Abstract
Immune checkpoint inhibitors (ICIs) have resulted in improved outcomes in non-small cell lung cancer (NSCLC) patients. However, data demonstrating the efficacy of ICIs in NSCLC brain metastases (NSCLCBM) is limited. We analyzed overall survival (OS) in patients with NSCLCBM treated with ICIs within 90 days of NSCLCBM diagnosis (ICI-90) and compared them to patients who never received ICIs (no-ICI). We reviewed 800 patients with LCBM who were diagnosed between 2010 and 2019 at a major tertiary care institution, 97% of whom received stereotactic radiosurgery (SRS) for local treatment of BM. OS from BM was compared between the ICI-90 and no-ICI groups using the Log-Rank test and Cox proportional-hazards model. Additionally, the impact of KRAS mutational status on the efficacy of ICI was investigated. After accounting for known prognostic factors, ICI-90 in addition to SRS led to significantly improved OS compared to no-ICI (12.5 months vs 9.1, p < 0.001). In the 109 patients who had both a known PD-L1 expression and KRAS status, 80.4% of patients with KRAS mutation had PD-L1 expression vs 61.9% in wild-type KRAS patients (p = 0.04). In patients without a KRAS mutation, there was no difference in OS between the ICI-90 vs no-ICI cohort with a one-year survival of 60.2% vs 54.8% (p = 0.84). However, in patients with a KRAS mutation, ICI-90 led to a one-year survival of 60.4% vs 34.1% (p = 0.004). Patients with NSCLCBM who received ICI-90 had improved OS compared to no-ICI patients. Additionally, this benefit appears to be observed primarily in patients with KRAS mutations that may drive the overall benefit, which should be taken into account in the development of future trials.
Subject terms: CNS cancer, Non-small-cell lung cancer
Introduction
Lung cancer is the most common primary tumor that metastasizes to the brain, accounting for approximately 50% of brain metastases (BM)1. For patients without actionable mutations, systemic chemotherapy has shown little intracranial efficacy, and traditionally, non-small cell lung cancer (NSCLC) BM have been managed with local treatments, such as resection and radiotherapy.
Exciting developments in cancer therapeutics targeting the PD-1 or PD-L1 axis have led to the approval of multiple immune checkpoint inhibitors (ICIs) as standard of care options in the first line setting2 and as salvage options after failure of first-line chemotherapy3. However, given the historically poor prognosis of patients with NSCLCBM, these patients were excluded from many of the initial trials unless they had previously treated and stable intracranial disease2–4. Recently, several retrospective series and a single center phase 2 clinical trial have demonstrated limited intracranial efficacy with ICIs alone, ranging from 12 to 40%5–7. Additionally, there is newer evidence that a combination approach with stereotactic radiosurgery (SRS) and ICI may be more beneficial for intracranial control, as this has been shown to have better intracranial response over either therapy alone8. Additionally, many researchers are interested in possible increased rates of the abscopal effect when fractional dose radiation and ICIs are given concordantly9.
KRAS mutations are present in approximately 30% of NSCLC adenocarcinomas and have been associated with a poorer prognosis when compared to wild-type tumors10–12. Until May 2021, there was no FDA approved targeted therapies towards KRAS mutations for patients with NSCLC. Patients frequently receive ICIs as standard-of-care treatments based on PD-L1 expression, regardless of KRAS mutation status. There is also evidence that KRAS mutations lead to increased expression of PD-L1, a known predictor of response to ICIs13,14; however, impact of KRAS mutation status on treatment outcome for patients with NSCLCBM has yet to be empirically demonstrated.
In this study, we analyzed a large population of patients with NSCLCBM who were treated at a single tertiary center. We compared the survival of those who received ICI after the diagnosis of BM to those who did not receive ICI within this timeframe. Additionally, the impact of KRAS mutation status on the efficacy of ICI was investigated. We hypothesized that patients treated with ICIs after the diagnosis of BM would have an improved survival and that within the ICI group, those with KRAS mutations would have improved survival over those with KRAS wild-type disease.
Methods
Patient selection and treatment
This retrospective cohort study included patients treated for NSCLCBM at a single tertiary care center from 2010 to 2019. This study was approved by the Cleveland Clinic Foundation institutional review board and received an informed consent waiver due to the retrospective nature of the study. All methods were carried out in accordance with relevant guidelines and regulations. Patient characteristics, diagnostic information, and treatment details were abstracted from the shared electronic medical record.
All the patients’ treatments were extracted after diagnosis of the primary cancer. This included SRS, whole-brain radiation therapy (WBRT), surgery, as well as any systemic therapies, including targeted therapies, chemotherapy, and ICI. Patients’ PD-L1 expression were extracted from pathology reports of tissue, primarily from the lung. Patients who had documented EGFR or ALK mutations who received a targeted therapy towards these mutations were excluded from this analysis. Patients who underwent resection of their intracranial tumors or diagnosed with small cell lung cancer were also excluded. All treatment decisions were made by the multidisciplinary team and were based on current national guideline standards at the time. Dosing schedule for Nivolumab was 3 mg/kg or 240 mg flat dose every other week and 2 mg/kg or 200 mg flat does every three weeks or 400 mg flat dose every six weeks for pembrolizumab.
Patients were divided into three groups based on if and when they were treated with ICIs. Those treated with ICIs within 90 days of the diagnosis of BM were classified as ICI-90, and those treated with ICI greater than 90 days after diagnosis of BM were classified as G90. Patients who did not receive ICI after diagnosis of BM at any point were classified as no-ICI.
Outcome measures
Overall survival (OS) was determined from the date of diagnosis of BM until the date of death. Patients who were still alive at the day of collection or lost to follow-up were censored at the last follow-up date.
Statistical analysis
All variables included in this analysis had < 5% missing except for Karnofsky Performance Status (KPS) at diagnosis (15% missing). Several numerical variables were also grouped as categorical and both their numerical and categorical forms were evaluated. This included age (< 65 and ≥ 65), number of SRS treatments (0–1 and ≥ 2) (only 3% of patients did not receive SRS), lesion number at diagnosis (1 and ≥ 2) and KPS (< 90 and ≥ 90). Patient demographic and clinical characteristics were compared using Chi-square test for categorical variables and Kruskal–Wallis test for numerical variables. If any differences between the three groups were detected, no-ICI and ICI-90 were then compared. Times from BM diagnosis to death or last contact were calculated for OS analysis. Patients who died after 2 years or followed more than 2 years were censored at 2 years.
Patient demographics and clinical characteristics that were potentially associated with OS (p < 0.05) among BM patients who did not have surgery were identified in univariate analysis using Kaplan–Meier method and simple Cox hazard models. Since identified factors potentially associated with OS were previously reported to associate with survival or commonly included in lung cancer BM studies15, they were included in the multivariate analysis of OS between ICI-90 and no ICI. A 1:1 propensity score (PS) matched analysis between no-ICI and ICI-90 was also performed to correct potential treatment selection biases. The matched pairs were identified from a logistic regression model with the Greedy method that included age, KPS, extracranial metastases (EC-mets), lesion number, and SRS number with exact match on EC-mets. Kaplan–Meier method was utilized to compare OS between matched ICI-90 and no-ICI group.
A secondary analysis explored ICI effect on OS by KRAS status. Among patients who had a known KRAS mutation status, Cox proportional hazard model with an ICI-KRAS interaction term was utilized to determine the impact of KRAS mutation status on ICI efficacy.
All analyses were performed using SAS version 9.4, two-sided p values are presented. A p < 0.05 was considered as statistically significant.
Results
Patient characteristics
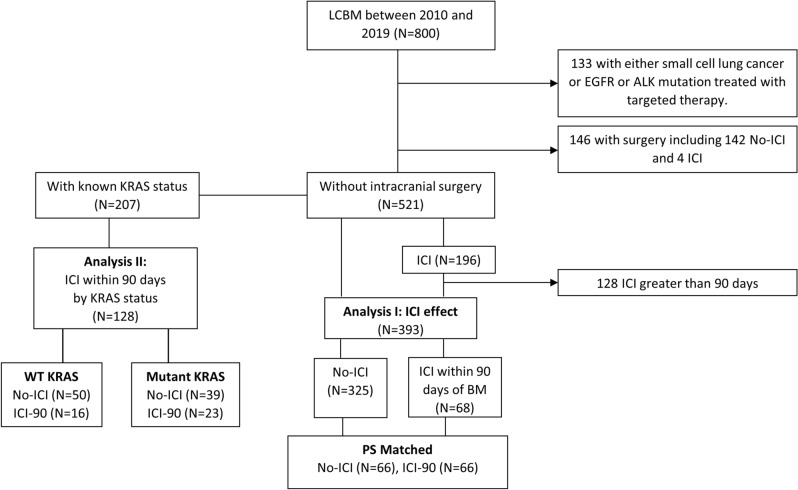
A total of 800 patients were diagnosed with lung cancer BM at our tertiary center between 2010 and 2019. Ninety-seven percent of the patients in this cohort received SRS for intracranial treatment of BM. One hundred and thirty-three patients with small cell lung cancer or NSCLC that was treated with targeted therapy against an EGFR mutation or ALK translocations were excluded from the analysis, leaving 667 patients. Additionally, 146 patients underwent an intracranial tumor resection, with four of these patients receiving ICIs versus 142 never receiving ICIs. Because of this heavily skewed distribution, these patients were excluded as well. This left 521 patients with the general characteristics outlined in Table 1. Twenty-five percent (n = 128) of patients received ICI greater than 90 days after the diagnosis of BM, 13% (n = 68) received ICI within 90 days of diagnosis of BM, and 62% (n = 325) of patients never received ICI (Fig. 1).
Table 1.
Patient characteristics at diagnosis of brain metastasis.
| Factor | Total (N = 521) | No-ICI (N = 325) | ICI-90 (N = 68) | G90 (N = 128) | p value overall/No-ICI vs IC-I90 |
|---|---|---|---|---|---|
| Age | 62.7 [56.9, 70.6] | 63.0 [57.1, 70.7] | 60.4 [54.1, 70.3] | 62.2 [57.1, 70.3] | 0.29 |
| Age, ≥ 65 | 214 (41.1) | 139 (42.8) | 22 (32.4) | 53 (41.4) | 0.28 |
| Sex: male | 262 (50.3) | 174 (53.5) | 32 (47.1) | 56 (43.8) | 0.15 |
| Race: white | 439 (84.3) | 272 (83.7) | 55 (80.9) | 112 (87.5) | 0.43 |
| KPS | 80.0 [80.0, 90.0] | 80.0 [70.0, 90.0] | 80.0 [70.0, 90.0] | 90.0 [80.0, 90.0] | < 0.001/0.86 |
| KPS ≥ 90 | < 0.001 | ||||
| < 90 | 221 (42.4) | 140 (43.1) | 38 (55.9) | 43 (33.6) | |
| ≥ 90 | 220 (42.2) | 112 (34.5) | 29 (42.6) | 79 (61.7) | |
| Unknown | 80 (15.4) | 73 (22.5) | 1 (1.5) | 6 (4.7) | |
| Lesion number | 2.0 [1.00, 3.0] | 1.00 [1.00, 3.0] | 2.0 [1.00, 3.0] | 2.0 [1.00, 3.0] | 0.27 |
| Lesion ≥ 2 | 261 (50.3) | 153 (47.4) | 37 (54.4) | 71 (55.5) | 0.23 |
| Number of SRS | 1.00 [1.00, 2.0] | 1.00 [1.00, 2.0] | 1.00 [1.00, 2.0] | 2.0 [1.00, 2.0] | < 0.001/0.64 |
| SRS ≥ 2 | 164 (32.2) | 81 (25.7) | 17 (25.4) | 66 (52.0) | < 0.001 |
| Histology: adenocarcinoma | 371 (73.9) | 216 (70.6) | 52 (76.5) | 103 (80.5) | 0.089 |
| EC-mets | 238 (46.6) | 139 (44.0) | 37 (55.2) | 62 (48.4) | 0.22 |
| WBRT | 131 (25.1) | 86 (26.5) | 11 (16.2) | 34 (26.6) | 0.19 |
| KRAS status | < 0.001/< 0.001 | ||||
| Negative | 109 (20.9) | 50 (15.4) | 16 (23.5) | 43 (33.6) | |
| Positive | 98 (18.8) | 39 (12.0) | 23 (33.8) | 36 (28.1) | |
| Missing | 314 (60.3) | 236 (72.6) | 29 (42.6) | 49 (38.3) | |
| PDL1 status | < 0.001/< 0.001 | ||||
| Negative | 30 (5.8) | 16 (4.9) | 4 (5.9) | 10 (7.8) | |
| Positive | 93 (17.9) | 27 (8.3) | 40 (58.8) | 26 (20.3) | |
| Missing | 398 (76.4) | 282 (86.8) | 24 (35.3) | 92 (71.9) |
Data not available for all subjects. Missing values: KPS = 80, lesion number = 2, srs number = 12, Histology = 19, Extracranial Metastases = 10. Statistics presented as Median [P25, P75] or N (column %). Significant differences (p < 0.05) are bolded.
Figure 1.
Flow chart of the study population. ICI = immune checkpoint inhibitor; BM = Brain Metastases; LCBM = lung cancer brain metastases.
Patient characteristics between those receiving ICI compared to those who never received ICI are outlined in Table 1. There was no statistical difference in age, sex, or race between the two groups (p > 0.05). The median KPS at diagnosis was 80 for both groups. The median number of lesions at the diagnosis of BM was 1 in no-ICI group and 2 in the ICI-90, however this variance was not statistically different (p = 0.27). Of the patients in the ICI-90 cohort, 55.2% had an EC-mets compared to only 44.0% in the no-ICI group.
Demographics and clinical factors that may associate with OS were first identified by univariate analysis across the 521 patients. KPS below 90 (p < 0.001), age older than 65 (p = 0.005), presence of EC-mets at diagnosis of BM (p = 0.02), adenocarcinoma histology (p < 0.001), and male sex (p = 0.02) were significant predictors of worse OS. However, the number of intracranial lesions at diagnosis of BM was not found to be statistically significant (p = 0.72) (Table 2).
Table 2.
Univariate analysis of demographics and clinical characteristics.
| Variable | N | Events | Median months | 1-Year survival % (95% CI) | log-rank p value | Cox univariate hazard ratio (95% CI) | Cox univariate Wald p-value |
|---|---|---|---|---|---|---|---|
| Sex | 0.023 | ||||||
| Female | 259 | 157 (61%) | 13.4 | 58.4 (52.3, 64.5) | – | ||
| Male | 262 | 178 (68%) | 11.1 | 48.6 (42.4, 54.8) | 1.28 (1.03, 1.59) | 0.024 | |
| Race | 0.75 | ||||||
| Non-white | 82 | 51 (62%) | 12.3 | 52.0 (40.9, 63.1) | – | ||
| White | 439 | 284 (65%) | 12.7 | 53.8 (49.0, 58.5) | 0.95 (0.71, 1.28) | 0.75 | |
| Age | 0.005 | ||||||
| < 65 | 307 | 186 (61%) | 13.8 | 56.8 (51.1, 62.4) | – | ||
| ≥ 65 | 214 | 149 (70%) | 10.5 | 48.8 (42.0, 55.6) | 1.36 (1.10, 1.69) | 0.005 | |
| KPS | < 0.001 | ||||||
| < 90 | 221 | 157 (71%) | 8.9 | 40.8 (34.2, 47.4) | 1.97 (1.55, 2.50) | < 0.001 | |
| ≥ 90 | 220 | 119 (54%) | 18.4 | 66.8 (60.5, 73.1) | – | ||
| Missing | 80 | 59 (74%) | 12.1 | 51.3 (40.0, 62.6) | 1.65 (1.21, 2.26) | 0.002 | |
| Lesion number | 0.49 | ||||||
| 1 | 258 | 160 (62%) | 13.3 | 55.7 (49.4, 61.9) | – | ||
| ≥ 2 | 261 | 173 (66%) | 12.3 | 51.4 (45.2, 57.6) | 1.08 (0.87, 1.34) | 0.49 | |
| SRS | < 0.001 | ||||||
| 0–1 | 345 | 245 (71%) | 9.8 | 43.4 (38.1, 48.8) | – | ||
| ≥ 2 | 164 | 82 (50%) | 21.7 | 75.1 (68.4, 81.8) | 0.45 (0.35, 0.58) | < 0.001 | |
| Histology | < 0.001 | ||||||
| Other | 131 | 100 (76%) | 9.1 | 38.7 (30.1, 47.3) | – | ||
| Adenocarcinoma | 371 | 221 (60%) | 15.0 | 58.4 (53.3, 63.5) | 0.57 (0.45, 0.72) | < 0.001 | |
| EC-mets | 0.018 | ||||||
| No | 273 | 166 (61%) | 13.9 | 57.7 (51.7, 63.6) | – | ||
| Yes | 238 | 162 (68%) | 10.5 | 47.9 (41.4, 54.5) | 1.30 (1.04, 1.61) | 0.018 | |
| WBRT | 0.11 | ||||||
| No | 390 | 248 (64%) | 12.2 | 51.0 (45.9, 56.1) | – | ||
| Yes | 131 | 87 (66%) | 14.9 | 60.7 (52.3, 69.1) | 0.82 (0.64, 1.05) | 0.11 | |
| Age | 521 | 335 (64%) | 12.7 | 53.5 (49.1, 57.8) | 1.014 (1.003, 1.025) | 0.012 | |
| KPS | 441 | 276 (63%) | 12.7 | 53.8 (49.1, 58.6) | 0.96 (0.95, 0.97) | < 0.001 | |
| Lesion number | 519 | 333 (64%) | 12.7 | 53.5 (49.1, 57.9) | 0.99 (0.96, 1.03) | 0.72 | |
| SRS | 509 | 327 (64%) | 12.7 | 53.8 (49.4, 58.2) | 0.65 (0.57, 0.75) | < 0.001 |
Significant differences (p < 0.05) are bolded.
ICI within 90 days
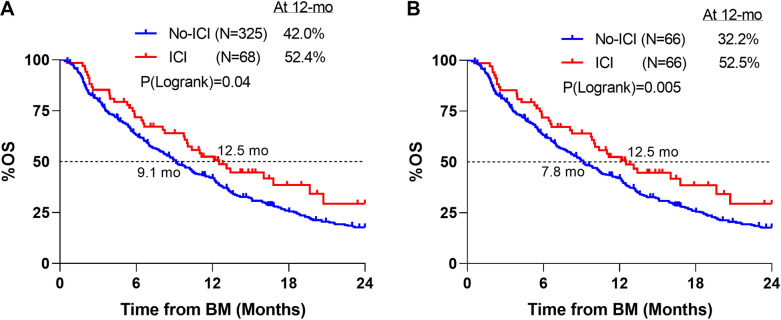
Overall survival from the time of diagnosis of BM was compared between those treated without ICI and those treated with ICI within the first 90 days. On univariable analysis, there was a significant difference in OS between the groups with median survival of 9.1 months and 12.5 months, respectively (p = 0.04) (Fig. 2A). In a multivariate analysis, treatment with ICI-90 led to significantly improved OS (p < 0.001) (Table 3). A similar analysis was performed excluding patients with missing KPS, and ICI-90 remained a significant predictor of OS (p = 0.001).
Figure 2.
(A) Overall survival comparing ICI-90 to no-ICI with the exclusion of patients who received surgery for brain metastases. (B) After a Greedy match was performed to balance the ICI-90 and no-ICI cohort after the removal of patients with intracranial surgery, overall survival from the diagnosis of brain metastases is graphed. Log-rank test was performed.
Table 3.
Multivariate analysis on the effect of ICI within 90 days.
| Variable | N | Events | Cox multivariable hazard ratio (95% CI) | Cox multivariable Wald p-value |
|---|---|---|---|---|
| ICI status | ||||
| No-ICI | 325 | 247 (76%) | 1.87 (1.30, 2.68) | < 0.001 |
| ICI-90 | 68 | 39 (57%) | – | |
| Sex | ||||
| Female | 187 | 130 (70%) | 1.02 (0.79, 1.32) | 0.87 |
| Male | 206 | 156 (76%) | – | |
| Age | ||||
| < 65 | 232 | 158 (68%) | – | |
| ≥ 65 | 161 | 128 (80%) | 1.302 (1.013, 1.674) | 0.039 |
| KPS | ||||
| < 90 | 178 | 138 (78%) | 1.69 (1.28, 2.23) | < 0.001 |
| ≥ 90 | 141 | 93 (66%) | – | |
| Unknown | 74 | 55 (74%) | 0.93 (0.64, 1.34) | 0.69 |
| SRS | ||||
| 0–1 | 284 | 218 (77%) | 2.08 (1.52, 2.85) | < 0.001 |
| ≥ 2 | 98 | 60 (61%) | – | |
| Histology | ||||
| Other | 106 | 88 (83%) | 1.39 (1.07, 1.82) | 0.014 |
| Adenocarcinoma | 268 | 184 (69%) | – | |
| EC-mets | ||||
| No | 207 | 144 (70%) | – | |
| Yes | 176 | 135 (77%) | 1.31 (1.02, 1.68) | 0.035 |
| Lesion number | ||||
| 1 | 201 | 140 (70%) | – | |
| ≥ 2 | 190 | 144 (76%) | 1.38 (1.07, 1.78) | 0.012 |
Significant differences (p < 0.05) are bolded.
Additionally, a PS matching was performed to select patients who did not receive ICIs with similar characteristics to the ICI-90 cohort. When both groups were matched to have similar age, KPS, EC-mets (exact match), number of lesions, and number of SRS treatments, there was almost a 5-month median survival advantage with ICIs within 90 days (12.5 vs 7.8 months, p = 0.005) (Fig. 2B).
Impact of KRAS mutation status on the efficacy of ICI
Given the emerging data regarding presence of KRAS mutation and response to ICI, we first analyzed the expression of PD-L1 based on KRAS mutation status. Out of 800 total patients diagnosed with LCBM, 109 patients had both a known PD-L1 expression and KRAS status. PD-L1 was expressed in 80.4% of patients with KRAS mutation vs. 61.9% of KRAS wild-type patients (p = 0.04).
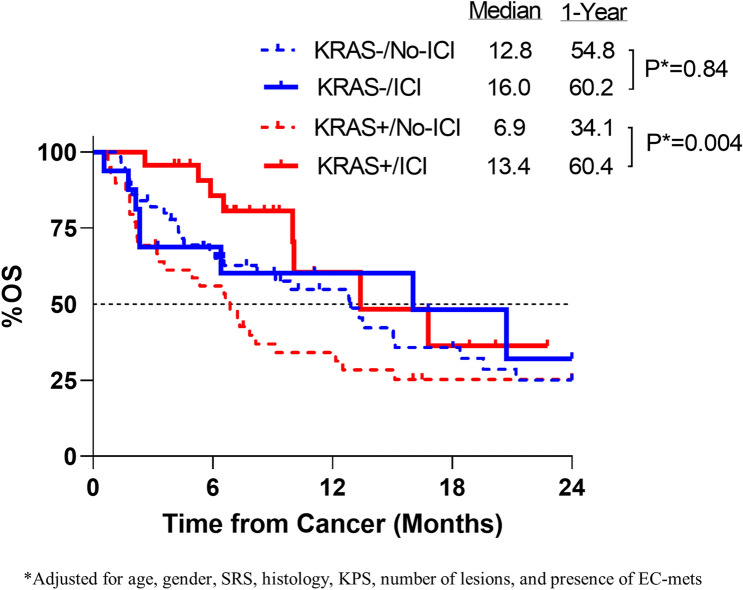
We then compared OS from the time of BM between those not treated with ICIs vs. those treated with ICIs (Fig. 3). In patients without KRAS mutations, there was no statistical difference in OS between the ICI vs. no ICI cohorts with a one-year survival of 60.2% vs 54.8% (adjusted p = 0.84). However, in patients with KRAS mutations, patient treated with ICIs had a 1-year survival of 60.4% vs. 34.1% in the no-ICI cohort. On multivariate analysis including age, gender, SRS, histology, KPS, number of lesions, and presence of EC-mets, these results remained consistent and statistically significant (p = 0.004). When comparing all patients who received ICIs, there was no difference in OS from BM between patients with vs without KRAS mutations.
Figure 3.
Impact of KRAS status on efficacy of ICI. Overall survival is plotted from the start of ICIs. Multivariate analysis which included age, gender, SRS, histology, KPS, number of lesions and presence of EC-mets.
Discussion
Patients with NSCLCBM have a poor prognosis and traditional systemic chemotherapies have provided limited clinical benefit. With advances in targeted therapies and ICI, this dogma is beginning to shift dramatically. While some clinical data is available suggesting the efficacy of ICIs in patients with stable, asymptomatic brain metastases, there is almost no prospective or retrospective data in patients with progressive or symptomatic disease. Our study retrospectively analyzed patients treated for NSCLCBM at a single tertiary care center and compared those treated with ICIs to those who were not. In agreement with the clinical trials, we observed an improvement in OS when patients were treated with ICIs within 90 days of the diagnosis of brain metastases (p < 0.0001)2–4. This data suggests that ICIs provide an OS benefit over traditional chemotherapy or local therapy alone.
Surgery has long been a mainstay of BM treatment, however, advances in SRS have decreased its utilization. While investigating the prevalence of surgical resection was not the primary goal of this study, when we compared our ICI-90 cohort to our no-ICI cohort we found a large difference in the proportion who underwent resection of a BM (29.4% vs 5.7%, p < 0.001) and therefore we excluded these patients from the remaining analyses. Advances in SRS technology more readily allow for the targeting of multiple intracranial lesions, deep-seated tumors, in patients who are too frail for surgery, have multiple medical co-morbidities, and is also commonly used in the salvage setting16,17. These improvements may account for the number of intracranial lesions not predicting OS within our cohort (hazard ratio of 0.99, p = 0.72). Newer prospective clinical trials are beginning to demonstrate that patients with multiple lesions can be treated with SRS and have improved intracranial control18. Our cohort provides additional retrospective evidence that number of lesions may not influence OS when all lesions are treated with SRS18,19. With that being said, other variables that have previously been reported, such as EC-mets, age and KPS all predicted OS15,20.
In order to identify patient populations who respond best to ICIs, we investigated the impact of KRAS mutation status. We found an improved OS in patients with a KRAS mutation who were treated with ICI within 90 days over no-ICI. This difference was not observed in the patients with wild-type KRAS. A similar result was seen in a meta-analysis of three clinical trials that compared the difference in response to ICIs based on KRAS status21. Additionally, another recently published study found that patients with KRAS mutations with advanced NSCLC had improved survival over patients with wild-type KRAS when treated with ICI22. These findings suggest that while ICIs do not directly target KRAS protein, they may provide significant clinical benefit to these patients. Additionally, the development of KRAS targeted therapies opens the possibility for combination therapy with ICIs23. KRAS mutation status should, therefore, be considered in the development of future clinical trials investigating ICIs and BM.
The retrospective nature of the study allows for errors in data collection from an electronic medical record not designed for research purposes. All our mutation and PD-L1 status was derived from the primary tumor and KRAS mutational subtypes were not collected. A 2015 study found that all patients with an activating KRAS mutation in the primary tumor shared this mutation in all clonally related cases24. Additionally, another recent study found a 75.5% concordance between PD-L1 expression in primary tumor and BM suggesting that while PD-L1 status of primary tumor has some predictive value, changes in intracranially do occur25. Additionally, although multivariable analysis was performed with known predictors of survival, the cohorts may have differed in other areas. We were unable to determine whether KRAS mutation status was an independent predictor of survival or if the increased PD-L1 expression in these patients accounts for the difference. The patients in the no-ICI group were not necessarily treated with any systemic chemotherapy and almost all received intracranial SRS. Finally, while our study investigated OS after BM, we did not specifically investigate intracranial or extracranial response, allowing the possibility that any difference in OS may be due to control (or lack thereof) of extracranial disease.
This study reports a significant survival benefit of ICIs over chemotherapy or local control alone in patients with NSCLCBM. This retrospective analysis of patients treated for NSCLCBM has also emphasized the shift away from surgery for management of BM in the age of ICIs. Finally, our data suggests the greatest benefit of ICIs are seen in patients with KRAS mutations in their tumors, which should be considered in future trials.
Author contributions
The experimental design was done by A.L., R.K. A.B., V.T., A.A., P.P., A.M., S.T.C., E.S.M., L.A., J.H.S., G.H.B., H.L., N.A.P., M.S.A. Data collection was performed by A.L., A.B., V.T., A.A. The analysis of the data was done by A.L., R.K. A.A. H.L. M.S.A. The writing of the original draft was done by A.L., R.K. M.S. All authors reviewed the manuscript.
Funding
This work was partially funded by the Ruth L. Kirschstein National Research Service Award (NRSA) Individual Fellowship F30CA250254 (AL).
Competing interests
Manmeet S. Ahluwalia: Receipt of grants/research supports: Astrazeneca, Abbvie, BMS, Bayer, Incyte, Pharmacyclics, Novocure, Merck, Mimivax, Novartis, Roswell Park Cancer Foundation, Velosano. Stock shareholder: Cytodyn, Doctible, Mimivax, Medinnovate Advisors LLC. Receipt of honoraria or consultation fees: Abbvie, AstraZeneca, BMS, Flatiron, Varian Medical Systems, Bayer, Cellularity, Elsevier, Forma therapeutics. GSK, Insightec, karyopharm, Kiyatec, Novocure Inc, Tocagen, VBI Vaccines, Wiley, Xoft, Nuvation, SDP Oncology, Appollomics, Prelude, Janssen. Rupesh Kotecha: Receipt of honoraria or consultation fees: Elsevier, Elekta AB, Accuray Inc, Novocure Inc, and Viewray Inc. Research funding from Medtronic Inc., Blue Earth Diagnostics, Novocure Inc., AstraZeneca, Exelixis, and Viewray Inc. Samuel Chao: Honorarium from Varian Medical Systems and research support from Blue Earth Diagnostics. Nathan A. Pennell: BMS, Merk, Astrazeneca, Eli Lilly, Inivata, Pfizer, Amgen, Mirati, Genentech, Xencor, Janssen, Viosera, G1 Therapeautics. Gene H. Barnett: consultant for Monteris Medical Inc, royalty interests for Roche. Alireza M. Mohammadi: consultant for Monteris Medical Company. John H. Suh: consultant for Neutron Therapeutics, Novocure Inc., Philips. Adam Lauko, Addison Barnett, Vineeth Tatineni, Assad Ali, Pradnya Patil, Erin S. Murphy, Lilyana Angelov, and Hong Li all declare no conflict of interest.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in the Funding section. “This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.” now reads: “This work was partially funded by the Ruth L. Kirschstein National Research Service Award (NRSA) Individual Fellowship F30CA250254 (AL).”
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/17/2022
A Correction to this paper has been published: 10.1038/s41598-022-05489-0
References
- 1.Suh JH, et al. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 2020;17:279–299. doi: 10.1038/s41571-019-0320-3. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi L, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N. Engl. J. Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 3.Borghaei H, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reck M, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 5.Crinò L, et al. P1.01-053 Italian Nivolumab Expanded Access Programme (EAP): Data from patients with advanced non-squamous NSCLC and brain metastases. J. Thorac. Oncol. 2017;12:S1915. doi: 10.1016/j.jtho.2017.09.707. [DOI] [Google Scholar]
- 6.Molinier O, et al. OA 17.05 IFCT-1502 CLINIVO: Real-life experience with nivolumab in 600 patients (Pts) with advanced non-small cell lung cancer (NSCLC) J. Thorac. Oncol. 2017;12:S1793. doi: 10.1016/j.jtho.2017.09.430. [DOI] [Google Scholar]
- 7.Goldberg SB, et al. Durability of brain metastasis response and overall survival in patients with non-small cell lung cancer (NSCLC) treated with pembrolizumab. J. Clin. Oncol. 2018;36:2009–2009. doi: 10.1200/JCO.2018.36.15_suppl.2009. [DOI] [Google Scholar]
- 8.Kotecha R, et al. The impact of sequencing PD-1/PD-L1 inhibitors and stereotactic radiosurgery for patients with brain metastasis. Neuro Oncol. 2019 doi: 10.1093/neuonc/noz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchwald ZS, et al. Radiation, immune checkpoint blockade and the abscopal effect: A critical review on timing, dose and fractionation. Front. Oncol. 2018;8:612. doi: 10.3389/fonc.2018.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guin, S. et al. Contributions of KRAS and RAL in non-small-cell lung cancer growth and progression. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer8, 1492–1501 (2013). [DOI] [PMC free article] [PubMed]
- 11.Chan BA, Hughes BGM. Targeted therapy for non-small cell lung cancer: Current standards and the promise of the future. Transl. Lung Cancer Res. 2015;4:36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood K, Hensing T, Malik R, Salgia R. Prognostic and predictive value in KRAS in non-small-cell lung cancer: A review. JAMA Oncol. 2016;2:805–812. doi: 10.1001/jamaoncol.2016.0405. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, et al. Clinicopathological analysis and prognostic significance of programmed cell death-ligand 1 protein and mRNA expression in non-small cell lung cancer. PLoS ONE. 2018;13:e0198634. doi: 10.1371/journal.pone.0198634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen N, et al. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol. Immunother. 2017;66:1175–1187. doi: 10.1007/s00262-017-2005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperduto PW, et al. Survival in patients with brain metastases: summary Report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020 doi: 10.1200/JCO.20.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotecha, R., Gondi, V., Ahluwalia, M. S., Brastianos, P. K. & Mehta, M. P. Recent advances in managing brain metastasis. F1000Research 7, (2018). [DOI] [PMC free article] [PubMed]
- 17.Kotecha R, et al. Three or more courses of stereotactic radiosurgery for patients with multiply recurrent brain metastases. Neurosurgery. 2017;80:871–879. doi: 10.1093/neuros/nyw147. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 19.Li J, et al. Stereotactic radiosurgery versus whole-brain radiation therapy for patients with 4–15 brain metastases: A phase III randomized controlled trial. Int. J. Radiat. Oncol. Biol. Phys. 2020;108:S21–S22. doi: 10.1016/j.ijrobp.2020.07.2108. [DOI] [Google Scholar]
- 20.Sperduto PW, et al. Estimating survival in patients with lung cancer and brain metastases: An update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA) JAMA Oncol. 2017;3:827–831. doi: 10.1001/jamaoncol.2016.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JH, Kim HS, Kim BJ. Prognostic value of KRAS mutation in advanced non-small-cell lung cancer treated with immune checkpoint inhibitors: A meta-analysis and review. Oncotarget. 2017;8:48248–48252. doi: 10.18632/oncotarget.17594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun L, et al. Association between KRAS variant status and outcomes with first-line immune checkpoint inhibitor-based therapy in patients with advanced non-small-cell lung cancer. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2021.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong DS, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N. Engl. J. Med. 2020;383:1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brastianos PK, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5:1164–1177. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camy, F. et al. Brain metastasis PD-L1 and CD8 expression is dependent on primary tumor type and its PD-L1 and CD8 status. J. Immunother. Cancer8, e000597 (2020). [DOI] [PMC free article] [PubMed]