Abstract
Massive hemoptysis may originate from injured pulmonary arteries, such as from pulmonary artery pseudoaneurysms (PAPs). A 93-year-old man, diagnosed with pneumonia, was hospitalized; he later developed a lung abscess (controlled with intravenous antibiotics). On post-hospitalization day 29, he suddenly developed hemoptysis. Multi-detector computed tomography angiography (MDCTA) showed an enhanced nodule, diagnosed as a PAP, inside the lung abscess. The hemoptysis resolved, without recurrence, following transcatheter arterial embolization (TAE) of the PAP and its feeding arteries. PAPs should be considered in patients with lung abscesses and delayed massive hemoptysis. In these patients, MDCTA and TAE are effective diagnostic and treatment modalities.
Keywords: Pulmonary artery pseudoaneurysm, Lung abscess, Multi-detector computed tomography angiography, Hemoptysis, Transcatheter arterial embolization
Highlights
-
•
Massive hemoptysis can originate from pulmonary artery pseudoaneurysms (PAPs).
-
•
PAPs should be considered in patients with delayed hemoptysis and lung abscesses.
-
•
Multi-detector computed tomography angiography can identify origin of hemoptysis.
-
•
It is useful for diagnosing and treating PAPs secondary to lung abscesses.
-
•
Transcatheter arterial embolization can treat PAPs secondary to lung abscesses.
Abbreviations
- PAP
pulmonary artery pseudoaneurysm
- MDCTA
multi-detector computed tomography angiography
- TAE
transcatheter arterial embolization
- CT
computed tomography
- WBC
white blood cell
- CRP
C-reactive protein
1. Introduction
Massive hemoptysis has a high mortality rate (50–100%) if not appropriately treated [1]. The origin of massive hemoptysis typically involves the systemic circulation, such as the bronchial circulation, but may also involve the pulmonary circulation (less than 10% of cases) [1]. In cases of hemoptysis derived from the pulmonary circulation, the central branches of pulmonary arteries are usually involved, and the hemoptysis is frequently (89% of cases) caused by congenital heart abnormalities or pulmonary hypertension [2]. In contrast, hemoptysis originating from the peripheral branches of the pulmonary arteries (11% of pulmonary circulation-involved cases) is usually caused by infection, inflammation, or trauma [3]. In cases of pulmonary artery pseudoaneurysms (PAPs) caused by pulmonary infection, PAPs related to non-tuberculous pulmonary infections are relatively rare [3] and require prompt diagnosis and treatment. In general, chest x-rays, computed tomography (CT), CT angiography, bronchoscopy, and conventional angiography are the diagnostic modalities of choice for patients exhibiting massive hemoptysis. One study showed that non-contrast multi-detector CT, chest x-rays, and bronchoscopy can identify the bleeding sites in 92.5%, 70%, and 52.5% of massive hemoptysis cases, respectively [4]. These same modalities are useful in determining the underlying causes of the massive hemoptysis in 60%, 25%, and 32.5% of cases, respectively [4]. Additionally, multi-detector CT angiography (MDCTA) is reportedly able to identify the vascular origins of massive hemoptysis in 76% of cases and can guide the subsequent therapeutic procedures [5]. Although MDCTA is a noninvasive and effective diagnostic modality for massive hemoptysis, its usefulness for diagnosing and guiding subsequent treatment is unclear for the rare cases of patients with PAPs secondary to lung abscesses.
2. Case presentation
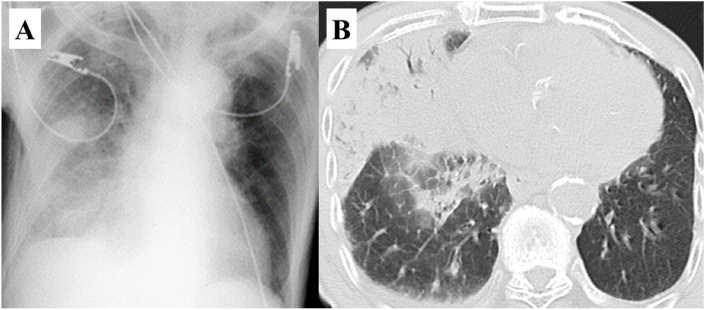
A 93-year-old Japanese man with moderate aortic stenosis, fever, and disturbed consciousness was transferred to the emergency department. His measured vital signs were: body temperature, 41.4 °C; blood pressure, 113/54 mmHg; pulse rate, 124 beats/min; oxygen saturation, 99% (under 90% oxygen inhalation); and Glasgow coma scale, E2V1M4 (seven points). A physical examination revealed crackles in his right lower chest, and laboratory findings revealed his white blood cell (WBC) count (14.8 × 103/μL; 74.4% neutrophils), C-reactive protein (CRP) concentration (13.0 mg/dL), prothrombin time-international normalized ratio (1.41), activated partial thromboplastin time (36 seconds), and fibrinogen concentration (475 mg/dL). Chest radiography showed homogeneous opacity consolidation in the right mid and lower zones (Fig. 1A), and chest CT showed consolidation, with an air bronchogram in the middle lobe of the right lung (Fig. 1B).
Fig. 1.
Chest x-ray image shows consolidation in the right mid and lower zones (A). Computed tomography shows consolidation and an air bronchogram in the middle lobe of the right lung (B).
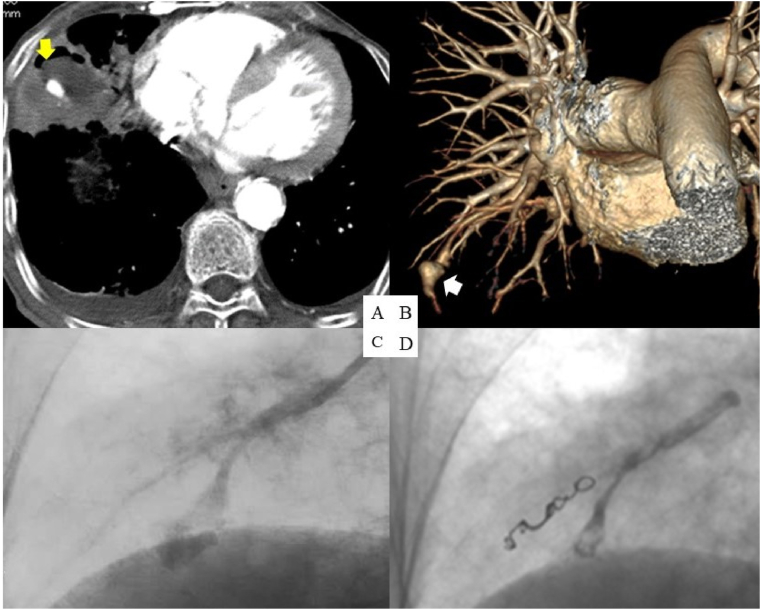
Hence, the patient was admitted with a diagnosis of pneumonia and treated with intravenous sulbactam/ampicillin (3 g every 8 h). A sputum culture obtained upon admission was positive for both Klebsiella pneumoniae and Pseudomonas aeruginosa; the latter was believed to have settled in his respiratory tract and not be pathogenic. On day 9, the patient's chest CT showed necrosis inside the consolidation; we continued intravenous sulbactam/ampicillin (3 g every 6 h) and diagnosed a lung abscess. Additionally, we administered intravenous ciprofloxacin (200 mg every 24 h), given that Pseudomonas aeruginosa was considered to be related to the lung abscess. Over the following days, the patient's general status improved, including defervescence of the fever with decreases in his WBC counts and CRP levels. On day 29, the patient suddenly demonstrated massive hemoptysis. The following day, MDCTA indicated an enhanced nodule inside the abscess, suggestive of a PAP (Fig. 2A and B). We urgently performed pulmonary angiography, which confirmed the presence of a PAP (Fig. 2C). We guided a 5-Fr GoodtecⓇ angiographic diagnostic catheter (Goodman Co., Ltd., Nagoya, Japan) and a 1.7-Fr NadeshikoⓇ micro-catheter (JMS, Shinagawa, Japan) to the PAP and subsequently performed embolization with two 0.018-inch embolization coils (3-2 and 4-2 mm coil diameters) (TornadoⓇ; Cook Medical, Bloomington, IN), HistoacrylⓇ (B. Braun Aesculap, Melsungen, Germany), and LipiodolⓇ Ultra Fluid (Guerbet, Villepinte, France) (Fig. 2D).
Fig. 2.
Multi-detector computed tomography angiography (MDCTA) on day 30 shows an enhanced nodule inside the lung abscess (yellow arrow) (A) and the pulmonary artery pseudoaneurysm (PAP) (white arrow) (B). Pulmonary angiography demonstrates the PAP (C). Blood flow almost disappears after successful transcatheter arterial embolization (D). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
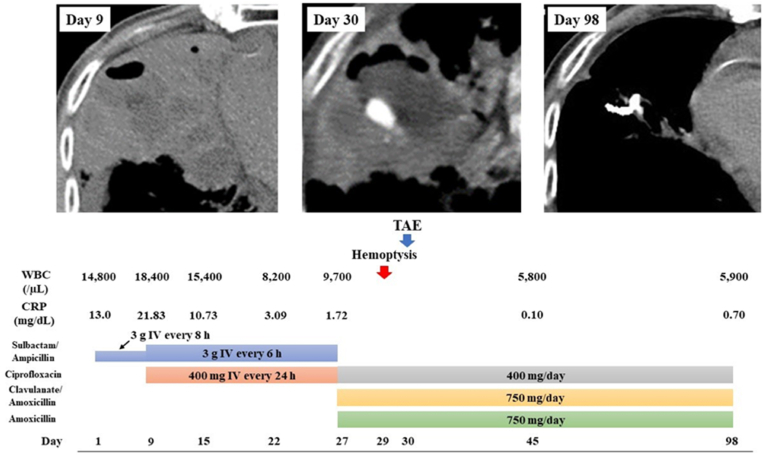
On day 35, MDCTA revealed a collapsed PAP, and the patient was discharged on day 45. Approximately 2 months after the embolization, the patient had not experienced recurrent hemoptysis, and a repeat chest CT was unable to detect the abscess (Fig. 3).
Fig. 3.
Patient's clinical course. During antibiotic treatment for the lung abscess, the patient suddenly developed massive hemoptysis on day 29. A pulmonary artery pseudoaneurysm was detected by multi-detector computed tomography angiography and was treated with transcatheter arterial embolization (TAE) on day 30 with no recurrence. The abscess was no longer detectable using chest computed tomography on day 98. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
This report highlights three clinical issues: 1) the potential development of a PAP, secondary to a lung abscess, which leads to massive hemoptysis; 2) the efficacy of MDCTA for diagnosing PAPs secondary to lung abscesses; and 3) the procedures for treating PAPs secondary to lung abscesses.
The reported incidence of pulmonary artery aneurysms or pseudoaneurysms is 0.007% [6]. Considering our patient's condition, we reviewed cases of non-tuberculosis-associated PAPs that arose secondary to lung abscesses. A search of the PubMed database revealed only five reports (in English) of PAPs secondary to lung abscesses since 1985. Table 1 summarizes the features of these 5 patients and the one described in this report [[7], [8], [9], [10], [11]]. Interestingly, all cases involved middle-aged or older Japanese men, suggesting a regional underdiagnosis of PAPs secondary to lung abscesses. Streptococcus constellatus, Staphylococcus aureus, K. pneumoniae, and P. aeruginosa were reported as having contributed to the abscesses. In concordance, our patient also presented with sputum samples positive for K. pneumoniae and P. aeruginosa. However, the isolates derived from our patient did not possess hypermucoviscosity (negative string test) or mucoid phenotypes. Additionally, among the reported cases, the median time from the diagnosis of a lung abscess to the development of hemoptysis was 14 days. In contrast, the analogous time for cases of bronchial artery-associated hemoptysis secondary to a lung abscess is unknown; therefore, distinguishing the arteries from which massive hemoptysis originated is challenging. Nevertheless, we should consider the possibility of PAPs in cases of delayed hemoptysis secondary to lung abscesses.
Table 1.
Pulmonary artery pseudoaneurysms associated with lung abscesses.
| Authors | Age (years) | Sex | Location | Comorbidities | Pathogen(s) | Antibiotics | Time from lung abscess to hemoptysis (days) | Tools to diagnose the PAP | Procedure | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Saito et al. [7] | 46 | Male | Japan | Depression | Staphylococcus aureus | NA | 0 | CTA | Lobectomy | A |
| Hamada et al [8] | 79 | Male | Japan | CIAf |
Not detected | Tazobactam/piperacillin | 7 | CTA | TAE | D1 |
| Oguma et al [9] | 66 | Male | Japan | Oropharyngeal Cancer |
Pseudomonas aeruginosa | Sulbactam/ampicillin | 16 | CTA | TAE | D2 |
| Morinaga et al. [10] | 63 | Male | Japan | Hypertension | Streptococcus constellatus | Levofloxacin | 30 | CTA | Lobectomy | A |
| Haranaga et al. [11] | 90 | Male | Japan | Hypertension Parkinsonism |
Not detected | Cefotaxime | 14 | CTA | None | A |
| This case | 93 | Male | Japan | Aortic stenosis |
Klebsiella pneumoniae Pseudomonas aeruginosa |
Ciprofloxacin Sulbactam/ampicillin |
30 | CTA | TAE | A |
CI, cerebral infarction; Af, artrial fibrillation; NA, not applicable; CTA, computed tomography angiography; TAE, transcatheter arterial embolization; A, alive; D1, death without relevance to TAE; D2, death with unknown cause.
MDCTA was used to diagnose all the PAPs described in Table 1. Conventional angiography is no longer used for diagnosing PAPs because of the limited information provided on a vascular lumen image, particularly pertaining to the surrounding anatomical structures [12]. Moreover, identifying PAPs in the peripheral branches of the pulmonary arteries or in the presence of a thrombus in the vascular lumen may not be possible [12]. MDCTA is also useful for diagnosing abnormal bronchial arteries and aortopulmonary artery shunts [3,13,14]. Therefore, this technique may guide endovascular procedures, minimize the contrast load and radiation exposure, and reduce the number of complications associated with angiography. In our case, we diagnosed a PAP, without abnormal bronchial arteries, using MDCTA before confirming using conventional angiography. Thus, MDCTA appears to be helpful for diagnosing PAPs secondary to lung abscesses.
PAPs are often treated with either TAE or surgery given that the mortality associated with ruptured PAPs is over 50% [15]; however, the criteria for selecting specific procedures have not been established. Urgent surgery is not recommended for PAPs in patients with poor respiratory function or in poor general condition as the procedure is associated with increased mortality in such patients [16]. However, surgery should be considered for patients with active pleural hemorrhage, recurrent hemoptysis, and active infections that are refractory to antibiotic treatment [17]. In contrast to surgery, TAE tends to be a safe and noninvasive procedure, even in urgent situations, as MDCTA can accurately identify PAPs and their associated feeding arteries; MDCTA can also be used to guide the TAE procedure [18]. TAE can also be performed to control hemorrhage in cases where surgery is to be performed [19,20]. In our review, TAE was successfully performed, without complications, in two patients, despite their poor respiratory [8] or general [9] conditions. Although TAE was initially planned in two surgical treatment cases, surgery was subsequently performed due to the difficulty associated with accessing the PAPs [7,10]. Accordingly, TAE of PAPs secondary to lung abscesses may be selected, if accessible; however, surgery should be considered in cases that are inappropriate for TAE. In our case, selective pulmonary angiography identified the PAP based on the MDCTA findings and TAE was successfully performed. We believed that the focal coagulation around the coils was inadequate because of the coagulation abnormality caused by the lung abscess and the liquid embolic materials filling the injured vessels. Hence, we first performed coil embolization of the distal segment of the pseudoaneurysm to avoid overflow of the n-butyl-2-cyanoacrylate (NBCA), which subsequently filled the injured vessel leading to the pseudoaneurysm. We believe that TAE should be considered for the control of massive hemoptysis caused by PAPs that form secondary to lung abscesses, regardless of whether surgery is performed.
4. Conclusions
-
•
PAP should be considered in the differential diagnosis of delayed hemoptysis secondary to a lung abscess.
-
•
MDCTA is effective for detecting PAPs and their feeding arteries.
-
•
TAE should be the first modality considered for the treatment of PAPs secondary to lung abscesses.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
All authors participated in the treatment of this case, and the first author drafted the manuscript. All authors read and approved the final manuscript.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal, upon request.
Ethical approval
This study was approved by the institutional review board and ethics committee of the Japanese Red Cross Ise Hospital (Permission number: ER2020-22).
Author statement
Hiroyuki Tanaka: Conceptualization, Methodology, Data curation, Writing - Original draft, Writing - Review and Editing, Visualization. Junji Uraki: Methodology. Motoaki Tanigawa: Supervision. Yuki Nakanishi: Conceptualization, Methodology, Writing - review & editing, Visualization. Hirokazu Toyoshima: Conceptualization, Methodology, Writing - review & editing, Visualization. Shigetoshi Sakabe: Supervision.
Declaration of competing interest
The authors state that they have no conflicts of interest.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Contributor Information
Hiroyuki Tanaka, Email: resident_1_2@yahoo.co.jp.
Junji Uraki, Email: junji70d@yahoo.co.jp.
Motoaki Tanigawa, Email: m.tanigawa@ise.jrc.or.jp.
Yuki Nakanishi, Email: y_nakanishi_127@yahoo.co.jp.
Hirokazu Toyoshima, Email: hirokazutoyoshima@gmail.com.
Shigetoshi Sakabe, Email: shigesakabe@yahoo.co.jp.
References
- 1.Khalil A., Parrot A., Nedelcu C., Fartoukh M., Marsault C., Carette M.F. Severe hemoptysis of pulmonary arterial origin: signs and role of multidetector row CT angiography. Chest. 2008;133:212–219. doi: 10.1378/chest.07-1159. [DOI] [PubMed] [Google Scholar]
- 2.Boyd L.J., McGavack T.H. Aneurysm of the pulmonary artery. Am. Heart J. 1939;18:562–578. doi: 10.1016/S0002-8703(39)90880-X. [DOI] [Google Scholar]
- 3.Ungaro R., Saab S., Almond C.H., Kumar S. Solitary peripheral pulmonary artery aneurysms. Pathogenesis and surgical treatment. J. Thorac. Cardiovasc. Surg. 1976;71:566–571. doi: 10.1016/S0022-5223(19)40180-3. [DOI] [PubMed] [Google Scholar]
- 4.Davoodi M., Kordi M., Gharibvand M.M., Shoushtari M.H., Borsi H., Bahadoram M. Hemoptysis: comparison of diagnostic accuracy of multi detector CT scan and bronchoscopy. Global J. Health Sci. 2015;7:373–377. doi: 10.5539/gjhs.v7n3p373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel-Ghany A.F., Nassef M.A., Osman N.M. Multidetector CT chest with bronchial and pulmonary angiography determining causes, site and vascular origin of bleeding in patients with hemoptysis, Egypt. J. Rad. Nucl. Med. 2013;44:769–778. doi: 10.1016/j.ejrnm.2013.07.011. [DOI] [Google Scholar]
- 6.Deterling R.A., Jr., Clagett O.T. Aneurysm of the pulmonary artery. Review of the literature and report of a case. Am. Heart J. 1947;34:471–499. doi: 10.1016/0002-8703(47)90527-9. [DOI] [PubMed] [Google Scholar]
- 7.Saito S., Kadota T., Gochi M. Pulmonary artery pseudoaneurysm caused by lung abscess. Am. J. Med. Sci. 2020;359:385–386. doi: 10.1016/j.amjms.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Hamada S., Nakano A., Tsukino M. Large lung abscess with pulmonary artery pseudoaneurysm. Arch. Bronconeumol. 2017;53:454–455. doi: 10.1016/j.arbres.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Oguma T., Morise M., Harada K., Tanaka J., Sato M., Horio Y., Takiguchi H., Tomomatsu H., Tomomatsu K., Takihara T., Niimi K., Hayama N., Aoki T., Urano T., Ito C., Koizumi J., Asano K. Pulmonary artery aneurysm/pseudoaneurysm, a delayed complication of lung abscess: a case report. Tokai J. Exp. Clin. Med. 2015;40:86–89. [PubMed] [Google Scholar]
- 10.Morinaga Y., Yanagihara K., Gyotoku H., Oshima K., Izumikawa K., Yamasaki N., Kakeya H., Hayashi T., Fukuoka J., Nagayasu T., Kohno S. Pulmonary artery pseudoaneurysm caused by Streptococcus constellatus. Int. J. Infect. Dis. 2013;17 doi: 10.1016/j.ijid.2013.03.013. e1064–e1066. [DOI] [PubMed] [Google Scholar]
- 11.Haranaga S., Teruya H., Nakamura H., Higa F., Tateyama M., Fujita J. Pulmonary artery pseudoaneurysm secondary to lung abscess. Intern. Med. 2009;48:2159–2160. doi: 10.2169/internalmedicine.48.2610. [DOI] [PubMed] [Google Scholar]
- 12.Guillaume B., Vendrell A., Stefanovic X., Thony F., Ferretti G.R. Acquired pulmonary artery pseudoaneurysms: a pictorial review. Br. J. Radiol. 2017;90:20160783. doi: 10.1259/bjr.20160783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noë G.D., Jaffé S.M., Molan M.P. CT and CT angiography in massive haemoptysis with emphasis on pre-embolization assessment. Clin. Radiol. 2011;66:869–875. doi: 10.1016/j.crad.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi H., Matsumoto T., Morimoto K., Osawa T., Tanaka Y., Yoshimori K., Kamei S., Yamamoto S., Kurosaki A., Hasebe T. Detection of shunting into pulmonary artery on multidetector row computed tomography arteriography before bronchial arterial embolization: a preliminary study. J. Comput. Assist. Tomogr. 2020;44:852–856. doi: 10.1097/RCT.0000000000001099. [DOI] [PubMed] [Google Scholar]
- 15.Vaideeswar P., Karande S., Yadav S., Pardeshi K. Pulmonary artery pseudoaneurysm: a rare cause of hemoptysis in a child. Pediatr. Dev. Pathol. 2016;19:146–149. doi: 10.2350/15-05-1642-CR.1. [DOI] [PubMed] [Google Scholar]
- 16.Koneru H., Roy S.B., Islam M., Abdelrazek H., Bandyopadhyay D., Madan N., Patil P.D., Panchabhai T.S. Pulmonary artery pseudoaneurysm: a rare cause of fatal massive hemoptysis. Case Rep. Pulmonol. 2018;2018:8251967. doi: 10.1155/2018/8251967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y., Gilman M.D., Humphrey K.L., Salazar G.M., Sharma A., Muniappan A., Shepard J.O., Wu C.C. Pulmonary artery pseudoaneurysms: clinical features and CT findings. AJR Am. J. Roentgenol. 2017;208:84–91. doi: 10.2214/AJR.16.16312. [DOI] [PubMed] [Google Scholar]
- 18.Shin T.B., Yoon S.K., Lee K.N., Choi J.S., Kim Y.H., Sung C.G., Kim Y.J., Kim C.W. The role of pulmonary CT angiography and selective pulmonary angiography in endovascular management of pulmonary artery pseudoaneurysms associated with infectious lung diseases. J. Vasc. Intervent. Radiol. 2007;18:882–887. doi: 10.1016/j.jvir.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Sakai T., Ikeda S., Yoshimura K., Mori M., Hoshino T., Yokota T. Treatment strategy for pulmonary artery pseudoaneurysm within lung abscess using trans-catheter arterial embolization: a case report. J. Jpn. Assoc. Chest Surg. 2017;31:950–956. doi: 10.2995/jacsurg.31.950. [DOI] [Google Scholar]
- 20.Sbano H., Mitchell A.W., Ind P.W., Jackson J.E. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am. J. Roentgenol. 2005;184:1253–1259. doi: 10.2214/ajr.184.4.01841253. [DOI] [PubMed] [Google Scholar]