Abstract
Introduction
Efficacious therapies are urgently required to tackle the coronavirus disease 2019 (COVID-19). This trial aims to evaluate the effects of atorvastatin in comparison with standard care for adults hospitalized with COVID-19.
Methods
We conducted a randomized controlled clinical trial on adults hospitalized with COVID-19. Patients were randomized into a treatment group receiving atorvastatin + lopinavir/ritonavir or a control group receiving lopinavir/ritonavir alone. The primary outcome of the trial was the duration of hospitalization. The secondary outcomes were the need for interferon or immunoglobulin, receipt of invasive mechanical ventilation, and O2 saturation (O2sat), and level of C-reactive protein (CRP) which were assessed at the onset of admission and on the 6th day of treatment.
Results
Forty patients were allocated and enrolled in the study with a 1 to 1 ratio in atorvastatin + lopinavir/ritonavir and lopinavir/ritonavir groups. Clinical and demographic characteristics were similar between the two groups. CRP level was significantly decreased in the lopinavir/ritonavir + atorvastatin group (P < 0.0001, Cohen’s d = 0.865) so that there was a significant difference in CRP level on the 6th day between the two groups (P = 0.01). Nevertheless, there was no significant difference in O2sat on day 6. Although the duration of hospitalization in the lopinavir/ritonavir + atorvastatin group was significantly reduced compared to the control group (P = 0.012), there was no significant difference in the invasive mechanical ventilation reception and the need for interferon and immunoglobulin.
Conclusion
Atorvastatin + lopinavir/ritonavir may be more effective than lopinavir/ritonavir in treating COVID-19 adult hospitalized patients.
Keywords: Coronavirus disease 2019, Atorvastatin, Lopinavir, Ritonavir, Inpatients
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic as a public health threat in the 21st century has affected the lives of millions of people around the world [1]. There is currently no drug that can definitively cure this disease [2]. Therefore, the reuse of approved drugs to reduce the severity of COVID-19 can be an effective way to control this crisis [3]. Statins are a low-cost, first-line therapy for dyslipidemia with tolerable side effects and are widely used around the world, including in developing countries. Anti-inflammatory and immune-regulating effects of statins propose that these drugs may be useful in confronting coronavirus infection, including SARS-CoV-2 [4], [5], [6]. Several studies have shown a considerable preservative effect of statins on ameliorating of proinflammatory cytokine release and immune cell functions among patients with viral and bacterial infections [6], [7], [8], [9]. Atorvastatin, the most common drug used to treat hypercholesterolemia, can block downstream processes in the biosynthesis of cholesterol, some of which are important factors in the virus infection. In the study of the antiviral ability of atorvastatin, by exposing cells infected with influenza A H1N1/New Jersey/76/76 virus to atorvastatin effective concentration (EC50), it was shown that atorvastatin in the treatment of the virus, by decreasing the virus titer and increasing cell viability, reduces the virus infection. By running MTT assay & hemagglutination endpoint test, significant anti-influenza properties of atorvastatin in cell culture were ascertained, which may interfere with inhibition of the early stage of virus replication [10]. A previous study has shown that combination therapy with atorvastatin + interferon pegylated alpha + ribavirin results in a high sustained virological response rate (SVR) of 95.83% in patients with genotype 3 chronic hepatitis C, and as a result, statin therapy can be performed with high safety in patients with chronic hepatitis C [11]. The Current guideline for the treatment of COVID-19 inpatients in Iran includes the administration of lopinavir/ritonavir (kaletra) [12], but atorvastatin has not been tested in hospitalized patients. Therefore, this study aimed to evaluate the effectiveness and safety of adding atorvastatin to the routine protocol of COVID-19 adult hospitalized patients.
2. Methods
2.1. Study design
A double-blind, randomized parallel-group, clinical trial with blinded outcome evaluation was operated on patients hospitalized with COVID‐19 infections from January 26, 2021, to February 17, 2021, at Razi referral hospital in Ghaemshahr, Mazandaran, Iran. This study was approved by the Ethical Committee (ID: IR.MAZUMS.REC.1399.7324) and the Research Council of Mazandaran University of Medical Sciences and was submitted and approved by the Iranian Registry of Clinical Trials (ID: IRCT20190727044343N2, the full trial protocol can be accessed at http://www.irct.ir).
2.2. Patients
Adults (between 20 and 50 years) hospitalized for<24 h with positive reverse transcription-polymerase chain reaction (RT-PCR) test, confirmed computed tomography (CT) scan findings for COVID-19, and respiratory symptoms for <10 days were evaluated to enter the trial. The exclusion criteria include cardiovascular diseases (such as coronary artery bypass grafting, coronary artery disease, rheumatic diseases, etc.), myositis, pregnancy, liver injury or problems, and use of statins, chloroquine, hydroxychloroquine, and lopinavir/ritonavir.
2.3. Randomization and masking
Patients were randomly assigned to either lopinavir/ritonavir plus atorvastatin or lopinavir/ritonavir groups using unstratified block randomization with a block size of four. A block randomization list was constructed using computer-generated random numbers. Allocation was concealed. Physicians, trial staff, and outcome appraisers were blinded and randomization with coding was done by a third person. Patients were blinded by receiving an envelope including atorvastatin 40 mg with lopinavir/ritonavir 400/100 mg tablets, or lopinavir/ritonavir 400/100 mg tablets with placebo tablets instead of atorvastatin which were similar in shape, size, and color to atorvastatin tablets. The placebo tablets were made by the Department of Pharmacology of Mazandaran University of Medical Sciences.
2.4. Interventions
All patients received lopinavir/ritonavir 400/100 mg tablets twice daily (Nordic Pharmaceutical Company, Sweden). The intervention group (lopinavir/ritonavir + atorvastatin) received a single daily 40 mg oral tablet of atorvastatin based on Patel et al. study [13] (Sobhan Darou Pharmaceutical Company, Iran) with lopinavir/ritonavir (400/100 mg tablets twice daily). Pharmaceutical companies were not involved in the design and financial support. The drugs used in the present study were purchased from official pharmacies in Iran. Pharmaceutical companies Nordic and Sobhan Darou did not have access to the study data either during the trial or before publication. The duration of treatment was five days according to our previous trial [14] and both patients and medical staff were unaware of the contents of the tablet.
2.5. Outcome measures
The primary outcome was the duration of hospitalization. The secondary outcomes included the need for interferon or immunoglobulin, receipt of invasive mechanical ventilation, O2 saturation (O2sat), and level of C-reactive protein (CRP). O2sat and CRP were both assessed at the onset of admission and on the 6th day of treatment.
2.6. Statistical analysis
Continuous variables normality was assessed by Kolmogorov-Smirnov and Shapiro-Wilk test and reported as mean ± standard deviation (SD) if normality could be assumed, or median ± interquartile range (IQR) otherwise. Qualitative values were reported as frequency (percentage). Independence t‐test (comparison of continuous variables between two groups), Paired t-test (comparison of continuous variables before and after treatment), Fisher's exact test, and Chi‐square test (comparing the categorical variables) were utilized for analysis. Cohen's d was also used to evaluate the effect size. For data analysis, the Statistical Package for the Social Sciences (SPSS Inc., version 22.0, Chicago, IL, USA) was used at a significance level of 0.05.
3. Results
3.1. Demographics and clinical characteristics
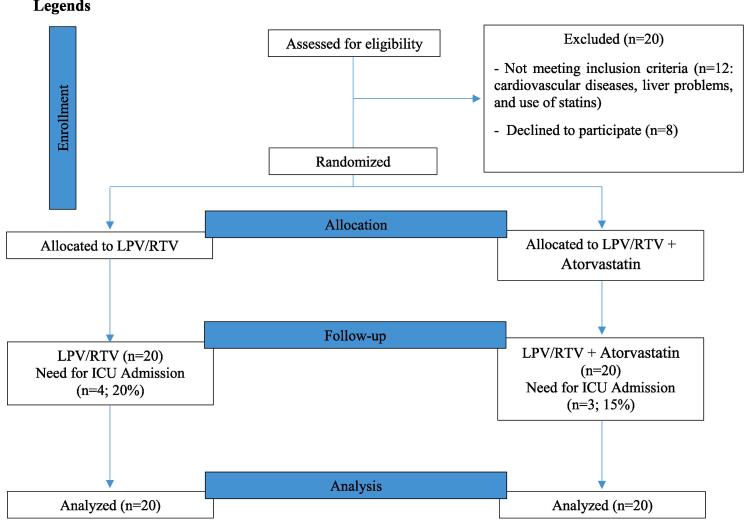
In total, 60 patients were appraised for eligibility, and finally, 20 were excluded from the study due to not meeting inclusion criteria (n = 12: cardiovascular diseases, liver problems, and use of statins) and declining to participate (n = 8). Finally, 40 patients in the two groups of lopinavir/ritonavir (n = 20) and lopinavir/ritonavir + atorvastatin (n = 20) were enrolled in the study (Fig. 1).
Fig. 1.
CONSORT 2010 flow diagram. LPV/RTV, lopinavir/ritonavir.
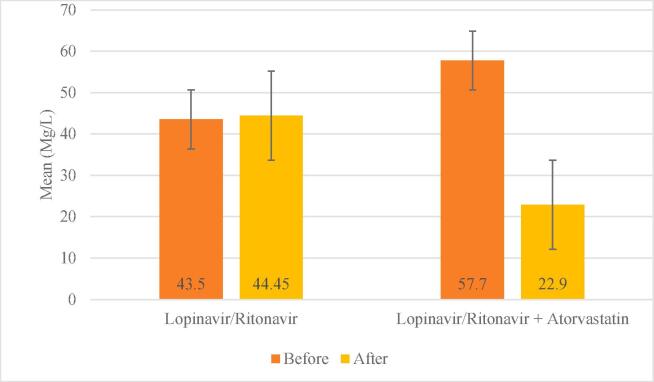
The demographic and clinical characteristics of patients at baseline are shown in Table 1. The mean age of patients was 46.02 ± 6.84 and 52.5% of patients were male. All of the patients had a fever. Dyspnea (72.5%), myalgia (80%), anosmia (77.5%), and diarrhea (32.5%) were recorded in the patients. Twenty-nine patients (72.5%) were current smokers. The White Blood Cell (WBC) counts in lopinavir/ritonavir + atorvastatin and control group were 7514.11 ± 377.55 109/L and 7732.51 ± 644.2 109/L, respectively. There was no significant difference between the lopinavir/ritonavir + atorvastatin and control arm in cholesterol levels, lymphocyte count, and respiratory rate. Body mass index (BMI), current smoking, and underlying diseases were similar across the treatment arm. CRP levels were significantly higher in the atorvastatin group than in the control group at baseline (57.70 ± 19.94 vs 43.50 ± 22.12, P = 0.040) (Table 1).
Table 1.
Demographics and clinical characteristics at baseline.
| Variables | lopinavir/ritonavir (n = 20) | lopinavir/ritonavir + atorvastatin (n = 20) | P-value |
|---|---|---|---|
| Gender, N (%) | 0.752* | ||
| Male | 11(55) | 10 (50) | |
| Female | 9 (45) | 10 (50) | |
| Age (year), Mean (SD) | 45.95 (6.98) | 46.10 (6.87) | 0.946** |
| BMI (Kg), Mean (SD) | 29 (42) | 30 (01) | 0.916** |
| Current smoking, N (%) | 14 (70) | 15 (75) | 0.723* |
| Underlying conditions, N (%) | 0.969* | ||
| Diabetes | 3 (15) | 2 (10) | |
| Hypertension | 4 (20) | 2 (10) | |
| Lung diseases | 2 (10) | 1 (5) | |
| Clinical features, N (%) | |||
| Fever | 20 (100) | 20 (100) | 1.000* |
| Dyspnea | 16 (80) | 13 (65) | 0.240* |
| Myalgia | 16 (80) | 16 (80) | 1.000* |
| Anosmia | 15 (75) | 16 (80) | 0.705* |
| Diarrhea | 7 (35) | 6 (30) | 0.736* |
| Cholesterol (mg/dL), Mean (SD) | 192.10 (20.21) | 201.61 (42.52) | 0.372** |
| Lymphocyte count (109/L), Mean (SD) | 745.70 (86.12) | 768.85 (67.35) | 0.349** |
| White Blood Cell count (109/L), Mean (SD) | 7732.51 (644.21) | 7514.11 (377.55) | 0.199** |
| Respiratory rate, Mean(SD) | 84.42 (7.13) | 87.71 (9.21) | 0.214** |
| C-Reactive protein (CRP) (mg/L), Mean (SD) | 43.50 (22.12) | 57.70 (19.94) | 0.040** |
SD: Standard deviation; N: Number; CRP: C-Reactive protein.
Chi-Square.
Independent T-Test.
3.2. Outcomes
3.2.1. Primary outcome
In the primary endpoint of the duration of hospitalization, the rate of hospitalization in lopinavir/ritonavir + atorvastatin was significantly lower than lopinavir/ritonavir (9.75 ± 2.29 vs. 7.95 ± 2.04 days; P = 0.012, Table 2). Seven patients were admitted to intensive unit care (ICU), three (15%) in the lopinavir/ritonavir + atorvastatin group, and four (20%) in the lopinavir/ritonavir group; no significant relationship was observed between the two groups (P = 0.5). Patients in the lopinavir/ritonavir + atorvastatin group had a better duration of hospitalization and ICU admissions.
Table 2.
Outcomes in lopinavir/ritonavir and lopinavir/ritonavir + atorvastatin treatments.
| Variables | Lopinavir/Ritonavir (n = 20) |
Lopinavir/Ritonavir + Atorvastatin (n = 20) |
P-value |
|---|---|---|---|
| Primary outcome | |||
| Duration of hospitalization (day), Mean (SD) | 9.75 (2.29) | 7.95 (2.04) | 0.012* |
| Secondary outcomes | |||
| Need for interferon or immunoglobulin, N (%) | 4 (20) | 3 (15) | 0.500*** |
| Receipt of invasive mechanical ventilation, N (%) | 1 (5) | 0 | NS |
| O2Sat (%), Mean (SD) | |||
| • Day 1 | 91.50 (1.79) | 91.50 (2.16) | 0.580* |
| • Day 6 | 91.50 (5.31) | 93.45 (3.22) | 0.168* |
| P-value | 1.000** | 0.030** | |
| CRP (mg/L), Mean (SD) | |||
| • Day 1 | 43.50 (22.12) | 57.70 (19.94) | 0.040* |
| • Day 6 | 44.45 (29.47) | 22.90 (19.31) | 0.010* |
| P-value | 0.909** | < 0.0001** |
SD: Standard deviation; N: Number; O2Sat: O2 saturation; CRP: C-Reactive protein; ICU: intensive unit care; NS: No Significant.
Independent T-Test between two groups.
Paired T-Test between day 1 and day 6.
Chi-Square.
3.2.2. Secondary outcomes
Seven patients needed interferon or immunoglobulin, which was higher in lopinavir/ritonavir but was not statistically significant (P = 0.5). One patient received invasive mechanical ventilation in the control group (5%), while in the lopinavir/ritonavir + atorvastatin group, no one needed the mechanical ventilation. This difference was non-significant (Table 2). The mean of O2sat before and after treatment was 91.50 ± 1.96 and 92.47 ± 4.44, respectively. Also, the mean of CRP before and after the treatment were 50.60 ± 21.99 and 33.67 ± 26.90, respectively. In the lopinavir/ritonavir + atorvastatin group, on the sixth day compared to the first day, O2sat increased and CRP decreased, which was statistically significant (P = 0.030). O2sat in group lopinavir/ritonavir was unchanged on the first and sixth days, but CRP increased on the sixth day. None of these changes were significant (P > 0.05, Table 2). There was no significant relationship between O2sat on the first day between the two groups, but on the sixth day, O2sat was significantly higher in the lopinavir/ritonavir + atorvastatin group. On the first day, CRP in the lopinavir/ritonavir + atorvastatin group was significantly higher, but on the sixth day, its level decreased significantly (P < 0.05; Cohen’s d = 0.865, Fig. 2, Table 2).
Fig. 2.
Comparison of mean CRP between before and after in two groups.
4. Discussion
Our results showed that the use of atorvastatin with lopinavir/ritonavir in hospitalized patients with COVID-19 may significantly reduce the number of hospitalization days in these patients. Also, the mean CRP in the atorvastatin + lopinavir/ritonavir group decreased significantly after 5 days. However, administration of atorvastatin had no efficacy on post-treatment O2sat. Moreover, there was no significant difference between the two groups in terms of invasive mechanical ventilation reception and the need for interferon or immunoglobulin.
It is rational to suppose that any effect demonstrated in the trial may be ascribed to atorvastatin. Current evidence suggests that in hospitalized patients with severe COVID-19, there was no benefit to lopinavir/ritonavir therapy compared to standard care [15]. In addition, recent results from the RECOVERY collaborative group have shown that lopinavir-ritonavir administration was not associated with diminutions in 28-day mortality, length of hospital stay, risk of progression to invasive mechanical ventilation, and death [16]. Currently, no drug can be definitively effective in treating patients with COVID-19 [17].
The results of studies on the use of statins in the treatment of patients with COVID-19 are polemical. An Indonesian meta-analysis, which analyzed 9 studies involving 3449 patients with COVID-19, showed that statin therapy had no effect on reducing mortality (OR 0.78, 95% CI: (0.50–1.21), p = 0.26, I2 = 0%, fixed-effect modelling) and severity (OR = 1.64, 95% CI: (0.51–5.23), P = 0.41, I2 = 93%, random-effect modelling) of COVID-19 [18]. In a retrospective cohort study of 717 patients admitted to Singapore's third COVID-19 infection center, logistic regression models showed that those who were treated with statins were less likely to be admitted to the ICU than those who were not (Average treatment effect of statins (ATET) Coeff (risk difference): − 0.12 (−0.23, − 0.01); p = 0.028). It was concluded that the use of statins independently was associated with a lower rate of hospitalization in the ICU [19]. These results may be due to the method utilized in statistical analysis. In another recent Iranian retrospective cohort of 150 patients admitted for COVID‑19 using Cox proportional-hazards regression models, statin use was associated with a lower risk of morbidity (HR = 0.85, 95% CI = (0.02, 3.93), P = 0.762) and mortality (HR = 0.76; 95% CI = (0.16, 3.72), P = 0.735). In addition, statins usage reduced the chances of progressing to mechanical ventilation (OR = 0.96, 95% CI = (0.61–2.99), P = 0.942). Patients consuming statins had more normal computed tomography (CT) scan results than others (OR = 0.41, 95% CI = (0.07–2.33), P = 0.312). However, none of these variables were statistically significant [20]. A single-center survey in California found that using statins significantly reduced the risk of severe COVID-19 (adjusted OR 0.29, 95 %CI 0.11 to 0.71, p < 0.01) and recovered faster in hospitalized patients without the severe form of the disease (adjusted HR for recovery 2.69, 95 %CI 1.36 to 5.33, p < 0.01) [21].
Our findings revealed that the addition of atorvastatin to the treatment regimen of patients admitted to the Razi COVID-19 referral center in Mazandaran province significantly reduces the number of days of hospitalization and level of CRP. However, there was no significant reduction in receipt of invasive mechanical ventilation, need for interferon or immunoglobulin, and ICU admission, which seems to be due to the small sample size of the present study. Some evidence has shown that statins usage in outpatients can reduce their mortality rate [22], [23]. It stands to reason that if statin therapy begins earlier in the course of the disease, better sequels would be acquired.
In the present study, although CRP levels decreased significantly, at discharge, CRP levels in patients in the atorvastatin group were higher than normal. This can be illustrated by the fact that due to the high volume of COVID-19 patients in the third peak of the disease in Iran and their referral to our medical center as a COVID-19 main center, further follow-up was not possible and as soon as observing improvement, patients were discharged. Hence, the duration of drug administration for patients was 5 days, as we set the 5 days treatment regimen in our previous trial in the first peak [14].
Several possible mechanisms have been suggested for the effect of statins on COVID-19. The causative agent of COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) enters cells by binding to the angiotensin-converting enzyme 2 (ACE2) and causes ACE downregulation. This converts angiotensin II to angiotensin, resulting in reduced protective effects. Statins cause ACE2 upregulation. Statins prevent the development of inflammatory cytokines by inhibiting the toll-like receptor – myeloid differentiation factor 88 – nuclear factor kappa light chain enhancer of activated B cells (TLR – MYD88 – NF-κB) pathway, the pathway by which coronaviruses trigger a proinflammatory response in the host. Statins may also be able to prevent SARS-CoV-2 from entering the cell by lowering the cholesterol content of the cell membrane [24]. There are reports of thrombosis in COVID-19 patients and its effect on the inflammatory factors elevation [25], [26], resulting in increased mortality and morbidity [25]. Due to the anti-inflammatory properties of statins and their effect on reducing inflammatory biomarkers such as CRP and IL-6 [27], it is expected that using statins will reduce the incidence of thrombosis in these patients.
Several limitations have been assumed in the current study. Primarily, to further measure the clinical effect of atorvastatin, clinical signs were not evaluated as outcomes. This trial was performed only on hospitalized adults because, in 2020 in Mazandaran province, where our research was conducted, most of the COVID-19 patients were under the age of 60 [14] and especially under 50 years [28]. Hence, the results may not be generalizable to other populations. Because the levels of CRP were higher at baseline in patients of the atorvastatin + lopinavir/ritonavir group, it could be a signal for imbalances in study randomization. This is a potential flaw of a small randomized study design. Furthermore, due to a large number of patients in our medical center, long-term follow-up was not possible. Also, since viral load tests are performed only by the Iranian government, these tests were not performed in the present study for a more accurate evaluation of treatment response. The small sample size of this study is another limitation of the present study. Therefore, future studies with larger sample sizes are necessary to reduce the risk of type II error.
5. Conclusion
In conclusion, among adults hospitalized with COVID-19, the use of atorvastatin + lopinavir/ritonavir reduces the number of hospitalization days and CRP levels after 5 days of consumption compared to lopinavir/ritonavir. Larger, well-designed surveys with longer follow-up periods are needed to confirm the efficacy of atorvastatin in COVID-19 hospitalized patients.
Authorship statement
All authors meet the ICMJE authorship criteria.
Funding statement
We did not receive any funds for this research.
Ethical statements
This project was conducted according to the World Medical Association Declaration of Helsinki and was performed after the approval of the Biomedical Research Ethics Committee of Mazandaran University of Medical Sciences. The consent form is approved by the Ethics Committee in Biomedical Research of Mazandaran University of Medical Sciences. Written informed consent was obtained from the patients for the publication of this article. A copy of the written consent is available for review by the editor-in-chief of this journal.
CRediT authorship contribution statement
Lotfollah Davoodi: Conceptualization, Methodology, Resources, Supervision, Validation, Data curation, Writing – original draft. Hamed Jafarpour: Conceptualization, Methodology, Resources, Writing – review & editing. Ziaeddin Oladi: Conceptualization, Methodology, Validation, Data curation, Writing – original draft. Zakaria Zakariaei: Conceptualization, Methodology, Validation, Data curation, Writing – original draft. Mohammad Tabarestani: Conceptualization, Resources, Methodology, Writing – review & editing. Bahareh Moayed Ahmadi: Conceptualization, Methodology, Resources. Alireza Razavi: Conceptualization, Methodology, Writing – review & editing, Supervision. Amirhossein Hessami: Conceptualization, Methodology, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Special thanks to the Student Research Committee of Mazandaran University of Medical Sciences for supporting us in this project.
References
- 1.Davoodi L., Oladi Z., Jafarpour H., Zakariaei Z., Soleymani E., Razavi A. A 33-year-old man with COVID-19 presented with subacute thyroiditis: A rare case report and literature review. New Microbes New Infect. 2021;41:100871. doi: 10.1016/j.nmni.2021.100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razavi A., Davoodi L., Shojaei L., Jafarpour H. COVID-19 in children: a narrative review. Open Access Macedonian J. Med. Sci. 2020;8(T1):23–31. [Google Scholar]
- 3.Zhang X.-J. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metabolism. 2020;32(2):176–187. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castiglione V., Chiriacò M., Emdin M., Taddei S., Vergaro G. Statin therapy in COVID-19 infection. Eur. Heart J.-Cardiovasc. Pharmacotherapy. 2020;6(4):258–259. doi: 10.1093/ehjcvp/pvaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dashti‐Khavidaki S., Khalili H. Considerations for statin therapy in patients with COVID-19. Pharmacotherapy: J. Human Pharmacol. DrugTherapy. 2020;40(5):484–486. doi: 10.1002/phar.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedson D.S. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99(3):417–435. doi: 10.1016/j.antiviral.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Papazian L. Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: a randomized clinical trial. JAMA. 2013;310(16):1692–1700. doi: 10.1001/jama.2013.280031. [DOI] [PubMed] [Google Scholar]
- 8.Pertzov B., Eliakim-Raz N., Atamna H., Trestioreanu A.Z., Yahav D., Leibovici L. Hydroxymethylglutaryl-CoA reductase inhibitors (statins) for the treatment of sepsis in adults–A systematic review and meta-analysis. Clin. Microbiol. Infect. 2019;25(3):280–289. doi: 10.1016/j.cmi.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Sapey E. Pulmonary infections in the elderly lead to impaired neutrophil targeting, which is improved by simvastatin. Am. J. Respir. Crit. Care Med. 2017;196(10):1325–1336. doi: 10.1164/rccm.201704-0814OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehrbod P., Ideris A., Omar A.R., Hair-Bejo M. Evaluation of antiviral effect of atorvastatin on H1N1 infection in MDCK cells. African J. Microbiol. Res. 2012;6(27):5715–5719. [Google Scholar]
- 11.Todorovska B. Atorvastatin in Combination with Pegylated Interferon and Ribavirin Provided High Rate of Sustained Virological Response in Patients with Genotype 3 Hepatitis C Virus. Open Access Macedonian J. Med. Sci. 2019;7(10):1641–1648. doi: 10.3889/oamjms.2019.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahmanzade R., Rahmanzadeh R., Hashemian S.M., Tabarsi P. Iran's Approach to COVID-19: Evolving Treatment Protocols and Ongoing Clinical Trials. Front. Public Health. 2020;8:523. doi: 10.3389/fpubh.2020.551889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel J.M. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial) Crit. Care. 2012;16(6):R231. doi: 10.1186/cc11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davoodi L. Febuxostat therapy in outpatients with suspected COVID-19: A clinical trial. Int. J. Clin. Pract. 2020;74(11) doi: 10.1111/ijcp.v74.1110.1111/ijcp.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao B. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horby P.W. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet. 2020;396(10259):1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davoodi L., Jafarpour H., Kazeminejad A., Soleymani E., Akbari Z., Razavi A. Hydroxychloroquine-induced Stevens–Johnson syndrome in COVID-19: a rare case report. Oxford Medical Case Rep. 2020;2020(6):omaa042. doi: 10.1093/omcr/omaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hariyanto T.I., Kurniawan A. Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metabolic Syndrome: Clin. Res. Rev. 2020;14(6):1613–1615. doi: 10.1016/j.dsx.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan W.Y., Young B.E., Lye D.C., Chew D.E., Dalan R. Statin use is associated with lower disease severity in COVID-19 infection. Sci. Rep. 2020;10(1):1–7. doi: 10.1038/s41598-020-74492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peymani P. Statins in patients with COVID-19: a retrospective cohort study in Iranian COVID-19 patients. Translat. Med. Commun. 2021;6(1):1–14. doi: 10.1186/s41231-021-00082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels L.B. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am. J. Cardiol. 2020;136:149–155. doi: 10.1016/j.amjcard.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta A. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-021-21553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedson D.S. Statin treatment of COVID-19. Am. J. Cardiol. 2020;136:171–173. doi: 10.1016/j.amjcard.2020.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minz M.M., Bansal M., Kasliwal R.R. Statins and SARS-CoV-2 disease: Current concepts and possible benefits. Diabetes & Metabolic Syndrome: Clin. Res. Rev. 2020.;14(6):2063–2067. doi: 10.1016/j.dsx.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanff T.C., Mohareb A.M., Giri J., Cohen J.B., Chirinos J.A. Thrombosis in COVID-19. Am. J. Hematol. 2020;95(12):1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davoodi L., Jafarpour H., Taghavi M., Razavi A. COVID-19 presented with deep vein thrombosis: an unusual presenting. J. Invest. Med. High Impact Case Rep. 2020;8 doi: 10.1177/2324709620931239. 2324709620931239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez A.L., Wojcik B.M., Wrobleski S.K., Myers D.D., Wakefield T.W., Diaz J.A. Statins, inflammation and deep vein thrombosis: a systematic review. J. Thromb. Thrombolysis. 2012;33(4):371–382. doi: 10.1007/s11239-012-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roozbeh F. Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial. J. Antimicrobial Chemotherapy. 2021;76(3):753–757. doi: 10.1093/jac/dkaa501. [DOI] [PMC free article] [PubMed] [Google Scholar]