Abstract
Background
Clinically, metastatic rectal cancer has been considered a subset of left-sided colon cancer. However, heterogeneity has been proposed to exist between high and middle/low rectal cancers. We aimed to examine the efficacy of anti-epidermal growth factor receptor (EGFR) treatment for middle/low rectal and left-sided colon cancers.
Methods
This study enrolled 609 patients with metastatic colorectal cancer who were treated with anti-EGFR therapy. They were divided into groups based on primary tumour locations: the right-sided colon, the left-sided colon or the middle/low rectum. The efficacy of first-line and non-first-line anti-EGFR treatment was analysed. Genomic differences in colorectal cancer data from The Cancer Genome Atlas (TCGA) were investigated and visualised with OncoPrint and a clustered heatmap.
Results
On first-line anti-EGFR treatment, patients with middle/low rectal tumours had significantly lower progression-free survival, overall survival, and overall response rates (6.8 months, 27.8 months and 43%, respectively) than those with left-sided colon cancer (10.1 months, 38.3 months and 66%, respectively). Similar outcomes were also identified on non-first-line anti-EGFR treatment. In TCGA analysis, rectal tumours displayed genetic heterogeneity and shared features with both left- and right-sided colon cancer.
Conclusions
Anti-EGFR treatment has lower efficacy in metastatic middle/low rectal cancer than in left-sided colon cancer.
Subject terms: Colorectal cancer, Colorectal cancer, Tumour biomarkers, Colorectal cancer
Introduction
Colorectal cancer (CRC) originating from the right and left side of the colon differs in embryologic, epidemiologic, genetic and molecular aspects [1–4]. In the era of targeted therapy, primary tumour location has been found to play an important role in predicting the treatment response and prognosis of metastatic CRC (mCRC). Patients with left-sided mCRC (tumours originating in the splenic flexure, the descending colon, the sigmoid colon, the rectosigmoid junction and sometimes even the rectum) have been demonstrated to have better survival benefits than patients with right-sided mCRC (tumours originating in the caecum, the ascending colon, the hepatic flexure and the transverse colon) [5–7]. Furthermore, the sidedness of primary tumours also determines the efficacy of targeted therapy in treating mCRC, as is the case for anti-epidermal growth factor receptor (EGFR) agents [8–11]. These results were obtained by retrospective analyses of CALGB/SWOG 80405, CRYSTAL and FIRE-3 [12, 13]. In recent years, a comprehensive pooled analysis of six large-scale clinical trials suggested the clear clinical benefit and superior treatment effect of first-line anti-EGFR therapy in patients with RAS-wild-type (RAS-wt) left-sided mCRC [14].
In terms of mCRC, the rectum has been commonly categorised as part of the left-sided colon in previous trials and research and treated according to the same treatment principles described in the ESMO and NCCN guidelines [15, 16]. However, with the advent of personalised medicine, differences in treatment performance between rectal and left-sided colon tumours have been identified. The left-sided colon and rectum are not merely anatomically and pathologically different [17, 18]. Tumour location (right-sided colon, left-sided colon and rectum) was suggested to serve as a prognostic factor in stage III CRC in a recent study [19]. Furthermore, in patients with mCRC treated with anti-EGFR agents, a higher overall response rate is achieved in left-sided colon tumours than in rectal tumours [20, 21].
The definition of rectal cancer itself, which could differ surgically, anatomically, and/or biologically, remains inconclusive [22, 23]. According to the latest ESMO guidelines, rectal cancer is divided into high, middle and low rectal cancer (>10–15, ≥5–10 and <5 cm from the anal verge, respectively) [15]. Moreover, data have suggested that high rectal tumours have similar characteristics to left-sided CRC, including different lymphatic drainage and vascular supply systems, distinguishing metastatic patterns, response to adjuvant chemotherapy and even survival outcomes [18, 24–28]. Therefore, whether tumours located 10–15 cm from the anal verge should be defined as colon or rectal tumours is still under debate. In clinical practice, during our multidisciplinary team meetings, middle/low rectal cancer was noted to be refractory to anti-EGFR therapy to some extent, and the efficacy of this therapy for middle/low rectal cancer was below expectations compared to left-sided colon cancer. However, there is no current large-scale research investigating this issue.
In our study, we aimed to compare the efficacy of anti-EGFR therapy in treating metastatic middle/low rectal cancers and left-sided colon cancers. Right-sided colon cancers were also added for a complete analysis of the impact of primary tumour locations. CRC data from The Cancer Genome Atlas (TCGA) were analysed to validate our clinical results at a molecular level.
Methods
Patients
This is a retrospective large-scale cohort study. Data were collected between May 2005 and December 2019 at the Taipei Veterans General Hospital, Taiwan. Patients with pathologically confirmed mCRC were eligible for enrolment, while those with RAS and BRAF mutations were excluded due to their unsuitability for anti-EGFR treatment. Patients were classified based on their primary tumour locations, including the right-sided colon (tumours originating in the caecum, ascending colon, hepatic flexure and transverse colon), the left-sided colon (tumours originating in the splenic flexure, descending colon, sigmoid colon, rectosigmoid junction and rectum >10 cm from the anal verge), and the middle/low rectum (≤10 cm from the anal verge). Patients with different primary tumour locations were treated with chemotherapy plus anti-EGFR (cetuximab or panitumumab) or anti-vascular endothelial growth factor (VEGF, bevacizumab) agents. They were divided into two groups based on their treatment sequences: one (the first-line group) receiving anti-EGFR first and the other (the non-first-line group) receiving anti-EGFR following anti-VEGF. All patients were administered at least two cycles of anti-EGFR regimens. The inclusion flowchart is presented in detail in Supplemental Fig. S1. Basic patient clinicopathological information, including age, sex, American Joint Committee on Cancer 7th Edition stage at first presentation (AJCC stage), metastasectomy (surgical resection of metastasis with curative intent during any stage of a patient’s metastatic course), pathology, histological grade, mucinous component, signet cell component, lymphovascular invasion status, perineural invasion status, carcinoembryonic antigen (CEA, in milligrams per decilitre) level and carbohydrate antigen 19-9 (CA199, in milligrams per decilitre) level, was collected. All materials and protocols in this study were compliant with the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocols were approved by the ethics committee and institutional review board of the Taipei Veterans General Hospital.
Treatment outcomes
Median overall survival (OS) was defined as the time from diagnosis of metastasis to cancer death or loss to follow-up. Median progression-free survival (PFS) represented the time from the beginning of anti-EGFR treatment to disease progression, as confirmed by radiological images (CT, PET/CT, MRI) or intolerable toxicity. The overall response rate (ORR; the proportion of patients with confirmed complete or partial response) and disease control rate (DCR; the proportion of patients with confirmed complete response, partial response or stable disease) of metastatic tumours were recorded based on the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 [29]. Patients who achieved partial or complete response were defined as treatment responders, and those who met only the criteria for stable disease or progressive disease were considered non-responders.
Gene expression analysis
In order to clarify the genomic differences among left-sided colon, right-sided colon and rectal cancers, TCGA colorectal data were utilised as the training set to explore the differential expression of genomic features between left- and right-sided colon cancer. The significantly different expression of genomic features in the training set was compared with the potentially preventive genomic features in rectal cancer as a validation model.
The clinical and molecular information of 594 colorectal tumour samples was acquired from the publicly available “TCGA Pan-Cancer Atlas” project on the cBioPortal TCGA website [30]. Patients with RAS or BRAF mutations, non-primary tumour samples or unspecified tumour locations were excluded from the evaluation (Supplemental Fig. S2). Samples were then divided into three groups based on their locations according to ICD 10 codes (C18.0, C18.2, C18.3 and C18.4 as right-sided colon, 161 samples; C18.5, C18.6, C18.7 and C19 as left-sided colon, 126 samples; and C20 as rectal cancer, 141 samples). Genomic and epigenomic differences regarding single-nucleotide polymorphisms (SNPs), the transcriptome (mRNA) and DNA methylation were analysed. Aggregated mutation information was obtained in a mutation annotation format, and the number and overlaps of 1–100% SNP mutations between the right-sided colon, left-sided colon and rectal cancer groups were calculated. Differentially methylated region (DMR) detection was utilised to identify possible genetic silencing caused by methylation between right- and left-sided colon tumours. The result was regarded as the control group (training set) and visualised with a volcano plot and a list of heatmaps. The rectal cancer group was then evaluated and compared with the control group. Significance was set to require beta-value differences <0.15 and an adjusted P value < 0.05. Differential mRNA expression analysis was conducted with the same method. The percentage differences in mRNA expression in right- and left-sided colon tumours were first collected in the control group. Rectal tumours were then added for comparison. A false discovery rate < 0.01 and log fold change > 1 were set as the cut-off points for significance. All data were processed with TCGAbiolinks and ComplexHeatmap [31–33].
Statistical analysis
Statistical comparisons were based on nonparametric tests. The correlations between clinicopathological variables and treatment responses were analysed using the chi-square test or Fisher’s exact test, where appropriate. PFS and OS curves were calculated through the Kaplan–Meier method. As for univariable and multivariable analyses, Cox proportional hazards regression was used to determine the effects of primary tumour locations and particular factors and to obtain hazard ratios (HRs) with corresponding two-tailed 95% confidence intervals (CIs). Cox regression models were conducted in the subgroup analysis to evaluate the impact of anti-EGFR treatment in primary tumour locations and sequences stratified by baseline variables. A two-sided P value < 0.05 was regarded as statistically significant. All statistical analyses were performed using SPSS 24.0 software (IBM Co., Armonk, NY).
Results
Patients
A total of 609 patients were included in this study. The baseline characteristics of patients in the right-sided colon (n = 125), the left-sided colon (n = 313) and the middle/low rectal (n = 171) tumour groups are presented in Table 1. Among them, 39% of right-sided colon, 43% of left-sided colon and 47% of middle/low rectal tumours were treated with first-line anti-EGFR therapy. Several variables, including sex, age, AJCC stage, metastasectomy, histological grade, mucinous component, signet cell component, CEA and CA199 level, led to imbalanced datasets due to the retrospective nature of the study. The characteristics of patients treated with first-line or non-first-line anti-EGFR therapy are listed in Supplemental Tables S1 and S2.
Table 1.
Baseline characteristics of the patientsa.
| Characteristic | Middle/low rectum (n = 171) | Left-sided colon (n = 313) | Right-sided colon (n = 125) | P value |
|---|---|---|---|---|
| Sex—no. (%) | 0.042 | |||
| Male | 107 (63) | 203 (65) | 65 (52) | |
| Female | 64 (37) | 110 (35) | 60 (48) | |
| Age—no. (%) | <0.001 | |||
| <70 years | 139 (81) | 239 (76) | 78 (62) | |
| ≥70 years | 32 (19) | 74 (24) | 47 (38) | |
| Line of therapy—no. (%)b | 0.429 | |||
| First-line | 80 (47) | 136 (43) | 49 (39) | |
| Non-first-line | 91 (53) | 177 (57) | 76 (61) | |
| AJCC stage—no. (%) | <0.001g | |||
| I | 12 (7) | 0 (0) | 0 (0) | |
| II | 13 (8) | 18 (6) | 4 (3) | |
| III | 42 (25) | 50 (16) | 14 (11) | |
| VI | 104 (61) | 245 (78) | 107 (86) | |
| Metastasectomy—no. (%) | 0.003 | |||
| Yes | 55 (32) | 140 (45) | 38 (30) | |
| No | 116 (68) | 173 (55) | 87 (70) | |
| Pathology—no. (%) | 0.256g | |||
| Adenocarcinoma | 163 (95) | 304 (97) | 117 (94) | |
| Mucinous adenocarcinoma | 7 (4) | 8 (3) | 8 (6) | |
| Carcinoma | 1 (1) | 1 (0) | 0 (0) | |
| Histological grade—no. (%)c,d | <0.001 | |||
| High | 19 (13) | 11 (4) | 25 (23) | |
| Low | 130 (87) | 267 (96) | 85 (77) | |
| Mucinous component—no. (%)c | 0.007 | |||
| Yes | 26 (21) | 41 (17) | 31 (32) | |
| No | 99 (79) | 204 (83) | 65 (68) | |
| Signet cell component—no. (%)c | 0.005g | |||
| Yes | 6 (5) | 4 (2) | 9 (9) | |
| No | 118 (95) | 240 (98) | 89 (91) | |
| Lymphovascular invasion—no. (%)c,e | 0.526 | |||
| Yes | 61 (49) | 134 (54) | 53 (55) | |
| No | 64 (51) | 112 (46) | 43 (45) | |
| Perineural invasion—no. (%)c,e | 0.052 | |||
| Yes | 41 (44) | 80 (42) | 19 (27) | |
| No | 52 (56) | 109 (58) | 51 (73) | |
| Baseline CEA level—no. (%)c,f | 0.004 | |||
| <6 mg/dL | 63 (40) | 76 (26) | 44 (37) | |
| ≥6 mg/dL | 93 (60) | 217 (74) | 76 (63) | |
| Baseline CA199 level—no. (%)c,f | 0.001 | |||
| <40 mg/dL | 97 (67) | 136 (48) | 59 (52) | |
| ≥40 mg/dL | 48 (33) | 145 (52) | 55 (48) | |
AJCC American Joint Committee on Cancer staging system, CEA carcinoembryonic antigen, CA199 carbohydrate antigen 19-9, EGFR epidermal growth factor receptor, VEGF vascular endothelial growth factor.
aPercentages may not add up to exactly 100% due to rounding.
bThe first-line group received anti-EGFR agents (cetuximab or panitumumab) first, and the non-first-line group received anti-EGFR agents after anti-VEGF agents (bevacizumab).
cThe sum of the groups within this variable may be not the same as the total number of patients due to incomplete pathology reports or serum biochemistry data for a variety of reasons.
dPoorly differentiated or undifferentiated tumours are counted as high-histological-grade tumours. Well-differentiated or moderately differentiated tumours are counted as low histological-grade tumours.
eBoth intramural and extramural invasions are counted.
fThe cut-offs for CEA and CA199 are based on the normal reference ranges for cancer surveys at our centre.
gP value by Fisher’s exact test.
Efficacy
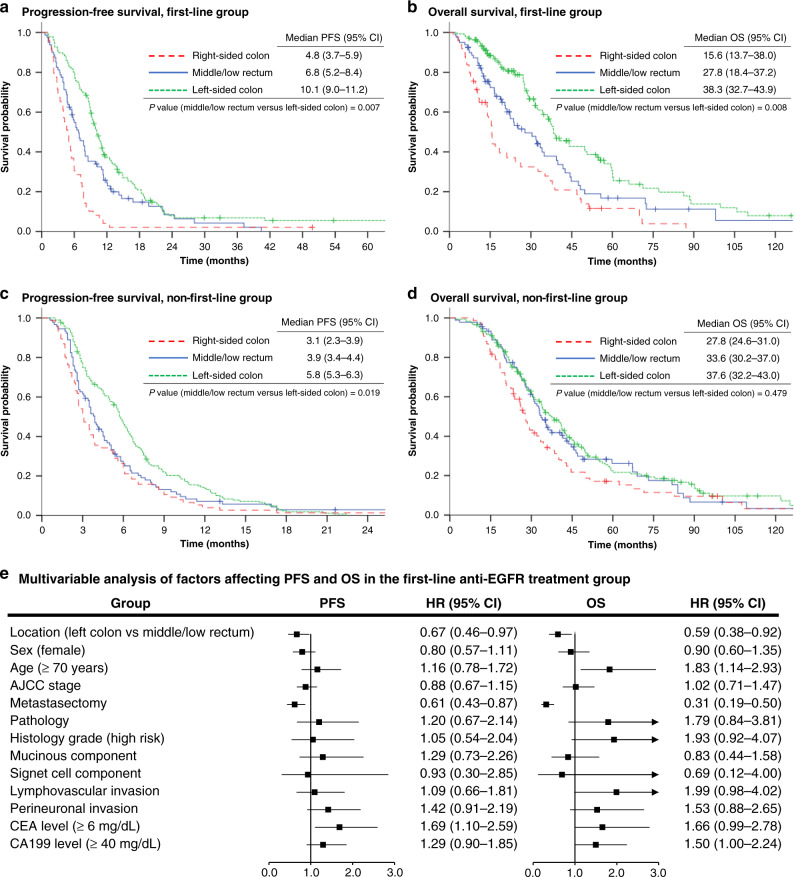
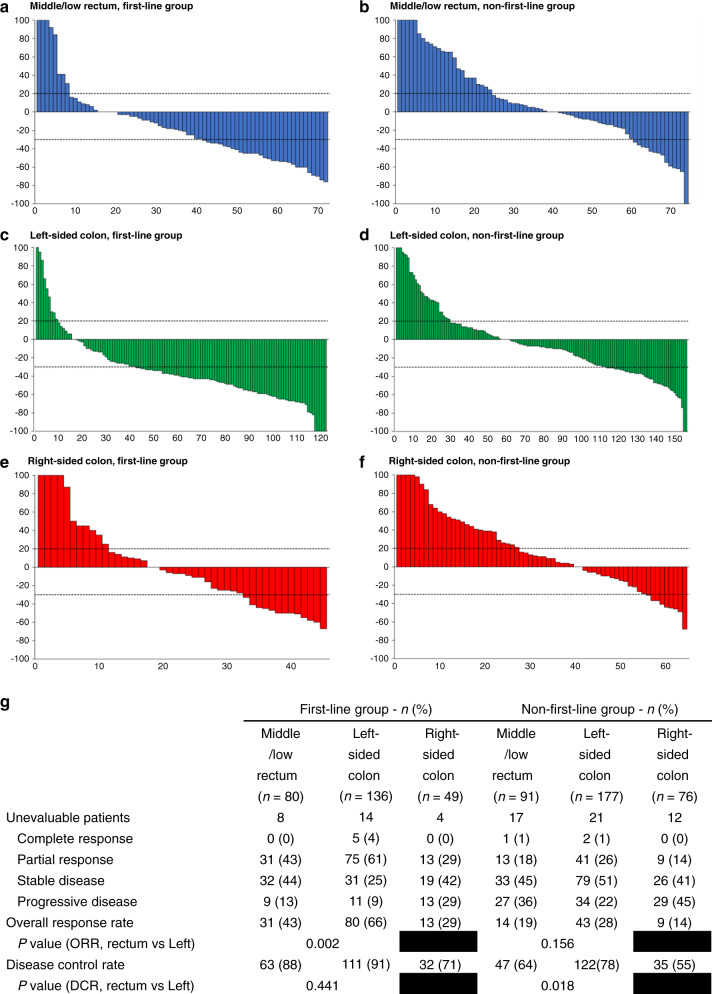
The PFS, OS, ORR and DCR results from different groups based on primary tumour location and lines of anti-EGFR treatment are presented in Figs. 1 and 2, with waterfall plots showing the best percentage change in metastatic lesions from baseline.
Fig. 1. Progression-free survival and overall survival.
a, b represent the progression-free survival (PFS) and overall survival (OS) of the first-line anti-epidermal growth factor receptor (EGFR) treatment group by different primary tumour locations. c, d represent the PFS and OS of the non-first-line anti-EGFR treatment group by different primary tumour locations. e shows the results of multivariate analyses of PFS and OS in patients treated with first-line anti-EGFR therapy. AJCC American Joint Committee on Cancer staging system, CEA carcinoembryonic antigen, CA199 carbohydrate antigen 19-9, HR hazard ratio, CI confidence interval.
Fig. 2. Best percentage change in the size of metastasis and best response in patients.
a–f show the waterfall plots of the best percentage change in the size of target lesions in each patient from the six groups. The analysis is based on Response Evaluation Criteria in Solid Tumours, version 1.1 (RECIST 1.1). The dashed lines at 20% and −30% represent progressive disease and partial response. The line at 0% means either no changes in metastatic sites or the development of a new lesion. g shows the tumour response in patients in different groups and is also evaluated based on RECIST 1.1. n number, ORR overall response rate, DCR disease control rate.
In terms of the first-line anti-EGFR therapy group, PFS (Fig. 1a: 10.1 months vs 6.8 months, HR: 0.67, 95% CI: 0.46–0.89, P = 0.007), OS (Fig. 1b: 38.3 months vs 27.8 months, HR: 0.62, 95% CI: 0.44–0.88, P = 0.008), and ORR (Fig. 2g: 66% vs 43%, P = 0.002) were significantly higher in the left-sided CRC group than in the middle/low rectal tumour group. Significantly longer PFS (Fig. 1c: 5.8 months vs 3.9 months, HR: 0.73, 95% CI: 0.56–0.95, P = 0.019) and DCR (Fig. 2g: 78% vs 64%, P = 0.018) were also observed in patients with left-sided tumours compared to middle/low rectal tumours under non-first-line anti-EGFR therapy. Conversely, a comparable OS outcome was observed between left-sided tumours and middle/low rectal tumours (Fig. 1d: 37.6 months vs 33.6 months, HR: 0.90, 95% CI: 0.68–1.20, P = 0.479) under non-first-line anti-EGFR therapy. The group of patients with right-sided CRC showed the worst PFS, OS, ORR and DCR in both first-line and non-first-line anti-EGFR treatment. The characteristics of treatment responders and non-responders among middle/low rectal cancer patients are summarised in Supplemental Table S3.
Further analysis of the traditionally defined rectal cancer group (with a cut-off of 15 cm from the anal verge) revealed no significant differences in PFS (8.0 months vs 9.3 months, P = 0.136) and DCR (90% vs 90%, P = 0.949) between the left-sided colon and rectum groups under first-line anti-EGFR treatment (Supplemental Tables S4 and S5).
Factors affecting PFS and OS in mCRC
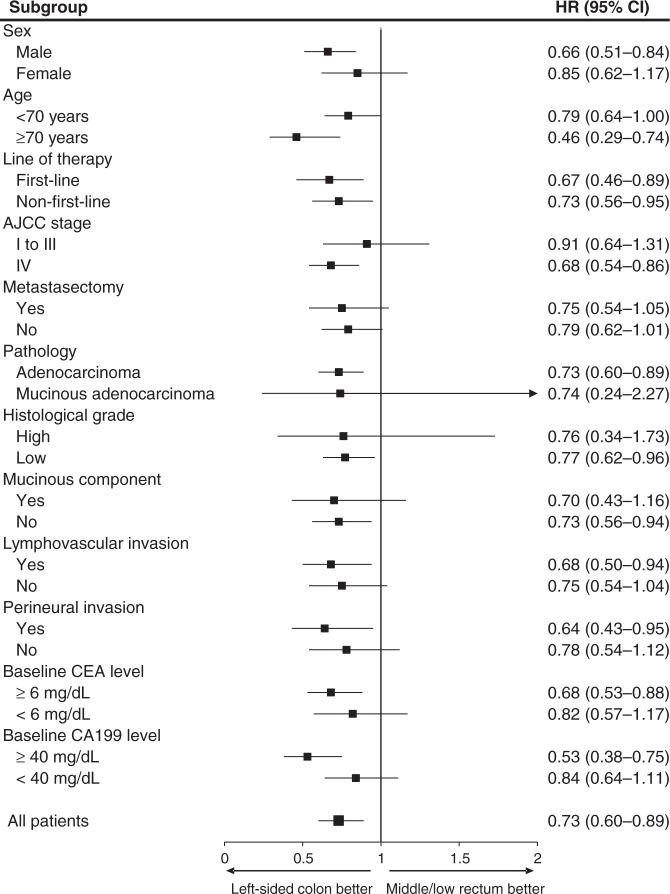
In order to classify the prognostic role of the primary tumour locations in patients with anti-EGFR treatment, univariate and multivariate analyses were performed (Fig. 1e and Supplemental Tables S6, S7, S8). After adjustment for confounding factors, the middle/low rectal tumour group was identified as an independent factor associated with worse treatment response and prognosis, including shorter PFS and OS under first-line therapy and shorter PFS under non-first-line therapy. In several subgroups, including the subgroups defined by male sex, age ≥70 years, AJCC stage IV, adenocarcinoma pathology, low histological grades, lack of a mucinous component, presence of lymphovascular or perineural invasion, and CEA ≥ 6 mg/dL or CA199 ≥ 40 mg/dL, patients with left-sided colon cancer had better progression-free survival than the equivalent middle/low rectal tumour subgroups (Fig. 3).
Fig. 3. Forest plot of progression-free survival for anti-EGFR treatment recipients with left-sided colon versus middle/low rectal tumours among different subgroups.
EGFR epidermal growth factor receptor, AJCC American Joint Committee on Cancer staging system, CEA carcinoembryonic antigen, CA199 carbohydrate antigen 19-9, HR hazard ratio, CI confidence interval.
OS according to primary tumour locations and treatment sequences in mCRC patients
Subgroup analysis of OS based on primary tumour locations and sequences of anti-EGFR treatment was also performed in all mCRC patients and the subpopulation of middle/low rectal cancer patients. For both groups, patients without metastasectomy showed superior prognostic results when treated with non-first-line therapy (Supplemental Figs. S3 and S4). Clinical details on patients undergoing metastasectomy in general and hepatic metastasectomy, in particular, are listed in Supplemental Tables S9 and S10.
Epigenomic and genomic difference analysis
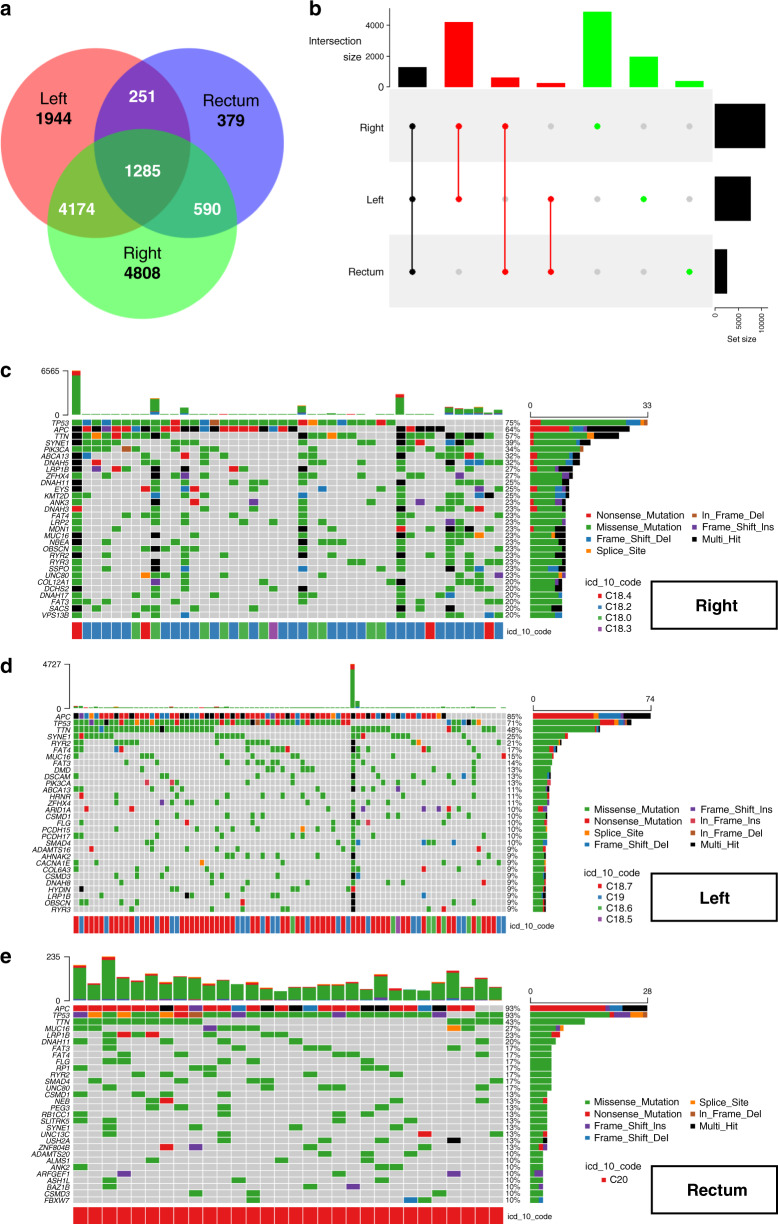
Genetic bioinformation in the mutation annotation format from 161 tumour samples with 1–100% SNP mutations was grouped by primary tumour location and visualised. The Venn diagram (Fig. 4a) and UpSet plot (Fig. 4b) revealed that right-sided colon tumours (n = 4808) had more SNP mutations than left-sided colon (n = 1944) or rectal (n = 379) tumours. There were more SNP overlaps between rectal and right-sided colon tumours (n = 590) than between rectal and left-sided colon tumours (n = 251). The genomic distribution and types of SNPs based on tumour locations are detailed in Fig. 4c–e. The top 30 most frequently mutated genes, along with their frequencies and mutation types, are reported in the columns.
Fig. 4. Overlaps and details of SNP mutations among different tumour locations.
A Venn diagram (a) and an UpSet plot (b) show the number of overlaps among different primary tumour locations. c–e represent the OncoPrints of the right-sided colon, left-sided colon, and rectal tumours, respectively. The top 30 mutated genes and their mutational types and percentages are visualised in detail.
For the epigenomic DNA methylation analysis, a volcano plot (Fig. 5a) and a list of heatmaps (Fig. 5b) revealed the DMRs among right- and left-sided colon tumours. Generally, differences in hyper- and hypomethylation of genes were easily observed between right- and left-sided colon cancer. Rectal cancer was introduced into the analysis, and its similarities and differences with right- and left-sided colon cancer are described in Fig. 5c and Supplemental Fig. S5. The same method of mRNA expression analysis was also performed and visualised in Supplemental Figs. S6 and S7. Comparisons across the three tumour groups are illustrated as a clustered heatmap (Fig. 5d) or a list of heatmaps (Supplemental Fig. S8). The rectal cancer group revealed a heterogeneous distribution, showing similarities in both DMR and differential mRNA expression with the left- and right-sided colon cancer groups. Other SNP, DMR and mRNA epigenomic and genomic details are listed in the Dataset file.
Fig. 5. Differences in DNA methylation and mRNA expression among different tumour locations.
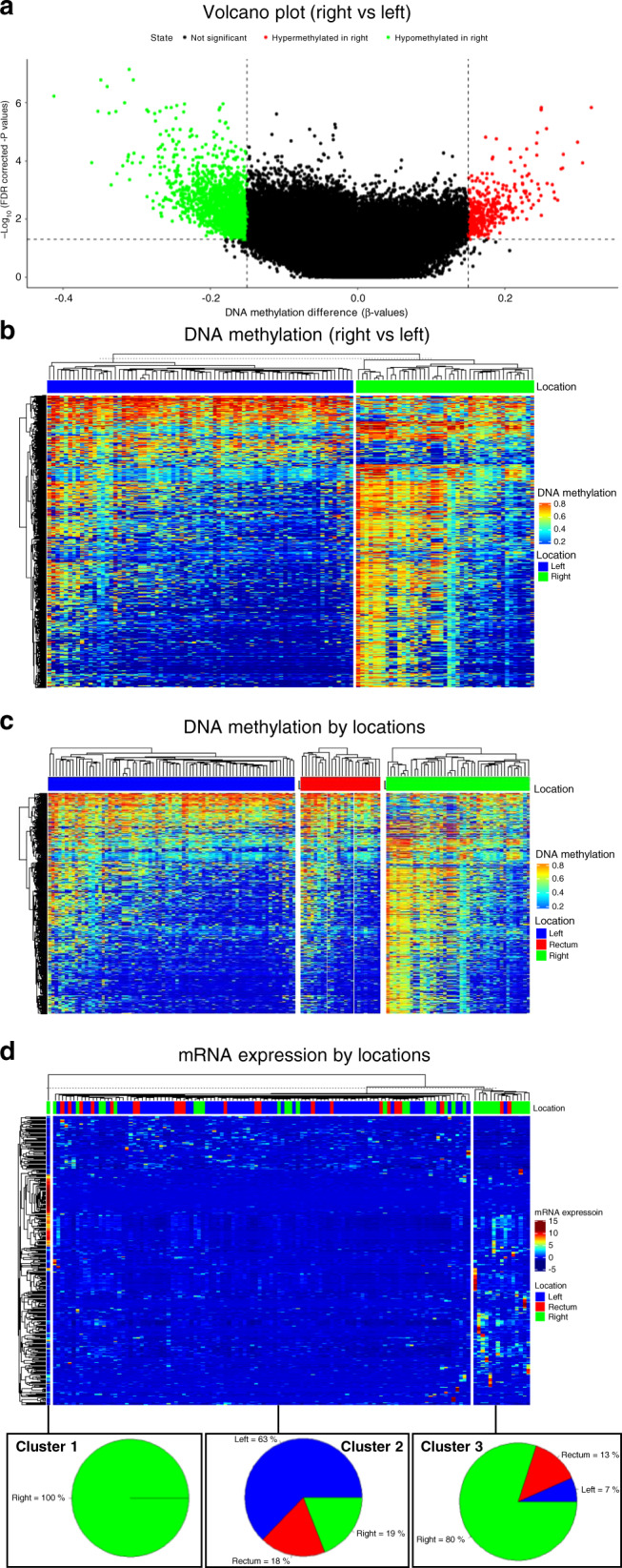
A volcano plot (a) and a list of heatmaps (b) were used to demonstrate the DMR between the right and left-sided colon. A list of heatmaps (c) was made after adding rectal tumours for comparison. A clustered heatmap (d) of mRNA expression differences was generated by k-means clustering (k = 3) based on the above steps.
Discussion
Primary tumour location has been considered a useful biomarker to predict the prognosis and the treatment effects of bioagents in mCRC. Although the rectum is routinely grouped in the left-sided colon in current treatment guidelines, this large-scale retrospective study, nevertheless, demonstrates that anti-EGFR therapy has lower efficacy in metastatic middle/low rectal cancer than in left-sided colon cancer, potentially leading to better clinical practice principles for mCRC management.
In our TCGA investigation, distinctions in a genomic level between the rectal and the left-sided colon tumours are identified. The molecular differences serve some possible explanations to our clinical findings. In Fig. 4a, b, the Venn diagram and UpSet plot demonstrate that rectal tumours have more SNP overlaps with right-sided than with left-sided colon tumours. In addition, the OncoPrint in Fig. 4c–e reveals that right-sided colon tumours (20–75%, median = 23%) have the highest mutation rates in their top 30 mutated genes, followed by rectal (10–93%, median = 13%) and left-sided colon (9–85%, median = 10%) tumours. These results indicate that the rectum is not substantially identical to the left-sided colon. From the following analyses of epigenetic DNA methylation and mRNA expression, a list of heatmaps and a clustered heatmap (k-means clustering, k = 3) are presented in Fig. 5c, d. Rectal cancer showed features resembling those of both left- and right-sided colon cancers. To our knowledge, the growing evidence supports the notion that genomic heterogeneity exists between the colon and the rectal tumours. For example, increased TOPO1 and ERCC1 expression, HER2/neu amplification, and KRAS mutation rates in rectal cancer compared with right- and left-sided colon cancer were reported [34, 35]. Lee et al. also described a linear tendency of the decreased mutation incidence for the TGF-β, PI3K and RTK-RAS pathways from the right-sided colon to rectal tumours [36].
Clinically, the differential efficacy of anti-EGFR therapy between the rectal and the left-sided colon tumours was proposed in the previous literature. The analysis of a Phase III trial of panitumumab (PICCOLO) reported similar PFS outcomes between rectal and right-sided colon cancers in RAS-wt mCRC patients and mentioned that the benefit of anti-EGFR agents against rectal cancers may be overestimated [37]. Loupakis et al. observed a reduced OS (19.6 vs 22.8 months, P = 0.028) and ORR (35.9% vs 48.9%, P = 0.019) of rectal tumours in the AVF2107g trial [20]. Another retrospective analysis of two Phase II Spanish trials described a reduced unconfirmed ORR (64% vs 80%, CI = 0.2–0.9) but similar PFS and OS in the rectal tumour group treated with anti-EGFR [21], which is different from our results to some degree. The main reason might be that different definitions of rectal cancer were used. Although the traditional classification of rectal cancer is within 15 cm of the anal verge, emerging studies regard the high rectum as a part of the left-sided colon in terms of pathological, physiological and clinical outcomes. A meta-analysis including 1196 patients with stage II or III rectal cancer suggested that tumours located within 10–15 cm from the anal verge might benefit from adjuvant chemotherapy, with higher disease-free survival and lower distant recurrence rate than middle/low rectal tumours [24]. Several retrospective studies also reported that OS and disease-free survival in high rectal cancer were similar to those in sigmoid colon cancer but superior to those in middle/low rectal cancer in non-metastatic CRC without adjuvant therapy [18, 26, 28]. Hence, in this study, tumours within 10 cm from the anal verge were assigned to the middle/low rectal tumour group, while those within 10–15 cm were assigned to the left-sided colon group. Our findings (Figs. 1 and 2) supported the assumption of worse PFS, OS and ORR among the middle/low rectal tumour group than among the left-sided colon group. The negative prognostic impact of middle/low rectal cancer in patients treated with first-line anti-EGFR was found to be independent in multivariate analyses of PFS and OS (Fig. 1e). However, regarding the clinical features of responding and non-responding middle/low rectal cancer patients under first-line anti-EGFR treatment, no significant differences were found (Supplemental Table S3).
Analyses of left-sided colon and rectal tumours were also performed using the traditional cut-off point (15 cm from the anal verge) with the aim of testing our hypothesis (Supplemental Tables S4 and S5). The results were similar to those reported by Loupakis et al. and Benavides et al., whereby rectal tumours were associated specifically with worse OS (32.1 months vs 38.3 months, P = 0.010) and ORR (49% vs 64%, P = 0.035) than left-sided colon tumours, while no significant differences in PFS (8.0 months vs 9.3 months, P = 0.136) and DCR (90% vs 90%, P = 0.949) were seen in the first-line anti-EGFR treatment group [20, 21]. Meanwhile, there were no significant differences in any of those variables in the non-first-line treatment group.
In several previous studies, anti-EGFR as a first-line treatment has shown higher efficacy and a greater survival benefit than anti-VEGF in treating left-sided, RAS-wt and mCRC patients [14]. However, there was a trend toward shorter OS in middle/low rectal cancer patients who received anti-EGFR treatment as the first line than in those who received non-first-line EGFR treatment (27.8 months vs 33.6 months, P = 0.124). Thus, the guiding principles for mCRC treatment selection according to primary tumour location, especially tumours in the middle/low rectum, need further evaluation and confirmation.
Subgroup analyses for all patients (Supplemental Fig. S3) and the limited population of patients with middle/low rectal cancer (Supplemental Fig. S4) indicate that patients without metastasectomy showed higher OS in the non-first-line anti-EGFR treatment group than in the first-line group. Metastasectomy has been proposed to benefit patients with mCRC, and anti-EGFR therapy was reported to produce an improved ORR and increased resectability of metastatic lesions [38–42]. However, in our study, a reduced ORR was observed in middle/low rectal cancer treated with anti-EGFR therapy, potentially leading to the unsatisfactory OS in first-line anti-EGFR treatment. We noted with interest that a recently published article from our centre described a 32% reduction in the death risk in the left-sided colon compared with right-sided colon cancer when hepatic metastasectomy was performed on patients with colorectal liver metastasis, whereas no OS difference was noted between the rectal and right-sided colon cancer groups [43]. The correlation between metastasectomy and the nature of primary tumour locations is worth investigating when targeted agents become an important confounding factor in the prognostic results.
This study was limited by its single-centre and retrospective nature. Some pathological data were not recorded due to the unresectable condition of the primary tumours. In addition, control arms consisting of mCRC patients treated with anti-VEGF therapy or chemotherapy only were not included in this study. The particular subgroup of interest in this study, namely, patients with middle/low rectal cancers, had only a modest sample size available for analysis, and the results need to be validated by future studies. The impact of bioagent treatment sequencing and efficacy by primary tumour location requires further investigation in the form of randomised controlled trials or head-to-head comparisons. Finally, we have evaluated only the bioinformatic differences among right-sided colon and left-sided colon cancer and rectal cancer. The molecular characteristics of different parts of the rectum (high vs middle/low) remain to be investigated. Specific genomic features affecting the efficacy and resistance rate of anti-EGFR therapy should also be explored to demonstrate their relationship with primary tumour locations.
In conclusion, the differential efficacy of anti-EGFR treatment between the middle/low rectal cancers and the left-sided colon cancers has been established. Moreover, TCGA genomic analysis partially supported our findings that rectal cancer is not a homogeneous group and that it shares features with both left-sided colon and right-sided colon cancer. The usefulness of primary tumour location for the basis of anti-EGFR treatment decisions warrants further exploration.
Supplementary information
Acknowledgements
Some of the data for this study were obtained from the Cancer Registry Database, Taipei Veterans General Hospital.
Author contributions
Y-TL, C-CL, H-HL, S-CH and H-HC helped write the manuscript and collected the data; W-SC, J-KJ, S-HY, H-SW, S-CC and YC designed the study and provided the materials; K-HL and H-WT designed and arranged this study and proofread the manuscript.
Funding
This study was funded by grants from the Ministry of Science and Technology, Taiwan (MOST 106-2314-B-075-062; MOST 109-2314-B-075-081-MY3); Taipei Veterans General Hospital (110DHA0100397, V110C-189, V109C-151 to H.W.T.); and the Taiwan Clinical Oncology Research Foundation.
Data availability
The datasets generated and/or analysed during this study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This retrospective study was conducted based on population-based data from Taipei Veterans General Hospital, Taiwan. This study followed the guidelines of the Declaration of Helsinki and was approved by the ethics committee and institutional review board of Taipei Veterans General Hospital, Taiwan. The requirement for informed consent was waived due to the retrospective nature of the study.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01470-2.
References
- 1.Missiaglia E, Jacobs B, D’Ario G, Di Narzo AF, Soneson C, Budinska E, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995–2001. doi: 10.1093/annonc/mdu275. [DOI] [PubMed] [Google Scholar]
- 2.Maus MKH, Hanna DL, Stephens C, Grimminger PP, Epstein M, Astrow SH, et al. Gene expression profiles and tumor locations in colorectal cancer (left vs. right vs. rectum) J Clin Oncol. 2013;31:3527. doi: 10.1200/jco.2013.31.15_suppl.3527. [DOI] [Google Scholar]
- 3.Li Y, Feng Y, Dai W, Li Q, Cai S, Peng J. Prognostic effect of tumor sidedness in colorectal cancer: a SEER-based analysis. Clin Colorectal Cancer. 2019;18:e104–e116. doi: 10.1016/j.clcc.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261–70. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- 5.Yahagi M, Okabayashi K, Hasegawa H, Tsuruta M, Kitagawa Y. The worse prognosis of right-sided compared with left-sided colon cancers: a systematic review and meta-analysis. J Gastrointest Surg. 2016;20:648–55. doi: 10.1007/s11605-015-3026-6. [DOI] [PubMed] [Google Scholar]
- 6.Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. 2017;3:211–9. doi: 10.1001/jamaoncol.2016.4227. [DOI] [PubMed] [Google Scholar]
- 7.Price T, Beeke C, Ullah S, Padbury R, Maddern G, Roder D, et al. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer. 2014;121:830–5. doi: 10.1002/cncr.29129. [DOI] [PubMed] [Google Scholar]
- 8.von Einem JC, Heinemann V, von Weikersthal LF, Vehling-Kaiser U, Stauch M, Hass HG, et al. Left-sided primary tumors are associated with favorable prognosis in patients with KRAS codon 12/13 wild-type metastatic colorectal cancer treated with cetuximab plus chemotherapy: an analysis of the AIO KRK-0104 trial. J Cancer Res Clin Oncol. 2014;140:1607–14. doi: 10.1007/s00432-014-1678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorich MJ, Wiese MD, Rowland A, Kichenadasse G, McKinnon RA, Karapetis CS. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol. 2015;26:13–21. doi: 10.1093/annonc/mdu378. [DOI] [PubMed] [Google Scholar]
- 10.Boeckx N, Koukakis R, Op de Beeck K, Rolfo C, Van Camp G, Siena S, et al. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: results from two randomized first-line panitumumab studies. Ann Oncol. 2017;28:1862–8. doi: 10.1093/annonc/mdx119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moretto R, Cremolini C, Rossini D, Pietrantonio F, Battaglin F, Mennitto A, et al. Location of primary tumor and benefit from anti-epidermal growth factor receptor monoclonal antibodies in patients with RAS and BRAF wild-type metastatic colorectal cancer. Oncologist. 2016;21:988–94. doi: 10.1634/theoncologist.2016-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3:194–201. doi: 10.1001/jamaoncol.2016.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venook AP, Niedzwiecki D, Innocenti F, Fruth B, Greene C, O’Neil BH, et al. Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance) J Clin Oncol. 2016;34:3504. doi: 10.1200/JCO.2016.34.15_suppl.3504. [DOI] [Google Scholar]
- 14.Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713–29. doi: 10.1093/annonc/mdx175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network. Colon Cancer (Version 2.2021), https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (2021). Accessed 25 March 2021.
- 17.Tamas K, Walenkamp AME, de Vries EGE, van Vugt MATM, Beets-Tan RG, van Etten B, et al. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat Rev. 2015;41:671–9. doi: 10.1016/j.ctrv.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Chiang JM, Hsieh PS, Chen JS, Tang R, You JF, Yeh CY. Rectal cancer level significantly affects rates and patterns of distant metastases among rectal cancer patients post curative-intent surgery without neoadjuvant therapy. World J Surg Oncol. 2014;12:197. doi: 10.1186/1477-7819-12-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shida D, Inoue M, Tanabe T, Moritani K, Tsukamoto S, Yamauchi S, et al. Prognostic impact of primary tumor location in Stage III colorectal cancer-right-sided colon versus left-sided colon versus rectum: a nationwide multicenter retrospective study. J Gastroenterol. 2020;55:958–68. doi: 10.1007/s00535-020-01706-7. [DOI] [PubMed] [Google Scholar]
- 20.Loupakis F, Yang D, Yau L, Feng S, Cremolini C, Zhang W, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107:1–9. doi: 10.1093/jnci/dju427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benavides M, Diaz-Rubio E, Carrato A, Abad A, Guillen C, Garcia-Alfonso P, et al. Tumour location and efficacy of first-line EGFR inhibitors in KRAS/RAS wild-type metastatic colorectal cancer: retrospective analyses of two phase II randomised Spanish TTD trials. ESMO Open. 2019;4:e000599. doi: 10.1136/esmoopen-2019-000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salerno G, Sinnatamby C, Branagan G, Daniels IR, Heald RJ, Moran BJ. Defining the rectum: surgically, radiologically and anatomically. Colorectal Dis. 2006;8:5–9. doi: 10.1111/j.1463-1318.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 23.Wu JS. Rectal cancer staging. Clin Colon Rectal Surg. 2007;20:148–57. doi: 10.1055/s-2007-984859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015;16:200–7. doi: 10.1016/S1470-2045(14)71199-4. [DOI] [PubMed] [Google Scholar]
- 25.Clancy C, Flanagan M, Marinello F, O’Neill BD, McNamara D, Burke JP. Comparative oncologic outcomes of upper third rectal cancers: a meta-analysis. Clin Colorectal Cancer. 2019;18:e361–e367. doi: 10.1016/j.clcc.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Cheng LJ, Chen JH, Chen SY, Wei ZW, Yu L, Han SP, et al. Distinct prognosis of high versus mid/low rectal cancer: a propensity score-matched cohort study. J Gastrointest Surg. 2019;23:1474–84. doi: 10.1007/s11605-018-04072-1. [DOI] [PubMed] [Google Scholar]
- 27.Swets M, Breugom AJ, Gelderblom H, van de Velde CJH. Should rectal cancer located 10-15 cm from the anal verge be defined as colon cancer. Ann Oncol. 2017;28:664–5. doi: 10.1093/annonc/mdw620. [DOI] [PubMed] [Google Scholar]
- 28.Park JS, Sakai Y, Simon NS, Law WL, Kim HR, Oh JH, et al. Long-term survival and local relapse following surgery without radiotherapy for locally advanced upper rectal cancer: an international multi-institutional study. Medicine. 2016;95:e2990. doi: 10.1097/MD.0000000000002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Chang K, Creighton CJ, Davis C, Donehower L, Drummond J, Wheeler D, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113–20. doi: 10.1038/ng.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva T, Colaprico A, Olsen C, D’Angelo F, Bontempi G, Ceccarelli M, et al. TCGA workflow: analyze cancer genomics and epigenomics data using Bioconductor packages [version 2; peer review: 1 approved, 2 approved with reservations]. F1000Res. 2016;5. 10.12688/f1000research.8923.2. [DOI] [PMC free article] [PubMed]
- 32.Colaprico A, Silva TC, Olsen C, Garofano L, Cava C, Garolini D, et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2015;44:e71. doi: 10.1093/nar/gkv1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32:2847–9. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 34.Salem ME, Weinberg BA, Xiu J, El-Deiry WS, Hwang JJ, Gatalica Z, et al. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget. 2017;8:86356–68. doi: 10.18632/oncotarget.21169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salem ME, Yin J, Weinberg BA, Renfro LA, Pederson LD, Maughan TS, et al. Clinicopathological differences and survival outcomes with first-line therapy in patients with left-sided colon cancer and rectal cancer: pooled analysis of 2879 patients from AGITG (MAX), COIN, FOCUS2, OPUS, CRYSTAL and COIN-B trials in the ARCAD database. Eur J Cancer. 2018;103:205–13. doi: 10.1016/j.ejca.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Lee D-W, Han S-W, Cha Y, Bae JM, Kim H-P, Lyu J, et al. Association between mutations of critical pathway genes and survival outcomes according to the tumor location in colorectal cancer. Cancer. 2017;123:3513–23. doi: 10.1002/cncr.30760. [DOI] [PubMed] [Google Scholar]
- 37.Seligmann JF, Elliott F, Richman S, Hemmings G, Brown S, Jacobs B, et al. Clinical and molecular characteristics and treatment outcomes of advanced right-colon, left-colon and rectal cancers: data from 1180 patients in a phase III trial of panitumumab with an extended biomarker panel. Ann Oncol. 2020;31:1021–9. doi: 10.1016/j.annonc.2020.04.476. [DOI] [PubMed] [Google Scholar]
- 38.Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31:1931–8. doi: 10.1200/JCO.2012.44.8308. [DOI] [PubMed] [Google Scholar]
- 39.Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 40.Heinemann V, Weikersthal LFV, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S-E, et al. Randomized comparison of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment of KRAS wild-type metastatic colorectal cancer: German AIO study KRK-0306 (FIRE-3) J Clin Oncol. 2013;31:LBA3506. [Google Scholar]
- 41.Yang YH, Lin JK, Chen WS, Lin TC, Yang SH, Jiang JK, et al. Comparison of cetuximab to bevacizumab as the first-line bio-chemotherapy for patients with metastatic colorectal cancer: superior progression-free survival is restricted to patients with measurable tumors and objective tumor response-a retrospective study. J Cancer Res Clin Oncol. 2014;140:1927–36. doi: 10.1007/s00432-014-1741-0. [DOI] [PubMed] [Google Scholar]
- 42.Aloia TA, Vauthey J-N, Loyer EM, Ribero D, Pawlik TM, Wei SH, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460–7. doi: 10.1001/archsurg.141.5.460. [DOI] [PubMed] [Google Scholar]
- 43.Chen T-H, Chen W-S, Jiang J-K, Yang S-H, Wang H-S, Chang S-C, et al. Effect of primary tumor location on postmetastasectomy survival in patients with colorectal cancer liver metastasis. J Gastrointest Surg. 2020;25:650–61. doi: 10.1007/s11605-020-04855-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during this study are available from the corresponding author on reasonable request.