Abstract
A 13-year-old, female, mixed-breed dog with a huge cranial mediastinal mass underwent radiotherapy (RT). On the following day, the dog presented with lethargy and anorexia. Hematological examination revealed elevated levels of blood urea nitrogen, creatinine, inorganic phosphorus, potassium, lactate dehydrogenase, creatine phosphokinase and aspartate aminotransferase, decreased calcium level, and metabolic acidosis. Urine output markedly decreased. The patient recovered with fluid therapy and diuretic therapy; however, died suddenly from an unknown cause 11 days after RT completion. Histopathological examination after necropsy showed thymoma in the cranial mediastinum and extensive tubular necrosis of both kidneys which may be due to RT-induced tumor lysis syndrome (TLS). This report suggests that the risk of TLS should be evaluated in dogs with thymoma who undergo RT.
Keywords: dog, radiotherapy, thymoma, tumor lysis syndrome (TLS)
Tumor lysis syndrome (TLS) is an oncologic emergency that results from rapid cell death caused by chemotherapy or radiotherapy (RT) [10]. In humans, TLS is caused by the intracellular contents (nucleic acid, uric acid, potassium, and phosphorus) released by lysed tumor cells into the blood, resulting in hyperuricemia, hyperphosphatemia, hyperkalemia, lactic acidosis, and hypocalcemia [2, 8]. The rapid occurrence of these phenomena results in the suppression of the circulatory system and acute kidney injury (AKI), which is attributed to acute tubular necrosis. TLS in humans was classified into laboratory TLS (LTLS) and clinical TLS (CTLS). LTLS based on abnormal values of two or more of the following: uric acid ≥8 mg/dl, potassium ≥6.0 mmol/l, inorganic phosphorus ≥4.5 mg/dl, and calcium ≤7 mg/dl, or a change of ˃25% above the reference value within 3 days prior to and up to 7 days after the initiation of cytotoxic therapy. CTLS based on these abnormal values plus renal insufficiency with serum creatinine (CRE) level of at least 1.5 times the upper limit of normal, arrhythmia or sudden death, or seizures. [2, 8]. In veterinary medicine, only a small number of reports on TLS in dogs have been published, limited to hematological malignancies treated with chemotherapy and/or RT, and angiosarcoma treated with surgical resection [1, 6, 7, 11]. Thus, it mainly has been reported in tumors of lymphoid origin that were predisposed to be induced apoptosis by cytotoxic treatments. However, there were no reports of TLS in thymoma, which might have a large number of lymphocytes, and the risk of TLS for RT for thymoma is not widely recognized. This report describes a first case of suspected TLS after RT on an inoperable thymoma in a dog.
A 13-year-old spayed female mixed-breed dog weighing 17.3 kg was referred to the Veterinary Medical Center of Osaka Prefecture University with a 2-week history of cough and anorexia for an evaluation for pleural effusion and mediastinal mass (1st day).
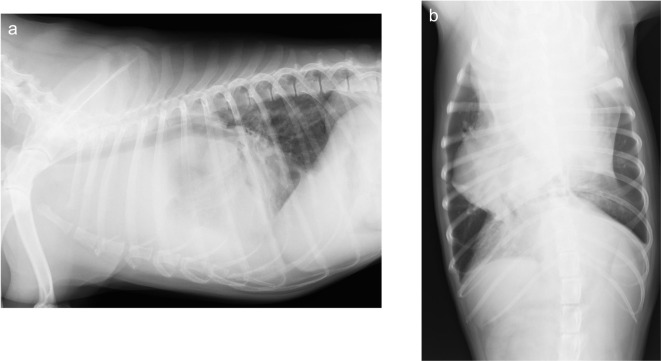
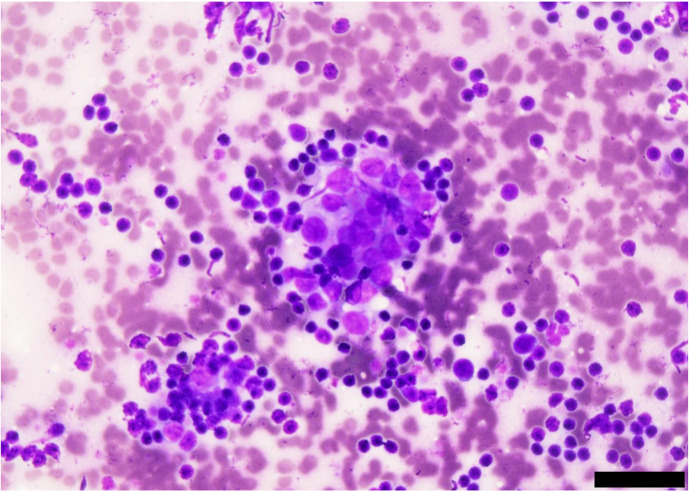
The patient had a weight of 17.3 kg, body temperature of 38.6°C, heart rate of 120 beats per minute, and respiratory rate of 48 breaths per min. Hematological examination revealed neutrophilia (26,900/µl) and thrombocytopenia (89,000/µl). Biochemical tests showed elevated alanine aminotransferase (ALT; 122 IU/l [reference range; 20–99 IU/l]), alkaline phosphatase (ALP; 376 IU/l [reference range; 49–298 IU/l]), C-reactive protein (CRP; 2.32 mg/dl [reference range; 0–1.0 mg/dl]), and calcium (12.3 mg/dl [reference range; 8.9–11.4 mg/dl]). X-ray examination revealed an opaque soft tissue mass (12 × 12 cm) in the cranial mediastinum (Fig. 1), and ultrasound-guided fine needle aspiration was performed for the mass. Cytology revealed a large number of lymphoid cells, mostly small lymphocytes with little atypia, and scattered thymic epithelial-like cells (Fig. 2). Based on the results, the cranial mediastinal mass was presumptively diagnosed as a thymoma, then prednisolone (1 mg/kg/day per oral) was administered for regression tumor by apoptosis of lymphocytes for 28 days.
Fig. 1.
Radiographic images identified a soft tissue mass (12 × 12 cm) in the cranial mediastinum. a. Right lateral radiographic image b. Ventrodorsal radiographic image
Fig. 2.
Cytological examination with Diff-Quik stain of the mass revealed small lymphoid cells and aggregated thymic epithelial-like cells (scale bar: 40 µm).
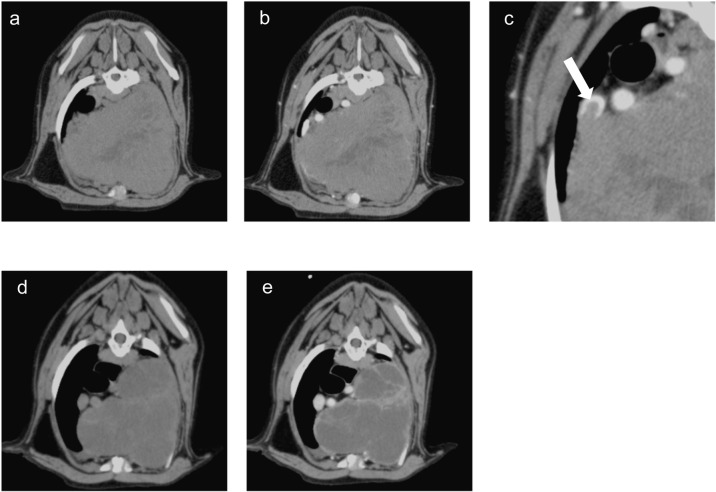
On the 29th day, X-ray examination showed few changes of the tumor volume, and there were no significant changes in physical examination and blood chemistry findings compared to the 1st day. Computed tomography (CT) examination revealed pleural effusion, and a 11 × 11.4 × 18 cm mass in cranila mediastinum, which had a pale contrast enhancement (Fig. 3a–c). The heart and aortic arch were displaced to the right by the mass. In addition, there was a tumor invasion into the cranial vena cava (Fig. 3c). Considering the tumor size and invasion into the vena cava, surgical treatment alone was anticipated high morbidity. Therefore, RT was started on the same day to reduce the tumor size prior to surgery. RT was performed with a 4-MeV linear accelerator (Canon Medical Systems, Ohtawara, Japan). RT planning was performed based on CT (Activion16; Canon Medical Systems) scans in prone position with a vacuum‐mattress immobilization device (Esform, Matsumoto, Japan). Treatment plans were created as three-dimensional conformal RT (3D-CRT) by treatment planning system (XiO, Elekta Japan, Tokyo, Japan). Gross tumor volume (GTV) was defined by the contrast‐enhancing area on CT images. There was no expansion margin of GTV for clinical target volume. Planning target volume (PTV) was defined by a margin of 0.5 cm around the CTV. The isocenter was determined by the PTV locations. The patient was administered 6 Gy per fraction, delivered to a total dose of 30 Gy with one fraction per week for a total of five fractions. The radiation dose prescription point was set at the isocenter. The planned dose and volume of the organs at risk were as follows: mean lung dose was 10.6 Gy, V20=24.8% for the lungs, mean heart dose was 18.5 Gy and V20=40.3% for the heart. CT examination was performed prior to treatment to confirm the location and size of the tumor. The RT planning was modified if more than 10% reduction was observed in the longest diameter of the tumor.
Fig. 3.
Transverse computed tomography (CT) images of the thorax on day 29 (before radiotherapy [RT]; a, b, and c) and 43 (last day of RT; d and e). a and d. plain CT axial view. b, c, and e. CT axial view in the portal venous phase. There was a mass with pale contrast enhancement in the cranial mediastinum (a and b), and tumor invasion into the vena cava indicated by white arrow (c). The size of the mass was reduced by half on the last day of RT, and the mass had no contrast enhancement (d and e).
On the next day (1 day post-RT), the patient visited our center and demonstrated loss of appetite and lethargy. Blood tests showed elevated levels of inorganic phosphorus, calcium, sodium, aspartate transaminase (AST), lactate dehydrogenase (LDH), and creatine phosphokinase (CPK) (Table 1). As TLS induced by RT was suspected based on these findings, RT was interrupted, and the patient was hospitalized. The patient was intravenously administered with dopamine (3 µg/kg/min), furosemide (1 mg/kg, bid), and saline (3 ml/kg/hr), and urine output was monitored.
Table 1. The results of the blood tests during the treatment period.
| Reference range | Day | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 29 | 30 | 31 | 32 | 33 | 36 | 43 | ||
| WBC (/µl) | 31,250 | 21,520 | 29,540 | 26,480 | 29,010 | 33,790 | 35,930 | 24,900 | |
| Stab (/µl) | 0–300 | 0 | 0 | 0 | 265 | 0 | 338 | 359 | 0 |
| Seg (/µl) | 3,000–11,500 | 26,875 | 17,216 | 28,063 | 24,097 | 26,689 | 32,101 | 32,337 | 22,161 |
| Lym (/µl) | 1,000–4,800 | 3,750 | 2,798 | 886 | 1,324 | 1,160 | 676 | 1,797 | 996 |
| Mon (/µl) | 150–1,350 | 625 | 646 | 591 | 794 | 1,160 | 676 | 1,437 | 996 |
| Eos (/µl) | Rare | 0 | 861 | 0 | 0 | 0 | 0 | 0 | 747 |
| PCV (%) | 39 | 47 | 51.7 | 52.4 | 48.2 | 43.2 | 35.6 | 38.7 | |
| PLT (×104/µl) | 20–50 | 8.9 | 20.4 | 15.2 | 9.5 | 9.4 | 9.8 | 5.3 | 13.1 |
| TP (g/dl) | 5.5–7.7 | 6.1 | 6.3 | 5.7 | 5.6 | 4.8 | 4.9 | 5.1 | 6.2 |
| Alb (g/dl) | 2.5–3.8 | 3.8 | 3.8 | 3.5 | 3.3 | 2.9 | 3 | 3.2 | 4.1 |
| ALP (U/l) | 49–298 | 376 | 725 | 998 | 1,288 | 1,220 | 1,075 | 2,011 | 3,304 |
| ALT (U/l) | 20–99 | 122 | 62 | 61 | 34 | 10 | 4 | 6 | 93 |
| AST (U/l) | 18–65 | 39 | 41 | 114 | 220 | 237 | 148 | 110 | 50 |
| T-bil (mg/dl) | 0–0.3 | 0.19 | 0.16 | 0.35 | 0.2 | 0.22 | 0.22 | 0.34 | 0.22 |
| BUN (mg/dl) | 6–31 | 14 | 21 | 44 | 90 | 141 | 132 | 42 | 48 |
| CRE (mg/dl) | 0.4–1.6 | 0.8 | 0.8 | 1.3 | 3 | 5.2 | 4.3 | 1.3 | 1.6 |
| Na (mEq/l) | 141–151 | 148.9 | 149.5 | 134.7 | 128.7 | 137.5 | 148.2 | 144.3 | 148.6 |
| K (mEq/l) | 3.5–5.4 | 4.56 | 4.14 | 4.93 | 7.56 | 7.22 | 3.82 | 4.15 | 4.49 |
| Cl (mEq/l) | 107–121 | 112.1 | 109.8 | 97.7 | 93.2 | 101.7 | 108.6 | 110.3 | 103.3 |
| Ca (mEq/l) | 8.9–11.4 | 12.3 | 10 | 8.4 | 8.2 | 7.4 | 7.1 | 7.7 | 9.4 |
| P (mg/dl) | 2–5.3 | 3 | 2.9 | 8 | 16.4 | 21.9 | 13.3 | 2.6 | 4.9 |
| CPK (U/l) | 50–170 | 80 | 131 | 1,021 | 3,655 | 6,508 | 2,257 | 695 | 109 |
| LDH (mg/dl) | 22–184 | 65 | 77 | 338 | 489 | 356 | 143 | 87 | 38 |
| CRP (mg/dl) | 0–1.0 | 2.32 | 2.89 | 3.06 | 4.67 | 2.99 | 2.62 | 1.58 | 0.7 |
WBC, white blood cell; PCV, packed cell volume; PLT, platelet; TP, total protein; Alb, albmin; ALP, alkaline phosphatase; ALT, alanin aminotranspherase; AST, aspartate transaminase; T-bil, total bilirubin; BUN, blood urea nitrogen; CRE, creatinine; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; CRP, C-reactive protein.
The urine output decreased (0.37 ml/kg/hr), and blood urine nitrogen (BUN), CRE, and potassium levels increased on the 31st day (2days post-RT). Meanwhile, there was no coagulation defects (prothrombin time=7.2 sec (reference range 7.1–7.4 sec), activated partial thromboplastin time=26.8 sec (reference range 13.7–25.6 sec), and fibrinogen=505 mg/dl (reference range 113–385 mg/dl). Furosemide was administered at a dose of 2 mg/kg, followed by additional doses of 1 mg/kg according to urine output. Since venous blood gas analysis showed metabolic acidosis ([HCO3-]=13.1 mmol/l [reference range; 20–24 mmol/l], pH=7.284 [reference range; 7.35–7.45], and BE= −14 mmol/l [reference range; −4–+4 mmol/l]), sodium bicarbonate was administered. In addition, effort ventilation and decreased consciousness were observed. A massive pleural effusion was confirmed by ultrasonography, and a 360 ml serosanguinous exudative pleural effusion (containing 49,320 nucleated cells/µl) was drained by thoracentesis.
On the 32nd day, (3 days post-RT), AKI started to improve. The serum potassium level decreased, consistent with increased urine output (4.9 ml/kg/hr) and decreased values of BUN and CRE. And, the patient’s consciousness improved. On the other hands, the patient continued to have a pleural effusion (300 ml/day), so a thoracic tube was placed.
From the 33rd day (4 days post-RT), pleural effusion was decreasing day by day, and appetite was improving, so the patient was discharged on the 36th day (7 days post-RT).
On the 43rd day (14 days post-1st RT), RT was resumed, when CT scan was performed again, and the treatment plan was revised because the tumor volume was decreased more than 10% (V20=26.0% for the lungs, V20=25.1% for the heart). RT was completed on the 64th day (total prescription radiation dose was 30 Gy). Compared with prior to RT, CT examination on the last day of RT showed a reduction in tumor size in half, with no contrast enhancement (Fig. 3d and 3e).
However, the patient suddenly died without any clinical signs, when his owner was away on the 75th day. A partial necropsy was performed on thoracic organs and kidneys, revealing small amounts of pleural effusion, courtesy of the owner.
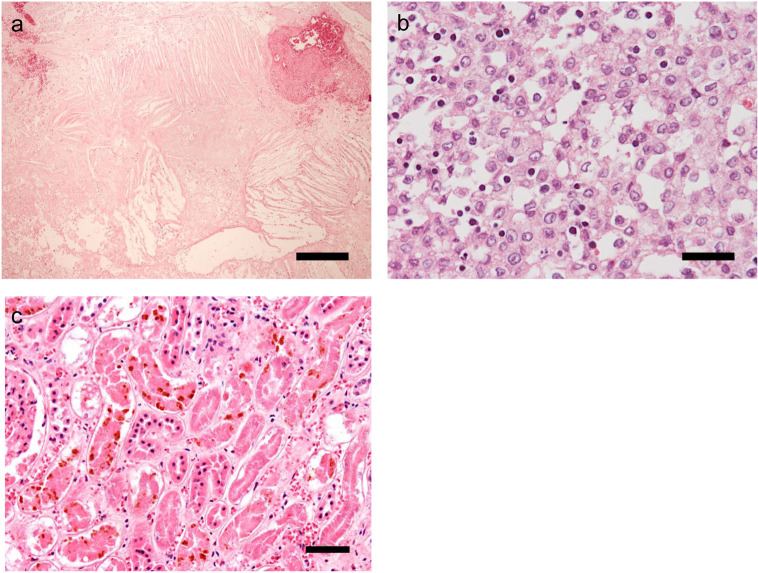
Histopathological examination after the necropsy revealed extensive necrosis, hemorrhage, and cholesterin crystals deposition in the tumor tissue. Calcium deposition of a basophilic substance was also observed (Fig. 4a). Diffuse epithelial neoplastic cells with large nuclei and eosinophilic granular cytoplasm were observed, with infiltration of small lymphocytes (Fig. 4b). Based on these findings, the cranial mediastinal mass was definitively diagnosed as thymoma. The kidneys showed extensive tubular necrosis and hemosiderin deposition in the tubular epithelial cells, which were possible causes of AKI (Fig. 4c). In the lungs, mild congestive edema was observed.
Fig. 4.
Histological examination by partial necropsy of the tumor tissue (a and b) and kidney tissue (c) with hematoxylin and eosin stain. a. Necrosis, hemorrhage, and cholesterin crystal depletion are observed over a wide area of the tumor (scale bar: 300 µm). b. Neoplastic cells with large nuclei and eosinophilic, granular cytoplasm proliferate diffusely and are accompanied by lymphocyte infiltration (scale bar: 30 µm). c. Extensive tubular necrosis and hemosiderin deposition in tubular epithelial cells are observed (scale bar: 50 µm).
Although the diagnostic criteria for TLS in humans have been defined, there are no detailed criteria in dogs, which are judged only based on the clinical history of the disease. This case showed hyperphosphatemia, hypocalcemia, hyperkalemia, and AKI, as observed in the previous reports [1, 7]. In addition, hyponatremia was observed, which might be due to massive pleural effusion. After the first RT, there was an increase in exudative pleural effusion and LDH, which might indicate tumor tissue lysis with lymphocytes infiltration. Based on these findings, we concluded that this patient had TLS after RT.
Risk factors for TLS include large tumor size, rapid growth, and high sensitivity to chemotherapy and RT [2]. Therefore, RT for thymoma, which contains a large number of lymphocytes, may be considered to have a high risk of TLS. However, there are no cases of TLS in the previous reports of RT for thymoma, and the risk of TLS has not been discussed. [3, 9] The current report was the first one regarding the development of TLS induced by RT for thymoma. In this case, 60% of the thoracic cavity was occupied by the tumor tissue. In addition, this tumor harbored many infiltrating normal lymphocytes, which were radiosensitive. Based on these findings, this case was considered to have a high risk for TLS. Altogether, we suggest that the risk for TLS should be considered when a large thymoma with a large number of infiltrating lymphocytes such as this case is treated with RT. In the future, correlation between occupancy of thymoma in the thoracic cavity and the proportion of lymphocyte in the tumor tissue and TLS occurrence need to be evaluated to clarify the risk factors of TLS.
The administration of uric acid inhibitors and uric acid-degrading enzymes, as well as infusion and diuretics, are recommended for uric acid and phosphate excretion, and AKI management in human TLS [2]. However, with the exception of Dalmatians and English Bulldogs, the role of uric acid in the development of TLS-associated AKI in dogs is limited, because uric acid is metabolized into water-soluble allantoin [4, 11]. Therefore, it is important for dogs with TLS to promote phosphate excretion by appropriate infusion and diuretic administration, and continuous renal replacement therapy might be needed to treat AKI [6]. In the present case, we considered that the patient developed TLS-induced AKI after RT. On the other hand, it was necessary to consider the possibility that the AKI was caused by contrast media or anesthesia. However, the possibility of contrast media or anesthesia seemed to be low, because AKI was not observed even after five anesthesia sessions after resumption of RT or administration of contrast media on the last day of treatment.
The current patient died suddenly 45 days after TLS development, but the cause was unknown. Based on the patient’s condition before death and histopathological findings, the dog was unlikely to die from renal failure or DIC. On the other hand, the influence of radiation on cardiovascular systems was not ruled out, although there were no significant pathological findings in the heart. In fact, several cases of heart-based tumor suddenly died after RT [5]. It is necessary to analyze more cases and evaluate the impact of radiation on cardiovascular function in the future. To our knowledge, this case is the first report of RT-associated TLS in dogs with thymoma. The risk of TLS needs to be evaluated in dogs with the tumors containing radiosensitive components and treated with RT, such as thymoma.
In RT of radiosensitive tumors, such as not only lymphoma but thymoma, it is necessary to consider the development of TLS. Once TLS occurs after RT in a tumor-bearing dog, intensive care and careful monitoring are needed.
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest with respect to the research and authorship.
Acknowledgments
We thank all staff of the Veterinary Medical Center in Osaka Prefecture University for their assistance.
REFERENCES
- 1.Chohan A. S., Greene S. A.2009. Anesthesia case of the month. Acute intraoperative tumor lysis. J. Am. Vet. Med. Assoc. 234: 746–749. doi: 10.2460/javma.234.6.746 [DOI] [PubMed] [Google Scholar]
- 2.Coiffier B., Altman A., Pui C. H., Younes A., Cairo M. S.2008. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J. Clin. Oncol. 26: 2767–2778. doi: 10.1200/JCO.2007.15.0177 [DOI] [PubMed] [Google Scholar]
- 3.Goto S., Murakami M., Kawabe M., Iwasaki R., Heishima K., Sakai H., Mori T.2017. Hypofractionated radiation therapy in the treatment of canine thymoma: Retrospective study of eight cases. Vet. Radiol. Ultrasound 58: 613–620. doi: 10.1111/vru.12525 [DOI] [PubMed] [Google Scholar]
- 4.Lulich J. P., Osborne C. A.2017. Lower Urinary Tract Urolithiasis in Dogs. pp 1996–2004. In: Textbook of Veterinary Internal Medicine, 8th ed. (Ettinger, S. J. and Feldman, E. C. eds), Elsevier, St Louis. [Google Scholar]
- 5.Magestro L. M., Gieger T. L., Nolan M. W.2018. Stereotactic body radiation therapy for heart-base tumors in six dogs. J. Vet. Cardiol. 20: 186–197. doi: 10.1016/j.jvc.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 6.Martin A., Acierno M. J.2010. Continuous renal replacement therapy in the treatment of acute kidney injury and electrolyte disturbances associated with acute tumor lysis syndrome. J. Vet. Intern. Med. 24: 986–989. doi: 10.1111/j.1939-1676.2010.0526.x [DOI] [PubMed] [Google Scholar]
- 7.Mylonakis M. E., Koutinas A. F., Papaioannou N., Lekkas S.2007. Acute tumour lysis syndrome in a dog with B-Cell multicentric lymphoma. Aust. Vet. J. 85: 206–208. doi: 10.1111/j.1751-0813.2007.00127.x [DOI] [PubMed] [Google Scholar]
- 8.Rahmani B., Patel S., Seyam O., Gandhi J., Reid I., Smith N., Khan S. A.2019. Current understanding of tumor lysis syndrome. Hematol. Oncol. 37: 537–547. doi: 10.1002/hon.2668 [DOI] [PubMed] [Google Scholar]
- 9.Rohrer Bley C., Meier V., Schneider U.2018. Dosimetric benefit of adaptive radiotherapy in the neoadjuvant management of canine and feline thymoma-An exploratory case series. Vet. Comp. Oncol. 16: 324–329. doi: 10.1111/vco.12382 [DOI] [PubMed] [Google Scholar]
- 10.Tumielewicz K. L., Hudak D., Kim J., Hunley D. W., Murphy L. A.2019. Review of oncological emergencies in small animal patients. Vet. Med. Sci. 5: 271–296. doi: 10.1002/vms3.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vickery K. R., Thamm D. H.2007. Successful treatment of acute tumor lysis syndrome in a dog with multicentric lymphoma. J. Vet. Intern. Med. 21: 1401–1404. doi: 10.1111/j.1939-1676.2007.tb01965.x [DOI] [PubMed] [Google Scholar]