Abstract
Optimal sequencing of complementary therapies can help improve symptom management through non-pharmacological approaches. A 12-week sequential multiple assignment randomized trial (SMART) comparing home-based reflexology and meditative practices on severity of fatigue and other symptoms was conducted among patients with cancer and their informal caregivers. Dyads were initially randomized to reflexology (N=150), meditative practices (N=150), or control (N=47). If patient’s fatigue did not improve (non-response) after 4 weeks of reflexology or meditative practices, the dyad was re-randomized to either add the other therapy or continue with the original therapy for weeks 5–8. Four decision rules (DRs) were compared: 1) Initiating reflexology, and if non-response on fatigue after 4 weeks, continue with reflexology for another 4 weeks, thus providing a higher dose; 2) Initiating reflexology, and if non-response on fatigue after 4 weeks, add meditative practices for the next 4 weeks; 3) Initiating meditative practices, and if non-response on fatigue after 4 weeks, continue meditative practices for another 4 weeks, thus providing a higher dose; and 4) Initiating meditative practices, and if non-response on fatigue after 4 weeks, add reflexology for the next 4 weeks. Symptoms were evaluated weekly using the M.D. Anderson Symptom Inventory (MDASI). Clinically, nurses can recommend either therapy since no differences were found among the 4 DRs, with the exception of lower severity for summed MDASI symptoms at week 8 for the use of reflexology only (DR-1) versus DR-2 (sequencing reflexology to meditative practices). Adding the other therapy for non-responders after 4 weeks may not be warranted.
Keywords: cancer, caregivers, meditative practices, reflexology, SMART design
Introduction
Symptom management is critical to maintaining medical adherence and quality of life during cancer treatment (Jacobs et al., 2019; Kim et al., 2017). Fatigue is the most commonly reported symptom during cancer treatment (Mustian et al., 2016). Other prevalent symptoms include pain, depression, anxiety, sleep disturbance, and examination of a broad range of symptoms is supported by the literature (Kim et al., 2017; Matzka et al., 2018; Reilly et al., 2013).
Many people facing cancer are interested in self-care through non-pharmacological interventions to alleviate their symptoms and are turning to complementary therapies (Link et al., 2013). In a survey of 17,638 people with cancer, 87% reported using at least one complementary therapy in the previous 12 months (Judson et al., 2017). The use was greatest among adult females (Morano et al., 2013), non-Hispanic whites, Caucasians, patients 60 to 69 years of age, and those who were married, had a higher level of education, and were employed (Judson et al., 2017).
Therapies that can be used at home throughout the duration of cancer treatment are particularly significant, and friend and family caregivers of patients with cancer are often willing to participate in care to help manage symptoms (Badger et al., 2020; Fletcher et al., 2012; Wyatt et al., 2017). The involvement of informal caregivers in the delivery of symptom management therapies is growing and adds to supportive care (Badger et al., 2020; Fletcher et al., 2012; Kim et al., 2015). It is recognized that the support provided by caregivers may influence the outcomes of their patient’s cancer treatment (Bevans & Sternberg, 2012). Caregivers have been successfully incorporated into complementary therapy delivery as key stakeholders in home-based symptom management via reflexology for patients with cancer. (Wyatt et al., 2017) and meditative practices (Atreya et al., 2018; Birnie et al., 2010). Because of the broad use of complementary therapies and caregiver willingness to participate in the delivery of these therapies, research is necessary to examine how best to tailor such therapies for patient needs.
Complementary therapies included in this study
Reflexology is a body-based therapy similar to massage in that soft tissue is manipulated for therapeutic purposes, but differs in its focus on special areas of the feet called reflexes and the use of a firm thumb-walking motion (Watson & Voner, 2008). Findings from randomized controlled trials (RCTs) of reflexology in cancer populations included: 1) lower fatigue and improved sleep quality during chemotherapy (Zengin & Aylaz, 2019); 2) decreased pain among patients with digestive cancers (Tsay et al., 2008); and 3) a significant relaxation response among post-surgical patients with breast cancer (Sharp et al., 2010). While limited cancer caregiver RCT-level research has been conducted with reflexology (Baglama & Bakir, 2019; Frambes, Sikorskii, et al., 2017; Stephenson et al., 2007), work in oncology has long involved informal caregivers in symptom management (Hu et al., 2019; Kim et al., 2015; Milbury et al., 2013). Further, therapy efficacy, based on 4 weekly sessions of reflexology, was established in two previous RCTs (Wyatt et al., 2012; Wyatt et al., 2017). This evidence base supported the efficacy of caregiver-delivered reflexology for fatigue and other cancer-related symptoms and provided a foundation for testing the sequencing of this therapy with meditative practices.
Meditative Practices are purposeful cognitively-mediated strategies aimed at building attentional capacities to be mindfully present to the current moment, including to one’s thoughts, emotions, bodily sensations, and the environment with nonjudgmental openness and acceptance (Victorson et al., 2015). Studies that incorporate meditation-based therapies in cancer have shown efficacy for fatigue as well as other related symptoms (Xie et al., 2020; Zhang et al., 2019). The earliest programs involving meditation in cancer studies incorporated the Mindfulness-Based Stress Reduction program, a group-based 8-week intervention (Kabat-Zinn, 2009). Meditation-based, health promoting interventions utilizing RCT designs have ranged in length from 2 to 12 weeks (Victorson et al., 2015). Studies evaluating abbreviated meditation protocols in cancer populations have demonstrated capacity to alter symptom perceptions and reduce distress (Ng et al., 2016). Our 4-week protocol was developed based on previous work (Lehto et al., 2015), support from the literature, and recognition that a sizeable proportion of participants would complete it.
Study purpose
At this point in the state of the science, dynamic tailoring of interventions to patient responses is needed to improve and personalize symptom management (Nahum-Shani et al., 2012b). Therefore the purpose of this study was to investigate the optimal sequencing of two evidence-based complementary therapies in order to determine whether it is best to use only one therapy or start with one and add another based on demonstrated need. We aimed to compare four decision rules with respect to the primary outcome of severity of fatigue, and secondary outcomes of summed index of severity of other symptoms, depression, and anxiety: 1) Starting with reflexology, and if no response on fatigue after 4 weeks, continue with reflexology for another 4 weeks; 2) Starting with reflexology, and if no response on fatigue after 4 weeks, add meditative practices for another 4 weeks; 3) Starting with meditative practices, and if no response on fatigue after 4 weeks, continue meditative practices for another 4 weeks; and 4) Starting with meditative practices, and if no response on fatigue after 4 weeks, add reflexology for another 4 weeks.
Methods
The trial was prospectively registered at clinicaltrials.gov.
Design
In this sequential multiple assignment randomized trial (SMART), patient-caregiver dyads were randomized initially to reflexology, meditative practices, or control. Reflexology and meditative practices were delivered by or practiced with the caregiver in the patient’s home setting. The control group and those randomized to interventions received usual care. Typically, a control group is not included in SMARTs as these trials are designed to investigate optimal sequencing of interventions that have already been shown to be efficacious (Almirall et al., 2014), as have reflexology and meditative practices. However, it is possible that two efficacious interventions may be too burdensome and produce worse outcomes in a sequence or combination than individually. Such a possibility was not likely with reflexology and meditative practices that have different mechanisms, but we added a control group in the design to compare the magnitude of the effects produced by intervention sequences versus the control group.
After the first 4 weeks in the two intervention groups (previously established dose for two therapies), patient’s response on fatigue was determined as described in detail below using weekly symptom assessments. Dyads with non-responding patients were randomized for the second time to either continue with the same therapy for 4 more weeks, thus increasing the dose, or add 4 weeks of the other therapy. Dyads with responding patients were free to continue using the therapy to which they were initially randomized. Because of the temporal nature of symptoms (Cleeland et al., 2013), they were assessed at baseline, weeks 1–8 during interventions or the same time frame for the control group, and at week 12.
Theoretical Framework
The study was guided by the adapted Barsevick symptom model (Barsevick et al., 2010). Fatigue is the most prevalent and often distressing symptom related to cancer and its treatment (Courtier et al., 2018; Pearson et al., 2018; So et al., 2021). According to Barsevick et al. (2010) and Cella et al. (2002), fatigue is known to affect quality of life and other outcomes (Choi et al., 2014; Huang et al., 2014; Mitchell & Berger, 2006; Nail, 2002; Piper et al., 1998). The biological changes due to chemotherapy and the resulting inflammatory processes may be responsible for the persistence of fatigue, as well as related symptoms.(Bower et al., 2011; Clevenger et al., 2012; Schrepf et al., 2013). Due to the high prevalence of fatigue and evidence showing efficacy of the two interventions for this symptom (Lehto & Wyatt, 2013; Wyatt et al., 2012), fatigue severity was the primary outcome. Evidence for both reflexology and meditative practices interventions supports their efficacy for the management of fatigue and other symptoms, either directly or due to the associations among multiple symptoms within patient (Wyatt et al., 2017; Xie et al., 2020; Zengin & Aylaz, 2019; Zhang et al., 2019). Therefore, these interventions were strong candidates to evaluate their effects on the primary outcome of fatigue and secondary outcomes of depression, anxiety, and the severity index of other symptoms.
Participants
Dyads (n = 471) of patients with solid tumor cancers and their friend/family caregivers were recruited nationally from three comprehensive cancer centers, one academic oncology setting, and one large community oncology clinic.
Patient inclusion criteria were: 1) age 21 or older; 2) solid tumor cancer diagnosis; 3) able to perform basic activities of daily living; 4) undergoing chemotherapy, hormonal therapy, or targeted therapy; 5) severity of ≥3 on fatigue using a 0–10 standardized scale at intake; 6) able to speak and understand English; 7) have telephone access; and 8) able to hear normal conversation. Exclusion criteria were: 1) diagnosis of major mental illness in medical record and verified by the recruiter; 2) nursing home resident; 3) bedridden; 4) currently involved with reflexology or meditative practices; or 5) deep vein thrombosis or painful foot neuropathy.
Caregiver inclusion criteria were: 1) age 18 or older; 2) able to speak and understand English; 3) access to a telephone; 4) able to hear normal conversation; 5) cognitively oriented to time, place and person (determined via recruiter); and 6) willing to be trained in reflexology and meditative practices.
Procedures for Recruitment
Study recruiters approached patients during their appointments and asked if they had a caregiver who would be willing to provide weekly reflexology sessions or participate in meditative practices sessions. If the patient did not identify a caregiver willing to participate, they were not recruited. For those who identified a caregiver, recruiters proceed to explain the study. If interested, signed consent was obtained from both patients and their caregivers prior to beginning the study. The study was approved by the investigators’ university Institutional Review Board and those of all participating agencies.
Procedures for Data Collection
After both patient and caregiver provided consent to participate, separate baseline telephone interviews were scheduled. Patients were called weekly during weeks 1–8 to assess their symptoms and number of completed intervention sessions in the past 7 days. Exit telephone interviews were completed with patients and caregivers separately at week 12. Interviewers were blinded to dyad’s group assignments. After the dyad completed the study, recruiters conducted health record reviews for participating patients to gather data on their cancer and its treatment during their 12-week participation in the study.
First randomization
The first randomization occurred after baseline interviews were completed by both patient and caregiver. By design, the odds of allocation to reflexology or meditative practices were the same, but odds of allocation to the control group was 3 times smaller (see power analysis). Randomization was performed using a computerized minimization procedure from the central study office to ensure allocation concealment and blinding of data collectors. The balancing factors were recruitment location, site of cancer (breast, lung, colon, prostate, other), stage of cancer (early, late), and treatment type (hormonal therapy alone or chemotherapy and/or targeted therapy).
Determination of response on fatigue during weeks 1–4
After the first 4 weeks, the patient response on fatigue was determined for reflexology or meditative practices, using the previously validated definition of improvement from moderate to mild or severe to moderate or mild fatigue between week 1 and week 4 (Given et al., 2008; Jeon et al., 2009). Based on established interference-based cut-points for 0–10 rating of fatigue severity, the mild fatigue category corresponds to a severity score of 1, moderate category corresponds to scores 2–4, and scores of 5–10 fall into the severe category. Dyads with responding patients continued with the intervention they were assigned in the first randomization.
Second randomization
Dyads with non-responding patients were re-randomized in 1:1 ratio to either continue with the intervention from the first randomization or add the other intervention. The technique for the second randomization was the same as for the first, with the same balancing factors.
Interventions
All intervention training and delivery took place in patient’s homes. For dyads randomized to reflexology, caregivers were trained by a study reflexology provider to deliver reflexology to the patient. The previously tested reflexology protocol included a brief warm water foot bath, followed by assuming a comfortable seated position for both the patient and caregiver, and stimulation of 9 reflexes on each foot for 15 minutes per foot for 4 weekly sessions (Wyatt et al., 2012; Wyatt et al., 2017). For dyads randomized to meditative practices both caregiver and patient were trained in meditative practices by a study meditation provider. The established meditative practices used in this study were conducted in a seated or lying position and included 4 weekly sessions of 30 minutes practicing three meditation components: focused breathing, gentle seated movements and a body scan (Bränström et al., 2010; Zhang et al., 2019).
Intervention Training & Delivery
Intervention protocol fidelity was assured through methods outlined by the NIH Treatment Fidelity Workgroup (Bellg et al., 2004). Experts in reflexology and meditative practices trained the study providers. Reflexology study providers were practicing reflexologists, and study meditation providers were health professionals. Both types of study providers passed a demonstration at ≥ 90% proficiency as judged by the experts’ score on a standardized protocol checklist for their respective therapy before they trained caregivers (Frambes, Lehto, et al., 2017). Thereafter, the study providers (practicing reflexologist and meditation providers) had biannual quality assurance checks on protocol fidelity.
Each study provider (reflexology and meditation) visited the home during the first two weeks of the intervention. The first visit for both therapies was for training. Patients participated in the meditative practices alongside the caregivers; whereas, for reflexology patients were passive as the caregiver performed the therapy. A 90% accuracy on the protocol checklist was expected on performance of the therapy the dyad was assigned through randomization. Dyads were left with written instructions and laminated diagrams of their therapy and asked to select a day and time each week for the home-based session. Dyads in the meditative practices group also received a recording to support the laminated diagrams of the three meditative components. The second study provider visit was to observe and correct any protocol errors. This method of caregiver involvement has established efficacy (Wyatt et al., 2017). The next sessions (weeks 3 and 4) were conducted independently, with a study provider phone number to call with questions.
At least one weekly session of reflexology and/or meditative practices (based on randomizations) was required. There was no restriction on conducting more than one session per week in the home-based setting, and data on the number of completed session were collected weekly during weeks1–8 and at the week 12 interview. In past research, there was no additional benefit for symptom reduction when more than one session was conducted per week (Rottman et al., 2020). When non-responding patients were re-randomized to the other therapy in the second randomization, training during weeks 5 and 6 mirrored training for the first therapy.
Measures
Brief Fatigue Inventory (BFI), primary outcome (Mendoza et al., 1999) (completed by patient at baseline & week 12). The instrument consists of nine items. Participants are asked to rate the severity of fatigue “right now,” at its “usual” level during the past 24 hours and at its “worst” level during the past 24 hours using the scale of 0 = no fatigue to 10 = as bad as you can imagine. The usual severity of fatigue was the primary outcome in this trial. The remaining six items assess how much fatigue interfered with: general activity, mood, walking ability, normal work, relations with other people, and enjoyment of life. Responses are on a 0 to 10 scale, where 0=does not interfere and 10=completely interferes. Alpha coefficient for the summed interference score exceeded .95.
Summed Symptom Severity Index, secondary outcome (Cleeland et al., 2000; Cleeland et al., 2013; Mendoza et al., 2011) (completed by patient at baseline & week 12 & week 1–8 calls). The expanded M.D. Anderson Symptom Inventory (MDASI) includes 19 symptoms: fatigue, pain, nausea, disturbed sleep, distress, shortness of breath, difficulty remembering, decreased appetite, drowsiness, dry mouth, sadness, vomiting, numbness/tingling, diarrhea, constipation, sore mouth, rash, hair loss, and cough, and the interference of these symptoms with daily life on the scale from 0 = symptom not present to 10 = worst imaginable. This instrument has established evidence of reliability and validity in samples of patients with cancer (Cleeland et al., 2000). It has been recently updated to include the most common symptoms experienced by patients undergoing current cancer treatments (Cleeland et al., 2013). Severity ratings of fatigue during weeks 1–8 were used as additional repeated measures of the usual fatigue (primary outcome). A single summed symptom severity index across 18 symptoms (without fatigue) was used as a secondary outcome. Alpha coefficient was not applicable to the collection of different symptoms.
Depression and Anxiety, secondary outcome. Patient Reported Outcomes Measurement Information System (PROMIS) short forms 4: depression and anxiety (Cella et al., 2010; PROMIS, 2012, 2013a, 2013b) (completed by patient at baseline and week 12). These two symptoms are not directly covered by the MDASI. Therefore, we administered the additional PROMIS measures for these symptoms during baseline and week 12 interviews.
Additional contextual measures:
Demographics(Dyads; baseline). Demographic data were collected from patient and caregiver during baseline interview.
Chronic Conditions (Bayliss et al., 2009) (Dyads; baseline) were assessed using the Bayliss tool that included a checklist of 20 comorbidities. The number of comorbidities was derived. Cronbach’s alpha is not applicable to a checklist.
Physical function (Cella et al., 2010) (completed by patient at baseline and week 12) was measured using the 4-item PROMIS short form that includes items reflecting one’s ability to carry out activities that require physical actions.
Cancer and Its Treatment (review of patient’s health record after week 12). Chart data included cancer diagnosis, staging, recurrence, and treatments received during 12 weeks in the study.
Statistical analysis
The characteristics of the participants and baseline values of the outcomes were summarized by study groups created by the first randomization. To address possible attrition bias, these were also compared by study group for drop-outs. All analyses included all participants who completed at least one post-baseline assessment regardless of completion of intervention sessions. Number of completed sessions across patient-weeks was summarized using descriptive statistics. To compare outcomes for groups created by the first randomization, repeated measures of the primary outcome of fatigue, and secondary outcome of summed severity index of other symptoms from the MDASI were entered into linear mixed effects models (LMEs), one model for each outcome. The covariates included week number as a class variable to model potentially non-linear patterns, study group from the first randomization, baseline version of the outcome, and balancing factors used in randomization. The LME models are a generalization of classical analysis of repeated measures and allow for data missing at random, so all patients with at least one non-missing post-baseline assessment were included in these analyses. In the absence of formal statistical tests for the missing at random assumption, baseline values of the outcomes of drop-outs were compared by randomized condition to evaluate the viability of this assumption. The least square (LS) means according to study group were output from the mixed models. These means reflected the main effect of study group (average over time), which accounts for the temporal nature of symptoms (Cleeland et al., 2013). To reflect key time points in the study design, we reported outcomes over weeks 1–4 and 5–12. In addition to the main (time-averaged) effects, we also analyzed outcomes at week 8 (end of intervention) and week 12 (follow-up) by adding group by time interaction to mixed models. Week 12 analyses were also performed for PROMIS depression and anxiety scores using general linear models as these were not assessed during weekly calls. The characteristics of responders and non-responders after 4 weeks of the first therapy were compared using t-tests, chi-square or Fisher’s exact tests as appropriate, separately for those initially randomized to reflexology and for those initially randomized to meditative practices.
Comparisons of groups created by the second randomization were performed separately for those initially randomized to reflexology and meditative practices. The modeling technique was the same as for Aim 1, but for outcomes at weeks 5–12 and study group from the second randomization as the key explanatory variable. Finally, analyses were performed for the comparison of 4 decision rules, defined as a combination of two decision points, one at baseline, and one at week 4. At these two time points, the choices were R=reflexology, and MP=meditative practices, resulting in 4 combinations: (R, R), (R, MP), (MP, R), (MP, MP). The methodology for these analyses was developed by Nahum-Shani and colleagues (Nahum-Shani et al., 2012a) and involved definition of weights reciprocal to the probability of receiving a particular intervention or sequence (2 for responders, and 4 for non-responders) and restructuring of the data to allow comparisons of four decision rules within the same general linear model. All statistical tests were two-sided and performed at .05 level of significance. All analyses were conducted in SAS 9.4.
Sample size considerations
Sample size considerations began with comparisons of groups created by the second randomization and required n = 55 per group to detect the effect size d = 0.54 (Sikorskii et al., 2017) adjusted for baseline and repeated measures, for power of 0.80 or greater in two-tailed tests at .05 level of significance. The expected response rate of 20% from past trial of reflexology with women with advanced breast cancer (Wyatt et al., 2017) was then used for planning purposes to determine n = 276 total randomized to reflexology or meditative practices (138 to each intervention). The sample size of n = 55 in the control group was selected for the comparison of the magnitude of effects produced by intervention sequences (not for formal tests of significance) to match the number per group in the second randomization. The total planned sample size available for analysis was n = 331, and we had planned to have 430 patient-caregiver dyads consented, based on the projected 23% attrition rate based on past work in symptom management for patients with solid tumor cancers (Sikorskii et al., 2018).
Results
The recruitment goal was exceeded with 471 consented dyads (Figure 1) to account for higher than anticipated attrition rate. The enrollment of participants over the initially planned sample size of 430 was approved by all IRBs. Among the 471 consented dyads, 347 were randomized. The majority of patients were female, and the majority of caregivers lived with the patients and were spouses or partners (Table 1). At baseline, the average usual fatigue was between 4 and 5 on a 0–10 rating scale (Table 2).
Figure 1.
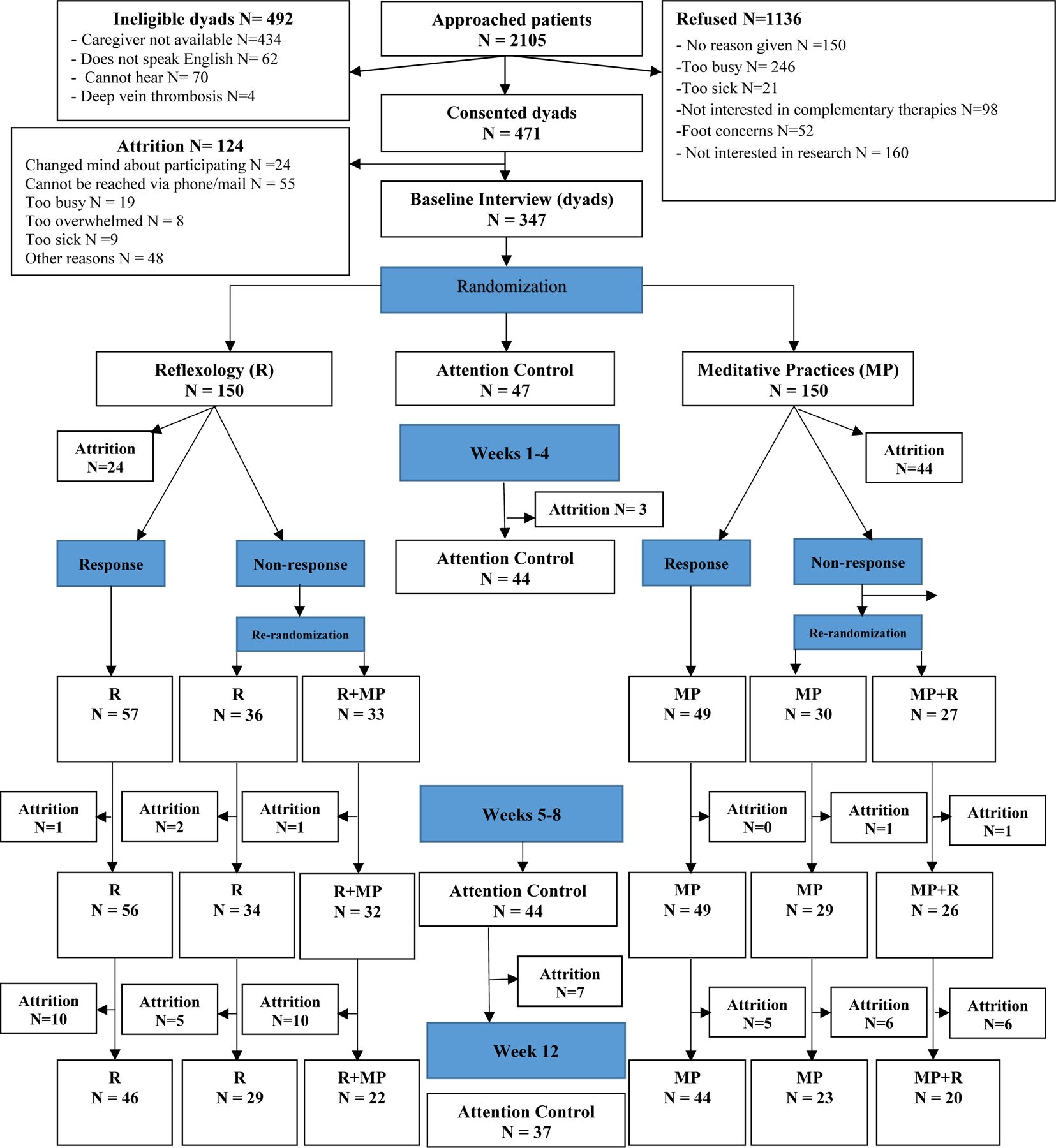
CONSORT chart
TABLE 1.
Demographic characteristics of groups created by the first randomization
| Characteristic | Patients | Caregivers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Reflexology, n = 150 |
Meditative
practices, n = 150 |
Control, n = 47 |
Reflexology, n = 150 |
Meditative
practices, n = 150 |
Control, n = 47 |
|||||||
|
|
||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
|
|
||||||||||||
| Sex | ||||||||||||
| Female | 116 | 77 | 115 | 77 | 34 | 72 | 58 | 40 | 61 | 44 | 19 | 42 |
| Male | 34 | 23 | 35 | 23 | 13 | 28 | 86 | 60 | 79 | 56 | 26 | 58 |
| Relationship: patient is | ||||||||||||
| Spouse | 85 | 59 | 94 | 64 | 30 | 64 | ||||||
| Adult child | 14 | 10 | 11 | 7 | 3 | 6 | ||||||
| Parent | 15 | 10 | 16 | 11 | 7 | 15 | ||||||
| Friend | 19 | 13 | 17 | 12 | 5 | 11 | ||||||
| Other | 12 | 8 | 9 | 6 | 2 | 4 | ||||||
| Living arrangement | ||||||||||||
| Together | 100 | 70 | 112 | 76 | 34 | 72 | ||||||
| Separately | 42 | 30 | 35 | 24 | 13 | 28 | ||||||
| Education | ||||||||||||
| High school or less | 2 | 2 | 4 | 4 | 0 | 0 | 25 | 17 | 18 | 12 | 6 | 12 |
| At least some college | 61 | 56 | 62 | 63 | 22 | 67 | 85 | 58 | 81 | 55 | 28 | 60 |
| Graduate/professional degree | 45 | 41 | 33 | 33 | 11 | 33 | 36 | 25 | 49 | 33 | 13 | 28 |
| Ethnicity | ||||||||||||
| Hispanic | 23 | 16 | 7 | 5 | 3 | 7 | 17 | 12 | 7 | 5 | 3 | 6 |
| Not Hispanic | 122 | 83 | 140 | 94 | 43 | 93 | 126 | 87 | 139 | 94 | 44 | 94 |
| Other | 2 | 1 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 0 |
| Race | ||||||||||||
| White Caucasian | 126 | 84 | 129 | 86 | 46 | 98 | 119 | 85 | 119 | 82 | 40 | 85 |
| Asian | 7 | 5 | 2 | 1 | 1 | 2 | 4 | 3 | 5 | 4 | 4 | 9 |
| Black or African American | 12 | 8 | 12 | 8 | 0 | 0 | 8 | 6 | 12 | 8 | 0 | 0 |
| Other | 5 | 3 | 7 | 5 | 0 | 0 | 8 | 6 | 9 | 6 | 3 | 6 |
| Employment | ||||||||||||
| Employed full time | 46 | 31 | 44 | 30 | 13 | 28 | 78 | 54 | 67 | 46 | 18 | 38 |
| Employed part time | 9 | 6 | 15 | 10 | 4 | 9 | 11 | 8 | 15 | 10 | 5 | 11 |
| Retired | 9 | 6 | 6 | 4 | 2 | 4 | 42 | 29 | 47 | 32 | 16 | 34 |
| Not employed | 83 | 57 | 81 | 56 | 28 | 59 | 14 | 9 | 18 | 12 | 8 | 17 |
| Income | ||||||||||||
| <=$24,999 | 18 | 12 | 8 | 5 | 1 | 2 | 13 | 9 | 9 | 7 | 4 | 9 |
| $25,000-$49,999 | 21 | 14 | 25 | 17 | 5 | 11 | 25 | 19 | 23 | 17 | 7 | 16 |
| $50,000-$99,999 | 30 | 21 | 28 | 19 | 7 | 15 | 32 | 24 | 42 | 31 | 6 | 14 |
| >=$100,000 | 17 | 12 | 24 | 16 | 11 | 24 | 24 | 18 | 20 | 14 | 11 | 26 |
| Other | 60 | 41 | 63 | 43 | 22 | 48 | 40 | 30 | 43 | 31 | 15 | 35 |
| Site of cancer | ||||||||||||
| Breast | 72 | 48 | 72 | 48 | 22 | 47 | ||||||
| Lung | 14 | 9 | 13 | 9 | 4 | 9 | ||||||
| Colon | 7 | 5 | 8 | 5 | 3 | 6 | ||||||
| Prostate | 6 | 4 | 7 | 5 | 2 | 4 | ||||||
| Other | 51 | 34 | 50 | 33 | 16 | 34 | ||||||
TABLE 2.
Descriptive statistics for patient outcomes and contextual factors at baseline by group from the first randomization
| Outcome | Reflexology, n = 150 |
Meditative
practices, n = 150 |
Control, n = 47 |
|||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean | StDev | Mean | StDev | Mean | StDev | |
|
|
||||||
| Patient age | 56.65 | 13.91 | 58.41 | 12.29 | 59.32 | 14.00 |
|
|
||||||
| Caregiver age | 56.07 | 14.84 | 55.56 | 14.58 | 54.59 | 13.42 |
|
|
||||||
| Patient number of comorbid conditions | 3.89 | 2.83 | 3.83 | 2.73 | 4.19 | 3.35 |
|
|
||||||
| Severity of worst fatigue | 5.00 | 3.24 | 5.16 | 3.07 | 4.85 | 3.15 |
| Severity of usual fatigue | 4.08 | 2.90 | 3.95 | 2.71 | 3.80 | 2.62 |
| Fatigue interference | 20.14 | 15.90 | 21.85 | 16.14 | 21.91 | 14.08 |
| Summed severity index of other symptoms | 38.61 | 29.00 | 40.43 | 28.24 | 37.23 | 22.46 |
| Symptom interference | 19.06 | 15.46 | 19.69 | 15.35 | 18.98 | 14.91 |
| PROMIS depression | 53.67 | 8.52 | 53.13 | 8.39 | 55.26 | 7.98 |
| PROMIS anxiety | 54.34 | 9.17 | 53.64 | 8.66 | 55.01 | 7.78 |
| Social support | 25.93 | 4.46 | 26.40 | 4.19 | 25.64 | 3.07 |
| PROMIS physical function | 39.97 | 7.79 | 40.17 | 8.58 | 39.91 | 7.01 |
Note. StDev = standard deviation; PROMIS = Patient Reported Outcomes Measurement Information System.
Attrition of participants who provided no post-baseline data was very low in the control arm (3/47, 6%), moderate (24/150, 16%) in the reflexology group, and highest (44/150, 29%) in the meditative practices group created by the first randomization. Among these drop-outs, there were no differences in demographics or baseline outcome values according to the first randomization (Supplemental Table 1). Because of lack of baseline differences among drop-outs by study group, the missing at random assumption was deemed reasonable for the analyses focused on group comparisons. All median values of the number of completed sessions across patient-weeks were equal to or greater than 1 required session per week during weeks 1–8, and the third quartile was 4 for each therapy that a participant was assigned to during the first 8 weeks. During the follow-up 4-week period (weeks 9–12), the median number of sessions was 2 (ranges 0–15 for reflexology, 0–40 for meditative practices).
There were no differences in primary or secondary outcomes between reflexology and meditative practices groups created by the first randomization during weeks 1–4 or 5–12 (Table 3), averaged over the second randomization. Differences between LS means for intervention sequences versus controls on severity of fatigue at week 8 were approximately 0.7 points for sequences starting with reflexology and 0.5 points for sequences starting with meditative practices, corresponding to the adjusted Cohen’s d effect sizes of 0.45 and 0.32, respectively.
TABLE 3.
Primary and secondary outcomes during weeks 1–12 for groups created by the first randomization: least square (LS) means and their standard errors (SEs), adjusted for baseline and balancing factors used in randomization
| Outcome | Reflexology | Meditative practices | Control | p for the difference between reflexology and meditative practices | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| LS Mean | SE | LS Mean | SE | LS Mean | SE | |||
|
|
||||||||
| Average severity of fatigue over weeks 1–4 | 4.17 | 0.17 | 4.08 | 0.18 | 4.32 | 0.26 | .72 | (−0.36, 0.52) |
| Average severity of fatigue over weeks 5–12 | 3.62 | 0.21 | 3.53 | 0.21 | 3.87 | 0.28 | .73 | (−0.42, 0.60) |
| Severity of fatigue at week 8 | 3.00 | 0.29 | 3.32 | 0.30 | 3.70 | 0.40 | .43 | (−1.12, 0.49) |
| Severity of fatigue at week 12 | 3.77 | 0.27 | 3.55 | 0.28 | 4.79 | 0.39 | .57 | (−0.51, 0.93) |
| Average summed severity index of other symptoms over weeks 1–4 | 29.99 | 1.27 | 29.32 | 1.30 | 32.52 | 1.87 | .68 | (−2.52, 3.86) |
| Average summed severity index of other symptoms over weeks 5–12 | 26.69 | 1.66 | 25.77 | 1.70 | 30.25 | 2.28 | .66 | (−3.19, 5.04) |
| Summed severity index of other symptoms at week 8 | 24.13 | 1.97 | 26.38 | 2.11 | 27.02 | 2.81 | .42 | (−7.72, 3.22) |
| Summed severity index of other symptoms at week 12 | 29.80 | 1.79 | 30.03 | 2.86 | 34.12 | 2.70 | .77 | (−5.59, 4.13) |
| PROMIS anxiety at week 12 | 52.64 | 0.84 | 51.96 | 0.89 | 52.96 | 1.24 | .53 | (−1.47, 2.83) |
| PROMIS depression at week 12 | 51.55 | 0.79 | 52.05 | 0.83 | 51.40 | 1.17 | .64 | (−2.50, 1.53) |
Note. LS = least square; SE = standard error; CI = confidence interval; PROMIS = Patient Reported Outcomes Measurement Information System.
The response rates to interventions after the initial 4 weeks were 45% (57 out of 126 post-attrition) in the reflexology group, and 46% (49 out of 106 post-attrition) in the meditative practices group. With drop-outs included in the denominator, the response rates were 38% (57 out of 150 randomized) for reflexology and 33% (49 out of 150 randomized) for meditative practices. There were virtually no differences between responders and non-responders to each therapy (Supplemental Table 2).
The lower than anticipated non-response rate to reflexology and meditative practices resulted in lower than planned counts in the non-responder groups who were eligible for the second randomization (Figure 1). The LS means for the primary and secondary outcomes for the groups created by the second randomization were not significantly different (Table 4).
TABLE 4.
Primary and secondary outcomes during weeks 5–12 for groups created by the second randomization: least square (LS) means and their standard errors (SEs), adjusted for baseline and balancing factors used in randomization
| 1st Randomization Weeks 1–4 |
Initial 4 weeks of Reflexology | Initial 4 weeks of Meditative Practices | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 2nd
Randomization Weeks 5–8 |
Continued reflexology only | Added MP | P-value | 95% CI | Continued MP only | Added reflexology | P-value | 95% CI | ||||
|
|
|
|||||||||||
| LS Mean | SE | LS Mean | SE | LS Mean | SE | LS Mean | SE | |||||
|
|
|
|||||||||||
| Outcomes | ||||||||||||
| Average severity of fatigue over weeks 5–12 | 3.99 | 0.35 | 3.74 | 0.38 | 0.57 | (−0.63, 1.14) | 4.03 | 0.42 | 4.52 | 0.52 | 0.39 | (−0.64, 1.63) |
| Severity of fatigue at week 8 | 3.00 | 0.50 | 3.55 | 0.63 | 0.43 | (−1.92 0.82) | 3.77 | 0.72 | 3.77 | 0.59 | 0.86 | (−1.72, 1.72) |
| Severity of fatigue at week 12 | 3.82 | 0.48 | 3.55 | 0.55 | 0.69 | (−1.07, 1.63) | 4.06 | 0.69 | 4.46 | 0.73 | 0.59 | (−1.16, 2.03) |
| Average summed severity index of other symptoms over weeks 5–12 | 28.02 | 3.57 | 29.96 | 3.97 | 0.67 | (−10.93, 7.04) | 30.33 | 3.04 | 32.18 | 3.76 | 0.66 | (−6.59, 10.29) |
| Summed severity index of other symptoms at week 8 | 19.48 | 4.94 | 27.43 | 5.41 | 0.23 | (−21.12, 5.21) | 31.68 | 4.23 | 31.58 | 3.49 | 0.99 | (−10.39, 10.57) |
| Summed severity index of other symptoms at week 12 | 30.19 | 4.72 | 28.94 | 5.27 | 0.71 | (−9.55, 6.54) | 36.37 | 3.78 | 35.56 | 3.27 | 0.87 | (−8.66, 10.27) |
| PROMIS anxiety at week 12 | 55.11 | 1.74 | 53.71 | (2.00) | 0.54 | (−3.15, 5.96) | 55.46 | 2.02 | 52.93 | (1.86) | 0.29 | (−2.22, 7.29) |
| PROMIS depression at week 12 | 53.66 | 1.34 | 52.54 | (1.54) | 0.52 | (−2.38, 4.60) | 52.98 | 2.07 | 51.75 | 1.90 | 0.61 | (−3.64, 6.09) |
Note. LS = least square; SE = standard error; CI = confidence interval PROMIS = Patient Reported Outcomes Measurement Information System.
Finally, comparisons for the four decision rules resulted in no significant differences, except for (R, R) and (R, MP) at week 8 on summed severity index of the MDASI symptoms other than fatigue. Participants who used reflexology for the full 8 weeks had lower summed severity index compared to those who started with reflexology and added meditative practices after the first 4 weeks : mean difference 7.04 points, 95% confidence interval [0.30, 13.78], p = .04 (Table 5).
TABLE 5.
Dynamic treatment regimes (decision rules: R=reflexology; MP=meditative practices): least square means over weeks 5–12 and their SEs, adjusted for baseline and balancing factors used in randomization
| Dynamic treatment regime | (R, R) | (R, MP) | (MP, R) | (MP, MP) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Outcome | LS Mean | SE | LS Mean | SE | LS Mean | SE | LS Mean | SE |
|
|
||||||||
| Average severity of fatigue over weeks 5–12 | 3.70 | 0.19 | 3.66 | 0.26 | 3.64 | 0.29 | 3.55 | 0.22 |
| Severity of fatigue at week 8 | 2.52 | 0.29 | 2.98 | 0.42 | 3.05 | 0.53 | 3.07 | 0.37 |
| Severity of fatigue at week 12 | 3.95 | 0.31 | 3.90 | 0.27 | 3.74 | 0.37 | 3.53 | 0.31 |
| Average severity index of other symptoms over weeks 5–12 | 27.50 | 1.16 | 28.78 | 2.40 | 28.58 | 2.49 | 26.69 | 1.61 |
| Summed severity index of other symptoms at week 8 | 18.12 | 2.51 1 | 25.15 | 3.371 | 19.02 | 2.24 | 22.20 | 2.43 |
| Summed severity index of other symptoms at week 12 | 30.43 | 2.41 | 28.30 | 2.92 | 30.52 | 2.88 | 29.50 | 1.96 |
| PROMIS anxiety at week 12 | 52.64 | 0.97 | 51.68 | 0.83 | 52.11 | 1.20 | 51.02 | 0.80 |
| PROMIS depression at week 12 | 51.16 | 0.56 | 50.42 | 0.88 | 51.60 | 1.12 | 51.07 | 0.85 |
Significant difference (p = .04) between two dynamic treatment regimens: (R, R) and (R, MP).
Note. LS = least square; SE = standard error; PROMIS = Patient Reported Outcomes Measurement Information System.
Discussion
The comparisons among intervention sequences and among decision rules resulted in no differences, except for lower summed severity index of symptoms other than fatigue at week 8 for those continuing with reflexology compared to those who had meditative practices added. Given the number of tests, chance is the best explanation for one observed significant difference. Similarly, there were no significant differences in characteristics of responders and non-responders. These findings lead to the conclusion that patient choice of a feasible and acceptable therapy is the main consideration in deciding between reflexology and meditative practices as supportive care during cancer treatment. Adding the second therapy for non-responders may not be warranted as it did not result in improved outcomes compared to continuing a single therapy. These findings lead to the possibility that adding the second therapy may be unmanageable, but this possibility was not supported by the data on the median number of sessions across patient-weeks that was one session per week specified in the protocol.
Because the SMART is designed for testing of sequencing of evidence-based therapies, the design does not typically involve a control group for several reasons. One reason is that prior efficacy testing does not need to be duplicated for individual therapies unless there is a possibility of two therapies being more burdensome than one. This was not hypothesized with reflexology and meditative practices that have different mechanisms for symptom reduction. While there are likely several psycho-physiological mechanisms that contribute to the positive outcomes from reflexology and meditative practices, the benefits derived from reflexology, a direct body manipulation, may be primarily physically-mediated (Zengin & Aylaz, 2019); whereas, the effects derived from training in meditative practices may be cognitively-mediated (Shapiro et al., 2006). Both reflexology and meditation may result in activation of a relaxation response (Mantoudi et al., 2020; Matchim et al., 2011; McVicar et al., 2007; Stussman et al., 2015), characterized by calmness, acceptance, and sensations of wellbeing, which may contribute to improved well-being (Satija & Bhatnagar, 2017).
Further, the SMART design often requires a large sample size because of multiple randomizations and stratification according to response to the initial therapy, resulting in smaller cell sizes at the end, as was seen in this trial. Inclusion of a large control group to ensure power in comparisons to it would have threatened study feasibility within the available research support and a reasonable timeframe. Therefore, in this trial, the control group was relatively small. It was included because, although unlikely, intervention sequences may have produced worse outcomes than individual interventions. This was not the case in this trial, based on estimated differences with the control group that were similar in magnitude to those seen in past trials with single therapies (Foley et al., 2010; Wyatt et al., 2012) and within the range of clinically meaningful differences based on the cut-off of d=0.33 proposed for patient-reported outcomes in cancer (Sloan et al., 2005). In the past trial formally powered for tests against controls, such a difference was statistically significant. In this trial, it was not because of the control group sample size. If point estimates indicated possibly worse outcomes, more research would have been needed to investigate why sequencing or combining reflexology and meditative practices might be problematic.
In future research, choice of therapy is an important consideration. In past research, the dose of reflexology and meditative practices was established (length, frequency and minimum). Further, whereas meditative practices can be practiced with or without an informal caregiver, reflexology has to be delivered by a caregiver in the home. In past research, the added task of hands-on delivery of reflexology to the patient did not hamper the patient-caregiver relationship (Holmstrom et al., 2015). Further, physical touch involved with a body-based therapy like reflexology may enhance social support (Mühlenpfordt et al., 2020) and strengthen the perceived reflexology experience. If caregiver delivery of reflexology is problematic given living arrangements or other factors, meditative practices may be a more feasible option without sacrifice in efficacy for symptom management.
The high attrition rate from meditative practices, however, suggests limitations in terms of its broad use. While these limitations were not specific to a particular population subgroup, the differential attrition rate across groups created by the first randomization warrants further examination of the elements of the meditative practices. In contrast to other studies incorporating meditation (Fjorback et al., 2011), this study included the relatively short training period required to adopt a new practice. Also, although the meditative practices used in this research are secular and grounded in scientific evidence, participants may have had concerns that the intervention is associated with Eastern spiritual practices (Bowen, Bergman, & Witkiewitz, 2015; Schlieter, 2017). In this regard, cognitive dissonance may occur if meditative practices are perceived to run counter to Christian spirituality, thus contributing to study attrition if not addressed proactively (under review, 2021). Our team developed a protocol for this study to address religious concerns when raised in regards to learning meditation (Lehto et al., 2021).
The higher than anticipated 20% response rate to reflexology and meditative practices supports the use of the two interventions for the management of fatigue and is consistent with a completed trial of reflexology against controls (Sikorskii et al., 2020). The sample in this study included people with solid tumor of any stage in contrast to women with advanced breast cancer in past reflexology trials. Regardless of site and stage of cancer, fatigue is a prevalent symptom with virtually no available pharmacological management means with the exception of fatigue related to anemia or neutropenia (Mustian et al., 2016; Wang & Woodruff, 2017). Because few complementary therapy options for the management of fatigue have been tested rigorously, it is difficult to gauge whether response rates of 33–46% are high or low. This range of response rates is consistent with rates seen for behavioral or psycho-educational interventions (Sikorskii et al., 2009). Magnitudes of reductions in fatigue severity with reflexology and meditative practices were similar to those seen with other interventions such as supervised exercise (Mustian et al., 2016).
The limitations of this study included deviations from the planned flow of participants. The attrition from meditative practices has raised questions about the protocol used since many studies have supported benefits achieved from interventions incorporating meditation training. The lower than anticipated non-response rate left fewer than expected participants in the non-responder cells for re-randomization within the SMART design. However, the point estimates for the means following re-randomizations were very close indicating that lack of statistically significant differences was not due to power, but potentially to lack of differences in outcomes produced by four decision rules. All primary and secondary outcomes were specified a priori, but comparisons among sequences, decision rules, and between responders and non-responders involved multiple statistical tests. Chance may be the best explanation for few significant differences that would not withstand adjustments for multiple tests. Other limitations included a sample make-up of a majority of white women consistent with the literature on people who are most interested in complementary therapies (Judson et al., 2017). Medical record data on supportive care medications were not available in a uniform manner across all recruitment sites. While all patients with cancer received usual care, and the use of these medications was equally distributed across trial arms, the examination of receipt of supportive care medications may be warranted in future studies. The same consideration apply to the completion or premature stoppage of cancer treatment.
In summary, sequences of reflexology and meditative practices were not different in symptom outcomes. The SMART design allowed for comparisons of different doses and sequencing of interventions that can be provided dynamically to individuals across time (Pelham et al., 2016). Based on this trial’s results, clinicians can encourage dyads to select either reflexology or meditative practices for home-based symptom management during cancer treatment.
Supplementary Material
Key message:
No differences were found among sequences of reflexology and meditative practices with respect to severity of fatigue and other symptoms. The magnitude of reduction in symptom severity by intervention sequences compared to control was similar to reductions observed in past studies with single therapies.
Acknowledgments:
This study was supported by grant 1 R01 CA193706 from the National Institutes of Health.
Disclosures and Acknowledgments
We would like to thank the patients and caregivers who contributed to this research. We would also like to thank Kristen Bilyea for clerical support in preparation of this manuscript.
Footnotes
Conflict of interest statement: The authors have declared that there is no conflict of interest.
Contributor Information
Gwen Wyatt, College of Nursing, Michigan State University, 1355 Bogue Street, East Lansing, MI 48824.
Rebecca Lehto, College of Nursing, Michigan State University, 1355 Bogue Street, East Lansing, MI 48824.
Pratim Guha Niyogi, Department of Statistics and Probability, Michigan State University, 619 Red Cedar Road, East Lansing, MI 48824.
Sarah Brewer, Department of Epidemiology and Biostatistics, Michigan State University, 909 Wilson Road, East Lansing, MI 48824.
David Victorson, Northwestern University Feinberg School of Medicine, Evanston, IL 60208.
Thaddeus Pace, University of Arizona College of Nursing, 1305 N. Martin Ave, PO Box 210203, Tucson, AZ 85721, Tucson, AZ 86721.
Terry Badger, University of Arizona College of Nursing, 1305 N. Martin Ave, PO Box 210203, Tucson, AZ 85721.
Alla Sikorskii, Department of Psychiatry, Michigan State University, 909 Wilson Road, Room 32, East Lansing, MI 48824.
References
- Almirall D, Nahum-Shani I, Sherwood NE, & Murphy SA (2014). Introduction to SMART designs for the development of adaptive interventions: With application to weight loss research. Transational Behavioral Medicine, 4(3), 260–274. 10.1007/s13142-014-0265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya CE, Kubo A, Borno HT, Rosenthal B, Campanella M, Rettger JP, Joseph G, Allen IE, Venook AP, Altschuler A, & Dhruva A (2018). Being Present: A single-arm feasibility study of audio-based mindfulness meditation for colorectal cancer patients and caregivers. PLoS One, 13(7), e0199423. 10.1371/journal.pone.0199423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger T, Segrin C, Sikorskii A, Pasvogel A, Weihs K, Lopez AM, & Chalasani P (2020). Randomized controlled trial of supportive care interventions to manage psychological distress and symptoms in Latinas with breast cancer and their informal caregivers. Psychology & Health, 35(1), 87–106. 10.1080/08870446.2019.1626395 [DOI] [PubMed] [Google Scholar]
- Baglama SS, & Bakir E (2019). Caregiver-Delivered foot reflexology: Effects on patients and caregivers. Holistic Nursing Practice, 33(6), 338–345. 10.1097/hnp.0000000000000351 [DOI] [PubMed] [Google Scholar]
- Barsevick AM, Cleeland CS, Manning DC, O’Mara AM, Reeve BB, Scott JA, & Sloan JA (2010). ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. Journal of Pain & Symptom Management, 39(6), 1086–1099. 10.1016/j.jpainsymman.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss EA, Ellis JL, & Steiner JF (2009). Seniors’ self-reported multimorbidity captured biopsychosocial factors not incorporated into two other data-based morbidity measures. Journal of Clinical Epidemiology, 62(5), 550–557.e551. 10.1016/j.jclinepi.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory MG, Ogedegbe G, Orwig D, Ernst D, & Czajkowski S (2004). Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology, 23(5), 443–451. 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- Bevans M, & Sternberg EM (2012). Caregiving burden, stress, and health effects among family caregivers of adult cancer patients. JAMA, 307(4), 398–403. 10.1001/jama.2012.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie K, Garland SN, & Carlson LE (2010). Psychological benefits for cancer patients and their partners participating in mindfulness-based stress reduction (MBSR). Psycho-oncology, 19(9), 1004–1009. 10.1002/pon.1651 [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, & Cole SW (2011). Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression and sleep disturbance share a common underlying mechanism? Journal of Clinical Oncology, 29(26), 3517–3522. 10.1200/JCO.2011.36.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bränström R, Kvillemo P, Brandberg Y, & Moskowitz JT (2010). Self-report mindfulness as a mediator of psychological well-being in a stress reduction for cancer patients - A randomized study. Annals of Behavioral Medicine, 39(2), 151–161. 10.1007/s12160-010-9168-6 [DOI] [PubMed] [Google Scholar]
- Cella D, Lai J, Chang C, Peterman A, & Slavin M (2002). Fatigue in cancer patients compared with fatigue in the general United States population. Cancer, 94(2), 528–538. 10.1002/cncr.10245 [DOI] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse DJ, Choi SW, Cook KF, DeVellis R, DeWalt D, Fries JF, Gershon R, Hahn E, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays RD, & Group., P. C. (2010). The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63(11), 1179–1194. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Tate J, Hoffman L, Schulz R, Ren D, Donahoe M, Given B, & Sherwood P (2014). Fatigue in family caregivers of adult intensive care unit survivors. Journal of Pain and Symptom Management, 48(3), 353–363. 10.1016/j.jpainsymman.2013.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, & Engstrom MC (2000). Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer, 89(7), 1634–1646. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Zhao F, Chang VT, Sloan JA, O’Mara AM, Gilman PB, Weiss M, Mendoza TR, Lee J-W, & Fisch MJ (2013). The symptom burden of cancer: Evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer, 119(24), 4333–4340. 10.1002/cncr.28376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger L, Schrepf A, Christensen D, DeGeest K, Bender D, Ahmed A, Goodheart MJ, Penedo F, Lubaroff DM, Sood AK, & Lutgendorf SK (2012). Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain Behav Immun, 26(7), 1037–1044. 10.1016/j.bbi.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtier N, Gaze S, Armes J, Smith A, Radley L, Armytage J, Simmonds M, Johnson A, Gambling T, & Hopkinson J (2018). ACTIVE - a randomized feasibility trial study protocol of a behavioural intervention to reduce fatigue in women undergoing radiotherapy for early breast cancer: Study protocol. Pilot and Feasibility Studies, 4(85). 10.1186/s40814-018-0275-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjorback LO, Arendt M, Ornbøl E, Fink P, & Walach H (2011). Mindfulness-based stress reduction and mindfulness-based cognitive therapy: a systematic review of randomized controlled trials. Acta psychiatrica Scandinavica, 124(2), 102–119. 10.1111/j.1600-0447.2011.01704.x [DOI] [PubMed] [Google Scholar]
- Fletcher BS, Miaskowski C, Given B, & Schumacher K (2012). The cancer family caregiving experience: an updated and expanded conceptual model. European Journal of Cancer, 16(8), 387–398. 10.1016/j.ejon.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley E, Baillie A, Huxter M, Price M, & Sinclair E (2010). Mindfulness-based cognitive therapy for individuals whose lives have been affected by cancer: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 78(1), 72–79. 10.1037/a0017566 [DOI] [PubMed] [Google Scholar]
- Frambes D, Lehto R, Sikorskii A, Tesnjak I, Given B, & Wyatt G (2017). Fidelity scorecard: Evaluation of a caregiver-delivered symptom management intervention. Journal of Advanced Nursing, 73(8), 2012–2021. 10.1111/jan.13266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frambes D, Sikorskii A, Tesnjak I, Wyatt G, Lehto R, & Given B (2017). Caregiver-Reported health outcomes: Effects of providing reflexology for symptom management to women with advanced breast cancer. Oncology Nursing Forum, 44(5), 596–605. 10.1188/17.onf.596-605 [DOI] [PubMed] [Google Scholar]
- Given B, Given CW, Sikorskii A, Jeon S, McCorkle R, Champion V, & Decker D (2008). Establising mild, moderate, and sever scores for cancer-related symptoms: How consistent and clinically meaningful are interference-based cut-points? Journal of Pain and Symptom Management, 35(2), 126–135. 10.1016/j.jpainsymman.2007.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom A, Wyatt G, Sikorskii A, Luo Z, Victorson D, & Tesnjak I (2015). Dyadic recruitment in complementary therapy studies: Experience from a clinical trial of caregiver-delivered reflexology. Applied Nursing Research, 29, 136–139. 10.1016/j.apnr.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Liu T, & Li F (2019). Association between dyadic interventions and outcomes in cancer patients: a meta-analysis. Supportive Care in Cancer, 27(3), 745–761. 10.1007/s00520-018-4556-8 [DOI] [PubMed] [Google Scholar]
- Huang H, Chen M, Liang J, & Miaskowski C (2014). Changes in and predictors of severity of fatigue in women with breast cancer: A longitudial study. International Journal of Nursing Studies, 51, 582–592. 10.1016/j.ijnurstu.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Jacobs JM, Ream ME, Pensak N, Nisotel LE, Fishbein JN, MacDonald J, Buzaglo J, Lennes IT, Safren SA, Pirl WF, Temel J, & Greer J (2019). Patient experiences with oral chemotherapy: Adherence, symptoms, and quality of life. Journal of the National Comprehensive Cancer Network: JNCCN, 17(3), 221–228. 10.6004/jnccn.2018.7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S, Given CW, Sikorskii A, & Given B (2009). Do interference-based cut-points differentiate mild, moderate, and severe levels of 16 cancer-related symptoms? Journal of Pain and Symptom Management, 37(2), 220–232. 10.1016/j.jpainsymman.2008.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson PL, Reem A, Xiong Y, Ebbert J, & Lancaster JM (2017). Complementary and alternative medicine use in individuals presenting for care at a comprehensive cancer center. Integrative Cancer Therapies, 16(1), 96–103. 10.117/1534735416660384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J (2009). Full Catastrophe Living: Using The Wisdom Of Your Body And Mind To Face Stress, Pain, and Illness (15 ed.). Delta Trade Paperbacks. [Google Scholar]
- Kim M, Kim K, & Lim C (2017). Symptom clusters and quality of life according to the survivorship stage in ovarian cancer survivors. Western Journal of Nursing Research, 40(9), 22. 10.1177/0193945917701688 [DOI] [PubMed] [Google Scholar]
- Kim Y, Carver CS, Shaffer KM, Gansler T, & Cannady RS (2015). Cancer caregiving predicts physical impairments: roles of earlier caregiving stress and being a spousal caregiver. Cancer, 121(2), 302–310. 10.1002/cncr.29040 [DOI] [PubMed] [Google Scholar]
- Lehto R, Sikorskii A, Marshall K, & Wyatt G (2021). Engaging patients in research that involves meditation: Religious concerns and nursing implications [Manuscript submitted for publication]. College of Nursing, Michigan State University. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto R, & Wyatt G (2013). Perceptions about using mindfulness therapy: A lung cancer focus group study. Cancer Nursing, 36(4), E51–E60. 10.1097/NCC.0b013e31826d2f16 [DOI] [PubMed] [Google Scholar]
- Lehto R, Wyatt G, Sikorskii A, Tesnjak I, & Kaufman VH (2015). Home-based mindfulness therapy for lung cancer symptom mamangement: A randomized feasibility trial. Psycho-oncology, 24(9), 1208–1212. 10.1002/pon.3755 [DOI] [PubMed] [Google Scholar]
- Link AR, Gammon MD, Jacobson JS, Abrahamson P, Bradshaw PT, Terry MB, Teitelbaum S, Neugut A, & Greenlee H (2013). Use of self-care and practitioner-based forms of Complementary and Alternative Medicine before and after a diagnosis of breast cancer. Evidence-Based Complementary and Alternative Medicine: eCAM, 2013, 301549. 10.1155/2013/301549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantoudi A, Parpa E, Tsilika E, Batistaki C, Nikoloudi M, Kouloulias V, Kostopoulou S, Galanos A, & Mystakidou K (2020). Complementary therapies for patients with cancer: Reflexology and relaxation in integrative palliative care. A randomized controlled comparative study. Journal of Alternative & Complementary Medicine, 26(9), 792–798. 10.1089/acm.2019.0402 [DOI] [PubMed] [Google Scholar]
- Matchim Y, Armer JM, & Stewart BR (2011). Effects of mindfulness-based stress reduction (MBSR) on health among breast cancer survivors. Western Journal of Nursing Research, 33(8), 996–1016. 10.1177/0193945910385363 [DOI] [PubMed] [Google Scholar]
- Matzka M, Kock-Hodi S, Jahn P, & Mayer H (2018). Relationship among symptom clusters, quality of life, and treatment specific optimisim in patients with cancer. Supportive Care in Cancer, 26(8), 8. 10.1007/s00520-018-4102-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVicar AJ, Greenwood CR, Fewell F, D’Arcy V, Chandrasekharan S, & Alldridge LC (2007). Evaluation of anxiety, salivary cortisol and melatonin secretion following reflexology treatement: A pilot study in healthy individuals. Complementary Therapies in Clinical Practice, 13(3), 137–145. 10.1016/j.ctcp.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Mendoza T, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, & Huber SL (1999). The rapid assessment of fatigue severity in cancer patients. Cancer, 85(5), 1186–1196. [DOI] [PubMed] [Google Scholar]
- Mendoza TR, Wang XS, Lu C, Palos GR, Liao Z, Mobley GM, Kapoor S, & Cleeland CS (2011). Measuring the symptom burden of lung cancer the validity and utility of the lung cancer module of the M.D. Anderson Symptom Inventory. Oncologist, 16(2), 217–227. 10.1634/theoncologist.2010-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbury K, Badr H, Fossella F, Pisters KM, & Carmack CL (2013). Longitudinal associations between caregiver burden and patient and spouse distress in couples coping with lung cancer. Supportive Care in Cancer, 21(9), 2371–2379. 10.1007/s00520-013-1795-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SA, & Berger AM (2006). Cancer-related fatigue: The evidence base for assessment and management. The Cancer Journal, 12(5), 374–387. 10.1097/00130404-200609000-0000 [DOI] [PubMed] [Google Scholar]
- Morano C, Giunta N, Parikh N, Panuska S, Fahs MC, & Gallo WT (2013). Mind-body techniques, race-ethnicity, and depression among urban senior center participants. Health & Social Work, 38(3), 167–172. 10.1093/hsw/hlt010 [DOI] [PubMed] [Google Scholar]
- Mühlenpfordt I, Stritter W, Bertram M, Ben-Arye E, & Seifert G (2020). The power of touch: External applications from whole medical systems in the care of cancer patients (literature review). Suppotive Care in Cancer, 28, 461–471. 10.1007/s00520-019-05172-7 [DOI] [PubMed] [Google Scholar]
- Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, Mohr D, Palesh OG, Peppone LJ, Piper BF, Scarpato J, Smith T, Sprod LK, & Miller SM (2016). Comparison of pharmaceutical, psychological, and exercise treatment for cancer-related fatigue: A meta-analysis. JAMA Oncology, 3(7), 961–968. 10.1001/jamaoncol.2016.6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Qian M, Almirall D, Pelham WE, Gnagy B, Fabiano GA, Waxmonsky JG, Yu J, & Murphy SA (2012a). Experimental design and primary data analysis methods for comparing adaptive interventions. Psychological Methods, 17(4), 457–477. 10.1037/a0029372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Qian M, Almirall D, Pelham WE, Gnagy B, Fabiano GA, Waxmonsky JG, Yu J, & Murphy SA (2012b). Q-learning: A data analysis method for constructing adaptive interventions. Psychological methods, 17(4), 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nail L (2002). Fatigue in patients with cancer. Oncol Nurs Forum, 29(3), 537. 10.1188/onf.537-546 [DOI] [PubMed] [Google Scholar]
- Ng CG, Lai KT, Tan SB, Sulaiman AH, & Zainal NZ (2016). The effect of 5 minutes of mindful breathing to the perception of distress and physiological responses in palliative care cancer patients: A randomized controlled study. Journal of Palliative Medicine, 19(9), 917–924. 10.1089/jpm.2016.0046 [DOI] [PubMed] [Google Scholar]
- Pearson E, Morris ME, di Stefano M, & McKinstry CE (2018). Interventions for cancer-related fatigue: A scoping review. European Journal of Cancer Care, 27(1). 10.1111/ecc.12516 [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA, Waxmonsky JG, Greiner AR, Gnagy EM, Pelham W. E. r., Coxe S, Verley J, Bhatia I, Hart K, Karch K, Konijnendijk E, Tresco K, Nahum-Shani I, & Murphy S (2016). Treatment sequencing for childhood ADHD: A multiple-randomization study of adaptive medication and behavioral interventions Journal of Clinical Child and Adolescent Psychology, 45(4), 19. 10.1080/15374416.2015.1105138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper B, Dibble S, Dodd M, Slaughter R, & Paul S (1998). The revised Piper Fatigue Scale: Psychometric evaluation in women with breast cancer. Oncology Nursing Forum, 25(4), 677–684. [PubMed] [Google Scholar]
- PROMIS. (2012). Adult Depression version 1.0 short form: a breif guide to the 8-item PROMIS Short Form v1.0-Depression 8b. https://assessmentcenter.net/
- PROMIS. (2013a). Adult physical function profile short forms. https://assessmentcenter.net/
- PROMIS. (2013b). Adult satisfaction with participation in social roles profile short forms. https://assessmentcenter.net/
- Reilly CM, Bruner DW, Mitchell SA, Minasian LM, Basch E, Dueck AC, Cella D, & Reeve B (2013). A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Supportive Care in Cancer, 21(6), 1525–1550. 10.1007/s00520-012-1688-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottman B, Wyatt G, Crane T, & Sikorskii A (2020). Expectany and utilisation of reflexology among women with advanced breast cancer. Applied Psychology: Health and Well-Being, 12(2), 493–512. 10.1111/aphw.12194 [DOI] [PubMed] [Google Scholar]
- Satija A, & Bhatnagar S (2017). Complementary therapies for symptom management in cancer patients. Indian Journal of Palliative Care, 23(4), 468–479. 10.4103/IJPC.IJPC_100_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlieter J (2017). Buddhist insight meditation (Vipassanā) and Jon Kabat-Zinn·s “Mindfulness-based Stress Reduction”: an example of dedifferentiation of religion and medicine? Journal of Contemporary Religion. 32(3), 447–463. 10.1080/13537903.2017.1362884 [DOI] [Google Scholar]
- Schrepf A, Clevenger L, Christensen D, DeGeest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L, Penedo F, Lucci JA 3rd, Ganjei-Azar P, Mendez L, Markon K, Lubaroff DM, Thaker PH, Slavich GM, Sood AK, & Lutgendorf SK (2013, March). Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain Behav Immun, 30 Suppl, S126–134. 10.1016/j.bbi.2012.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SF, Carlson LE, Astin JA, & Freedman B (2006). Mechanisms of mindfulness. Journal of Clinical Psychology 62, 373–386. 10.1002/jclp.20237 [DOI] [PubMed] [Google Scholar]
- Sharp DM, Walker MB, Chaturvedi A, Upadhyay S, Hamid A, Walker AA, Batemen JS, Braid F, Ellwood K, Hebblewhite C, Hope T, Lines M, & Walker LG (2010). A randomised, controlled trial of the psychological effects of reflexology in early breast cancer. European Journal of Cancer, 46(2), 312–322. 10.1016/j.ejca.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Sikorskii A, Given CW, Given B, Vachon E, Krauss JC, Rosenzweig M, McCorkle R, Champion V, Banik A, & Majumder A (2018). An automated intervention did not improve adherance to oral oncolytic agents while managing symptoms: Results from a two-arm radomized controlled trial. Journal of Pain and Symptom Management, 56(5), 727–735. 10.1016/j.painsymman.2018.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorskii A, Given CW, You M, Jeon S, & Given B (2009). Response analysis for multiple symptoms revealed differences between arms of a symptom management trial. Journal of Clinical Epidemiology, 62(7), 716–724. 10.1016/j.jclinepi.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorskii A, Guha Niyogi P, Victorson D, Tamkus D, & Wyatt G (2020). Symptom response analysis of a randomized controlled trial of reflexology for symptom management among women with advanced breast cancer. Supportive Care in Cancer, 28(3), 1395–1404. 10.1007/s00520-019-04959-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorskii A, Wyatt G, Lehto R, Victorson D, Badger T, & Pace T (2017). Using SMART design to improve symptom management among cancer patients: a study protocol. Research in Nursing & Health, 40(6), 501–511. 10.1002/nur.21836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan JA, Cella D, & Hays RD (2005). Clinical significance of patient-reported questionnaire data: Another step toward consensus. Journal of Clinical Epidemiology, 58(12), 1217–1219. 10.1016/j.jclinepi.2005.07.009 [DOI] [PubMed] [Google Scholar]
- So W, Law B, Ng M, He X, Chan D, Chan C, & McCarthy AL (2021). Symptom clusters experienced by breast cancer patients at various treatment stages: A systematic review. Cancer Medicine, 00, 1–35. 10.1002/cam4.3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson N, Swanson M, Dalton J, Keefe FJ, & Engelke M (2007). Partner-delivered reflexology: Effects on cancer pain and anxiety. Oncology Nursing Forum, 34(1), 127–132. 10.1188/07.ONF.127-132 [DOI] [PubMed] [Google Scholar]
- Stussman BJ, Black LI, Barnes PM, Clarke TC, & Nanhin RL (2015). Wellness-related use of common complementary health approaches among adults: United States, 2012. National Health Statistics Reports(85), 1–12. [PubMed] [Google Scholar]
- Tsay SL, Chen HL, Chen SC, Lin HR, & Lin KC (2008). Effects of reflexotherapy on acute postoperative pain and anxiety among patients with digestive cancer. Cancer Nursing, 31(2), 109–115. 10.1097/01.NCC.0000305694.74754.7b [DOI] [PubMed] [Google Scholar]
- Victorson D, Kentor M, Maletich C, Lawton RC, Kaufman VH, Borrero M, Languido L, Lewett K, Pancoe H, & Berkowitz C (2015). Mindfulness meditation to promote wellness and manage chronic disease: A systematic review and meta-analysis of mindfulness-based randomized controlled trials relevant to lifestyle medicine. American Journal of Lifestyle Medicine, 9(3). 10.1177/1559827614537789 [DOI] [Google Scholar]
- Wang XS, & Woodruff JF (2017). Cancer-related and treatment-related fatigue. Gynecologic Oncology, 136(3), 446–452. 10.1016/j.ygyno.2014.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S, & Voner V (2008). Practical Reflexology: Interpretation and Techniques. McGraw-Hill Companies, Incorporated. http://books.google.com/books?id=UM6eAAAACAAJ [Google Scholar]
- Wyatt G, Sikorskii A, Rahbar M, Victorson D, & You M (2012, November ). Health-related quality of life outcomes: A reflexology trial with patients with advanced-stage breast cancer. Oncology Nursing Forum, 39(6), 568–577. 10.1188/12.ONF.568-577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt G, Sikorskii A, Tesnjak I, Frambes D, Holmstrom A, Luo Z, Tamkus D, & Victorson D (2017). A randomized clinical trial of caregiver-delivered reflexology for symptom management during breast cancer treatment. Journal of Pain and Symptom Management, 54(5), 670–679. 10.1016/j.jpainsymman.2017.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Dong B, Wang L, Jing X, Wu Y, Lin L, & Tian L (2020). Midfulness-based stress reduction can alleviate cancer-related fatigue: A meta-analysis. Journal of Psychosomatic Research, 130, 109916. 10.1016/j.jpsychores.2019.109916 [DOI] [PubMed] [Google Scholar]
- Zengin L, & Aylaz R (2019). The effects of sleep hygiene education and reflexology on sleep quality and fatigue in patients receving chemotherapy. European Journal of Cancer Care, 28(3). 10.1111/ecc.13020 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhao H, & Zheng Y (2019). Effectiveness of mindfulness-based stress reduction (MBSR) on symptom variables and health-related quality of life in breast cancer patients-a systematic review and meta-analysis. Supportive Care in Cancer, 27, 771–781. 10.1007/s00520-018-4570-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


