Abstract
Bisphenol A (BPA) is used as a monomer in a number of consumer products, including baby bottles and sippy cups. Some jurisdictions around the world (including Canada) have regulated the production, advertising or selling polycarbonate baby bottles with BPA. Following the ban, makers have opted for alternative materials to BPA [named BPA analogues, BPAAs], which may not be as safe as promoted. The objective of this project was to conduct a migration study in baby bottles and sippy cups, and analyze 16 BPAAs, as a follow-up on the BPA migration study conducted by Health Canada in 2009. Baby bottles (20 brands) and sippy cups (13 brands) were tested for migration of BPAAs. The most commonly detected analytes in baby bottles were BPS, BPA, BPF, BPAF, BPM and BPTMC with detection frequency (DF) of more than 50%. In sippy cups, only BPA, BPS and BPF were frequently detected. The mean concentration of BPA in baby bottle leachate was 31.5 ng/L in water simulant whereas a 1.4-fold increase was seen in 50% EtOH simulant. Similarly, a 1.4-fold increase was seen in the mean concentration of BPS in 50% EtOH simulant, when compared to the mean concentration of 2.33 ng/L in water simulant. Increasing median concentration was observed for BPA as the ethanol content of the simulant increased (water<10% EtOH<50% EtOH). The concentration of BPS and BPA was higher in sippy cups than that in their matched brand of baby bottles with the 50% EtOH simulant. Although most of the target analytes were detected in baby bottles, their concentrations were low and no migration was observed for any of the analytes with increasing incubation time. Therefore, it is likely that known BPA analogues are not present in the polymers used in the manufacture of most of the baby bottle brands sold in Canada.
Keywords: Bisphenol A, Bisphenol A analogues, Baby bottles, Sippy cups, Migration
Graphical abstract
Highlights
-
•
Sensitive and selective UPLC-MS/MS method was developed for simultaneous determination of 16 bisphenols.
-
•
Migration study was conducted on baby bottles and sippy cups purchased on Canadian market.
-
•
BPA and BPS were detected in all baby bottles and sippy cups.
-
•
The study suggests that repeated use of the baby bottles will not increase the leaching of BPA analogues.
1. Introduction
Bisphenol A (2,2-bis(4-hydroxyphenyl) propane; BPA) is an established anthropogenic endocrine disruptor (EDC) and one of the highest production volume (HPV) chemicals worldwide (Vandenberg et al., 2007). It is used in the production of polycarbonate (PC) plastics and epoxy resins, as well as in many consumer products including food containers, paper products, toys, medical equipment and electronics (Vandenberg et al., 2007). Widespread use of BPA in the plastic industry led to its extensive distribution in the environment, and hence unavoidable human exposure to the chemical via dietary and non-dietary sources (Geens et al., 2012; Usman and Ahmad, 2016). Hence, occurrence of BPA has been reported in human serum, urine, umbilical cord blood and breast milk, thus reflecting a more global human exposure (Geens et al., 2012; Rochester, 2013). Adverse effects of BPA on reproduction and development, cardiovascular, neurological, metabolic and immune systems have been well documented in in vitro and in vivo studies (Bonefeld-Jorgensen et al., 2007; Richter et al., 2007; vom Saal et al., 2007). The non-traditional dose response of BPA associated with its endocrine disrupting nature, prompted European Food Safety Authority (EFSA) to reduce the reference dose, also, the concern over health effects led to stronger restrictions and regulations on the production and usage of BPA in North America and the European Union (EU) (Resnik and Elliott, 2015). In 2010, the Canadian Government prohibited the import and sale of PC baby bottles and children's drinking cups containing BPA (Canada, 2010). The EU followed and prohibited the use of PC plastics in infant feeding bottles (Chen et al., 2016). The regulations imposed by governments along with public concerns over BPA, led to the development and production of alternative substances to replace BPA in various applications (Chen et al., 2016). A number of chemicals bearing structural similarity to BPA have been used in manufacture of plastics products and epoxy resins (Table 1). These chemicals share a common structure of two hydroxyphenyl functionalities and are thus, collectively referred to as “bisphenol A analogues (BPAAs)”. The main substitutes of BPA in the manufacture of PC plastics are BPS (4-hydroxyphenyl sulfone), BPF (4,4′-methylenediphenol) and BPAF (4,4′-hexafluoroisopropylidene) diphenol (Chen et al., 2016). The annual production of BPS was estimated to be 1–10 million pounds in US since 2011 (USEPA, 2018). There is a lack of data on production and usage of all the BPAAs; however, numerous studies have suggested that their production and application is on the rise globally as can be evidenced from their occurrence in environmental media, foods, food containers, consumer products and human biospecimens (Chen et al., 2016; Jin and Zhu, 2016; Liao et al., 2012a, Liao et al., 2012b; Naderi et al., 2014; Pivnenko et al., 2015; Wang et al., 2020a, Wang et al., 2020b; Zhang et al., 2019). Although studies on the toxicity of BPAAs are limited; nevertheless, reports show that for certain endpoints, BPAAs may have similar or higher adverse effects than BPA (Chen et al., 2016; Ji et al., 2013; Lee et al., 2013; Rochester and Bolden, 2015; Spewak et al., 2016; Usman and Ahmad, 2016). However, for the alternatives used in the manufacture of the baby bottles and drinking cups, either as monomers or as additives, only limited information is available on their potential leaching from these consumer products. Since infants have a lower bodyweight compared to adults, their exposure to food contact substances is higher; therefore, it is important to verify the absence of these BPA alternatives for safe bottle-feeding. There have been studies reporting migration of BPAAs from plastic food contact materials and water bottle (Bach et al., 2013; Hwang et al., 2018; Russo et al., 2019a, Russo et al., 2019b; Wang et al., 2020a, Wang et al., 2020b; Wang et al., 2019). However, there are limited studies on baby bottles and/or drinking cups.
Table 1.
Compound information, acronym, polarity, multiple reactions monitoring (MRM) transitions, MS scan parameters and method detection limit (MDL) for BPA and fifteen bisphenol analogues.
| Compounds | CAS # | Acronym | aLogP | bpKa | Scan Group | CV(V) | Quantifier, CE (eV) | Qualifier, CE (eV) | MDL (ng/L) |
|---|---|---|---|---|---|---|---|---|---|
| Bisphenol S (13C12) | NA | 13C-BPS | NA | NA | I | 42 | 260.5 > 113.7, 26 | NA | – |
| Bisphenol S | 80-09-1 | BPS | 1.65 | 7.42-8.03 | I | 42 | 248.5 > 107.6, 26 | 248.5 > 91.7, 36 | 0.10 |
| Bisphenol F (13C12) | NA | 13C-BPF | NA | NA | I | 36 | 210.9 > 98.7, 22 | NA, | – |
| Bisphenol F | 620-92-8 | BPF | 2.91 | 9.84-10.45 | I | 36 | 199.0 > 92.7, 20 | 199.0 > 104.7, 22 | 0.27 |
| Bisphenol E | 2081-08-5 | BPE | *3.90 | 9.81-10.42 | I | 38 | 213.0 > 197.6, 18 | 213.0 > 118.8, 22 | 0.20 |
| Bisphenol A (13C12) | NA | 13C-BPA | NA | NA | I | 42 | 238.7 > 223.7, 20 | NA, | – |
| Bisphenol A | 80-05-7 | BPA | 3.32 | 9.78-10.39 | I | 38 | 226.8 > 211.7, 18 | 226.8 > 132.8, 20 | 0.31 |
| Bisphenol B (13C12) | NA | 13C-BPB | NA | NA | II | 40 | 252.5 > 223.5, 20 | NA, | – |
| Bisphenol B | 77-40-7 | BPB | 4.13 | 9.77-10.38 | II | 40 | 241.2 > 211.7, 20 | 241.2 > 146.9, 27 | 0.23 |
| Bisphenol AF (13C12) | NA | 13C-BPAF | NA | NA | II | 34 | 346.5 > 276.8, 22 | NA | – |
| Bisphenol AF | 1478-61-1 | BPAF | 4.50 | 9.13-9.74 | II | 30 | 334.9 > 264.7, 20 | 334.9 > 196.8, 36 | 0.12 |
| Bisphenol AP | 1571-75-1 | BPAP | *4.40 | 9.66-10.27 | II | 50 | 288.8 > 273.7, 20 | 288.8 > 210.7, 28 | 0.15 |
| Bisphenol C | 79-97-0 | BPC | *4.70 | NA | II | 32 | 255.2 > 239.8, 18 | 255.2 > 146.9, 28 | 0.60 |
| Bisphenol Z | 843-55-0 | BPZ | *5.40 | 9.76-10.37 | II | 36 | 267.1 > 172.9, 30 | 267.1 > 222.9, 32 | 0.33 |
| Bisphenol BP | 1844-01-5 | BPBP | *5.60 | NA | III | 58 | 345.1 > 273.4, 22 | 345.1 > 257.8, 24 | 0.18 |
| Bisphenol M | 13595-25-0 | BPM | *6.10 | NA | III | 54 | 345.1 > 251.3, 28 | 345.1 > 132.8, 40 | 0.23 |
| Bisphenol G | 127-54-8 | BPG | *6.30 | NA | III | 55 | 311.2 > 295.0, 30 | 311.2 > 174.8, 30 | 0.16 |
| Bisphenol P | 2167-51-3 | BPP | *6.10 | 9.78-10.38 | III | 54 | 345.1 > 314.8, 38 | 345.1 > 329.7, 28 | 0.22 |
| Bisphenol TMC | 129188-99-4 | BPTMC | *6.30 | NA | III | 56 | 309.0 > 214.8, 28 | 309.0 > 199.7, 36 | 0.16 |
| Bisphenol PH | 24038-68-4 | BPPH | *7.30 | NA | III | 60 | 379.0 > 208.9, 38 | 379.0 > 362.8, 36 | 0.27 |
a: LogKow, experimental value cited from PubChem; *: computed values by XLogP3 3.0 (PubChem Release, 2019.06.18); b: computed values of pKa1-pKa2 by ChemAxon (Regueiro, 2015); NA = not available; scan group I: 0.00–6.25 min; scan group II: 6.25–8.00 min; scan group III: 8.00 to 2.00 min; CV = cone voltage; CE = collision energy. Reference: Regueiro, J., Breidbach, A., Wenzl, T. Rapid Commun. Mass Spectrom. 2015, 29, 1473–1484, https://doi.org/10.1002/rcm.7242.
The objective of the present study was to analyze selected 16 bisphenols that may be migrating/leaching from baby bottles and sippy cups, adopting experimental protocol recommended by the US Food and Drug Administration's (USFDA) Guidance for Industry Preparation of Pre-Market Submissions for Food Contact Substances: Chemistry Recommendations (2007) to cover repeated normal and repetitive use scenarios. The migration study was conducted on different brands of baby bottles and sippy cups available on the Canadian market.
2. Materials and methods
2.1. Standards and reagents
Bisphenol A (BPA) and BPA analogue standards (>98% purity) were purchased from AccuStandard (New Haven, CT, USA). Labelled BPAA standards (>98% purity) were obtained from Cambridge Isotope Laboratories (Tewksbury, MA, USA). The list of standards and relevant compound information are summarized in Table 1. HPLC-grade ethanol was obtained from VWR International (Mississauga, ON, Canada). HPLC-grade water and LC-MS grade methanol were purchased from EMD Millipore Corporation (Billerica, MA, USA). All standard stock solutions were prepared in LC-MS grade methanol.
2.2. Migration protocol
In total, twenty brands of baby bottles and thirteen brands of sippy cups were purchased online or from local retail stores in Eastern Ontario (Canada) in 2019. Food simulants were used in this study as outlined in US FDA (2007) guidance for industry: Preparation of Premarket Submissions for Food Contact Substances (Chemistry Recommendations) (FDA, 2007). In brief, HPLC-grade water was used to simulate drinking water and aqueous foods, 10% ethanol/HPLC water solution (v/v) to simulate foods that have hydrophilic properties such as acidic foods (e.g., juice products), and 50% ethanol/HPLC water solution (v/v) to simulate foods that have lipophilic properties, including covers of dairy and non-dairy fatty foods (e.g., infant formula). The migration testing was carried out at 40 °C for the following incubation time period: 2, 24, 96 and 240 h. The 10-day (240 h) testing period was included in order to mimic repetitive use of baby bottles or sippy cups. Time point zero corresponds to 2 h, although no incubation was required for time point of zero but 2 h was needed to allow the content of the bottle to be cooled down to the room temperature before sample (simulant) extraction.
In this study, all new bottles were used once and only the inside body (sans nipple) was tested. In addition, for each brand and migration period, only one baby bottle or sippy cup was used. Before the test, bottles were rinsed with HPLC-grade water three times to flush out any potential residual target chemical, and air dried. Rinsing food contact materials (FCMs) prior to testing is normally not recommended, as many potential target analytes may be also washed out in the process. However, it was done so to mimic real life scenario, where a bottle would be rinsed prior to first time use. The weights of empty and bottles filled with the simulants, were recorded. To mimic the home sterilization process, each tested bottle was filled instantly with hot boiling water or hot simulant prior to incubation. The bottles filled with simulant were placed in an incubator at 40 °C for the designated time to investigate potential time-dependent migration.
2.3. Sample preparation
Target analytes were extracted from the samples (simulants) as per the method described previously with minor modifications (Kubwabo et al., 2009). In brief, bottles with each testing simulant were taken out from incubator and rested for 2 h to reach the room temperature. Then, 20 μL of 1 μg/mL of internal standard mixture (containing five isotope labelled compounds: 13C-BPS, 13C-BPF, 13C-BPA, 13C-BPB and 13C-BPAF) was spiked into each bottle and mixed well prior to SPE extraction (HLB, 200mg/5 mL, glass tube, Waters). HLB cartridges were conditioned with methanol and water (6 mL each) prior to the extraction process. Each sample was loaded onto the SPE cartridge under vacuum at a flow rate of approximately 5 mL/min. The cartridges were then rinsed with 6 mL of 5% methanol and target analytes were eluted with 6 mL of methanol. The extracts were concentrated to near dryness under a gentle stream of nitrogen at 40 °C in a water bath, reconstituted in 200 μL of 50% methanol, vortexed and transferred to amber vials for LC-MS/MS analysis.
2.4. UPLC-MS/MS
The separation and quantitation of bisphenol A analogues were performed on a Waters Acquity Ultra Performance Liquid Chromatography (UPLC) system (Milford, MA, USA) coupled to a Waters Xevo TQD triple quadrupole mass spectrometer (Milford, MA, USA). Ten μL of sample extract was injected in full loop and the separation was performed at 30 °C on an Acquity UPLC BEH C18 column (1.7 μm, 2.1 mm × 50 mm, Waters) with a Van Guard BEH C18 pre-column (1.7 μm, 2.1 × 5 mm, Waters). The mobile phase consisted of (A): 5% methanol in water and (B): 2 mM NH4OH in methanol. The gradient program was as follows: initial gradient 100% (A) hold for 1min, to 55% (B) in 1 min, to 100% (B) in 10 min, back to 100% (A) in 0.5 min, and hold for 3.5 min at 100% (A). Flow rate was set at 0.2 mL/min. The MS/MS was operated in multiple reaction monitoring (MRM) using electrospray ionization in negative ion mode (ESI-). The MRM transitions and MS parameters were obtained and optimized using Waters IntelliStart (Waters, MA, USA). MRM transitions and optimized MS parameters are summarized in Table 1. Source temperature, desolvation temperature, and gas flow were set at 150 °C, 350 °C and 600 L/h, respectively. Capillary voltage was set at 2.5 kV and extractor voltage at 10 V.
3. Results and discussion
3.1. Method optimisation and performance
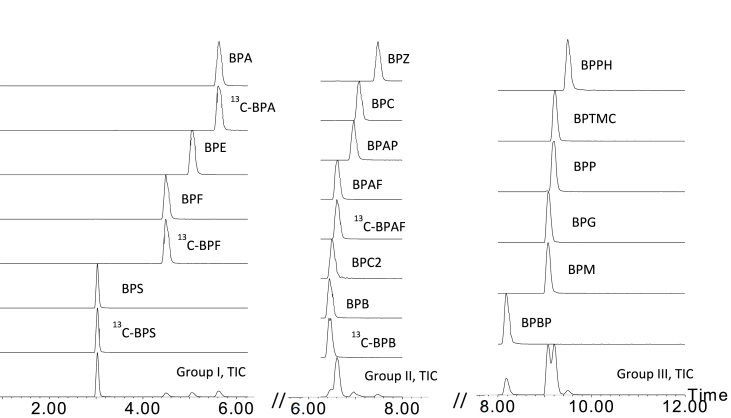
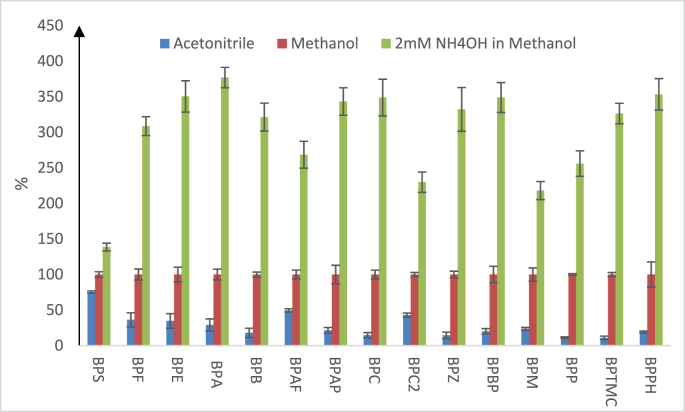
The MRM transitions were divided into three scan groups according to respective retention time, which significantly increased individual analyte's dwell time. Sixteen target BPAAs were separated in 12 min. For co-eluted compounds (i.e., pairs of native and labelled compounds) or those with limited LC resolution (with similar polarity or pairs of isomers), resolving power of MRM allowed their identification regardless of LC resolution, which is clearly demonstrated by the MRM chromatogram in Fig. 1. Considering the weak acidic property of BPAAs, basic pH conditions in theory should facilitate targets' ionization efficiency, which can lead to improved LC-MS/MS sensitivity. In this study, the commonly used solvents (1) acetonitrile and (2) methanol were tested first, and the results suggested significant higher response of BPAAs with methanol as mobile phase. Furthermore, methanol with 2 mM NH4OH was tested to investigate the effect of basic condition. All the tests employed same gradient as described above in materials and methods. Tests were conducted by injecting 50 ng/mL of a mixture of target analytes in triplicate for each condition. By taking average peak area of individual compound in 100% methanol, relative percentile response in other conditions was calculated for comparison study. As presented in Fig. 2, it clearly indicates that acetonitrile significantly reduced response to 28.1% (10.9%–75.3%) in average when compared to that in methanol. On the other hand, 2 mM NH4OH in methanol significantly increased BPAAs' response to 301.4% on average. However, basic condition resulted in variable effect among individual target analytes. Most likely, pKa value plays key role in causing such differences. BPA analogues are group of weak acid compounds, according to their structure and available computed pKa value (Table 1). In general, ionization efficiency of a compound is positively correlated to its dissociation degree, which depends on its dissociation constant (pKa) and solvent property. For weak acids, NH4+ can facilitate equilibrium toward dissociation and that lead to increased LC-MS/MS response. In this study, it was noticed the weaker the acidic nature or dissociation tendency (lower pKa value) of a compound, the larger was the degree of increased response in basic condition. It should be mentioned that dissociation constant of BPA analogues in methanol (or mixture of water and methanol) is not available, the listed computed pKa value in Table 1 can only be used to compare their relative acidity or dissociation tendency in methanol. Out of sixteen BPA analogues analysed, BPS is the most acidic compound, based on its pKa value (pKa1 = 7.42) and therefore it is the compound with highest dissociation degree in methanol, which could explain the response increase of 38.6% in basic condition. Compared to BPS, as listed in Table 1, pKa1 value of other BPA analogues ranged from 9.13 (BPAF) to 9.84 (BPF), which suggest much lower dissociation degree in methanol and furthermore higher response increase in basic conditions. In this study, mobile phase A was 5% methanol, and NH4OH concentration in mobile phase B was tested at concentration of 2 mM, 5 mM and 20 mM in methanol. However, 5 mM of NH4OH and 20 mM of NH4OH in methanol did not further increase the response of target analytes. However, 20 mM NH4OH caused significant BPS retention time shifting (early shift) and tailing peak shape. Based on these test results, it is recommended that the concentration of NH4OH in methanol should be optimized to maximize instrument sensitivity but not compromising UPLC separation performance.
Fig. 1.
A typical LC-MS/MS MRM chromatograms obtained at injection of 100 ng/mL BPAAs standard mixture prepared in 50% methanol.
Fig. 2.
Effect of different solvents or additives to BPAAs' LC-MS/MS response. All tests were conducted by triplicate injections of 50 ng/mL standard mixture at same LC gradient. Average peak area of individual compound in methanol was considered as 100%. Mean and standard deviation (SD) of relative percentile response was plotted among sixteen individual compounds.
The method detection limits (MDL), presented in Table 1, was determined according to the EPA Regulation 40 CFR part 136 method, whereby the standard deviation associated with eight replicate analyses of different simulants spiked with target analytes at 0.2 ng/L per sample and processed through the entire analytical procedure was multiplied by the Student's t value of 2.998 (99% confidence level) (USEPA, 2016). The relative percent recoveries were based on the recoveries of the labelled internal standards, which was greater than 80% for all the target analytes. Quantitation was conducted by using matrix-matched extracted calibration curves established in 250 mL of three different simulants:100% water, 10% ethanol/water and 50% ethanol/water respectively, and multiple isotope labelled internal standards was applied, which compensates for loss of targets during sample preparation and possible sample matrix effect. The matrix-matched calibration curves were linear over a concentration range from 0.5 ng/mL to 500 ng/mL with coefficient of correlation (r2) greater than 0.998 for all of the compounds of interest. Intra-day reproducibility and inter-day variability was investigated and it was less than 10%. Extracted calibration samples, blank and quality control samples were prepared in pre-cleaned glass bottle and processed in the same way as the samples (simulants). Spiking level of BPAAs in quality control (QC) samples was 20 ng/L. Level in blank was subtracted to calculate the final test results.
3.2. Concentrations in baby bottles and sippy cups
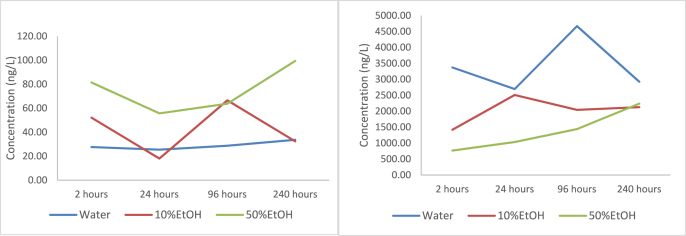
Twenty brands of baby bottles and thirteen brands of sippy cups were tested. The most frequently detected bisphenols were BPS, BPA, BPF, BPAF, BPM and BPTMC in baby bottles with a detection frequency (DF) of more than 50%, whereas in sippy cups only BPA, BPS and BPF were detected with a DF > 50%. For the three incubation matrices (Water, 10% EtOH, 50% EtOH), the highest concentration for the most detected analytes was observed at the 2-h time point. No increasing trend in concentration of the bisphenol A analogues was observed for the 4-different incubation periods (2 h, 24 h, 96 h and 240 h), thus, the concentrations discussed are for the 2-h incubation time. The detection frequency, mean concentration and median concentration of the target analytes for the baby bottles and sippy cups are presented in Table 2, Table 3, respectively. The mean concentration of BPA in baby bottles was 31.5 ng/L in water simulant whereas a 1.4-fold increase was observed in 50% EtOH simulant. Similarly, 1.4-fold increase was seen in the mean concentration of BPS in 50% EtOH simulant, when compared to the mean concentration of 2.33 ng/L in water simulant. Increasing median concentration was seen for BPA in the presence of fatty food simulants (water<10% EtOH<50% EtOH). As previously mentioned, no increase in leaching of target analytes was seen after the 2 h' time point. Similar observation has been reported in a non targeted analysis of BPA analogue from plastic baby bottles conducted in Belgium (Onghena et al., 2014). In a Korean study conducted on food contact materials, none of the BPA analogues was detected (Hwang et al., 2018). Recently, a migration study on PET and PC bottled water reported the detection of BPS, BPAP and BPAF in PC bottled water, whereas BPE and BPF were detected in PET bottles (Wang et al., 2020a, Wang et al., 2020b). Although, the experimental conditions in the study by Wang et al., 2020a, Wang et al., 2020b were not similar to the current study conditions, it is interesting to note the difference in leaching/migration of the BPA analogues in the two plastic materials (i.e., PC and PET bottles). In a study conducted in Italy, eleven brands of baby bottles were tested for the migration of BPA and BPS, and none of the brands showed detectable levels of BPS (Russo et al., 2018), with the levels in most of the baby bottles were below their limit of detection. However, the highest level of BPA reported in the Italian study was 102.18 ng/mL, whereas the highest level in the present study was 0.22 ng/mL for similar simulant. In a study conducted in Jordan on 15 baby bottles labelled as ‘BPA-free’, although no residual BPA was found in the bottles before testing, results from migration test showed that the concentration of BPA was high, with a mean concentration of 1890 ng/L (Ali et al., 2018). The high BPA level in the Jordan study suggests that the ‘BPA-free’ claim is misleading (Rochester and Bolden, 2015). Therefore, caution should be exercised as drinking bottles labelled ‘BPA-free’ with recycling symbol ‘7’ are often freely distributed in various events. Although, a number of unrelated plastics can carry a code 7, including Tritan, polycarbonate, nylon and even the newer bio-plastics (Eastman Chemical Company, 2021). The mean concentration of BPA at 24 h for water and 50% EtOH in the migration study in 2009, was 3.2 times and 33 times higher when compared to the concentration of BPA for the same time-point in this study, suggesting that regulations for the manufacture of baby bottles in Canada are respected (Kubwabo et al., 2009). In the present study, data suggest that bisphenol A and its analogues remained on the surface of the bottles during the manufacturing process of baby bottles made in non-PC materials. In another migration study conducted in Canada on 30 reusable plastic bottles, no BPA or its analogues were detected in the food simulants which was an indication that all tested bottles were free of BPA, and also that bisphenol analogues were not used as BPA replacements in the manufacture of the bottles (Tian et al., 2019). In this study, there was no correlation between increasing exposure (incubation) time and the concentrations of BPAAs in the simulants. However, there was one brand where we noticed an increase in the concentration of BPAP when 50% EtOH simulant was used. For the same brand an increase was also observed in the concentration of BPA, from the 2-h time point to 240-h time point; however, the trend for BPA was not as significant as was for BPAP (Fig. 3). A 1.3-fold, 1.9-fold and 2.9-fold increase in BPAP concentration was observed for the 3-time points, when compared with the concentration at 2-h incubation. For BPA, a 1.04-fold increase for 96 h and 1.21-fold increase for 240-h incubation was recorded. In a previous study by Kubwabo et al. (2009) on PC baby bottles, a clear trend in the migration of BPA in baby bottles was seen with time and temperature (Kubwabo et al., 2009), which was not the case in this study for any brand of baby bottle. It will be worth mentioning here, that the migration study on baby bottles conducted in 2007, was done prior to the ban on the usage of BPA by Government of Canada in 2008.
Table 2.
Detection frequency, mean and median of bisphenol analogues in baby bottles (N = 20).
| Compound | Detection Frequency (%) |
Mean (ng/L) |
Median (ng/L) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | 10% EtOH | 50% EtOH | Water | 10% EtOH | 50% EtOH | Water | 10% EtOH | 50% EtOH | |
| BPS | 100 | 100 | 100 | 2.33 | 0.65 | 3.19 | 1.97 | 0.49 | 2.49 |
| BPF | 80 | 90 | 35 | 4.70 | 1.09 | 1.41 | 1.43 | 0.69 | <LOD |
| BPA | 100 | 100 | 100 | 31.50 | 23.33 | 45.33 | 13.88 | 15.13 | 48.01 |
| BPAF | 25 | 100 | 95 | 0.18 | 0.34 | 2.07 | <LOD | 0.31 | 1.93 |
| BPPH | 40 | 0 | 0 | 5.08 | ND | ND | <LOD | ND | ND |
| BPM | 55 | 0 | 0 | 3.23 | ND | ND | 0.52 | ND | ND |
| BPG | 35 | 0 | 0 | 1.44 | ND | ND | <LOD | ND | ND |
| BPTMC | 55 | 0 | 0 | 3.96 | ND | ND | 0.36 | ND | ND |
| BPAP | 15 | 10 | 5 | 0.16a | N/Aa | N/Aa | <LOD | <LOD | <LOD |
| BPE | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
| BPB | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
| BPC | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
| BPZ | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
| BPBP | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
There was one brand where the level of BPAP was extremely high, therefore, it was not included in calculating the mean.
Table 3.
Detection frequency, mean and median of bisphenol analogues in sippy cups (N = 13).
| Compound | Detection Frequency (%) |
Mean (ng/L) |
Median (ng/L) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | 10% EtOH | 50% EtOH | Water | 10% EtOH | 50% EtOH | Water | 10% EtOH | 50% EtOH | |
| BPS | 100 | 100 | 100 | 0.42 | 0.66 | 3.56 | 0.28 | 0.50 | 1.54 |
| BPF | 54 | 69 | 73 | 1.35 | 0.53 | 7.18 | 0.51 | 0.50 | 1.36 |
| BPA | 100 | 100 | 100 | 16.42 | 37.27 | 54.94 | 7.20 | 42.80 | 55.17 |
| BPAF | 8 | 100 | 64 | <LOD | 0.34 | 1.24 | <LOD | 0.31 | 1.52 |
| BPPH | 38 | 0 | 0 | 0.82 | ND | ND | <LOD | ND | ND |
| BPM | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
| BPG | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
| BPTMC | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
| BPE | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
| BPB | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
| BPC | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
| BPZ | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
| BPBP | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
| BPAP | 0 | 0 | 0 | ND | ND | ND | ND | ND | ND |
Fig. 3.
Levels of BPA (a) and BPAP (b) in different time-points for the three simulants. Data here is only for one brand of baby bottle (brand #5).
For sippy cups, the mean concentration of BPS and BPA was highest for 50% EtOH. An 8.5-fold increase was observed for the mean concentration of BPS in 50% EtOH simulant when compared to the mean concentration in water simulant. The increasing order of the mean as well as median concentration was observed for BPA for the 3 simulants as follows: Water <10% EtOH <50% EtOH. To the best of the authors' knowledge, this study reports for the first time bisphenol A and its analogues migration data from sippy cups. Albeit the levels are low, nevertheless, it is important to note that toddlers often drink fruit juices and water from sippy cups and the cumulative effect of bisphenols leaching from the cups along with usage of other plastic utensils might result in more intake of these chemicals, as was also suggested by another research group (Ali et al., 2018). Substitutes to polycarbonates have entered EU market include polypropylene (PP), silicone, polyamide (PA), polyethersulphone (PES) and a new co-polyester named ‘Tritan’ (Simoneau, Van den Eede and Valzacchi, 2012). Similar substitutes to PC can be expected to be used globally. For these materials, monomers or additives can migrate from the plastic to the simulant (Guart et al., 2013). Of the 20 brands analysed in this study, 10 were made of polypropylene, one was made from Tritan and for the remaining nine baby bottles were made of material kept confidential; most likely those bottles were made from a polypropylene copolymer material. In all the 20 brands investigated, BPA analogues were detected at low concentration. Similar results were reported in a study by Onghena et al. (2014) on baby bottles made of PP (Onghena et al., 2014). For sippy cup, during sample preparation, it was observed that 3 extracts were coloured, and the coloration was similar to the colour of the cup. Usage of pigments as additives in many products made of plastic has been reported (Hahladakis et al., 2018). However, what led to the development of the colour was not explored.
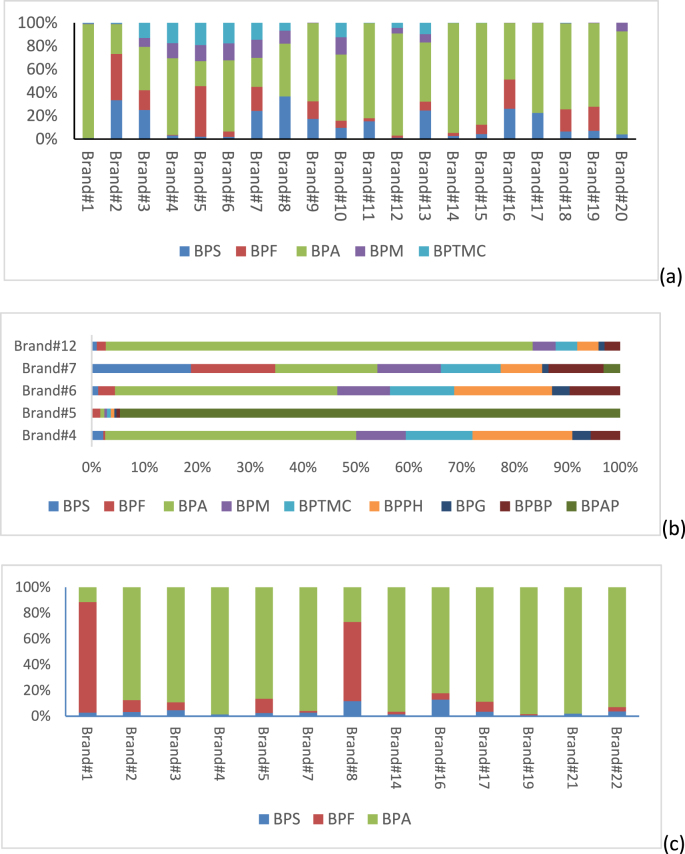
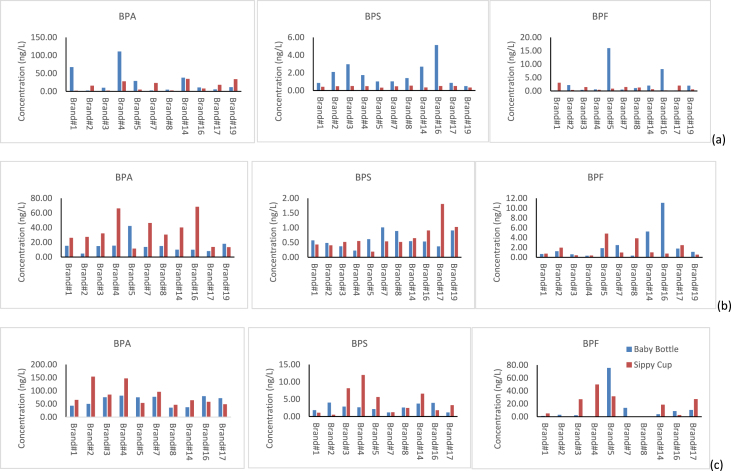
3.3. Contribution pattern (%) in different brands of baby bottles
The concentration pattern of the BPA analogues for baby bottles and sippy cups in water simulant is presented in Fig. 4. It was observed that the concentrations (%) of the analytes varied quite considerably among the 20 baby bottle brands. For brand #2, BPS, BPA and BPM were the major constituents, whereas for brand #5, BPF was the major constituent and for brand #1 it was BPA. Although, BPA still represents one of the most abundant xenoestrogen studied in food matrices, other analogues have been detected at higher concentrations indicating that industries are switching to other bisphenols (Russo et al., 2019a, Russo et al., 2019b). In this study, BPA was not the major contributor for some baby bottle brands. However, in 50% EtOH, BPA was found to be the major contributor for all the 20 brands, which can be explained by the leaching strength of the simulant. For five brands under investigation (brand #4, 5, 6, 7, 12), nine bisphenols (BPA, BPA, BPF, BPM, BPTMC, BPPH, BPG, BPBP and BPAP) were detected above their respective limits of quantitation. The contributing pattern (%) is presented in Fig. 4. In the case of sippy cups, the major contributor was BPA, followed by BPS. However, for 2 brands (brands #1 and #8) the major contributor was BPF.
Fig. 4.
Contribution (%) of the bisphenols in (a) all 20 brands of baby bottles; (b) the five brands with the highest number of BPAA detects; (c) all 13 brands of sippy cups.
3.4. Comparison of levels in matched baby bottles and sippy cups
Of the 20 brands of baby bottles and 13 brands of sippy cups analysed, there were eleven paired brands of baby bottles and sippy cups (i.e., same brand name). When the levels of the bisphenols were compared in matched baby bottles and sippy cups, it was observed that for water simulant the level of BPS was higher in baby bottles than in sippy cups and the difference ranged from 1.5 to 10 fold (Fig. 5). It is interesting to note that, when the simulant was 50% EtOH, the level of BPS was higher in sippy cups than in baby bottles. In the case of BPA, no difference was observed for water. However, a clear difference was noticed when the simulant was 10% EtOH and 50% EtOH, where the level of BPA was higher in sippy cups than the baby bottles, suggesting stringent quality control during the process of manufacturing baby bottles, and likely less control in the manufacture of sippy cups. It is really difficult to make a valid conclusion since no information is available about the manufacturers of ‘paired’ baby bottles and sippy cups.
Fig. 5.
Comparison of BPA, BPS and BPF in matched brand of baby bottle and sippy cup. (a): Water simulant; (b):10% EtOH simulant; (c) 50% EtOH simulant.
4. Conclusion
A sensitive and selective UPLC-MS/MS method was developed for the analysis of replacement chemicals to bisphenol A. In this migration study conducted on 20 brands of baby bottles and 13 brands of sippy cups, BPA and BPS were detected in the simulants for all the brands tested. Most of the BPA analogues were found to be present in bottles made of polypropylene, albeit in low concentration. However, because of the scarcity of toxicity data the potential health significance of the low concentration of the chemicals detected remains unknown. The present migration study also suggests that repeated use of the baby bottles does not increase the leaching of BPA analogues. Also, it can be deduced that bisphenol A and its analogues measured could be a contamination during the manufacturing process and therefore remained on the surface of the bottles. Exposure to the chemicals from other sources like water, maternal exposure, ambient air/dust has to be taken into account to assess the risk of these chemicals to infants. Although this study suggests, low leaching of bisphenol analogues into the simulants, nevertheless they warrant a regular and accurate monitoring of these chemicals, which also act as endocrine disruptors because of the vulnerability of the exposed group. As the newborns and infants have reduced metabolic capabilities, the bioaccumulation of these chemicals can be greater and much more detrimental as compared to adult organisms. Regular use drinking water bottles, used by both adolescents and adults, are often made with plastic materials, this study also calls for an investigation of the leaching of BPA analogues in those bottles.
CRediT authorship contribution statement
Shabana Siddique: Sample preparation, Writing – original draft, preparation. Gong Zhang: Formal analysis, Sample preparation, instrument analysis and data processing. Kaela Coleman: Conceptualization, sample preparation, Writing – review & editing. Cariton Kubwabo: Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors acknowledge the financial contribution of Health Canada. The authors also thank Gordon Barrett of Risk Assessment Bureau (CHPSD), Health Canada and Dharani Das of Environmental Health Science and Research Bureau, Health Canada for their valuable comments.
References
- Ali M., Jaghbir M., Salam M., Al-Kadamany G., Damsees R., Al-Rawashdeh N. Testing baby bottles for the presence of residual and migrated bisphenol A. Environ. Monit. Assess. 2018;191(1):7. doi: 10.1007/s10661-018-7126-0. [DOI] [PubMed] [Google Scholar]
- Bach C., Dauchy X., Severin I., Munoz J.F., Etienne S., Chagnon M.C. Effect of temperature on the release of intentionally and non-intentionally added substances from polyethylene terephthalate (PET) bottles into water: chemical analysis and potential toxicity. Food Chem. 2013;139(1–4):672–680. doi: 10.1016/j.foodchem.2013.01.046. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen E.C., Long M., Hofmeister M.V., Vinggaard A.M. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ. Health Perspect. 2007;115(Suppl. 1):69–76. doi: 10.1289/ehp.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada . Vol. 144. Government of Canada; 2010. p. 7.http://www.chemicalsubstanceschimiques.gc.ca/challeng-defi/batch-lot-2/bisphenol-a/bpa-risk_hazard-eng.php (Government of Canada. Order Amending Schedule I to the Hazardous Products Act (Bisphenol A), Part II). Retrieved from. [Google Scholar]
- Chen D., Kannan K., Tan H., Zheng Z., Feng Y.L., Wu Y., Widelka M. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity-A review. Environ. Sci. Technol. 2016;50(11):5438–5453. doi: 10.1021/acs.est.5b05387. [DOI] [PubMed] [Google Scholar]
- FDA U. 2007. Guidance for Industry: Preparation of Premarket Submissions for Food Contact Substances.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-preparation-premarket-submissions-food-contact-substances-chemistry#iid1c [Google Scholar]
- Geens T., Aerts D., Berthot C., Bourguignon J.P., Goeyens L., Lecomte P. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012;50(10):3725–3740. doi: 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Guart A., Wagner M., Mezquida A., Lacorte S., Oehlmann J., Borrell A. Migration of plasticisers from Tritan and polycarbonate bottles and toxicological evaluation. Food Chem. 2013;141(1):373–380. doi: 10.1016/j.foodchem.2013.02.129. [DOI] [PubMed] [Google Scholar]
- Hahladakis J.N., Velis C.A., Weber R., Iacovidou E., Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard Mater. 2018;344:179–199. doi: 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Hwang J., Bae I.A., Lee C., Lee S., Choi J.C., Park S.J. Simultaneous analysis and exposure assessment of migrated bisphenol analogues, phenol, and p-tert-butylphenol from food contact materials. Food Addit. Contam. Part A Chem Anal Control Expo Risk Assess. 2018;35(11):2270–2278. doi: 10.1080/19440049.2018.1523571. [DOI] [PubMed] [Google Scholar]
- Ji K., Hong S., Kho Y., Choi K. Effects of bisphenol s exposure on endocrine functions and reproduction of zebrafish. Environ. Sci. Technol. 2013;47(15):8793–8800. doi: 10.1021/es400329t. [DOI] [PubMed] [Google Scholar]
- Jin H., Zhu L. Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China. Water Res. 2016;103:343–351. doi: 10.1016/j.watres.2016.07.059. [DOI] [PubMed] [Google Scholar]
- Kubwabo C., Kosarac I., Stewart B., Gauthier B.R., Lalonde K., Lalonde P.J. Migration of bisphenol A from plastic baby bottles, baby bottle liners and reusable polycarbonate drinking bottles. Food Addit. Contam. Part A Chem Anal Control Expo Risk Assess. 2009;26(6):928–937. doi: 10.1080/02652030802706725. [DOI] [PubMed] [Google Scholar]
- Lee S., Liu X., Takeda S., Choi K. Genotoxic potentials and related mechanisms of bisphenol A and other bisphenol compounds: a comparison study employing chicken DT40 cells. Chemosphere. 2013;93(2):434–440. doi: 10.1016/j.chemosphere.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Liao C., Liu F., Guo Y., Moon H.B., Nakata H., Wu Q., Kannan K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ. Sci. Technol. 2012;46(16):9138–9145. doi: 10.1021/es302004w. [DOI] [PubMed] [Google Scholar]
- Liao C., Liu F., Moon H.B., Yamashita N., Yun S., Kannan K. Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: spatial and temporal distributions. Environ. Sci. Technol. 2012;46(21):11558–11565. doi: 10.1021/es303191g. [DOI] [PubMed] [Google Scholar]
- Naderi M., Wong M.Y., Gholami F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat. Toxicol. 2014;148:195–203. doi: 10.1016/j.aquatox.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Onghena M., van Hoeck E., Vervliet P., Scippo M.L., Simon C., van Loco J., Covaci A. Development and application of a non-targeted extraction method for the analysis of migrating compounds from plastic baby bottles by GC-MS. Food Addit. Contam. Part A Chem Anal Control Expo Risk Assess. 2014;31(12):2090–2102. doi: 10.1080/19440049.2014.979372. [DOI] [PubMed] [Google Scholar]
- Pivnenko K., Pedersen G.A., Eriksson E., Astrup T.F. Bisphenol A and its structural analogues in household waste paper. Waste Manag. 2015;44:39–47. doi: 10.1016/j.wasman.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Resnik D.B., Elliott K.C. Bisphenol A and risk management ethics. Bioethics. 2015;29(3):182–189. doi: 10.1111/bioe.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C.A., Birnbaum L.S., Farabollini F., Newbold R.R., Rubin B.S., Talsness C.E. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24(2):199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester J.R. Bisphenol A and human health: a review of the literature. Reprod. Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Rochester J.R., Bolden A.L. Bisphenol S and F: a systematic review and comparison of the hormonal activity of Bisphenol A substitutes. Environ. Health Perspect. 2015;123(7):643–650. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo G., Barbato F., Cardone E., Fattore M., Albrizio S., Grumetto L. Bisphenol A and Bisphenol S release in milk under household conditions from baby bottles marketed in Italy. J Environ Sci Health B. 2018;53(2):116–120. doi: 10.1080/03601234.2017.1388662. [DOI] [PubMed] [Google Scholar]
- Russo G., Barbato F., Mita D.G., Grumetto L. Occurrence of Bisphenol A and its analogues in some foodstuff marketed in Europe. Food Chem. Toxicol. 2019;131:110575. doi: 10.1016/j.fct.2019.110575. [DOI] [PubMed] [Google Scholar]
- Russo G., Varriale F., Barbato F., Grumetto L. Are canned beverages industries progressively switching to Bisphenol AF? J. Food Sci. 2019;84(11):3303–3311. doi: 10.1111/1750-3841.14833. [DOI] [PubMed] [Google Scholar]
- Simoneau C., Van den Eede L., Valzacchi S. Identification and quantification of the migration of chemicals from plastic baby bottles used as substitutes for polycarbonate. Food Addit. Contam. Part A Chem Anal Control Expo Risk Assess. 2012;29(3):469–480. doi: 10.1080/19440049.2011.644588. [DOI] [PubMed] [Google Scholar]
- Spewak M.B., Williamson R.S., Mertens A.C., Border W.L., Meacham L.R., Wasilewski-Masker K.J. Yield of screening echocardiograms during pediatric follow-up in survivors treated with anthracyclines and cardiotoxic radiation. Pediatr. Blood Canc. 2016 doi: 10.1002/pbc.26367. [DOI] [PubMed] [Google Scholar]
- Tian L., Lin L., Bayen S. Optimization of the post-acquisition data processing for the non-targeted screening of trace leachable residues from reusable plastic bottles by high performance liquid chromatography coupled to hybrid quadrupole time of flight mass spectrometry. Talanta. 2019;193:70–76. doi: 10.1016/j.talanta.2018.09.070. [DOI] [PubMed] [Google Scholar]
- USEPA . 2016. Definition and Procedure for the Determination of the Method Detection Limit, Revision 2.https://www.epa.gov/sites/production/files/2016-12/documents/mdl-procedure_rev2_12-13-2016.pdf Retrieved from. [Google Scholar]
- Usman A., Ahmad M. From BPA to its analogues: is it a safe journey? Chemosphere. 2016;158:131–142. doi: 10.1016/j.chemosphere.2016.05.070. [DOI] [PubMed] [Google Scholar]
- Vandenberg L.N., Hauser R., Marcus M., Olea N., Welshons W.V. Human exposure to bisphenol A (BPA) Reprod. Toxicol. 2007;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- vom Saal F.S., Akingbemi B.T., Belcher S.M., Birnbaum L.S., Crain D.A., Eriksen M. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 2007;24(2):131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liu Z.H., Tang Z., Zhang J., Yin H., Dang Z. Bisphenol analogues in Chinese bottled water: quantification and potential risk analysis. Sci. Total Environ. 2020;713:136583. doi: 10.1016/j.scitotenv.2020.136583. [DOI] [PubMed] [Google Scholar]
- Wang H., Liu Z.H., Zhang J., Huang R.P., Yin H., Dang Z. Human exposure of bisphenol A and its analogues: understandings from human urinary excretion data and wastewater-based epidemiology. Environ. Sci. Pollut. Res. Int. 2020;27(3):3247–3256. doi: 10.1007/s11356-019-07111-9. [DOI] [PubMed] [Google Scholar]
- Wang H., Song S., Shao M., Gao Y., Yang C., Li Y. Determination of bisphenol analogues in food-contact plastics using diode array detector, charged aerosol detector and evaporative light-scattering detector. Ecotoxicol. Environ. Saf. 2019;186:109778. doi: 10.1016/j.ecoenv.2019.109778. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang Y., Li J., Yang M. Occurrence and exposure assessment of bisphenol analogues in source water and drinking water in China. Sci. Total Environ. 2019;655:607–613. doi: 10.1016/j.scitotenv.2018.11.053. [DOI] [PubMed] [Google Scholar]