Abstract
Background
Abiraterone and enzalutamide use is associated with significant cardiovascular (CV) morbidity in clinical trials, but the magnitude and clinical relevance of this association in real-world prostate cancer (PC) population remain unknown.
Materials and methods
We retrospectively reviewed the MarketScan claims databases (1 January 2013 to 30 September 2018) to identify adults with diagnosis of metastatic PC who received treatment with androgen deprivation therapy (ADT) and novel antiandrogen agents (abiraterone or enzalutamide). The primary CV outcome measure was composite outcome of acute myocardial infarction (MI) or stroke. Secondary outcomes were individual risks of MI or stroke. We used an intention-to-treat approach to analyze the CV outcomes associated with drug exposure among patients with metastatic PC. Cox regression model was used to estimate the independent association of two drugs with CV risk after adjustment for age, baseline atrial fibrillation, and Charlson Comorbidity Index.
Results
A total of 6294 patients with metastatic PC who were treated with ADT and either abiraterone or enzalutamide were included in the final analysis. Of these, 4017 (63.8%) patients used abiraterone and 2217 (32.2%) patients used enzalutamide. During the study period, 255 (6.3%) primary endpoint events occurred, resulting in an incidence rate of 4.3 per 100 patient-years. In multivariable analysis, abiraterone use was associated with a 31% increased risk of MI or stroke compared to enzalutamide (hazard ratio 1.31; 95% confidence interval 1.05-1.63; P = 0.01). The incidence rate was similar in patients who switched initial therapy from abiraterone to enzalutamide or vice versa (5.0 versus 5.6 per 100 patient-years, respectively).
Conclusions
To our knowledge, this is the first real-world assessment of MI and stroke among metastatic PC patients receiving novel anti-androgens. Our findings of increased MI and stroke risk with abiraterone compared with enzalutamide are consistent with data from clinical trials and suggest that enzalutamide may be preferable for prostate cancer patients at high CV risk.
Key words: metastatic prostate cancer, cardiovascular toxicity, abiraterone, enzalutamide, stroke, myocardial infarction
Highlights
-
•
Abiraterone and enzalutamide have comparable efficacy but substantial differences in CV toxicity.
-
•
We identified metastatic PC patients treated with ADT and abiraterone or enzalutamide from insurance claims-based database.
-
•
Abiraterone use was associated with a 31% increased risk for MI or stroke when compared to enzalutamide.
-
•
Enzalutamide may be preferable in patients with baseline high CV risk.
Introduction
Castrate-resistant prostate cancer (CRPC) is a lethal state of advanced prostate cancer (PC) resulting from tumor adaptation to a low testosterone milieu. Typically, patients who develop metastatic CRPC do so after 3-8 years of response to androgen deprivation therapy (ADT).1 Prolonged ADT exposure in an aging population is associated with increased cardiovascular (CV) morbidity.2 Novel antiandrogen therapies like abiraterone and enzalutamide also target the hormonal axis; therefore, concern about potential increased CV mortality with these therapies is justified.3
Enzalutamide is a second-generation androgen receptor (AR) inhibitor, and abiraterone is a 17α-hydroxylase/c-17,20-lyase (CYP17) inhibitor that reduces adrenal and intratumoral androgen synthesis.4,5 Abiraterone combined with low-dose prednisone was first approved by US Food and Drug Administration (FDA) in April 2011 to treat patients with metastatic CRPC who have received prior docetaxel chemotherapy.6 Enzalutamide was FDA-approved for the same indication in August 2012.7 Since then, both drugs have expanded FDA approval for earlier stages of PC, where the duration of treatment may extend to several years.8, 9, 10, 11 CV toxicity has emerged as an important side-effect of these therapies given that most patients are older and have other baseline CV comorbidities. Data on CV adverse effects from ADT have conflicting evidence.2 Safety data from seminal phase III clinical trials suggest increased relative risk of CV toxicity with both novel anti-androgens in comparison to ADT alone.6, 7, 8, 9, 10, 11 A meta-analysis comparing CV toxicities of abiraterone and enzalutamide showed that abiraterone was associated with significantly increased risk of grade ≥3 hypertension (HTN) and CV toxicities when compared to placebo.12 On the other hand, enzalutamide increases the risk of grade 3 HTN without any significantly increased risk for other cardiac events. However, a major limitation of these is the lack of specific incidence of clinically relevant CV events like myocardial infarction (MI) and stroke in a large majority of these studies. Given the importance of CV outcomes among cancer patients,13 it is crucial to understand the comparative CV risks of abiraterone or enzalutamide among CRPC patients concurrently treated with ADT, to make informed treatment decisions. In this analysis, we used individual patient-level data from a large national insurance claims database to test the hypothesis that abiraterone versus enzalutamide is associated with greater risk of MI and stroke, among metastatic CRPC patients concurrently treated with ADT.
Materials and methods
Study population
IBM MarketScan® Commercial Claims and Encounter and Medicare Supplemental and Coordination of Benefits databases for calendar years 2013 through 2018 were used in the present analysis. These administrative databases contain individual-level, de-identified, Health Insurance Portability and Accountability Act of 1996 (HIPAA)-compliant, health care claims information from US employers, health plans, hospitals, and Medicare programs. Individual-level identifiers are used to link data across enrollment records and inpatient, outpatient, ancillary, and drug claims. The University of Minnesota Institutional Review Board deemed this research exempt from review. In the past, our group has successfully used MarketScan database for large-scale pharmacoepidemiologic studies.14, 15, 16
In the present analysis, we included patients aged 18-99 years who were enrolled in the database at any point between 1 January 2013 and 30 September 2018. Patients with a diagnosis of PC were identified by at least one inpatient claim or two outpatient claims 7-365 days apart using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 185 or ICD-10-CM C61 code in any position. Metastatic PC was identified with the following ICD-9-CM codes: 198.1 (bladder or urethra), 198.5 (bone and bone marrow metastases), 197.0 (lung metastases), 196.6 (intrapelvic lymph nodes such as iliac or sacral), 197.7 (liver metastases), and 198.3 (brain and spinal cord metastases). ICD-10-CM codes used to identify metastatic PC were C79.11 (bladder), C79.19 (urinary organs), C79.51 (bone), C79.52 (bone marrow), C78.00 (unspecified lung), C78.01 (right lung), C78.02 (left lung), C77.5 (intrapelvic lymph nodes), C78.7 (liver and intrahepatic bile duct), C79.31 (brain), and C79.32 (cerebral meninges). Using ICD-9-CM codes for metastatic PC identification is reliable and has previously demonstrated a sensitivity, specificity, positive predicted value (PPV), and negative predicted value (NPV) of 95%, 100%, 100%, and 98.7%, respectively.17 ICD-10-CM codes were cross-walked to ICD-9 codes and reviewed for face validity.
Patients with at least one ADT drug claim and at least one outpatient concurrent drug claim for abiraterone or enzalutamide after the first metastatic PC claim and ≥90 days of continuous enrollment after their first prescription of abiraterone or enzalutamide were included. Patients with MI or stroke within 3 months of the PC diagnosis were excluded from the analysis. An overview of the methods is shown in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100261.
Ascertainment of exposure to PC treatments
Pharmacy claims data were used to identify prescription fills. ADT exposure in these patients were identified by drug injection claims (for leuprolide, goserelin, triptorelin, buserelin, histrelin, degarelix) or orchiectomy procedure with current procedure terminology (CPT code 54520, 54530, 54535), ICD9 62.4, 62.4X, or IC10 0VTC0ZZ or 0VTC4ZZ before initiation of abiraterone or enzalutamide. In the primary analysis, assignment of a subject to either abiraterone or enzalutamide cohort was based on the drug that was first exposed before the end of follow-up (i.e. outcome date or death, disenrollment, or end of study period). An exploratory sub-group analysis was done in the subset of patients who switched from enzalutamide to abiraterone or vice versa. Patients who started the ‘second drug’ before the primary outcome or the final date of the study period were considered ‘switchers’.
Primary and secondary outcomes
The primary outcome is a composite CV endpoint of MI and stroke. MI was identified with a hospital discharge diagnosis code of acute MI (ICD-9 code 410.x excluding 410.x2, ICD-10 I21.XX excluding I21.AX) in any position. Stroke was defined with a hospital discharge diagnosis code of ischemic or hemorrhagic stroke (ICD-9-CM 430, 431, 432.x, 433.x1, 434.x1, 435.x, 436.x, 437.1x, 437.9x, 434.x1; ICD-10 I67.81, I67.82, I67.89) in the primary or secondary position. Secondary outcomes are the individual CV endpoints of MI and stroke. Use of ICD-9-CM codes for identification of MI and stroke has been successfully validated in several pharmacoepidemiologic studies and high rates of sensitivity, specificity, PPV, and NPV have been reported.18
Covariate assessment
Baseline (at study entry) demographic variables, presence of atrial fibrillation (Afib) (yes/no), and comorbidities were evaluated with Charlson Comorbidity Index (CCI).19 CCI has been shown to be an independent predictor of mortality in patients with MI and stroke.20,21 CCI is a comprehensive scoring tool validated for assessment of comorbidities. The pre-determined covariates were identified based on inpatient claims that were before the first prescription of the drug. The components of CCI include age, history of MI, congestive heart failure (CHF), peripheral vascular disease (PVD), cerebrovascular disease (CVA) or transient ischemic attack (TIA), dementia, chronic obstructive pulmonary disorder (COPD), connective tissue disease, peptic ulcer disease, liver disease, diabetes mellitus (DM), hemiplegia, moderate-to-severe chronic kidney disease (CKD), solid tumor, leukemia, lymphoma, and acquired immunodeficiency syndrome. These CCI components were divided into CV variables (MI, CHF, PVD, CVA/TIA, DM, hemiplegia) and non-CV variables (all others). We used ICD-9 code 427.3X and ICD-10 code I148.X to evaluate the presence of Afib at baseline. In addition, data on another important CV variable, i.e. HTN, was also collected.
Statistical analysis
This retrospective cohort used a ‘new user’ design, focusing on who initiated abiraterone or enzalutamide, in addition to ADT, for treatment of metastatic CRPC. In order to emulate a randomized controlled trial (RCT), the primary analysis followed an intent-to-treat (ITT) protocol, whereby participants remained on the drug they were first prescribed (i.e. abiraterone or enzalutamide) for the full analysis. This approach is consistent with pharmacoepidemiology recommended practices.22 Person-time was calculated from the date of the first prescription for abiraterone or enzalutamide until a CV outcome, disenrollment, or administrative censoring at the end of the study period. Baseline characteristics are described based on clinical characteristics of patients before the first abiraterone or enzalutamide prescription. Numeric variables were tested with analysis of variance and categorical variables tested with the chi-square tests. Cumulative incidence of the outcomes was created with time-to-event analysis. Cox regression models were used to compare CV outcomes among those on abiraterone versus enzalutamide, in a univariate model and after adjusting for age at diagnosis, Afib at study entry, and Charlson score (CV and non-CV components). All analyses were carried out using R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria). P values <0.05 were considered statistically significant. Similarly, an exploratory sub-group analysis of the CV outcomes in the ‘switchers’ group was carried out comparing abiraterone switchers to enzalutamide switchers.
Results
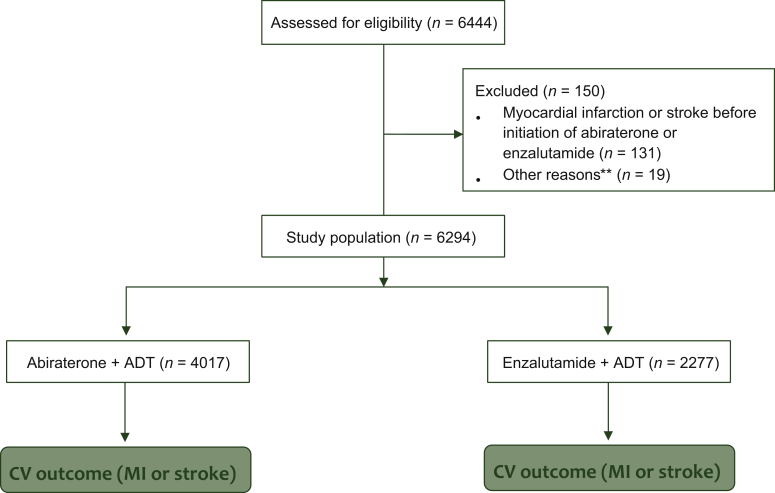
Among eligible patients with metastatic PC, we identified 6444 patients with at least one inpatient or two outpatient claims on different days that contained a PC ICD-9-CM diagnosis claim in any position and were also prescribed ADT. Of these, 150 patients were excluded for the following reasons: 131 patients who did not use either abiraterone or enzalutamide before the first outcome date, 14 patients with survival time (time interval between date of outcome, disenrollment, or end of study and start date of abiraterone or enzalutamide less than zero) and 5 patients who started both drugs on the same day. The final analysis included 6294 patients (Figure 1).
Figure 1.
CONSORT diagram of study population: metastatic prostate cancer patients treated with ADT and abiraterone or enzalutamide.
ADT, androgen deprivation therapy; CV, cardiovascular; MI, myocardial infarction.
∗∗Fourteen patients were excluded who had survival time (time interval between date of outcome, disenrollment, or end of study and start date of abiraterone or enzalutamide start date less than zero) and five patients were excluded who started both enzalutamide and abiraterone on the same day.
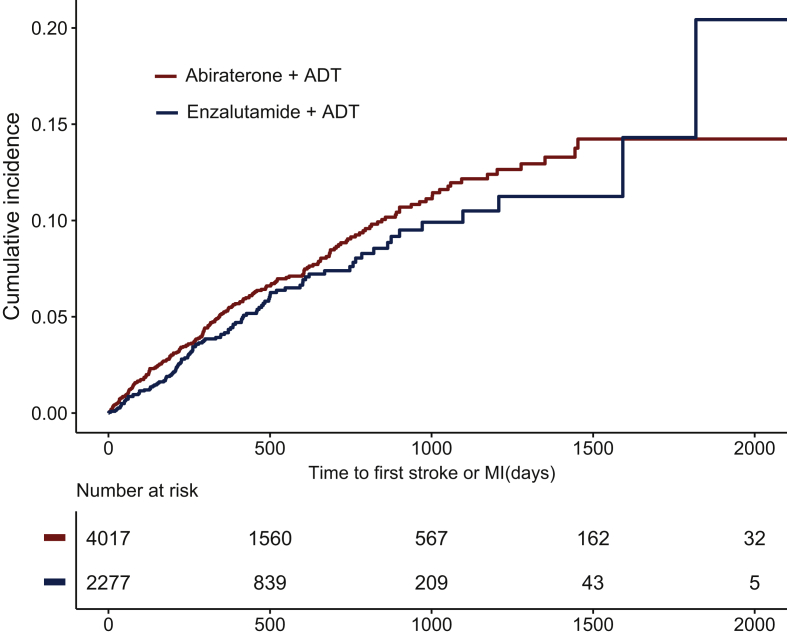
Of those included in our analysis, 4017 patients received ADT in combination with abiraterone and 2277 patients received ADT in combination with enzalutamide. Median follow-up was 12.3 months [interquartile range (IQR) 6.4-23.2 months] in the abiraterone cohort and 11.7 months (IQR 6.2-21.3 months) in the enzalutamide cohort. Median age was 69 years (IQR 61-78 years) and 70 years (IQR 61-78 years) in the abiraterone and enzalutamide cohorts, respectively. Baseline Afib was more frequent in the enzalutamide cohort (12.2%) than in the abiraterone cohort (9.5%) (Table 1). Primary composite outcome of MI or stroke occurred in 255 (6.3%) and 166 (5.1%) subjects in the abiraterone and enzalutamide cohorts, respectively (Table 2 and Figure 2). Individual outcomes of MI occurred in 124 (3.1%) and 67 (2.9%) subjects in the abiraterone and enzalutamide cohorts, respectively (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100261). Similarly, stroke outcome occurred in 142 (3.5%) and 57 (2.5%) subjects in the abiraterone and enzalutamide cohorts, respectively (Table 2).
Table 1.
Clinical and demographic characteristics of patients with metastatic CRPC treated with ADT and abiraterone or enzalutamide from MarketScan database from January 2013 to September 2018
| Abiraterone (n = 4017) | Enzalutamide (n = 2277) | P valuea | |
|---|---|---|---|
| Age at diagnosis | |||
| Mean (SD) | 69.1 (10.8) | 69.6 (10.9) | 0.102 |
| Median (range) | 69.0 (34.0-98.0) | 70.0 (35.0-97.0) | |
| IQR | 61-78 | 61-78 | |
| DM | 201 (5.0%) | 207 (9.1%) | <0.001 |
| CKD | 183 (4.6%) | 139 (6.1%) | 0.007 |
| HTN | 1674 (41.7%) | 1181 (51.9%) | <0.001 |
| Afib | 382 (9.5%) | 278 (12.2%) | <0.001 |
| Charlson score | |||
| Mean (SD) | 8.90 (1.53) | 9.09 (1.67) | <0.001 |
| Median (range) | 9 (6-20) | 9 (6-21) | |
| Charlson score (CVD) | |||
| Mean (SD) | 0.16 (0.55) | 0.23 (0.63) | <0.001 |
| Median (range) | 0 (0-7) | 0 (0-7) | |
| Charlson score (non-CVD) | |||
| Mean (SD) | 8.74 (1.27) | 8.86 (1.35) | <0.001 |
| Median (range) | 9 (6-15) | 9 (6-17) | |
| Orchiectomy | 74 (1.8%) | 39 (1.7%) | 0.710 |
| Follow-up time | <0.001 | ||
| Mean (SD) | 16.8 (14.3) | 15.3 (12.2) | |
| Median (range) | 12.3 (0.1-72.0) | 11.7 (0.1-72.0) | |
| IQR | 6.4-23.2 | 6.2-21.3 |
ADT, androgen deprivation therapy; Afib, Atrial fibrillation; CKD, chronic kidney disease; CRPC, castrate-resistant prostate cancer; CVD, cardiovascular disease; DM, diabetes mellitus; SD, standard deviation; HTN, hypertension; IQR, interquartile range.
Statistical testing—numeric variables tested with analysis of variance and categorical variables tested with chi-square.
Table 2.
Cumulative incidence of primary outcome and individual outcomes for MI and stroke in patients with metastatic CRPC treated with ADT and abiraterone or enzalutamide from MarketScan database from January 2013 to September 2018
| Abiraterone (n = 4017) | Enzalutamide (n = 2277) | Total (N = 6294) | P valuea | |
|---|---|---|---|---|
| Primary outcomeb | 255 (6.3%) | 116 (5.1%) | 371 (5.9%) | 0.042 |
| MI | 124 (3.1%) | 67 (2.9%) | 191 (3.0%) | 0.748 |
| Stroke | 142 (3.5%) | 57 (2.5%) | 199 (3.2%) | 0.025 |
ADT, androgen deprivation therapy; CRPC, castrate-resistant prostate cancer; MI, myocardial infarction; SD, standard deviation.
Statistical testing—numeric variables tested with analysis of variance and categorical variables tested with chi-square.
Primary outcome is composite outcomes of MI and stroke.
Figure 2.
Cumulative incidence plot of the composite primary outcome (MI and stroke) in metastatic prostate cancer patients treated with ADT and abiraterone or enzalutamide.
ADT, androgen deprivation therapy; MI, myocardial infarction.
In univariate analysis, those on abiraterone showed a trend toward increased risk for composite primary CV outcome [hazard ratio (HR) 1.17; 95% confidence interval (CI) 0.94-1.47; P = 0.15]. In multivariable analysis, after adjustment for age, Afib, and CCI, patients in the abiraterone cohort were associated with increased risk of composite primary outcomes of MI and stroke compared to enzalutamide (HR 1.31; 95% CI 1.05-1.63; P = 0.01) (Table 3). For MI as an individual secondary outcome, the risk was not significantly different in the two patient populations (HR 0.99; 95% CI 0.73-1.33; P = 0.92). However, for stroke as an individual secondary outcome, on multivariable analysis, the risk was significantly higher in the abiraterone group (HR 1.52; 95% CI 1.06-1.96; P = 0.008) (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100261).
Table 3.
Unadjusted and adjusted Cox regression analysis of the primary outcome in the metastatic CRPC patients receiving ADT with abiraterone versus enzalutamide
| HR (95% CI) | P value | |
|---|---|---|
| Abiraterone (versus enzalutamide)a | 1.17 (0.94-1.47) | 0.154 |
| Adjustedb | 1.31 (1.05-1.63) | 0.018 |
| Age at diagnosis | 1.04 (1.02-1.05) | <0.001 |
| Afib at baseline | 1.86 (1.43-2.43) | <0.001 |
| Charlson score (CV) | 1.49 (1.30-1.66) | <0.001 |
| Charlson score (non-CV) | 1.86 (1.00-1.28) | 0.043 |
Primary outcome is composite outcomes of myocardial infarction and stroke.
CV variables include history of myocardial infarction (MI), congestive heart failure (CHF), peripheral vascular disease (PVD), cerebrovascular accident/transient ischemic attack (CVD/TIA), diabetes mellitus (DM), and hemiplegia.
Non-CV variables include age, dementia, chronic obstructive pulmonary disorder (COPD), connective tissue disease, peptic ulcer disease, liver disease, moderate-to-severe chronic kidney disease (CKD), solid tumor, leukemia, lymphoma, and acquired immunodeficiency syndrome (AIDS).
ADT, androgen deprivation therapy; Afib, atrial fibrillation; CRPC, castrate-resistant prostate cancer.
Unadjusted analysis.
Adjusting for age at dx and Charlson score [cardiovascular (CV) and non-CV].
Exploratory analysis
Among patients who switched treatment, more patients in the abiraterone cohort switched to enzalutamide than vice versa [1533 (38.2%) versus 570 (25%)]. The median duration of treatment with the first drug was similar in the two cohorts. Enzalutamide switchers (who first received enzalutamide) had a higher prevalence of DM and HTN at baseline (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100261). Primary composite outcome of MI or stroke occurred in 77 (5.0%) and 32 (5.6%) subjects in abiraterone switchers (initially received abiraterone) and enzalutamide switchers (initially received enzalutamide), respectively. Incidence of MI was 2.1% and 3.0% and that of stroke was 3.1% and 2.8% in the abiraterone and the enzalutamide switcher groups, respectively (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100261).
Discussion
The current study utilized individual patient-level data from real-world claim-based database to compare incidence and risk of MI and stroke among users of abiraterone versus enzalutamide in patients with metastatic PC. The study showed a 31% increased risk for the composite CV outcome of MI and stroke with use of abiraterone compared to enzalutamide. The risk was independent of common CV comorbidities of Afib, HTN, CKD, and DM and was consistent across all sub-groups. The risk seemed to be driven predominantly by a significantly higher risk of stroke in the abiraterone group. Despite the higher baseline prevalence of Afib in the enzalutamide cohort, the risk for stroke was higher the abiraterone cohort. We speculate that increased risk of stroke may be related to CYP17 inhibition by abiraterone leading to decreased cortisol synthesis and increased synthesis of mineralocorticoid precursors through stimulation of adrenocorticotrophic hormone. In pre-clinical animal models, increase in levels of mineralocorticoids is associated with increase in vessel wall thickness, wall: lumen ratio, and decreased lumen and outer diameters of major blood vessels in the brain.23
Abiraterone and enzalutamide have never been compared head to head in the context of a randomized phase III clinical trial; however, both drugs are approved in similar settings and generally have similar cancer-related outcomes. Our study directly compares the two agents in a real-world setting and provides a more refined assessment of risk specific for MI and stroke. This is especially important for oncologists who need to consider baseline CV comorbidities when determining an individualized approach for selecting appropriate therapy for a given patient. In this study, we only focused on MI and stroke as they are clinically meaningful markers of CV disease that share common risk factors and can be identified with high validity from claims data. Our study did not assess risk for HTN or heart failure specifically. While HTN and heart failure share some risk factors, reliable identification of patients with medication-induced exacerbation of HTN or heart failure from claims data can be challenging due to modest sensitivity.24,25 In our analysis, we did not assess the use of anticoagulants in the context of Afib. While anticoagulants are important for stroke prevention in Afib, <60% of those with prevalent Afib are on anticoagulation.26 There is significant variability in the types of anticoagulation used for Afib and we believe that there is insufficient power to analyze the use of different anticoagulation types for this study.
In the largest meta-analyses of RCTs published thus far, abiraterone was consistently associated with increased risk for CV toxicities compared to placebo whereas enzalutamide did not consistently increase the risk of CV toxicities but increased the risk of HTN.12,27 For enzalutamide, the findings from the AFFIRM and PREVAIL trials showed that the risk for high-grade CV toxicities was not statistically significant compared to placebo7,11 as opposed to the TERRAIN trial that showed statistically increased risk for high-grade CV toxicities when compared to bicalutamide.28 Possible explanations for this discrepancy are the overall small number of events, heterogeneity in patient population, and different comparison groups across the three trials. Thus far, only one phase II trial directly compared abiraterone and enzalutamide in newly diagnosed metastatic CRPC.29 This trial showed higher rate of grade 3-4 HTN for patients receiving first-line abiraterone (23%) compared to enzalutamide (13%). However, this trial was limited by the small sample size (n = 101 in each group), and no specific incidence for MI and stroke was documented.
RCT for PC therapies may underestimate CV risk for several reasons. These trials are subject to strong selection bias and do not define CV toxicities in a standardized way compared to large prospective CV outcome trials. In addition, these trials are not sufficiently powered to look for differences in CV toxicities.
The results of our analyses are overall consistent with the literature and are likely a true estimate of the CV morbidity. Similar to our study, another observational study from the Surveillance, Epidemiology, and End Result (SEER) database in advanced PC patients (N = 2845) with pre-existing CV disease (including MI and stroke) receiving abiraterone showed that compared to pre-treatment period, the hospitalization incidence post-abiraterone increased by 58% and the crude risk of 6-month overall mortality was between 21.4% and 25.6%.30 In our study, mortality data were not available in these patients. However, observational studies may potentially lead to an overestimation of CV risk due to susceptibility to confounding, outcome reporting bias, and lack of information on treatment adherence. Prospective clinical trials evaluating CV outcomes in high-risk patients are underway.31
The primary analysis was ITT and focused on new users of abiraterone or enzalutamide. This approach is consistent with pharmacoepidemiology recommended practices and has been successfully implemented previously.14,16,22 There are several reasons to justify this ITT approach. Firstly, a focus on medication initiation provides results that were more likely to answer questions relevant to most patients, which involve decisions regarding initiation of a drug. Secondly, studying initiation simulates more closely what would be done in an RCT, in which patients are randomly assigned to initiate the use of a new medication or control therapy. Lastly, studying drug initiators (instead of prevalent users) is associated with less bias and provides estimates that are closer to those obtained in randomized trials.32, 33, 34 In an exploratory analysis focused on patients who switched therapy from one drug to the other, we found that the incidence and risk for MI and stroke was similar. However, we cannot ascertain the exact reason for switching therapy (adverse effect or disease progression).
In conclusion, our study in >6000 patients with metastatic PC showed that, compared to enzalutamide, abiraterone was associated with a 31% increased risk for MI or stroke. Despite the higher CV risk, abiraterone may still be a good option for patients for multiple reasons including other non-CV toxicities with enzalutamide such as memory loss. Sequencing considerations may also factor into the choice of initial treatment—a small phase II crossover trial showed that, compared to abiraterone, enzalutamide as a second-line treatment led to a longer time to second prostate-specific antigen progression for the sequence of abiraterone followed by enzalutamide than with the opposite treatment sequence.29 We recommend that oncologists must be mindful of baseline CV risk parameters including HTN and heart failure when selecting novel anti-androgens in addition to ADT for the management of metastatic CRPC. Enzalutamide may be preferred for patients at high risk for stroke or MI.
Acknowledgments
Funding
None declared.
Disclosure
CJR reports the following: research funding: Pfizer; advisory board: Exelixis. AR reports the following: honoraria from: Lilly, Bayer, Sanofi, and Cardinal Health; consulting or advisory role: Lilly; speakers' bureau: Bristol-Myers Squibb; research funding: Clovis Oncology, Pfizer/Astellas, Seattle Genetics/Astellas, and Lilly. The remaining authors have declared no conflicts of interest.
Supplementary data
References
- 1.Harris W.P., Mostaghel E.A., Nelson P.S., Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6(2):76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veccia A., Maines F., Kinspergher S., Galligioni E., Caffo O. Cardiovascular toxicities of systemic treatments of prostate cancer. Nat Rev Urol. 2017;14(4):230–243. doi: 10.1038/nrurol.2016.273. [DOI] [PubMed] [Google Scholar]
- 3.Hu J.R., Duncan M.S., Morgans A.K. Cardiovascular effects of androgen deprivation therapy in prostate cancer: contemporary meta-analyses. Arterioscler Thromb Vasc Biol. 2020;40(3):E55–E64. doi: 10.1161/ATVBAHA.119.313046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran C., Ouk S., Clegg N.J. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attard G., Reid A.H.M., Yap T.A. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 6.de Bono J.S., Logothetis C.J., Molina A. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scher H.I., Fizazi K., Saad F. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 8.Ryan C.J., Smith M.R., de Bono J.S. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fizazi K., Massard C., Bono P. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol. 2014;15(9):975–985. doi: 10.1016/S1470-2045(14)70240-2. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong A.J., Szmulewitz R.Z., Petrylak D.P. Arches: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974–2986. doi: 10.1200/JCO.19.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beer T.M., Armstrong A.J., Rathkopf D.E. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iacovelli R., Ciccarese C., Bria E. The cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourin Cancer. 2018;16(3):e645–e653. doi: 10.1016/j.clgc.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Sturgeon K.M., Deng L., Bluethmann S.M. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–3897. doi: 10.1093/eurheartj/ehz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutsey P.L., Zakai N.A., MacLehose R.F. Risk of hospitalised bleeding in comparisons of oral anticoagulant options for the primary treatment of venous thromboembolism. Br J Haematol. 2019;185(5):903–911. doi: 10.1111/bjh.15857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutsey P.L., Walker R.F., MacLehose R.F., Alonso A., Adam T.J., Zakai N.A. Direct oral anticoagulants and warfarin for venous thromboembolism treatment: trends from 2012 to 2017. Res Pract Thromb Haemost. 2019;3(4):668–673. doi: 10.1002/rth2.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutsey P.L., Norby F.L., Zakai N.A. Oral anticoagulation therapy and subsequent risk of venous thromboembolism in atrial fibrillation patients. Curr Med Res Opin. 2019;35(5):837–845. doi: 10.1080/03007995.2018.1541445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolan M.T., Kim S., Shao Y.H., Lu-Yao G.L. Authentication of algorithm to detect metastases in men with prostate cancer using ICD-9 codes. Epidemiol Res Int. 2012;2012:970406. doi: 10.1155/2012/970406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cozzolino F., Montedori A., Abraha I. A diagnostic accuracy study validating cardiovascular ICD-9-CM codes in healthcare administrative databases. The Umbria Data-Value Project. PLoS One. 2019;14(7):e0218919. doi: 10.1371/journal.pone.0218919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson M., Szatrowski T.P., Peterson J., Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 20.Nuñez J.E., Núñez E., Fácila L. Prognostic value of Charlson comorbidity index at 30 days and 1 year acute myocardial infarction. Rev Esp Cardiol. 2004;57(9):842–849. [PubMed] [Google Scholar]
- 21.Jiménez Caballero P.E., López Espuela F., Portilla Cuenca J.C., Ramírez Moreno J.M., Pedrera Zamorano J.D., Casado Naranjo I. Charlson comorbidity index in ischemic stroke and intracerebral hemorrhage as predictor of mortality and functional outcome after 6 months. J Stroke Cerebrovasc Dis. 2013;22(7):e214–e218. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Hernán M.A., Alonso A., Logan R. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorrance A.M., Rupp N.C., Nogueira E.F. Mineralocorticoid receptor activation causes cerebral vessel remodeling and exacerbates the damage caused by cerebral ischemia. Hypertension. 2006;47:590–595. doi: 10.1161/01.HYP.0000196945.73586.0d. [DOI] [PubMed] [Google Scholar]
- 24.Saczynski J.S., Andrade S.E., Harrold L.R. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):129–140. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu K., Campbell N.R., Chen Z.L., Cauch-Dudek K.J., McAlister F.A. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1(1):e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 26.Ogilvie I.M., Newton N., Welner S.A., Cowell W., Lip G.Y.H. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123(7):638–645.e4. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Lee H.Y., Chen H.L., Teoh J.Y.C. Abiraterone and enzalutamide had different adverse effects on the cardiovascular system: a systematic review with pairwise and network meta-analyses. Prostate Cancer Prostatic Dis. 2021;24(1):244–252. doi: 10.1038/s41391-020-00275-3. [DOI] [PubMed] [Google Scholar]
- 28.Shore N.D., Chowdhury S., Villers A. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17(2):153–163. doi: 10.1016/S1470-2045(15)00518-5. [DOI] [PubMed] [Google Scholar]
- 29.Khalaf D.J., Annala M., Taavitsainen S. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20(12):1730–1739. doi: 10.1016/S1470-2045(19)30688-6. [DOI] [PubMed] [Google Scholar]
- 30.Lu-Yao G., Nikita N., Keith S. Abstract 4469: clinical outcomes among patients treated with abiraterone acetate for advanced prostate cancer with pre-existing cardiovascular conditions. Cancer Res. 2019;79:4469. [Google Scholar]
- 31.Shore N.D., Saad F., Cookson M.S. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382:2187–2196. doi: 10.1056/NEJMoa2004325. [DOI] [PubMed] [Google Scholar]
- 32.Ray W.A. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 33.Johnson E.S., Bartman B.A., Briesacher B.A. The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf. 2013;22(1):1–6. doi: 10.1002/pds.3334. [DOI] [PubMed] [Google Scholar]
- 34.Danaei G., Tavakkoli M., Hernán M.A. Bias in observational studies of prevalent users: Lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012;175(4):250–262. doi: 10.1093/aje/kwr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.