Graphical abstract
Abbreviations: MetS, Metabolic Syndrome; DM, Diabetes Mellitus; IR, Insulin Resistance; CVD, Cardiovascular disease; CRP, C-reactive protein; HGS, Healthy Growth Study; WC, Waist Circumference; BP, Arterial Blood Pressure; SBP, Systolic Blood Pressure; DPB, Diastolic Blood Pressure; TC, Total Cholesterol; HDL-C, High-density Lipoprotein Cholesterol; LDL-C, Low-density Lipoprotein Cholesterol; TG, Triglycerides
Keywords: Adipokines, Childhood obesity, Metabolic syndrome, C-reactive protein
Highlights
-
•
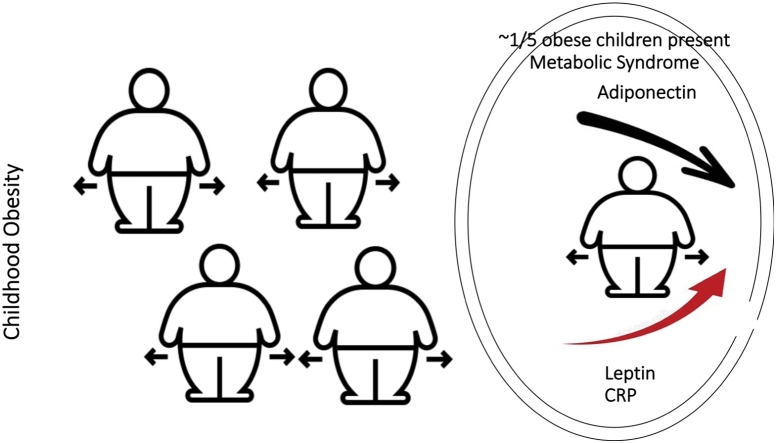
MetS occurs in a 1 out of 4 obese children and increases the future risk of serious health complications.
-
•
The serum level of adiponectin decreases while leptin increases as body weight increases, in the understudy children group.
-
•
The serum level of the inflammatory marker CRP increases significantly as body weight increases in the understudy population.
-
•
Adipokines and CRP can distinguish the children with Metabolic Syndrome as compared to children with no Metabolic Syndrome.
Abstract
Background
Metabolic syndrome (MetS) occurs in a proportion of overweight and obese children and increases their future risk of serious health complications, even in adolescence and young adulthood. We aimed to explore the role of certain adipokines and inflammatory markers in identifying children with MetS.
Methods
This study is a secondary analysis of data coming from the Healthy Growth Study, a cross-sectional study conducted with schoolchildren in Greece. The present study included data from a representative sample of 1376 schoolchildren (mean age: 11.19 ± 0.66 years), recruited from 77 primary schools in four large regions in Greece. Anthropometric, clinical and biochemical data were recorded. Children’s body weight status and the presence of MetS were determined and their correlation with the serum levels of leptin, adiponectin and C-reactive protein (CRP) was explored.
Results
The prevalence of the MetS was 21.7 % and 3.7 % in obese and overweight children, respectively. The balance of adipokines was disturbed in obesity, as the serum level of adiponectin decreased as body weight increased, while the serum level of leptin increased. The serum level of the inflammatory marker CRP increased significantly as body weight increased. Discriminant analysis showed that these factors could distinguish the children with MetS as compared to children with no MetS.
Conclusions
In the under study Mediterranean childhood population, monitoring of the levels of adipokines and CRP could identify the overweight and obese children with MetS. Appropriate individualized dietary and lifestyle interventions can be applied in these children to prevent health complications associated with MetS.
1. Background
Childhood obesity is a major health issue, increasing in global prevalence and with detrimental consequences [1,2], among which is metabolic syndrome (MetS). To date, there is no clear consensus regarding the definition of MetS, as well as the utility of its diagnosis in paediatric populations, due to the fact that its construct is challenging to define and its implications for clinical care are still unclear. Nevertheless, the term of MetS refers to clustering of the syndrome’s cardiometabolic risk factors [3]. Clinical features of MetS in childhood include obesity, dyslipidemia, hypertension and type 2 diabetes mellitus (DM) [4,5] and it has a serious impact on morbidity and mortality [[6], [7], [8]].
The pathophysiology of MetS is complex and is associated with various hormonal changes, including impaired regulation of adipokines and inflammatory markers [4,9]. Adipokines such as leptin and adiponectin are thought to be a link between obesity and other disturbances related to MetS [4]. Specifically in adults, adiponectin plays a protective role against insulin resistance (IR) and cardiovascular disease (CVD) [10], and low levels are observed in various conditions associated with MetS, including hypertension, type 2 DM and IR [4]. Leptin, in contrast, has pro-inflammatory effects, and high levels of leptin are associated with the development of IR and CVD [4,11]. Hypoadiponectinemia and hyperleptinemia are observed in obese individuals, both adults and children [[11], [12], [13], [14]], and the leptin/adiponectin (L/A) ratio has been proposed as a sensitive marker of MetS in children and adolescents [12,13,15,16]. In addition to the abnormal levels of adipokines, inflammation is also exacerbated in individuals with MetS [17]. More specifically increased levels of inflammatory markers, including C-reactive protein (CRP) [18], are detected in both obese adults [19] and children [[19], [20], [21]] with MetS. Recent data propose the use of impaired inflammatory (CRP) and insulin-adipogenic indices for stratifying senescent populations with MetS [22,23].
A significant body of research has been focused on the interrelations between adipokines, inflammation and MetS in obese children, but no relevant information is available for Mediterranean populations. The aim of this study, was to explore these associations and their implications for the identification of MetS in children, for the first time in the Mediterranean area.
2. Methods
2.1. Sampling and participants
The Healthy Growth Study (HGS) was a large-scale cross-sectional epidemiological study initiated in May 2007 and completed in June 2009. Approval to conduct the study was granted by the Greek Ministry of National Education and the Ethical Committee of Harokopio University of Athens (16/19.12.2006). The study population was representative of the 9–13 year-old school children living in the four counties under study, which are scattered throughout Greek territory, covering the northern (i.e., Thessaloniki), central (i.e., Attica), western (i.e., Aitoloakarnania), and southern (i.e., Iraklio-Crete) parts of Greece (indicating potential representativeness at a national level). The sampling of schools participating in the HGS was random, multistage, and stratified by parents’ educational level and total population of students attending schools within municipalities of these counties. A detailed letter explaining the aims of the study and a consent form for conducting full measurements was provided to all parents or guardians (“parents” hereinafter) with a child in these schools. Parents who responded positively were asked to sign the consent form and provide their contact details. Data from children and their parents were collected by face-to-face interviews and clinical assessments conducted at school sites. Of the 4145 children who were eligible to participate, 2656 were enrolled in the study (64.1 % response) after the parents signed consent on behalf of the children. Detailed methodology has been published elsewhere [22]. Of these 2656 children, a subsample of 1376 children who had full data on the variables which determine the existence of MetS, were used for the analyses.
2.2. Anthropometric data
Schoolchildren participating in the study underwent a physical examination by two trained members of the research team. The protocol and equipment used were the same in all schools. Weight was measured to the nearest 10 g using a digital scale (Seca Alpha, model 770;Seca, Hamburg, Germany). Children were weighed without shoes and wearing the minimum clothing possible. Height was measured to the nearest 0·1 cm using a commercial stadiometer (Leicester Height Measure; Invicta Plastics, Oadby, UK) with each child standing barefoot, keeping shoulders in a relaxed position, arms hanging freely and head in the Frankfort horizontal plane [24]. Weight and height were converted to body mass index (BMI) using Quetelet’s equation (weight (kg)/height2 (m2)). Using the International Obesity Task Force (IOTF) cut-off points [[25], [26], [27]], students were categorized as “underweight”, "normal weight", "overweight" or "obese". Waist circumference (WC) was measured to the nearest 0.1 cm with the use of a non-elastic tape (Hoechstmass, Germany). Each student was advised to stand, at the end of a gentle expiration and the measuring tape was placed around the trunk, at the level of umbilicus midway, between the lower rib margin and the iliac crest [28]. For the classification of central obesity, waist circumference percentiles were used (≥90th percentile) [29]. In addition, a well-trained an experienced pediatrician in each prefecture determined the pubertal stage (Tanner stage) of each subject, after a thorough visual inspection of breast development in girls and genital development in boys [30]. Finally, each girl was asked by the paediatrician about her menstruation status and age of menarche. Details of the proxedure could be found elsewhere [28].
2.3. Clinical indices
Using a valid automatic Omron M6 Blood Pressure Monitor (Omron Healthcare Europe BV, Hoofddorp, The Netherlands) [31], arterial blood pressure (BP) was recorded twice, with a 2-minute interval, in the right arm, with the participant seated, and after a 5-minute rest. Prior to measurement, mid-upper arm circumference was assessed and different cuff sizes were used. In case of a difference of over 10 mmHg between the first two measurements, an additional third measurement was taken. The average values of the two measurements of systolic (SBP) and diastolic BP (DBP) of each participant were recorded and used in analysis [28].
2.4. Biochemical indices
Participating schoolchildren visited the laboratory between 08.30 and 10.30 in the morning and their blood samples were obtained after an overnight fast (12 h). The previous day, reminders were given both to parents and children regarding compliance with fasting. Part of the blood of each sample was collected in test tubes with no added anticoagulant, where it was allowed to clot for approximately 2 h, in order to achieve serum separation. Clotted blood was centrifuged at 3.000 rpm for 15 min and the collected serum was divided into aliquots and stored at −80 °C, as described in depth elsewhere [[32], [33], [34]].
2.4.1. Lipidemic profile
Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were determined twice, using commercially available enzymatic colorimetric assays (Roche Diagnostics SA, Vasilia, Switzerland) on an automated analyzer (Roche/Hitachi Modular). For the calculation of low-density lipoprotein cholesterol (LDL-C), the Friedewald equation was used [35]. The LDL-C/HDL-C and the TC/HDL-C ratios were estimated, as described elsewhere [32]. Dyslipidemias were defined using the National Cholesterol Education Program (NCEP) cut-off points for blood lipids [36].
2.4.2. Glycemic profile
Plasma glucose was determined with a commercially available enzymatic colorimetric assay (Roche Diagnostics SA, Vassilia, Swiss), and serum insulin was determined by a Chemiluminescence immunoassay (Kyowa Medex Ltd, Minami-Ishiki, Japan, for Siemens Diagnostics USA) [33].
2.4.3. Adipokine profile
Serum leptin level was measured by human leptin ELISA, Clinical Range kit (BioVendor Research and Diagnostic products, Karasek, Czech Republic) and was reported in ng/mL.. Adiponectin was measured by a Human Adiponectin/Acrp30 DuoSet ELISA kit (R&D Systems, Minneapolis, MN, USA) [34] and reported in _g/mL..
2.4.4. CRP profile
As marker of inflammation, CRP was measured by human CRP ELISA kit (R&D Systems, Minneapolis, MN, USA) and was reported in nmol/L [31].
2.5. Determination of MetS
For the purposes of the study, the International Diabetes Federation (IDF) criteria for MetS for children aged 10–16 years were used, which include the presence of central obesity plus the presence of at least two of the following components: raised triglyceride level, low HDL-cholesterol level, high BP and/or raised plasma glucose level [37].
2.6. Statistical analysis
Prior to analysis the assumption of normal distribution for the examined variables was tested. Given that Kolmogorov-Smirnov test was statistically significant for adipokines and CRP, a log-transformation was used to better approximate the normal distribution. Analysis of variance (ANOVA) was performed to explore possible differences between children of the three weight categories across all depended variables. Chi-square test of independence was used for the examination of possible association between weight category and MetS. Discriminant function analysis was conducted to investigate whether the levels of adipokine and CRP could correctly assign children in MetS and no MetS groups. For this analysis, children of underweight or normal weight who demonstrated one or two of the IDF criteria (N = 610) were excluded. All analyses were performed with SPSS Statistics 19 (SPSS Inc., Chicago, IL).
3. Results
3.1. Study population characteristics
Among the 1376 study participants, 685 were boys (49.8 %) and 691 were girls (50.2 %), with a mean age of 11.19 ± 0.66 years. Most of the children lived in an urban area (64.8 %), and the rest in a semi-urban (14.8 %) or rural (20.3 %) area.
Table 1 shows the anthropomorphic characteristics of the children in the study. Regarding body weight status, 819 children (59.5 %) were normal weight or underweight, 405 children (29.4 %) were overweight and 152 children (11 %) were obese (Table 1).
Table 1.
Anthropomorphic characteristics of the sample of primary schoolchildren in the Healthy Growth Study.
|
Boys (n = 685) |
Girls (n = 691) |
Total (n = 1376) |
||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Age (years) | 11.20 | 0.66 | 11.18 | 0.66 | 11.19 | 0.66 |
| Weight group | ||||||
| Underweight/Normal | 393 (57.4 %) | 426 (61.6 %) | 819 (59.4 %) | |||
| Overweight | 208 (30.3 %) | 197 (28.5 %) | 405 (29.4 %) | |||
| Obese | 84 (12.3 %) | 68 (9.8 %) | 152 (11 %) | |||
3.2. Presence of MetS
According to the IDF criteria for the definition of the MetS in children and adolescents [37], only 3.5 % (48 of 1376) children demonstrated central obesity and fulfilled the criteria for MetS: 30 had a low level of HDL (62.5 %), and 13 had a raised level of triglycerides (27 %), 18 raised plasma glucose (37.5 %) and 42 had high BP (87.5 %).
Table 2 shows the characteristics of the children with MetS.
Table 2.
Study group’s characteristics related to weight categories and metabolic syndrome.
| Normal/Underweight (n = 819) |
Overweight (n = 405) |
Obese (n = 152) |
Total (n = 1376) |
|||||
|---|---|---|---|---|---|---|---|---|
| No MS | MS | No MS | MS | No MS | MS | No MS | MS | |
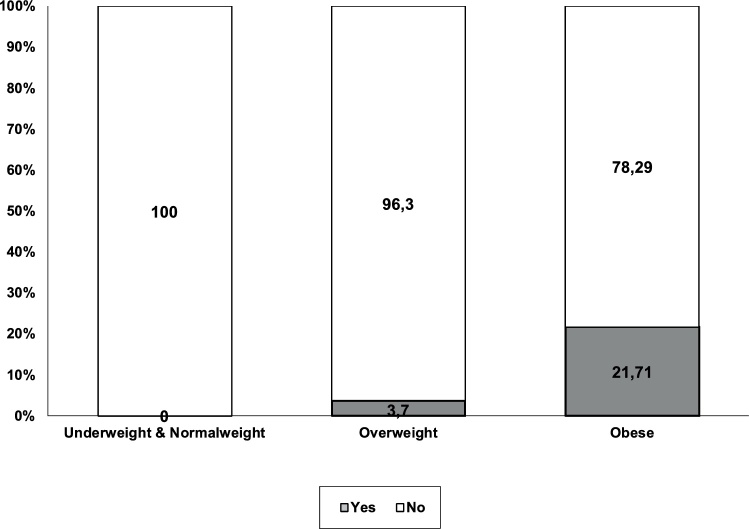
| Percentage (%) | 100 | 0 | 96.3 | 3.7 | 78.29 | 21.71 | 96.5 | 3.5 |
| Central obesity presence (cases, %) | 0 (0%) | – | 82 (20.2 %) | 15 (3.7 %) | 104 (68.4 %) | 33 (21.7 %) | 186 (13.5 %) | 48 (3.5 %) |
| HDL normal (cases, %) | 786 (95.9 %) | 0 (0%) | 358 (88.4 %) | 7 (1.7 %) | 113 (74.3 %) | 11 (7.4 %) | 1257 (91.3 %) | 18 (1.3 %) |
| HDL < 40 mg/dl (cases, %) | 33 (4.1 %) | 0 (0%) | 32 (7.9 %) | 8 (2%) | 6 (3.8 %) | 22 (14.5 %) | 71 (5.2 %) | 30 (2.2 %) |
| TG normal (cases, %) | 817 (99.7 %) | 0 (0%) | 386 (95.3 %) | 13 (3.2 %) | 118 (77.6 %) | 22 (14.5 %) | 1321 (96 %) | 35 (2.5 %) |
| TG ≥ 150 mg/dl (cases, %) | 2 (0.3 %) | 0 (0%) | 4 (0.1 %) | 2 (0.5 %) | 1 (0.7 %) | 11 (7.2 %) | 7 (0.5 %) | 13 (1%) |
| BP normal (cases, %) | 682 (83.27 %) | 0 (0%) | 267 (65.9 %) | 2 (0,5%) | 70 (46 %) | 4 (2.6 %) | 1019 (74 %) | 6 (0.4 %) |
| BP elevated (cases, %) | 137 (16.73 %) | 0 (0%) | 123 (30.4 %) | 13 (3.2 %) | 49 (32.2 %) | 29 (19.1 %) | 309 (22.5 %) | 42 (3.1 %) |
| GL normal (cases, %) | 722 (88.15 %) | 0 (0%) | 336 (83 %) | 7 (1.7 %) | 110 (72.4 %) | 23 (15.1 %) | 1168 (84.9 %) | 30 (2.2 %) |
| GL ≥100 mg/dl (cases, %) | 97 (11.85 %) | 0 (0%) | 54 (13.3 %) | 8 (2%) | 9 (5.9 %) | 10 (6.6 %) | 160 (11.6 %) | 18 (1.3 %) |
MS: Metabolic Syndrome, TG: Triglycerides, BP: Blood Pressure elevated: as estimated by Systolic Blood Pressure (≥130 mm Hg) and Diastolic Blood Pressure (≥85 mm Hg), GL: Plasma Glucose.
MetS in the study children was significantly associated with obesity (χ2 (2, N = 1376) = 179.57, p = < 0.001, Cramer’s V = 0.361). Specifically, MetS was observed in 21.71 % of the obese children, in contrast to only 3.7 % of the overweight children, and there was no case of MetS among the normal weight and underweight children (Fig. 1).
Fig. 1.
Relationships between weight category and metabolic syndrome.
3.3. Weight and adipokine levels
Possible differences to the log transformation of the serum levels of leptin, adiponectin and L/A ratio were explored among children from the three different weight categories (underweight/normal weight, overweight and obese). Significant differences were found between the three groups in all the depended variables (p < .001) (Table 3). The highest value of the levels of adiponectin were noticed in underweight/normal weight children (.80 ± .20) and lower values in overweight (.74 ± .20) and obese children (.68 ± .19). Conversely, regarding the levels of leptin, the highest value was noticed in the obese category (1.18 ± .19), the lowest in the underweight/normal weight category (.63 ± .35) whereas the overweight category demonstrated a mean value between the other two categories (1.08 ± .26). Consequently regarding the L/A ratio, underweight/normal category demonstrated the lowest value (.82 ± .54) the overweight category demonstrated a higher value than this (1.54 ± .56) and the obese category the highest (2.20 ± .78). Post hock tests revealed that the three groups differed in all the aforementioned depended variables at least at the 0.05 level (Table 3).
Table 3.
Descriptive statistics and differences between the three weight categories in the log transformation of the serum levels of leptin, adiponectin, CRP and L/A ratio (n = 1376).
| Under /normal weight |
Overweight |
Obese |
||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | Sig. | |
| Adiponectin (ng/mL) | .80a | .20 | .74b | .20 | .68c | .19 | 24.33 | .000 |
| Leptin (ng/mL) | .63a | .35 | 1.08b | .26 | 1.38c | .19 | 416.09 | .000 |
| L/A ratio | .82a | .54 | 1.54b | .56 | 2.20c | .78 | 324.87 | .000 |
| CRP (ng/mL) | 2.54a | .57 | 2.90b | .53 | 3.28c | .38 | 112.37 | .000 |
Note: Values sharing different subscripts are significant different at least at the 0.05 level.
3.4. Weight and CRP level
The serum levels of CRP were considerably higher in children of greater body weight. Specifically, the lowest value was noticed in underweight/normal weight children (2.54 ± .57), the highest in obese children (3.28 ± .38), while the overweight children were placed between these two groups (2.90 ± .53) (Table 3). All groups differed significantly at the 0.001 level.
3.5. Prediction of MetS
Discriminant analysis was conducted, excluding 610 children of low or normal weight, but with one or two of the IDF criteria. These children were excluded in order to examine if adipokines levels could sufficiently recognize children with MetS and undoubtedly healthy children in other words without any of the IDF criteria for MetS. As shown in Table 4, discriminant analysis revealed that the level of adipokines and CRP could distinguish the children of the two categories (discriminant function Wilks’ Lambda = .880, χ2(3, 890) = 77.80, p < .001). The leptin and CRP levels were the main contributors to this discrimination (Table 4a). The predictive accuracy of the model was good, with 96.1 % of the cross validated group cases being correctly classified (Table 4b).
Table 4.
Greek 5th and 6th grade primary schoolchildren. (a) Discriminant analysis of children with, and those without* metabolic syndrome, according to serum level of leptin, adiponectin and C-reactive protein (CRP), and (b)classification results.
| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Standardized | Discriminant | Metabolic syndrome |
Normal |
|||||
| Coefficients | Loading | M | SD | M | SD | F | Significance | |
| Leptin | 0.778 | 0.908 (1) | 1.40 | 0.22 | 0.77 | 0.39 | 68.63 | 0.000 |
| Adiponectin | −.314 | −0.370 (3) | 0.66 | 0.22 | 0.78 | 0.19 | 11.40 | 0.001 |
| CRP | 0.291 | 0.609 (2) | 3.29 | 0.48 | 2.65 | 0.58 | 30.86 | 0.000 |
| M: mean; SD: standard deviation *under- and normal weight children with one or two Metabolic syndrome criteria were excluded | ||||||||
| (b) | |||
|---|---|---|---|
| Actual Group | No. of cases | Predicted group membership |
|
| Normal | Metabolic syndrome | ||
| Normal | 583 | 583 (100 %) | 0 (0%) |
| Metabolic syndrome | 27 | 24 (88.9 %) | 3 (11.1 %) |
Note: 96.1 % of the original group cases were correctly classified.
4. Discussion
The present study managed to identify sufficiently well between 10−12-year-old children with and without MetS, based on the serum levels of selected adipokines (leptin and adiponectin) and the inflammatory index CRP.
In line with previous research, MetS was observed exclusively in obese and overweight children, although some of the normal and underweight children presented one or two of the IDF criteria. In a systematic review of 85 studies, the median prevalence of MetS in children was 3.3 %, ranging from 0-1% in non-obese and non-overweight populations, while in overweight children the prevalence was 11.9 %, and in obese children 29.2 % [38]. In our sample of Greek children, the corresponding rates in overweight and obese children were 3.7 % and 21.71 %. In view of the fact that MetS has been reported to track from childhood and adolescence to adulthood, where it has been associated with a higher likelihood of type 2 Diabetes and cardiovascular disease [6,7], the findings of the present study give rise to concern. As the definitions and criteria used for MetS in children and young people vary [5], we proceeded with investigation of the possible relationship with serum levels of adipokines and CRP.
We show herein that the mean level of leptin and the L/A ratio were significantly higher in overweight and obese children than in their under- and normal weight peers, and the level of adiponectin declined significantly as body weight increased. Current documentation [12] highlights the importance of adipokine levels as a diagnostic marker for MetS in young, especially as hyperleptinemia and hypoadiponectinemia have been correlated with an increased risk of indications of metabolic imbalance, including IR and cardiometabolic dysfunction in overweight and obese children [12]. In addition, decreased levels of adiponectin are reported to enhance the inflammatory process in adipose tissue [39].
In an analogous way, the level of CRP levels, which is an established inflammatory marker, showed a significant rise with increase in body weight, in line with previous studies [[18], [19], [20]]. For this reason, regular monitoring of CRP is recommended for overweight individuals, as it could assist identifying those at increased cardiovascular risk [40].
Taking into consideration the varying definitions for MetS in childhood, the use of a range of markers, such as body weight and serum levels of leptin, adiponectin and CRP, can facilitate the diagnostic process. Not all obese or overweight children are at high risk of MetS, so it is important to use markers early to distinguish those at greater risk. We propose monitoring the levels of leptin, adiponectin and CRP levels in overweight and obese children in order to identify those with MetS. For these children appropriate individualized lifestyle and dietary strategies can be developed for weight management, as a preventive measure.
5. Strengths and limitations
The Healthy Growth Study was a large-scale epidemiological study, in which a representative sample of children from four main regions of Greece was recruited. The cross-sectional design of the study is an important limitation as the data cannot provide a casual pathway on the observed associations. Moreover, the relatively small number of the MetS group, in comparison to the normal weight group of children, can be considered as an additional limitation.
6. Conclusions
Based on the findings of this study, the researchers propose future focused research on identifying the cut-off levels of adipokines and CRP, to be used as potential markers for distinguishing between overweight and obese children with MetS and those without MetS. The complex nature of obesity and its profound effects on the overall health status prompts the use of a variety of different markers for optimum risk assessment and application of individualized therapeutic intervention. MetS is interrelated with serious health complications, and its diagnosis should lead to therapeutic dietary and lifestyle strategies, individualized according to personal clinical history, and food and lifestyle preferences.
Source of funding
This study was co-financed by the European Union (European Social Fund e ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) - Research Funding Program: Heraclitus II. Investing in knowledge society through the European Social Fund. The funders had no involvement in any decision made regarding the study (design, data collection, interpretation, writing of the paper or manuscript submission).
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank the “Healthy Growth Study” group for the valuable contribution to the completion of the study.
Handling Editor: Dr. Aristidis Tsatsakis
Contributor Information
Yannis Manios, Email: manios@hua.gr.
George Moschonis, Email: g.moschonis@latrobe.edu.au.
References
- 1.Abarca-Gómez L. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;(390):2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blüher M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019;15(May (5)):288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 3.Magge S.N., Goodman E., Armstrong S.C., COMMITTEE ON NUTRITION, SECTION ON ENDOCRINOLOGY, SECTION ON OBESITY The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics. 2017;140(August (2)) doi: 10.1542/peds.2017-1603. [DOI] [PubMed] [Google Scholar]
- 4.Kumari R., Kumar S., Kant R. An update on metabolic syndrome: metabolic risk markers and adipokines in the development of metabolic syndrome. Diabetes Metab. Syndr. Clin. Res. Rev. 2019;13(July (4)):2409–2417. doi: 10.1016/j.dsx.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hamad D., Raman V. Metabolic syndrome in children and adolescents. Transl. Pediatr. 2017;6(4):397–407. doi: 10.21037/tp.2017.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison J., Friedman L., Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the princeton lipid research clinics follow-up study. Pediatrics. 2007;120(August (2)):340–345. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 7.Morrison J., Friedman L., Wang P., Glueck C. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J. Pediatr. 2008;152(2):201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Mottillo S., Filion K.B., Genest J., Joseph L., Pilote L., Poirier P. The metabolic syndrome and cardiovascular risk. J. Am. Coll. Cardiol. 2010;56(September (14)):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Zorena K., Jachimowicz-Duda O., Ślęzak D., Robakowska M., Mrugacz M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. IJMS. 2020;21(May (10)):3570. doi: 10.3390/ijms21103570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuzawa Y., Funahashi T., Kihara S., Shimomura I. Adiponectin and metabolic syndrome. ATVB. 2004;24(January (1)):29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 11.Fasshauer M., Blüher M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015;36(July (7)):461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Frithioff‐Bøjsøe C., Lund M.A.V., Lausten‐Thomsen U., Hedley P.L., Pedersen O., Christiansen M. Leptin, adiponectin, and their ratio as markers of insulin resistance and cardiometabolic risk in childhood obesity. Pediatr. Diabetes. 2020;21(March (2)):194–202. doi: 10.1111/pedi.12964. [DOI] [PubMed] [Google Scholar]
- 13.Li G., Xu L., Zhao Y., Li L., Fu J., Zhang Q. Leptin-adiponectin imbalance as a marker of metabolic syndrome among Chinese children and adolescents: the BCAMS study. PLoS One. 2017;12(October (10)) doi: 10.1371/journal.pone.0186222. Chen Y-C, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Jaramillo P., Gómez-Arbeláez D., López-López J., López-López C., Martínez-Ortega J., Gómez-Rodríguez A. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm. Mol. Biol. Clin. Investig. 2014;18(January (1)) doi: 10.1515/hmbci-2013-0053. [DOI] [PubMed] [Google Scholar]
- 15.Nappo A., González-Gil E.M., Ahrens W., Bammann K., Michels N., Moreno L.A. Analysis of the association of leptin and adiponectin concentrations with metabolic syndrome in children: results from the IDEFICS study. Nutr. Metab. Cardiovasc. Dis. 2017;27(June (6)):543–551. doi: 10.1016/j.numecd.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Diamond Jr.F.B., Cuthbertson D., Hanna S., Eichler D. Correlates of adiponectin and the Leptin/Adiponectin ratio in obese and non-obese children. J. Pediatr. Endocrinol. Metab. 2004;17(January (8)) doi: 10.1515/jpem.2004.17.8.1069. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland J.P., McKinley B., Eckel R.H. The metabolic syndrome and inflammation. Metab. Syndr. Relat. Disord. 2004;2(2):82–104. doi: 10.1089/met.2004.2.82. [DOI] [PubMed] [Google Scholar]
- 18.Devaraj S., Singh U., Jialal I. Human C-reactive protein and the metabolic syndrome. Curr. Opin. Lipidol. 2009;20(June (3)):182–189. doi: 10.1097/MOL.0b013e32832ac03e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi J., Joseph L., Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis: obesity and CRP in various populations J. Choi et al. Obes. Rev. 2013;14(March (3)):232–244. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 20.Mărginean C.O., Meliţ L.E., Ghiga D.V., Mărginean M.O. Early inflammatory status related to pediatric obesity. Front. Pediatr. 2019;7(June):241. doi: 10.3389/fped.2019.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitsios K., Papadopoulou M., Kosta K., Kadoglou N., Papagianni M., Tsiroukidou K. High-sensitivity C-Reactive protein levels and metabolic disorders in obese and overweight children and adolescents. J. Clin. Res. Pediatr. Endocrinol. 2013;5(March (1)):44–49. doi: 10.4274/Jcrpe.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gradinaru D., Khaddour H., Margina D., Ungurianu A., Borsa C., Ionescu C. Insulin-leptin Axis, cardiometabolic risk and oxidative stress in elderly with metabolic syndrome. Exp. Clin. Endocrinol. Diabetes. 2018;126(July (07)):445–452. doi: 10.1055/s-0043-123825. [DOI] [PubMed] [Google Scholar]
- 23.Gradinaru D., Margina D., Borsa C., Ionescu C., Ilie M., Costache M. Adiponectin: possible link between metabolic stress and oxidative stress in the elderly. Aging Clin. Exp. Res. 2017;29(August (4)):621–629. doi: 10.1007/s40520-016-0629-z. [DOI] [PubMed] [Google Scholar]
- 24.Moschonis G., Kaliora A., Karatzi K., Michaletos A., Lambrinou C.-P., Karachaliou A. Perinatal, sociodemographic and lifestyle correlates of increased total and visceral fat mass levels in schoolchildren in Greece: the Healthy Growth Study. Public Health Nutr. 2017;20(4):660–670. doi: 10.1017/S1368980016002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole T., Bellizzi M., Flegal K., Dietz W. Vol. 320. 2000. p. 1240. (Establishing a Standard Definition for Child Overweight and Obesity Worldwide: International Survey). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cameron N. Vol. 335. 2007. pp. 166–167. (Body Mass index Cut Offs to Define Thinness in Children and Adolescents). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole T.J., Flegal K.M., Nicholls D., Jackson A.A. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ. 2007;335(July (7612)):194. doi: 10.1136/bmj.39238.399444.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manios Y., Karatzi K., Protogerou A.D., Moschonis G., Tsirimiagou C., Androutsos O. Prevalence of childhood hypertension and hypertension phenotypes by weight status and waist circumference: the Healthy Growth Study. Eur. J. Nutr. 2018;57(April (3)):1147–1155. doi: 10.1007/s00394-017-1398-y. [DOI] [PubMed] [Google Scholar]
- 29.Fernández J., Redden D., Pietrobelli A., Allison D. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J. Pediatr. 2004;145(4):439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Tanner J.M. 1955. Growth at Adolescence. [Google Scholar]
- 31.El Assaad M.A., Topouchian J.A., Asmar R.G. Evaluation of two devices for self-measurement of blood pressure according to the international protocol: the Omron M5-I and the Omron 705IT. Blood Press. Monit. 2003;8(June (3)):127–133. doi: 10.1097/00126097-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Moschonis G., Mavrogianni C., Karatzi K., Iatridi V., Chrousos G.P., Lionis C. Increased physical activity combined with more eating occasions is beneficial against dyslipidemias in children. The Healthy Growth Study. Eur. J. Nutr. 2013;52(April (3)):1135–1144. doi: 10.1007/s00394-012-0424-3. [DOI] [PubMed] [Google Scholar]
- 33.Androutsos O., Moschonis G., Mavrogianni C., Roma-Giannikou E., Chrousos G.P., Kanaka-Gantenbein C. Identification of lifestyle patterns, including sleep deprivation, associated with insulin resistance in children: the Healthy Growth Study. Eur. J. Clin. Nutr. 2014;68(March (3)):344–349. doi: 10.1038/ejcn.2013.280. [DOI] [PubMed] [Google Scholar]
- 34.Karatzi K., Moschonis G., Polychronopoulou M.C., Chrousos G.P., Lionis C., Manios Y. Cutoff points of waist circumference and trunk and visceral fat for identifying children with elevated inflammation markers and adipokines: the Healthy Growth Study. Nutrition. 2016;32(October (10)):1063–1067. doi: 10.1016/j.nut.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Friedewald W., Levy R., Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 36.American Academy of Pediatrics National Cholesterol Education Program. Report of the expert panel on blood cholesterol levels in children and adolescents. Pediatrics. 1992;89:525–584. [PubMed] [Google Scholar]
- 37.Zimmet P., Alberti G., Kaufman F. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr. Diabetes. 2007:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 38.Friend A., Craig L., Turner S. The prevalence of metabolic syndrome in children: a systematic review of the literature. Metab. Syndr. Relat. Disord. 2013;11(April (2)):71–80. doi: 10.1089/met.2012.0122. [DOI] [PubMed] [Google Scholar]
- 39.Wittcopp C., Conroy R. Metabolic syndrome in children and adolescents. Pediatr. Rev. 2016;37(May (5)):193–202. doi: 10.1542/pir.2014-0095. [DOI] [PubMed] [Google Scholar]
- 40.Halcox J.P., Roy C., Tubach F., Banegas J.R., Dallongeville J., De Backer G. C-reactive protein levels in patients at cardiovascular risk: EURIKA study. BMC Cardiovasc. Disord. 2014;14(December (1)):25. doi: 10.1186/1471-2261-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]