Abstract
Background
Elective colorectal surgery can involve formation of bowel anastomoses, which may be complicated by postoperative anastomotic leaks. Routine intra‐operative drain placement aims to help clinicians diagnose and treat postoperative leaks. There is little agreement on the prophylactic use of drains for elective colorectal anastomoses. Once anastomotic leakage has occurred, it is generally agreed that drains should be used for therapeutic purposes. However, on prophylactic use no such agreement exists.
Objectives
To assess the effectiveness and safety of a prophylactic drain after elective colorectal anastomosis.
Search methods
We searched the Cochrane Colorectal Cancer Group's Specialized Register (February 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 2), Ovid MEDLINE (1950 to February 2015) and Ovid EMBASE (1974 to February 2015). We also searched trial registers for ongoing and registered trials, Clinicaltrials.gov and the World Health Organization (WHO) search platform International Clinical Trials Registry Platform.
Selection criteria
We included randomised controlled trials (RCTs) comparing drainage with non‐drainage regimens after anastomoses in elective colorectal surgery.
Data collection and analysis
Two review authors independently performed selection of studies, assessment of trial quality and extraction of relevant data; a third review author resolved disagreements. We used GRADE methods to evaluate the quality of evidence.
Main results
Of the 908 participants enrolled (three RCTs), 454 were allocated for drainage and 454 for no drainage. We found no new RCTs for this review update. Two trials reported the primary outcome measure of anastomotic dehiscence. There was no statistically significant difference in anastomotic dehiscence in participants treated with intra‐abdominal drainage routinely compared to no treatment (risk ratio (RR) 1.40, 95% confidence intervals (CI) 0.45 to 4.40; I2 = 0%; 2 RCTs; 809 participants). There was no statistically significant difference in mortality (RR 0.77, 95% CI 0.41 to 1.45; I2 = 0%; 3 RCTs; 908 participants); surgical re‐intervention (RR 1.11, 95% CI 0.67 to 1.82; I2 = 29%; 3 RCTs; 908 participants); radiological dehiscence (RR 0.85, 95% CI 0.39 to 1.83; I2 = 0%; 2 RCTs; 809 participants) and wound infection (RR 0.82, 95% CI 0.45 to 1.51; I2 = 0%; 3 RCTs; 908 participants) in participants treated with routine prophylactic drainage compared to no treatment undergoing elective colorectal surgery. The quality of evidence was low according to GRADE method assessment.
Authors' conclusions
There was insufficient evidence for the use of prophylactic drains after elective colorectal anastomoses. The conclusions of this review were limited due to the nature of the available clinical data; The three included RCTs performed different interventions with relatively small sample sizes of eligible participants.
Plain language summary
Prophylactic anastomotic drainage for colorectal surgery
Background
We designed this review to compare drainage with non‐drainage in people having colorectal surgery (a procedure that is used to repair damage to the colon, rectum, anus and muscle of the lower belly) with bowel anastomosis (where two ends of the bowel are joined to each other). Elective colorectal surgery (where surgery is planned in advance rather than carried out as an emergency) often involves removal of part of the large bowel for a variety of diagnoses with subsequent anastomosis. There is the option for the surgeon to place a drain at the time of surgery to prevent leakage from the anastomosis. This is called a prophylactic drain. Cochrane review authors assessed the evidence for the routine use of prophylactic drain placement after the formation of colorectal anastomoses.
Study characteristics
We included three clinical trials involving 908 participants. The trials were conducted in Germany and France. All trials compared routine anastomotic drainage versus no anastomotic drainage after elective colorectal surgery. The evidence was current to February 2015.
Key results
This review showed no apparent difference in anastomotic leak, death, radiological (x‐ray) evidence of anastomotic leak, wound infection or need for re‐operation. We found insufficient evidence to support the use of routine prophylactic drains after elective colorectal anastomosis. We based our conclusion on limited evidence with relatively small numbers of participants; this means that it is difficult to detect differences between treatment groups that may be present.
Quality of the evidence
The quality of the evidence was low, making it impossible to draw firm conclusions about the use of routine prophylactic drains after elective colorectal anastomosis. Additional studies are needed to strengthen the conclusion drawn by this systematic review and to provide further analysis using modern colorectal surgery. We found no new evidence since the previous version of our systematic review of 2004.
Summary of findings
Summary of findings for the main comparison. Intra‐abdominal drainage compared with no drainage for elective colorectal surgery.
| Intra‐abdominal drainage compared with no drainage for elective colorectal surgery | |||||
|
Patient or population: unselected population undergoing elective colorectal anastomosis Settings: high resource settings Intervention: intra‐abdominal drainage Comparison: no drainage | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No drainage | Intra‐abdominal drainage | ||||
| Anastomotic dehiscence | Unselected population undergoing elective colorectal anastomosis | RR 1.40 (0.45 to 4.40) | 809 (2) | ⊕⊕⊝⊝ low | |
| 12 per 1000* |
17 per 1000 (5 to 52.8) |
||||
| Mortality | Unselected population undergoing elective colorectal anastomosis | RR 0.77 (0.41 to 1.45) | 908 (3) | ⊕⊕⊝⊝ low | |
| 46 per 1000* |
47 per 1000 (19 to 67) |
||||
| *The basis for the assumed risk (e.g. the median control group risk) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence (www. gradepro.org) High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Assumed risk: anastomotic dehiscence occurred 5 times in 406 participants in the no drainage (control) group. Mortality occurred 21 times in 454 participants in the no drainage (control) group.
The evidence was downgraded to low quality according to GRADE methods; there was substantial clinical heterogeneity and further research in current laparoscopic colorectal practice is very likely to have an important impact on our confidence in the estimate of effect of drainage. In addition, there was a low event rate for anastomotic leak and mortality within the studies assessed, which may not accurately reflect current clinical estimates.
Background
Description of the condition
Formation of colorectal anastomoses is common in people undergoing colorectal surgery. A large number of intra‐abdominal conditions requiring bowel resection result in the requirement for a bowel anastomosis, for example, colorectal carcinoma and inflammatory bowel disease. There are varying techniques for anastomosis formation, including hand‐sewn, stapled and, less commonly, tissue adhesive techniques (Sajid 2012; Slieker 2013; Vakalopoulos 2013). Complications of bowel anastomoses include anastomotic leak (AL), bleeding, fistulae and strictures (Davis 2013). Although a consensus definition of AL is lacking, most surgeons agree that extravasation of contrast on imaging and faecal material seen in drains constitutes a clinical leak (Adams 2013). In simple terms, AL can be defined as the leak of bowel contents from a surgical join between two hollow viscera. Computed tomography (CT) imaging is often used to confirm clinical suspicion of the diagnosis; however, overall sensitivity of CT scanning to diagnose leakage is estimated at 0.68 (95% confidence interval (CI) 0.59 to 0.75) for colonic resection (Kornmann 2013).
AL can be divided into generalised (gross abdominal faecal contamination) or local (localised faecal contamination in the peri‐anastomotic space). Practically, there is a large difference between the management of a generalised versus local leak. A generalised leak will result in diffuse peritonitis, acute sepsis and necessitates immediate surgical re‐intervention. This will be performed as an emergency procedure (laparoscopic or open) to wash out the contaminated abdomen, take down or reinforce the anastomosis, defunction the person using a stoma to divert faeces from the leak, or a combination of these.
A small contained localised leak in the peri‐anastomotic space may produce a localised collection requiring radiological drainage. If the person has already been defunctioned with a stoma at the time of the initial resection, there may be little clinical sequelae. Small contained leaks can lead to enterocutaneous fistula formation or anastomotic stricturing with a significant negative impact on outcome.
AL is associated with prolonged hospital stay; increased morbidity (Montedori 2010; Tsujinaka 2011); and, more pertinently, increased local recurrence, reduced survival following cancer resections and mortality (Mirnezami 2011). A number of studies have used multivariate analysis of participant cohorts to identify risk factors for AL: these are male sex, history of radiotherapy, increased intra‐operative times, peri‐operative blood transfusion, a high American Society of Anethesiologists (ASA) score and body mass index greater than 30 kg/m2(Boccola 2011; Klein 2012; Kube 2010; McDermott 2015). The incidence of AL varies from 2% to 39% and is inversely proportional to the distance of the anastomosis from the anal verge (Montedori 2010; Vignali 1997). A variety of clinical markers are currently being developed to predict and diagnose AL accurately and as early as possible. These include elevated cytokines, metallo‐proteinases, serum C‐reactive protein and reduced intra‐operative rectal stump blood flow as measured by laser Doppler (Almeida 2012; Cini 2013; Kao 2012; Vignali 2000).
AL remains a major and frequent complication following colorectal surgery, and is associated with significant postoperative morbidity and mortality. The incidence of AL after colon surgery is reported to be 1% to 3%, and after colorectal surgery around 10% (Alberts 2003; Borowski 2010). Mortality increases significantly after AL, with AL associated with a 16% mortality compared to 3% without AL (Bakker 2014). It still remains a feared complication in colorectal surgery. Surgical strategies to minimise the risk of AL include creating an anastomosis under no tension, with an adequate blood supply and in a surgical field free of infection.
Description of the intervention
The use of routine prophylactic drains when performing an elective colorectal anastomosis has been debated since the late 1980s. The usefulness of the drain to act as an early indicator of AL and a means of localised drainage to prevent AL remain key points in this debate. This is weighed against drain‐related complications including drain‐site wound infection, pain, herniation and bleeding. There is the suggestion that drain placement may itself be a risk factor for AL as placement of the drain in close proximity to the anastomosis may mechanically disrupt the tissue or blood supply to the join (Tsujinaka 2011).
How the intervention might work
The prophylactic use of a drain adjacent to the anastomosis could evacuate peri‐anastomotic fluid collections, reducing the risk of sepsis and pressure in and around the anastomosis and thus decrease the risk of AL.
The purpose of this review was to compare outcomes after elective colorectal surgery with and without the use of prophylactic anastomotic drains.
Why it is important to do this review
This review assessed and summarised the current evidence regarding the efficacy of routine abdominal drainage for colorectal anastomosis. This review is an update of a previous Cochrane review (Jesus 2004). The comparisons within the review should assist people and their clinicians in choosing an appropriate surgical procedure with a better knowledge of the current evidence. The review serves to highlight the current limitations in the literature and to highlight the need for further research.
Objectives
To assess the effectiveness and safety of a prophylactic drain after elective colorectal anastomosis.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) and cluster‐RCTs. We excluded non‐randomised and quasi‐randomised trials as they are associated with a high risk of bias.
Types of participants
All people undergoing elective primary colorectal anastomosis.
Types of interventions
We included trials if they compared an intra‐abdominal drain with placebo (e.g. blind ended drain) or no treatment.
Types of outcome measures
Primary outcomes
Anastomotic dehiscence (as defined by the trialists).
Secondary outcomes
Mortality (30‐day all‐cause mortality).
Surgical re‐intervention (return to theatre for washout or intervention for AL (re‐operation)).
Radiological anastomotic dehiscence (as defined by the trialists).
Wound infection (as defined by the trialists).
Search methods for identification of studies
We searched for all published and unpublished RCTs, without language restriction and in consultation with the Colorectal Cancer Review Group Trials Search Co‐ordinator (Marija Barbateskovic).
Electronic searches
We searched the following electronic databases, trial registers and websites:
Cochrane Colorectal Cancer Group's Specialized Register (February 2015);
Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 2) (Appendix 1);
Ovid MEDLINE (1950 to February 2015) (Appendix 2);
Ovid EMBASE (1974 to February 2015) (Appendix 3).
Other electronic searches that we performed included:
trial registers for ongoing and registered trials: ClinicalTrials.gov (clinicaltrials.gov) and World Health Organization (WHO) search platform International Clinical Trials Registry Platform (www.who.int/trialsearch/Default.aspx);
Citation indexes (scientific.thomson.com/products/sci);
PubMed (www.ncbi.nlm.nih.gov/pubmed/);
OpenGrey database (opengrey.eu/) and Google for grey literature;
Conference abstracts in the Web of Knowledge (wokinfo.com).
Searching other resources
We handsearched reference lists of articles retrieved by the search and contacted experts in the field to obtain additional data. In addition, we handsearched relevant journals and conference abstracts that were not covered in the Colorectal Cancer Review Group Trials register, in liaison with the Trials Search Co‐ordinator (UK colorectal and general surgery conference proceedings (Association of Surgeons in Training (ASiT), Association of Surgeons of Great Britain and Ireland (ASGBI), Association of Coloproctology of Great Britain and Ireland (ACPGBI)) and newsletters were reviewed since 2011).
Data collection and analysis
Selection of studies
Three review authors (RR, SA and JD) performed an initial screen of the titles and abstracts retrieved by the search. We retrieved the full texts of all potentially eligible studies. Two review authors (RR and JD) independently examined these full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We included all relevant trials irrespective of whether they reported on measured outcome data. We corresponded with study investigators, as required, to clarify study eligibility or to seek further data where necessary. We resolved disagreements about study eligibility by discussion or by a third review author (RN). See Figure 1 for the selection process in the PRISMA flow chart.
1.
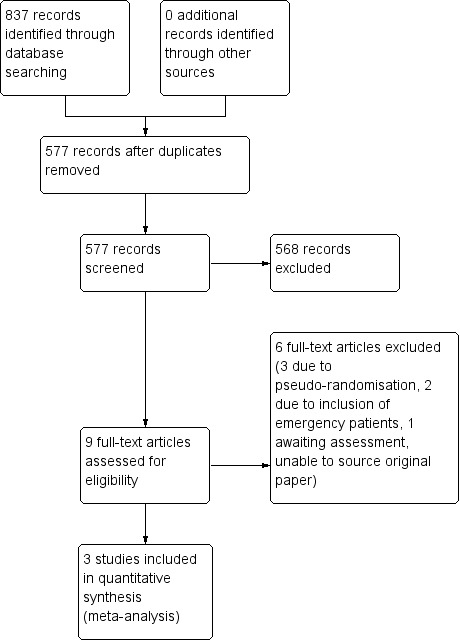
PRISMA study flow diagram.
Data extraction and management
Two review authors (RR and JD) independently extracted the data from eligible studies using a data extraction form designed and pilot‐tested by the review authors. We resolved any disagreements by discussion or by a third review author (PN). Data extracted included study characteristics and outcome data. Where studies had multiple publications, we used the main trial report as the reference and derived additional details from the secondary papers. We corresponded with study investigators for further data on methods and results, as required.
Assessment of risk of bias in included studies
Review authors (RR and JD) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool in the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Bias Methods Group; Higgins 2011) to assess:
selection bias: allocation (random sequence generation and allocation concealment);
performance/detection bias: blinding of participants and personnel, blinding of outcome assessors;
attrition bias: incomplete outcome data;
reporting bias: selective reporting;
other bias (differences in baseline characteristics, sample size calculations).
We judged each domain as high, low or unclear risk of bias according to criteria used in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection bias
Was the allocation sequence adequately generated?
Score 'low risk' if a random component in the sequence generation process was described (e.g. referring to a random number table).
Score 'high risk' when a non‐random method is used (e.g. performed by date of admission).
Score 'unclear risk' if not specified in the paper.
Was the allocation adequately concealed?
Score 'low risk' if participants and investigators enrolling participants could not foresee assignment (e.g. because used a centralised randomisation scheme, an on‐site computer system or sealed opaque envelopes).
Score 'high risk' if non‐randomised studies.
Score 'unclear risk' if not specified in the paper.
Performance/detection bias
Was knowledge of the allocated interventions adequately prevented during the study?
Score 'low risk' if the authors stated explicitly that the primary outcome variables were assessed blindly, or the outcomes were objective (e.g. length of hospital stay).
Score 'high risk' if the outcomes were not assessed blindly.
Score 'unclear risk' if not specified in the paper.
Attrition bias
Were incomplete outcome data adequately addressed?
Score 'low risk' if missing outcome measures were unlikely to bias the results (e.g. reasons for missingness unlikely to be related to true outcome, missing outcome data balanced in numbers across intervention groups with similar reasons for missing data or missing data were imputed using appropriate methods).
Score 'high risk' if missing outcome data were likely to bias the results.
Score 'unclear risk' if not specified in the paper.
Reporting bias
Were reports of the study free from selective outcome reporting?
Score 'low risk' if there is no evidence that outcomes were selectively reported (e.g. the study had a protocol pre‐specifying the outcomes, or all relevant outcomes in the methods section were reported in the results section).
Score 'high risk' if some pre‐specified outcomes were subsequently omitted from the results.
Score 'unclear risk' if not specified in the paper.
Were reports of the study free from selective analysis reporting?
Score 'low risk' for each outcome if there was no evidence that analyses were selectively reported (e.g. analyses were defined in the methods section of the protocol or paper).
Score 'high risk' if there was evidence of selective analysis reporting (e.g. multiple adjusted analyses were carried out and only one reported, or unusual cut‐points were used for categorising an outcome).
Score 'unclear' risk if unclear from the paper.
We resolved disagreements by discussion or by a third review author (RN). We described all the judgements fully and presented the conclusions in the 'Risk of bias' table. We used sensitivity analyses to incorporate the risk of bias into the interpretation of review findings. We took care to search for within trial selective reporting, such as trials failing to report obvious outcomes or reporting them in insufficient detail to allow inclusion. We sought published protocols and compared the outcomes between the protocol and the final published study.
Measures of treatment effect
All outcomes were dichotomous. We used the numbers of events in the control and intervention groups of each study and calculated risk ratios (RRs). We planned to reverse the direction of effect of individual studies, if required, to ensure consistency across trials. We presented 95% CI for all outcomes. We compared the magnitude and direction of effect reported by studies with how they are presented in the review, taking into account the clinical significance of these differences.
Unit of analysis issues
All included RCTs were parallel in design, therefore the unit of analysis was the individual participant. Should we identify any cluster‐randomised trials in future updates, we will include cluster RCTs in the analyses along with individually randomised trials. We will adjust their sample sizes or standard errors using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
Dealing with missing data
We analysed studies on an intention‐to‐treat basis, as far as possible. If data were unclear or missing, we contacted the authors of the studies for further details. If there were no further data or details made available from the contacted authors, we assessed the studies on the data provided and excluded them from overall analysis if they did not meet the inclusion criteria.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity using the I2 statistic. An I2 value greater than 50% indicated substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. Some types of reporting bias (e.g. publication bias, multiple publication bias, language bias) reduce the likelihood that all studies eligible for a review will be retrieved. If all eligible studies are not retrieved, the review may also be biased. In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise the potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. We sought published protocols and compared the outcomes between the protocol and the final published study. As the review included only three RCTs, the use of funnel plots to evaluate publication bias was not appropriate.
Data synthesis
When studies were sufficiently similar, we combined the data using a fixed‐effect model. The trials were clinically heterogeneous; however, there were not enough to inform the distribution of effects in a random‐effects model, therefore the choice of a fixed‐effect model was due to the small number of trials. An increase in the RRs of a particular outcome, which is detrimental to the participant (e.g. AL) is displayed graphically in the meta‐analysis to the right of the centre line and a decrease in the RR of an outcome to the left of the centre line.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis to determine if the incidence of AL in the two drainage groups was affected by the level of colorectal anastomosis (intra‐ or extra‐peritoneal) and type of drainage (active or passive drains).
Sensitivity analysis
We conducted sensitivity analyses for the primary outcome to determine whether the conclusions were robust to arbitrary decisions made regarding the eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if we restricted eligibility to studies without high risk of bias.
Summary of findings
We assessed the quality of evidence for the outcomes anastomotic dehiscence and mortality for intra‐abdominal drainage versus no drainage for elective colorectal surgery using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach and created a 'Summary of findings' table using the GRADEpro Guideline Development tool (GRADEpro).
The GRADE approach classifies the quality of evidence in one of four grades:
high: further research is very unlikely to change our confidence in the estimate of effect;
moderate: further research is likely to have an impact on our confidence in the estimate of effect and may change the estimate;
low: further research is very likely to have an important impact on our confidence on the estimate of effect and is likely to change the estimate;
very low: any estimate of effect is very uncertain.
The quality of evidence can be downgraded by one (serious concern) or two levels (very serious concern) for the following reasons: risk of bias, inconsistency (unexplained heterogeneity, inconsistency of results), indirectness (indirect population, intervention, control or outcomes) and imprecision (wide CIs, single trial). The quality can also be upgraded by one level due to a large summary effect estimate.
Results
Description of studies
Results of the search
The search resulted in a total of 837 records. After deleting duplicates, we screened 577 records. We excluded 568 clearly irrelevant records leaving nine potentially eligible studies of which we retrieved full texts (Mennigen 1989; Merad 1998; Merad 1999; Brown 2001; Hoffmann 1987; Johnson 1989; Sagar 1993; Sagar 1995; Hagmuller 1990). We excluded five studies for reasons listed in the Characteristics of excluded studies table (Brown 2001; Hoffmann 1987; Johnson 1989; Sagar 1993; Sagar 1995), and listed one study as awaiting classification (Hagmuller 1990) as we were unable to retrieve the original paper from authors (see Characteristics of studies awaiting classification table). Finally, three studies met our inclusion criteria (Mennigen 1989; Merad 1998; Merad 1999). Figure 1 shows the study flow diagram.
See: Characteristics of included studies table, Characteristics of excluded studies table and Characteristics of ongoing studies table.
Included studies
Study design and setting
The review included three RCTs (Mennigen 1989; Merad 1998; Merad 1999). One trial was conducted in a single centre (Mennigen 1989), and two trials were conducted in multiple centres (Merad 1998; Merad 1999). The trials were conducted in France (Merad 1998; Merad 1999), and Germany (Mennigen 1989).
Participants
The studies randomised 908 adults (Mennigen 1989; Merad 1998; Merad 1999). Participant's ages ranged from 15 to 98 years. Male to female ratios were approximately balanced. The majority of participants underwent colorectal resection for carcinoma. The other reasons for resection included benign tumours, diverticular disease and inflammatory bowel disease.
Interventions
One study used silicone passive drain (Mennigen 1989), one study used closed suction and silicone passive drains (Merad 1998), and one study used closed suction drain (Merad 1999). Drains were positioned at the site of anastomosis. They used silk sutures to secure the drain.
Primary outcome
Anastomotic leak
All trials reported anastomotic dehiscence. Anastomotic dehiscence was determined clinically by clinical signs and radiological imaging. Definitions of outcome measures were consistent between the three studies. However, radiological anastomotic dehiscence was subject to radiologist interpretation on each of the sites and, therefore, may have differed between the studies in reporting rates of AL. AL usually presents within seven to 10 days postoperatively; however, reporting for AL is at 30 days postoperatively.
Secondary outcomes
All trials reported the following secondary outcomes:
mortality;
surgical re‐intervention;
radiological anastomotic dehiscence;
wound infection (not standardised across trials).
The participants assigned to the drainage group compared with the participants assigned to non‐drainage group showed:
anastomotic dehiscence: 4% (18/454) participants compared to 4% (18/454) participants;
mortality: 3.5% (16/454) participants compared to 6.6% (30/454) participants;
surgical intervention: 6.8% (31/454) participants compared to 6.2% (28/454) participants;
radiological anastomotic dehiscence: 2.4% (11/454) participants compared to 2.9% (13/454) participants;
wound infection: 4% (18/454) participants compared to 4.8% (22/454) participants.
Excluded studies
The review excluded six trials for the following reasons:
Brown 2001, Hoffmann 1987, and Johnson 1989 were quasi‐randomised. These studies stated "drawing of sealed envelopes" as the method of sequence generation for randomisation. We judged this as pseudo‐randomisation as the investigators were taking from a receptacle. There was no description of random sequence generation and the authors did not provide further information, for example sequentially numbered envelopes. If future assignments can be anticipated, either by predicting them (number of envelopes left in receptacle) or knowledge of envelope contents (previous envelope used again in same receptacle), then selection bias can arise due to the selective enrolment and non‐enrolment of participants into the study (Section 8.9.1; Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)).
Sagar 1993 and Sagar 1995 included people having emergency surgery;
Hagmuller 1990 awaiting classification. Review authors awaiting paper for detailed analysis (requested from German authors).
Risk of bias in included studies
See Characteristics of included studies table.
Allocation
All included trials were at low risk of selection bias related to sequence generation as they used random number tables. None of the included trials described the allocation concealment method used and were at unclear risk of selection bias related to allocation concealment.
Blinding
We considered that blinding of participants and outcome observers would not influence the objective primary or secondary outcomes. It would not be possible to blind the personnel involved in surgical interventions. It would have been challenging to blind study participants. It would have been possible to blind outcome assessors following drain removal. None of the included trials stated whether participants and outcome assessors were blinded.
Incomplete outcome data
All trials reported their exclusions and losses to follow‐up. The studies were at low risk of attrition bias. The reported drop‐out rates were low for all three studies, 1/100 (1%) (Mennigen 1989), and lost to follow‐up rates were 2/317 (less than 1%) (Merad 1998), and 2/492 (less than 1%). An assumption was made that the five participants who dropped out were unlikely to bias the results (e.g. reasons for the drop‐out unlikely to be related to true outcome measures).
Selective reporting
We were unable to identify published protocols for the included studies. The included RCTs reported all outcomes that were pre‐stated in the methods section. All included RCTs reported adverse events.
Other potential sources of bias
No studies reported substantial baseline differences in the treatment and control groups and in sample size calculation.
Effects of interventions
See: Table 1
Primary outcome
Anastomotic dehiscence (30‐day postoperative)
There was no statistically significant difference in anastomotic dehiscence in participants treated with intra‐abdominal drainage compared with no treatment (RR 1.40, 95% CI 0.45 to 4.40; 2 RCTs; 809 participants) (Analysis 1.1). There was a low degree of heterogeneity (I2 = 0%); however, this has little value due to the low number of included trials. When we carried out the pre‐defined subgroup analysis, anastomotic dehiscence was not affected by the level of colorectal anastomosis or drain type (Analysis 1.1; Analysis 2.1). We were unable to carry out the pre‐defined sensitivity analysis, as all included trials were associated with a high risk of bias.
1.1. Analysis.

Comparison 1 All studies stratified by type of drain, Outcome 1 Clinical anastomotic dehiscence.
2.1. Analysis.
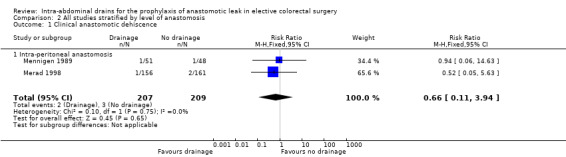
Comparison 2 All studies stratified by level of anastomosis, Outcome 1 Clinical anastomotic dehiscence.
Secondary outcomes
Mortality (30‐day postoperative)
All trials reported 30‐day mortality. There was no statistically significant difference in anastomotic dehiscence in participants treated with intra‐abdominal drainage compared with no treatment (RR 0.77, 95% CI 0.41 to 1.45; 3 RCTs; 908 participants) (Analysis 1.2). There was a low degree of heterogeneity (I2 = 0%).
1.2. Analysis.
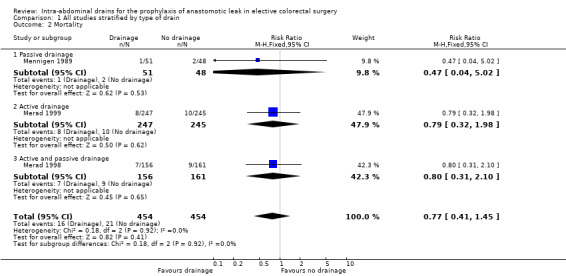
Comparison 1 All studies stratified by type of drain, Outcome 2 Mortality.
Surgical re‐intervention (30‐day postoperative)
All trials reported surgical re‐intervention. There was no statistically significant difference in anastomotic dehiscence in participants treated with intra‐abdominal drainage compared with no treatment (RR 1.11, 95% CI 0.67 to 1.82; 3 RCTs; 908 participants) (Analysis 1.3). There was a low degree of heterogeneity (I2 = 29%).
1.3. Analysis.
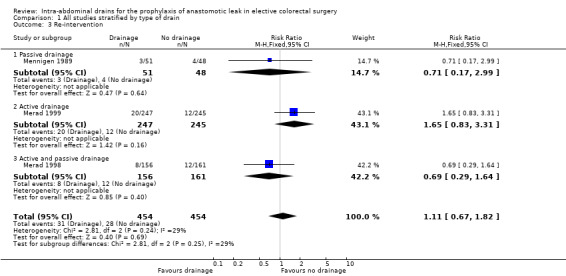
Comparison 1 All studies stratified by type of drain, Outcome 3 Re‐intervention.
Radiological anastomotic dehiscence (30‐day postoperative)
All trials reported radiological anastomotic dehiscence. AL was assessed clinically and with radiological imaging. There was no statistically significant difference in anastomotic dehiscence in participants treated with intra‐abdominal drainage compared with no treatment (RR 0.85, 95% CI 0.39 to 1.83; 3 RCTs; 908 participants) (Analysis 1.4). There was a low degree of heterogeneity (I2 = 0%).
1.4. Analysis.
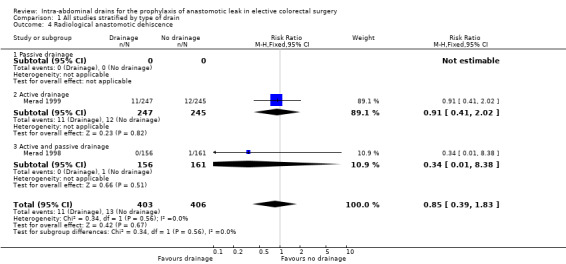
Comparison 1 All studies stratified by type of drain, Outcome 4 Radiological anastomotic dehiscence.
Wound infection (30‐day postoperative)
All trials reported wound infection. Trials did not use a validated classification system to define wound infection. The definition of wound infection was based on clinical signs and elevated laboratory blood markers for infection. There was no statistically significant difference in wound infection in participants treated with intra‐abdominal drainage compared with no treatment (RR 0.82, 95% CI 0.45 to 1.51; 3 RCTs; 908 participants) (Analysis 1.5). There was a low degree of heterogeneity (I2 = 0%).
1.5. Analysis.
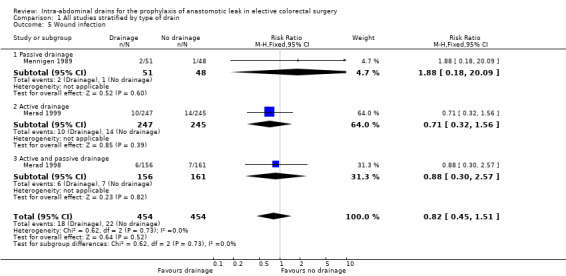
Comparison 1 All studies stratified by type of drain, Outcome 5 Wound infection.
Discussion
The purpose of this review was to compare outcomes after elective colorectal surgery with and without the use of prophylactic anastomotic drains.
Meta‐analysis of three RCTs showed no statistically significant difference in the incidence of clinically or radiologically determined AL associated with the use of drains (Mennigen 1989; Merad 1998; Merad 1999). There was no detectable difference in mortality between the drainage and non‐drainage groups (Mennigen 1989; Merad 1998; Merad 1999). Re‐operation rates were consistent between the two groups, suggesting that in the event of AL, a drain placed at the time of surgery may not have a therapeutic benefit or change the outcome. However, the power of the analyses in this review was low, therefore, no firm conclusions can be made based on current evidence (Mennigen 1989; Merad 1998; Merad 1999).
The incidence of AL in the two drainage groups was also not affected by whether the anastomosis was intra‐ or extra‐peritoneal, there was no evidence of a greater benefit in drains covering low rectal anastomoses (Merad 1999). This subgroup analysis was limited in its conclusion due to limited participant numbers and a very low event rate of AL (4%); low participant numbers makes it difficult for analyses to detect differences between groups that may be present. Many surgeons routinely place drains after mid‐ to low‐rectal resections with a view to draining pelvic haematomas postoperatively rather than detecting or preventing ALs. Further high‐quality large RCTs are required to address this topic in greater detail.
There may be several reasons why drains placed at surgery appear to be of no benefit. Following surgery, drains will often become blocked, eradicating their therapeutic potential (Averbach 1995). Drains may also become displaced from their original placement site. Furthermore, the studies employed in this meta‐analysis used various types of drain. The differing properties of the drainage systems used could have affected the results and needs to be investigated further. The reasoning against the use of drains in colorectal surgery includes concerns about potential negative effects of drains. Indeed, the routine use of drains is not recommended in colorectal enhanced recovery programmes, which have become widespread in recent years, due to concerns regarding increased pain, difficulty with mobilisation and subsequent complications (Lassen 2009).
The conclusions of this review were limited by the heterogeneous nature of the operations performed in the included studies, which included both intra‐ and extra‐peritoneal anastomoses. The lack of recruitment of sufficient sample sizes in the included studies means that the conclusions may be limited due to inadequate power and, therefore, a type II error. In particular, the studies included are relatively dated and there was no new evidence for this review since 2004. The available evidence was insufficient to draw firm conclusions regarding the benefit or harm from routine drainage in colorectal resections as the two main results from the pooled data were contradictory and inconsistent. Further trials are required, not only to determine the safety and efficacy of drains, but also their role in diagnosing anastomotic leakage, which has been one of the purported benefits of routine drain placement. In addition, trials are needed to investigate the effects of drainage in the emergency colorectal surgical setting which is not addressed in this review.
Summary of main results
There was insufficient evidence to support or refute the routine use of abdominal drainage for elective colorectal anastomosis. There were no statistical significant differences in the primary outcome, postoperative anastomotic dehiscence, or secondary outcomes including mortality, surgical re‐intervention, radiological anastomotic dehiscence and wound infection.
Overall completeness and applicability of evidence
The trials recruited participants considered at low anaesthetic and surgical risk, therefore, the applicability of this review to inform practice in high‐risk people undergoing surgery is limited. There was insufficient evidence to draw any conclusions regarding differences in efficacy for people with benign disease compared with people with malignancy. All trials recruited participants within high resource settings, which limits the applicability of this research to inform practice within low resource settings. All included studies reported the primary and secondary outcomes but there was substantial clinical heterogeneity. Clinically, colorectal surgery has moved towards minimally invasive laparoscopic operations and enhanced recovery pathways. The studies included did not address the effectiveness of drains in current colorectal surgical practice.
Quality of the evidence
The quality of the evidence for effectiveness outcomes was low, denoting that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. The methodological bias was low in the included trials as methods of randomisation were described, and it was unclear if blinding was likely to affect the objective primary and secondary outcome measures. There was sufficient evidence to reach preliminary conclusions regarding primary and secondary outcomes. Lack of power calculations in the included studies means that the conclusions drawn may be subject to type II errors or due to problems in participant recruitment to the trial.
Potential biases in the review process
The strengths of this systematic review include its robust search strategy (guided and developed by the Colorectal Cancer Review Group) and the methodological design and statistical analysis (developed with reference to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)). The protocol was peer‐reviewed, published and freely available. We applied no limitations such as a publication type, language or date restrictions. Two review authors independently conducted study selection, risk of bias assessment and data collection. We resolved disagreements by discussion or by a third review author (RN or PN). All review authors have stated that there are no conflicts of interest. Systematic reviews are not without limitations. The comprehensive search strategy identified only three trials, making extrapolation difficult. Notably, two of the studies are from the same research group, which may be a potential source of bias. The studies included are relatively dated in surgical practice and do not account for advances in laparoscopic surgical approaches. This may limit the applicability of the conclusions to current practice.
Agreements and disagreements with other studies or reviews
The results from this systematic review were in agreement with previous systematic reviews on this topic, which also concluded that there is insufficient evidence to support the use of routine drainage in elective colorectal surgery (Karliczek 2006; Urbach 1999; Jesus 2004). We found no new evidence for this review compared to the first published version of the systematic review in 2004 (Jesus 2004).
The findings of this review agree with additional systematic reviews on the routine use of drains for abdominal surgery including gastrectomy, liver resection, cholecystectomy and incisional hernia repair (Gurusamy 2007a; Gurusamy 2007b; Gurusamy 2013; Wang 2011). There remains a paucity of high‐quality RCTs in this field and large‐scale RCTs are required to provide robust scientific evidence in current surgical practice.
Authors' conclusions
Implications for practice.
There remains no firm evidence for the prophylactic use of drains in preventing anastomotic leak after colorectal surgery, although the review also does not reveal any significant contraindication to the use of drains. The conclusions of this review were based on evidence from three studies all performed in the 1980s and 1990s with no new studies since the previous systematic review in 2004 (Jesus 2004). The applicability of the conclusions based on the included studies to current minimally invasive colorectal surgery with enhanced recovery programmes is unclear and remains a significant limitation of the review.
Implications for research.
Uncertainty about the effects of drains in colorectal surgery remains. A large‐scale randomised controlled trial would help to resolve these uncertainties. This trial should be performed in the context of a modern enhanced recovery programme following elective laparoscopic resection, with the drainage group having a standardised passive closed drain. All participants would need to be randomised to drainage or no drainage with both groups receiving enhanced recovery programmes. Blinding participants and clinicians to the intervention would not be possible. The endpoints should be clearly defined, and in addition to the endpoints used in this review, include measurements of postoperative pain, and specific drain‐related complications such as herniation, wound infection and quality of life scores. A prospective protocol with consensus outcome definitions should be published. In particular, radiological anastomotic leak as an outcome measure requires a consensus opinion between three independent radiologists to confirm an anastomotic leak to prevent subjective reporting. Given the relatively high leak rates after low rectal anastomosis, this should form a separate subgroup for analysis. In addition, separate trials should be conducted to investigate the effects of drains in emergency colorectal surgery.
What's new
| Date | Event | Description |
|---|---|---|
| 16 March 2016 | Amended | No new RCTs included in this update. Methodology reported according to required MECIR standards. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 22 December 2015 | Feedback has been incorporated | Editorial comments incorporated |
| 25 March 2015 | New citation required but conclusions have not changed | Updated Prisma Flow Chart with new search results. 2 authors have verified no new studies for inclusion from new search results.Conclusions remain unchanged in this update. |
| 6 April 2014 | Amended | Updated discussion and references |
| 27 July 2013 | New search has been performed | Updated text and results |
| 22 January 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to acknowledge Jesus EC, Karliczek A, Matos D, Castro AA and Atallah AN, authors of the previous Cochrane review (Jesus 2004). Thanks to the Cochrane Colorectal Cancer Group (Henning Keinke Andersen and Marija Barbateskovic) for their invaluable support, editors and peer referees, and the Cochrane Copy Edit Support (Anne Lawson) for careful copy editing of this updated review.
Appendices
Appendix 1. CENTRAL search strategy
Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 2) #1 MeSH descriptor: [Colorectal Surgery] explode all trees #2 MeSH descriptor: [Digestive System Surgical Procedures] explode all trees #3 MeSH descriptor: [Colorectal Neoplasms] explode all trees #4 MeSH descriptor: [Rectum] explode all trees #5 MeSH descriptor: [Colon] explode all trees #6 MeSH descriptor: [Abdomen] explode all trees #7 ((colorect* or colon* or rect* or anal or anus or intestin* or bowel* or abdom*) and (surger* or resec* or operat*)):ti,ab,kw #8 (#1 or #2 or #3 or #4 or #5 or #6 or #7) #9 MeSH descriptor: [Anastomosis, Surgical] explode all trees #10 (anastomo*):ti,ab,kw #11 (#9 or #10) #12 MeSH descriptor: [Drainage] explode all trees #13 (drain* or dren* or suction*):ti,ab,kw #14 (#12 or #13) #15 (#8 and #11 and #14)
Appendix 2. MEDLINE search strategy
Ovid MEDLINE (1950 to 6 February 2015) 1. exp Colorectal Surgery/ 2. exp Digestive System Surgical Procedures/ 3. exp Colorectal Neoplasms/ 4. exp Rectum/ 5. exp Colon/ 6. exp Abdomen/ 7. ((colorect* or colon* or rect* or anal or anus or intestin* or bowel* or abdom*) and (surger* or resec* or operat*)).mp. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. exp Anastomosis, Surgical/ 10. anastomo*.mp. 11. 9 or 10 12. exp Drainage/ 13. (drain* or dren* or suction*).mp. 14. 12 or 13 15. 8 and 11 and 14 16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. randomized.ab. 19. placebo.ab. 20. clinical trial.sh. 21. randomly.ab. 22. trial.ti. 23. 16 or 17 or 18 or 19 or 20 or 21 or 22 24. humans.sh. 25. 23 and 24 26. 15 and 25
Appendix 3. EMBASE search strategy
Ovid EMBASE (1974 to 6 February 2015) 1. exp colorectal surgery/ 2. exp abdominal surgery/ 3. exp colon tumor/ 4. exp rectum tumor/ 5. exp rectum/ 6. exp colon/ 7. ((colorect* or colon* or rect* or anal or anus or intestin* or bowel* or abdom*) and (surger* or resec* or operat*)).mp. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. exp anastomosis/ 10. anastomo*.mp. 11. 9 or 10 12. exp surgical drainage/ 13. (drain* or dren* or suction*).mp. 14. 12 or 13 15. 8 and 11 and 14 16. CROSSOVER PROCEDURE.sh. 17. DOUBLE‐BLIND PROCEDURE.sh. 18. SINGLE‐BLIND PROCEDURE.sh. 19. (crossover* or cross over*).ti,ab. 20. placebo*.ti,ab. 21. (doubl* adj blind*).ti,ab. 22. allocat*.ti,ab. 23. trial.ti. 24. RANDOMIZED CONTROLLED TRIAL.sh. 25. random*.ti,ab. 26. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27. (exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.) 28. 26 not 27 29. 15 and 28
Data and analyses
Comparison 1. All studies stratified by type of drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical anastomotic dehiscence | 2 | 809 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.45, 4.40] |
| 1.1 Active drainage | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.50, 7.84] |
| 1.2 Active and passive drainage | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.63] |
| 2 Mortality | 3 | 908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.41, 1.45] |
| 2.1 Passive drainage | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.04, 5.02] |
| 2.2 Active drainage | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.32, 1.98] |
| 2.3 Active and passive drainage | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.31, 2.10] |
| 3 Re‐intervention | 3 | 908 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.67, 1.82] |
| 3.1 Passive drainage | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.17, 2.99] |
| 3.2 Active drainage | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.83, 3.31] |
| 3.3 Active and passive drainage | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.29, 1.64] |
| 4 Radiological anastomotic dehiscence | 2 | 809 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.39, 1.83] |
| 4.1 Passive drainage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Active drainage | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.41, 2.02] |
| 4.3 Active and passive drainage | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.38] |
| 5 Wound infection | 3 | 908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.45, 1.51] |
| 5.1 Passive drainage | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.18, 20.09] |
| 5.2 Active drainage | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.32, 1.56] |
| 5.3 Active and passive drainage | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.30, 2.57] |
Comparison 2. All studies stratified by level of anastomosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical anastomotic dehiscence | 2 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 3.94] |
| 1.1 Intra‐peritoneal anastomosis | 2 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 3.94] |
| 2 Mortality | 2 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.30, 1.80] |
| 2.1 Intra‐peritoneal anastomosis | 2 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.30, 1.80] |
| 3 Re‐intervention | 2 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.31, 1.62] |
| 3.1 Intra‐peritoneal anastomosis | 2 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.31, 1.62] |
| 4 Radiological anastomotic dehiscence | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.38] |
| 4.1 Intra‐peritoneal anastomosis | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.38] |
| 5 Wound infection | 2 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.39, 2.66] |
| 5.1 Intra‐peritoneal anastomosis | 2 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.39, 2.66] |
2.2. Analysis.
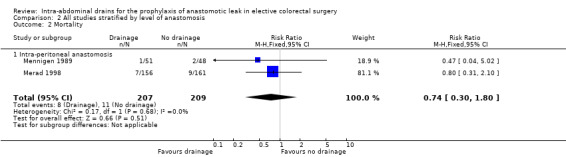
Comparison 2 All studies stratified by level of anastomosis, Outcome 2 Mortality.
2.3. Analysis.
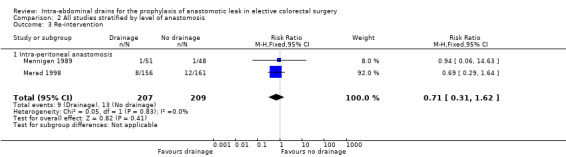
Comparison 2 All studies stratified by level of anastomosis, Outcome 3 Re‐intervention.
2.4. Analysis.

Comparison 2 All studies stratified by level of anastomosis, Outcome 4 Radiological anastomotic dehiscence.
2.5. Analysis.
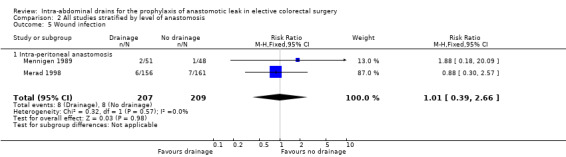
Comparison 2 All studies stratified by level of anastomosis, Outcome 5 Wound infection.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Mennigen 1989.
| Methods | Sequence generation: random number tables Allocation concealment: not described Blinded: not described |
|
| Participants | Inclusion criteria: elective resection, intra‐abdominal anastomosis Exclusion criteria: obstruction, perforation or sepsis present at time of performing anastomosis, i.e. emergency operation Type of disease: 77/100 carcinoma, 6/100 adenoma, 7/100 diverticular disease, 9/100 inflammatory colon disease, 1/100 type of disease not reported (for 1 drop‐out) Type of anastomosis: 59/100 left hemicolectomy, 33/100 right hemicolectomy, 7/100 subtotal colectomy, 1/100 not described Number of participants: 100 Age range: 15‐87 years Gender: 49 male, 50 female Place of study: Germany (single centre) Time of study: June 1984 to November 1986 |
|
| Interventions | Treatment: silicone drain Control: no drain |
|
| Outcomes | Primary outcome: anastomotic dehiscence Secondary outcomes: mortality, surgical re‐intervention, radiological anastomotic dehiscence and wound infection |
|
| Notes | Publication: journal article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out 1/100 (1%) |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes that were pre‐stated in the methods section |
| Other bias | Unclear risk | No studies reported substantial baseline differences in the treatment and control groups. We found no other potential sources of bias within the included studies Sample size calculation not described. Source of bias |
Merad 1998.
| Methods | Sequence generation: random number tables Allocation concealment: not described Blinded: not described |
|
| Participants | Inclusion criteria: elective resection, intra‐abdominal anastomosis Exclusion criteria: emergency operation, infection at time of inclusion, resection without anastomosis or with pelvic anastomosis Type of disease: 223/317 carcinoma, 13/317 sigmoid diverticular disease, 34/317 benign tumour, 18/317 Crohn's disease, 27/317 other, 2/317 (information not provided in the study) Type of anastomosis: 222/317 ileocolica, 95/317 colocolica Methods of anastomosis: 124/317 manual, 193/317 stapled Number of eligible participants: 319 Gender: 135 male and 184 female Number of participants randomised: 317 (two participants excluded due to protocol violations); 156 patients were randomized to the abdominal drainage group and 161 to the no abdominal drainage group. Age range: 21‐95 years Place of study: France (15 centres) Time of study: 1990‐1995 |
|
| Interventions | Treatment: drain (82/156 suction ‐ multi‐perforated tubular 14F polyvinylchloride catheter; 74/156 non‐suction ‐ silicone multi‐tubular 10 mm) Control: no drain |
|
| Outcomes | Primary outcome: anastomotic dehiscence Secondary outcomes: mortality, surgical re‐intervention, radiological anastomotic dehiscence and wound infection |
|
| Notes | Publication: journal article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up 2/317 (< 1%) |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes that were pre‐stated in the methods section |
| Other bias | Unclear risk | No studies reported substantial baseline differences in the treatment and control groups Sample size calculation not described. Source of bias |
Merad 1999.
| Methods | Sequence generation: random number tables Allocation concealment: not described Blinded: not described |
|
| Participants | Inclusion criteria: elective resection with pelvic anastomosis Exclusion criteria: intra‐abdominal anastomosis, infection, inadequate haemostasis, emergency operation Type of disease: carcinoma: 304/492, sigmoid diverticular disease: 123/492, benign tumour: 23/492, Crohn's disease: 13/492, other: 26/492, 3/492 (information not provided in the study) Type of anastomosis: 360/492 supraperitoneal rectum, 115/492 infraperitoneal rectum, 17/492 anus Methods of anastomosis: 360/492 manual, 132/492 stapled Number of participants: 492 Age range: 24‐98 years Gender: 248 male, 244 female Place of study: France (18 centres) Time of study: September 1990 to June 1995 |
|
| Interventions | Treatment: multi‐perforated polyvinyl chloride F14 suction drains Control: no drain |
|
| Outcomes | Primary outcome: anastomotic dehiscence Secondary outcomes: mortality, surgical re‐intervention, radiological anastomotic dehiscence and wound infection |
|
| Notes | Publication: journal article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | High risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up 2/492 (< 1%) |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes that were pre‐stated in the methods section |
| Other bias | Unclear risk | No reported substantial baseline differences in the treatment and control groups Sample size calculation not described. Source of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brown 2001 | Quasi‐randomisation |
| Hoffmann 1987 | Quasi‐randomisation |
| Johnson 1989 | Quasi‐randomisation |
| Sagar 1993 | Participants undergoing emergency surgery |
| Sagar 1995 | Participants undergoing emergency surgery |
Characteristics of studies awaiting assessment [ordered by study ID]
Hagmuller 1990.
| Methods | Randomised prospective clinical study |
| Participants | Prophylactic drainage for elective resection of the colon (intra‐peritoneal) |
| Interventions | Capillar drainage (easy‐flow‐drainage) (n = 60) vs. no drainage(n = 53) |
| Outcomes | Anastomotic leakage; impaired wound healing; re‐laparotomy |
| Notes | Unable to source original paper from German authors; awaiting assessment |
n: number of participants.
Contributions of authors
RR: Took the lead in writing the review, was involved in selecting trials for inclusion, performed independent data extraction and quality assessment of the included trials, and was responsible for statistical analysis and interpretation of the data.
JD: Involved in selecting trials for inclusion, performed independent data extraction and quality assessment of the included trials, and was responsible for statistical analysis and interpretation of the data.
SA: Involved in selecting trials and quality assessment of included trials.
PN: Commented on drafts of the review, and added clinical expertise to the discussion.
RN: Commented on drafts of the review, and added clinical expertise to the discussion.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR Cochrane Incentive Award, Reference Number 14/175/53, UK.
This project was supported by the National Institute for Health Research, via Cochrane Incentive Award funding to the Cochrane Colorectal Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Mennigen 1989 {published data only}
- Mennigen R, Kusche J, Troidl H. Prophylaktische drainage von kolonanastomosen. Coloproctology 1989;11(2):76‐80. [Google Scholar]
Merad 1998 {published data only}
- Merad F, Yahchouchi E, Hay JM, Fingerhut A, Laborde Y, Langlois‐Zantain O, for the French Associations for Surgical Research. Prophylactic abdominal drainage after elective colonic resection and suprapromontory anastomosis. Archives of Surgery 1998;133(3):309‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Merad 1999 {unpublished data only}
- Merad F, Hay JM, Fingerhut A, Yahchouchi E, Laborde Y, Pelissier E, et al. Is prophylactic pelvic drainage useful after elective rectal or anal anastomosis? A multicenter controlled randomized trial. Surgery 1999;125:529‐35. [PubMed] [Google Scholar]
References to studies excluded from this review
Brown 2001 {published data only}
- Brown SR, Seow‐Choen F, Eu KW, Heah SM, Tang CL. A prospective randomised study of drains in infra‐peritoneal rectal anastomoses. Techniques in Coloproctology 2001;5:89‐92. [DOI] [PubMed] [Google Scholar]
Hoffmann 1987 {published data only}
- Hoffmann J, Shokouh‐Amiri MH, Damm P, Jersen R. A prospective, controlled study of prophylactic drainage after colonic anastomoses. Diseases of the Colon and Rectum 1987;30(6):449‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnson 1989 {published data only}
- Johnson CD, Lamont PM, Orr N, Lennox M. Is a drain necessary after colonic anastomosis?. Journal of the Royal Society of Medicine 1989;82(11):661‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sagar 1993 {published data only}
- Sagar PM, Couse N, Kerin N, May J, MacFie J. Randomized trial of drainage of colorectal anastomosis. British Journal of Surgery 1993;80:769‐71. [DOI] [PubMed] [Google Scholar]
Sagar 1995 {published data only}
- Sagar PM, Hartley MN, MacFie J, Mancey‐Jones B, Sedman P, May J. Randomized trial of pelvic drainage after rectal resection. Diseases of the Colon and Rectum 1995;38(3):254‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Hagmuller 1990 {published data only}
- Hagmuller E, Lorenz D, Werthmann K, Trede M. Uses and risks of drainage following elective colon resection. A prospective, randomized and controlled clinical study. Chirurgie 1990;61(4):266‐71. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
Adams 2013
- Adams K, Papagrigoriadis S. Little consensus in either definition or diagnosis of a lower gastro‐intestinal anastomotic leak amongst colorectal surgeons. International Journal of Colorectal Disease 2013;28(7):967‐71. [DOI] [PubMed] [Google Scholar]
Alberts 2003
- Alberts JCJ, Parvaiz A, Moran BJ. Predicting risk and diminishing the consequences of anastomotic dehiscence following rectal resection. Colorectal Disease 2003;5:478‐82. [DOI] [PubMed] [Google Scholar]
Almeida 2012
- Almeida AB, Faria G, Moreira H, Pinto‐de‐Sousa J, Correia‐da‐Silva P, Maia JC. Elevated serum C‐reactive protein as a predictive factor for anastomotic leakage in colorectal surgery. International Journal of Surgery 2012;10(2):87‐91. [DOI] [PubMed] [Google Scholar]
Averbach 1995
- Averbach AM, Sugarbaker PH. The use of drains in elective surgery for colorectal cancer: always, never or selectively?. Tumori 1995;81(3 Suppl):89‐97. [PubMed] [Google Scholar]
Bakker 2014
- Bakker IS, Grossmann I, Henneman D, Havenga K, Wiggers T. Risk factors for anastomotic leakage and leak‐related mortality after colonic cancer surgery in a nationwide audit. British Journal of Surgery 2014;101(4):424‐32. [DOI] [PubMed] [Google Scholar]
Boccola 2011
- Boccola MA, Buettner PG, Rozen WM, Siu SK, Stevenson AR, Stitz R, et al. Risk factors and outcomes for anastomotic leakage in colorectal surgery: a single‐institution analysis of 1576 patients. World Journal of Surgery 2011;35(1):186‐95. [DOI] [PubMed] [Google Scholar]
Borowski 2010
- Borowski DW, Bradburn DM, Mills SJ, Bharathan B, Wilson RG, Ratcliffe AA, et al. Volume‐outcome analysis of colorectal cancer‐related outcomes. British Journal of Surgery 2010;97:1416‐30. [DOI] [PubMed] [Google Scholar]
Cini 2013
- Cini C, Wolthuis A, D'Hoore A. Peritoneal fluid cytokines and matrix‐metalloproteinases as early markers of anastomotic leakage in colorectal anastomosis. A literature review and meta‐analysis. Colorectal Disease 2013;15(9):1070‐7. [DOI] [PubMed] [Google Scholar]
Cochrane Bias Methods Group
- Cochrane Bias Methods Group. Assessing risk of bias in included studies, 2013. bmg.cochrane.org/assessing‐risk‐bias‐included‐studies (accessed 23 March 2016).
Davis 2013
- Davis B, Rivadeneira DE. Complications of colorectal anastomoses: leaks, strictures, and bleeding. Surgical Clinics of North America 2013;93(1):61‐87. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- McMaster University. GRADEpro. McMaster University, 2015.
Gurusamy 2007a
- Gurusamy KS, Samraj K, Davidson BR. Routine abdominal drainage for uncomplicated liver resection. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006232.pub2] [DOI] [PubMed] [Google Scholar]
Gurusamy 2007b
- Gurusamy KS, Samrak K, Mullerat P, Davidson BR. Routine abdominal drainage for uncomplicated laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006004.pub3] [DOI] [PubMed] [Google Scholar]
Gurusamy 2013
- Gurusamy KS, Allen VB. Wound drains after incisional hernia repair. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD005570.pub4] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kao 2012
- Kao LS, Millas SG. Predicting the risk of anastomotic leakage in left‐sided colorectal surgery using a Colon Leakage Score. Journal of Surgical Research 2012;173(2):246‐8. [DOI] [PubMed] [Google Scholar]
Karliczek 2006
- Karliczek A, Jesus EC, Matos D, Castro AA, Atallah AN, Wiggers T. Drainage or nondrainage in elective colorectal anastomosis: a systematic review and meta‐analysis. Colorectal Disease 2006;8(4):259‐65. [DOI] [PubMed] [Google Scholar]
Klein 2012
- Klein M, Gögenur I, Rosenberg J. Postoperative use of non‐steroidal anti‐inflammatory drugs in patients with anastomotic leakage requiring reoperation after colorectal resection: cohort study based on prospective data. BMJ 2012;26:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kornmann 2013
- Kornmann VN, Treskes N, Hoonhout LH, Bollen TL, Ramshorst B, Boerma D. Systematic review on the value of CT scanning in the diagnosis of anastomotic leakage after colorectal surgery. International Journal of Colorectal Disease 2013;28(4):437‐45. [DOI] [PubMed] [Google Scholar]
Kube 2010
- Kube R, Mroczkowski P, Granowski D, Benedix F, Sahm M, Schmidt U, et al. Quality assurance in primary colorectal carcinoma [Qualitätssicherung Kolon/Rektum‐Karzinome (Primärtumor)]. European Journal of Surgical Oncology 2010;36(2):120‐4. [DOI] [PubMed] [Google Scholar]
Lassen 2009
- Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Archives of Surgery 2009;144(10):961‐9. [DOI] [PubMed] [Google Scholar]
McDermott 2015
- McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. British Journal of Surgery 2015;102(5):462‐79. [DOI] [PubMed] [Google Scholar]
Mirnezami 2011
- Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta‐analysis. Annals of Surgery 2011;253(5):890‐9. [DOI] [PubMed] [Google Scholar]
Montedori 2010
- Montedori A, Cirocchi R, Farinella E, Sciannameo F, Abraha I. Covering ileo‐ or colostomy in anterior resection for rectal carcinoma. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD006878.pub2] [DOI] [PubMed] [Google Scholar]
Sajid 2012
- Sajid MS, Rafay M, Siddiqui MR, Baig MK. Single layer versus double layer suture anastomosis of the gastrointestinal tract. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD005477.pub4] [DOI] [PubMed] [Google Scholar]
Slieker 2013
- Slieker JC, Daams F, Mulder IM, Jeekel J, Lange JF. Systematic review of the technique of colorectal anastomosis. Journal of the American Medical Association of Surgery 2013;148(2):190‐201. [DOI] [PubMed] [Google Scholar]
Tsujinaka 2011
- Tsujinaka S, Konishi F. Drain vs no drain after colorectal surgery. Indian Journal of Surgical Oncology 2011;2(1):3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Urbach 1999
- Urbach DR, Kennedy ED, Cohen MM. Colon and rectal anastomoses do not require routine drainage: a systematic review and meta‐analysis. Annals of Surgery 1999;229(2):174‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vakalopoulos 2013
- Vakalopoulos KA, Daams F, Wu Z, Timmermans L, Jeekel JJ, Kleinrensink GJ, et al. Tissue adhesives in gastrointestinal anastomosis: a systematic review. Journal of Surgical Research 2013;180(2):290‐300. [DOI] [PubMed] [Google Scholar]
Vignali 1997
- Vignali A, Fazio VW, Lavery IC, Milsom JW, Church JM, Hull TL, et al. Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1014 patients. Journal of the American College of Surgeons 1997;185(2):113‐21. [DOI] [PubMed] [Google Scholar]
Vignali 2000
- Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Carlo V. Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Diseases of the Colon and Rectum 2000;43(1):76‐82. [DOI] [PubMed] [Google Scholar]
Wang 2011
- Wang Z, Chen J, Su K, Dong Z. Abdominal drainage versus no drainage post gastrectomy for gastric cancer. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD008788.pub3] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jesus 2004
- Jesus EC, Karliczek A, Matos D, Castro AA, Atallah AN. Prophylactic anastomotic drainage for colorectal surgery. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD002100.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


