Abstract
A growing body of evidence indicates that neutrophil elastase (NE) is involved in the pathogenesis of respiratory infectious diseases, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This study aimed to analyze the dynamic changes in serum levels of NE associated with inflammation, disease activity, and mortality rate in patients with COVID-19. We measured the serum concentrations of NE, C-Reactive protein (CRP), interleukin (IL)- 4, IL-6, IL-8, IL-10, and vitamin D levels in 83 ICU and 69 non-ICU patients compared with 82 healthy subjects (HS) in three-time points (T1-T3). Serum levels of NE, IL-6, IL-8, and CRP in ICU and non-ICU patients were significantly higher than HS (P < 0.001) in three-time points. Also, serum levels of NE, IL-6, IL-8, and CRP in ICU patients were significantly higher than in non-ICU patients (P < 0.05). On the day of admission (T1), the levels of NE, CRP, IL-6, IL-8 were gradually decreased from T1 to T3. At the same time, IL-4 and IL-10 were gradually increased from T1 to T2 and then reduced to T3. Further analyses demonstrated that the levels of NE, IL-6, and IL-8 in deceased patients were significantly higher than in recovered patients (P < 0.05). The ROC curve analysis demonstrated that markers, including NE, IL-6, and IL-8, were valuable indicators in evaluating the activity of COVID-19. Overall, our results signify the critical role of NE in the pathogenesis of COVID-19, and also, further support that NE has a potential therapeutic target for the attenuation of COVID-19 severity.
Keywords: COVID-19, SARS-CoV-2, Neutrophil elastase, Inflammation, Disease activity
1. Introduction
Recently, the pandemic coronavirus called SARS-CoV-2 prompted an intimidating disease, which is characterized by high mortality, economic, and well-being burden on the world society [1], [2], [3]. SARS-CoV-2 is the causative agent of COVID-19, which was first identified in Wuhan, Hubei province, China, and then was spread worldwide [4], [5], [6], [7]. In addition to its critical respiratory destruction, COVID-19 provoked thromboembolic issues and multi-organ collapse, including kidneys, heart, and liver [8], [9]. These outcomes may be due to overactive innate immune reactions [10] and a cytokine storm generated by lymphocyte and macrophage activation induced by SARS-CoV-2 [11], [12]. The neutrophils work as an essential component of the immune defense barrier linking innate and adaptive immunity among the innate immune cells. They cooperate to remove exogenous pathogens and endogenous cell debris and perform a crucial function in the pathogenesis of numerous respiratory disorders, such as viral respiratory infections [13], [14], [15], [16].
Neutrophils have an armory of defensive tactics that comprise neutrophil extracellular traps (NETs), discharge of antimicrobial granules, and NE (a serine protease) [13], [17]. The histone-DNA ingredients of falling neutrophils are described as NETs, which are involved in host protection against pathogens [13], [17]. An investigation reported that two markers of NETs, including cell-free DNA and myeloperoxidase (MPO)-DNA, were elevated in hospitalized COVID-19 cases compared with 30 controls and related to CRP, D-dimer, lactate dehydrogenase, and total neutrophil number [18]. Besides, in recent work performed on ambulatory cases, it has been found that myeloperoxidase-DNA concentration is increased in the initial phase of SARS-CoV-2 infection [19]. Also, another crucial antimicrobial compound released from neutrophils is NE, which is thought to possess antimicrobial properties either by its proteolytic activity or the production of ROS [20]. NE is found in the serum and airways in hospitalized infants infected with the respiratory syncytial virus (RSV) [21], [22]. This finding indicates that during RSV infection, activated neutrophils are recruited and enter the lung. However, the putative function of neutrophils in prophylaxis versus pathogenesis is still opaque. [23]. A recent study in the field of inflammation indicated that air hydrogen sulfide stimulates macrophage extracellular traps to exacerbate inflammatory damage via the control of miR-15b-5p on MAPK and insulin signals in the trachea of chickens [24]. Another finding showed that environmental factors including lithium [25] and contaminant ammonia [26] promote the occurrence of inflammatory responses.
Further evidence has highlighted the crucial function of NE in pathologic conditions, including chronic obstructive pulmonary disease and lung injury [27], [28], [29]. Besides, recently, it has been noticed that NE may be implicated in the pathogenesis of COVID-19 [30]. Therefore, the present study evaluated the association between dynamic changes in NE levels associated with inflammatory markers (IL-6 and IL-8), anti-inflammatory markers (IL-4, IL-10), and vitamin D levels on the three-time points (T1-T3) in ICU and non-ICU COVID-19 patients.
2. Methods
2.1. Subjects
A case-control study was intended to analyze whether the dynamic alterations in the levels of NE are associated with dynamic changes in the levels of inflammatory markers (CRP, IL-6, and IL-8), anti-inflammatory markers (IL-4 and IL-10), and vitamin D levels in ICU and non-ICU patients with COVID-19 in T1 to T3. Also, we addressed the correlation of dynamic changes in these factors with the disease severity. In this study, COVID-19 patients were recruited and referred to Firouzgar Hospital, Tehran, Iran, between November 2020 and April 2021. In order to obtained the legal and ethical authority for collecting the specimens, informed consent was obtained from all individuals who participated in this study. Additionally, this research was confirmed by the ethics committee of the Iran University of Medical Sciences (IUMS) (ECIUMS; IR.IUMS.REC.1399.175). A total number of 152 patients with COVID-19 were admitted to Firouzgar Hospital and classified into two groups according to the criteria established by Li and colleagues; the first group comprised 83 cases with COVID-19 (severe patients hospitalized in ICU), the second group consisted of 69 patients with COVID-19 (moderate patients). Also, in this study, 82 healthy subjects were enrolled as the control group.
2.2. Laboratory validation and treatment
The study was performed on three time points as follows: T1: day of hospitalization, T2: 3 days after hospitalization, T3: 6 days after hospitalizations. At each time point, 5 ml peripheral blood was collected from each patient and quickly following the sample gathering, serum samples were isolated by centrifugation and kept at − 70C° until use. For RNA extraction from sputum and throat swab specimens, the QIAamp Viral RNA Mini Kit (Qiagen, Germany) was utilized according to the manufacturer’s instructions. Then, the real-time RT-PCR assay was applied for the detection of RNA of SARS-CoV-2. The routine biochemical parameters of sera obtained from confirmed COVID-19 patients were analyzed on the day of admission by standard automated methods in a Technicon Dax autoanalyzer (Technicon Instruments, CO, USA).
2.3. The enzyme-linked immunosorbent assay
The levels of inflammatory (CRP; BOSTER BIOLOGICAL TECHNOLOGY, EK7040, IL-6; Abcam # ab46027, IL-8; Abcam # ab46032), and anti-inflammatory (IL-4; IBL International GmbH # BE58041, IL-10; IBL International GmbH # BE58101) markers were assessed according to the manufacturer's structure. We used a quantitative chemiluminescent immunometric assay for the assessment of Vit D levels in serum specimens (DiaSorin, spA, Via Crescentino, Vercelli, Italy). All specimens were examined in duplicate, and the mean values were used for the statistical interpretations.
2.4. Statistical methods
Continuous and categorical variables were presented as median (IQR) and n (%), respectively. In order to compare the differences between experimental groups, the Wilcoxon rank-sum test, χ2 test, or Fisher’s exact test were applied, where appropriate. In addition, the relationship between laboratory measurements was calculated using the Pearson correlation coefficient. Regarding the total number of Non-ICU cases in this study (n = 69) and to avoid overfitting in the model, eight variables were chosen for multivariate analysis based on previous findings and clinical constraints. A two-sided α of<0·05 was regarded statistically significant. Statistical analyses were performed using R version 4.0.4 (2021–02-15).
3. Results
3.1. Demographic characteristics
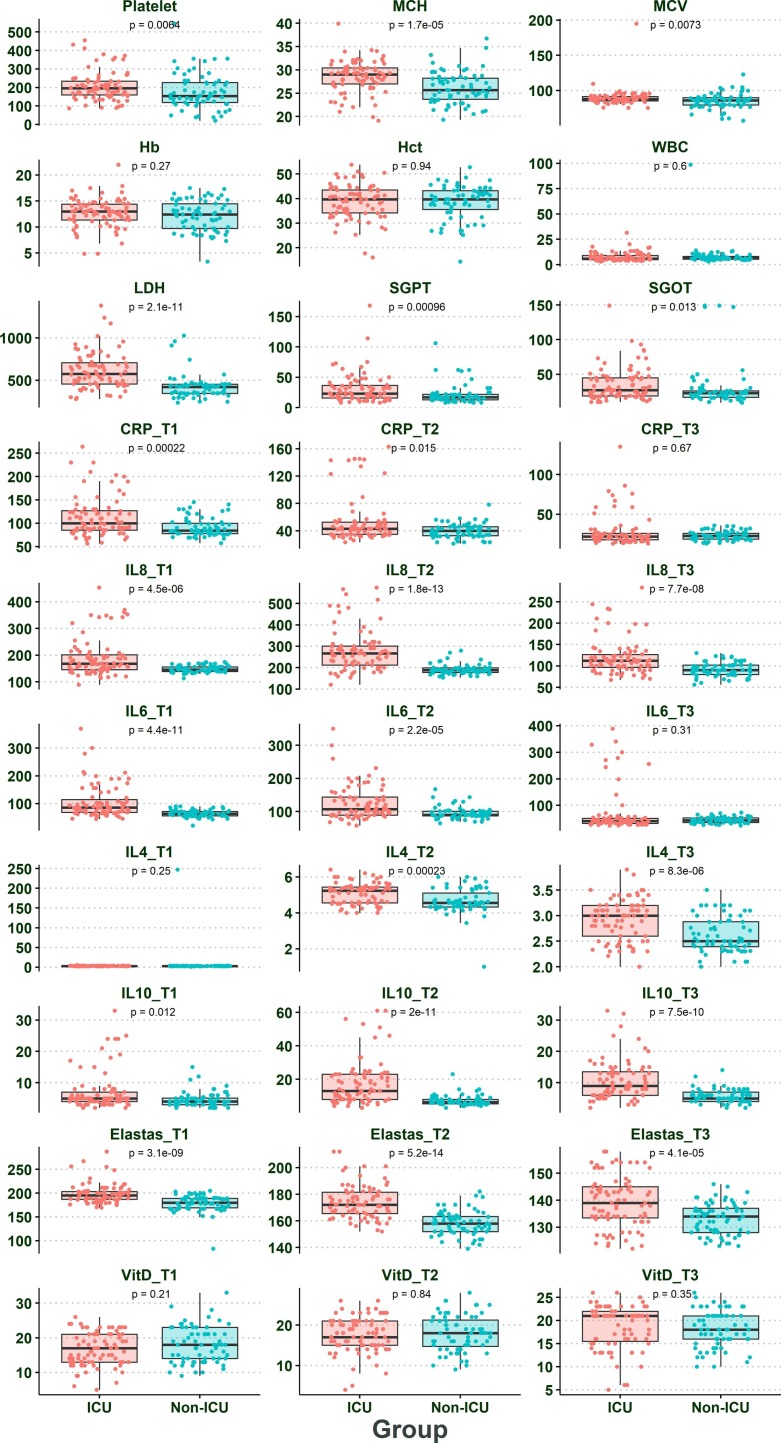
Table 1 summarized the demographics and laboratory characteristics of COVID-19 patients and HS. The results showed that the sex and age of individuals infected with COVID-19 were significantly different (P < 0.05). Besides, Table 2 and Fig. 1 indicated the difference between demographics and laboratory findings in ICU and non-ICU patients. Some factors, such as hospital discharge (duration time of hospitalization), death rate, mean cell hemoglobin (MCH), lactate dehydrogenase (LDH), glutamic-oxaloacetic transaminase (SGOT), serum glutamic-pyruvic transaminase (SGPT), smoking were significantly higher in ICU patients than in non-ICU patients (P < 0.05). Table 3 illustrated that some risk factors such as age, lung disease, CRP, IL-4, IL-6, IL-8, IL-10, vitamin D were significantly different in deceased patients than alive patients. Further details are displayed in Table 3. Furthermore, we used multivariate regression analysis to determine the impact of confounding factors, such as age and sex. The results of multivariate regression analysis revealed that age and sex had no significant effects on the obtained results (P > 0.05) Table 2.1 and Table 3.1 .Fig. 2.
Table 1.
Demographic and laboratory markers of patients with COVID-19 and HS.
| Variable | N | Overall, N = 2341 |
Group |
p-value2 | ||
|---|---|---|---|---|---|---|
| Control, N = 821 | ICU, N = 831 | Non-ICU, N = 691 | ||||
| Sex | 234 | 0.011 | ||||
| Female | 98 (42%) | 34 (41%) | 44 (53%) | 20 (29%) | ||
| Male | 136 (58%) | 48 (59%) | 39 (47%) | 49 (71%) | ||
| Age | 234 | 54 (37, 65) | 46 (36, 59) | 58 (40, 70) | 53 (36, 63) | 0.006 |
| CRP_T1 | 234 | 79 (2, 100) | 2 (1, 2) | 100 (85, 127) | 84 (78, 100) | <0.001 |
| IL8_T1 | 209 | 145 (49, 167) | 32 (23, 43) | 168 (146, 202) | 146 (139, 156) | <0.001 |
| IL6_T1 | 234 | 59 (23, 78) | 21 (19, 24) | 86 (68, 114) | 63 (56, 72) | <0.001 |
| IL4_T1 | 234 | 2.48 (1.30, 3.10) | 1.20 (0.90, 1.30) | 3.00 (2.50, 3.25) | 2.80 (2.45, 3.14) | <0.001 |
| IL10_T1 | 234 | 4.0 (3.0, 5.0) | 3.0 (2.2, 3.2) | 5.0 (4.0, 7.0) | 4.0 (3.0, 5.1) | <0.001 |
| Elastas_T1 | 234 | 178 (105, 192) | 93 (84, 112) | 195 (187, 204) | 180 (169, 189) | <0.001 |
| VitD_T2 | 151 | 18 (15, 21) | NA (NA, NA) | 17 (15, 21) | 18 (15, 21) | 0.8 |
n (%); Median (IQR)
Pearson's Chi-squared test; Kruskal-Wallis rank sum test
Table 2.
Demographic and clinical parameters of ICU and non-ICU patients.
| Variable | N | Overall, N = 1521 |
Group |
p-value2 | |
|---|---|---|---|---|---|
| ICU, N = 831 | Non-ICU, N = 691 | ||||
| Sex | 152 | 0.003 | |||
| Female | 64 (42%) | 44 (53%) | 20 (29%) | ||
| Male | 88 (58%) | 39 (47%) | 49 (71%) | ||
| Age | 152 | 56 (38, 68) | 58 (40, 70) | 53 (36, 63) | 0.047 |
| Platelet | 152 | 175 (140, 232) | 196 (160, 234) | 154 (119, 227) | 0.006 |
| MCH | 152 | 27.9 (25.0, 29.9) | 29.0 (27.0, 30.4) | 25.7 (23.7, 28.2) | <0.001 |
| MCV | 152 | 87 (83, 91) | 88 (85, 91) | 86 (79, 90) | 0.007 |
| Hb | 152 | 12.75 (10.60, 14.43) | 13.00 (11.35, 14.40) | 12.40 (9.70, 14.50) | 0.3 |
| Hct | 152 | 40 (34, 43) | 40 (34, 44) | 40 (36, 43) | >0.9 |
| WBC | 152 | 6.30 (5.10, 8.65) | 5.90 (4.85, 9.05) | 6.30 (5.40, 8.20) | 0.6 |
| LDH | 152 | 453 (374, 650) | 576 (456, 708) | 420 (345, 452) | <0.001 |
| SGPT | 152 | 19 (15, 32) | 23 (16, 36) | 17 (13, 22) | <0.001 |
| SGOT | 152 | 24 (18, 41) | 27 (19, 45) | 23 (17, 26) | 0.013 |
| Cigarette | 152 | 0.014 | |||
| Negative | 132 (87%) | 67 (81%) | 65 (94%) | ||
| Positive | 20 (13%) | 16 (19%) | 4 (5.8%) | ||
| Opium | 152 | >0.9 | |||
| Negative | 148 (97%) | 81 (98%) | 67 (97%) | ||
| Positive | 4 (2.6%) | 2 (2.4%) | 2 (2.9%) | ||
| Alcohol | 152 | >0.9 | |||
| Negative | 145 (95%) | 79 (95%) | 66 (96%) | ||
| Positive | 7 (4.6%) | 4 (4.8%) | 3 (4.3%) | ||
| Kidney.failure | 152 | 0.10 | |||
| Negative | 131 (86%) | 68 (82%) | 63 (91%) | ||
| Positive | 21 (14%) | 15 (18%) | 6 (8.7%) | ||
| Dialysis | 152 | 0.5 | |||
| Negative | 141 (93%) | 78 (94%) | 63 (91%) | ||
| Positive | 11 (7.2%) | 5 (6.0%) | 6 (8.7%) | ||
| Lung.disease | 152 | 0.2 | |||
| Negative | 131 (86%) | 69 (83%) | 62 (90%) | ||
| Positive | 21 (14%) | 14 (17%) | 7 (10%) | ||
| Heart.disease | 152 | 0.6 | |||
| Negative | 127 (84%) | 68 (82%) | 59 (86%) | ||
| Positive | 25 (16%) | 15 (18%) | 10 (14%) | ||
| Discharg | 152 | 7 (5, 8) | 8 (7, 9) | 6 (5, 6) | <0.001 |
| Death | 152 | 0.002 | |||
| Negative | 142 (93%) | 73 (88%) | 69 (100%) | ||
| Positive | 10 (6.6%) | 10 (12%) | 0 (0%) | ||
| CRP_T1 | 152 | 90 (79, 120) | 100 (85, 127) | 84 (78, 100) | <0.001 |
| CRP_T2 | 152 | 43 (34, 48) | 43 (35, 52) | 40 (33, 46) | 0.015 |
| CRP_T3 | 152 | 22 (19, 26) | 22 (18, 26) | 23 (19, 26) | 0.7 |
| IL8_T1 | 152 | 155 (140, 174) | 168 (146, 202) | 146 (139, 156) | <0.001 |
| IL8_T2 | 152 | 204 (184, 276) | 267 (212, 301) | 188 (178, 199) | <0.001 |
| IL8_T3 | 152 | 101 (87, 116) | 112 (96, 126) | 90 (80, 102) | <0.001 |
| IL6_T1 | 152 | 72 (59, 90) | 86 (68, 114) | 63 (56, 72) | <0.001 |
| IL6_T2 | 151 | 98 (87, 123) | 107 (89, 144) | 90 (87, 101) | <0.001 |
| IL6_T3 | 151 | 43 (33, 53) | 41 (32, 51) | 44 (36, 53) | 0.3 |
| IL4_T1 | 152 | 3.00 (2.50, 3.20) | 3.00 (2.50, 3.25) | 2.80 (2.45, 3.14) | 0.3 |
| IL4_T2 | 152 | 4.71 (4.43, 5.40) | 5.23 (4.56, 5.43) | 4.56 (4.33, 5.10) | <0.001 |
| IL4_T3 | 151 | 2.80 (2.44, 3.10) | 3.00 (2.60, 3.20) | 2.50 (2.39, 2.89) | <0.001 |
| IL10_T1 | 152 | 5.0 (3.7, 6.0) | 5.0 (4.0, 7.0) | 4.0 (3.0, 5.1) | 0.012 |
| IL10_T2 | 151 | 8 (6, 14) | 13 (8, 23) | 6 (5, 8) | <0.001 |
| IL10_T3 | 152 | 7 (5, 10) | 9 (6, 14) | 5 (4, 7) | <0.001 |
| Elastas_T1 | 152 | 189 (178, 199) | 195 (187, 204) | 180 (169, 189) | <0.001 |
| Elastas_T2 | 151 | 165 (158, 176) | 172 (166, 182) | 158 (152, 163) | <0.001 |
| Elastas_T3 | 151 | 136 (130, 142) | 139 (134, 145) | 134 (128, 137) | <0.001 |
| VitD_T1 | 152 | 17 (13, 21) | 17 (13, 21) | 18 (14, 23) | 0.2 |
| VitD_T2 | 151 | 18 (15, 21) | 17 (15, 21) | 18 (15, 21) | 0.8 |
| VitD_T3 | 151 | 20 (16, 22) | 21 (16, 22) | 18 (16, 21) | 0.4 |
| Ct_value | 151 | 29 (24, 33) | 25 (24, 33) | 32 (24, 34) | 0.15 |
n (%); Median (IQR)
Pearson's Chi-squared test; Wilcoxon rank sum test; Fisher's exact test
Fig. 1.
The laboratory finding and dynamic changes in CRP levels, IL-4, IL-6, IL-8, IL-10, and vitamin D in ICU and non-ICU patients.
Table 3.
Demographic and clinical parameters of deceased patients versus alive patients.
| Variable | N | Overall, N = 1521 |
Death |
p-value2 | |
|---|---|---|---|---|---|
| Negative, N = 1421 | Positive, N = 101 | ||||
| Group | 152 | 0.002 | |||
| ICU | 83 (55%) | 73 (51%) | 10 (100%) | ||
| Non-ICU | 69 (45%) | 69 (49%) | 0 (0%) | ||
| Sex | 152 | >0.9 | |||
| Female | 64 (42%) | 60 (42%) | 4 (40%) | ||
| Male | 88 (58%) | 82 (58%) | 6 (60%) | ||
| Age | 152 | 56 (38, 68) | 54 (37, 66) | 77 (72, 83) | <0.001 |
| Platelet | 152 | 175 (140, 232) | 178 (142, 234) | 160 (126, 174) | 0.2 |
| MCH | 152 | 27.9 (25.0, 29.9) | 27.8 (24.9, 29.9) | 28.4 (27.3, 29.3) | 0.5 |
| MCV | 152 | 87 (83, 91) | 87 (83, 91) | 87 (84, 88) | 0.6 |
| Hb | 152 | 12.75 (10.60, 14.43) | 12.70 (10.40, 14.30) | 14.75 (11.88, 15.88) | 0.068 |
| Hct | 152 | 40 (34, 43) | 40 (34, 43) | 41 (35, 48) | 0.4 |
| WBC | 152 | 6.30 (5.10, 8.65) | 6.30 (5.30, 8.75) | 6.05 (4.85, 8.38) | 0.7 |
| LDH | 152 | 453 (374, 650) | 453 (368, 622) | 586 (450, 656) | 0.15 |
| SGPT | 152 | 19 (15, 32) | 19 (15, 32) | 18 (15, 30) | >0.9 |
| SGOT | 152 | 24 (18, 41) | 24 (18, 41) | 24 (22, 58) | 0.7 |
| Cigarette | 152 | >0.9 | |||
| Negative | 132 (87%) | 123 (87%) | 9 (90%) | ||
| Positive | 20 (13%) | 19 (13%) | 1 (10%) | ||
| Opium | 152 | >0.9 | |||
| Negative | 148 (97%) | 138 (97%) | 10 (100%) | ||
| Positive | 4 (2.6%) | 4 (2.8%) | 0 (0%) | ||
| Alcohol | 152 | 0.4 | |||
| Negative | 145 (95%) | 136 (96%) | 9 (90%) | ||
| Positive | 7 (4.6%) | 6 (4.2%) | 1 (10%) | ||
| Kidney.failure | 152 | 0.6 | |||
| Negative | 131 (86%) | 123 (87%) | 8 (80%) | ||
| Positive | 21 (14%) | 19 (13%) | 2 (20%) | ||
| Dialysis | 152 | 0.2 | |||
| Negative | 141 (93%) | 133 (94%) | 8 (80%) | ||
| Positive | 11 (7.2%) | 9 (6.3%) | 2 (20%) | ||
| Lung.disease | 152 | 0.033 | |||
| Negative | 131 (86%) | 125 (88%) | 6 (60%) | ||
| Positive | 21 (14%) | 17 (12%) | 4 (40%) | ||
| Heart.disease | 152 | 0.060 | |||
| Negative | 127 (84%) | 121 (85%) | 6 (60%) | ||
| Positive | 25 (16%) | 21 (15%) | 4 (40%) | ||
| Discharg | 152 | 7 (5, 8) | 7 (6, 8) | 0 (0, 0) | <0.001 |
| CRP_T1 | 152 | 90 (79, 120) | 89 (79, 110) | 195 (189, 208) | <0.001 |
| CRP_T2 | 152 | 43 (34, 48) | 42 (34, 46) | 143 (126, 145) | <0.001 |
| CRP_T3 | 152 | 22 (19, 26) | 22 (18, 26) | 70 (59, 78) | <0.001 |
| IL8_T1 | 152 | 155 (140, 174) | 154 (140, 168) | 349 (342, 359) | <0.001 |
| IL8_T2 | 152 | 204 (184, 276) | 200 (182, 266) | 489 (465, 536) | <0.001 |
| IL8_T3 | 152 | 101 (87, 116) | 100 (86, 113) | 206 (197, 234) | <0.001 |
| IL6_T1 | 152 | 72 (59, 90) | 70 (59, 86) | 205 (190, 264) | <0.001 |
| IL6_T2 | 151 | 98 (87, 123) | 97 (87, 111) | 204 (191, 252) | <0.001 |
| IL6_T3 | 151 | 43 (33, 53) | 41 (33, 49) | 274 (247, 321) | <0.001 |
| IL4_T1 | 152 | 3.00 (2.50, 3.20) | 3.00 (2.50, 3.20) | 2.65 (2.50, 3.08) | 0.5 |
| IL4_T2 | 152 | 4.71 (4.43, 5.40) | 4.69 (4.41, 5.40) | 5.12 (4.52, 5.42) | 0.6 |
| IL4_T3 | 151 | 2.80 (2.44, 3.10) | 2.77 (2.44, 3.10) | 3.10 (3.02, 3.10) | 0.018 |
| IL10_T1 | 152 | 5.0 (3.7, 6.0) | 4.4 (3.0, 5.8) | 22.5 (9.0, 24.0) | <0.001 |
| IL10_T2 | 151 | 8 (6, 14) | 8 (6, 13) | 48 (29, 55) | <0.001 |
| IL10_T3 | 152 | 7 (5, 10) | 6 (5, 9) | 22 (12, 27) | <0.001 |
| Elastas_T1 | 152 | 189 (178, 199) | 189 (178, 198) | 235 (190, 256) | 0.005 |
| Elastas_T2 | 151 | 165 (158, 176) | 165 (158, 172) | 190 (175, 201) | 0.001 |
| Elastas_T3 | 151 | 136 (130, 142) | 135 (130, 142) | 140 (130, 150) | 0.3 |
| VitD_T1 | 152 | 17 (13, 21) | 18 (14, 21) | 10 (8, 12) | <0.001 |
| VitD_T2 | 151 | 18 (15, 21) | 18 (15, 21) | 12 (8, 15) | <0.001 |
| VitD_T3 | 151 | 20 (16, 22) | 20 (16, 22) | 14 (7, 16) | 0.002 |
| Ct_value | 151 | 29 (24, 33) | 29 (24, 33) | 24 (24, 34) | 0.7 |
n (%); Median (IQR)
Fisher's exact test; Wilcoxon rank sum test
Table 2.1.
Multivariate logistic regression results between ICU and non-ICU patients.
| Characteristic | OR | 95% CI | p-value |
|---|---|---|---|
| Age | 1.00 | 0.98, 1.03 | 0.8 |
| Sex | |||
| Female | 1.00 | ||
| Male | 0.47 | 0.18, 1.20 | 0.12 |
| Cigarette | |||
| Negative | 1.00 | ||
| Positive | 5.97 | 1.35, 36.3 | 0.029 |
| CRP_T1 | 1.02 | 1.00, 1.04 | 0.12 |
| IL8_T1 | 1.01 | 0.99, 1.04 | 0.4 |
| IL6_T1 | 1.06 | 1.02, 1.10 | 0.002 |
| IL10_T1 | 1.12 | 0.94, 1.35 | 0.2 |
| Elastas_T1 | 1.09 | 1.05, 1.14 | <0.001 |
Table 3.1.
Multivariate logistic regression results between deceased and alive patients.
| Characteristic | OR | 95% CI | p-value |
|---|---|---|---|
| Age | 1.00 | 0.98, 1.03 | 0.7 |
| Sex | |||
| Female | 1.00 | ||
| Male | 0.42 | 0.18, 0.95 | 0.040 |
| Lung. Disease | |||
| Negative | 1.00 | ||
| Positive | 0.71 | 0.19, 2.74 | 0.6 |
| CRP_T1 | 1.02 | 1.01, 1.04 | 0.003 |
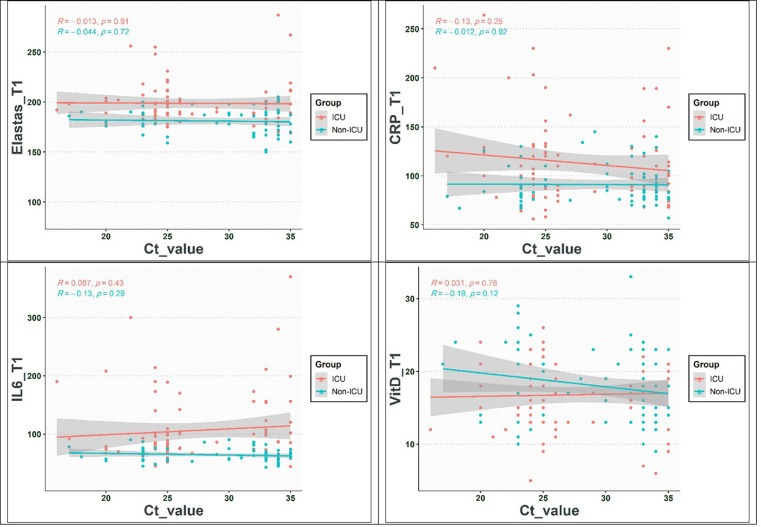
Fig. 2.
The correlation between NE, IL-6, CRP, and vitamin D with CT value.
3.2. Dynamic changes in the CRP, IL-4, IL-6, IL-8, 1L-10, NE, and vitamin D levels in ICU versus non-ICU patients
The levels of CRP, IL-4, IL-6, IL-8, 1L-10, NE, and Vitamin D in COVID-19 patients and HS are depicted in Table 2 and Fig. 1. Also, the levels of mentioned markers as well as demographic and laboratory results in ICU and non-ICU patients, were illustrated in Table 2. According to Table 2, our results revealed that the CRP, IL-4, IL-6, IL-8, IL-10, and NE were significantly higher in ICU patients than non-ICU patients (P < 0.05). Further analyses point out that the levels of IL-4, IL-6, IL-8, IL-10, and NE on the day of admission to T2 (3 days after admission) were gradually increased in both ICU and non-ICU patients (P < 0.05), and also these markers were gradually reduced from T2 to T3 (6 days after admission) (P < 0.05). Concurrently, the CRP levels from the day of admission to T3 were gradually diminished (P < 0.05). We did not found significant differences in terms of vitamin D levels in ICU patients when compared with non-ICU patients (P > 0.05). Further details are shown in Table 2.
3.3. Dynamic alterations in the levels of CRP, IL-4, IL-6, IL-8, 1L-10, NE, and vitamin D in deceased patients in comparison with alive patients
The levels of CRP, IL-4, IL-6, IL-8, 1L-10, NE, and vitamin D in deceased patients versus alive patients were shown in Table 3. As shown in Table 2, the concentrations of the above marker were significantly higher in deceased patients than alive patients (P < 0.05). However, the vitamin D levels were significantly lower in deceased patients than in alive patients in T1 to T3 (P < 0.05). Similar to results obtained from the comparison between ICU and non-ICU patients, the serum concentrations of IL-4, IL-6, IL-8, IL-10, and NE on T1 were gently increased to T2 and then declined from T2 to T3. Further details are demonstrated in Table 3.
3.4. The impact of smoking on the dynamic changes in CRP, IL-4, IL-6, IL-8, 1L-10, NE, and vitamin D levels in patients with COVID-19
The results indicated that the frequency of smoking history in ICU patients was significantly higher than in non-ICU patients (P < 0.05). Also, further analyses showed that smoking history did not show any effects on the changes of CRP levels, IL-4, IL-6, IL-8, 1L-10, NE in comparison with those who were not smoker (P > 0.05). Of note, the only factor that was affected by smoking was vitamin D (P < 0.05). Smoking history was significantly associated with cardiovascular diseases, discharge from the hospital, alcohol consumption, and addiction (P < 0.05). Further details are displayed in Table 4 .
Table 4.
Demographic and laboratory findings among smoker and non-smoker patients.
| Variable | N | Overall, N = 1521 |
Cigarette |
p-value2 | |
|---|---|---|---|---|---|
| Negative, N = 1321 | Positive, N = 201 | ||||
| Group | 152 | 0.014 | |||
| ICU | 83 (55%) | 67 (51%) | 16 (80%) | ||
| Non-ICU | 69 (45%) | 65 (49%) | 4 (20%) | ||
| Sex | 152 | 0.8 | |||
| Female | 64 (42%) | 55 (42%) | 9 (45%) | ||
| Male | 88 (58%) | 77 (58%) | 11 (55%) | ||
| Age | 152 | 56 (38, 68) | 55 (37, 67) | 62 (47, 69) | 0.2 |
| Platelet | 152 | 175 (140, 232) | 174 (138, 228) | 202 (174, 250) | 0.072 |
| MCH | 152 | 27.9 (25.0, 29.9) | 27.8 (24.8, 29.7) | 27.9 (26.3, 30.2) | 0.7 |
| MCV | 152 | 87 (83, 91) | 87 (83, 91) | 88 (84, 96) | 0.3 |
| Hb | 152 | 12.75 (10.60, 14.43) | 12.80 (10.60, 14.43) | 12.55 (10.88, 14.25) | >0.9 |
| Hct | 152 | 40 (34, 43) | 40 (35, 43) | 37 (33, 45) | 0.5 |
| WBC | 152 | 6.30 (5.10, 8.65) | 6.15 (5.10, 8.53) | 6.95 (5.23, 10.12) | 0.4 |
| LDH | 152 | 453 (374, 650) | 453 (374, 594) | 626 (412, 733) | 0.14 |
| SGPT | 152 | 19 (15, 32) | 19 (15, 32) | 22 (17, 32) | 0.3 |
| SGOT | 152 | 24 (18, 41) | 24 (18, 41) | 22 (18, 34) | 0.8 |
| Opium | 152 | <0.001 | |||
| Negative | 148 (97%) | 132 (100%) | 16 (80%) | ||
| Positive | 4 (2.6%) | 0 (0%) | 4 (20%) | ||
| Alcohol | 152 | 0.049 | |||
| Negative | 145 (95%) | 128 (97%) | 17 (85%) | ||
| Positive | 7 (4.6%) | 4 (3.0%) | 3 (15%) | ||
| Kidney.failure | 152 | >0.9 | |||
| Negative | 131 (86%) | 113 (86%) | 18 (90%) | ||
| Positive | 21 (14%) | 19 (14%) | 2 (10%) | ||
| Dialysis | 152 | >0.9 | |||
| Negative | 141 (93%) | 122 (92%) | 19 (95%) | ||
| Positive | 11 (7.2%) | 10 (7.6%) | 1 (5.0%) | ||
| Lung.disease | 152 | 0.2 | |||
| Negative | 131 (86%) | 116 (88%) | 15 (75%) | ||
| Positive | 21 (14%) | 16 (12%) | 5 (25%) | ||
| Heart.disease | 152 | 0.025 | |||
| Negative | 127 (84%) | 114 (86%) | 13 (65%) | ||
| Positive | 25 (16%) | 18 (14%) | 7 (35%) | ||
| Discharg | 152 | 7 (5, 8) | 6 (5, 8) | 8 (6, 9) | 0.023 |
| Death | 152 | >0.9 | |||
| Negative | 142 (93%) | 123 (93%) | 19 (95%) | ||
| Positive | 10 (6.6%) | 9 (6.8%) | 1 (5.0%) | ||
| CRP_T1 | 152 | 90 (79, 120) | 90 (79, 120) | 100 (87, 121) | 0.2 |
| CRP_T2 | 152 | 43 (34, 48) | 43 (34, 48) | 42 (33, 51) | 0.9 |
| CRP_T3 | 152 | 22 (19, 26) | 22 (19, 26) | 22 (17, 26) | 0.6 |
| IL8_T1 | 152 | 155 (140, 174) | 156 (140, 172) | 154 (140, 186) | >0.9 |
| IL8_T2 | 152 | 204 (184, 276) | 200 (184, 278) | 226 (194, 270) | 0.4 |
| IL8_T3 | 152 | 101 (87, 116) | 100 (87, 114) | 110 (90, 126) | 0.2 |
| IL6_T1 | 152 | 72 (59, 90) | 72 (59, 89) | 72 (62, 94) | 0.6 |
| IL6_T2 | 151 | 98 (87, 123) | 98 (87, 122) | 100 (87, 126) | 0.8 |
| IL6_T3 | 151 | 43 (33, 53) | 43 (34, 53) | 37 (30, 51) | 0.3 |
| IL4_T1 | 152 | 3.00 (2.50, 3.20) | 2.94 (2.49, 3.20) | 3.05 (2.50, 3.25) | 0.4 |
| IL4_T2 | 152 | 4.71 (4.43, 5.40) | 4.71 (4.40, 5.40) | 4.92 (4.50, 5.40) | 0.8 |
| IL4_T3 | 151 | 2.80 (2.44, 3.10) | 2.77 (2.44, 3.10) | 3.05 (2.70, 3.24) | 0.060 |
| IL10_T1 | 152 | 5.0 (3.7, 6.0) | 5.0 (4.0, 6.0) | 4.5 (3.0, 5.2) | 0.7 |
| IL10_T2 | 151 | 8 (6, 14) | 8 (6, 16) | 8 (6, 11) | 0.5 |
| IL10_T3 | 152 | 7 (5, 10) | 7 (5, 11) | 6 (5, 7) | 0.2 |
| Elastas_T1 | 152 | 189 (178, 199) | 188 (178, 198) | 198 (186, 206) | 0.074 |
| Elastas_T2 | 151 | 165 (158, 176) | 165 (158, 174) | 170 (160, 183) | 0.2 |
| Elastas_T3 | 151 | 136 (130, 142) | 135 (129, 142) | 138 (134, 143) | 0.3 |
| VitD_T1 | 152 | 17 (13, 21) | 17 (13, 21) | 21 (17, 23) | 0.035 |
| VitD_T2 | 151 | 18 (15, 21) | 17 (14, 21) | 20 (17, 21) | 0.082 |
| VitD_T3 | 151 | 20 (16, 22) | 19 (15, 21) | 21 (18, 23) | 0.026 |
| Ct_value | 151 | 29 (24, 33) | 29 (24, 33) | 25 (24, 33) | 0.5 |
n (%); Median (IQR)
Pearson's Chi-squared test; Wilcoxon rank sum test; Fisher's exact test
3.5. The correlation between NE levels and pro/anti-inflammatory markers in ICU and non-ICU patients
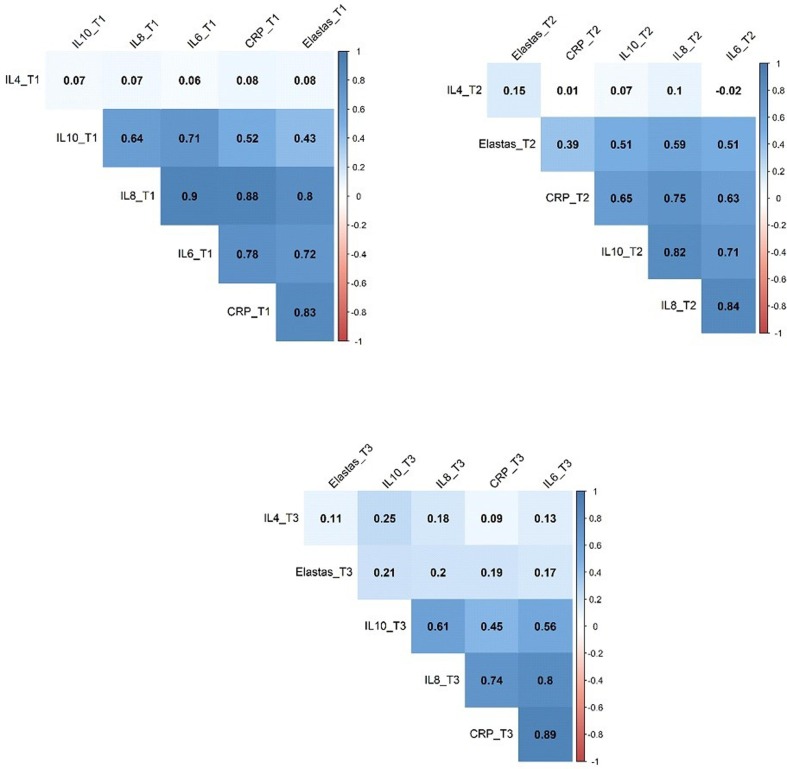
In order to determine the correlation between serum NE levels and the levels of pro/anti-inflammatory markers, we used the matrix model of relationship. The results showed that on the day of admission (T1), the patterns of association between NE and pro/anti-inflammatory markers from low to high orders were IL-4 < IL-10 < IL-6 < IL-8 < CRP. Also, the correlation patterns on T2 and T3 from low to high orders were indicated as follows T2: IL-4 < CRP < IL-10 < IL-8 < IL-6, and T3: IL-4 < IL-6 < CRP < IL-8 < IL10, respectively (Fig. 3 ).Fig. 4.
Fig. 3.
The matrix correlation between NE and pro/anti-inflammatory markers.
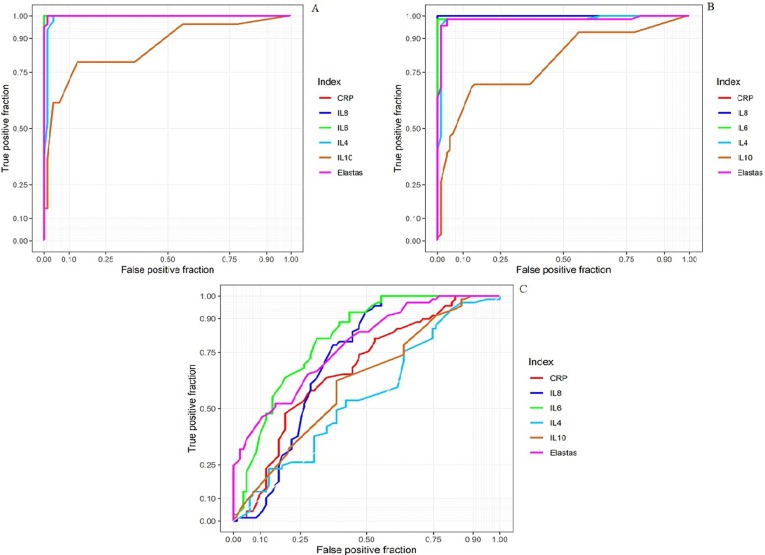
Fig. 4.
ROC curve analysis between, A: Healthy people vs. ICU patients, B: Healthy people vs. Non-ICU patients, and C: Non-ICU patients vs. ICU patients. AUC: Area under the curve.
3.6. The value of NE compared to other markers in prognosis of patients with COVID‑19
The ROC analysis demonstrated that NE and other indices, such IL-4, IL-6, IL-8, IL-10, and CRP were significantly different between COVID-19 cases and HS. Also, according to the ROC curve analysis, area under the curve (AUC) of NE and other markers, including CRP, IL-4, IL-6, IL-8, and IL-10, on the first day between ICU and non-ICU patients were 0.7795, 0.6745, 0.5542, 0.8109, 0.7163, and 0.6174, respectively. Overall, the ROC results indicated that NE alone or in combination with IL-6 and IL-8 might be suitable candidates for monitoring the disease activity in patients with COVID-19.
4. Discussion
Neutrophils are frontier cells of innate immunity in protecting against pathogens and throughout the acute phase of inflammatory responses [31]. In current years, experimental data have recommended that there may be different subtypes of neutrophils with distinct functions in infection, cancer, and autoimmunity [32].
The participation of neutrophils in defense against various bacterial and fungal diseases is well established. However, the function of neutrophils in responses to viruses (which replicate intracellularly) has been less investigated [23]. Recently, accumulating evidence points out the critical role of neutrophils in the pathogenesis of COVID-19, especially in those with acute disease phases [31], [33], [34], [35]. Therefore, this study investigated the possible correlation between NE and inflammatory markers to elucidate whether a dynamic change in NE levels correlates with inflammation and patient outcomes.
Our results noted that the levels of NE were significantly higher in patients with COVID-19 than HS. Also, our study findings displayed that NE levels in ICU patients were significantly elevated than in non-ICU patients. Our results were in line with a recent study conducted by Louis Guéant et al. [30] who revealed that the NE levels were significantly elevated in COVID-19 patients. The recent evidence has represented the pathogenic role of NE in respiratory viral infections [36] and lung injury [27], [37], [38]. NE can lead to lung damage; however, this protease has not evolved to cause lung tissue injury; the elastin-rich connective tissue framework of the lungs seems to be especially sensitive to the function of elastolytic proteases. Assuming that NE most likely represents a function in the immigration of neutrophils toward a site of inflammation and degeneration of proteins from attacking organisms or other inflammatory response results, it is the performance of inhibitors of this protease to preserve normal tissues from its impacts [29]. These results may reflect the event in which the excessive activation of neutrophils, followed by the elevated levels of NE which can result in lung injury via serine protease effects.
The axial role of cytokine storm in the pathogenesis of COVID-19 has profoundly been appreciated [39], [40], [41], [42]. Our data show that the levels of inflammatory indices, such as CRP, IL-6, and IL-8, were significantly enhanced in cases with COVID-19 than in HS. Additional analyses revealed that the serum concentrations of these inflammatory markers in ICU patients was significantly higher than in non-ICU patients. We have also showed that the levels of IL-6 and IL-8 were gradually increased from day 0 to day 3 in both ICU and non-ICU patients. However, the CRP levels were gently reduced from day 0 to day 6 in both groups of patients. Our finding confirms previous investigations demonstrating that the IL-6 [43], [44], [45], [46], [47], [48], [49], [50], IL-8 [51], [52], [53], [54], and CRP [55], [56] were significantly elevated in patients with COVID-19. Besides, our results revealed that the concentrations of IL-4 and IL-10 were significantly elevated in cases with COVID-19 than in HS.
Additional analyses indicated that the levels of IL-4 and IL10 were significantly higher in ICU patients than non-ICU patients. Our findings were consistent with several studies that showed the levels of IL-4 [57] and IL-10 [43], [58], [59], [60] elevated in patients with COVID-19. Recently, in infected alveolar macrophages, it has been appreciated that IL-6 and IL-8 are generated at the same time of NF-κB activation through mechanisms that plausibly involve Bruton tyrosine kinase (BTK). This is represented by the encouraging clinical outcomes provided by the BTK inhibitor acalabrutinib in critical COVID‐19 patients [61]. IL‐8 acts as neutrophil‐activating chemokine when bound to CXCR2, which is a essential chemokine receptor of neutrophils [62]. Hence, a notable rise in NE in critical COVID‐19 cases may be correlated to the activation of neutrophils through the IL‐8/CXCR2 pathways [63].
The vast majority of observational research has verified a low vitamin D state associated with the occurrence of the upper respiratory viral infections [64], [65], [66], [67]. Our results revealed that Vit D levels were significantly lower in COVID-19 cases than in HS. Additionally, there was no statistically significant difference in serum vitamin D levels between ICU and non-ICU patients. However, serum vitamin D levels were significantly lower in deceased patients than in alive patients. Our findings, in agreement with similar studies, indicate that COVID-19 patients had lower Vit D levels [67], [68], [69], [70].
Finally, the ROC curve report shows that AUC from high to low is: IL-6 > NE > IL-8 > CRP > IL-10 > IL-4. Similarly, matrix correlation showed the NE has a high correlation with CRP, IL-6, and IL-8. Overall, these findings implying that NE, along with IL-6 and IL-8, is a reliable indicator in differentiating severe SARS-CoV2 infection (ICU patients) cases from moderate ones (non-ICU patients).
5. Conclusion
Overall, we provided further significant supports for the pathogenic role of NE in COVID-19. Also, we have addressed a possible link between NE dynamic changes with inflammatory cytokines, implying that this enzyme alone or in combination with other markers, such as IL-6 and IL-8, could be employed for monitoring the disease activity. Ultimately, the elucidation of the interaction between NE and inflammatory cytokines may improve our knowledge to shed light on this enzyme's role in health and disease state. We have previously mentioned that NE is a serine proteinase that can damage lung tissue when the balance between protease and anti-proteases is disturbed, so in the case of COVID-19, the application of NE inhibitors may open up a therapeutic window for the treatment of patients with COVID-19.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
On behalf of the authors, we sincerely thank patients, healthy subjects, and clinicians (Firouzgar Hospital, Tehran, Iran) for their collaborations.
Ethical approval
The Iran University of Medical Sciences Ethics Review Board approved this study (No. IR.IUMS.FMD.REC.1399.624(.
References
- 1.Goodarzi P., Mahdavi F., Mirzaei R., Hasanvand H., Sholeh M., Zamani F., Sohrabi M., Tabibzadeh A., Jeda A.S., Niya M.H.K., Coronavirus disease Coronavirus disease 2019 (COVID-19): Immunological approaches and emerging pharmacologic treatments. Int Immunopharmacol. 2020 doi: 10.1016/j.intimp.2020.106885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. Jama. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 3.Keyvani H., Zahednasab H., Sholeh M., Mirzaei R., Esghaei M., Karampoor S. Gender Preponderance Might be Associated with the Severity of COVID-19 Infection. J Clin Cell Immunol. 2020;11:598. [Google Scholar]
- 4.Mirzaei R., Mohammadzadeh R., Mahdavi F., Badrzadeh F., Kazemi S., Ebrahimi M., Soltani F., Kazemi S., Jeda A.S., Darvishmotevalli M. Overview of the current promising approaches for the development of an effective severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine. Int Immunopharmacol. 2020;106928 doi: 10.1016/j.intimp.2020.106928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H., Liu S.-M., Yu X.-H., Tang S.-L., Tang C.-K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. International journal of antimicrobial agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirzaei R., Karampoor S., Sholeh M., Moradi P., Ranjbar R., Ghasemi F. A contemporary review on pathogenesis and immunity of COVID-19 infection. Molecular biology reports. 2020;47(7):5365–5376. doi: 10.1007/s11033-020-05621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China, The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region. Italy, Jama. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azkur A.K., Akdis M., Azkur D., Sokolowska M., van de Veen W., Brüggen M.C., O’Mahony L., Gao Y., Nadeau K., Akdis C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmunity reviews. 2020;19(6) doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H., Xiong Y., Cheng Z., Gao S., Liang K. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nature Reviews Immunology. 2018;18(2):134. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 14.Liu J., Pang Z., Wang G., Guan X., Fang K., Wang Z., Wang F. Advanced role of neutrophils in common respiratory diseases. Journal of immunology research. 2017;2017 doi: 10.1155/2017/6710278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camp J.V., Jonsson C.B. A role for neutrophils in viral respiratory disease. Frontiers in immunology. 2017;8:550. doi: 10.3389/fimmu.2017.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang S.-C., Tsai Y.-F., Pan Y.-L., Hwang T.-L. Understanding the role of neutrophils in acute respiratory distress syndrome. Biomedical Journal. 2020 doi: 10.1016/j.bj.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. Journal of Experimental Medicine. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuo Y., Srilakshmi Y., Hui S., Kelsey G. Melanie Zet al, Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11) doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guéant J.L., Fromonot J., Guéant-Rodriguez R.M., Lacolley P., Guieu R., Regnault V. Blood myeloperoxidase-DNA, a biomarker of early response to SARS-CoV-2 infection? Allergy. 2020 doi: 10.1111/all.14533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borregaard N. Development of neutrophil granule diversity. Annals of the New York Academy of Sciences. 1997;832(1):62–68. doi: 10.1111/j.1749-6632.1997.tb46237.x. [DOI] [PubMed] [Google Scholar]
- 21.Emboriadou M., Hatzistilianou M., Magnisali C., Sakelaropoulou A., Exintari M., Conti P., Aivazis V. Human neutrophil elastase in RSV bronchiolitis. Annals of Clinical & Laboratory Science. 2007;37(1):79–84. [PubMed] [Google Scholar]
- 22.Abu-Harb M., Bell F., Finn A., Rao W., Nixon L., Shale D., Everard M. IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. European Respiratory Journal. 1999;14(1):139–143. doi: 10.1034/j.1399-3003.1999.14a23.x. [DOI] [PubMed] [Google Scholar]
- 23.Johansson C., Kirsebom F.C. Neutrophils in respiratory viral infections. Mucosal Immunology. 2021:1–13. doi: 10.1038/s41385-021-00397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song N., Wang W., Wang Y., Guan Y., Xu S., Guo M.-Y. Hydrogen sulfide of air induces macrophage extracellular traps to aggravate inflammatory injury via the regulation of miR-15b-5p on MAPK and insulin signals in trachea of chickens. Science of The Total Environment. 2021;771 doi: 10.1016/j.scitotenv.2021.145407. [DOI] [PubMed] [Google Scholar]
- 25.Jing H., Zhang Q., Gao X.-J. Excessive lithium of water induced a toxic effect on kidney via oxidative damage and inflammation in carp. Aquaculture. 2021;535 [Google Scholar]
- 26.Jing H., Wang S., Wang Y., Shen N., Gao X.-J. Environmental contaminant ammonia triggers epithelial-to-mesenchymal transition-mediated jejunal fibrosis with the disassembly of epithelial cell-cell contacts in chicken. Science of The Total Environment. 2020;726 doi: 10.1016/j.scitotenv.2020.138686. [DOI] [PubMed] [Google Scholar]
- 27.Polverino E., Rosales-Mayor E., Dale G.E., Dembowsky K., Torres A. The role of neutrophil elastase inhibitors in lung diseases. Chest. 2017;152(2):249–262. doi: 10.1016/j.chest.2017.03.056. [DOI] [PubMed] [Google Scholar]
- 28.Galani I.E., Andreakos E. Neutrophils in viral infections: current concepts and caveats. Journal of leukocyte biology. 2015;98(4):557–564. doi: 10.1189/jlb.4VMR1114-555R. [DOI] [PubMed] [Google Scholar]
- 29.Sandhaus R.A., Turino G. Neutrophil elastase-mediated lung disease. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2013;10(sup1):60–63. doi: 10.3109/15412555.2013.764403. [DOI] [PubMed] [Google Scholar]
- 30.Gueant J.L., Rodriguez-Gueant R.M., Fromonot J., Oussalah A., Louis H., Chery C., Gette M., Gleye S., Callet J., Raso J. Elastase and Exacerbation of Neutrophil Innate Immunity are Involved in Multi-Visceral Manifestations of COVID-19. Authorea Preprints. 2020 doi: 10.1111/all.14746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reusch N., De Domenico E., Bonaguro L., Schulte-Schrepping J., Baßler K., Schultze J.L., Aschenbrenner A.C. Neutrophils in COVID-19. Frontiers in Immunology. 2021;12 doi: 10.3389/fimmu.2021.652470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng L.G., Ostuni R., Hidalgo A. Heterogeneity of neutrophils. Nature Reviews Immunology. 2019;19(4):255–265. doi: 10.1038/s41577-019-0141-8. [DOI] [PubMed] [Google Scholar]
- 33.Dennison D., Al Khabori M., Al Mamari S., Aurelio A., Al Hinai H., Al Maamari K., Alshekaili J., Al Khadouri G. Circulating activated neutrophils in COVID-19: An independent predictor for mechanical ventilation and death. International Journal of Infectious Diseases. 2021;106:155–159. doi: 10.1016/j.ijid.2021.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aschenbrenner A.C., Mouktaroudi M., Kraemer B., Oestreich M., Antonakos N., Nuesch-Germano M., Gkizeli K., Bonaguro L., Reusch N., Baßler K. Disease severity-specific neutrophil signatures in blood transcriptomes stratify COVID-19 patients. Genome Medicine. 2021;13(1):1–25. doi: 10.1186/s13073-020-00823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meizlish M.L., Pine A.B., Bishai J.D., Goshua G., Nadelmann E.R., Simonov M., Chang C.-H., Zhang H., Shallow M., Bahel P. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood advances. 2021;5(5):1164–1177. doi: 10.1182/bloodadvances.2020003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang B.M., Shojaei M., Teoh S., Meyers A., Ho J., Ball T.B., Keynan Y., Pisipati A., Kumar A., Eisen D.P. Neutrophils-related host factors associated with severe disease and fatality in patients with influenza infection. Nature communications. 2019;10(1):1–13. doi: 10.1038/s41467-019-11249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawabata K., Hagio T., Matsuoka S. The role of neutrophil elastase in acute lung injury. European journal of pharmacology. 2002;451(1):1–10. doi: 10.1016/s0014-2999(02)02182-9. [DOI] [PubMed] [Google Scholar]
- 38.Boxio R., Wartelle J., Nawrocki-Raby B., Lagrange B., Malleret L., Hirche T., Taggart C., Pacheco Y., Devouassoux G., Bentaher A. Neutrophil elastase cleaves epithelial cadherin in acutely injured lung epithelium. Respiratory research. 2016;17(1):1–15. doi: 10.1186/s12931-016-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cron R.Q. COVID-19 cytokine storm: targeting the appropriate cytokine, The Lancet. Rheumatology. 2021;3(4):e236–e237. doi: 10.1016/S2665-9913(21)00011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Frontiers in immunology. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine & growth factor reviews. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Frontiers in immunology. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhar S.K., Vishnupriyan K., Damodar S., Gujar S., Das M. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: results from meta-analysis and regression. Heliyon. 2021;7(2) doi: 10.1016/j.heliyon.2021.e06155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fraser D.D., Cepinskas G., Slessarev M., Martin C., Daley M., Miller M.R., O'Gorman D.B., Gill S.E., Patterson E.K., Dos Santos C.C. Inflammation profiling of critically ill coronavirus disease 2019 patients. Critical care explorations. 2020;2(6) doi: 10.1097/CCE.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasonov E., Samsonov M. The role of Interleukin 6 inhibitors in therapy of severe COVID-19. Biomedicine & Pharmacotherapy. 2020;110698 doi: 10.1016/j.biopha.2020.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavalli G., Larcher A., Tomelleri A., Campochiaro C., Della-Torre E., De Luca G., Farina N., Boffini N., Ruggeri A., Poli A. Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study. The Lancet Rheumatology. 2021;3(4):e253–e261. doi: 10.1016/S2665-9913(21)00012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J., Hao Y., Ou W., Ming F., Liang G., Qian Y., Cai Q., Dong S., Hu S., Wang W. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: a cohort study. Journal of translational medicine. 2020;18(1):1–8. doi: 10.1186/s12967-020-02571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guirao J.J., Cabrera C.M., Jiménez N., Rincón L., Urra J.M. High serum IL-6 values increase the risk of mortality and the severity of pneumonia in patients diagnosed with COVID-19. Molecular Immunology. 2020;128:64–68. doi: 10.1016/j.molimm.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun H., Guo P., Zhang L., Wang F. Serum Interleukin-6 Concentrations and the Severity of COVID-19 Pneumonia: A Retrospective Study at a Single Center in Bengbu City, Anhui Province, China, in January and February 2020. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2020;26:e926941–e926951. doi: 10.12659/MSM.926941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karampoor S., Zahednasab H., Farahmand M., Mirzaei R., Zamani F., Tabibzadeh A., Bouzari B., Ajdarkosh H., Nikkhah M., Hashemi M.R. A possible pathogenic role of Syndecan-1 in the pathogenesis of coronavirus disease 2019 (COVID-19) Int Immunopharmacol. 2021;97:107684. doi: 10.1016/j.intimp.2021.107684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma A., Zhang L., Ye X., Chen J., Yu J., Zhuang L., Weng C., Petersen F., Wang Z., Yu X. High Levels of Circulating IL-8 and Soluble IL-2R Are Associated With Prolonged Illness in Patients With Severe COVID-19. Frontiers in immunology. 2021;12:12. doi: 10.3389/fimmu.2021.626235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darif D., Hammi I., Kihel A., Saik I.E.I., Guessous F., Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microbial Pathogenesis. 2021;104799 doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.H. Codina, I. Vieitez, A. Gutierrez-Valencia, V. Skouridou, C. Martínez, L. Patiño, M. Botero-Gallego, M. Trujillo-Rodríguez, A. Serna-Gallego, E. Muñoz-Muela, Elevated anti-SARS-CoV-2 antibodies and IL-6, IL-8, MIP-1β, early predictors of severe COVID-19, bioRxiv (2021). [DOI] [PMC free article] [PubMed]
- 54.Li H., Zhang J., Fang C., Zhao X., Qian B., Sun Y., Zhou Y., Hu J., Huang Y., Ma Q. The prognostic value of IL-8 for the death of severe or critical patients with COVID-19. Medicine. 2021;100(11) doi: 10.1097/MD.0000000000023656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahnach M., Zbiri S., Nejjari S., Ousti F., Elkettani C. C-reactive protein as an early predictor of COVID-19 severity. Journal of Medical Biochemistry. 2020;39(4):500. doi: 10.5937/jomb0-27554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L. C-reactive protein levels in the early stage of COVID-19. Medecine et maladies infectieuses. 2020;50(4):332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.C.B.V. de Paula, M.L.V. de Azevedo, S. Nagashima, A.P.C. Martins, M.A.S. Malaquias, A.F.R. dos Santos Miggiolaro, J.d.S.M. Júnior, G. Avelino, L.A.P. do Carmo, L.B. Carstens, IL-4/IL-13 remodeling pathway of COVID-19 lung injury, Scientific Reports 10(1) (2020) 1-8. [DOI] [PMC free article] [PubMed]
- 58.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., Zhang P., Liu X., Gao G., Liu F. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerging microbes & infections. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu L., Zhang H., Dauphars D.J., He Y.-W. A Potential Role of Interleukin-10 in COVID-19 Pathogenesis. Trends in Immunology. 2020 doi: 10.1016/j.it.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.J. Li, L. Rong, R. Cui, J. Feng, Y. Jin, Y. Yu, X. Chen, R. Xu, Dynamic changes in serum IL-6, IL-8, and IL-10 are associated with the outcome of patients with severe COVID-19 in ICU, (2020). [DOI] [PubMed]
- 61.Roschewski M., Lionakis M.S., Sharman J.P., Roswarski J., Goy A., Monticelli M.A., Roshon M., Wrzesinski S.H., Desai J.V., Zarakas M.A. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Science immunology. 2020;5(48) doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uddin M., Watz H., Malmgren A., Pedersen F. NETopathic inflammation in chronic obstructive pulmonary disease and severe asthma. Frontiers in immunology. 2019;10:47. doi: 10.3389/fimmu.2019.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Athanasios D. COVID-19 Hyperinflammation: What about Neutrophils? mSphere. 2020;5(3) doi: 10.1128/mSphere.00367-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bryson K., Nash A., Norval M. Does vitamin D protect against respiratory viral infections? Epidemiology & Infection. 2014;142(9):1789–1801. doi: 10.1017/S0950268814000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Balla M., Merugu G.P., Konala V.M., Sangani V., Kondakindi H., Pokal M., Gayam V., Adapa S., Naramala S., Malayala S.V. Back to basics: review on vitamin D and respiratory viral infections including COVID-19. Journal of community hospital internal medicine perspectives. 2020;10(6):529–536. doi: 10.1080/20009666.2020.1811074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Charan J., Goyal J.P., Saxena D., Yadav P. Vitamin D for prevention of respiratory tract infections: A systematic review and meta-analysis. Journal of pharmacology & pharmacotherapeutics. 2012;3(4):300. doi: 10.4103/0976-500X.103685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jain A., Chaurasia R., Sengar N.S., Singh M., Mahor S., Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Scientific reports. 2020;10(1):1–8. doi: 10.1038/s41598-020-77093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Merzon E., Tworowski D., Gorohovski A., Vinker S., Golan Cohen A., Green I., Frenkel-Morgenstern M. Low plasma 25 (OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. The FEBS journal. 2020;287(17):3693–3702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carpagnano G.E., Di Lecce V., Quaranta V.N., Zito A., Buonamico E., Capozza E., Palumbo A., Di Gioia G., Valerio V.N., Resta O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. Journal of endocrinological investigation. 2021;44(4):765–771. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panagiotou G., Tee S.A., Ihsan Y., Athar W., Marchitelli G., Kelly D., Boot C.S., Stock N., Macfarlane J., Martineau A.R. Low serum 25-hydroxyvitamin D (25 [OH] D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clinical endocrinology. 2020;93(4):508–511. doi: 10.1111/cen.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]