Abstract
Background
This study compared the surgical and oncological outcomes of open and minimally invasive pelvic exenteration.
Methods
Patients who underwent pelvic exenterations for primary locally advanced rectal cancers with invasion of the urogenital organs (central and anterior disease) between August 2013 and September 2020 were reviewed retrospectively. Patients were categorized as undergoing open or minimally invasive surgery (MIS) and these groups were compared for perioperative outcomes and 3-year survival (overall, recurrence-free and local relapse-free survival). Multivariable Cox regression analysis was performed to assess the independent influence of approach of surgery and cancer features on recurrence-free survival (RFS).
Results
Of the 158 patients who underwent pelvic exenteration, 97 (61.4 per cent) had open exenterations and 61 (38.6 per cent) patients had an MIS resection (44 patients (72 per cent) using laparoscopy and 17 (28 per cent) using robotic surgery). There were 96 (60.8 per cent) total pelvic exenterations and 62 (39.2 per cent) posterior pelvic exenterations. MIS exenterations had significantly longer operative times (MIS versus open: 640 mins versus 450 mins; P < 0.001) but reduced blood loss (MIS versus open: 900 ml versus 1600 ml; P < 0.001) and abdominal wound infections (MIS versus open: 8.2 versus 17.5 per cent; P = 0.020) without a difference in hospital stay (MIS versus open: 11 versus 12 days; P = 0.620). R0 resection rates and involvement of circumferential resection margins were similar (MIS versus open: 88.5 versus 91.8 per cent, P = 0.490 and 13.1 versus 8.2 per cent, P = 0.342 respectively). At a median follow-up of 29 months, there were no differences in 3-year overall survival (MIS versus open: 79.4 versus 60.2 per cent; P = 0.251), RFS (MIS versus open: 51.9 versus 47.8 per cent; P = 0.922) or local relapse-free survival (MIS versus open: 89.7 versus 75.2 per cent; P = 0.491. On multivariable analysis, approach to surgery had no bearing on RFS, and only known distant metastasis, aggressive histology and inadequate response to neoadjuvant radiation (pathological tumour regression grade greater than 3) predicted worse RFS.
Conclusion
MIS exenterations documented longer procedures but resulted in less blood loss and fewer wound infections compared with open surgeries. In the setting of an experienced centre, the hospital stay, R0 resection rates and oncological outcomes at 3 years were similar to those of open exenterations.
Minimally invasive exenterations are oncologically safe operations for primary rectal cancers in specialized centres, with outcomes comparable to those of open exenterations. Minimally invasive surgeries were associated with longer operative times but reduced blood loss and abdominal wound infections.
Introduction
Locally advanced rectal cancers comprise 25–30 per cent of all rectal cancers1, however this rate has been documented at up to 45–50 per cent in the Indian subcontinent2. Although minimally invasive surgery (MIS) for rectal cancers is well established in clinical practice with benefits in short-term outcomes, the available evidence has led to scepticism concerning its oncological equivalence3–6.
T4 tumours requiring extended and beyond total mesorectal excision (TME) operations were excluded by all the randomized trials of MIS rectal resections3,4,6–8. Few centres have reported on the short-term results, and comparative studies with open exenterations have yielded equivalent results9–11.
A pelvic exenteration, with its two ostomies, is associated with a reduction in quality of life (QoL), which could be a significant issue in younger patients12. Total pelvic exenteration (TPE) is associated with initial worsening of QoL but returns to preoperative levels in the majority of patients after 3–6 months12.
A previous report demonstrated similar short-term outcomes for MIS exenterations in terms of perioperative and pathological outcomes in a small set of patients13. The present study was aimed at comparing the perioperative and short-term oncological outcomes of pelvic exenterations performed by MIS approaches in primary locally advanced rectal cancers with those obtained by open surgery.
Methods
Patient selection
A retrospective analysis of all patients who underwent exenterative, multivisceral procedures for rectal adenocarcinoma between August 2013 and September 2020 was performed from a single tertiary referral cancer centre. Surgeries performed for recurrent rectal cancers and those for histology other than adenocarcinoma were excluded. Patients with extensive posterior and lateral disease requiring bone or vascular resections were also excluded. Non-multivisceral procedures and resections of lesser intent, including posterior vaginal wall excision, seminal vesicle excisions, prostatic shave, presacral fascia and removal of hypogastric nerves, were not included. All patients were operated with curative intent. Thus, only patients with central and anterior diseases involving the urogenital organs were included. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Treatment
All patients were discussed in the colorectal multidisciplinary team (MDT) meetings. MRI was used for local staging of the disease and response assessment. Height of the lower edge of the tumour was measured from the anal verge on sigmoidoscopy. Tumours were labelled as rectosigmoid when the upper edge reached greater than 15 cm from the anal verge. Neoadjuvant long-course chemoradiation (CTRT) (50.4 Gy/28 days) was preferred unless there was known distant metastasis or patients were considered to be at very high risk of systemic disease. In these cases, short-course radiation (SCRT) (25 Gy/5 days) was administered followed by consolidation chemotherapy. After CTRT a minimum of 6 weeks was allowed before restaging MRI and definitive surgery was planned if R0 resection was deemed possible, otherwise consolidation chemotherapy was initiated. Upper rectal tumours were operated after SCRT, whereas rectosigmoid tumours usually underwent upfront surgery or neoadjuvant SCRT followed by surgery based on the MDT decision. Pretreatment staging laparoscopy was performed if faecal diversion was necessary for symptoms of bowel obstruction or an ovarian transposition was required in younger patients14. Radiological response was assessed using the RECIST 1.1 criteria while pathological response was reported using the tumour regression grade (TRG) scaled using the Mandard’s classification15.
Surgery
For the first 2.5 years of the study period, only open exenterations were performed, after which patients were planned for MIS exenterations unless there were prior open abdominal surgeries or logistical difficulties (availability of laparoscopic/robotic carts). TPE was performed for extensive involvement of the urinary bladder, prostate or urethra. Posterior pelvic exenteration (PPE) removed the uterus, cervix and part of vagina with the rectum because of their direct involvement. The techniques for laparoscopic and robotic TPE and PPE have been described previously16–19. Urinary reconstruction was performed by non-continent ileal or sigmoid conduit. The ureteric anastomosis was performed extracorporeally in the vast majority of cases. The anal sphincter was preserved when technically and oncologically feasible. After the perineal phase of resection, soft tissue and skin defects were reconstructed using V–Y gluteal advancement flaps and same was used for vaginal reconstruction. Adjuvant therapy and follow-up after curative intent treatment were performed according to National Comprehensive Cancer Network (NCCN) guidelines. All recurrent disease in the pelvis at the site of anastomosis, along the pelvic side walls or in the presacral space was considered to be local recurrence.
Outcome measures
Perioperative results included: operative time; blood loss; 30-day complications, assessed using the Clavien–Dindo classification20; abdominal wound infections; surgical-site infections, defined using the Centers for Disease Control and prevention (CDC) criteria21; ileus, defined as non-passage of flatus/stool for 24 hours, two or more episodes of nausea or vomiting or inability to tolerate oral diet after postoperative day 422; urinary reconstruction complications; duration of hospital stay; and 90-day readmissions.
Surgical specimens were compared for the nodal yield, the R0 resection rate and circumferential resection margin (CRM). A negative CRM was determined on final histology, defined as absence of viable tumour within 1 mm of the non-peritonealized surface of the rectum.
Oncological outcomes included 3-year survival rate. Overall survival (OS) was calculated from completion of treatment to death from any cause. Recurrence-free survival (RFS) was calculated from the date of treatment completion to recurrence. Follow-up was conducted using 3-monthly visits for clinical examination and carcinoembryonic antigen (CEA) tests with 6-monthly chest, abdomen and pelvic CT scans for 2 years; after this, the interval of surveillance was increased to every 6 months for 5 years. Colonoscopy was performed at 1 and 3 years after resection and then every 5 years.
Statistical analysis
Data were recorded in the SPSS platform and analysed using SPSS® version 25 (IBM, Armonk, New York, USA). For continuous variables, means, median and interquartile range were calculated and comparisons were done using Student’s t test for means and Mann–Whitney U test for medians. Normality of the data was assessed using the Shapiro Wilk test. Categorical data were described using proportions, and comparisons were made using the χ2 test. Median follow-up times were calculated using reverse Kaplan–Meier method. Survivals were calculated using Kaplan–Meier analysis and compared using the log rank method. Uni- and multivariable Cox regression analyses were used to evaluate the association between approach of surgery and RFS using hazard ratios and including the following co-variables: age, location of tumour, histological subtype (poorly differentiated/signet ring), nodal stage, presence of lateral pelvic nodes, distant metastasis, preoperative bowel obstruction, neoadjuvant therapy and tumour response, preoperative BMI, haemoglobin and albumin levels, pathological variables (TRG, CRM, lymphovascular and perineural invasion) and perioperative outcomes (duration of operation, blood loss, duration of hospital stay and complications).
Univariable filtering of all potential variables was carried out. The backward elimination method was used for creation of a multivariable model until convergence was achieved or until likelihood ratio test of the model was maximized. The log likelihood test was used to assess goodness of fit of the Cox regression model using STATA (StataCorp. 2015). A value of P ≤ 0.050 was considered statistically significant.
Ethics
The data of the present study were collected in the course of common clinical practice, and, accordingly, the signed informed consent was obtained from each patient for any surgical and clinical procedure. The study protocol was in accordance with the ethical standards of the institutional research committee and based on the retrospective design of the study and the use of anonymized data.
Results
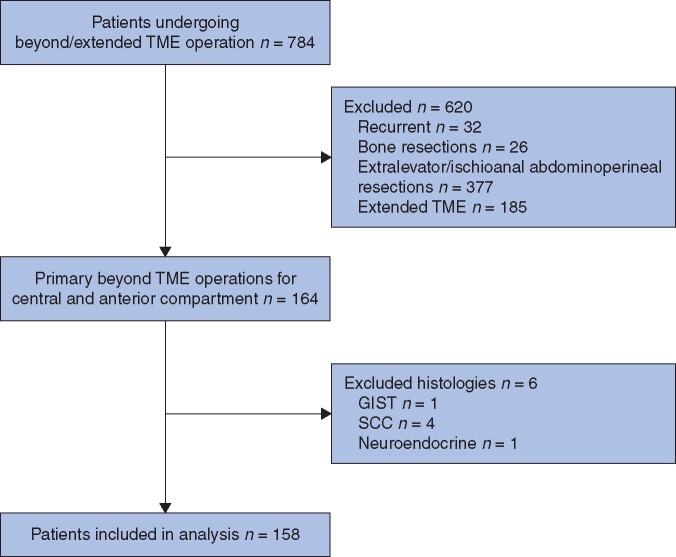
A total of 599 patients underwent beyond TME operations in the defined study period. Of these 158 were total or posterior pelvic exenteration for primary rectal adenocarcinoma without extensive involvement of the lateral or posterior compartments (Fig. 1). Of the 158 patients, 97 (61.4 per cent) had open exenterations and 61 (38.6 per cent) patients had minimally invasive surgery (MIS). Within the MIS cohort, 44 patients (72.1 per cent) had laparoscopic resections and 17 (27.9 per cent) had robotic surgery. Of the entire cohort of 158 patients, 96 (60.8 per cent) underwent TPE and 62 (39.2 per cent) had PPE. TPE and PPE resections according to the MIS or open approaches are detailed in Table 1. Conversions to open surgery took place in two patients (3 per cent), both from laparoscopic exenteration and none from the robotic group. Both conversions occurred low in the pelvis after majority of the surgery was completed laparoscopically, due to difficulties in pelvic side wall dissection. These patients were analysed with the MIS cohort.
Fig. 1.
Patient selection
TME, total mesorectal excision; GIST, gastrointestinal stromal tumour; SCC, squamous cell carcinoma
Table 1.
Distribution of total pelvic exenteration and posterior pelvic exenteration by approach
| Total pelvic exenteration | Posterior pelvic exenteration | Total | |
|---|---|---|---|
| (n = 96) | (n = 62) | (n = 158) | |
| Open | 55 (57.3) | 42 (67.7) | 97 (61.4) |
| Laparoscopic | 31 (32.3) | 13 (20.9) | 44 (27.8) |
| Robotic | 10 (10.4) | 7 (11.3) | 17 (10.8) |
Values in parentheses are percentages.
Median age of the entire cohort was 44 years and 55 per cent were male patients. Average distance of the lower edge of tumour from anal verge was 4 cm. Some 32.2 per cent of tumours had poorly differentiated or signet ring cell histology and 11 per cent had distant metastasis. The majority (147 patients) received neoadjuvant radiation and 65 per cent had consolidation chemotherapy with overall local progression of disease in 18.4 per cent of cases, as determined by radiology. All patients were clinically T4 tumours and only 4.4 per cent were radiologically node negative while 34.2 per cent had lateral pelvic nodes considered significant by MRI criteria23. Final histology after neoadjuvant therapy and surgery revealed complete response in 16.5 per cent while 40.5 per cent had inadequate tumour regression (TRG >3). Except for pathological TRG, all other factors were similar between the MIS and the open exenteration cohorts (Table 2).
Table 2.
Baseline clinical, treatment and pathological characteristics
| Characteristic | Overall | MIS | Open | P# |
|---|---|---|---|---|
| (n = 158) | (n = 61) | (n = 97) | ||
| Age (years) | 44 (35–56) | 45 (35–54) | 43 (35–57) | 0.861 |
| Sex | ||||
| Male | 87 (55.1) | 37 (61) | 50 (52) | 0.262 |
| Female | 71 (44.9) | 24 (39) | 47 (49) | |
| Tumour site | ||||
| Rectum | 144 (91.1) | 58 (95) | 86 (89) | 0.173 |
| Rectosigmoid | 14 (8.9) | 3 (5) | 11 (11) | |
| Distance from anal verge (cm)* | 4 (1–7) | 3 (1–6) | 4 (2–8) | 0.090 |
| ⩽5 cm from anal verge | 104 (65.8) | 45 (74) | 59 (61) | |
| >5cm from anal verge | 54 (34.1) | 16 (26) | 38 (39) | |
| Histology | ||||
| Well to moderately differentiated | 107 (67.8) | 44 (72) | 63 (65) | 0.333 |
| Poorly differentiated/signet ring | 51 (32.2) | 17 (28) | 34 (35) | |
| CEA (ng/ml)* | 6.3 (3.0–21.8) | 4.5 (2.7–20.0) | 9.3 (3.3–29.6) | 0.232 |
| Clinical nodal stage | ||||
| N0 | 7 (4.4) | 5 (8) | 2 (2) | 0.162 |
| N1 | 96 (60.8) | 34 (55) | 62 (64) | |
| N2 | 55 (34.8) | 22 (37) | 33 (34) | |
| Clinical M1 | 18 (11.4) | 5 (8) | 13 (13) | 0.321 |
| Lateral pelvic nodes | 54 (34.2) | 22 (36) | 32 (33) | 0.752 |
| Preoperative luminal obstruction | 47 (29.6) | 14 (22) | 33 (34) | 0.130 |
| Preoperative radiation | ||||
| None | 11 (7.0) | 1 (2) | 10 (10) | 0.081 |
| Short-course RT | 22 (13.9) | 7 (11) | 15 (15) | |
| Long-course chemo-RT | 125 (79.1) | 53 (87) | 72 (74) | |
| Interval between RT and surgery (weeks)† | 21.14 | 19.14 | 21.93 | 0.132 |
| Preoperative chemotherapy | 103 (65.2) | 34 (56) | 69 (71) | 0.061 |
| Response to preoperative treatment § ‡ | ||||
| Partial | 90 (61.2) | 38 (63) | 52 (60) | 0.331 |
| Stable | 30 (20.4) | 14 (23) | 16 (18) | |
| Progression | 27 (18.4) | 8 (13) | 19 (22) | |
| ASA | ||||
| 1 | 105 (66.5) | 39 (64) | 66 (68) | 0.590 |
| ≥2 | 53 (33.5) | 22 (36) | 31 (32) | |
| BMI (kg/m2)* | 22.5 (19.6–25) | 22.3 (20–24) | 22.8 (19.3–29) | 0.532 |
| BMI ⩽25 kg/m2‡ | 74.8 | 78 | 73 | |
| BMI >25 kg/m2‡ | 25.2 | 22 | 27 | |
| Haemoglobin (g/dl)* | 11.2 (10.2–12.4) | 11.2 (10.2–12.4) | 11.2 (10.2–12.3) | 0.422 |
| Haemoglobin ⩽12 g/dl‡ | 68.4 | 72 | 66 | |
| Haemoglobin >12 g/dl‡ | 31.6 | 28 | 34 | |
| Albumin (g/dl)* | 3.8 (3.5–4.1) | 4 (3.7–4.2) | 3.7 (3.4–4) | 0.193 |
| Albumin <3.5 g/dl‡ | 27.2 | 21 | 31 | |
| Albumin >3.5 g/dl‡ | 72.8 | 79 | 69 | |
| Pathological T stage | ||||
| T0 | 26 (16.5) | 7 (12) | 19 (20) | 0.714 |
| T1 | 3 (1.9) | 1 (2) | 2 (2) | |
| T2 | 14 (8.9) | 6 (10) | 8 (8) | |
| T3 | 41 (25.9) | 18 (30) | 23 (24) | |
| T4 | 74 (46.8) | 29 (48) | 45 (46) | |
| Pathological nodal stage | ||||
| N0 | 94 (59.5) | 33 (54) | 61 (63) | 0.442 |
| N1 | 43 (27.2) | 20 (33) | 23 (24) | |
| N2 | 21 (13.3) | 8 (13) | 13 (13) | |
| Tumour regression grade >3§ | 60 (40.8) | 17 (28) | 43 (49) | 0.020 |
| Lymphovascular invasion | 32 (20.3) | 11 (18) | 21 (22) | 0.471 |
| Perineural invasion | 30 (19) | 13 (21) | 17 (18) | 0.672 |
Values in parentheses are percentages unless indicated otherwise
*values are median (i.q.r.)
values are median
values are percentages
Available for 147 patients. MIS, minimally invasive surgery; RT, radiation therapy; CEA, carcinoembryonic antigen
P vlaues obtained from Mann-Whitney U test for medians and Fisher’s χ2 test for proportions.
Sphincter preservation was performed in 30.4 per cent of patients and lateral pelvic lymph node dissection in 35.4 per cent (Table 3). Preferred method of urinary reconstruction after TPE was the ileal conduit (90 patients, 93.8 per cent) and a sigmoid conduit was constructed for six patients (6.2 per cent) for pre-existing transverse colostomy. Plastic surgical procedures for vaginal and perineal reconstruction were required in 35.2 per cent of patients, and 44.5 per cent of patients required vaginal resection or perineal excision.
Table 3.
Surgical, postoperative and oncological outcomes
| Outcome | All patients | MIS | Open | P # |
|---|---|---|---|---|
| (n = 158) | (n = 61) | (n = 97) | ||
| Surgery type | ||||
| Total pelvic exenteration | 96 (60.8) | 41 (67) | 55 (57) | 0.212 |
| Posterior exenteration | 62 (39.2) | 20 (33) | 42 (43) | |
| Sphincter preservation | 48 (30.4) | 13 (21) | 35 (36) | 0.049 |
| Pelvic node dissection | 56 (35.4) | 27 (44) | 29 (30) | 0.066 |
| Urinary reconstruction (n = 96) | ||||
| Ileal conduit | 90 (93.8) | 37 (90) | 53 (96) | 0.461 |
| Sigmoid conduit | 6 (6.2) | 4 (10) | 2 (4) | |
| Vaginal reconstruction (n = 71) | 25 (35.2) | 8 (33) | 17 (36) | 0.812 |
| Perineal reconstruction (n = 110) | 49 (44.5) | 30 (63) | 19 (31) | 0.0009 |
| Operative duration (mins)* | 500 (410–650) | 640 (500–690) | 450 (360–540) | <0.0001 |
| Blood loss (ml)* | 1450 (700–2200) | 900 (600–1700) | 1600 (1100–2200) | <0.0001 |
| Hospital stay (days)* | 12 (10–19) | 11 (10–16) | 12 (10–20) | 0.620 |
| Nodal yield † | 15 (9–18) | 16 (10–20) | 14.5 (8–17) | 0.823 |
| Positive circumferential margin | 16 (10.1) | 8 (13) | 8 (8) | 0.342 |
| Resections | ||||
| R0 | 143 (90.5) | 54 (89) | 89 (92) | 0.490 |
| R1 | 14 (8.9) | 7 (12) | 7 (7) | |
| R2 | 1 (0.6) | 0 (0) | 1 (1) | |
| Complications (Clavien–Dindo grade) | ||||
| 0 | 69 (43.7) | 24 (39) | 45 (46) | 0.152 |
| I | 14 (8.9) | 9 (15) | 5 (5) | |
| II | 38 (24.1) | 10 (16) | 28 (29) | |
| IIIA | 16 (10.1) | 8 (13) | 8 (8) | |
| IIIB | 18 (11.4) | 9 (15) | 9 (9) | |
| IV | 2 (1.3) | 1 (2) | 1 (1) | |
| V | 1 (0.6) | 0 (0) | 1 (1) | |
| Complications grade IIIA or more | 37 (23.4) | 18 (30) | 19 (20) | 0.152 |
| Surgical-site infection | 46 (29.1) | 19 (31) | 27 (28) | 0.621 |
| Abdominal wound infections | 22 (13.9) | 5 (8) | 17 (18) | 0.020 |
| Ileus | 20 (12.7) | 7 (11) | 13 (14) | 0.583 |
| Urinary reconstruction complications ¶ | 8 (8.4) | 3 (7) | 5 (9) | 0.783 |
| 90-day readmissions | 18 (11.4) | 10 (16) | 8 (8) | 0.104 |
| Adjuvant therapy | 127 (80.4) | 48 (79) | 79 (81) | 0.601 |
| Recurrences | ||||
| All | 64 (40.5) | 20 (33) | 44 (45) | |
| Local | 6 (3.8) | 1 (2) | 5 (5) | |
| Distant | 54 (34.2) | 18 (30) | 36 (37) | |
| Local + distant | 4 (2.5) | 1 (2) | 3 (3) | |
| Distant recurrence sites | ||||
| Lung | 27 (46.5) | 9 (35) | 18 (46) | |
| Peritoneum | 11 (18.9) | 4 (15) | 7 (18) | |
| Nodes | 11 (18.9) | 2 (8) | 9 (23) | |
| Liver | 5 (8.6) | 1 (4) | 4 (10) | |
| Bone | 4 (6.9) | 3 (12) | 1 (3) | |
| Deaths | 42 (26.6) | 8 (13) | 34 (35) | |
| 3-year local recurrence-free survival ‡ | 79.1 | 90 | 75 | 0.491 |
| 3-year recurrence-free survival§ (median) | 48.7 (35 months) | 52(37 months) | 48 (35 months) | 0.922 |
| 3-year overall survival ‡ | 64 | 79 | 60 | 0.251 |
Values in parentheses are percentages unless indicated otherwise
values are median (i.q.r.)
values are mean (i.q.r.)
values are percentages
values are percentage (median).
Available for 96 patients.
P values obtained from Mann-Whitney U test for medians and Fisher’s χ2 test for proportions.
Perioperative outcomes
Median operative time was longer in MIS cohorts and blood loss was less (MIS versus open: 640 versus 450 mins (P < 0.001); and 900 versus 1600 ml (P < 0.001) respectively). 30-day postoperative complications were similar except for abdominal wound infections which were more frequent in the open group (MIS versus open: 8.2 versus 17.5 per cent; P = 0.020). Grade IIIA complications or more complications occurred in 23.4 per cent (MIS versus open: 29.5 per cent versus 19.6 per cent; P = 0.152) of patients. There was one postoperative death (0.6 per cent) due to urinary leak from the ileal conduit. This patient underwent reoperation but died due to abdominal sepsis. No difference in duration of hospital stay (median 12 days) was noted between the groups. Similar rates of 90-day hospital readmissions and delivery of adjuvant therapy were found in the MIS and open exenterations.
There were no differences in nodal yield, R0 resections and CRM-positive rate between the MIS and open operations. Pathological CRM was positive in 16 patients (10.1 per cent) and the distal margin was involved in one of the 48 patients who underwent sphincter preservation (2 per cent). Only one patient had gross residual disease (R2) due to nodal plaque involving the common iliac vessels (Table 3).
Oncological outcomes
At a median follow-up of 29 months, 64 patients had relapsed (40.5 per cent) and 42 (26.6 per cent) deaths were recorded. The majority of recurrences were distant recurrences (58 patients, 36.7 per cent), and only 10 local recurrences (6.3 per cent) were detected. The most common site of relapse was the lung (27 patients) followed by peritoneum and nodes outside the pelvis (18.9 per cent of all distant recurrences). Three-year local recurrence-free survival (LRFS), RFS and OS were 79.1, 48.7 and 64 per cent respectively (Fig. 2), with no differences between MIS or open groups. Median RFS was 35 months while the median for OS or LRFS was not reached (Table 3).
Fig. 2.
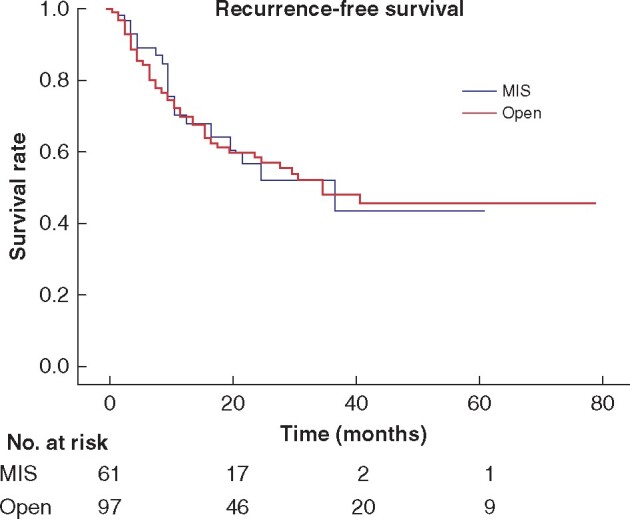
Recurrence-free survival by surgical approach
MIS, minimally invasive surgery.
In the multivariable Cox proportional hazard model for RFS, poorly differentiated or signet-ring cell histology, metastatic disease at presentation and pathological TRG greater than 3 were the only significant factors with nearly equal hazard ratios (hazard ratio 2.2) (Table 4). Approach to surgery (MIS or open) did not impact RFS in univariable Cox regression (hazard ratio 0.97; P = 0.932) and addition of this factor to the multivariable model did not improve the log-likelihood ratio and thus the predictive power of the model.
Table 4.
Cox regression for recurrence free survival
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| Hazard ratio | P | Hazard ratio | P # | |
| Age | 0.984 (0.956, 1.004) | 0.121 | ||
| Distance from anal verge >5 cm | 0.947 (0.883, 1.016) | 0.133 | ||
| Histology – poorly differentiated/signet ring | 2.339 (1.404, 3.895) | 0.0011 | 2.210 (1.242, 3.930) | 0.007 |
| Node positive | 2.324(1.419, 3.805) | 0.0008 | 1.504 (0.830, 2.726) | 0.182 |
| Lateral pelvic nodes | 1.312 (0.792, 2.176) | 0.294 | ||
| M1 at presentation | 2.159 (1.121, 4.157) | 0.020 | 2.219 (1.061, 4.643) | 0.031 |
| Consolidation chemotherapy | 1.412 (0.704, 2.831) | 0.331 | ||
| Progression on preoperative treatment | 1.374 (0.769, 2.455) | 0.281 | ||
| Preoperative bowel obstruction | 1.267 (0.766, 2.096) | 0.372 | ||
| Body mass index >25 kg/m2 | 1.052 (0.601, 1.839) | 0.863 | ||
| Haemoglobin >12 g/dl | 0.897 (0.532, 1.514) | 0.612 | ||
| Albumin >3.5 g/dl | 1.058 (0.607, 1.845) | 0.851 | ||
| Circumferential margin positive | 3.001 (1.593, 5.651) | 0.0007 | 1.754 (0.840, 3.663) | 0.132 |
| Tumour regression grade >3 | 2.706 (1.467, 4.993) | 0.0014 | 2.214 (1.124, 4.36)0 | 0.02 |
| Lymphovascular invasion | 2.843 (1.64, 4.928) | 0.0002 | 1.663 (0.847, 3.264) | 0.143 |
| Perineural invasion | 1.693 (0.952, 3.01) | 0.073 | 1.275 (0.585, 2.778) | 0.543 |
| MIS | 0.974 (0.571, 1.661) | 0.932 | 0.976 (0.524, 1.818) | 0.942 |
| Blood loss | 1.001 (0.999, 1.002) | 0.124 | ||
| Operation duration | 1.002 (0.998, 1.004) | 0.132 | ||
| Duration of hospital stay | 1.060 (0.96, 1.171) | 0.250 | ||
| Complications (Clavien–Dindo grade IIIA or more) | 1.425 (0.866, 2.344) | 0.161 | ||
Values in parentheses are 95 per cent confidence intervals. MIS, minimally invasive surgery.
P values obtained from Cox regression test.
Discussion
In this series, the short-term benefits of MIS are reflected by the reduction in blood loss and abdominal wound infections. Approach to surgery had no influence on recurrence and only aggressive histology, distant metastasis and unsatisfactory response to neoadjuvant therapies predicted worse RFS.
For the present comparative analysis, only central and anterior compartments were included since posterior and lateral compartments requiring bone and vascular resections were operated upon by open approach exclusively. Of note, inferior compartment involvement requiring extralevator or ischioanal abdominoperineal resections did not present additional challenges during the abdominal phase of operation and differed from standard TME only during the perineal dissections. Recurrent tumours were also excluded since all recurrent rectal cancers requiring exenteration were operated by open surgery. These exclusions were anticipated to balance the baseline characteristics of the open and MIS cohorts. On comparison of preintervention variables, all were insignificantly different and pathological TRG was the only dissimilar factor and is not strictly a presurgical characteristic. Radiological (MRI) TRG was not recorded however, local progression of disease was indicated and there were no differences in non-responders in the MIS and open surgery patients. The other difference noted was the higher proportion of upper rectal/rectosigmoid tumours in the open surgery arm. This accounted for higher sphincter preservation in the open surgery group where a lower proportion of patients required perineal resection and consequent reconstruction.
When comparing the short-term outcomes, operation duration was longer in the MIS group. The larger number of perineal excisions and reconstructions in the MIS patients increased the operation time and this partly accounted for the differences. Blood loss was less in the MIS patients, however greater absolute difference was anticipated which was offset by the perineal and reconstructive blood loss. Separate abdominal and perineal phase operative times and blood loss should have been recorded to allow accurate variations between the groups. Duration of hospital stay was similar for both approaches and this is explained in part by the mandatory 9-day hospital stay until ureteric stents are removed in TPE patients24. In the PelvEx meta-analysis of 37 patients undergoing MIS and 133 open exenterations, blood loss and hospital stay were less, while operating times were greater. No differences in postoperative complications were found25. In another propensity-matched analysis of 137 T4 rectal cancers comparing open and MIS approaches, reduced time to first flatus, decreased duration of hospital stay and faster return to normal diet were recorded for the MIS patients26. However, multivisceral and exenterative surgeries were performed in only 26.3 per cent of MIS and 30 per cent of open operations. The present study found no significant differences in 30-day postoperative complications or 90-day hospital readmissions. Only abdominal wound infections were fewer in patients operated by MIS (8.2 versus 17.5 per cent; P = 0.020). Rates of ileus, surgical-site infections and complications related to urological reconstruction were similar.
R0 resections were achieved in over 90 per cent of patients in the present study, without significant variations by operative approach. This is non-inferior to the global standard defined by the PelvEx database of 80 per cent1. Other reports of comparative studies of MIS and open exenterations have achieved R0 resections in 66.7–100 per cent of surgeries9–11,27. Conversion to open operations occurred only in two patients in the present study (3 per cent). For T4 rectal cancers, conversion rates range from 18–22 per cent26,28. Conversions, when performed due to non-progress of surgery rather than for complications, have not led to worse outcomes28. It is clear that MIS should not be pursued at the risk of positive margins and planned conversions reflect sound judgement depending on operator experience rather than a complication. Some 16.5 per cent patients had ypT0 tumours after resection and it is not uncommon to have fixed growths without viable tumour on pathology. With residual masses they are neither candidate for watch and wait nor lesser resections. Besides, watch and wait is yet to be established for T4 tumours despite complete clinical response29.
At a median follow-up of 2.5 years, local control was achieved by pelvic exenterations (local recurrence (LR) 6.3 per cent) and 3-year LRFS was 79.1 per cent with comparable results for the MIS and open surgeries. Even though numerically different, the 3-year OS for MIS and open exenterations (79.4 and 60.2 per cent respectively) did not attain statistical significance. These figures appear comparable to those in international collaborative PelvEx data where 3-year OS for R0 resections was 56.4 per cent1. In a matched analysis of T4 rectal cancers, no difference was found in 3-year OS for laparoscopic and open resections (66.7 versus 64.1 per cent)26. Similar findings in multiple small retrospective review of all T4 tumours, where multivisceral resections and exenterations constituted a minority, were observed30. In a recent report of robotic resections for cT4 rectal cancers, 52 patients had cT4b disease and 3-year LR was 4 per cent and disease-free survival 70.4 per cent31.
In another study, M1 status and node positivity predicted survival and not the approach to T4 rectal cancer operations26. Signet-ring and poorly differentiated cancers are universally associated with higher stage at presentation and early relapses32. Aggressive histology of tumours not only predicts larger and chemoradiation-resistant tumours but also worse prognosis (hazard ratio 2.2), yet MIS was found to be safe for signet-ring cancers in previous studies33. In the present analysis, TRG greater than 3 took precedence over most of the other pathological features: nodal metastasis, and lymphovascular and perineural invasion. These inadequately responding tumours have inherently worse biology and perform poorly irrespective of the approach to surgery. The herein reported cohort differed from the patients in the RAPIDO trial and the present reported approach to total neoadjuvant therapy was heterogeneous, but consolidation chemotherapy did not improve RFS in this study unlike the results of the RAPIDO trial34.
Since the median was reached only for RFS, the Cox regression was computed for this endpoint. If LRFS or OS were chosen, regression models would have lost power and become biased if multiple independent variables were included due to smaller number of events.
The other caveat is that the results may not be generalizable to all centres. A large amount of experience in standard TME is required before attempting beyond TME operations. Surgeons at Tata Memorial Hospital, Mumbai had an experience of 45 open TPE and 40 laparoscopic pelvic node dissections before the first MIS exenteration was attempted. At present, over 300 MIS rectal resections are performed annually and prior open abdominal explorations are the only relative contraindications for MIS exenterations. The choice between robotic and laparoscopic operations for central diseases requiring exenteration was largely based on the logistical availability of the robot. Only 28 per cent of all MIS exenterations were robotic and thus comparisons with laparoscopic procedures were not made in the present study. Second, the proportion of MIS exenterations being performed in the authors’ centre have increased over the years24 and adjustments for the learning effect have not been made. Some variables were not recorded and the exact reasons for choosing one approach over the other were not clarified.
With surgical trials providing conflicting reports regarding the safety of MIS for rectal cancers, a randomized trial for T4 rectal cancers is unlikely in the near future and these results show the benefits of MIS in selected patients by dedicated colorectal units.
Acknowledgements
The data that support the findings of this study are available on request from the corresponding author on reasonable request. The data are not publicly available due to privacy or ethical restrictions.
Disclosures. The authors declare no conflict of interest.
Contributor Information
M Kazi, Department of Colorectal Surgical Oncology, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India.
N A N Kumar, Department of Surgical Oncology, Manipal Comprehensive Cancer Care Centre, Kasturba Medical College, Manipal Academy of Higher Education (MAHE), Manipal, India.
J Rohila, Department of Colorectal Surgical Oncology, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India.
V Sukumar, Department of Colorectal Surgical Oncology, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India.
R Engineer, Department of Radiation Oncology, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India.
S Ankathi, Department of Radiology, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India.
A Desouza, Department of Colorectal Surgical Oncology, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India.
A Saklani, Department of Colorectal Surgical Oncology, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India.
References
- 1. PelvEx Collaborative. Surgical and survival outcomes following pelvic exenteration for locally advanced primary rectal cancer: results from an international collaboration. Ann Surg 2019;269:315–321. [DOI] [PubMed] [Google Scholar]
- 2. Patil PS, Saklani A, Gambhire P, Mehta S, Engineer R, De'Souza A et al. Colorectal cancer in India: an audit from a tertiary center in a low prevalence area. Indian J Surg Oncol 2017;8:484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA 2015;314:1346–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stevenson ARL, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ et al. ; ALaCaRT Investigators. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: The ALaCaRT Randomized Clinical Trial. JAMA 2015;314:1356–1363. [DOI] [PubMed] [Google Scholar]
- 5. Jeong S-Y, Park JW, Nam BH, Kim S, Kang S-B, Lim S-B et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 2014;15:767–774. [DOI] [PubMed] [Google Scholar]
- 6. Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MHGM, de Lange-de Klerk ESM et al. ; COLOR II Study Group. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015;372:1324–1332. [DOI] [PubMed] [Google Scholar]
- 7. Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AMH et al. ; UK MRC CLASICC Trial Group. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol 2007;25:3061–3068. [DOI] [PubMed] [Google Scholar]
- 8. Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: The ROLARR Randomized Clinical Trial. JAMA 2017;318:1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang K, Cai L, Yao L, Zhang Z, Zhang C, Wang X et al. Laparoscopic total pelvic exenteration for pelvic malignancies: the technique and short-time outcome of 11 cases. World J Surg Oncol 2015;13:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogura A, Akiyoshi T, Konishi T, Fujimoto Y, Nagayama S, Fukunaga Y et al. Safety of laparoscopic pelvic exenteration with urinary diversion for colorectal malignancies. World J Surg 2016;40:1236–1243. [DOI] [PubMed] [Google Scholar]
- 11. Uehara K, Nakamura H, Yoshino Y, Arimoto A, Kato T, Yokoyama Y et al. Initial experience of laparoscopic pelvic exenteration and comparison with conventional open surgery. Surg Endosc 2016;30:132–138. [DOI] [PubMed] [Google Scholar]
- 12. Steffens D, Solomon MJ, Young JM, Koh C, Venchiarutti RL, Lee P et al. Cohort study of long‐term survival and quality of life following pelvic exenteration. BJS Open 2018;2:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar NA, Sasi SP, Shinde RS, Verma K, Sugoor P, Desouza A et al. Minimally invasive surgery for pelvic exenteration in primary colorectal cancer. JSLS 2020;24:e2020.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kazi M, Nekkanti SS, Rohila J, Patel S, Sukumar V, Desouza A et al. Impact of surgical staging for aggressive histology rectal cancers: a retrospective review. ANZ J Surg 2021;91:E119–E122. [DOI] [PubMed] [Google Scholar]
- 15. Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680–2686. [DOI] [PubMed] [Google Scholar]
- 16. Kammar P, Sasi S, Kumar N, Rohila J, deSouza A, Saklani A. Robotic posterior pelvic exenteration for locally advanced rectal cancer – a video vignette. Colorectal Dis 2019;21:606. [DOI] [PubMed] [Google Scholar]
- 17. Kammar P, Bakshi G, Verma K, Sugoor P, Saklani A. Robotic total pelvic exenteration for locally advanced rectal cancer – a video vignette. Colorectal Dis 2018;20:731. [DOI] [PubMed] [Google Scholar]
- 18. Bhamre R, Pokharkar A, Shinde R, Saklani A. Laparoscopic total pelvic exenteration for locally advanced carcinoma of the rectum – a video vignette. Colorectal Dis 2018;20:161–162. [DOI] [PubMed] [Google Scholar]
- 19. Pokharkar A, Kammar P, D'Souza A, Bhamre R, Sugoor P, Saklani A. Laparoscopic pelvic exenteration for locally advanced rectal cancer, technique and short-term outcomes. J Laparoendosc Adv Surg Tech A 2018;28:1489–1494. [DOI] [PubMed] [Google Scholar]
- 20. Clavien PA, Barkun J, de Oliveira ML. The Clavien–Dindo classification of surgical complications. Ann Surg 2009;240:250–e252. [DOI] [PubMed] [Google Scholar]
- 21. Surgical Site Infection. 2021;39. https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf.
- 22. Vather R, Trivedi S, Bissett I. Defining postoperative ileus: results of a systematic review and global survey. J Gastrointest Surg 2013;17:962–972. [DOI] [PubMed] [Google Scholar]
- 23. Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Curvo-Semedo L et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2018;28:1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kazi M, Rohila J, Kumar NAN, Bankar S, Engineer R, Desouza A et al. Urinary reconstruction following total pelvic exenteration for locally advanced rectal cancer: complications and factors affecting outcomes. Langenbecks Arch Surg 2021;406:329–337. [DOI] [PubMed] [Google Scholar]
- 25. PelvEx Collaborative. Minimally invasive surgery techniques in pelvic exenteration: a systematic and meta-analysis review. Surg Endosc 2018;32:4707–4715. [DOI] [PubMed] [Google Scholar]
- 26. de’Angelis N, Landi F, Vitali GC, Memeo R, Martínez-Pérez A, Solis A et al. Multicentre propensity score-matched analysis of laparoscopic versus open surgery for T4 rectal cancer. Surg Endosc 2017;31:3106–3121. [DOI] [PubMed] [Google Scholar]
- 27. Winters BR, Mann GN, Louie O, Wright JL. Robotic total pelvic exenteration with laparoscopic rectus flap: initial experience. Case Rep Surg 2015;2015:835425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bretagnol F, Dedieu A, Zappa M, Guedj N, Ferron M, Panis Y. T4 colorectal cancer: is laparoscopic resection contraindicated? Colorectal Dis 2011;13:138–143. [DOI] [PubMed] [Google Scholar]
- 29. Chadi SA, Malcomson L, Ensor J, Riley RD, Vaccaro CA, Rossi GL et al. Factors affecting local regrowth after watch and wait for patients with a clinical complete response following chemoradiotherapy in rectal cancer (InterCoRe consortium): an individual participant data meta-analysis. Lancet Gastroenterol Hepatol 2018;3:825–836. [DOI] [PubMed] [Google Scholar]
- 30. Kumar NAN, Kammar P, Saklani A. Minimal invasive approach for beyond total mesorectal excision/extended resections in rectal cancer. Mini-invasive Surg 2018;2:19 doi: 10.20517/2574-1225.2018.26. [DOI] [Google Scholar]
- 31. Yamaoka Y, Shiomi A, Kagawa H, Hino H, Manabe S, Kato S et al. Robotic surgery for clinical T4 rectal cancer: short- and long-term outcomes. Surg Endosc 2021. 10.1007/s00464-020-08241-9 [DOI] [PubMed] [Google Scholar]
- 32. Vallam KC, Desouza A, Bal M, Patil P, Engineer R, Saklani A. Adenocarcinoma of the rectum – a composite of three different subtypes with varying outcomes? Clin Colorectal Cancer 2016;15:e47–e52. [DOI] [PubMed] [Google Scholar]
- 33. Raghavan S, Singh DK, Rohila J, DeSouza A, Engineer R, Ramaswamy A et al. Outcomes of definitive treatment of signet ring cell carcinoma of the rectum: is minimal invasive surgery detrimental in signet ring rectal cancers? Indian J Surg Oncol 2020;11:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM-K et al. ; RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29–42. [DOI] [PubMed] [Google Scholar]