by an uncuffed endotracheal tube after induction. The size of an endotracheal tube was chosen according to size of bird to avoid leakage of an anesthetic agent. After intubation, intubation was stabilized by spontaneous ventilation with 2 to 2.5% isoflurane saturated in 100% oxygen (3 l/min). Medical imaging at anesthetic status in a sequence of ultrasonography, computed tomography and skull radiography were performed.
Ultrasonographic examinations and biometry
Ultrasonography was performed with a B-mode ocular unit (Xario, SSA 660A, Toshiba Inc., Japan) with 8 MHz convex transducer. The eyes of raptors were anesthesized by 0.5% proparacaine hydrochloride (Alcaine®, Alcon, Puurs, Belgium) and scanned bilaterally in dorsal plane (oculus dexter (OD), right eye; oculus sinister (OS), left eye). The transducer was positioned directly on the cornea, with ultrasound transmission gel (Fany Sonic®, Taiheung Medical Co., Korea) for contact. Standoff pad was not used (Fig. 1). Additionally, the anterior eye chamber was scanned in the transverse plane with transducer placed at temporal area. With a routine sonography, biometric measurements of the axial length (WL) of lens, length of pecten oculi (LP), depth of the anterior chamber (AC), the axial globe length (LB), and the vitreous chamber (VC) were performed. The images of the transversal scan at 6 hr were used to determine LP. All measurements were taken in dorsal plane at level of lens maximal diameter. During this, ocular surfaces were aligned along with an eye’s central optical axis symmetrically. Measurement were stored on the computer and documented for results.
Fig. 1.
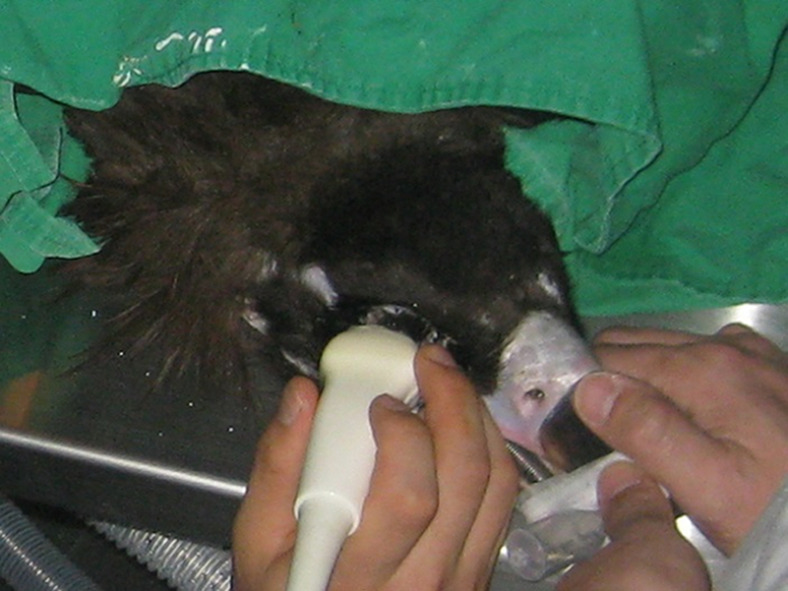
Ultrasonographic examination of the right eye of a cinereous vulture (Aegypius monachus). Standoff pad did not used.
Computed Tomographic examinations and biometric measurement
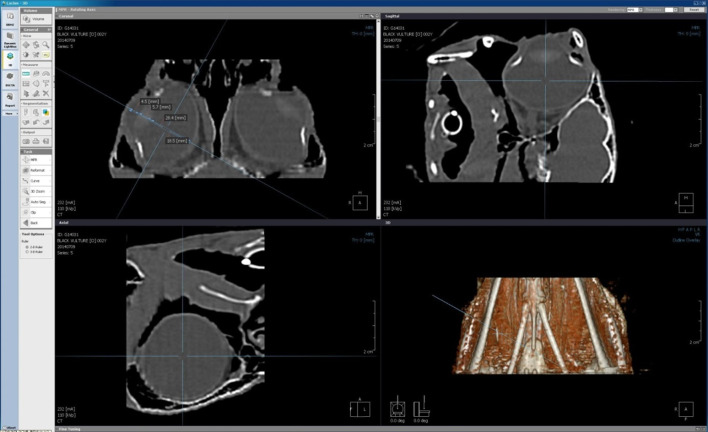
All the CT examinations were measured in sternal recumbency having general anesthesia with isoflurane using two channel multi-detector row CT (MDCT) scanner (Somatom Emotion, Siemens Medical System, Erlangen, Germany) (Fig. 2). An orbital area was scanned in axial planes with 1 mm slice thickness, 110 kVp, evaluated in soft tissue, and 200 mAs (200 window width, 40 window level). In CT, the whole examination took about 5 to 10 min. The data was stored in hard drive of computer. All data were analyzed after CT examination of the birds. Biometric measurements of anterior chambers, the vitreous chamber, the axial length of lens, length of axial globe of both eyes were performed. Biometry was performed in an appropriate selected cross section image that reconstructed from the original axial CT scan with clinically used PC-based software called multiplanar reformatting (MPR) program (LUCION®, Infinitt Technology, Seoul, Korea) (Fig. 3). The CT images were compared with the sonographic appearance.
Fig. 2.
Computed tomographic examination of the eyes of a cinereous vulture (Aegypius monachus) under general anesthesia with isoflurane.
Fig. 3.
Multiplanar reformatting images of the eyes and volume rendering figure of the CT scanned area are reconstructed from the original axial CT scan images. A proper cross section image was selected when along the optical axis on scans on which the nucleus lentis appeared in its maximal size.
Radiographic examination
Radiographs of the eyes including heads were taken in right lateral and dorsoventral positions. If necessary, oblique views under general anesthesia with isoflurane were also performed.
Ocular volumetric measurement in CT
Images of two channel multi-detector row CT (MDCT) scanner (Somatom Emotion, Siemens Medical System) were converted to dorsal and sagittal plane with clinically used PC-based software i.e., multiplanar reformatting (MPR) program (LUCION®, Infinitt Technology). The imaging parameters were 110 kVp, 200 mAs, 1 mm section thickness, and continuous slices. The anterior border of globe of the eye was determined as first CT slice with visible maxillary sinus and posterior border as an apex of the orbit. The areas in each axial CT slice were measured with measuring tool mentioned earlier as LUCION®. With slice thickness of the CT scan, known volumes could be calculated automatically.
Statistical analyses
The IBM SPSS Statistics 21® software program (IBM Corp., Armonk, NY, USA) has been used for statistical analysis of data. Kolmogorov-Smirnov test was implemented to investigate Gaussian distribution of qualitative variables. Obtained values were expressed as mean ± SD. Correlation of measurements between OS and OD eyes along with correlation between CT and US measurements of the various ocular structures were calculated by using the Pearson’s correlation coefficient. Difference between right and left eyes as well as differences between US and CT measurements of LB, WL, LP, AC, VC were investigated by paired t-test, when these are parametric, and by Wilcoxon signed rank test, for the nonparametric values. P-values <0.05 were considered significant statistically.
RESULTS
Ultrasonographic appearance of eye
According to ultrasonographic examination, all 26 eyes and their structures had similar appearance. The corneal echo was curvilinear and super reflective, while anterior chamber of the eye was anechoic. The lens was appeared as double hyperechoic curved lines presenting an anterior and posterior echogenicity in every side of lens. The ossicles of scleral ring were hyperechoic and appeared on medial and lateral wall of the eye. These ossicles caused the distal shadowing (Fig. 4), that cause hindrance in measuring the transverse diameter of bulbus. Pecten was moderately echogenic structure, while extending from optic nerve to vitreous chamber was anechoic (Fig. 5). The posterior eye wall including choroid, retina, and sclera were slightly curvilinear but super reflective echo. Posterior wall of globe could not be differentiated from retrobulbar tissue clearly. The optic nerve was appeared as hypoechoic structure, very close to the pecten insertion, within hyperechoic retrobulbar tissue.
Fig. 4.
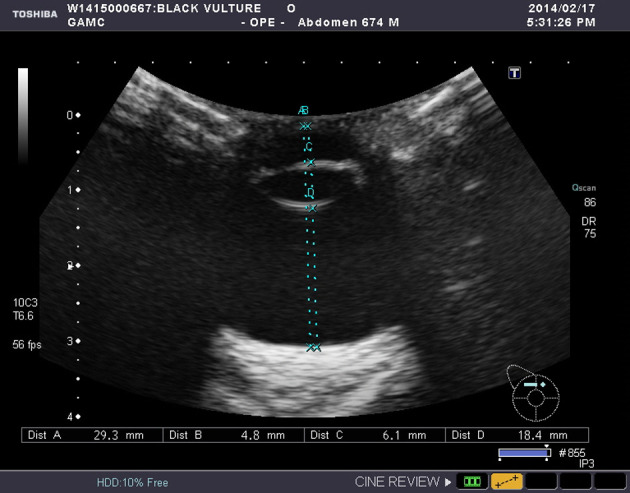
Ultrasonographic appearance and biometric measurements of the left eye of a cinereous vulture taken on dorsal plane.
Fig. 5.

Ultrasonographic appearance of the long pecten in a cinereous vulture. The anterior chamber was deformed because of the slightly angled beam to visualize the pectin.
Computed tomographic appearance of eye
The cornea and periocular tissues appeared as hyper attenuating. Anterior eye chamber and vitreous chamber was appeared as soft tissue opacity and homogeneous. In CT, it was impossible to differentiate cornea from the anterior eye chamber in cinereous vulture. The lens was consisted to hyper attenuating capsule and hyper attenuating nucleus (Fig. 6). A slightly less hyper attenuating capsule or peripheral zone was seen that was interpreted as cortex of lens. Two hyper attenuating slim profiles framing bulbus presented the scleral ring. No imaging artifacts were detected that could be caused by these bony structures. As compared to US, LP could not be measured because of no differentiation. In US and more specifically CT, bulbus was appeared in globular shape and length of the axial lens was one fourth to entire axial bulbus depth.
Fig. 6.

Normal computed tomography appearance of the head of a cinereous vulture in axial plane. Left side, sagittal section image of topogram. Right side, image at the level of the nucleus lentis: Note the typical globular shape of the bulbus, the lens, the nucleus lentis, and the bony scleral ossicle.
Biometric measurements of eye
The US and CT measurements can be found in Table 1, showing comparison results between both eyes and between the 2 measurements. Most of paired data did not show any significant difference between both eyes and the CT and US measurements. However, there were significant differences (P<0.05) between CT and US measurement of VC, AC, and WL in right eyes, among WL, VC, LB, LP, and AC dimensions. In addition, there was also a significant difference (P<0.05) in measurement of VC in the CT for between both eyes.
Table 1. Biometric results in ultrasonography (US) and CT in cinereous vultures (Aegypius monachus) .
| Area | Device | N | ODǂ (mm) | OS† (mm) | Paired t (P-value) b |
|---|---|---|---|---|---|
| AC | US | 13 | 4.52 ± 0.34 (3.8–5.2) | 4.43 ± 0.32 (3.9–5.1) | 1.951 (0.075) |
| CT | 13 | 4.33 ± 0.23 (3.7–4.6) | 4.37 ± 0.18 (3.9–4.6) | −1.328 (0.209) | |
| Paired t (P-value) a | 3.850 (0.002) ** | 1.120 (0.285) | |||
| WL | US | 13 | 5.83 ± 0.15 (5.6–6.2) | 5.84 ± 0.21 (5.6–6.3) | −0.291 (0.776) |
| CT | 13 | 5.75 ± 0.11 (5.6–6.0) | 5.74 ± 0.08 (5.6–5.9) | 0.617 (0.549) | |
| Paired t (P-value) a | 3.333 (0.006) ** | 2.142 (0.053) | |||
| VC | US | 13 | 18.21 ± 0.53 (17.4–18.9) | 18.22 ± 0.47 (17.5–18.8) | −0.313 (0.760) |
| CT | 13 | 18.35 ± 0.52 (17.7−19.2) | 18.25 ± 0.51 (17.5−19.0) | 3.338 (0.006) ** | |
| Paired t (P-value) a | −2.992 (0.011) * | −0.519 (0.613) | |||
| LB | US | 13 | 28.61 ± 0.78 (27.4−29.7) | 28.57 ± 0.81 (27.3−29.9) | 0.357 (0.727) |
| CT | 13 | 28.55 ± 0.62 (27.7−29.5) | 28.50 ± 0.72 (27.0−29.7) | 0.689 (0.504) | |
| Paired t (P-value) a | 0.746 (0.470) | 0.574 (0.576) | |||
| LP | US | 13 | 11.52 ± 0.75 (10.6−13.3) | 11.47 ± 0.81 (10.5−13.5) | 0.321 (0.754) |
| CT | 13 | - | - | - | |
Mean ± standard deviation (min–max). ǂ OD, right eye; † OS, left eye. N, number; SD, standard deviation; Max, highest measurement; Min, lowest measurement; WL, axial length of the lens; AC, depth of anterior chamber; VC, depth of vitreous chamber; LB, axial length of the globe; LP, length of the pecten. aPaired t-test for comparing US and CT. bPaired t-test for comparing OD and OS. *indicates P<0.05. **indicates P<0.01.
Tables 2 and 3 show the correlations between the left and right eye and the measurements took place in dorsal planes in CT and US. The depth and width of the anterior chamber, vitreous chamber, and lens as well as the axial length of bulbus and pecten had strong correlation between both eyes and between the measurements of the US and CT. Ocular volumetric measurement values were shown in Table 4.
Table 2. Correlations in left (OS) and right (OD) eye biometric measurement in ultrasonography (US) and CT of the whole examined cinereous vultures (Aegypius monachus) (n=13).
| AC | WL | VC | LB | LP | |
|---|---|---|---|---|---|
| US | |||||
| Pearson’s correlation coefficient | 0.866 | 0.924 | 0.943 | 0.882 | 0.780 |
| P-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 |
| CT | |||||
| Pearson’s correlation coefficient | 0.891 | 0.608 | 0.979 | 0.923 | - |
| P-value | 0.000 | 0.028 | 0.000 | 0.000 | - |
A Pearson’s correlation coefficient (r) of more than 0.8 means very strong correlation, 0.60<r<0.79 means strong correlation, and 0.40<r<0.59 means moderate correlation. A P-value <0.05 is considered a significant correlation. x, length of the pecten in CT not visible and therefore not measured; WL, axial length of the lens; AC, depth of anterior chamber; VC, depth of vitreous chamber; LB, axial length of the globe; LP, length of the pecten; US, ultrasonography; CT, computed tomography.
Table 3. Correlations of the biometric measurements in different imaging modalities (ultrasonography and CT) in the left (OS) and right (OD) eye in the whole examined cinereous vultures (Aegypius monachus) (n=13).
| AC | WL | VC | LB | LP | |
|---|---|---|---|---|---|
| OD* | |||||
| Pearson’s correlation coefficient | 0.869 | 0.834 | 0.944 | 0.956 | - |
| P-value | 0.000 | 0.000 | 0.000 | 0.000 | - |
| OS† | |||||
| Pearson’s correlation coefficient | 0.822 | 0.713 | 0.907 | 0.846 | - |
| P-value | 0.001 | 0.006 | 0.000 | 0.000 | - |
* OD, right eye; † OS, left eye. A Pearson’s correlation coefficient (r) of more than 0.8 means very strong correlation, 0.60<r<0.79 means strong correlation, and 0.40<r<0.59 means moderate correlation. A P-value <0.05 is considered a significant correlation. x, length of the pecten in CT not visible and therefore not measured; WL, axial length of the lens; AC, depth of anterior chamber; VC, depth of vitreous chamber; LB, axial length of the globe; LP, length of the pecten; US, ultrasonography; CT, computed tomography.
Table 4. Volume of the globe in cinereous vulture (Aegypius monachus) (n=10).
| Globe Volume (n=10 randomized eyes) | |||
|---|---|---|---|
| Mean (cc) | SD (cc) | Min (cc) | Max (cc) |
| 13.48 | 0.38 | 13.03 | 14.12 |
Multiplanar reformatting (MPR) program (LUCION®, Infinitt Technology, Seoul, Korea) were used for volumetric calculation.
Radiographic appearance of head and eyes
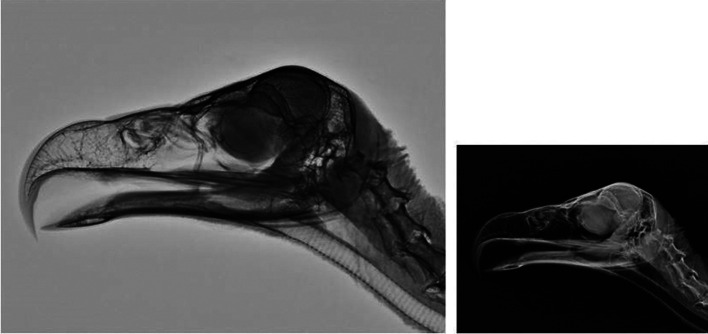
Right lateral (Fig. 7) and ventrodorsal (Fig. 8) skull radiographs showed cranium of cinereous vultures contained many connects to the sinuses. An osseous scleral ring is more visible in radiographs but interlobular septum between both eyes is hardly visible.
Fig. 7.
Lateral xeroradiograph and radiograph of the head of a normal cinereous vulture (Aegypius monachus).
Fig. 8.
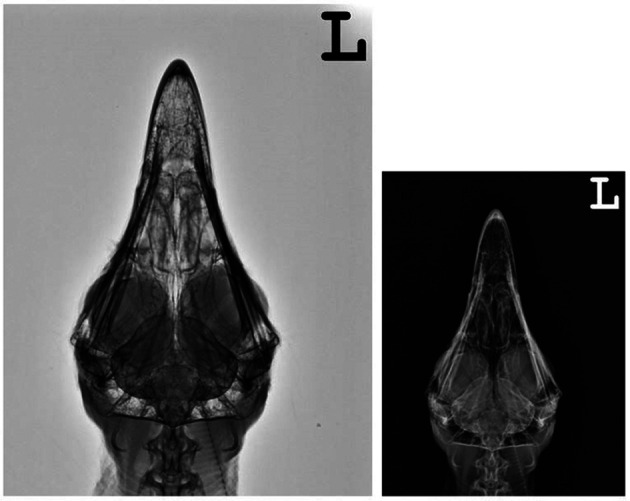
Ventrodorsal xeroradiograph and radiograph of the head of a normal cinereous vulture (Aegypius monachus).
DISCUSSION
In veterinary medicine, first biometric study was evaluated canine eyes by A-mode US [22] and published in 1982. So, ocular biometry became an interesting object in studies of several species [25]. It has been described in several species e.g., cat, dog, goat, rabbit, cattle, horse, guinea pig, capybear, rhesus monkey, ferret, one humped camel, striped owl, elephant, and human. So far, in few studies, there are different anatomic configurations regarding shape and size of various avians eyes [4, 7, 25].
Being an endangered species, cinereous vultures (Aegypius monachus) were examined in this study. Especially in the winter, they have been presented often in wildlife rescue center due to emaciation, poisoning, skeletal fractures, and poor nutritional conditions [10, 18]. There are most common diseases being found in cinereous vultures i.e., retinal degeneration, cataract, corneal lesions, glaucoma, traumatic uveitis, retinal dysplasia, and ciliary body malformations [1, 3, 7]. Because of these debilities, these suffer from weakened eyesight even blindness, sometimes it can lead to the death.
It is difficult and dangerous to capture and restraint the raptors as compare to other animals because they get more stressed during their examination. As described in previous study of striped owls, ocular medical imaging examination was assessed without need of chemical sedation [25]. While, in another study, ocular ultrasound was performed in a colony of Screech owls was performed with help of the general anesthesia using isoflurane [7].
B-mode ultrasonography is an inexpensive, precise, and easy method for eye measurements, performed in various studies of ocular biometry [4, 7, 8, 16, 20, 21, 25, 26]. In certain studies, there was strong correlation between B-mode US and CT ocular measures. If eye surfaces are not aligned symmetrically along eye’s central optic axis, there is chance of inaccuracy of the measurement by the ultrasound [2, 6]. In this study, it was performed corneal contact technique that provides sufficient visualization of the entire globus along with cornea. While, comparing to immersion technique, the superior anatomic definition of posterior pole of the eye was obvious in previous study [5].
The ocular morphology of cinereous vulture (Aegypius monachus) was very similar to other raptors. As described in a previous study, usefulness of US is limited to evaluate optic nerve, fibrous tunics, and orbit of eyes due to artifacts and distal shadowing by scleral ossicles [5]. Similar observations were stated in different studies along with this study during examination in US [4, 7, 25]. This limitation can be resolved by using CT along with US.
In the present study, CT allowed proper visualization of lens, anterior eye chamber, poster eye call, cornea, scleral ring, ocular nerve, retrobulbar space, and whole skull except for examination of pecten as found in previous studies [4, 17]. It shows that US is a useful tool having ability to detect pecten anomalies even in opacification of eye’s anterior segment.
Other studies about various avian species revealed that dorsal plane images obtained from CT presented a satisfactory overview of both eyes at the same time [4, 25]. On the other hand, bulbi were distorted in sagittal plane but it was barely possible to find central axis. In this study, an orbital area was scanned in axial planes with 1 mm slice thickness. Biometry was performed in MPR slice in which nucleus lentis appeared in its maximal size along the optical axis. With consideration of anatomical differences of head and eye position of cinereous vulture (Aegypius monachus) with other avians, it showed promising results.
The axial LB means were 28.57 mm and 28.61 mm in US for left and right eye consecutively. These values are higher than other studies in small raptors like striped owl, screech owls or tawny owls having 23.76 mm for left eye, 24.25 mm for right eye, or 24.70 ± 0.82 mm respectively [4, 7, 25]. In other species, authors found no significant difference (P<0.05) between right and left eye’s axial length and other intraocular structures dimensions in CT and US [4, 7, 25]. Most of the paired data of biometry did not show significantly difference among US and CT. In the current study, most of biometric results showed strong correlation between left and right eyes in CT and US. But, some paired values in CT showed significant differences (P<0.05). The selection of cross section image which reconstructed from original axial CT scan with MPR program is supposed to affect the value of measurement.
The pecten of variable shapes, extending from optic nerve to vitreous chamber, is highly pigmented, nonsensory structure. Length of pecten in US ranged from 11.47 mm to 13.50 mm in cinereous vulture (Aegypius monachus) that is much longer to values of pecten found in striped owl (4.49 mm to 6.73 mm) and tawny owl (4.40 mm to 6.60 mm) [4, 25]. In US, the posterior wall was seemed to be under estimated because of hyperechoic retrobulbar space and weak differentiation of hyperechoic presentation of wall. However, it was not possible to differentiate the previous wall efficiently in common kestrels and many of barn owls. This was distinguished more easily in CT due to adjustable window levelling. The retrobulbar fat was appeared to be hypo attenuating compared to posterior wall.
In this study, all of examined birds were normal but ocular diseases were common findings, however, microphthalmia was most common disease among 16 raptors in a previous study [1]. Informations and measurements for both eyes for every species were highly valuable for diagnosing the affected eyes.
It can be concluded that variable ocular medical imaging techniques of these raptors provides useful data of biometry and morphology. Especially, when ophthalmoscopic examinations are not available because of opacity in anterior segment of eyes, CT or US is essential examination of definitive diagnosis in veterinary ophthalmology. These results showed good correlation between CT and US. The US is a safe procedure and easy to be implemented in raptor’s eyes. Even though, this species has same ultrasonographic aspects to other species but it is essential to carry out a correct record of eye examination and biometric values for cinereous vulture (Aegypius monachus).
Although CT requires anesthesia, but it provides specific measurements of bony scleral ring along with proper visualization of size and shape of intraocular and extraocular structures. It may be helpful in situations of bone affection and unclear conditions in cinereous vulture (Aegypius monachus). As the advantages and shortcomings of the US and CT are supplementary to each other, it would be best to perform ocular examinations with both the US and the CT. However, our study shows that these two diagnostic methods show good correlation in results. Hence, if one is highly trained in both examination methods and could come up with an accurate diagnosis, one can reach an accurate ocular examination and biometric measurements with the use of just one of these methods.
CONFLICT OF INTEREST
The authors declare no conflicts of interest and there was no financial support of manufacturer associated with the products used in this study.
Acknowledgments
This study was supported by the BK21 PLUS Program for Creative Veterinary Science Research and the Research Institute for Veterinary Science (RIVS) of Seoul National University, Korea. The study was also partially supported by the Research Institute for Veterinary Science, Seoul National University.
References
- 1.Buyukmihci N. C., Murphy C. J., Schulz T.1988. Developmental ocular disease of raptors. J. Wildl. Dis. 24: 207–213. doi: 10.7589/0090-3558-24.2.207 [DOI] [PubMed] [Google Scholar]
- 2.Cottrill N. B., Banks W. J., Pechman R. D.1989. Ultrasonographic and biometric evaluation of the eye and orbit of dogs. Am. J. Vet. Res. 50: 898–903. [PubMed] [Google Scholar]
- 3.Cousquer G.2005. Ophthalmological findings in free-living tawny owls (Strix aluco) examined at a wildlife veterinary hospital. Vet. Rec. 156: 734–739. doi: 10.1136/vr.156.23.734 [DOI] [PubMed] [Google Scholar]
- 4.Gumpenberger M., Kolm G.2006. Ultrasonographic and computed tomographic examinations of the avian eye: physiologic appearance, pathologic findings, and comparative biometric measurement. Vet. Radiol. Ultrasound 47: 492–502. doi: 10.1111/j.1740-8261.2006.00168.x [DOI] [PubMed] [Google Scholar]
- 5.Hager D. A., Dziezyc J., Millichamp N. J.1987. Two-dimensional realtime ocular ultrasonography in the dog. Vet. Radiol. Ultrasound 28: 60–65. doi: 10.1111/j.1740-8261.1987.tb01726.x [DOI] [Google Scholar]
- 6.Hamidzada W. A., Osuobeni E. P.1999. Agreement between A-mode and B-mode ultrasonography in the measurement of ocular distances. Vet. Radiol. Ultrasound 40: 502–507. doi: 10.1111/j.1740-8261.1999.tb00382.x [DOI] [PubMed] [Google Scholar]
- 7.Harris M. C., Schorling J. J., Herring I. P., Elvinger F., Bright P. R., Pickett J. P.2008. Ophthalmic examination findings in a colony of Screech owls (Megascops asio). Vet. Ophthalmol. 11: 186–192. doi: 10.1111/j.1463-5224.2008.00618.x [DOI] [PubMed] [Google Scholar]
- 8.Hernández-Guerra A. M., Rodilla V., López-Murcia M. M.2007. Ocular biometry in the adult anesthetized ferret (Mustela putorius furo). Vet. Ophthalmol. 10: 50–52. doi: 10.1111/j.1463-5224.2007.00500.x [DOI] [PubMed] [Google Scholar]
- 9.Jenkins J. R.1991. Use of computed tomography (CT) in pet bird practice. Proc. Annu. Conf. Assoc. Avian Vet. 276–279.
- 10.Jung K., Kim Y., Lee H., Kim J. T.2009. Aspergillus fumigatus infection in two wild Eurasian black vultures (Aegypius monachus Linnaeus) with carbofuran insecticide poisoning: a case report. Vet. J. 179: 307–312. doi: 10.1016/j.tvjl.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 11.Kern T. J.1997. Disorders of the special sense. pp. 563–589. Avian Med. Surg., 1st ed., W.B. Saunders, Philadelphia. [Google Scholar]
- 12.Kostka V., Krautwald-Junghanns M. E., Tellhelm B.1991. Radiology of the avian skull. Zentralbl. Veterinärmed. A 38: 175–186. doi: 10.1111/j.1439-0442.1991.tb00999.x [DOI] [PubMed] [Google Scholar]
- 13.Krautwald-Junghanns M. E., Kostka V., Dorsch B.1998. Comparative studies of the diagnostic value of conventional radiography and computed tomography in evaluating the heads of psittacine and raptorial birds. J. Avian Med. Surg. 12: 149–157. [Google Scholar]
- 14.Lee S. D.2013. Distribution and abundance of wintering raptors in the Korean peninsula. J. Ecol. Environ. 36: 211–216. doi: 10.5141/ecoenv.2013.211 [DOI] [Google Scholar]
- 15.Lindley D. M., Hathcock J. T., Miller W. W., DiPinto M. N.1988. Fractured scleral ossicles in a red tail hawk. Vet. Radiol. 29: 209–212. doi: 10.1111/j.1740-8261.1988.tb01501.x [DOI] [Google Scholar]
- 16.Montiani-Ferreira F., Truppel J., Tramontin M. H., Vilani R. G., Lange R. R.2008. The capybara eye: clinical tests, anatomic and biometric features. Vet. Ophthalmol. 11: 386–394. doi: 10.1111/j.1463-5224.2008.00663.x [DOI] [PubMed] [Google Scholar]
- 17.Morgan R. V., Donnell R. L., Daniel G. B.1994. Magnetic resonance imaging of the normal eye and orbit of a screech owl (Otus asio). Vet. Radiol. Ultrasound 35: 362–367. doi: 10.1111/j.1740-8261.1994.tb02055.x [DOI] [Google Scholar]
- 18.Nam D. H., Lee D. P.2009. Abnormal lead exposure in globally threatened Cinereous vultures (Aegypius monachus) wintering in South Korea. Ecotoxicology 18: 225–229. doi: 10.1007/s10646-008-0275-0 [DOI] [PubMed] [Google Scholar]
- 19.Paul M., Jr, Koblik P. D., Stein G., Penninck D. G.1990. Psittacine skull radiography. Anatomy, radiographic technic, and patient application. Vet. Radiol. 31: 218–224. doi: 10.1111/j.1740-8261.1990.tb01818.x [DOI] [Google Scholar]
- 20.Potter T. J., Hallowell G. D., Bowen I. M.2008. Ultrasonographic anatomy of the bovine eye. Vet. Radiol. Ultrasound 49: 172–175. doi: 10.1111/j.1740-8261.2008.00345.x [DOI] [PubMed] [Google Scholar]
- 21.Rogers M., Cartee R. E., Miller W.1986. Evaluation of the extirpated equine eye using B-mode ultrasonography. Vet. Radiol. Ultrasound 27: 24–29. doi: 10.1111/j.1740-8261.1986.tb00616.x [DOI] [Google Scholar]
- 22.Schiffer S. P., Rantanen N. W., Leary G. A., Bryan G. M.1982. Biometric study of the canine eye, using A-mode ultrasonography. Am. J. Vet. Res. 43: 826–830. [PubMed] [Google Scholar]
- 23.Smith B. J., Smith S. A., Flammer K., Spaulding K. A., Smallwood J. E.1990. The normal xeroradiographic and radiographic anatomy of the orangewinged amazon parrot (Amazona amazonica amazonica). Vet. Radiol. 31: 114–124. doi: 10.1111/j.1740-8261.1990.tb01849.x [DOI] [Google Scholar]
- 24.Smith S. A., Smith B. J.1991. Normal xeroradiographic and radiographic anatomy of the great horned owl (Bubo virginianus), with special reference to the barn owl (Tyto alba). Vet. Radiol. 32: 6–16. doi: 10.1111/j.1740-8261.1991.tb00075.x [DOI] [Google Scholar]
- 25.Squarzoni R., Perlmann E., Antunes A., Milanelo L., de Moraes Barros P. S.2010. Ultrasonographic aspects and biometry of Striped owl’s eyes (Rhinoptynx clamator). Vet. Ophthalmol. 13Suppl: 86–90. doi: 10.1111/j.1463-5224.2010.00819.x [DOI] [PubMed] [Google Scholar]
- 26.Williams D. L.2004. Lens morphometry determined by B-mode ultrasonography of the normal and cataractous canine lens. Vet. Ophthalmol. 7: 91–95. doi: 10.1111/j.1463-5224.2004.04005.x [DOI] [PubMed] [Google Scholar]