Abstract
The ribosomal proteins (RPs) of Saccharomyces cerevisiae are encoded by 137 genes that are among the most transcriptionally active in the genome. These genes are coordinately regulated: a shift up in temperature leads to a rapid, but temporary, decline in RP mRNA levels. A defect in any part of the secretory pathway leads to greatly reduced ribosome synthesis, including the rapid loss of RP mRNA. Here we demonstrate that the loss of RP mRNA is due to the rapid transcriptional silencing of the RP genes, coupled to the naturally short lifetime of their transcripts. The data suggest further that a global inhibition of polymerase II transcription leads to overestimates of the stability of individual mRNAs. The transcription of most RP genes is activated by two Rap1p binding sites, 250 to 400 bp upstream from the initiation of transcription. Rap1p is both an activator and a silencer of transcription. The swapping of promoters between RPL30 and ACT1 or GAL1 demonstrated that the Rap1p binding sites of RPL30 are sufficient to silence the transcription of ACT1 in response to a defect in the secretory pathway. Sir3p and Sir4p, implicated in the Rap1p-mediated repression of silent mating type genes and of telomere-proximal genes, do not influence such silencing of RP genes. Sir2p, implicated in the silencing both of the silent mating type genes and of genes within the ribosomal DNA locus, does not influence the repression of either RP or rRNA genes. Surprisingly, the 180-bp sequence of RPL30 that lies between the Rap1p sites and the transcription initiation site is also sufficient to silence the Gal4p-driven transcription in response to a defect in the secretory pathway, by a mechanism that requires the silencing region of Rap1p. We conclude that for Rap1p to activate the transcription of an RP gene it must bind to upstream sequences; yet for Rap1p to repress the transcription of an RP gene it need not bind to the gene directly. Thus, the cell has evolved a two-pronged approach to effect the rapid extinction of RP synthesis in response to the stress imposed by a heat shock or by a failure of the secretory pathway. Calculations based on recent transcriptome data and on the half-life of the RP mRNAs suggest that in a rapidly growing cell the transcription of RP mRNAs accounts for nearly 50% of the total transcriptional events initiated by RNA polymerase II. Thus, the sudden silencing of the RP genes must have a dramatic effect on the overall transcriptional economy of the cell.
The Saccharomyces cerevisiae cell invests enormous resources in the synthesis of ribosomes. In a rapidly growing cell, the 100 or more rRNA genes are responsible for at least 60% of total transcription (59). The 137 ribosomal protein (RP) genes are among the most active in the genome (57). As a result, the cell has evolved mechanisms that tightly control the synthesis of the components of the ribosome. The 78 RPs are synthesized in nearly equimolar amounts that are matched to the synthesis of rRNA. Furthermore, the synthesis of these components is coordinately responsive to many changes within or outside the cell, including heat shock, carbon source, and nutrient availability (reviewed in references 44 and 59).
The primary level of regulation is at transcription (7, 16, 24). Most RP genes have similar promoter motifs, consisting of two Rap1p binding sites in tandem arrangement 250 to 400 bp upstream of the transcription initiation site recently compiled by Lascaris et al. (26), followed by one or two T-rich regions, and then a region of about 180 bp that includes the putative TATA element. Serial promoter deletions of RP genes, including RPS14A (CRY1), RPL25, and RPL28 (CYH2), have shown that the Rap1p sites are responsible for about 90% of promoter activity, with the rest due to the T-rich regions (41, 45, 47). Deletions downstream of the T-rich elements have little effect on the rate of transcription but can affect the initiation site (47). For a small minority of RP genes, including RPL3, RPL4A, RPL4B, and RPS28A, a single Abf1p site replaces the two Rap1p sites (8, 13). Nevertheless, such genes appear to be regulated coordinately with the others. (In this paper, we have used the new nomenclature for RPs and their genes that was recently adopted (32, 64a). Thus, L30 was previously known as L32, L3 was previously known as Tcm1p, and L8 was previously known as L4.)
Rap1p is an essential, abundant DNA-binding protein that has several functions in the cell. It is a context-dependent transcription regulator (50), responsible for activating the transcription of many of the most highly transcribed genes, encoding not only the RPs and other translation factors but also the very abundant glycolytic enzymes (reviewed in reference 49). Rap1p also acts as a transcriptional silencer in at least two contexts. At the silent mating type loci, Rap1p cooperates with Abf1p and the origin of replication complex (ORC) to repress transcription (1). At the telomeres, Rap1p binds to the [C1-3A]n repeats (63), leading to the silencing of genes adjacent to telomeres. In both cases Rap1p recruits multiple copies of Sir3p and Sir4p that participate in the silencing, perhaps by interacting with the tails of histones H3 and H4 (38). A mutant allele, rap1s, relieves the repression at silent mating type loci. The overexpression of Sir3p or Sir4p can restore the repression (53).
When yeast cells growing at 23°C are shifted to 37°C, both growth and protein synthesis continue unabated; indeed, depending on strain background, they sometimes increase (11). Furthermore, mRNA synthesis, measured by the incorporation of [3H]uracil into poly(A)+ RNA, is unaffected (24). Yet, during the first 20 min after such a shift the level of RP mRNAs declines by about 80% and then recovers to the original level by about 60 min (11, 15). This down-regulation of RP mRNA has been attributed to a temporary transcriptional silencing of RP genes (24) and to an increased turnover of RP mRNAs (15). In neither case have the elements responsible been identified (15, 45).
Recently, we have shown that a defect at any of several points in the secretory pathway down-regulates the synthesis of ribosomes, through the repression of transcription of both rRNA and RP genes (28, 36). Inhibitors that disrupt the secretory pathway, such as brefeldin A and tunicamycin, also repress ribosome synthesis. A similar effect on rRNA transcription was observed in a sec23 mutant (29). These results suggest that there exists an intracellular signal transduction pathway between the secretory apparatus and the master control of ribosome biosynthesis.
To identify the elements of an RP gene that makes it subject to repression by a temperature shock and by the failure of the secretory pathway, we have carried out experiments which demonstrate (i) that the rapid decline in mRNA levels is due entirely to the rapid repression of transcription, coupled to a normal, rapid turnover of the mRNA (this repression does not depend on the presence of Sir2p, Sir3p, or Sir4p) and (ii) that the repression of transcription is mediated by two cis elements, the Rap1p binding sites and a 180-bp sequence just upstream of the transcription initiation site. Either of these elements alone can mediate at least 75% of repression.
MATERIALS AND METHODS
Strains.
The strains used in this study are listed in Table 1. Unless otherwise stated, cultures were grown on 2% yeast extract–1% Bacto Peptone–2% glucose (YPD) at 23°C. Cultures were shifted from 23 to 37°C by pouring them into a large, prewarmed flask shaking in a water bath. Cultures were shifted from YPGal to YPD by filtration onto a Millipore filter which was immediately transferred to the new medium. The whole operation took less than 20 s.
TABLE 1.
S. cerevisiae strains used in these experiments
| Strain | Genotype | Reference or source |
|---|---|---|
| W303a | MATa ade2-1 leu2-3,112 ura3-1 his3-11,15 trp1-1 can1-100 ssd1-1 | 54 |
| 169ts | MATα ade2-1 leu2-3,112 ura3-1 his3-11,15 can1-100 ssd1-1 ypt6-1 | 28 |
| JV7-2a | As W303, rpl30Δ::HIS3 (pYE: CEN, URA3 GAL1-RPL30) | 58 |
| BL17 | MATa/MATα YPT6/ypt6-1 rpl30Δ::HIS3/RPL30 his3-11,15/his3-11,15 ura3-1/ura3-1 leu2-3,112/leu2-3,112 ade2-1/ade2-1 can1-100/cam1-100 ssd1-1/ssd1-1 (pRS316: CEN, URA3 ACT1-RPL30) | This study |
| BL174 | ypt6-1 rpl30Δ::HIS3 (pRS316: CEN, URA3 ACT1-RPL30 [construct A]) | This study |
| BL175 | ypt6-1 rpl30Δ::HIS3 (pRS316: CEN, URA3 ACT1-RPL30 [construct B]) | This study |
| BL176 | ypt6-1 rpl30Δ::HIS3 (pRS316: CEN, URA3 ACT1-RPL30 [construct C]) | This study |
| BL177 | ypt6-1 rpl30Δ::HIS3 (pRS316: CEN, URA3 ACT1-RPL30 [construct D]) | This study |
| Y260 | MATa ura3-52 leu2 rpb1-1 | 42 |
| JW1201 | MATα ypt6-1 ade2-1 leu2-3,112 ura3-1 his3-11,15 can1-100 ssd1-1 act1Δ::URA3 RPL30-ACT1 | This study |
| YDS2 | MATa leu2 ade2 his3 trp1 ura3 (∼W303) | D. Shore |
| YDS714 | MATα leu2 ade2 his3 trp1 ura3 sir2Δ::HIS3 | D. Shore |
| JW1210 | MATα leu2 ade2 his3 trp1 ura3 sly1-1 sir2Δ::URA3 | This study |
| JW1211 | MATα leu2 ade2 his3 trp1 ura3 sly1-1 hst1Δ::URA3 | This study |
| YDS430 | MATα leu2 ade2 his3 trp1 ura3 sir3Δ::LEU2 | D. Shore |
| BL180 | YPT6 SIR3 | This study |
| BL181 | YPT6 sir3Δ::LEU2 | This study |
| BL182 | ypt6-1 SIR3 | This study |
| BL183 | ypt6-1 sir3Δ::LEU2 | This study |
| YDV122 | MATα leu2 ade2 his3 trp1 ura3 sir4Δ::LEU2 hmrΔ::TRP1 | D. Shore |
| BL185 | YPT6 SIR4 | This study |
| BL186 | YPT6 sir4Δ::LEU2 | This study |
| BL187 | ypt6-1 SIR4 | This study |
| BL188 | ypt6-1 sir4Δ::LEU2 | This study |
Yeast total RNA isolation.
Generally, 10-ml volumes of cultures in log phase were harvested by pouring them on crushed ice (46). Cells were collected by centrifugation, resuspended in 400 μl of AE buffer (50 mM NaAc [pH 5.3], 1 mM EDTA), and transferred to microcentrifuge tubes. Forty microliters of 10% sodium dodecyl sulfate was added, and the mixture was vortexed. An equal volume of fresh, AE buffer-saturated phenol was added, and the mixture was vortexed for 2 min. The samples were then incubated at 65°C for 4 min and chilled rapidly in dry ice-ethanol until phenol crystals appeared. The samples were centrifuged at maximum speed for 10 min, and the aqueous layer was transferred to another tube, extracted with phenol-chloroform at room temperature for 5 min, and then centrifuged for 5 min. Total RNA was precipitated with ethanol, washed with 70% ethanol, dried, and resuspended in 50 μl of sterile water.
RNA gel and Northern analysis.
Ten micrograms of total RNA was fractionated on 1.5% agarose gels containing 6% formaldehyde. The RNA was transferred to Nytran (Schleicher & Schuell) nylon membranes, cross-linked with UV light, and then baked in a vacuum for 1 h at 80°C. Northern blot analysis was performed as described previously (10). 32P-labelled antisense RNA probes and oligodeoxynucleotides were used to detect mRNAs (36). rRNA was labelled with [C3H3]methionine (60) and analyzed by fluorography (36).
Construction of fused genes with site-directed mutagenesis.
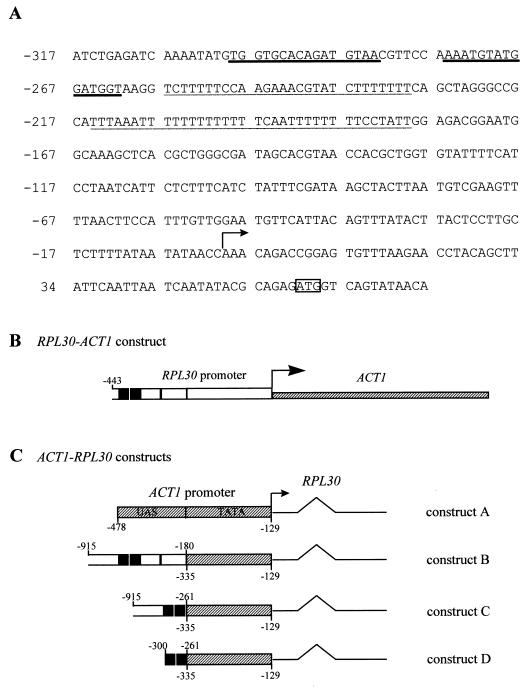
For the ACT1 gene constructs, the A of ATG in the coding region is at position +1. For RPL30, the transcription initiation site is considered position +1 and is 58 nucleotides upstream of ATG. To fuse the RPL30 promoter to the ACT1 transcription unit, the URA3 gene was first cloned into pMDJ8, which contains a 1.8-kb fragment with the complete RPL30 sequence, from −443 to +1520. The new plasmid, containing divergently expressed URA3 and RPL30 genes, each from its own promoter, was then used as a template for PCR. The left-hand primer contained ACT1 sequences from −410 to −364 and the reverse complement of URA3 sequences from +915 to +890. (The URA3 open reading frame [ORF] is 803 bp). The right-hand primer contained ACT1 sequences from −96 to −141 and RPL30 sequences from −1 to −26. PCR amplified a fragment in which the entire URA3 gene and RPL30 promoter sequences (to the transcription start site at +1) were bracketed by ACT1 sequences. The PCR fragment was integrated into the chromosomal ACT1 locus of strain 169ts or JW142 by homologous recombination, selecting for URA3. The integration reconstituted the ACT1 transcription unit, under the control of the RPL30 promoter (Fig. 4B). The correct integration was verified by PCR. The correct initiation of transcription was verified by primer extension. The resulting strain was designated JW1201 (Table 1).
FIG. 4.
(A) The promoter of RPL30. The start codon is boxed. The two Rap1p binding sites are indicated by a heavy underline, and the two T-rich regions are indicated by a light underline. The initiation of transcription, termed +1 and marked with an arrow, is 58 nucleotides upstream of ATG. (B) The RPL30-ACT1 fusion gene. See Materials and Methods for details. (C) Constructing the fused gene with the ACT1 promoter driving the RPL30 transcript (constructs A to D); see Materials and Methods. The stippled area represents sequences from ACT1. Nucleotides from the RPL30 promoter were fused to the ACT1 TATA region to form constructs B, C, and D. The hatched boxes are ACT1 sequences; the open boxes are RPL30 sequences; the black boxes represent the RPL30 Rap1p binding sites. The line represents the L30 transcript. The nucleotide boundaries of the RPL30 sequences are shown above the constructs, and those of the ACT1 sequences are shown below the constructs. Because the ACT1 gene has multiple sites of transcription initiation, the numbering is in reference to ATG of the coding region.
Constructs A to D (Fig. 4C) were made starting with a pUC19-based plasmid carrying a 2.2-kb EcoRI fragment from RPL30. The promoter region was replaced with a fragment containing the promoter of ACT1 (construct A; Fig. 4B). From this construct, the upstream activating sequence (UAS) of ACT1 was replaced with portions of the UAS of RPL30 (constructs B, C, and D; Fig. 4C). For use in yeast, the constructs were subcloned into the CEN/URA3 plasmid, pRS316 (51). Primer extension revealed that about 50% of the transcripts initiated at the normal site for RPL32, and 50% initiated nine nucleotides downstream.
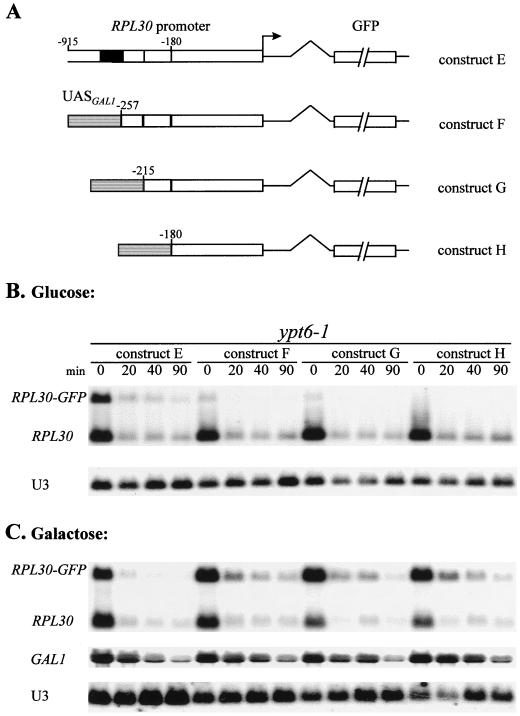
Constructs E to H (Fig. 9A) were made, starting with the vector pRS316 carrying RPL30. A large portion of the ORF downstream of the intron was replaced by the ORF encoding the green fluorescent protein (GFP), generating construct E (see text for details). From construct E, different portions of the RPL30 promoter were replaced with a 500-bp fragment containing the four Gal4p binding sites from GAL1 (nucleotides −829 to −324; with the start codon numbered +1), generating constructs F to H.
FIG. 9.
(A) Construction of fused genes between GAL1 and RPL30-GFP (constructs E to H). The stippled area represents sequences from the GAL1 UAS. The nucleotide boundaries of the RPL30 sequences are shown, and numbering follows the conventions described in the legend to Fig. 3A. The black boxes represent the RPL30 Rap1p binding sites. (B) The 180-bp cis element mediates the repression of RP gene transcription in a sec mutant. ypt6-1 cells carrying constructs E to H were grown to log phase in uracil-free medium containing 2% glucose at 23°C. An aliquot was harvested, the rest of the cultures were shifted to 37°C, and aliquots were harvested at intervals. Total RNA was isolated and analyzed as described previously. mRNAs for RPL30 and RPL30-GFP were detected with an RNA probe that is complementary to the first exon of RPL30. The snoRNA U3 was detected with an oligonucleotide probe. (C) The same as panel B except that cells were grown in 2% galactose to induce the GAL1 UAS. GAL1 mRNA was detected with an antisense RNA probe.
Deletion mutations were constructed by PCR (18). A circular plasmid carrying the gene of interest was used as a template for PCR catalyzed by Tli DNA polymerase (Promega), which has proofreading ability. PCR products were purified from an agarose gel with a DNA extraction kit (Qiagen). The purified DNA was used directly for self-ligation with the standard protocol, except that 10 U of polynucleotide kinase was included in the ligation reaction. The deletion constructs were sequenced.
mRNA half-life measurement.
The strains to be tested were grown overnight at 23°C to log phase. Cell cultures were concentrated fivefold and then shifted to 37°C (43). At the indicated time points 2-ml aliquots of the cultures were quickly collected and frozen in dry ice-ethanol. Total RNA was isolated and analyzed as described above. RNA levels were quantified relative to the U3 internal loading standard by using PhosphorImager (Molecular Dynamics) analysis.
RESULTS
Repression of RP gene expression.
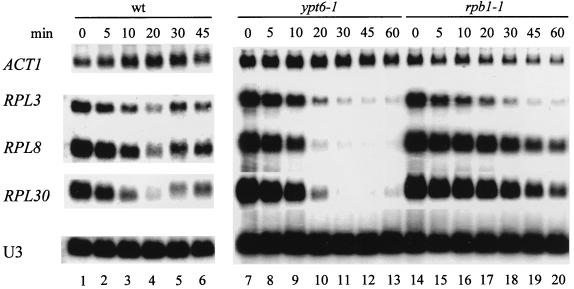
The mRNAs encoding RPs appear to be particularly sensitive to environmental changes. As shown in Fig. 1, a temperature shift from 23 to 37°C leads to a rapid decline in RP mRNA levels that is temporary in wild-type (wt) cells (lanes 1 to 6) but permanent in cells with a temperature-sensitive (ts) mutation in the secretory pathway, exemplified by ypt6-1 (lanes 7 to 13). This is not true of most non-RP genes, e.g., ACT1, or of the stable small nucleolar RNA (snoRNA) U3. These results could be explained by the hypothesis that the transcription of RP genes is extinguished after the temperature shift, temporarily in wt cells and permanently in cells with a defect in the secretory pathway. However, it has also been suggested that the loss of RP mRNA after a heat shock is due instead to an accelerated turnover of RP mRNA (15).
FIG. 1.
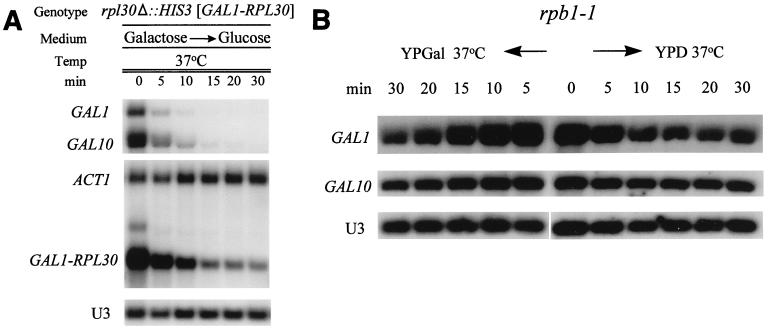
The level of RP mRNAs is down-regulated in W303 (wild type), 169ts (ypt6-1), and Y260 (rpb1-1) strains. The indicated strains (Table 1) were grown to log phase in YPD at 23°C. An aliquot was harvested, the rest of the culture was shifted to 37°C, and aliquots were harvested as indicated. Total RNA was isolated, and 10 μg of RNA was analyzed by Northern blotting. Individual RNAs were detected by using either antisense RNA probes, for RPL30 and ACT1, or oligonucleotide probes, for RPL3, RPL8, and U3 snoRNA, as described in Materials and Methods.
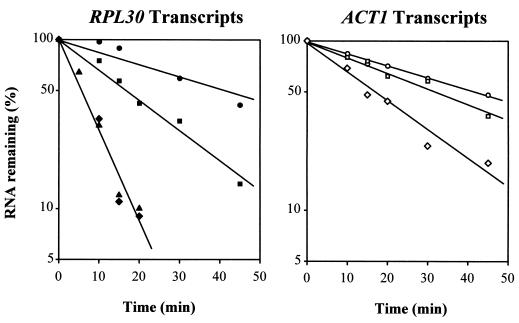
In an attempt to distinguish between these alternatives we have determined the half-life (t1/2) of an RP mRNA when its transcription is halted in two distinct ways. In one case we employed strain Y260, which carries rpb1-1, a ts allele of the largest subunit of RNA polymerase II (Pol II); in such cells, RNA Pol II-dependent transcription is reduced to less than 10% within 2 min after a shift to 37°C (42, 43). When Y260 is transferred to the nonpermissive temperature, the mRNA derived from most RP genes declines with a t1/2 of about 20 min (Fig. 1, lanes 14 to 20; Fig. 2; Table 2). The contrast between the effects of a secretory mutant (Fig. 1, lanes 7 to 13) and a polymerase mutant (lanes 14 to 20) (Fig. 2) supports the hypothesis that the turnover of RP mRNAs is accelerated due to heat shock in wt or sec cells.
FIG. 2.
A portion of the data used to generate Table 2. Cultures were handled as described in the text. The values represent the levels of the indicated RNA measured by PhosphorImager analysis, normalized to the amount of U3 RNA in that lane of the gel, and further normalized to 100% at the start of the experiment. Note that the repression of the GAL1-L30 transcripts by glucose is indistinguishable from the repression of the RPL30 transcripts by the secretory defect. The transcripts shown are as follows, with the method of repression in parentheses: ACT1-L30 (rpb1-1) (●), RPL30 (rpb1-1) (■), RPL30 (ypt6-1) (⧫), GAL1-L30 (glucose) (▴), ACT1 (rpb1-1) (○), RPL30-ACT1 (rpb1-1) (□), and RPL30-ACT1 (ypt6-1) (◊).
TABLE 2.
t1/2 of mRNA in strains of various genotypes at 37°Ca
| Genotype |
t1/2 of mRNA (min)
|
||
|---|---|---|---|
| wtb | ypt6-1 | rpb1-1 | |
| RPL3c | 9.0 | 9.5 | 11 |
| RPL8 | 8.5 | 7.5 | 20 |
| RPS6 | 9.0 | 8.0 | 20 |
| RPS28c | 8.5 | 8.0 | 20 |
| RPL30 | 7.5 | 7.0 | 20 |
| GAL1-L30d | 5.5 | ||
| GAL1-L30d (23°C) | 11 | ||
| GAL1d | 3.0 | >20 | |
| GAL10d | 2.5 | >20 | |
| ACT1 | 23e | 40 | |
| RPL30-ACT1 | 16 | 34 | |
The data are the averages of at least two but in most cases four experiments. They are reproducible within about ±10% except for the very short t1/2 mRNAs, for which the determinations are sensitive to small variations in the way the experiment is carried out. These are reproducible to about ±1 min.
t1/2 in the first 30 min following a shift from 23 to 37°C (see the text).
These promoters have Abf1p-binding sites (see the text).
After shifting from YPGal to YPD (4% dextrose).
From reference 14, carried out at 30°C; it would be shorter at 37°C.
To halt transcription in a way that does not depend on a temperature shift we employed modified RPL30 in which the UAS was replaced with that of GAL1. When this gene is used as the sole source of L30, cells will grow only in the presence of galactose. In that case substantial RPL30 mRNA is present (Fig. 3A). Once the galactose is replaced with glucose, transcription is immediately repressed (20, 30); the mRNA derived both from GAL1-RPL30 (Fig. 2) and from the GAL1 and GAL10 genes themselves declines rapidly (Fig. 3A), with a t1/2 of 5 to 7 min for the transcript encoding L30 and 3 to 5 min for the GAL genes (Fig. 3A and Table 2), as observed previously (3). ACT1 mRNA and U3 RNA are unaffected. This result suggests that the t1/2 for the RPL30 mRNA is artificially extended in the rpb1-1 strain. Indeed, when the rpb1-1 strain is grown on galactose and shifted to the nonpermissive temperature, the t1/2 of the GAL1 and GAL10 mRNAs is increased greatly, independent of whether the galactose has been replaced with glucose (Fig. 3B). Thus, the influence of rpb1-1 on the measured t1/2 of mRNAs is not limited to the transcripts of the RP genes.
FIG. 3.
(A) Direct measurement of the t1/2 of the mRNA encoding L30. Strain JV7-2a (rpl30Δ::HIS3 [pYE: CEN, URA3 GAL1-RPL30]) was grown at 37°C in YPGal medium and shifted to YPD (4% dextrose) as described in Materials and Methods. Cells were harvested just before and at intervals after the shift, and RNA was prepared and analyzed by Northern blotting as described previously. After PhosphorImager analysis, the t1/2 of the GAL1, GAL10, and GAL-RPL30 mRNAs was determined by using the stable U3 RNA as a loading control (Table 2). Note that the same experiment was carried out at 23°C, in which case the t1/2 of the mRNAs was about twice as long. (B) Extended t1/2 of the GAL1 and GAL10 mRNAs in an rpb1-1 strain. Strain Y260 (rpb1-1) was grown in YPGal medium at 23°C. At zero time, one sample was taken; half the remaining culture was shifted to 37°C, and the other half was filtered and resuspended in YPD (4% dextrose), prewarmed, and maintained at 37°C. Samples were taken at the indicated times, and RNA was prepared and analyzed as for panel A.
Integrating the data of Fig. 1, 2, and 3 and Table 2 suggests the following scenario. A temperature shift from 23 to 37°C leads to an immediate, but temporary, repression of the transcription of RP genes. In wt cells, the transcription of RP genes resumes after about 20 min. In a sec mutant, however, the transcription of RP genes does not resume, and RP mRNA is reduced to a very low level. These are likely to be two separate phenomena because either the ablation of the protein kinase C pathway (40) or mutation of the silencing domain of Rap1p (35) relieves the repression of transcription due to a secretory defect without affecting the repression due to heat shock. The immediate kinetics of the repression due to a defect in the secretory pathway are obscured by the cell’s response to heat shock. Nevertheless, we have found that the inhibition of the endoplasmic reticulum-Golgi communication with brefeldin A appears to repress the transcription of RP genes within 15 min (36), while direct stress on the plasma membrane, with the intercalating drug chlorpromazine, leads to repression almost immediately (40).
These data suggest that there is no need to invoke accelerated turnover (15) to explain the response of RP mRNA to a temperature shift. The t1/2 of RP mRNAs observed in response to a temperature shift in either a wt or a sec cell is the same as that observed at 37°C when transcription is halted due to glucose repression of the GAL1 promoter (Fig. 3A). However, the extended t1/2 of both the RP and the GAL mRNAs when transcription is extinguished by the inactivation of RNA polymerase II suggests that this experimental approach may be having broader physiological effects on the RNA metabolism of the cell.
RP promoter mediates repression.
The experiments whose results are shown in Fig. 1 and 2 demonstrate an almost instantaneous and complete repression of RP gene transcription. What sequence elements of the RP genes are responsible for this repression? The promoter of RPL30 (Fig. 4A) resembles the promoters of most RP genes, with two Rap1p binding sites as the major UAS, and one or two T-rich regions which have some promoter activity (41, 45, 47), followed by a less well defined region that contains the putative TATA box. Previous work has implicated the Rap1p binding sites as the elements mediating the regulation of transcription, in the response to a carbon source shift (7, 16), to amino acid starvation (37), and to cyclic AMP (cAMP) (25, 39), but not in the response to a temperature shift (41, 45).
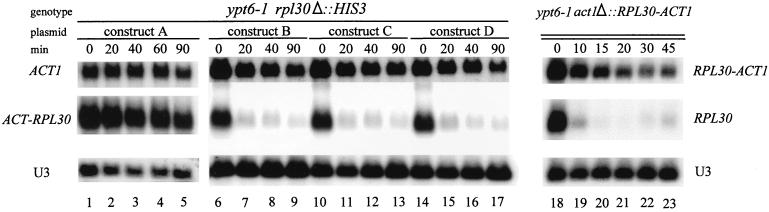
To determine which of the elements in the promoter of RP genes mediate the repression of transcription in response to a defect in the secretory pathway we made two promoter swap constructs, in which the RPL30 promoter drives the ACT1 transcript (Fig. 4B) and the ACT1 promoter drives the RPL30 transcript (Fig. 4C). Primer extension demonstrated that the resulting ACT1 transcripts were initiated at the same site as that for the endogenous ACT1; about half of the RPL30 transcripts were initiated at the correct site, with the rest being initiated nine nucleotides downstream (data not shown). The ACT1-RPL30 construct is not responsive to the ypt6-1 mutation (Fig. 5, lanes 1 to 5). The RPL30-ACT1 construct is responsive to the ypt6-1 mutation, with the level of its transcript decreasing monotonically from the time of the temperature shift (Fig. 5, lanes 18 to 23, and Fig. 2). The rate of decline in RPL30-ACT1 mRNA is lower than that of RPL30, with a t1/2 of about 16 min, presumably because the intrinsic stability of the ACT1 transcript is greater. Once again, the t1/2 measured here is substantially shorter than that measured with the rpb1-1 mutation (Fig. 2 and Table 2), reinforcing the suggestion that rpb1-1 has a broad effect on mRNA stability.
FIG. 5.
Rap1p binding sites mediate the repression of RP mRNAs. ypt6-1 rpl30Δ::HIS3 strains containing constructs A to D (strains BL174 to BL177; Table 1) and strain JW1201, in which RPL30-ACT1 is the only source of actin sequences, were grown to log phase at 23°C and then shifted to 37°C. Aliquots were harvested at the indicated times, and RNA was prepared and analyzed as described previously.
Rap1p binding sites mediate RP gene repression.
If the RPL30-ACT1 construct is repressed while the ACT1-RPL30 transcript is not, the influence of the secretory pathway on RPL30 must depend solely on sequences upstream of the transcription initiation site. In an attempt to identify which sequences are involved, the UAS of the ACT1 promoter of construct A was replaced with fragments of the RPL30 promoter (constructs B, C, and D shown in Fig. 4C). Plasmids carrying these genes were used to transform BL17, a diploid strain with the genotype RPL30/rpl30Δ::HIS3 YPT6/ypt6-1. Following sporulation and dissection of tetrads, strains carrying ypt6-1 and with constructs A to D as the only source of L30 were identified (strains BL174 to BL177 in Table 1).
At the permissive temperature, the level of mRNA derived from each of the constructs was about the same (Fig. 5, zero time), suggesting that the two Rap1p sites (construct D) are sufficient to substitute for the UAS of ACT1. Upon a shift to the nonpermissive temperature, there is a rapid decline in the levels of mRNA derived from constructs B, C, and D. Clearly the presence of Rap1p binding sites, either with (construct B) or without (construct D) T-rich regions, makes the test gene responsive to the secretion defect. These results suggest that in this context the 40-bp sequence containing the two Rap1p binding sites is a sufficient cis element to effect repression in response to a defect in the secretory pathway.
It has been reported that Rap1p is degraded in cells depleted of cAMP (39), resulting in the reduced transcription of RP genes. To determine if limiting Rap1p lies behind the repression of RP transcription in response to a defect in the secretory pathway, we overexpressed Rap1p in both W303 and 169ts, by transforming each strain with a 2μm plasmid carrying wt RAP1. The shift of the transformants from 23 to 37°C led to a loss of RP mRNA that was indistinguishable from that shown in Fig. 1 and 5 (data not shown), suggesting that the repression of RP gene transcription is not due to limiting Rap1p.
The SIR complex is not involved in the repression induced by secretion defects.
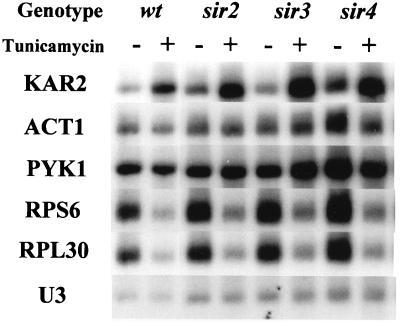
While Rap1p is a major transcriptional activator, it is also a major transcriptional silencer, at the silent mating type loci and at telomeric regions (reviewed in reference 49). In both cases, Rap1p recruits Sir3p and Sir4p to form a complex that inhibits the transcription of the adjacent genes (38). Since our results implicate Rap1p in mediating the repression of RP gene transcription, we asked if Sir3p and Sir4p are involved in the repression. The effects of tunicamycin on the mRNA levels of RP genes and others were determined in strains lacking components of the SIR complex (Fig. 6). It is apparent that the presence of tunicamycin leads to a substantial repression of the transcripts of both RPS6 and RPL30, with little effect on ACT1 or PYK1. The deletion of SIR2, SIR3, or SIR4 does not alter the repression of the RP genes. The induction of KAR2 is an expression of the “unfolded protein response” demonstrating that the tunicamycin is active on these cells (4).
FIG. 6.
The SIR complex does not mediate the repression of RP genes in response to a defect in the secretory pathway. Cultures of strains YDS2 (wt), YDS714 (sir2Δ), YDS430 (sir3Δ), and YDV122 (sir4Δ) were grown in YPD at 30°C. One half of each culture was treated with 1 μg of tunicamycin per ml for 2 h. All eight cultures were harvested on crushed ice, and RNA was prepared and subjected to Northern analysis, by using several probes.
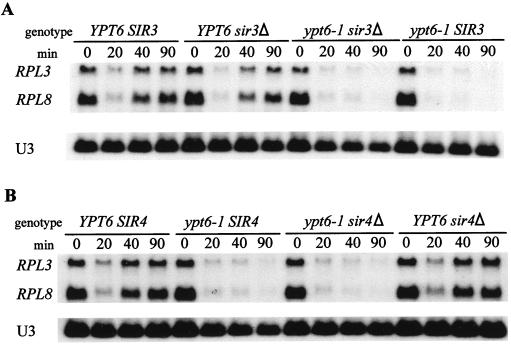
As a more direct test, strains containing null alleles of either SIR3 (YDS430) or SIR4 (YDV122) were crossed with 169ts, the strain carrying ypt6-1. From each diploid, we selected a tetrad that provided the four combinations of the YPT6 and SIR3 alleles (strains BL180 to BL183; Table 1) or the SIR4 allele (strains BL185 to BL188; Table 1). If either Sir3p or Sir4p were essential for the repression, its absence would eliminate the repression of RP mRNAs in cells carrying the ypt6-1 allele. As shown in Fig. 7, the repression of the transcription of RP genes, induced either in YPT6 cells by heat shock or in ypt6-1 cells by a failure in the secretory pathway, depends neither on Sir3p nor on Sir4p.
FIG. 7.
Neither Sir3p (A) nor Sir4p (B) is involved in the repression of RP gene transcription in a sec mutant or during heat shock. Strains of the indicated genotypes (Table 1) were grown to log phase in YPD at 23°C. An aliquot was harvested, the cultures were shifted to 37°C, and aliquots were harvested at the indicated times. RNA was prepared and analyzed as described previously, except that all RNAs were detected with 32P-labelled oligonucleotide probes.
Sir2p is not necessary for repression of either rRNA or RP genes.
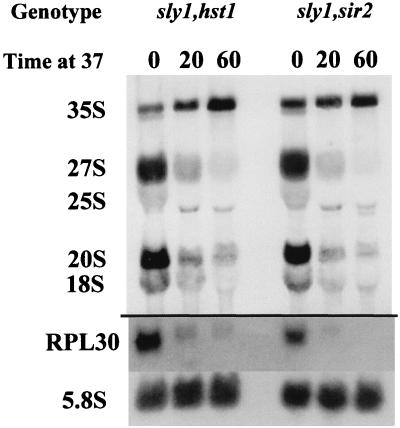
Sir2p is another participant in the repression of the silent mating type loci, although its relationship to Rap1p is less clear than that of Sir3p and Sir4p. Surprisingly, Sir2p has recently been shown to participate in the silencing of a Pol II-transcribed gene within the ribosomal DNA locus (52), although it has not been implicated directly in the control of rRNA transcription. To determine whether Sir2p plays a role in the repression of ribosome synthesis, we deleted SIR2 or its close relative HST1, which can partially substitute for SIR2 in silencing (5), from a strain carrying a ts mutation in sly1, an essential component of the secretory pathway (36), yielding strains JW1210 and JW1211. The results of shifting these two strains to the nonpermissive temperature are shown in Fig. 8. In this case, the cells were grown in a medium lacking methionine and pulsed for 3 min with [C3H3]methionine as a measure of rRNA transcription. RNA prepared from the cells was separated on an acrylamide gel, the upper portion was subjected to fluorography, and the lower portion was subjected to Northern analysis. From the latter it is apparent that the mRNA for L30 disappears rapidly, just as in the ypt6-1 strain shown in Fig. 1. C3H3 groups are incorporated predominantly into 35S rRNA, which is then processed through the intermediate 27S and 20S species to the mature 25S and 18S rRNAs (56). In a 3-min pulse (Fig. 8, 0-min lanes), most of the radioactivity has already passed into the 27S and 20S intermediates, and some is in mature 18S rRNA. We have previously shown that the transfer to the nonpermissive temperature causes a rapid repression of rRNA transcription in sly1-1 cells, with little effect on wt cells (36). Similarly, the transfer of the sly1-1 sir2Δ or the sly1-1 hst1Δ double mutant strains to the nonpermissive temperature leads to a strong inhibition of the incorporation of C3H3 into rRNA (Fig. 8, 20- and 60-min lanes), just as for the cells carrying sly1-1 alone. What little 35S RNA is formed is processed slowly if at all, presumably due to a lack of RPs. We conclude that neither Sir2p nor Hst1p is involved in the repression of ribosome synthesis in response to a defect in the secretory pathway.
FIG. 8.
Neither Sir2p nor Hst1p is involved in the repression of ribosome synthesis in response to a defect in the secretory pathway. Cultures of strains JW1210 and JW1211 were grown in methionine-free dropout medium at the permissive temperature of 23°C. At zero time an aliquot was labelled with 60 μCi [C3H3]-methionine for three minutes and poured onto crushed ice. The remainder of the culture was shifted to 37°C, and aliquots were similarly pulsed with [C3H3]-methionine after 20 and 60 min. RNA was prepared, and equal amounts were subjected to polyacrylamide gel electrophoresis. The upper portion of the gel was impregnated with En3Hance and subjected to fluorography to show the incorporation of C3H3 groups into rRNA. The lower portion was used for Northern analysis with probes against RPL30 and against 5.8S rRNA as a loading control (5.8S rRNA has no methyl groups).
Non-Rap1p binding sites also mediate RP gene repression.
Although Rap1p binding sites contribute most of the transcriptional activation of RP genes, some activity remains after the deletion of the Rap1p binding site, due to the T-rich elements (41, 45, 47). This residual activity of the RP genes still responds to heat shock, implicating sequences other than the Rap1p sites in the regulation of transcription of these genes. To identify such sequences and to determine if they are also involved in the response to a secretion defect, we developed a series of constructs based on the reporter gene RPL30-GFP, in which the ORF of GFP replaced the ORF of L30, starting with amino acid 4 in the second exon of RPL30 (Fig. 9A, construct E). The spliced transcripts contain the 5′ untranslated region of RPL30, seven nucleotides from the RPL30 ORF, the GFP ORF, and the 3′ untranslated region of RPL30. Note that the repression of RPL30 is independent of sequences downstream of the transcription initiation site (ACT-RPL30; Fig. 5, lanes 1 to 5). Increasing portions of the RPL30 promoter were replaced with four Gal4p binding sites derived from the GAL1 promoter, generating constructs F, G, and H (Fig. 9A). The reporter gene constructs, on CEN-based plasmids, were transformed into cells carrying the ypt6-1 allele. The necessary L30 protein is supplied by the genomic RPL30. Since the GFP ORF is 400 nucleotides longer, the RPL30 and RPL30-GFP mRNAs can be compared directly on Northern blots by using a probe complementary to the first exon of RPL30.
For the first set of experiments the cells were grown in glucose to repress transcription from the GAL1 region, thereby revealing the transcriptional contribution of the residual RPL30 sequences (Fig. 9B). In a ypt6-1 background, following a temperature shift, construct E (RPL30-GFP) is repressed as severely as endogenous RPL30, confirming that the promoter of the RP genes mediates the repression caused by a failure in the secretory pathway. Deletion of the two Rap1p binding sites, leaving the T-rich domains (construct F), leads to the loss of 90% of the transcriptional activity. The 10% of the activity remaining is repressed as severely as endogenous RPL30. The presence of a single T-rich domain (construct G) has only 5% of normal transcription, also repressible. Deletion of both Rap1 sites and both T-rich domains (construct H) leads to a loss of any detectable transcription. These results confirm that the T-rich region can activate some transcription of RPL30, consistent with the observations for the other RP genes RPL28, RPS14, and RPL25 (41, 45, 47). Nevertheless, upon a temperature shift whatever residual transcription that remains is repressed in the ypt6-1 mutant (Fig. 9B). This result, in the absence of Rap1p sites, implicates the remaining downstream elements of the RPL30 promoter in the repression of transcription.
Growth of these strains in a medium containing galactose permits Gal4p binding to the sites present in constructs F, G, and H, activating transcription, at the permissive temperature, to slightly higher levels than with the RPL30 promoter itself (constructs F, G, and H; Fig. 9C). Nevertheless, when the cells are shifted from 23 to 37°C, the transcription of constructs F, G, and H is strongly repressed. The level of GAL1 transcripts is not dramatically affected, at least at 20 and 40 min after the temperature shift (Fig. 9C). Since the t1/2 of GAL1 transcripts is <5 min (3) (Fig. 3A), Gal4p-promoted transcription must be continuing. Comparison of construct E with constructs F, G, and H suggests that a defect in the secretory pathway can largely, though not completely, repress the transcription of an RP gene that is driven by a novel activator, in this case Gal4p. As the results for constructs F and H barely differ, we again conclude that the T-rich regions play little role in the repression of transcription. Thus, the repression of construct H suggests that the 180 bp that lie between the T-rich elements and the origin of transcription of RPL30 contain sequence elements that are sufficient for a major proportion of the sec-dependent repression of transcription of an RP gene. Comparing the data from Fig. 5 and 9 leads us to conclude that either the Rap1p sites or the 180-bp region will respond to the repression effected by a failure in the secretory pathway. In a different context, Neuman-Silberberg et al. (39) also concluded that Rap1p-binding sites and downstream sequences could play separate roles in the regulation of RP transcription.
Is TAF145 responsible for the repression of RP gene transcription?
A recent report suggests that the transcription of many of the RP genes is particularly vulnerable to a mutation in the transcription factor TAFII145 (48). Perhaps TAFII145 is responsible for the effects of the 180-nucleotide region implicated by construct H (Fig. 9B), since this region contains the TATA box with which TAFII145 is presumably associated. However, while both IPP1 (48) and ACT1 (our unpublished data) are severely repressed by ts mutants of TAFII145, they are not repressed by a ts mutant in the secretory pathway (IPP1; data not shown). Finally, a genome-wide analysis of the transcriptional effects of a ts allele of TAFII145 suggests little specificity for RP genes (17). Thus, it appears that TAFII145 is not the agent responsible for the repression of transcription of the RP genes under these conditions.
DISCUSSION
Turnover of RP mRNA.
The determination of the intrinsic t1/2 of an mRNA presents several experimental uncertainties. The most direct but most demanding method is the approach to equilibrium, which requires not only sensitive hybridization methods but also the determination of the approach to equilibrium of the nucleotide pools (12, 23). Alternate methods require turning off the transcription of all mRNA, using either inhibitors or the ts allele of rpb1 (reviewed in reference 2). The former have proved inconsistent in yeast. The latter involves three perturbations of the cell: the raising of the temperature, which brings the heat shock response into play, the inhibition of any mRNA that might play a direct role in the t1/2 of a specific mRNA, and the gradual loss of all the cell’s mRNAs, which can change the dynamic of translation and, consequently, of turnover. A more specific approach is the use of a repressible promoter, such as GAL1, with which one can turn off the expression of a limited number of genes.
It is clear from Fig. 1, 2, and 3, as well as from Table 2, that the decline of RP mRNA after a temperature shift, in either wt or sec cells, is similar to that observed when the RP mRNA is under the control of the GAL1 promoter that is suddenly repressed. This result has two major implications. It suggests that a heat shock temporarily represses and a sec mutant permanently represses RP transcription to nearly the same degree as glucose does the Gal4p-driven genes. It also suggests that the intrinsic t1/2 of RP mRNAs can be estimated from the decline of the mRNA after a heat shock; for most RP mRNAs that value would be <10 min at 37°C (9, 61). For some genes we have made an independent determination of the t1/2, based on approach-to-equilibrium labelling; for RPL3 it is 13 min, and for RPL30 it is 16.6 min (23). (Note that those two genes were known as Rp1 and Rp73, respectively, at that time.) This was carried out on cells growing in a synthetic medium at 23°C with a doubling time of 132 min, and thus the values are likely to be an underestimate compared to those based on cells growing in YPD at 37°C. Indeed, in our studies the t1/2 of the GAL1-RPL30 mRNA increases from 5 to 11 min as the temperature is lowered from 37 to 23°C (Table 2).
We suggest, therefore, that the intrinsic t1/2 of the mRNA encoding L30 is between 5 and 7 min at 37°C. Thus, the decline of this mRNA after cells are shifted from 23 to 37°C can be ascribed solely to a repression of transcription, without the need to invoke an activation of turnover (15).
The data shown in Fig. 1 to 3 also suggest that the use of the rpb1-1 mutant may lead to severely misleading estimates of the t1/2 of mRNAs. This could be either a general effect due to the pleiotropic consequences of halting all Pol II transcription or a specific effect on the genes subject to severe repression.
One might ask why the t1/2 of RP mRNAs is so short, since replacing them at frequent intervals seems an unnecessary use of resources. Indeed, the t1/2s of the mRNAs encoding the abundant glycolytic enzymes are much longer (14). An explanation may be that the level of production of RPs must be closely monitored (27). Because the RPs participate in the assembly of a complex structure and because they are generally strong RNA-binding proteins, an excess of an RP may be far more deleterious to the cell than an excess of a glycolytic enzyme. Therefore, it seems likely that there is selective pressure to maintain a short t1/2 for RP mRNAs in order to more closely control the relative production of the many RPs.
General considerations regarding transcription of RP genes.
Before discussing our data regarding the control of transcription of RP genes, we argue on two grounds, magnitude and coordination, for the potential special nature of RP gene transcription and thus for the likelihood that it has unusual features.
(i) The transcription of ribosomal proteins is a major portion of the Pol II activity of the cell. As measured by SAGE analysis (57), RP genes account for 20 of the 30 most abundant mRNAs; each RP gene is represented, on average, by about 30 to 50 mRNAs per cell (our analysis of data provided by the authors of reference 57). The 137 RP genes, therefore, would account for >4,000 of the mRNAs in the cell. A more direct measurement has recently been reported by Holstege et al. (17). Based on an estimate of 15,000 mRNAs per cell, they determined that 132 of the 137 RP genes contributed 4,437 mRNAs, about 30% of the total. This is a reasonable number because the ∼15,000,000 ribosomal proteins (∼200,000 ribosomes/cell × 78 proteins/ribosome) make up about 15% of the protein mass of the cell, and even more of the protein number, since they average only ∼150 amino acids in length. As shown in Table 2, the t1/2 of an RP mRNA is 5 to 7 min. This value is consistent with our observations of the effect of heat shock on the mRNA levels of many RP genes (11). It is also consistent with the data recently reported by Eisen et al. (9), in which the mRNA level for most RP genes had declined to less than 20% of normal by 20 min after a heat shock. Yet, mRNAs encoding most other genes have a t1/2 of >15 min (17) (based on the use of the rpb1-1 allele, which admittedly may be misleading). Thus, if RP mRNAs account for 30% of the total mRNAs yet have a t1/2 that is substantially shorter than those of most other mRNAs, we are led to conclude that the RP genes account for nearly 50% of all Pol II initiation events.
(ii) As would be expected for genes encoding components of a molecular machine, the 137 RP genes appear to be regulated in lockstep (reviewed in reference 44). This is true of responses to heat shock (11, 24), to a defect in the secretory pathway (34 and this paper), to growth conditions such as C source (16, 22), to levels of cAMP (25, 39), and to the deprivation of amino acids (37, 62). During the growth cycle, RP genes are repressed as the cells enter late log phase (6, 21) and are induced dramatically within 10 min after stationary cells are diluted into a fresh medium (unpublished data). By using classical methods no exceptions have been found among the 20 or so proteins whose mRNAs have been studied or among the 50 or so proteins whose synthesis has been studied in a few situations (11). Very recent data from a genome-wide analysis of a few conditions, e.g., heat shock (9) and diauxie (6), suggest that none of the RP genes escapes from this coordinate regulation, although a few genes with apparently intermediate results will require more direct analysis.
It is intriguing to consider why S. cerevisiae has evolved to utilize transcription as its primary method to regulate the production of RPs, while both eubacteria (reviewed in reference 64) and vertebrates (reviewed in reference 33) have chosen to regulate RP synthesis largely at translation, albeit in very different ways. Indeed, a recent transcriptome analysis of mouse fibroblasts during the transition from stationary to growth phase found almost no change in the levels of RP mRNAs (19), although there is a substantial increase in the rate of RP synthesis (55).
Role of Rap1p in the repression of RP gene transcription.
Rap1p is the major factor activating the transcription of nearly all of the RP genes, as well as many other genes with abundant transcripts, such as those encoding elongation factor 1α (EF1α) and the enzymes of the glycolytic pathway. Rap1p is also the primary element of the complex that silences both the silent mating type loci and genes adjacent to telomeres (49). Therefore, Rap1p is a likely candidate for effecting the silencing of the RP genes in response to stress. Indeed, we find that just 40 bp containing the two Rap1p binding sites of the RPL30 gene are sufficient to make the ACT1 promoter respond like an RP gene to a defect in the secretory pathway (Fig. 5, construct D). Furthermore, in cells carrying the rap1-17 allele, which encodes a truncated form of Rap1p that retains both its DNA binding domains and its activation domain but not its silencing domain, the RP genes are no longer silenced in response to a defect in the secretory pathway (35), although temporary silencing in response to a temperature shift still occurs. This effect of the rap1-17 allele is also true for genes that have no Rap1p binding sites, such as RPL3 (35) and construct G shown in Fig. 9A (data not shown). This observation not only implicates Rap1p in the pathway between the secretory system and the RP genes but also suggests that a different pathway is used for the repression of RP gene transcription in response to a temperature shift.
Yet several facts suggest that conventional silencing by Rap1p is not responsible for the repression of RP genes. Abf1p, rather than Rap1p, is the major transcription factor for several RP genes that are also repressed in response to a defect in the secretory pathway, e.g., RPL3 (Fig. 1). In addition, Rap1p-mediated silencing at telomeres and at silent mating type loci requires Sir3p and Sir4p (31), neither of which is necessary for the silencing of RP genes (Fig. 6). Finally, sequences downstream of the Rap1p binding sites, adjacent to the promoter of RPL30, will silence the transcription driven by Gal4p, in response to a temperature shift or to a defect in the secretory pathway (Fig. 9). These sequences contain the putative TATA box and presumably bind TBP and its associated TAFs, as well as the sequences adjacent to the transcription initiation site.
Thus, our attempts to identify the cis-acting sequences of RP genes that mediate the repression of transcription have led to an apparent contradiction. Either the Rap1p binding sites or the promoter-proximal sequences can play such a role. Yet the effect of the rap1-17 allele suggests that Rap1p is necessary for silencing in response to a defect in the secretory pathway. Our conclusion, then, is that for Rap1p to activate the transcription of an RP gene it must bind to upstream sequences, yet for Rap1p to repress the transcription of an RP gene it need not bind to the gene directly. It remains to be seen how this fascinating protein can pull off such a trick.
Whatever the mechanism, the sudden silencing of the RP genes, which account for 50% of Pol II activity, must have a dramatic effect on the overall transcriptional economy of the cell. What influence does this sudden release of transcriptional potential have on the transcription of other genes?
ACKNOWLEDGMENTS
We are grateful to Josep Vilardell for a close reading of the manuscript, to Ian Willis, Roy Parker, Allan Sachs, Christine Brown, and Allan Jacobson for useful discussions, to Jung-Hoon Sohn for communicating unpublished data, to David Shore, Art Lustig, and Myra Derbyshire for strains and plasmids, and to Mary Studeny and Saqui Huq for technical assistance.
This research was partially supported by NIH grants GM25532 to J.R.W. and CA13330 to the Albert Einstein Cancer Center.
REFERENCES
- 1.Brand A H, Micklem G, Nasmyth K. A yeast silencer contains sequences that can promote autonomous plasmid replication and transcriptional activation. Cell. 1987;51:709–719. doi: 10.1016/0092-8674(87)90094-8. [DOI] [PubMed] [Google Scholar]
- 2.Caponigro G, Parker R. Mechanisms and control of mRNA turnover in Saccharomyces cerevisiae. Microbiol Rev. 1996;60:233–249. doi: 10.1128/mr.60.1.233-249.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavalli G, Thoma F. Chromatin transitions during activation and repression of galactose-regulated genes in yeast. EMBO J. 1993;12:4603–4613. doi: 10.1002/j.1460-2075.1993.tb06149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox J S, Shamu C E, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 5.Derbyshire M K, Weinstock K G, Strathern J. HST1, a new member of the SIR2 family of genes. Yeast. 1996;12:631–640. doi: 10.1002/(SICI)1097-0061(19960615)12:7%3C631::AID-YEA960%3E3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 6.DeRisi J L, Iyer V R, Brown P O. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 7.Donovan D M, Pearson N J. Transcriptional regulation of ribosomal proteins during a nutritional upshift in Saccharomyces cerevisiae. Mol Cell Biol. 1986;6:2429–2435. doi: 10.1128/mcb.6.7.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorsman J C, Doorenbosch M M, Maurer C T, de Winde J H, Mager W H, Planta R J, Grivell L A. An ARS/silencer binding factor also activates two ribosomal protein genes in yeast. Nucleic Acids Res. 1989;17:4917–4923. doi: 10.1093/nar/17.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisen M B, Spellman P T, Brown P O, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eng F J, Warner J R. Structural basis for the regulation of splicing of a yeast messenger RNA. Cell. 1991;65:797–804. doi: 10.1016/0092-8674(91)90387-e. [DOI] [PubMed] [Google Scholar]
- 11.Gorenstein C, Warner J R. Coordinate regulation of the synthesis of eukaryotic ribosomal proteins. Proc Natl Acad Sci USA. 1976;73:1547–1551. doi: 10.1073/pnas.73.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg J R. High stability of messenger RNA in growing cultured cells. Nature. 1972;240:102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- 13.Hamil K G, Nam H G, Fried H M. Constitutive transcription of yeast ribosomal protein gene TCM1 is promoted by uncommon cis- and trans-acting elements. Mol Cell Biol. 1988;8:4328–4341. doi: 10.1128/mcb.8.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrick D, Parker R, Jacobson A. Identification and comparison of stable and unstable mRNAs in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:2269–2284. doi: 10.1128/mcb.10.5.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herruer M H, Mager W H, Raue H A, Vreken P, Wilms E, Planta R J. Mild temperature shock affects transcription of yeast ribosomal protein genes as well as the stability of their mRNAs. Nucleic Acids Res. 1988;16:7917–7929. doi: 10.1093/nar/16.16.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herruer M H, Mager W H, Woudt L P, Nieuwint R T, Wassenaar G M, Groeneveld P, Planta R J. Transcriptional control of yeast ribosomal protein synthesis during carbon-source upshift. Nucleic Acids Res. 1987;15:10133–10144. doi: 10.1093/nar/15.24.10133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holstege F C P, Jennings E G, Wyrick J J, Lee T I, Hengartner C J, Green M R, Golub T R, Lander E S, Young R A. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- 18.Imai Y, Matsushima Y, Sugimura T, Terada M. A simple and rapid method for generating a deletion by PCR. Nucleic Acids Res. 1991;19:2785. doi: 10.1093/nar/19.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer V R, Eisen M B, Ross D T, Schuler G, Moore T, Lee J C F, Trent J M, Staudt L M, Hudson J, Boguski M S, Lashkari D A, Shalon D, Botstein D, Brown P O. The transcriptional program in response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 20.Johnston M, Carlson M. Regulation of carbon and phosphate utilization. In: Jones E W, Pringle J R, Broach J R, editors. The molecular and cellular biology of the yeast Saccharomyces: gene expression. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 193–281. [Google Scholar]
- 21.Ju Q, Warner J R. Ribosome synthesis during the growth cycle of Saccharomyces cerevisiae. Yeast. 1994;10:151–157. doi: 10.1002/yea.320100203. [DOI] [PubMed] [Google Scholar]
- 22.Kief D R, Warner J R. Coordinate control of syntheses of ribosomal ribonucleic acid and ribosomal proteins during nutritional shift-up in Saccharomyces cerevisiae. Mol Cell Biol. 1981;1:1007–1015. doi: 10.1128/mcb.1.11.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim C H, Warner J R. Messenger RNA for ribosomal proteins in yeast. J Mol Biol. 1983;165:79–89. doi: 10.1016/s0022-2836(83)80243-5. [DOI] [PubMed] [Google Scholar]
- 24.Kim C H, Warner J R. Mild temperature shock alters the transcription of a discrete class of Saccharomyces cerevisiae genes. Mol Cell Biol. 1983;3:457–465. doi: 10.1128/mcb.3.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein C, Struhl K. Protein kinase A mediates growth-regulated expression of yeast ribosomal protein genes by modulating RAP1 transcriptional activity. Mol Cell Biol. 1994;14:1920–1928. doi: 10.1128/mcb.14.3.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lascaris R F, Mager W H, Planta R J. DNA-binding requirements of the yeast protein Rap1p as selected in silico from ribosomal gene promoter sequences. Bioinformatics. 1999;15:267–277. doi: 10.1093/bioinformatics/15.4.267. [DOI] [PubMed] [Google Scholar]
- 27.Li B, Vilardell J, Warner J R. An RNA structure involved in feedback regulation of splicing and of translation is critical for biological fitness. Proc Natl Acad Sci USA. 1996;93:1596–1600. doi: 10.1073/pnas.93.4.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B, Warner J R. Mutation of the Rab6 homologue of Saccharomyces cerevisiae, YPT6, inhibits both early Golgi function and ribosome biosynthesis. J Biol Chem. 1996;271:16813–16819. doi: 10.1074/jbc.271.28.16813. [DOI] [PubMed] [Google Scholar]
- 29.Liang S, Lacroute F, Kepes F. Multicopy STS1 restores both protein transport and ribosomal RNA stability in a new yeast sec23 mutant allele. Eur J Cell Biol. 1993;62:270–281. [PubMed] [Google Scholar]
- 30.Lohr D, Venkov P, Zlatanova J. Transcriptional regulation in the yeast GAL gene family: a complex genetic network. FASEB J. 1995;9:777–787. doi: 10.1096/fasebj.9.9.7601342. [DOI] [PubMed] [Google Scholar]
- 31.Lustig A J. Mechanisms of silencing in Saccharomyces cerevisiae. Curr Opin Genet Dev. 1998;8:233–239. doi: 10.1016/s0959-437x(98)80146-9. [DOI] [PubMed] [Google Scholar]
- 32.Mager W H, Planta R J, Ballesta J P, Lee J C, Mizuta K, Suzuki K, Warner J R, Woolford J L., Jr A new nomenclature for the cytoplasmic ribosomal proteins of Saccharomyces cerevisiae. Nucleic Acids Res. 1997;25:4872–4875. doi: 10.1093/nar/25.24.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyuhas O, Avni D, Shama S. Translational control of ribosomal protein mRNAs in eukaryotes. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 363–388. [Google Scholar]
- 33a.MIPS. 26 January 1998, posting date. Yeast—a new nomenclature for the cytoplasmic ribosomal proteins of Saccharomyces cerevisiae. [Online.] http://www.mips.biochem.mpg.de/proj/yeast/reviews/rib_nomencl.html. [8 June 1999, last date accessed.]
- 34.Mizuta K, Hashimoto T, Suzuki K, Otaka E. Yeast ribosomal proteins. XII. YS11 of Saccharomyces cerevisiae is a homologue to E. coli S4 according to the gene analysis. Nucleic Acids Res. 1991;19:2603–2608. doi: 10.1093/nar/19.10.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizuta K, Tsujii R, Warner J R, Nishiyama M. The C-terminal silencing domain of Rap1p is essential for the repression of ribosomal protein genes in response to a defect in the secretory pathway. Nucleic Acids Res. 1998;26:1063–1069. doi: 10.1093/nar/26.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizuta K, Warner J R. Continued functioning of the secretory pathway is essential for ribosome synthesis. Mol Cell Biol. 1994;14:2493–2502. doi: 10.1128/mcb.14.4.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moehle C M, Hinnebusch A G. Association of RAP1 binding sites with stringent control of ribosomal protein gene transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:2723–2735. doi: 10.1128/mcb.11.5.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moretti P, Freeman K, Coodly L, Shore D. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes Dev. 1994;8:2257–2269. doi: 10.1101/gad.8.19.2257. [DOI] [PubMed] [Google Scholar]
- 39.Neuman-Silberberg F S, Bhattacharya S, Broach J R. Nutrient availability and the RAS/cyclic AMP pathway both induce expression of ribosomal protein genes in Saccharomyces cerevisiae but by different mechanisms. Mol Cell Biol. 1995;15:3187–3196. doi: 10.1128/mcb.15.6.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nierras C R, Warner J R. Protein kinase C enables the regulatory circuit that connects membrane synthesis to ribosome synthesis in S. cerevisiae. J Biol Chem. 1999;274:13235–13241. doi: 10.1074/jbc.274.19.13235. [DOI] [PubMed] [Google Scholar]
- 41.Nieuwint R T, Mager W H, Maurer K C, Planta R J. Mutational analysis of the upstream activation site of yeast ribosomal protein genes. Curr Genet. 1989;15:247–251. doi: 10.1007/BF00447039. [DOI] [PubMed] [Google Scholar]
- 42.Nonet M, Scafe C, Sexton J, Young R. Eukaryotic RNA polymerase conditional mutant that rapidly ceases mRNA synthesis. Mol Cell Biol. 1987;7:1602–1611. doi: 10.1128/mcb.7.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker R, Herrick D, Peltz S W, Jacobson A. Measurement of mRNA decay rates in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:415–423. doi: 10.1016/0076-6879(91)94032-8. [DOI] [PubMed] [Google Scholar]
- 44.Planta R J. Regulation of ribosome synthesis in yeast. Yeast. 1997;13:1505–1518. doi: 10.1002/(SICI)1097-0061(199712)13:16<1505::AID-YEA229>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 45.Rotenberg M O, Woolford J L., Jr Tripartite upstream promoter element essential for expression of Saccharomyces cerevisiae ribosomal protein genes. Mol Cell Biol. 1986;6:674–687. doi: 10.1128/mcb.6.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt M E, Brown T A, Trumpower B L. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990;18:3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwindinger W F, Warner J R. Transcriptional elements of the yeast ribosomal protein gene CYH2. J Biol Chem. 1987;262:5690–5695. [PubMed] [Google Scholar]
- 48.Shen W-C, Green M R. Yeast TAFII145 functions as a core promoter selectivity factor, not a general coactivator. Cell. 1997;90:615–624. doi: 10.1016/s0092-8674(00)80523-1. [DOI] [PubMed] [Google Scholar]
- 49.Shore D. RAP1: a protean regulator in yeast. Trends Genet. 1994;10:408–412. doi: 10.1016/0168-9525(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 50.Shore D, Nasmyth K A. Purification and cloning of a DNA binding protein from yeast that binds to both silencer and activator elements. Cell. 1987;51:721–732. doi: 10.1016/0092-8674(87)90095-x. [DOI] [PubMed] [Google Scholar]
- 51.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith J S, Boeke J D. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 1997;11:241–254. doi: 10.1101/gad.11.2.241. [DOI] [PubMed] [Google Scholar]
- 53.Sussel L, Shore D. Separation of transcriptional activation and silencing functions of the RAP1-encoded repressor/activator protein 1: isolation of viable mutants affecting both silencing and telomere length. Proc Natl Acad Sci USA. 1991;88:7749–7753. doi: 10.1073/pnas.88.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas B J, Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- 55.Tushinski R J, Warner J R. Ribosomal proteins are synthesized preferentially in cells commencing growth. J Cell Physiol. 1982;112:128–135. doi: 10.1002/jcp.1041120119. [DOI] [PubMed] [Google Scholar]
- 56.Udem S A, Warner J R. Ribosomal RNA synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972;65:227–242. doi: 10.1016/0022-2836(72)90279-3. [DOI] [PubMed] [Google Scholar]
- 57.Velculescu V E, Zhang L, Zhou W, Vogelstein J, Basrai M A, Bassett D E, Jr, Hieter P, Vogelstein B, Kinzler K W. Characterization of the yeast transcriptome. Cell. 1997;88:243–251. doi: 10.1016/s0092-8674(00)81845-0. [DOI] [PubMed] [Google Scholar]
- 58.Vilardell J, Warner J R. Regulation of splicing at an intermediate step in the formation of the spliceosome. Genes Dev. 1994;8:211–220. doi: 10.1101/gad.8.2.211. [DOI] [PubMed] [Google Scholar]
- 59.Warner J R. Synthesis of ribosomes in Saccharomyces cerevisiae. Microbiol Rev. 1989;53:256–271. doi: 10.1128/mr.53.2.256-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warner J R. Labeling of RNA and phosphoproteins in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:423–428. doi: 10.1016/0076-6879(91)94033-9. [DOI] [PubMed] [Google Scholar]
- 61.Warner J R, Gorenstein C. The synthesis of eucaryotic ribosomal proteins in vitro. Cell. 1977;11:201–212. doi: 10.1016/0092-8674(77)90331-2. [DOI] [PubMed] [Google Scholar]
- 62.Warner J R, Gorenstein C. Yeast has a true stringent response. Nature. 1978;275:338–339. doi: 10.1038/275338a0. [DOI] [PubMed] [Google Scholar]
- 63.Wright J H, Gottschling D E, Zakian V A. Saccharomyces telomeres assume a non-nucleosomal chromatin structure. Genes Dev. 1992;6:197–210. doi: 10.1101/gad.6.2.197. [DOI] [PubMed] [Google Scholar]
- 64.Zengel J M, Lindahl L. Diverse mechanisms for regulating ribosomal protein synthesis in Escherichia coli. Prog Nucleic Acid Res Mol Biol. 1994;47:331–370. doi: 10.1016/s0079-6603(08)60256-1. [DOI] [PubMed] [Google Scholar]