Abstract
Symptomatic adjacent segment disease (ASD) is a common challenge after anterior cervical discectomy and fusion (ACDF). The objective of this study was to compare the biomechanical effects of a second ACDF and laminoplasty for the treatment of ASD after primary ACDF. We developed a finite element (FE) model of the C2-T1 based on computed tomography images. The FE models of revision surgeries of ACDF and laminoplasty were simulated to treat one-level and two-level ASD after primary ACDF. The range of motion (ROM) and intradiscal pressure (IDP) of the adjacent segments, and stress in the cord were analyzed to investigate the biomechanical effects of the second ACDF and laminoplasty. The results indicated that revision surgery of one-level ACDF increased the ROM and IDP at the C2–C3 segment, whereas two-level ACDF significantly increased the ROM and IDP at the C2–C3 and C7-T1 segments. Furthermore, no significant changes in the ROM and IDP of the laminoplasty models were observed. The stress in the cord of the re-laminoplasty model decreased to some extent, which was higher than that of the re-ACDF model. In conclusion, both ACDF and laminoplasty can relieve the high level of stress in the spinal cord caused by ASD after primary ACDF, whereas ACDF can achieve better decompression effect. Revision surgery of the superior ACDF or the superior and inferior ACDF after the primary ACDF increased the ROM and IDP at the adjacent segments, which may be the reason for the high incidence of recurrent ASD after second ACDF.
Keywords: adjacent segment degeneration, finite element analysis, revision surgery, anterior cervical discectomy and fusion, laminoplasty
Introduction
Anterior cervical discectomy and fusion (ACDF) is generally accepted as the standard surgical treatment for cervical degenerative diseases (Oglesby et al., 2013; Kelly et al., 2018). ACDF is recognized as a comparatively safe procedure associated with few complications. However, adjacent segment disease (ASD), defined as new symptoms at nerve roots or myelopathy and new radiographic evidence of degenerative changes at adjacent segments of previously fused segments, is one of the major problems after ACDF (Hilibrand and Robbins, 2004). In a retrospective study of 177 patients who underwent ACDF, radiographic and clinical ASD were found in 92.1 and 19.2% of patients, respectively; approximately 7% of the patients required revision surgery (Chung et al., 2014). Another study reported an incidence of 2.4% per year of revision surgery at adjacent segments after primary surgery, and the authors estimated that 22% of patients would require second surgery due to symptomatic ASD within a decade (Lee et al., 2015).
ASD can occur in the superior, inferior, or both adjacent levels, depending on the levels affected. Considering the clinical situation and secondary preoperative imaging findings, ASD can be treated by second ACDF, laminoplasty, and posterior fusion (Wang F. et al., 2017; Cao et al., 2020). ACDF decompresses the nerve roots and myelopathy by removing the herniated disc and posterior osteophytes, followed by restoration of the disc height and cervical lordosis by cages and bone graft (Schroeder et al., 2016; Muzević et al., 2018). Cervical laminoplasty was considered to be an effective method for the treatment of cervical degenerative stenosis as it expands the stenosed spinal canal (Yeh et al., 2014; Kurokawa and Kim, 2015). Posterior decompression and fusion can decompress the spinal cord and achieve immediate stabilization, thereby, preventing the occurrence of kyphotic (Du et al., 2014). Therefore, the appropriate surgical approaches for the treatment of ASD after ACDF still need to be studied.
The finite element (FE) analysis is an important method to study the spinal biomechanics (Nikkhoo et al., 2019; Cai et al., 2020b; Mesbah and Barkaoui, 2020). The range of motion (ROM), intradiscal pressure (IDP), facet joint stress, and stress in the cord can be calculated and analyzed to evaluate the biomechanical effects of different spine surgeries (Mesbah et al., 2020; Nikkhoo et al., 2020; Srinivasan et al., 2021). FE analysis can also be used to assess the risk of complications of spinal surgery, such as degeneration and internal implants fractures. However, the biomechanical evaluation of different surgical approaches for the treatment of ASD after ACDF has not been reported. In the present study, FE models with superior and two-level ASD after C4-C6 ACDF were conducted. The aim of this study was to compare the biomechanical effects of a second ACDF and laminoplasty for the treatment of ASD after primary ACDF.
Materials and Methods
Construction of Intact Cervical Model (C2–T1)
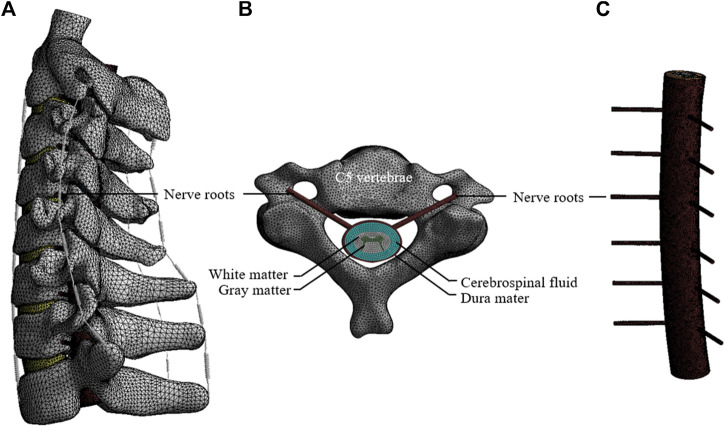
In this study, a three-dimensional FE model of C2–T1 segments was developed based on the computed tomography (CT) images of a healthy volunteer (male, 25 years old, 64 kg, and 176 cm). This study was approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology. Written informed consent was obtained from the volunteer. The CT images of the participant were obtained at intervals of 0.625 mm (Dual Source CT; Siemens, Munich, Germany). Mimics Research 20.0 software (Materialize, Leuven, Belgium) was used to reconstruct the geometric structure of the vertebrae. Hypermesh (Altair Engineering, Troy, Michigan, United States) was used to mesh and build the FE models of C2-T1 vertebrae. Afterwards, the FE models were analyses by ANSYS (ANSYS Ltd., Canonsburg, Pennsylvania, United States). This C2–T1 FE model could be divided into cancellous bone, cortical bone, intervertebral disc (IVD), facet joints, and ligaments (Figure 1A). The cortical bone was constructed as a shell with the thickness of 0.4 mm (Mo et al., 2017). The IVD consisted of annulus fibrosus and nucleus pulposus with the volume ratio to be 6:4. The IVD was considered as an elastic material referring to the previous studies (Wu et al., 2019; Li et al., 2021). The endplates were constructed as a shell with the thickness of 0.5 mm. The facet joints were assumed with 0.5-mm thick cartilage with nonlinear, surface-to-surface, frictionless sliding contact (Li et al., 2017). The ligaments consisted of anterior longitudinal ligament, posterior longitudinal ligament, ligamentum flavum, interspinous ligament, supraspinous ligament, capsular ligament, and intertransverse ligament. These ligaments were established using nonlinear tension-only Spring element (Guo et al., 2021; Lin et al., 2021). The material properties of the model are listed in Table 2 (Cai et al., 2020a; Hua et al., 2020).
FIGURE 1.
The FE model of the C2–T1. (A) The FE model of the cervical spine and spinal cord. (B) Axial view of the spinal cord at the C5 vertebrae. (C) Lateral view of the spinal cord model.
TABLE 2.
Material properties of the spinal cord.
| Materials | Material model | Material parameters |
|---|---|---|
| White matter | Hyperelastic (Ogden) | μ = 4.0 kPa, α= 12.5 |
| Gray matter | Hyperelastic (Ogden) | μ = 4.1 kPa, α= 14.7 |
| Dura mater and nerve roots | Elastic | E = 80 MPa, ν= 0.49 |
| Cerebrospinal fluid | Newtonian fluid | Viscosity = 0.001 Pa s |
In addition, the spinal cord was reconstructed according to the geometry of the cervical column and human spinal cord. The spinal cord model included white matter, gray matter, dura mater, nerve roots, and cerebrospinal fluid (CSF) layers (Figures 1B,C). The dural sheath was placed approximately 2.5 mm from the cord, since the CSF layer in the human cervical spine was reported to be 1.5–4.0 mm in a previous literature (Holsheimer et al., 1994). The white and gray matter were assumed as Hyperelastic element based on study (Ichihara et al., 2001). The dura mater and nerve roots were constructed with elastic element according to study (Persson et al., 2010). CSF was assumed as Newtonian fluid according to the viscosity of CSF (Brydon et al., 1995). A one-way Fluid-Solid Interaction analysis method was used to couple the interaction between the fluid and solid material. Material properties of the spinal cord model are listed in Table 1.
TABLE 1.
Material properties of the spinal structures.
| Component/materials | Young’s modulus E (MPa) | Poisson’s ratio | Element type |
|---|---|---|---|
| Cortical bone | 12000 | 0.29 | Shell93 |
| Cancellous bone | 450 | 0.29 | Solid187 |
| Posterior element | 3500 | 0.29 | Solid187 |
| Facet cartilage | 10.4 | 0.4 | Solid187 |
| Endplate | 500 | 0.4 | Shell93 |
| Nucleus pulposus | 1 | 0.49 | Solid187 |
| Annulus fibrosus | 3.4 | 0.4 | Solid187 |
| Anterior longitudinal Ligament | 30 | 0.3 | Spring (tension only) |
| Posterior longitudinal Ligament | 20 | 0.3 | Spring (tension only) |
| Ligamentum flavum | 1.5 | 0.3 | Spring (tension only) |
| Capsular Ligament | 20 | 0.3 | Spring (tension only) |
| Interspinous Ligament | 1.5 | 0.3 | Spring (tension only) |
| Supraspinous Ligament | 1.5 | 0.3 | Spring (tension only) |
| Intertransverse Ligament | 20 | 0.3 | Spring (tension only) |
| PEEK | 3000 | 0.3 | Solid187 |
| Bone graft | 450 | 0.29 | Solid187 |
| Titanium alloy | 110,000 | 0.3 | Solid187 |
| Degenerative annulus fibrosus | 4 | 0.45 | Solid187 |
| Degenerative nucleus pulposus | 4 | 0.49 | Solid187 |
| Osteophytes | 450 | 0.23 | Solid187 |
One-Level and Two-Level ASD Models After C4-C6 ACDF
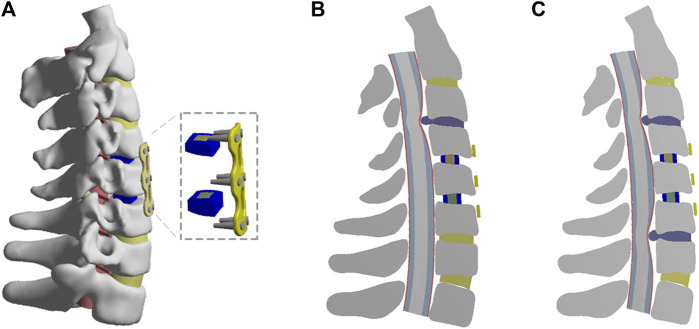
A recent study demonstrated that patients treated with one- or two-segment anterior cervical arthrodesis were more likely to develop ASD than those treated with three or more segments (Lee et al., 2015). Therefore, the ASD models after primary surgery were based on the C4-C6 ACDF model (Figure 2A). The C4-C6 ACDF model was constructed according to a previous study (Hua et al., 2020). In brief, annulus fibrosus and nucleus pulposus were partly resected and the polyetheretherketone (PEEK) cages with bone graft were placed in the intervertebral space. Then, solid fusion was achieved with anterior titanium alloy plates and titanium alloy screws. The one-level and two-level ASD models after C4-C6 ACDF were shown in Figures 2B,C. A moderate degeneration in the adjacent segment was modified to simulate ASD according to study (Cai et al., 2020a). The disc height was reduced by 50% relative to the height of the normal model. An osteophyte, one quarter of the size of the herniated disc, was constructed to simulate intervertebral disc calcification. An occupying ratio of 40% was assumed to simulate the spinal cord compression by ASD. The occupying ratio was defined as the ratio of the thickness of herniated disc to the anterior–posterior diameter of the spinal canal. The material properties of PEEK cages, bone graft, titanium alloy and degenerative intervertebral disc were also listed in Table 2.
FIGURE 2.
The FE models of C4-C6 ACDF and one-level or two-level ASD after primary ACDF. (A) The FE model of C4-C6 ACDF. (B) Cross-sectional views of the FE model of one-level ASD. (C) Cross-sectional views of the FE model of two-level ASD.
Anterior Surgical Models for One-Level or Two-Level ASD After C4-C6 ACDF
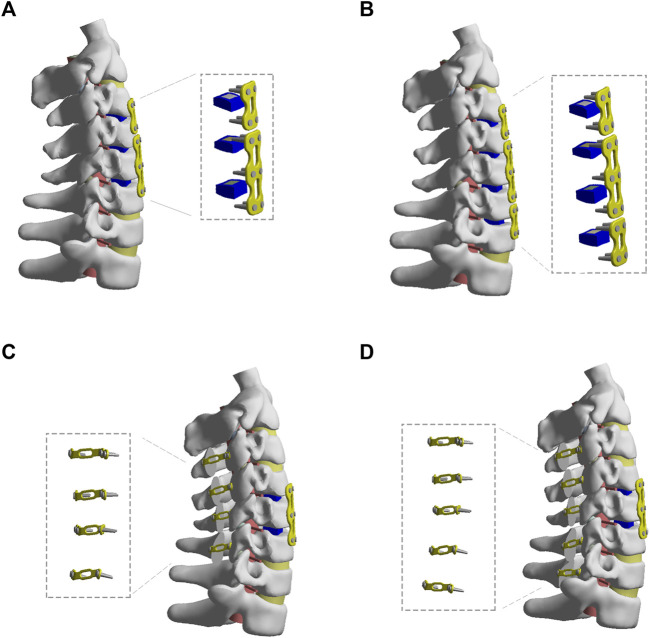
As shown in Figure 3A, for the treatment of one-level ASD after C4-C6 ACDF, an additional ACDF (re-ACDF) at C3-C4 level was constructed. As shown in Figure 3B, the second ACDF at C3-C4 and C6-C7 levels was simulated to treat two-level ASD after C4-C6 ACDF. The steps of ACDF are described above.
FIGURE 3.
The FE models of different revision surgeries after C4-C6 ACDF. (A) The revision surgery of C2-C3 ACDF. (B) The revision surgery of C3-C4 and C6-C7 ACDF. (C) The revision surgery of C3-C6 laminoplasty. (D) The revision surgery of C3-C7 laminoplasty.
Posterior Surgical Models for One-Level or Two-Level ASD After C4-C6 ACDF
As shown in Figures 3C,D, C3-C6 or C3-C7 laminoplasty (re-laminoplasty) was simulated to treat one-level or two-level ASD after C4-C6 ACDF, respectively. The laminoplasty models were developed based on conventional surgical protocols (Hirabayashi et al., 2010). Firstly, a longitudinal groove of 3 mm width was constructed between the lamina and lateral mass at hinge side of the lamina. Then, an opening width of 12 mm was made at the open side. The lamina was fixed using titanium alloy plates and screws.
Boundary and Loading Conditions
All models were fixed at the inferior surface of the T1 vertebrae. A follower load of 73.6 N combined with a moment of 1.0 Nm was applied over the superior surface of C2 to simulate the spinal motions of flexion, extension, lateral bending, and axial rotation (Mo et al., 2017; Zhao et al., 2018). The ROM, IDP, and maximum von-Mises stress in the cord were analyzed to investigate the biomechanical effects of the second ACDF and laminoplasty for the treatment of ASD after primary ACDF.
Results
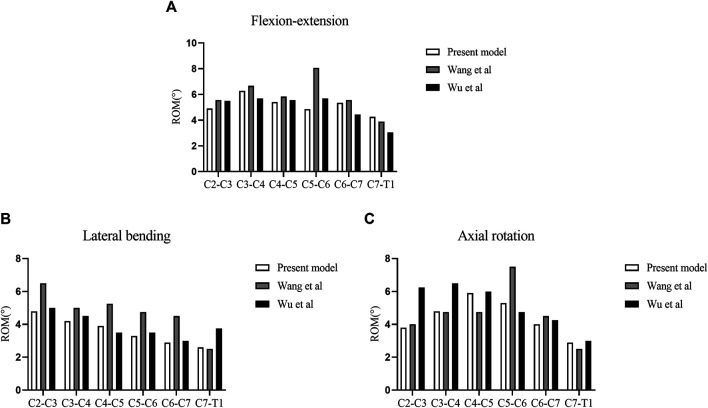
Model Validation
The FE model of the intact cervical spine used in this study was validated by comparison with previous biomechanical models (Wang K. et al., 2017; Wu et al., 2018). The ROM and IDP of each segment were consistent with those of previous studies (Figure 4; Supplementary Figure S1).
FIGURE 4.
Validation of the intact cervical model. (A) ROM in flexion-extension. (B) ROM in lateral bending. (C) ROM in axial rotation.
Analyses of the Biomechanical Effects of Different Surgical Approaches for the Treatment of One-Level ASD After Primary ACDF
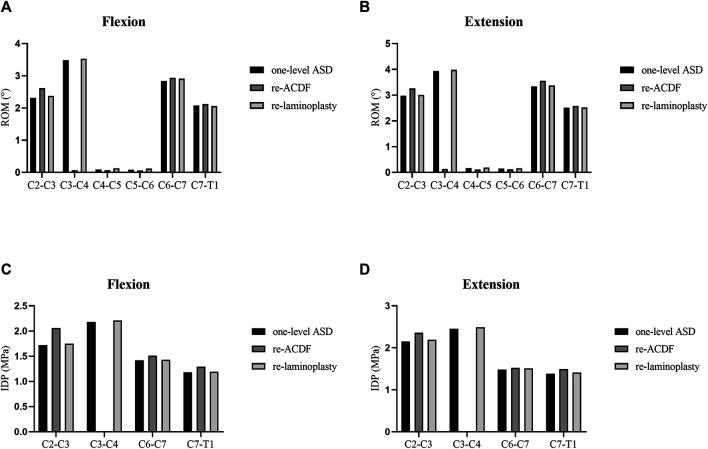
The segmental ROM and IDP of the FE models of different surgical approaches for the treatment of one-level ASD after primary ACDF were shown in Figure 5. The ROM at the C2–C3 segment of re-ACDF model increased than that of the one-level ASD model (Figures 5A,B). Similarly, the IDP at the C2–C3 segment of re-ACDF model was larger than that of the one-level ASD model (Figures 5C,D). Furthermore, no significant changes in the ROM and IDP of re-laminoplasty model were observed compared to the one-level ASD model (Figure 5).
FIGURE 5.
ROM and IDP of different revision surgeries for the treatment of one-level ASD. (A) ROM in flexion. (B) ROM in extension. (C) IDP in flexion. (D) IDP in extension.
Analyses of the Biomechanical Effects of Different Surgical Approaches for the Treatment of Two-Level ASD After Primary ACDF
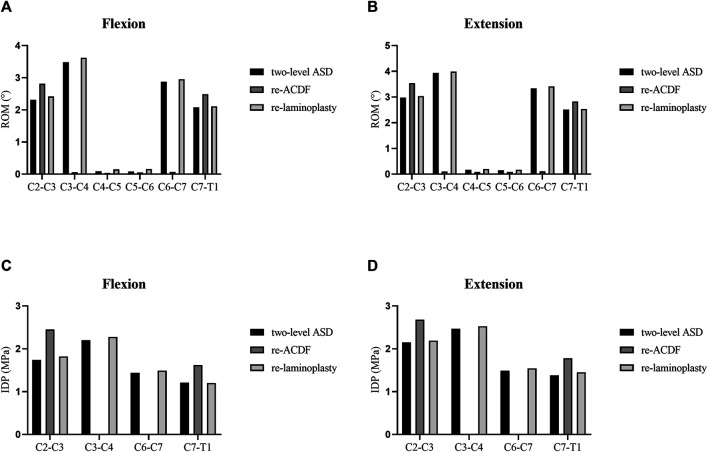
The segmental ROM and IDP of the FE models of different surgical approaches for the treatment of two-level ASD after primary ACDF were shown in Figure 6. The ROM at the C2–C3 and C7-T1 segments of re-ACDF model increased significantly than that of the two-level ASD model (Figures 6A,B). Similarly, the IDP at the C2–C3 and C7-T1 segments of re-ACDF model was significantly larger than that of the two-level ASD model (Figures 6C,D). The ROM and IDP of re-laminoplasty model increased slightly but the difference was not statistically significant (Figure 6).
FIGURE 6.
ROM and IDP of different revision surgeries for the treatment of two-level ASD. (A) ROM in flexion. (B) ROM in extension. (C) IDP in flexion. (D) IDP in extension.
Analyses of the Stress in the Spinal Cord of Different Surgical Approaches for the Treatment of One-Level or Two-Level ASD After Primary ACDF
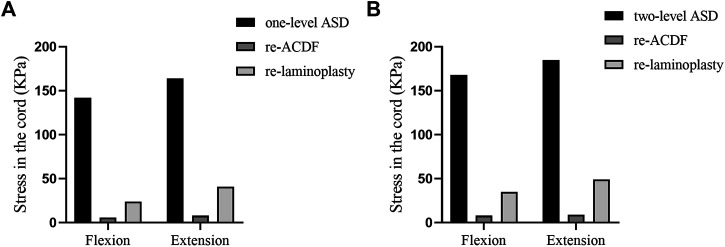
The maximum von-Mises stress in the spinal cord of the FE models of different surgical approaches for the treatment of one-level or two-level ASD after primary ACDF were shown in Figure 7. The maximum von-Mises stress in the cord of the re-ACDF model was greatly reduced compared to the one-level or two-level ASD model. The stress in the cord of the re-laminoplasty model decreased to some extent, although it was higher than that of the re-ACDF model. The stress distribution in the spinal cord in the sagittal plane of different surgical approaches for the treatment of one-level or two-level ASD after primary ACDF were shown in Figures 8, 9. The peak stress occurred where the cord and dural sheath attached. Anterior and posterior surgical approaches all decreased the stress in the spinal cord caused by ASD after the first surgery.
FIGURE 7.
Maximum von-Mises stress in the cord of re-ACDF and re-laminoplasty models. (A) Stress in the cord of different revision surgeries for the treatment of one-level ASD. (B) Stress in the cord of different revision surgeries for the treatment of two-level ASD.
FIGURE 8.
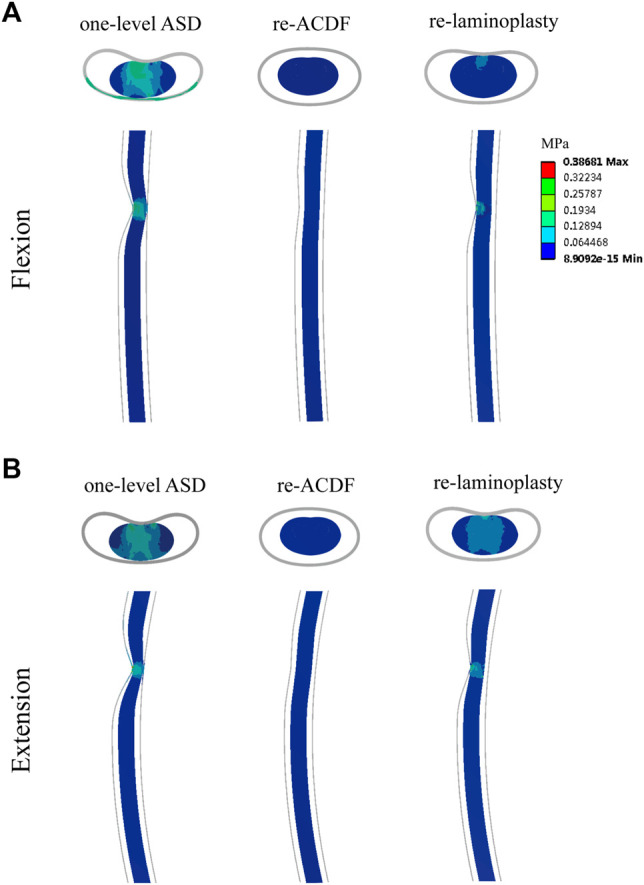
Distribution of von-Mises stress in the spinal cord of re-ACDF and re-laminoplasty models for the treatment of one-level ASD. (A) Stress distribution in flexion. (B) Stress distribution in extension.
FIGURE 9.
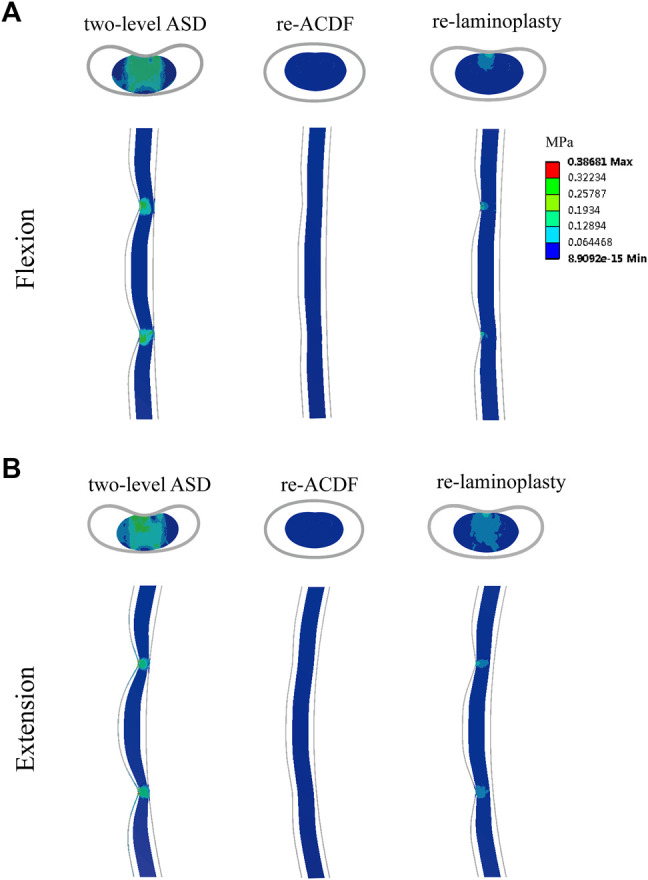
Distribution of von-Mises stress in the spinal cord of re-ACDF and re-laminoplasty models for the treatment of two-level ASD. (A) Stress distribution in flexion. (B) Stress distribution in extension.
Discussion
The purpose of this study was to compare the biomechanical effects of second ACDF and laminoplasty for the treatment of one-level or two-level ASD after primary ACDF. The biomechanical results indicated that both ACDF and laminoplasty can relieve the increased stress in the spinal cord caused by ASD after primary ACDF, whereas ACDF can achieve a better decompression effect than laminoplasty regardless of the level of ASD. Revision surgery of the superior ACDF or the superior and inferior ACDF after primary ACDF both increased the ROM and IDP at the adjacent segments. Furthermore, laminoplasty after primary ACDF had no significant effect on the biomechanical stability of the spine.
Recently, symptomatic ASD has become a major problem after spinal fusion surgeries Some experts think that ASD is the result of a natural history, while others believe that ASD is due to compensatory pressure on adjacent discs following vertebral fusion (Hilibrand and Robbins, 2004; Kavadi and Badve, 2019). If ASD occurs, conservative treatment is often the first choice for many patients. However, a revision surgery should be considered for the patients with obvious clinical manifestation and poor effect of conservative treatment. There remains some debate on the appropriate surgical approaches for the treatment of ASD after ACDF. Revision surgery via anterior approach was reported to be effective for patients who underwent primary ACDF for symptomatic ASD (Li et al., 2016; O'Neill et al., 2016). However, the incidence of radiculopathy and ASD recurrence after anterior revision surgery was higher than that undergoing posterior approached (Xu et al., 2014). Furthermore, posterior revision surgery could result in greater blood loss and a longer hospital stay (Steinhaus et al., 2020). When patients developed spinal stenosis at the initial surgical levels or ossification of the posterior longitudinal ligament, a revision surgery with an anterior approach cannot easily resolve the issue, but a posterior approach can achieve extensive decompression (Cabraja et al., 2010; Cole et al., 2015).
ACDF and laminoplasty were reported to be effective in treating ASD after primary ACDF (Wang and Green, 2003; Basques et al., 2017). The clinical outcomes of the two surgical approaches have also been compared. The ACDF was reported to reduce intraoperative bleeding and better preserve cervical lordosis, while laminoplasty retained more ROM (Montano et al., 2019). Recently, Mohamed et al. reported a higher incidence of dysphagia, new-onset cervicalgia, and increased incidence of recurrence in patients with ACDF compared to those with laminoplasty (Mesregah et al., 2021). In a prospective cohort study of 60 patients with lordotic cervical spine, Liang et al. reported similar sagittal alignment results between ACDF and laminoplasty, while ACDF was associated with poor cervical lordosis preservation (Liang et al., 2019). Another study reported that both ACDF and laminoplasty can achieve favorable clinical results in patients with multilevel cervical spondylotic myelopathy (Chen et al., 2019). Compared with laminoplasty, ACDF has the advantage of less trauma and may be more suitable for elderly patients with poor surgical tolerance. However, the biomechanical evaluation of ACDF and laminoplasty for the treatment of ASD after ACDF is limited.
In the present study, the FE models of the one-level and two-level ASD based on C4-C6 ACDF were constructed to simulate the postoperative degeneration after primary ACDF. Revision surgeries of ACDF and laminoplasty were stimulated to compare the biomechanical effect of different surgical approaches for the treatment of ASD after primary ACDF. The biomechanical results suggested that revision surgery of the superior ACDF or the superior and inferior ACDF after the primary ACDF both increased the ROM and IDP at the adjacent segments. Increased IDP at the adjacent segments of the fused surgeries was supposed to be an important factor in the development of ASD (Eck et al., 2002). Xu et al. (2014) reported that patients who underwent a second ACDF after primary ACDF had a higher chance of developing recurrent ASD, up to 25%. The increased ROM and IDP at the adjacent segments of the re-ACDF model in our study may be a possible reason for the high incidence of recurrent ASD after second ACDF. Furthermore, no significant changes in the ROM and IDP of the re-laminoplasty model were observed, which is similar to the finding of a previous study (Xu et al., 2014). Decompression of the spinal cord is the main objective of revision surgery and it determines the outcome. The biomechanical results indicated that both ACDF and laminoplasty can decrease the stress in the spinal cord caused by ASD after primary ACDF, but ACDF can achieve a better decompression effect than laminoplasty regardless of the level of ASD. Compared with ACDF, laminoplasty serves as a motion-preserving procedure that allows for indirect decompression, which may be safer than direct decompression (Bakhsheshian et al., 2017).
Taken together, our results suggested that both ACDF and laminoplasty were effective for the treatment of ASD after primary ACDF. Although ACDF can achieve a better decompression effect, laminoplasty retained more ROM of the surgical segments. For decompression of one-level ASD after primary ACDF, both ACDF and laminoplasty were feasible and open to consideration. As for the superior and inferior ASD, multilevel laminoplasty may be a suitable choice, while ACDF could significantly increase the ROM and IDP at the adjacent segments. The biomechanical results of this study provided guidance for surgical decisions for the treatment ASD after primary ACDF, but the actual situation should also be considered in clinical practice.
There are some limitations in the present study. First, only linear elastic materials were used for the vertebral body and IVD, which ignored the anisotropic properties of materials. Second, the muscles and collagen fibers were not considered in this study, which may affect the stability of cervical spine. Third, the ligaments were considered as nonlinear Spring element with no effect on compression. Furthermore, the model was constructed based on the data from a single volunteer. Although it had the advantage of making comparisons between different conditions and treatments, the results were somewhat haphazard. However, the simplified model can objectively reflect the biomechanics of the spine and has certain clinical guiding value for the evaluation of different surgical methods. Meanwhile, more accurate FE model and clinical studies are needed to explore the effect of different surgical methods in the future.
Conclusion
In conclusion, ACDF and laminoplasty can relieve the high level of stress in the spinal cord caused by ASD after primary ACDF, whereas ACDF can achieve a better decompression effect than laminoplasty regardless of the level of ASD. Revision surgery of the superior ACDF or the superior and inferior ACDF after primary ACDF increased the ROM and IDP at the adjacent segments, which may be the reason for the high incidence of recurrent ASD after second ACDF. Due to some defects in finite element analysis, it may not fully represent the real situation in vivo. The biomechanical results of this study provided guidance for surgical decisions for the treatment ASD after primary ACDF, but the actual situation should also be considered in clinical practice.
Acknowledgments
We would like to thank SDL, YS, RJL, ZWL, and GCL for their help in revising the manuscript.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology. Written informed consent was obtained from the volunteer.
Author Contributions
WCK, CC, and BJW designed the study, analyzed the data, and wrote the manuscript. WBH, LM, YSS, KW, and SL participated in the design of the study and analyzed the data. SDL, YS, RJL, ZWL, and GCL helped in revising the manuscript. CY, YKZ, and XHW collected the clinical data, helped in writing the manuscript, and instructed on the surgical technique. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFB1105700), National Natural Science Foundation of China (Grant no. 81904020, 81772401, and 81803917), Natural Science Foundation of Hubei Province (2019CFB305), and the Fundamental Research Funds for the Central Universities (2019kfyXMBZ063).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2021.718996/full#supplementary-material
Validation of IDP of the intact cervical model.
References:
- Bakhsheshian J., Mehta V. A., Liu J. C. (2017). Current Diagnosis and Management of Cervical Spondylotic Myelopathy. Glob. Spine J. 7, 572–586. 10.1177/2192568217699208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basques B. A., Ondeck N. T., Geiger E. J., Samuel A. M., Lukasiewicz A. M., Webb M. L., et al. (2017). Differences in Short-Term Outcomes between Primary and Revision Anterior Cervical Discectomy and Fusion. Spine (Phila Pa 1976) 42, 253–260. 10.1097/brs.0000000000001718 [DOI] [PubMed] [Google Scholar]
- Brydon H. L., Hayward R., Harkness W., Bayston R. (1995). Physical Properties of Cerebrospinal Fluid of Relevance to Shunt Function. 1: The Effect of Protein upon CSF Viscosity. Br. J. Neurosurg. 9, 639–644. 10.1080/02688699550040927 [DOI] [PubMed] [Google Scholar]
- Cabraja M., Abbushi A., Koeppen D., Kroppenstedt S., Woiciechowsky C. (2010). Comparison between Anterior and Posterior Decompression with Instrumentation for Cervical Spondylotic Myelopathy: Sagittal Alignment and Clinical Outcome. Foc 28, E15. 10.3171/2010.1.focus09253 [DOI] [PubMed] [Google Scholar]
- Cai X.-Y., Sang D., Yuchi C.-X., Cui W., Zhang C., Du C.-F., et al. (2020a). Using Finite Element Analysis to Determine Effects of the Motion Loading Method on Facet Joint Forces after Cervical Disc Degeneration. Comput. Biol. Med. 116, 103519. 10.1016/j.compbiomed.2019.103519 [DOI] [PubMed] [Google Scholar]
- Cai X.-Y., Yuchi C.-X., Du C.-F., Mo Z.-J. (2020b). The Effect of Follower Load on the Range of Motion, Facet Joint Force, and Intradiscal Pressure of the Cervical Spine: a Finite Element Study. Med. Biol. Eng. Comput. 58, 1695–1705. 10.1007/s11517-020-02189-7 [DOI] [PubMed] [Google Scholar]
- Cao J., Qi C., Yang Y., Lei T., Wang L., Shen Y. (2020). Comparison between Repeat Anterior and Posterior Decompression and Fusion in the Treatment of Two-Level Symptomatic Adjacent Segment Disease after Anterior Cervical Arthrodesis. J. Orthop. Surg. Res. 15, 308. 10.1186/s13018-020-01834-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Qin M., Chen F., Ni B., Guo Q., Han Z. (2019). Comparison of Outcomes between Anterior Cervical Decompression and Fusion and Posterior Laminoplasty in the Treatment of 4-Level Cervical Spondylotic Myelopathy. World Neurosurg. 125, e341–e347. 10.1016/j.wneu.2019.01.075 [DOI] [PubMed] [Google Scholar]
- Chung J.-Y., Kim S.-K., Jung S.-T., Lee K.-B. (2014). Clinical Adjacent-Segment Pathology after Anterior Cervical Discectomy and Fusion: Results after a Minimum of 10-year Follow-Up. Spine J. 14, 2290–2298. 10.1016/j.spinee.2014.01.027 [DOI] [PubMed] [Google Scholar]
- Cole T., Veeravagu A., Zhang M., Azad T. D., Desai A., Ratliff J. K. (2015). Anterior versus Posterior Approach for Multilevel Degenerative Cervical Disease. Spine 40, 1033–1038. 10.1097/brs.0000000000000872 [DOI] [PubMed] [Google Scholar]
- Du W., Zhang P., Shen Y., Zhang Y.-Z., Ding W.-Y., Ren L.-X. (2014). Enlarged Laminectomy and Lateral Mass Screw Fixation for Multilevel Cervical Degenerative Myelopathy Associated with Kyphosis. Spine J. 14, 57–64. 10.1016/j.spinee.2013.06.017 [DOI] [PubMed] [Google Scholar]
- Eck J. C., Humphreys S. C., Lim T.-H., Jeong S. T., Kim J. G., Hodges S. D., et al. (2002). Biomechanical Study on the Effect of Cervical Spine Fusion on Adjacent-Level Intradiscal Pressure and Segmental Motion. Spine 27, 2431–2434. 10.1097/00007632-200211150-00003 [DOI] [PubMed] [Google Scholar]
- Guo X., Zhou J., Tian Y., Kang L., Xue Y. (2021). Biomechanical Effect of Different Plate-To-Disc Distance on Surgical and Adjacent Segment in Anterior Cervical Discectomy and Fusion - a Finite Element Analysis. BMC Musculoskelet. Disord. 22, 340. 10.1186/s12891-021-04218-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilibrand A. S., Robbins M. (2004). Adjacent Segment Degeneration and Adjacent Segment Disease: the Consequences of Spinal Fusion?. Spine J. 4, 190S–194S. 10.1016/j.spinee.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Hirabayashi S., Yamada H., Motosuneya T., Watanabe Y., Miura M., Sakai H., et al. (2010). Comparison of Enlargement of the Spinal Canal after Cervical Laminoplasty: Open-Door Type and Double-Door Type, European Spine Journal, 19, 1690–1694. 10.1007/s00586-010-1369-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsheimer J., Den Boer J. A., Struijk J. J., Rozeboom A. R. (1994). MR Assessment of the normal Position of the Spinal Cord in the Spinal Canal. AJNR Am. J. Neuroradiol 15, 951–959. [PMC free article] [PubMed] [Google Scholar]
- Hua W., Zhi J., Ke W., Wang B., Yang S., Li L., et al. (2020). Adjacent Segment Biomechanical Changes after One- or Two-Level Anterior Cervical Discectomy and Fusion Using Either a Zero-Profile Device or Cage Plus Plate: A Finite Element Analysis. Comput. Biol. Med. 120, 103760. 10.1016/j.compbiomed.2020.103760 [DOI] [PubMed] [Google Scholar]
- Ichihara K., Taguchi T., Shimada Y., Sakuramoto I., Kawano S., Kawai S. (2001). Gray Matter of the Bovine Cervical Spinal Cord Is Mechanically More Rigid and Fragile Than the white Matter. J. neurotrauma 18, 361–367. 10.1089/08977150151071053 [DOI] [PubMed] [Google Scholar]
- Kavadi N., Badve S. (2019). Commentary on: Risk Factors of Second Surgery for Adjacent Segment Disease Following Anterior Cervical Discectomy and Fusion: A 16-year Cohort Study. Int. J. Surg. 69, 165. 10.1016/j.ijsu.2019.07.010 [DOI] [PubMed] [Google Scholar]
- Kelly M. P., Eliasberg C. D., Riley M. S., Ajiboye R. M., Soohoo N. F. (2018). Reoperation and Complications after Anterior Cervical Discectomy and Fusion and Cervical Disc Arthroplasty: a Study of 52,395 Cases. Eur. Spine J. 27, 1432–1439. 10.1007/s00586-018-5570-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa R., Kim P. (2015). Cervical Laminoplasty: The History and the Future, Neurol. Med. Chir.(Tokyo), 55, 529–539. 10.2176/nmc.ra.2014-0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Lee S.-H., Peters C., Riew K. D. (2015). Adjacent Segment Pathology Requiring Reoperation after Anterior Cervical Arthrodesis. Spine 40, E571–E577. 10.1097/brs.0000000000000846 [DOI] [PubMed] [Google Scholar]
- Li J., Tong T., Niu R., Shen Y. (2016). A Study on the Clinical Outcomes of Patients with Revision Surgery for Adjacent Segment Disease after 10-year's Anterior Cervical Spine Surgery. J. Orthop. Surg. Res. 11, 5. 10.1186/s13018-016-0341-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fogel G. R., Liao Z., Tyagi R., Zhang G., Liu W. (2017). Biomechanical Analysis of Two-Level Cervical Disc Replacement with a Stand-Alone U-Shaped Disc Implant. Spine (Phila Pa 1976) 42, E1173–E1181. 10.1097/brs.0000000000002128 [DOI] [PubMed] [Google Scholar]
- Li Z., Liu H., Yang M., Zhang W. (2021). A Biomechanical Analysis of Four Anterior Cervical Techniques to Treating Multilevel Cervical Spondylotic Myelopathy: a Finite Element Study. BMC Musculoskelet. Disord. 22, 278. 10.1186/s12891-021-04150-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G., Liang C., Zheng X., Xiao D., Zeng S., Yin D., et al. (2019). Sagittal Alignment Outcomes in Lordotic Cervical Spine. Spine (Phila Pa 1976) 44, E882–E888. 10.1097/brs.0000000000003016 [DOI] [PubMed] [Google Scholar]
- Lin M., Shapiro S. Z., Doulgeris J., Engeberg E. D., Tsai C.-T., Vrionis F. D. (2021). Cage-screw and Anterior Plating Combination Reduces the Risk of Micromotion and Subsidence in Multilevel Anterior Cervical Discectomy and Fusion-A Finite Element Study. Spine J. 21, 874–882. 10.1016/j.spinee.2021.01.015 [DOI] [PubMed] [Google Scholar]
- Mesbah M., Barkaoui A. (2020). Biomechanical Investigation of the Effect of Pedicle-Based Hybrid Stabilization Constructs: A Finite Element Study. Proc. Inst. Mech. Eng. H 234, 931–941. 10.1177/0954411920934956 [DOI] [PubMed] [Google Scholar]
- Mesbah M., Barkaoui A., Chiali H., Bendoukha M. (2020). Posterior Dynamic Topping-Off Fusion Stabilization System in Lumbosacral Spine: a Review of Different Instrumentation Techniques. Ser. Biomech. 34, 41–47. [Google Scholar]
- Mesregah M. K., Formanek B., Liu J. C., Buser Z., Wang J. C. (2021). Perioperative Complications of Surgery for Degenerative Cervical Myelopathy: A Comparison between 3 Procedures. Glob. Spine J 2192568221998306, 2192568221998306. 10.1177/2192568221998306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z., Li Q., Jia Z., Yang J., Wong D. W.-C., Fan Y. (2017). Biomechanical Consideration of Prosthesis Selection in Hybrid Surgery for Bi-level Cervical Disc Degenerative Diseases. Eur. Spine J. 26, 1181–1190. 10.1007/s00586-016-4777-9 [DOI] [PubMed] [Google Scholar]
- Montano N., Ricciardi L., Olivi A. (2019). Comparison of Anterior Cervical Decompression and Fusion versus Laminoplasty in the Treatment of Multilevel Cervical Spondylotic Myelopathy: A Meta-Analysis of Clinical and Radiological Outcomes. World Neurosurg. 130, 530–536. 10.1016/j.wneu.2019.06.144 [DOI] [PubMed] [Google Scholar]
- Muzević D., Splavski B., Boop F. A., Arnautović K. I. (2018). Anterior Cervical Discectomy with Instrumented Allograft Fusion: Lordosis Restoration and Comparison of Functional Outcomes Among Patients of Different Age Groups. World Neurosurg. 109, e233–e243. 10.1016/j.wneu.2017.09.146 [DOI] [PubMed] [Google Scholar]
- Nikkhoo M., Cheng C.-H., Wang J.-L., Khoz Z., El-Rich M., Hebela N., et al. (2019). Development and Validation of a Geometrically Personalized Finite Element Model of the Lower Ligamentous Cervical Spine for Clinical Applications. Comput. Biol. Med. 109, 22–32. 10.1016/j.compbiomed.2019.04.010 [DOI] [PubMed] [Google Scholar]
- Nikkhoo M., Cheng C.-H., Wang J.-L., Niu C.-C., Parnianpour M., Khalaf K. (2020). The Biomechanical Response of the Lower Cervical Spine Post Laminectomy: Geometrically-Parametric Patient-specific Finite Element Analyses. J. Med. Biol. Eng. 41, 59–70. 10.1007/s40846-020-00579-8 [DOI] [Google Scholar]
- O'neill K. R., Wilson R. J., Burns K. M., Mioton L. M., Wright B. T., Adogwa O., et al. (2016). Anterior Cervical Discectomy and Fusion for Adjacent Segment Disease: Clinical Outcomes and Cost Utility of Surgical Intervention. Clin. Spine Surg. 29, 234–241. 10.1097/BSD.0b013e31828ffc54 [DOI] [PubMed] [Google Scholar]
- Oglesby M., Fineberg S. J., Patel A. A., Pelton M. A., Singh K. (2013). Epidemiological Trends in Cervical Spine Surgery for Degenerative Diseases between 2002 and 2009. Spine 38, 1226–1232. 10.1097/brs.0b013e31828be75d [DOI] [PubMed] [Google Scholar]
- Persson C., Evans S., Marsh R., Summers J. L., Hall R. M. (2010). Poisson's Ratio and Strain Rate Dependency of the Constitutive Behavior of Spinal Dura Mater. Ann. Biomed. Eng. 38, 975–983. 10.1007/s10439-010-9924-6 [DOI] [PubMed] [Google Scholar]
- Schroeder G. D., Kurd M. F., Millhouse P. W., Vaccaro A. R., Hilibrand A. S. (2016). Performing an Anterior Cervical Discectomy and Fusion. Clin. Spine Surg. 29, 186–190. 10.1097/bsd.0000000000000383 [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Kumar S D., R S., Jebaseelan D D., Yoganandan N., Rajasekarans (2021). Effect of Heterotopic Ossification after bryan-cervical Disc Arthroplasty on Adjacent Level Range of Motion: A Finite Element Study. J. Clin. Orthopaedics Trauma 15, 99–103. 10.1016/j.jcot.2020.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhaus M. E., York P. J., Bronheim R. S., Yang J., Lovecchio F., Kim H. J. (2020). Outcomes of Revision Surgery for Pseudarthrosis after Anterior Cervical Fusion: Case Series and Systematic Review. Glob. Spine J. 10, 559–570. 10.1177/2192568219863808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Wang P., Miao D.-C., Du W., Shen Y. (2017a). Different Surgical Approaches for the Treatment of Adjacent Segment Diseases after Anterior Cervical Fusion. Medicine (Baltimore) 96, e7042. 10.1097/md.0000000000007042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Wang H., Deng Z., Li Z., Zhan H., Niu W. (2017b). Cervical Traction Therapy with and without Neck Support: A Finite Element Analysis. Musculoskelet. Sci. Pract. 28, 1–9. 10.1016/j.msksp.2017.01.005 [DOI] [PubMed] [Google Scholar]
- Wang M. Y., Green B. A. (2003). Laminoplasty for the Treatment of Failed Anterior Cervical Spine Surgery. Neurosurg. Focus 15, E7. 10.3171/foc.2003.15.3.7 [DOI] [PubMed] [Google Scholar]
- Wu T. K., Meng Y., Liu H., Wang B. Y., Hong Y., Rong X., et al. (2019). Biomechanical Effects on the Intermediate Segment of Noncontiguous Hybrid Surgery with Cervical Disc Arthroplasty and Anterior Cervical Discectomy and Fusion: a Finite Element Analysis. Spine J. 19, 1254-–1263. 10.1016/j.spinee.2019.02.004 [DOI] [PubMed] [Google Scholar]
- Wu W.-k., Yan Z. j., Zhang T.-f., Liao C.-g., Liang K.-l., Chen L., et al. (2018). Biomechanical Influences of Transcorporeal Tunnels on C4 Vertebra under Physical Compressive Load under Flexion Movement: A Finite Element Analysis. World Neurosurg. 114, e199–e208. 10.1016/j.wneu.2018.02.140 [DOI] [PubMed] [Google Scholar]
- Xu R., Bydon M., Macki M., De La Garza-Ramos R., Sciubba D. M., Wolinsky J.-P., et al. (2014). Adjacent Segment Disease after Anterior Cervical Discectomy and Fusion. Spine 39, 120–126. 10.1097/brs.0000000000000074 [DOI] [PubMed] [Google Scholar]
- Yeh K.-T., Yu T.-C., Chen I.-H., Peng C.-H., Liu K.-L., Lee R.-P., et al. (2014). Expansive Open-Door Laminoplasty Secured with Titanium Miniplates Is a Good Surgical Method for Multiple-Level Cervical Stenosis. J. Orthop. Surg. Res. 9, 49. 10.1186/s13018-014-0049-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Chen J., Liu J., Elsamaloty L., Liu X., Li J., et al. (2018). Biomechanical Analysis on of Anterior Transpedicular Screw-Fixation after Two-Level Cervical Corpectomy Using Finite Element Method. Clin. Biomech. 60, 76–82. 10.1016/j.clinbiomech.2018.09.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation of IDP of the intact cervical model.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.