Abstract
Purpose
Dietary guidelines recommend to limit egg consumption to 4 servings per week but the relation between egg intake and health outcomes is still controversial. To evaluate the association of egg consumption and mortality risk in Italian adults and to investigate nutritional factors and serum lipids as potentially explaining such associations.
Methods
Longitudinal analysis on 20,562 men and women aged ≥ 35y, free from cardiovascular disease (CVD) and cancer belonging to the Moli-sani Study cohort (enrolled 2005–2010) followed up for a median of 8.2 years.
Results
In multivariable-adjusted analysis as compared to low intake (> 0 ≤ 1 egg/week), eating > 4 eggs/week led to an increased risk of all-cause (Hazard ratio [HR] = 1.50; 95%CI 1.13–1.99), CVD (HR = 1.75; 1.07–2.87) and cancer mortality (HR = 1.52; 0.99–2.33). Similarly, an intake of 2–4 eggs/week was associated with higher all-cause (HR = 1.22; 1.01–1.46) and CVD mortality risk (HR = 1.43; 1.03–1.97). An increase of 1 egg per week was associated with higher mortality risk among high-risk individuals, such as those with hypertension and hyperlipidaemia. Dietary cholesterol explained about 43.0% and 39.3% (p values < 0.0001) of the association of eggs with all-cause and CVD mortality, respectively, while serum lipids (e.g., total cholesterol) accounted for a small proportion of egg-mortality relation.
Conclusions
Among Italian adults, high egg consumption leads to an increased risk of all-cause and CVD mortality, with the risk being evident even at the recommended intake of 2–4 eggs per week. A substantial part of this association was likely due to the egg contribution to dietary cholesterol. Our findings suggest limiting the consumption of eggs in the diet and these results should be considered in the development of dietary guidelines and updates.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-021-02536-w.
Keywords: Eggs, Dietary cholesterol, Mortality risk, Mediterranean diet
Introduction
Eggs are a common food in every diet and are used in many basic and formulated preparations. Eggs are affordable and nutrient-dense food items, containing high-quality protein with low levels of saturated fatty acids, and are rich in several micronutrients including vitamins and minerals, some of which (vitamin E, carotenoids) are reported to have antioxidant properties [1].
On the other side, eggs are also major sources of dietary cholesterol, a potential risk factor for cardiovascular health [2]. Dietary guidelines for egg consumption have changed several times over the past few decades, and vary among health agencies [3, 4]. In 2010, the Dietary Guidelines for Americans recommended cholesterol intake to be limited to no more than 300 mg per day [5], but the 2015–2020 Dietary Guidelines Advisory Committee did not bring forward this recommendation, due to the lack of evidence of an appreciable relationship between consumption of dietary cholesterol and serum cholesterol, a decision consistent with the conclusions of the American Heart Association/American College of Cardiology report [6].
Based on their high cholesterol content, the Mediterranean Diet Foundation recommends to consume up to 4 eggs per week, as a healthy alternative to fish or meat [7], and the same amount (2–4 eggs per week) was indicated in the latest Italian dietary guidelines [8]. In the Eatwell Guide issued by the National Health Service in the UK there is no recommended limit on how many eggs people should eat [9].
Epidemiological studies analysing the association of eggs with health outcomes have provided controversial results [10–12]. Several meta-analyses and an umbrella review of observational studies failed to report significant associations between egg intake and cardiovascular outcomes, even highlighting a downward trend association with stroke risk [13–16].
Data on the association of egg intake with mortality risk is limited and controversial [17–19], and the scientific evidence for the recommendations on dietary cholesterol and eggs is inconsistent and lacking in Mediterranean countries where the association between eggs and health has been seldom explored [10, 20, 21].
In light of this, our study first aimed to longitudinally evaluate the association of egg consumption with all-cause and cause-specific mortality in a large Italian population of adult men and women; second, to investigate whether dietary cholesterol and other nutritional factors may account for the association between egg intake and mortality risk.
Materials and methods
Study population
Data are from the Moli-sani Study cohort established in 2005–2010 in the Molise region, a Mediterranean area in Italy, that recruited 24,325 men and women aged ≥ 35 years [22].
For the purpose of the present analyses, we excluded subjects with a history of cardiovascular disease (CVD; n = 1537) or missing data on CVD (n = 360), with a history of cancer (n = 777) or missing data on cancer (n = 89), those individuals with missing data for egg intake (n = 100), those reporting implausible energy (< 800 kcal/d in men and < 500 kcal/d in women or > 4000 kcal/d in men and > 3500 kcal/d in women; n = 771) or egg intakes (≥ 14 eggs/week; n = 2), dietary or medical questionnaires judged as unreliable by interviewers (n = 955 and n = 235, respectively), subjects lost to follow-up (n = 23) and missing information on cause-specific death (n = 45).
We finally analysed 20,562 individuals (84.5% of the study sample).
The Moli-sani Study cohort was followed up until December 31st 2015 with the main outcome of interest being mortality. Overall and cause-specific mortality was assessed by the Italian mortality registry (ReNCaM registry), validated by Italian death certificates (ISTAT form) and coded according to the International Classification of Diseases (ICD-9 version).
CVD mortality included deaths from diseases of the circulatory system when the underlying cause of death included ICD9 codes 390–459. ICD-9 codes 430–438 were used to define the specific cause of death for cerebrovascular disease, ICD-9 codes 410–414 and 429 for ischemic heart disease (IHD). Cancer death was considered when the underlying cause of death included ICD9 codes 140–208. Non cardiovascular/non cancer causes of death were included in ‘other cause mortality’ group.
The Moli-sani Study complies with the Declaration of Helsinki and was approved by the ethical committee of the Catholic University Medical School in Rome, Italy. All participants provided written informed consent.
Dietary assessment
Dietary intake was assessed by a trained interviewer-administered semi-quantitative EPIC food frequency questionnaire (FFQ) validated and adapted to the Italian population to assess participants’ diet during the past 12 months.
The FFQ includes 188 food items, classified into 74 predefined food groups on the basis of similar nutrient characteristics or culinary usage [23]. Participants were asked to indicate the number of times a given item was consumed (per day, week, month or year) from which the frequency of consumption was calculated. The quantity of food consumed was assessed by asking the participant to select one among several images of different food portions or a predefined standard portion when no image was available.
Frequencies and quantities of each food were then linked to Italian Food Tables [24], using a specifically designed software [25], to obtain quantitative estimates of daily intake of macro- and micro-nutrients plus energy.
Total egg consumption from various food sources (e.g. whole egg, omelette) was defined as number per week (we used 50 g as the standard weight for one medium-sized egg) and categorized as up to 1 egg/week, > 1 ≤ 2 eggs/week, > 2 ≤ 4 eggs/week and > 4 eggs/week or used as a continuous variable as a 1-egg increment per week.
Adherence to the traditional Mediterranean diet (MD) was evaluated by the Mediterranean Diet Score (MDS) developed by Trichopoulou et al. [26].
Assessment of covariates
At baseline, information on socio-demographic variables, lifestyles and medical history were obtained by interviewer-administered questionnaires.
Participants were considered to have diabetes, hypertension or hyperlipidaemia at baseline if they were taking disease-specific drugs.
Leisure-time physical activity (PA) was calculated for sport activity, walking and gardening, and then dichotomized as < or ≥ 30 min/d. Bodyweight and height were measured with a column mechanical scale with a telescopic measuring rod (SECA 700, Hamburg, Germany), in subjects wearing no shoes and only light indoor clothing. Body mass index (BMI) was calculated as kg/m2 and then grouped into three categories as normal (≤ 25 kg/m2), overweight (25–30 kg/m2) or obese (≥ 30 kg/m2) [27].
Subjects were classified as never-smokers, current smokers or former smokers (quit at least 1 year ago). Education was based on the highest qualification attained and was categorized as up to lower secondary (approximately ≤ 8 years of study), upper secondary school (8–13 years of study) and postsecondary education (> 13 years of study).
Urban or rural environments were defined on the basis of the urbanization level as described by the European Institute of Statistics (EUROSTAT definition) and obtained by the tool ‘Atlante Statistico dei Comuni’ provided by the Italian National Institute of Statistics [28].
Venous blood samples were obtained from participants who had fasted overnight and had refrained from smoking for at least 6 h.
Serum lipids (total cholesterol, HDL-cholesterol, triglycerides) were assayed by enzymatic reaction methods using an automatic analyzer (ILab 350, Instrumentation Laboratory (IL), Milan, Italy).
Low-density lipoprotein (LDL) cholesterol was calculated according to Friedewald.
Quality control (high and low levels) for lipids was obtained by a commercial standard provided by the IL and an in-house serum standard pool. The coefficients of variability were respectively for high, medium and low values 4.9%, 5.2% and 4% for blood cholesterol; 3.2%, 3% and 4.5% for HDL-cholesterol; 5.2%, 5.3% and 5% for triglycerides.
Statistical analysis
Baseline characteristics of the study population by categories of egg intake or by survival status at the end of follow-up were presented as means with standard deviation (SD), or number and percentages.
Risk estimates for all-cause and cause-specific deaths were expressed as hazard ratios (HR) with 95% confidence intervals (95% CI) and calculated using Cox regression models with time-on-study on the time scale and adjusting for baseline age as covariate in the model.
Based on previous literature and biological plausibility, two multivariable models were fitted to assess the association between egg intake and mortality: Model 1 was adjusted for age (continuous), sex and energy intake (kcal; continuous); Model 2 as in model 1 further controlled for education (up to lower secondary school; upper secondary school; postsecondary/higher), household income (≤ 10.000; > 10.000 ≤ 25.000; > 25.000 ≤ 40.000; > 40.000 EUR/y), residence (urban, rural), leisure-time PA (< 30 or ≥ 30 min/d), smoking status (never, current, former), BMI (normal, overweight, obese), diabetes (no, yes, missing), hyperlipidaemia (no, yes, missing), hypertension (no, yes, missing); Model 3 as in model 2 further controlled for MDS (continuous). Missing values for diabetes (n = 241), hypertension (n = 131) and hyperlipidaemia (n = 147) were included in the models as dummy variables, similar to the way valid categories were represented. For education, BMI and smoking (less than 1% of missing values) missing values were imputed to the modal value.
Main macronutrients contained in eggs (saturated fat and protein, g/d), dietary cholesterol (mg/d), dietary vitamin E (mg/d) and beta-carotene (µg/d) were tested as potential mediators of the association between egg intake and mortality.
For the mediation analysis, we used the publicly available %MEDIATE macro in SAS software [29] to calculate the point and interval estimates of the percent of exposure effect (PTE) explained by one or more intermediate variables, with 95% confidence interval and P values. Nutritional factors and serum lipids were entered into the mediation analysis as continuous variables and positively skewed variables (triglycerides) were log transformed before analysis. Mediation analyses with biomarkers were restricted to 20,146 subjects after exclusion of those individuals with missing data on any of the biomarker.
To increase the applicability of the study results we calculated risk estimations for each additional 186 mg of dietary cholesterol per day, 6.2 g of dietary proteins per day, 1.6 g of saturated fats per day, 0.56 mg of vitamin E per day and 42 µg per day of beta-carotene since these are the amounts of nutrients present in 1 egg [30].
Subgroup analyses according to levels of intake of each food group included into the MDS (above/below the study population median) and by various baseline risk factors were conducted.
Appropriate multiplicative terms for testing interaction were included in the multivariable-adjusted models to test for a difference of the effect of egg intake across subgroups.
Statistical tests were two-sided, and p values < 0.05 were considered to indicate statistical significance. The data analysis was generated using SAS/STAT software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of the population
The analysed population consisted of 10,905 women (53%) and 9657 men (47%) with an average age of 54 years (± 11 years, range 35 to 94 years). The majority of participants were low educated (51.1%), non-smokers (50.3%) and overweight (42.9%) (Table 1).
Table 1.
Baseline characteristics of the study sample by categories of egg consumption (n = 20,562)
| Whole sample | Egg consumption (n of eggs/week) | ||||
|---|---|---|---|---|---|
| > 0 ≤ 1 | > 1 ≤ 2 | > 2 ≤ 4 | > 4 | ||
| N of subjects (%) | 20,562 | 5517 (26.8) | 7143 (34.8) | 6526 (31.7) | 1376 (6.7) |
| Number of eggs/week, median (IQR) | 1.57 (0.97–2.46) | 0.63 (0.39–0.85) | 1.47 (1.23–1.64) | 2.55 (2.20–3.07) | 4.60 (4.19–5.26) |
| Age, years | 54 (11) | 56 (11) | 54 (11) | 53 (11) | 53 (11) |
| Men (%) | 47.0 | 47.6 | 47.1 | 45.9 | 48.8 |
| Educational level (%) | |||||
| Up to lower secondary | 51.1 | 52.8 | 50.7 | 49.7 | 52.8 |
| Upper secondary | 35.7 | 33.7 | 36.2 | 36.9 | 35.6 |
| Postsecondary | 13.2 | 13.5 | 13.1 | 13.4 | 11.6 |
| Income categories (%) | |||||
| ≤ 10.000 | 5.4 | 6.6 | 5.2 | 4.6 | 5.9 |
| > 10.000 ≤ 25.000 | 30.5 | 29.7 | 30.1 | 31.1 | 32.4 |
| > 25.000 ≤ 40.000 | 21.1 | 20.7 | 21.5 | 21.0 | 20.5 |
| > 40.000 | 12.5 | 12.5 | 13.0 | 12.3 | 10.8 |
| Missing | 30.5 | 30.5 | 30.2 | 31.0 | 30.4 |
| Urban residence (%) | 67.0 | 66.4 | 66.9 | 66.7 | 70.0 |
| Smoking status (%) | |||||
| Non-smokers | 50.3 | 49.9 | 50.4 | 50.6 | 49.7 |
| Current smokers | 23.8 | 22.9 | 23.3 | 24.4 | 27.0 |
| Former smokers | 25.9 | 27.2 | 26.2 | 25.0 | 23.3 |
| Physical activity > 30 min/d (%) | 64.0 | 63.8 | 63.5 | 63.8 | 68.3 |
| BMI, kg/m2 | 27.9 (4.7) | 28.1 (4.8) | 28.0 (4.7) | 27.7 (4.7) | 27.7 (4.6) |
| BMI, kg/m2 (%) | |||||
| Normal (≤ 25) | 28.4 | 27 | 27.1 | 30.4 | 30.7 |
| Overweight (25–30) | 42.9 | 42.4 | 43.9 | 42.5 | 41.7 |
| Obese (≥ 30) | 28.7 | 30.5 | 29.0 | 27.1 | 27.6 |
| Diabetes (%) | 4.0 | 4.6 | 4.2 | 3.4 | 3.1 |
| Hypertension (%) | 25.2 | 28.9 | 25.6 | 22.4 | 21.4 |
| Hyperlipidaemia (%) | 5.3 | 7.4 | 5.5 | 4.0 | 2.3 |
| Total blood cholesterol, mg/dL | 213.8 (40.8) | 215.6 (41.8) | 213.7 (40.3) | 212.5 (40.3) | 210.4 (40.5) |
| HDL-cholesterol, mg/dL | 58.0 (14.7) | 57.6 (14.9) | 57.5 (14.6) | 57.9 (14.7) | 57.4 (14.7) |
| LDL-cholesterol, mg/dL | 131.3 (34.6) | 132.7 (35.5) | 131.4 (34.2) | 130.3 (34.4) | 129.3 (34.5) |
| Triglycerides, mg/dL | 122.4 (64.1) | 126.8 (65.1) | 123.9 (64.9) | 121.2 (62.6) | 118.4 (62.6) |
1 egg = 50 g
Values are means (SDs) unless otherwise stated
Analyses for serum lipids were run on 20,146 participants
Mean consumption was 1.8 eggs per week (± 1.3), while overall mean dietary cholesterol intake was 322 mg/d (± 108). Egg contributed to 14.6% of total cholesterol intake in the diet.
Participants with a greater egg intake (> 4 eggs/week) were younger, had lower socioeconomic status, less cardiovascular risk factors (i.e. BMI, diabetes, hypertension and hyperlipidaemia), including lower levels of blood lipids than participants consuming lower amounts of egg (Table 1). High egg consumers tended to have a lower baseline MD and consumed less fruits and nuts, cereals, and more meat and meat products. The contribution of proteins and fats to total energy intake increased across categories of egg consumption, whereas that of carbohydrates and fibre decreased; dietary cholesterol increased according to egg intake, as well as vitamin E levels while no substantial differences were observed for beta-carotene levels (Supplementary Table 1).
Egg consumption and mortality risk
The cohort of 20,562 participants was followed-up for a median of 8.3 years (interquartile range = 7.4–9.3 years; 170,032 person-years) during which 838 deaths were ascertained and validated; 271 from CVD, of which 153 were from IHD/cerebrovascular disease, 334 from cancer, and 233 from other causes (Supplementary Table 2).
In a multivariable-adjusted model including also MDS, as compared to participants reporting lower egg consumption, consuming more than 4 eggs/week was associated with increased risk of all-cause (HR = 1.50; 95%CI 1.13–1.99), CVD (HR = 1.75; 1.07–2.87) and cancer mortality (HR = 1.52; 0.99–2.33); an upward trend of risk with IHD/cerebrovascular disease mortality associated with increased egg intake was also observed (HR = 1.58; 0.79–3.17), although statistical significance was not hold (Table 2, models 3). The recommended intake of 2–4 eggs/week also led to increased all-cause (HR = 1.22; 1.01–1.46) and CVD mortality risk (HR = 1.43; 1.03–1.97) (Table 2, models 3).
Table 2.
Hazard ratios (HR) with 95% confidence intervals (95%CI) for all-cause and cause-specific mortality associated with egg consumption in the Moli-sani Study cohort (n = 20,562)
| Egg consumption (n of eggs/week) | 1 Egg/week increment | ||||||
|---|---|---|---|---|---|---|---|
| > 0 ≤ 1 | > 1 ≤ 2 | > 2 ≤ 4 | > 4 | p for trend | HR (95%CI) | p Value | |
| N of subjects (%) | 5517 (26.8) | 7143 (34.8) | 6526 (31.7) | 1372 (6.7) | – | – | |
| All-cause mortality | |||||||
| N of deaths | 234 | 281 | 256 | 67 | – | – | – |
| Person-years | 44,712 | 58,776 | 54,630 | 11,914 | – | – | – |
| Event rates per 10,000 person-years | 52.3 | 47.8 | 46.9 | 56.2 | – | – | – |
| Model 1 | -1- | 1.13 (0.95–1.35) | 1.23 (1.03–1.48) | 1.55 (1.17–2.06) | 0.0018 | 1.07 (1.01–1.13) | 0.018 |
| Model 2 | -1- | 1.14 (0.96–1.37) | 1.21 (1.01–1.46) | 1.50 (1.13–1.99) | 0.0043 | 1.06 (1.00–1.12) | 0.037 |
| Model 3 | -1- | 1.14 (0.96–1.36) | 1.22 (1.01–1.46) | 1.50 (1.13–1.99) | 0.0044 | 1.06 (1.00–1.12) | 0.039 |
| CVD mortality | |||||||
| N of deaths | 71 | 90 | 87 | 23 | – | – | – |
| Event rates per 10,000 person-years | 15.9 | 15.3 | 15.9 | 19.3 | – | – | – |
| Model 1 | -1- | 1.23 (0.90–1.69) | 1.43 (1.03–1.97) | 1.80 (1.10–2.94) | 0.0076 | 1.11 (1.01–1.22) | 0.025 |
| Model 2 | -1- | 1.29 (0.94–1.77) | 1.43 (1.03–1.97) | 1.75 (1.07–2.88) | 0.011 | 1.10 (1.01–1.21) | 0.036 |
| Model 3 | -1- | 1.29 (0.94–1.77) | 1.43 (1.03–1.97) | 1.75 (1.07–2.87) | 0.010 | 1.10 (1.01–1.21) | 0.036 |
| IHD/ cerebrovascular mortality | |||||||
| N of deaths | 42 | 51 | 49 | 11 | – | – | – |
| Event rates per 10,000 person-years | 9.4 | 8.7 | 9.0 | 9.2 | – | – | – |
| Model 1 | -1- | 1.24 (0.82–1.87) | 1.47 (0.96–2.25) | 1.69 (0.85–3.38) | 0.046 | 1.13 (1.00–1.28) | 0.054 |
| Model 2 | -1- | 1.26 (0.83–1.90) | 1.44 (0.94–2.21) | 1.58 (0.79–3.17) | 0.072 | 1.11 (0.98–1.26) | 0.090 |
| Model 3 | -1- | 1.25 (0.83–1.90) | 1.44 (0.94–2.21) | 1.58 (0.79–3.17) | 0.072 | 1.11 (0.98–1.26) | 0.090 |
| Cancer mortality | |||||||
| N of deaths | 94 | 104 | 106 | 30 | – | – | – |
| Event rates per 10,000 person-years | 21.0 | 17.7 | 19.4 | 25.2 | – | – | – |
| Model 1 | -1- | 0.99 (0.75–1.32) | 1.19 (0.89–1.58) | 1.59 (1.04–2.45) | 0.039 | 1.06 (0.97–1.15) | 0.19 |
| Model 2 | -1- | 0.98 (0.74–1.31) | 1.16 (0.88–1.54) | 1.52 (0.99–2.33) | 0.070 | 1.05 (0.96–1.14) | 0.29 |
| Model 3 | -1- | 0.98 (0.74–1.30) | 1.16 (0.87–1.54) | 1.52 (0.99–2.33) | 0.070 | 1.05 (0.96–1.14) | 0.29 |
| Other causes mortality | |||||||
| N of deaths | 69 | 87 | 63 | 14 | – | – | – |
| Event rates per 10,000 person-years | 15.8 | 14.2 | 11.4 | 11.8 | – | – | – |
| Model 1 | -1- | 1.22 (0.89–1.68) | 1.07 (0.75–1.52) | 1.16 (0.64–2.11) | 0.66 | 1.02 (0.92–1.14) | 0.69 |
| Model 2 | -1- | 1.24 (0.90–1.71) | 1.07 (0.75–1.53) | 1.16 (0.64–2.11) | 0.68 | 1.02 (0.92–1.14) | 0.71 |
| Model 3 | -1- | 1.24 (0.90–1.71) | 1.07 (0.75–1.53) | 1.14 (0.63–2.08) | 0.68 | 1.02 (0.92–1.13) | 0.73 |
Model 1 adjusted for age (continuous), sex and energy intake (continuous)
Model 2 as in model 1 further adjusted for educational level (categorical), household income (categorical), residence (categorical), smoking (categorical), BMI (categorical), leisure-time PA (categorical), baseline diabetes (categorical), hypertension (categorical), hyperlipidaemia (categorical)
Model 3 as in model 2 further adjusted for the Mediterranean diet score (continuous)
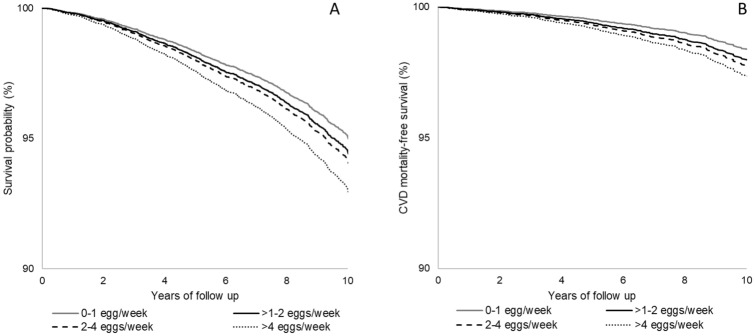
Multivariable-adjusted Kaplan–Meier estimates for all-cause mortality and CVD mortality for the four categories of egg consumption are separated (Figs. 1a, b) and showed increased mortality risk associated with higher egg intake (p = 0.030 and p = 0.083 respectively).
Fig. 1.
Multivariable adjusted Kaplan–Meier estimates for all-cause (a) and cardiovascular mortality (b) for increasing number of weekly egg consumption in the Moli-sani Study cohort (n = 20,562). Estimates were obtained from the multivariable-adjusted model including age, sex, energy intake, educational level, household income, residence, smoking, leisure-time PA, BMI, presence at baseline of diabetes, hyperlipidaemia, hypertension, and the Mediterranean diet score
Each additional egg per week was associated with a higher risk of all-cause (HR = 1.06; 1.00–1.12) and CVD mortality (HR = 1.10; 1.01–1.21) (Table 2, models 3).
Dietary fats and mortality
Dietary cholesterol positively correlated with saturated fat in the diet (Spearman correlation coefficient r = 0.83; p < 0.0001, data not shown).
Each additional 186 mg of dietary cholesterol consumed per day was associated with a higher risk of all-cause (HR = 1.28; 95% CI 1.07–1.51) and CVD mortality (HR = 1.39; 95% CI 1.03–1.88; Supplementary Table, 3, model 2) although the strength of the association was reduced after inclusion of other nutrients into the model. Similarly, saturated fat intake was associated with increased risk of all-cause and CVD mortality, although adjustment for other dietary factors mitigated the magnitude of the association (Supplementary Table, 3).
Mediation analysis
Dietary cholesterol explained 43.0% (p < 0.0001) and 39.3% (p < 0.0001) of the association of egg intake with all-cause and CVD mortality risk, respectively; saturated fats accounted from 11.6 to 13.1%, while dietary protein offered little contribution (Table 3). Antioxidant content did not attenuate the magnitude of the egg-mortality association.
Table 3.
Nutritional factors and serum lipids as possible mediators of the association between egg intake with all-cause and cardiovascular mortality in the Moli-sani Study cohort (n = 20,146)
| Mediator | All-cause mortality | CVD mortality | ||
|---|---|---|---|---|
| Proportion mediated (95% CI) | p value | Proportion mediated (95% CI) | p value | |
| Dietary cholesterol (mg/d) | 43.0% (22.8–65.7%) | < 0.0001 | 39.3% (16.6–67.9%) | < 0.0001 |
| Saturated fat (g/d) | 13.1% (7.7–21.4%) | < 0.0001 | 11.6% (5.8–21.8%) | < 0.0001 |
| Dietary protein (g/d) | 7.2% (3.8–13.0%) | < 0.0001 | 4.8% (1.6–13.6%) | 0.016 |
| Dietary vitamin E (mg/d) | Null | – | Null | – |
| Dietary beta-carotene (µg/d) | Null | – | Null | – |
| Dietary cholesterol, saturated fat, protein | 3.0% (0.0–78.5%) | 0.33 | 10.5% (1.3–50.4%) | 0.14 |
| Serum lipids* | 6.3% (3.3–11.9%) | < 0.0001 | 3.8% (1.6–8.9%) | 0.0009 |
Proportion of effect explained by intermediate variables with 95% CI and relevant p-value as produced by the %MEDIATE macro are reported for each potential mediator, in multivariable-adjusted Cox PH model controlled for sex, age (continuous), energy intake (continuous), educational level (categorical), household income (categorical), residence (categorical), smoking (categorical), BMI (categorical), leisure-time PA (categorical), baseline diabetes (categorical), hypertension (categorical), hyperlipidaemia (categorical), and the Mediterranean diet score (continuous)
The proportion refers to the high (> 4 eggs/week) vs low egg intake (> 0 ≤ 1 egg/week)
Null = not mediating the effect
*Mediation analysis restricted to 20,146 participants after exclusion of those individuals with missing data on any of the biomarker. Serum lipids include blood cholesterol (mg/dL), HDL-cholesterol (mg/dL), LDL-cholesterol (mg/dL), triglycerides (mg/dL; logarithm)
Dietary cholesterol inversely correlated with total serum cholesterol levels (Spearman correlation coefficients r = − 0.02, p = 0.005), HDL-cholesterol (r = − 0.02, p = 0.0005) and triglycerides (r = − 0.04, p < 0.0001) and was not related to LDL-cholesterol (r = 0.001, p = 0.92; data not shown).
Baseline differences in serum lipids across categories of egg intake marginally accounted for the association of eggs with mortality, explaining 6.3% (p < 0.0001) and 3.8% (p = 0.0009) of the relation between high egg consumption and all-cause and CVD mortality risk (Table 3).
Sub-group analysis
Sub-group analyses indicated that overall diet quality, as measured by the MDS, and single food groups were not effect modifiers of the relation between eggs and mortality risk (all p values for interaction > 0.05; Supplementary Table 4), although an increased risk of CVD mortality associated with 1-egg increment per week was found among low fruit consumers as compared to high consumers (p for interaction = 0.0034, Supplementary Table 4).
Among baseline risk factors, hyperlipidaemia and hypertension were likely to modify the magnitude of the association between egg consumption (1-egg increment per week) and CVD mortality risk, resulting to be stronger in those using lipid-lowering drugs (p for interaction = 0.010) and among participants taking antihypertensive medications (p for interaction = 0.042; Table 4).
Table 4.
Sub-group analysis for the association of 1 egg/week increment and all-cause and CVD mortality in the Moli-sani Study cohort (n = 20,562)
| All-cause mortality | CVD mortality | |||||
|---|---|---|---|---|---|---|
| N of deaths/n of subjects | HR (95%CI) | p for interaction | N of deaths | HR (95%CI) | p for interaction | |
| Whole sample | 838/20,562 | 1.06 (1.00–1.12) | – | 271 | 1.10 (1.01–1.20) | – |
| Aged < 65 years | 258/16,550 | 1.05 (0.95–1.16) | 0.37 | 58 | 1.08 (0.89–1.32) | 0.44 |
| Aged ≥ 65 years | 580/4012 | 1.09 (1.03–1.17) | 213 | 1.16 (1.05–1.28) | ||
| Women | 299/10,905 | 1.03 (0.94–1.14) | 0.19 | 104 | 1.19 (1.04–1.36) | 0.50 |
| Men | 539/9657 | 1.11 (1.04–1.18) | 167 | 1.11 (0.98–1.24) | ||
| Up to lower secondary education | 606/10,510 | 1.06 (1.00–1.13) | 0.88 | 205 | 1.11 (1.01–1.23) | 0.64 |
| Upper secondary/postsecondary | 232/10,052 | 1.05 (0.94–1.16) | 66 | 1.09 (0.88–1.34) | ||
| Non-smokers | 647/15,665 | 1.06 (1.00–1.13) | 0.89 | 212 | 1.09 (0.99–1.21) | 0.56 |
| Smokers | 191/4897 | 1.05 (0.94–1.17) | 59 | 1.07 (0.88–1.29) | ||
| Physical activity ≤ 30 min/d | 306/7402 | 1.07 (0.97–1.17) | 0.49 | 101 | 1.10 (0.94–1.28) | 0.81 |
| Physical activity > 30 min/d | 532/13,160 | 1.06 (0.99–1.13) | 170 | 1.11 (0.99–1.24) | ||
| Normal/overweight | 549/14,655 | 1.07 (1.00–1.14) | 0.81 | 165 | 1.12 (1.00–1.26) | 0.69 |
| Obese | 289/5907 | 1.07 (0.98–1.17) | 106 | 1.09 (0.94–1.26) | ||
| Free from diabetes | 701/19,501 | 1.06 (1.00–1.12) | 0.82 | 224 | 1.09 (0.99–1.20) | 0.20 |
| Subjects with diabetes | 113/820 | 1.09 (0.93–1.28) | 34 | 1.26 (0.95–1.65) | ||
| Free from hyperlipidaemia | 762/19,323 | 1.06 (1.00–1.12) | 0.97 | 250 | 1.07 (0.97–1.18) | 0.010 |
| Subjects with hyperlipidaemia | 66/1092 | 1.06 (0.86–1.32) | 18 | 1.66 (1.21–2.26) | ||
| Free from hypertension | 435/15,256 | 1.03 (0.96–1.11) | 0.35 | 107 | 1.00 (0.85–1.18) | 0.042 |
| Subjects with hypertension | 400/5175 | 1.08 (1.00–1.17) | 163 | 1.15 (1.03–1.28) | ||
| Excluding early deaths (follow up > 2 years) | 740/20,464 | 1.06 (1.00–1.12) | – | 233 | 1.14 (1.01–1.22) | – |
Hazard ratios with 95% CI from the multivariable model adjusted for sex, age (continuous), energy intake (continuous), educational level (categorical), household income (categorical), residence (categorical), smoking (categorical), BMI (categorical), leisure-time PA (categorical), baseline diabetes (categorical), hypertension (categorical), hyperlipidaemia (categorical), and the Mediterranean diet score (continuous)
Missing data: diabetes (n = 241), hypertension (n = 131) and hyperlipidaemia (n = 147)
Discussion
In a large cohort of Italian adults, eating more than 4 eggs per week was associated with an increased risk of all-cause and CVD mortality, in comparison with a lower intake (up to 1 egg per week) and independently of overall diet quality as reflected by adherence to the Mediterranean diet. Of interest, we found that even moderate egg intake of 2–4 servings per week, which is generally recommended by international dietary guidelines, led to an increased risk of all-cause and CVD mortality by 22% and 43%, respectively.
Egg and dietary cholesterol intakes in our cohort were similar to that reported in European countries from EPIC study [31] and in some US cohorts [32, 33], but egg consumption in our cohort was slightly lower than that documented in other Mediterranean cohorts, which reported an average intake of 3–4 eggs per week [9, 19].
Our findings expand the scarce literature on this topic in Mediterranean countries. Consumption of 10 g of egg per day (corresponding to approximately 1 and a half egg per week) was found associated with increased risk of total (31%) and CVD death (54%) in patients with diabetes from the Greek arm of the EPIC study [21], while in the Spanish cohort of EPIC no association between egg consumption (up to 7 eggs/week) and all-cause, CVD and IHD of mortality was found; yet, inverse associations between egg intake and risk of death from other causes (24% reduction) and from the nervous system diseases (41%) were documented [20]. In the Spanish SUN cohort, no association was found between egg consumption and the incidence of CVD [9].
Studies from non-Mediterranean cohorts also yielded inconsistent findings. In a recent analysis from the NHANES cohort no significant associations between egg consumption and mortality in US adults were observed [34]; on the contrary, pooled data from 6 US-based cohorts showed that each additional half an egg consumed per day was significantly associated with 6% higher risk of incident CVD and 8% of all-cause mortality [32].
Moreover, recent findings from nine European countries showed that higher egg consumption was associated with a higher risk of haemorrhagic stroke (25%) and an upward trend of increased ischaemic stroke risk [31], and in a Japanese cohort of women, a direct association was reported between egg intake (≥ 2 eggs/d vs 1 egg/d) and total mortality [17].
A meta-analysis of 14 studies involving 320,778 subjects identified a dose–response positive association between egg consumption and the risk of CVD and diabetes [12], but more recently an analysis from three large US cohorts and an updated meta-analysis including 28 prospective studies showed that moderate egg consumption (up to 7 eggs per week) was not associated with cardiovascular disease risk overall [35].
Recently, a dose–response meta-analysis of prospective cohort studies found no association with risk of cardiovascular outcomes following the habitual consumption of one egg per day compared to no intake, with exception of the risk of heart failure, which resulted higher especially in men from US cohorts [36].
Inconsistencies across studies may be due to differences in population characteristics, sample sizes, cooking methods for eggs, or differences in dietary patterns related to different amounts of egg consumption and also different adjustments for confounders.
Our study relied on a comprehensive assessment of nutritional factors (e.g. saturated fats and overall diet quality) and also on a number of other potential confounding variables, such as lifestyles and socio-demographic factors.
We also observed an increased risk of cancer mortality associated with eating more than 4 eggs per week and this is in line with a dose–response meta-analysis of prospective observational studies showing that eating ≥ 5 eggs per week may be associated with a modestly elevated risk of breast, ovarian and fatal prostate cancers [37]. Similarly, Japanese women consuming more than 2 eggs per day, as compared to those having 1 egg/d, tripled their risk of dying from cancer [17].
Furthermore, we found that the association between egg consumption and CVD mortality looked much stronger among subjects with hyperlipidaemia, suggesting that egg consumption should be strongly discouraged in this high-risk subgroup, even despite the use of lipid-lowering medications.
Generally, eggs are a controversial food because of their saturated fat (about 3 g/100 g) and cholesterol content (about 370 mg/100 g) [30] and on this basis experts have produced mounting evidence against frequent egg consumption due to their potential association with CVD [32].
Indeed, pooled data from 6 US-based cohorts recently showed that each additional 300 mg of dietary cholesterol consumed per day was significantly associated with 17% and 18% higher risk of incident CVD and all-cause mortality, respectively [32], while others found that the dietary cholesterol-mortality relation likely depends on the baseline intake levels, with an inverse association in those with lower intake levels (< 250 mg/day) but a positive association in those with higher intake levels (≥ 250 mg/day) [34].
In our study, we found that a substantial part of the excess risk of all-cause and CVD mortality associated with egg intake was accounted for by dietary cholesterol that, in turn, was associated with an increased risk of all-cause and CVD mortality, although the strength of the relation was reduced in multivariable-adjusted models. Our data are in accordance with observational data from US cohorts reporting that dietary cholesterol largely explained the association of eggs with increased CVD mortality [32].
However, the potential health risk of high dietary cholesterol levels has been questioned [38, 39] and more recently a meta-analysis concluded that available evidence is too heterogeneous and actually lacks methodologic rigor to draw any definitive conclusion regarding the influence of dietary cholesterol on CVD risk [38].
Since cholesterol-containing foods are usually rich in saturated fat and animal protein, which have been associated with increased CVD mortality risk in previous reports [40, 41], we also accounted for such nutrients but found that proteins were unlikely to attenuate the relationship between eggs and mortality risk, while saturated fats played a limited role. These results should be interpreted in light of the fact that eggs contain high-quality protein with minimal saturated fatty acids.
Differences at baseline in serum lipids among study participants were unlikely to explain the excess of CVD risk associated with higher egg intake while explaining about 7% of the relation with all-cause mortality. Individuals eating eggs more frequently tended to have lower levels of serum lipids; this may be counter-intuitive, but epidemiological evidence on a direct association between serum lipids and disease/death risk is inconsistent and not fully elucidated [42, 43].
Strengths and limitations
To our knowledge, this is one of the largest prospective cohort studies evaluating the association between egg consumption and mortality in a Mediterranean population, and one of the few examining the role of the nutrient content of eggs in the egg- mortality relation.
Major strengths of this study include a large community-based cohort, its prospective design, detailed information of dietary intake and the considerable number of covariates allowing to minimize sources of bias and confounding.
However, this study also suffers from several limitations: first, the observational nature of the study cannot allow to fully rule out residual confounding or confounding by unmeasured factors. Second, cause-specific mortality analysis in this dataset is limited by the small number of deaths and the relatively short period of follow up; also, the rather small number of subjects and events in the highest egg consumption category has to be acknowledged.
Furthermore, information on dietary intake was self-reported and can lead to under- or over-estimates; moreover, subjects’ dietary information was collected at baseline only, thus life-course changes possibly occurred during the follow-up period, may have influenced the strength of our findings. Also, we do not have data on preferred cooking methods which may likely influence the egg-mortality association.
Finally, participants lived in Molise, a region located between central and southern Italy, that its traditionally Mediterranean in culture; thus caution is needed in extending our results to other geographical and cultural contexts, although the main characteristics of our population sample are comparable to those of the Italian Cardiovascular Epidemiological Observatory and therefore representative of at least the Italian population [44].
Conclusions
Our findings report an increased risk of all-cause, CVD and cancer mortality associated with intake of more than 4 eggs per week in a large cohort of Italians from the general population. Increased death risk was also observed at lower intakes, namely 2–4 servings per week, which correspond to the egg intake recommended by many health bodies and international dietary guidelines.
The adverse health effects of eggs were found to be independent of the overall quality of the diet, while part of the excess of all-cause and CVD mortality risk was likely due to the high content of cholesterol and saturated fats of eggs, which in turn were found predictive of higher mortality risk in our cohort. As dietary cholesterol was not directly related to serum lipids, its adverse health effect may likely pass through biological mechanisms other than cholesterol-related ones.
Although we do acknowledge the limitations of our observational study, our findings are unlikely to be supportive of the current dietary guidelines most of which recommending a safe use up to 4 eggs per week, or even no type of restriction.
Finally, caution should be taken especially for high-risk individuals, such as those with hypercholesterolemia and hypertension.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The Moli-sani Study research group thanks the Associazione Cuore Sano Onlus (Campobasso, Italy) for its financial and cultural support, and is grateful to the BIOMARCARE Consortium. Moli-sani Study Investigators. The enrolment phase of the Moli-sani Study was conducted at the Research Laboratories of the Catholic University in Campobasso (Italy), the follow up of the Moli-sani cohort is being conducted at the Department of Epidemiology and Prevention of the IRCCS Neuromed, Pozzilli, Italy. Steering Committee: Licia Iacoviello*°(Chairperson), Giovanni de Gaetano* and Maria Benedetta Donati*. Scientific secretariat: Marialaura Bonaccio*, Americo Bonanni*, Chiara Cerletti*, Simona Costanzo*, Amalia De Curtis*, Augusto Di Castelnuovo§, Francesco Gianfagna°§, Mariarosaria Persichillo*, Teresa Di Prospero* (Secretary). Safety and Ethical Committee: Jos Vermylen (Catholic Univesity, Leuven, Belgio) (Chairperson), Ignacio De Paula Carrasco (Accademia Pontificia Pro Vita, Roma, Italy), Antonio Spagnuolo (Catholic University, Roma, Italy). External Event adjudicating Committee: Deodato Assanelli (Brescia, Italy), Vincenzo Centritto (Campobasso, Italy). Baseline and Follow-up data management: Simona Costanzo* (Coordinator), Marco Olivieri (Associazione Cuore Sano, Campobasso, Italy), Teresa Panzera*. Data Analysis: Augusto Di Castelnuovo§ (Coordinator), Marialaura Bonaccio*, Simona Costanzo*, Simona Esposito*, Alessandro Gialluisi*, Francesco Gianfagna°§, Emilia Ruggiero*. Biobank and biochemical laboratory: Amalia De Curtis* (Coordinator), Sara Magnacca§. Genetic laboratory: Benedetta Izzi* (Coordinator), Annalisa Marotta*, Fabrizia Noro*, Roberta Parisi*, Alfonsina Tirozzi*. Recruitment staff: Mariarosaria Persichillo* (Coordinator), Francesca Bracone*, Francesca De Lucia (Associazione Cuore Sano, Campobasso, Italy), Cristiana Mignogna°, Teresa Panzera*, Livia Rago*. Communication and Press Office: Americo Bonanni*. Regional Health Institutions: Direzione Generale per la Salute—Regione Molise; Azienda Sanitaria Regionale del Molise (ASReM, Italy); Molise Dati Spa (Campobasso, Italy); Offices of vital statistics of the Molise region. Hospitals: Presidi Ospedalieri ASReM: Ospedale A. Cardarelli—Campobasso, Ospedale F. Veneziale—Isernia, Ospedale San Timoteo—Termoli (CB), Ospedale Ss. Rosario—Venafro (IS), Ospedale Vietri—Larino (CB), Ospedale San Francesco Caracciolo—Agnone (IS); Casa di Cura Villa Maria—Campobasso; Ospedale Gemelli Molise—Campobasso; IRCCS Neuromed—Pozzilli (IS). *Department of Epidemiology and Prevention, IRCCS Neuromed, Pozzilli, Italy. °Department of Medicine and Surgery, University of Insubria, Varese, Italy. §Mediterranea Cardiocentro, Napoli, Italy. Baseline Recruitment staff is available at https://www.moli-sani.org/?page_id=173.
Authors' contribution
ER, MB, and LI contributed to the design of the study and interpretation of data; SC, ADeC and MP managed data collection; ER, MB and ADiC analysed the data; ER and MB wrote the manuscript; MBD, CC, GdG and LI originally inspired the research and critically reviewed the manuscript. All Authors have read and approved the manuscript.
Funding
Open access funding provided by Università degli Studi dell'Insubria within the CRUI-CARE Agreement. The enrolment phase of the Moli-sani Study was supported by unrestricted research grants from the Pfizer Foundation (Rome, Italy), the Italian Ministry of University and Research (MIUR, Rome, Italy)–Programma Triennale di Ricerca, Decreto no.1588 and Instrumentation Laboratory, Milan, Italy. The follow-up phase of the Moli-sani Study (assessment of incident cases) was partially supported by AIRC “5xMILLE” (HYPERCAN Study, n. 12237) and the Italian Ministry of Health (PI GdG, CoPI SC; grant n. RF-2018-12367074). ER was supported by a Fondazione Umberto Veronesi Fellowship. The present analyses were partially supported by a grant to MB of the Italian Ministry of Health 2013 [Grant number GR-2013-02356060], by the Hypercan Study, AIRC “5xMILLE” n.12237 and by POR FESR 2014-2020: DD n. 459 del 27/11/2018. SATIN: Sviluppo di Approcci Terapeutici INnovativi per patologie neoplastiche resistenti ai trattamenti and by a grant to LI as a partner of BiomarCaRE (Biomarkers for Cardiovascular Risk Assessment in Europe) by European Commission Seventh Framework Programme FP7/2007-2013 [HEALTH-F2-2011-278913]. Funders had no role in study design, collection, analysis, and interpretation of data; in the writing of the manuscript and in the decision to submit the article for publication. All Authors were and are independent from funders.
Declarations
Conflict of interest
None of the authors has conflicts of interest to disclose.
Footnotes
Moli-sani Study investigators are listed in the Acknowledgements section and in Supplementary material.
Contributor Information
Licia Iacoviello, Email: licia.iacoviello@moli-sani.org.
the Moli-sani Study Investigators:
Licia Iacoviello, Giovanni de Gaetano, Maria Benedetta Donati, Marialaura Bonaccio, Americo Bonanni, Chiara Cerletti, Simona Costanzo, Amalia De Curtis, Augusto Di Castelnuovo, Francesco Gianfagna, Mariarosaria Persichillo, Teresa Di Prospero, Jos Vermylen, Ignacio De Paula Carrasco, Antonio Spagnuolo, Deodato Assanelli, Vincenzo Centritto, Simona Costanzo, Marco Olivieri, Teresa Panzera, Augusto Di Castelnuovo, Marialaura Bonaccio, Simona Costanzo, Simona Esposito, Alessandro Gialluisi, Francesco Gianfagna, Emilia Ruggiero, Amalia De Curtis, Sara Magnacca, Benedetta Izzi, Annalisa Marotta, Fabrizia Noro, Roberta Parisi, Alfonsina Tirozzi, Mariarosaria Persichillo, Francesca Bracone, Francesca De Lucia, Cristiana Mignogna, Teresa Panzera, Livia Rago, and Americo Bonanni
References
- 1.Nimalaratne C, Wu J. Hen egg as an antioxidant food commodity: a review. Nutrients. 2015;7(10):8274–8293. doi: 10.3390/nu7105394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shekelle RB, Stamler J. Dietary cholesterol and ischaemic heart disease. Lancet (London, England) 1989;1(8648):1177–1179. doi: 10.1016/s0140-6736(89)92759-1. [DOI] [PubMed] [Google Scholar]
- 3.BHCA (2009) Cholesterol fact sheet. http://www.betterhealth.vic.gov.au/bhcv2/bhcarticles.nsf/pages/Cholesterol_explained. Accessed Sept 2020
- 4.Food and Agricultural Organization of the United Nations. Food based dietary guidelines by country (2009) http://www.fao.org/ag/humannutrition/nutritioneducation/fbdg/en/. Accessed Sept 2020
- 5.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102(18):2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 6.https://health.gov/dietaryguidelines/2015-scientific-report/pdfs/scientific-report-of-the-2015-dietary-guidelines-advisory-committee.pdf. Accessed Sept 2020
- 7.Mediterranean Diet Foundation (2010) What’s the Mediterranean diet? Internet: http://dietamediterranea.com/en/nutrition. Accessed Sept 2020
- 8.https://www.crea.gov.it/web/alimenti-e-nutrizione/-/linee-guida-per-una-sana-alimentazione-2018. Accessed Sept 2020
- 9.https://www.nhs.uk/live-well/eat-well/eggs-nutrition/. Accessed Sept 2020
- 10.Zazpe I, Beunza JJ, Bes-Rastrollo M, Warnberg J, de la Fuente-Arrillaga C, Benito S, Vázquez Z, Martínez-González MA, SUN Project Investigators Egg consumption and risk of cardiovascular disease in the SUN Project. Eur J Clin Nutr. 2011;65(6):676–682. doi: 10.1038/ejcn.2011.30. [DOI] [PubMed] [Google Scholar]
- 11.Qin C, Lv J, Guo Y, Bian Z, Si J, Yang L, Chen Y, Zhou Y, Zhang H, Liu J, Chen J, Chen Z, Yu C, Li L. Associations of egg consumption with cardiovascular disease in a cohort study of 0.5 million Chinese adults. Heart (British Cardiac Society) 2018;104(21):1756–1763. doi: 10.1136/heartjnl-2017-312651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Zhou C, Zhou X, Li L. Egg consumption and risk of cardiovascular diseases and diabetes: a meta-analysis. Atherosclerosis. 2013;229(2):524–530. doi: 10.1016/j.atherosclerosis.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Rong Y, Chen L, Zhu T, Song Y, Yu M, Shan Z, Sands A, Hu FB, Liu L. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ (Clinical research ed) 2013;346:e8539. doi: 10.1136/bmj.e8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin JY, Xun P, Nakamura Y, He K. Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(1):146–159. doi: 10.3945/ajcn.112.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L, Lam TH, Jiang CQ, Zhang WS, Zhu F, Jin YL, Woo J, Cheng KK, Thomas GN. Egg consumption and the risk of cardiovascular disease and all-cause mortality: Guangzhou Biobank Cohort Study and meta-analyses. Eur J Nutr. 2019;58(2):785–796. doi: 10.1007/s00394-018-1692-3. [DOI] [PubMed] [Google Scholar]
- 16.Marventano S, Godos J, Tieri M, Ghelfi F, Titta L, Lafranconi A, Gambera A, Alonzo E, Sciacca S, Buscemi S, Ray S, Del Rio D, Galvano F, Grosso G. Egg consumption and human health: an umbrella review of observational studies. Int J Food Sci Nutr. 2020;71(3):325–331. doi: 10.1080/09637486.2019.1648388. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura Y, Okamura T, Kita Y, Okuda N, Kadota A, Miura K, Okayama A, Ueshima H, NIPPON DATA90 Research Group Re-evaluation of the associations of egg intake with serum total cholesterol and cause-specific and total mortality in Japanese women. Eur J Clin Nutr. 2018;72(6):841–847. doi: 10.1038/s41430-017-0051-4. [DOI] [PubMed] [Google Scholar]
- 18.Farvid MS, Malekshah AF, Pourshams A, Poustchi H, Sepanlou SG, Sharafkhah M, Khoshnia M, Farvid M, Abnet CC, Kamangar F, Dawsey SM, Brennan P, Pharoah PD, Boffetta P, Willett WC, Malekzadeh R. Dietary protein sources and all-cause and cause-specific mortality: the Golestan cohort study in Iran. Am J Prev Med. 2017;52(2):237–248. doi: 10.1016/j.amepre.2016.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dehghan M, Mente A, Rangarajan S, Mohan V, Lear S, Swaminathan S, Wielgosz A, Seron P, Avezum A, Lopez-Jaramillo P, et al. Association of egg intake with blood lipids, cardiovascular disease, and mortality in 177,000 people in 50 countries. Am J Clin Nutr. 2020;111(4):795–803. doi: 10.1093/ajcn/nqz348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamora-Ros R, Cayssials V, Cleries R, Redondo ML, Sánchez MJ, Rodríguez-Barranco M, Sánchez-Cruz JJ, Mokoroa O, Gil L, Amiano P, Navarro C, Chirlaque MD, Huerta JM, Barricarte A, Ardanaz E, Moreno-Iribas C, Agudo A. Moderate egg consumption and all-cause and specific-cause mortality in the Spanish European Prospective into Cancer and Nutrition (EPIC-Spain) study. Eur J Nutr. 2019;58(5):2003–2010. doi: 10.1007/s00394-018-1754-6. [DOI] [PubMed] [Google Scholar]
- 21.Trichopoulou A, Psaltopoulou T, Orfanos P, Trichopoulos D. Diet and physical activity in relation to overall mortality amongst adult diabetics in a general population cohort. J Intern Med. 2006;259(6):583–591. doi: 10.1111/j.1365-2796.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- 22.Di Castelnuovo A, Costanzo S, Persichillo M, Olivieri M, de Curtis A, Zito F, Donati MB, de Gaetano G, Iacoviello L, MOLI-SANI Project Investigators Distribution of short and lifetime risks for cardiovascular disease in Italians. Eur J Prevent Cardiol. 2012;19(4):723–730. doi: 10.1177/1741826711410820. [DOI] [PubMed] [Google Scholar]
- 23.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(Suppl 1):S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 24.Salvini S, Parpinel M, Gnagnarella P, Maissoneuve P, Turrini A. Banca dati composizione degli alimenti per studi epidemiologici in Italia. Milano: European Institute of Oncology; 1998. [Google Scholar]
- 25.Pala V, Sieri S, Palli D, Salvini S, Berrino F, Bellegotti M, Frasca G, Tumino R, Sacerdote C, Fiorini L, Celentano E, Galasso R, Krogh V. Diet in the Italian EPIC cohorts: presentation of data and methodological issues. Tumori. 2003;89(6):594–607. doi: 10.1177/030089160308900603. [DOI] [PubMed] [Google Scholar]
- 26.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599e608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 27.Iacoviello L, Bonanni A, Costanzo S, De Curtis A, Di Castelnuovo A, Olivieri M, Zito F, Donati M, de Gaetano G, on behalf of the Moli-sani project Investigators The Moli-Sani Project, a randomized, prospective cohort study in the Molise region in Italy; design, rationale and objectives. Italian J Public Health. 2007;4:110–118. doi: 10.2427/5886. [DOI] [Google Scholar]
- 28.ISTAT: Istituto Nazionale di Statistica, Atlante statistico dei comuni. Edizione 2014. https://www.istat.it/it/archivio/113712. Accessed Sept 2020
- 29.Hertzmark E, Pazaris M, Spiegelman D. The SAS MEDIATE Macro. Boston: Harvard T.H. Chan School of Public Health; 2012. [Google Scholar]
- 30.Food Composition Database for Epidemiological Studies in Italy” by Gnagnarella P, Salvini S, Parpinel M. Version 1. 2015 Website http://www.bda-ieo.it/. Accessed Sept 2020
- 31.Tong TYN, Appleby PN, Key TJ, Dahm CC, Overvad K, Olsen A, Tjønneland A, Katzke V, Kühn T, Boeing H, et al. The associations of major foods and fibre with risks of ischaemic and haemorrhagic stroke: a prospective study of 418 329 participants in the EPIC cohort across nine European countries. Eur Heart J. 2020;41(28):2632–2640. doi: 10.1093/eurheartj/ehaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong VW, Van Horn L, Cornelis MC, Wilkins JT, Ning H, Carnethon MR, Greenland P, Mentz RJ, Tucker KL, Zhao L, Norwood AF, Lloyd-Jones DM, Allen NB. Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA. 2019;321(11):1081–1095. doi: 10.1001/jama.2019.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djoussé L, Gaziano JM. Egg consumption in relation to cardiovascular disease and mortality: the physicians' health study. Am J Clin Nutr. 2008;87(4):964–969. doi: 10.1093/ajcn/87.4.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia PF, Pan XF, Chen C, Wang Y, Ye Y, Pan A. Dietary intakes of eggs and cholesterol in relation to all-cause and heart disease mortality: a prospective cohort study. J Am Heart Assoc. 2020;9(10):e015743. doi: 10.1161/JAHA.119.015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drouin-Chartier JP, Chen S, Li Y, Schwab AL, Stampfer MJ, Sacks FM, Rosner B, Willett WC, Hu FB, Bhupathiraju SN. Egg consumption and risk of cardiovascular disease: three large prospective US cohort studies, systematic review, and updated meta-analysis. BMJ (Clinical research ed) 2020;368:m513. doi: 10.1136/bmj.m513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godos J, Micek A, Brzostek T, Toledo E, Iacoviello L, Astrup A, Franco OH, Galvano F, Martinez-Gonzalez MA, Grosso G (2020) Egg consumption and cardiovascular risk: a dose-response meta-analysis of prospective cohort studies. Eur J Nutr. 10.1007/s00394-020-02345-7. Advance online publication. 10.1007/s00394-020-02345-7 [DOI] [PMC free article] [PubMed]
- 37.Keum N, Lee DH, Marchand N, Oh H, Liu H, Aune D, Greenwood DC, Giovannucci EL. Egg intake and cancers of the breast, ovary and prostate: a dose-response meta-analysis of prospective observational studies. Br J Nutr. 2015;114(7):1099–1107. doi: 10.1017/S0007114515002135. [DOI] [PubMed] [Google Scholar]
- 38.Soliman GA. Dietary cholesterol and the lack of evidence in cardiovascular disease. Nutrients. 2018;10(6):780. doi: 10.3390/nu10060780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lecerf JM, de Lorgeril M. Dietary cholesterol: from physiology to cardiovascular risk. Br J Nutr. 2011;106(1):6–14. doi: 10.1017/S0007114511000237. [DOI] [PubMed] [Google Scholar]
- 40.Wu JH, Zheng M, Catterall E, Downs S, Thomas B, Veerman L, Barendregt JJ. Contribution of trans-fatty acid intake to coronary heart disease burden in Australia: a modelling study. Nutrients. 2017;9(1):77. doi: 10.3390/nu9010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tharrey M, Mariotti F, Mashchak A, Barbillon P, Delattre M, Fraser GE. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: the Adventist Health Study-2 cohort. Int J Epidemiol. 2018;47(5):1603–1612. doi: 10.1093/ije/dyy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonaccio M, Gianfagna F, de Gaetano G, Iacoviello L. Too many individuals are unaware of their blood lipid levels, but might still get health benefit from the Mediterranean diet through lipid-independent mechanisms. Eur J Prevent Cardiol. 2019;26(18):1953–1956. doi: 10.1177/2047487319867782. [DOI] [PubMed] [Google Scholar]
- 43.Tsoupras A, Lordan R, Zabetakis I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients. 2018;10(5):604. doi: 10.3390/nu10050604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giampaoli S, Palmieri L, Donfrancesco C, Panico S, Pilotto L, Addis A, Boccanelli A, Di Pasquale G, Brignoli O, Filippi A, Vanuzzo D, Gruppo di Ricerca dell'Osservatorio Epidemiologico Cardiovascolare La valutazione del rischio cardiovascolare globale assoluto: confronto tra carta e punteggio del Progetto CUORE [Assessment of the absolute global cardiovascular risk comparison between the risk chart and the individual score of the CUORE Project] G Ital Cardiol. 2006;7(5):359–364. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.