Abstract
Provoked vestibulodynia (PVD) is a chronic vulvar pain disorder characterized by hypersensitivity and severe pain with pressure localized to the vulvar vestibule. Knowledge regarding pathophysiological mechanisms contributing to the etiology and production of symptoms in PVD remains incomplete but is considered multifactorial. Using a cross-sectional observational study design, data from untargeted metabolomic profiling of vaginal fluid and plasma in women with PVD and healthy women was combined with pain testing and brain imaging in women with PVD to test the hypotheses that women with PVD compared to healthy women show differences in vaginal and plasma metabolites involved in steroid hormone biosynthesis. Steroid hormone metabolites showing group differences were correlated with vulvar vestibular pain and vaginal muscle tenderness and functional connectivity of brain regions involved in pain processing in women with PVD to provide insight into the functional mechanisms linked to the identified alterations. Sensitivity analyses were also performed to determine the impact of hormonal contraceptive use on the study findings. Women with PVD compared to healthy controls had significant reductions primarily in vaginal fluid concentrations of androgenic, pregnenolone and progestin metabolites involved in steroidogenesis, suggesting localized rather than systemic effects in vagina and vulvar vestibule. The observed reductions in androgenic metabolite levels showed large effect size associations with increased vulvar vestibular pain and vulvar muscle tenderness and decreases in androgenic and progestin metabolites were associated with decreased connectivity strength in primary sensorimotor cortices. Women with PVD showed symptom-associated reductions in vaginal fluid concentrations of metabolites involved in the biosynthesis of steroid hormones previously shown to affect the integrity of vulvar and vaginal tissue and nociceptive processing. Deficiency of certain steroids may be an important mechanism contributing to the pathophysiology of symptoms in PVD may provide potential diagnostic markers that could lead to new targets for therapeutic intervention.
Keywords: Dyspareunia, metabolomics, pain, provoked vestibulodynia, steroid hormone biosynthesis vaginal metabolites, vulvodynia
Introduction
Provoked vestibulodynia (PVD) is a chronic pain disorder characterized by local hypersensitivity of the vestibule and severe pain with vaginal penetration. PVD affects approximately 7 to 16% of the female population at some time in their lives and is the leading cause of painful intercourse in reproductive-aged women.1 The etiology of PVD remains poorly understood. It has been linked to multiple factors including inflammation, vulvovaginal infections, mucosal nerve fiber proliferation, hormonal alterations, pelvic floor muscle dysfunction, central pain mechanisms, and genetic factors (i.e. gene polymorphism related to sensitivity to pain, regulation of inflammatory response and androgen receptor).2–4
Neuroimaging evidence supports the notion that structural and functional alterations of the central nervous system are closely associated with symptoms, and may be responsible for the hypersensitivity to pain, or are secondary responses to the chronic pain experienced in PVD.5–11 Across all multimodal magnetic resonance imaging (MRI) studies in PVD, the most consistently reported findings are symptom-associated structural and functional alterations in regions comprising sensorimotor networks (including thalamus and basal ganglia) which are involved in pain processing and modulation.
The role of sex steroid alterations related to estrogen in PVD has been investigated in previous studies ranging from vestibular tissue immunohistochemistry to circulating microRNA.12–15 Androgens have also been implicated in the pathophysiology of PVD based primarily on epidemiological studies of women taking combined oral contraceptives.13–19 The use of oral contraceptives is known to increase sex hormone binding globulin and decrease circulating androgen levels, and insufficiency of sex steroid hormones have been identified in some studies as risk factors for the development of PVD.16–19 While estrogen has been shown to affect the structural integrity and thickness of vaginal and vulvar tissues, androgens including testosterone and dehydroepiandrosterone (DHEA) can influence genital mucosal epithelial integrity and function.20–23 Regardless of oral contraceptive use, vestibular mucosa measured by ultrasound was thinner in women with PVD compared with healthy controls and correlated with degree of vestibular burning pain.23 Interestingly, research based largely on experimental animal models indicates that steroid hormones can modulate pain perception suggesting that changes detected in plasma and vaginal fluid could result in mechanical allodynia.20,21,24–27
Metabolomics is a discovery based analytic profiling technique that indexes current cellular processes by measuring the biochemical products of molecular events downstream of genomic, transcriptomic, and proteomic systems in tissues or body fluids.28 Because these metabolites are small molecular endpoints and intermediates, they may serve as important tools for identifying biochemical pathways and metabolites (e.g., small molecules) that may be altered in disease states. Metabolomics assessment of biological samples (e.g., biofluids, tissue) has emerged as an important precision medicine tool for drug and biomarker discovery, determining drug response phenotypes, and identifying new pathophysiological mechanisms underlying chronic disease28–31 including vaginal health,32–34 risk for preterm birth,35,36 and chronic pain conditions including endometriosis.37–39 Ultimately, metabolomics may serve as a complementary tool to existing experimental approaches that can shed further light on the role sex steroid hormone alterations in PVD.
The aim of this exploratory study was to investigate whether women with PVD compared to healthy women show differences in metabolites involved in steroid hormone biosynthesis in the vagina, proximal to the area of pain, and peripherally in the plasma. Specifically, we examine group differences in steroid metabolites involved in steroid hormone biosynthesis pathways (i.e., androgen, progestin, pregnenolone, and corticosteroids) that were identified using an untargeted liquid chromatography–mass spectrometry (LC–MS) metabolomics assessment of vaginal fluid and plasma samples (Estrogenic steroids are not generally measurable in the untargeted assessment). We then examine the association of metabolites altered in PVD with vulvar vestibular pain and vaginal muscle tenderness, and functional connectivity of brain regions involved in pain processing to provide insight into the functional mechanisms linked to the identified alterations. Based on the extant literature, it was expected that women with PVD compared to healthy controls (HC) would have alterations in concentrations of vaginal fluid and plasma metabolites involved in steroid hormone biosynthesis. Given the potential for steroids to modify peripheral and central nociceptive processing, it was anticipated that alterations in sex steroids would be associated with increased vulvar vestibular pain and vaginal muscle tenderness as well as alterations in functional connectivity strength of pain processing regions in women with PVD.
Materials and methods
Subjects
Participants were recruited between May 2015 to December 2017 via advertisement on social media, Craigslist, campus wide emails via the Registrar’s office at UCLA, onsite recruiting by the study coordinator at the UCLA Division of Digestive Diseases general GI clinics and OB/GYN clinics, and the UCLA and other local college newspapers. Premenopausal women with PVD and HC, ages 18 to 50, were identified. A diagnosis of PVD was confirmed by clinical examination by a gynecologist or specialized nurse practitioner, both with recognized expertise and examination of women with vulvar vestibular pain. All subjects provided written informed consent to participate and were compensated for participating in the study. The study was approved by the University of California, Los Angeles (UCLA) Institutional Review Board (IRB#13–001113) and was conducted in accordance with the institutional guidelines regulating human subject research. A total of 60 women with PVD and 49 HC were enrolled in the study providing the statistical power to detect moderate effect size differences (Cohen’s d∼=.50), if they existed, in metabolite concentrations between PVD and HC.
Inclusion/exclusion criteria
Inclusion criteria for women with PVD included: at least 6 months of vulvar vestibular pain, at least 4 out of 10 in severity during intercourse or other activities involving vestibular pressure (e.g., tampon use), and findings on cotton swab exam consistent with provoked vestibulodynia. Infections (such as candida, bacterial vaginosis or herpes simplex), estrogen deficiency or dermatological disorders were ruled out by history, visual inspection, vaginal pH and saline and potassium hydroxide slide prep. Speculum examination of the vagina and bimanual pelvic examination were performed to exclude other potential etiologies or pathology such as vaginal or pelvic masses or inflammation. We excluded patients who met criteria for generalized vulvodynia and those with only spontaneous but not provoked vestibulodynia.1 All subjects were asked to abstain from intercourse for 7 days prior to the visit, and to avoid the use of intravaginal products, including douches, sprays, tampons, spermicides, gels, foams, and diaphragms. All subjects were between 18–50 years of age and were excluded from the study if they were post-menopausal, pregnant, breast-feeding or postpartum less than 4 months. In addition, subjects were excluded if they have received systemic or vaginal antimicrobial or probiotic therapy within 1 month before the study visit. Inclusion/exclusionary criteria for healthy control subjects were the same as for those with PVD, with the exception of inclusion for pain with vaginal penetration and vestibular allodynia.
Study design
Participants meeting inclusion criteria were scheduled for an on-site study visit. The subject menstrual history was obtained during phone screening so that the study visit could be scheduled during the mid to late follicular phase (days 5–14 of cycle) or if taking hormonal contraceptives, day 5–14 after the first day of last menstrual period. During the study visit HCs and women with PVD met with the study nurse, provided biological samples and filled out study questionnaires. Women with PVD but not HCs also underwent pain testing with the study nurse practitioner and brain imaging assessments.
Clinical assessment
During clinical examination detailed information was obtained regarding vulvar vestibular pain for the PVD patients including pain duration, whether in addition to provoked pain, pain might also arise spontaneously (unprovoked) and whether the onset of symptoms was primary or secondary (at or after first introital penetration, respectively).1
Pain testing
Pain testing of the vestibule was performed using a cotton swab, which is the main diagnostic tool for PVD.16 PVD subjects were mapped for pain in the vulvar vestibule by touching the vestibule perpendicularly with the cotton end of swab (enough to indent the mucosa to a depth of less than 1/3 of the cotton end) for 1 second at 5, 6, 7 (posterior vestibule), 10, 12 and 2 o’clock (anterior vestibule peri-urethral). Participants were asked to rate the pain at each site using a numeric rating scale (NRS) from 0–10/10 (0, none −10, most severe pain imaginable). Pain scores across all sites on the cotton swab test were summed for a total vulvar vestibular pain score (0–60). Vaginal muscle pain was assessed in PVD subjects with a single lubricated digit inserted into the vagina, applying approximately 2 kg of pressure for 2 seconds. The examiner’s finger pressure was calibrated with a handheld digital algometer (Pain Test™ Wagner Instruments) just before the vaginal exam. The right and left levator ani muscles and the perineal complex (at the vaginal entrance) were assessed. The participant was asked to rate the pain severity each site from 0–10/10 Scores were summed across all locations to compute a total vaginal muscle tenderness score (0–30, NRS).40
Study questionnaires
For all subjects, we obtained information on age, body mass index, ethnicity and race. Detailed data was obtained on current use of contraceptives or hormones which was used to categorize hormone use as “none” (i.e., condoms, withdrawal, rhythm, abstinence), “local” (e.g., levonorgestrel containing intrauterine device (IUD), estrogen or testosterone vaginal cream), or “systemic” (e.g., oral contraceptive pills, vaginal contraceptive ring, transdermal contraceptive patch, etonogestrel sub-dermal contraceptive implant).
In women with PVD, Female sexual functioning was assessed using the Female Sexual Functioning Index (FSFI). The FSFI is a multi-dimensional self-report instrument consisting of six subscales: desire (two items), arousal (four items), lubrication (four items), orgasm (three items), global satisfaction (three items) and pain (three items). Items are summed to indicate a total “sexual function” score, where higher scores indicate greater sexual function, and where women who score below the clinical cut-off of 26 are considered at-risk for sexual dysfunction (Rosen et al., 2000).41
Brain imaging
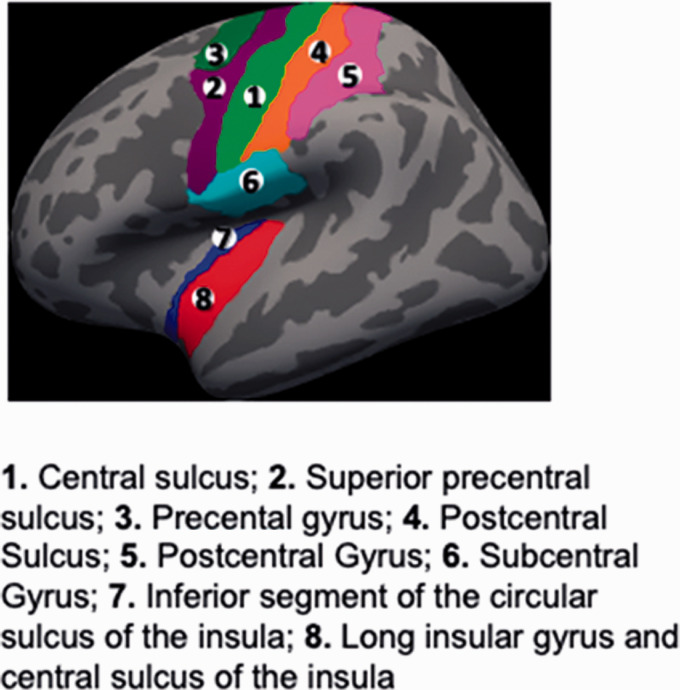
Using a 3.0 T MRI scanner (Siemens Trio; Siemens, Erlangen, Germany), we obtained a high resolution structural brain images and a ten minute 6 sec eyes-closed resting state scan in women with PVD. From this data, we applied graph theory to compute the connectivity strength of or global influence of regions of interest (ROIs) on brain network functioning. We used the Destrieux (cortical)42 and Harvard-Oxford Subcortical Atlases43 to define a priori ROIs based on their known roles in the processing and modulation pain. As shown in Figure 1, cortical ROIs included sensorimotor cortices (precentral gyrus, inferior part of the precentral sulcus, superior part of the precentral sulcus, paracentral lobule and sulcus, subcentral sulci and gyrus (central operculum) and sulci, central sulcus, postcentral sulcus, postcentral gyrus) and the posterior insula (long insular gyrus and central sulcus of the insula, and the inferior segment of the circular sulcus of the insula). We also included subcortical regions including the amygdala, hippocampus, basal ganglia (nucleus accumbens, caudate nucleus, globus pallidus, putamen), thalamus and brainstem. Strength represents the weight sum of connections (i.e., Fisher Z transform correlations >.30) for a given ROI. High values for strength indicate greater centrality or influence on the global state of brain network functioning. For details on image acquisition, preprocessing, and computation of connectivity strength, see Supplemental Methods I.
Figure 1.
Cortical regions of interest.
Biological sample collection and metabolomics
Plasma samples were obtained in 60 women with PVD and 49 HC women. From these women, vaginal swab samples were obtained from 51 of the 60 PVD women providing plasma samples and all 49 HC women. Additionally, 3 women with PVD only provided vaginal swab samples. Vaginal swab and plasma samples were collected during the mid to late follicular phase based on last menstrual period in women not on hormonal contraceptives or in days 5–14 of the active pill phase, considering day 1 as the first day of bleeding.
Vaginal fluid samples
When swabs arrive from the manufacturer, the study coordinator labeled each sterile tube containing the swabs with two cryo-freezer safe labels. One label was for patient study ID and date of sample, and the second label was for PRE and POST collection weight, in grams. The coordinator weighed each dry swab and container and provided PRE gram weight on the swab label. At Visit 1, the physician/nurse practitioner collected the vaginal sample after gently retracting the labia with gloved fingers and inserting a sterile swab approximately 1 inch into the vaginal vault. Swab was rotated 360 degrees, removed, and inserted into the sterile tube (without liquid medium). This procedure was repeated for the remaining 2 swabs. The swabs were immediately weighed post collection (using the same gram scale) and the POST weight was documented on the cryo-freezer label. The samples were immediately placed into a small lab cooler filled with ice and brought to the lab where they were stored in a −80 degree Celsius freezer within 20 minutes of collection. Samples were shipped on dry ice with collection log (vaginal sample log) to Metabolon for further processing and analysis on their global metabolomics and bioinformatics platform (Metabolon, 617 Davis Drive, Durham, NC).
Plasma samples
Using standard venipuncture procedure, blood was collected in EDTA tubes from individuals after an overnight fast. Plasma was centrifuged, aliquoted to 6 (1 ml) sterile polypropylene cryo tubes, and immediately stored at −80°C until shipped to Metabolon.
Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectroscopy (UPLC-MS/MS) was used to perform discovery-based, untargeted global metabolomic profiling to characterize biochemical pathways in vaginal fluid and plasma samples that were altered in PVD compared to HC (Metabolon, 617 Davis Drive, Durham, NC). Specific details on the Metabolomics platform can be found in Supplemental Materials and Methods II. Datasets provided by Metabolon identified a total of 824 compounds of known identity (named biochemicals) detected in plasma, and 952 in vaginal swabs.
Statistical analysis
Global untargeted metabolomic profiling resulted in two datasets (vaginal and plasma samples) comprising a total of 824 compounds of known identity (named biochemicals) in plasma, and 952 in vaginal swabs. From this data set, we extracted metabolites known to be involved in steroid hormone biosynthesis. This included a total of 21 validated biochemicals in vaginal fluid and 33 in plasma samples belonging to androgen, progestin, pregnenolone, and corticosteroids subpathways. No estrogenic steroids were detected in either plasma or vaginal fluid. Following log transformation and imputation of any missing values, with the minimum observed value for each compound, Welch’s two-sample t-test was used to test for differences between PVD and HCs in these steroid hormone metabolites. Because steroid hormone metabolite data was obtained via untargeted metabolomics profiling and the exploratory nature of the analysis, conservative control for false positives (Type I errors) was realized by calculating the false discovery rate(FDR)-adjusted p value (q), based on the total number of metabolites identified during global metabolomic profiling for each sample type (952 in vaginal swabs, and 824 in plasma samples). A q < .10 was used as a threshold for reporting significance.
Because there were differences in use of hormone contraceptives in the sample, we performed an additional sensitivity analysis to explore the influence of taking systemic hormonal contraceptives on observed differences in the steroid hormone metabolites. Specifically, we subgrouped women by hormone usage (no hormones (NH), systemic hormones (SH)) and performed contrast analyses within the framework of the general linear model44–46 testing the following contrasts of interest between-diagnostic groups: PVD NH – HC NH and PVD SH – HC SH, and with-in diagnostic groups: 3) PVD SH – PVD NH, and HC SH – HC NH. Because very few individuals reported use local hormone therapy, women taking local hormones were excluded from the sensitivity analyses.
In women with PVD not taking hormones, Spearman’s correlation was used to estimate the association between untransformed concentrations of altered metabolites with vulvar vestibular pain and vaginal muscle tenderness scores and functional connectivity strength of pain processing brain regions.
We used t-tests and 2 sided Fisher’s exact tests to compare groups by biological sample type on continuous and categorical descriptive variables. The general linear model was employed to test for differences between hormone subgroups on sexual function, pain duration, total vulvar vestibular pain and total vaginal muscle tenderness scores.
Results
Table 1 presents the demographic characteristics and contraceptive hormonal intake of the PVD and HC subjects. The mean age for women with PVD (∼28 y) was about 3 years older than the mean age for HC (∼25 y). About half the sample report no hormone contraceptive use [∼47%, PVD (Nplasma = 29, Nvaginal = 25), 60% HC (N = 29) . Less than half of participants were taking systemic hormonal contraceptives [PVD (∼40%, Nplasma = 23; Nvaginal = 22), HC (29%, N = 14)]. Of these, 12 HCs and 18 women with PVD were using oral contraceptive pills or a nuvaring with the remainder endorsing etonorgestrel implant. Few women were using a local hormone [13% PVD (Nplasma = 8, Nvaginal = N = 7), 13% (HC N = 6)]. Of these, 3 PVD and 2 HC were using estrogen or testosterone cream, and the remainder endorsed use of levonorgestrel containing intrauterine device.
Table 1.
Characteristics of the participants providing plasma and vaginal swab samples.
|
Vaginal swab sample |
Plasma sample |
Vaginal and plasma samples |
PVDV vs. HC |
PVDP vs. HC |
||
|---|---|---|---|---|---|---|
|
PVD N = 54 |
PVD N = 60 |
HCN = 49 |
Fisher’s Exact test | Fisher’s Exact test | ||
| N(%) | N(%) | N(%) | p=.11 | p=.37 | ||
| Hormone type | None | 25 (46.3%) | 29(48.3%) | 29 (59.2%) | ||
| Local | 7 (13.0%) | 8 (13.3%) | 6 (12.2%) | |||
| Systemic | 22 (40.7%) | 23 (38.3%) | 14 (28.6%) | |||
| Ethnicity | Hispanic | 6(11.1) | 6(10) | 8(16.3) | ||
| Race | Amer Ind | 0 | 0 | 2(4.1) | ||
| Asian | 10(18.5) | 12(20) | 25(51) | |||
| Black | 3(5.6) | 2(3.3) | 4(5.6) | |||
| Hawaiian | 0 | 1(2) | 1(2) | |||
| White | 46(85.2) | 51(85) | 25(51) | |||
| Multiracial | 5(9.3) | 5(8.3) | 4(8.1) | |||
|
Mean(SD) range |
Mean(SD)range |
Mean(SD)range |
||||
| Age | 27.5 (4.9)20−42 | 27.6(6.0)18−50 | 24.6(6.1)18−46 | t(101) = 2.62, p = .01 | t(107) = 2.55, p = .012 | |
| Body mass index | 22.9(3.6)14.9−31.5 | 22.8(3.5)14.9−31.5 | 23.0(3.7)16.1−32.4 | t(101) = −0.14, p = 0.89 | t(107) = −0.27, p = .79 |
HC: healthy controls; N: sample size; PVD: provoked vestibulodynia; SD: standard deviation; t: independent t-test.
Table 2 contains the clinical characteristic of the women with PVD by biological sample type. There were no differences between the hormone subgroups in pain duration, sexual functioning by FSFI, total vulvar vestibule pain score on exam, or total vaginal muscle tenderness.
Table 2.
Provoked vestibulodynia-specific sample characteristics.
| Vaginal swab (V) N = 54 | Plasma sample (P) N = 60 | |
|---|---|---|
| N(%) | N(%) | |
| Unprovokeda | 8(14.8) | 9 (15) |
| Onset | ||
| Primary | 27 (50) | 28 (47) |
| Secondary | 27 (50) | 32 (53) |
|
Mean (SD), range |
Mean (SD), range |
|
| Pain durationb | 81.5 (47.8), 36–144 | 78 (.047.0), 26–144 |
| Total vulvar vestibular pain on examc | 44.6 (15.4), 17–77 | 44.2 (14.5),17–77 |
| Total vaginal muscle tenderness scored | 13.87 (6.9), 0–25 | 13.2 (6.9), 0–25 |
| Female sexual functioning indexe | 13.26 (6.07), 1.33–24.9 | 12.80 (6.20), 1.33–24.9 |
N=sample size, =standard deviation.
a8 patients with both provoked and unprovoked vestibulodynia.
bpain duration in months.
c2 missing datapoints in plasma.
d1 missing data point in vaginal swab samples, 4 missing data points in plasma samples.
e3 missing data points in vaginal swab samples, 6 missing data point in plasma samples.
Steroid hormones pathway alterations in PVD
As shown in Table 3 (All Subjects), women with PVD compared to HC had lower vaginal concentrations of steroid in pathways involving pregnenolone (21-hydroxypregnenolone disulfate, pregnenediol disulfate), progestin (5alpha-pregnan-3beta,20beta-diol monosulfate (1), 5alpha-pregnan-3beta,20alpha-diol disulfate, pregnanediol-3-glucuronide) and androgens (dehydroisoandrosterone sulfate (DHEA-S), 16a-hydroxy DHEA 3-sulfate, androstenediol (3beta,17beta) disulfate (1), androstenediol (3beta,17beta) disulfate (2), andro steroid monosulfate
Table 3.
Vaginal concentrations of steroid pathway metabolites.
| All subjects |
Subgroup analysis |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
PVD (n = 54)/HC(n = 49) |
No hormone subgroup: PVD(n = 25)/HC(n = 29) |
Systemic hormone subgroupPVD (N = 22)/HC(n = 14) |
PVD Systemic/PVD No hormone |
HCSystemic/HC No hormone |
||||||||||||
| Subpathways | Biochemical | Fold Change | P | q | Fold Change | p-value | q-value | Fold Change | p-value | q-value | Fold Change | p-value | q-value | Fold Change | p-value | q-value |
| Pregnenolone steroids | 17alpha-hydroxypregnanolone glucuronide | 0.38 | 0.093 | 0.125 | 0.55 | 0.049 | 0.117 | 1.20 | 0.63 | 0.74 | 0.71 | 0.28 | 0.82 | 0.33 | 0.002 | 0.129 |
| 21-hydroxypregnenolone disulfate | 0.27 | 0.011 | 0.039 | 0.41 | 0.008 | 0.061 | 0.87 | 0.73 | 0.79 | 1.16 | 0.68 | 0.96 | 0.55 | 0.124 | 0.422 | |
| pregnenediol disulfate (C21H34O8S2)* | 0.20 | 0.002 | 0.020 | 0.22 | 0.001 | 0.035 | 0.65 | 0.43 | 0.63 | 1.37 | 0.50 | 0.92 | 0.47 | 0.149 | 0.454 | |
| Progestin steroids | 5alpha-pregnan-3beta,20beta-diol monosulfate (1) | 0.50 | 0.001 | 0.016 | 0.50 | 0.011 | 0.063 | 0.63 | 0.17 | 0.48 | 1.11 | 0.71 | 0.97 | 0.88 | 0.679 | 0.845 |
| 5alpha-pregnan-3beta,20alpha-diol disulfate | 0.16 | 0.032 | 0.069 | 0.53 | 0.007 | 0.057 | 0.97 | 0.90 | 0.85 | 0.98 | 0.93 | 0.99 | 0.54 | 0.026 | 0.254 | |
| pregnanediol-3-glucuronide | 0.18 | 0.009 | 0.036 | 0.26 | 0.003 | 0.045 | 1.01 | 0.99 | 0.86 | 0.89 | 0.80 | 0.98 | 0.23 | 0.007 | 0.173 | |
| Cortico-steroids | Cortisone | 1.09 | 0.135 | 0.154 | 1.06 | 0.663 | 0.483 | 1.21 | 0.29 | 0.56 | 0.82 | 0.19 | 0.73 | 0.72 | 0.057 | 0.314 |
| Androgenicsteroids | 11-ketoetiocholanolone glucuronide | 0.52 | 0.232 | 0.214 | 0.68 | 0.243 | 0.265 | 1.14 | 0.75 | 0.79 | 0.86 | 0.68 | 0.96 | 0.52 | 0.093 | 0.375 |
| dehydroisoandrosterone sulfate (DHEA-S) | 0.35 | 0.053 | 0.090 | 0.42 | 0.020 | 0.080 | 1.01 | 0.98 | 0.86 | 1.40 | 0.38 | 0.90 | 0.59 | 0.221 | 0.525 | |
| 16a-hydroxy DHEA 3-sulfate | 0.22 | 0.006 | 0.029 | 0.30 | 0.003 | 0.045 | 0.83 | 0.71 | 0.78 | 0.83 | 0.66 | 0.96 | 0.30 | 0.012 | 0.216 | |
| androsterone glucuronide | 0.63 | 0.551 | 0.358 | 0.64 | 0.241 | 0.264 | 1.50 | 0.39 | 0.61 | 0.83 | 0.63 | 0.95 | 0.36 | 0.022 | 0.246 | |
| epiandrosterone sulfate | 0.66 | 0.581 | 0.373 | 0.68 | 0.249 | 0.267 | 1.35 | 0.47 | 0.65 | 1.08 | 0.83 | 0.98 | 0.55 | 0.126 | 0.423 | |
| androsterone sulfate | 0.46 | 0.337 | 0.267 | 0.48 | 0.089 | 0.152 | 1.36 | 0.57 | 0.70 | 1.05 | 0.91 | 0.99 | 0.37 | 0.055 | 0.314 | |
| etiocholanolone sulfate | 0.68 | 0.517 | 0.343 | 0.56 | 0.172 | 0.218 | 1.16 | 0.77 | 0.80 | 1.22 | 0.67 | 0.96 | 0.59 | 0.287 | 0.592 | |
| etiocholanolone glucuronide | 0.65 | 0.692 | 0.409 | 0.69 | 0.361 | 0.333 | 1.42 | 0.48 | 0.65 | 0.75 | 0.51 | 0.93 | 0.37 | 0.038 | 0.283 | |
| androstenediol (3beta,17beta) monosulfate (1) | 0.23 | 0.143 | 0.160 | 0.70 | 0.051 | 0.118 | 0.98 | 0.92 | 0.85 | 1.18 | 0.39 | 0.90 | 0.85 | 0.443 | 0.706 | |
| androstenediol (3beta,17beta) disulfate (1) | 0.41 | 0.020 | 0.051 | 0.28 | 0.005 | 0.049 | 0.84 | 0.76 | 0.79 | 1.75 | 0.25 | 0.80 | 0.57 | 0.297 | 0.599 | |
| androstenediol (3beta,17beta) disulfate (2) | 0.31 | 0.014 | 0.043 | 0.28 | 0.006 | 0.054 | 0.84 | 0.76 | 0.79 | 1.31 | 0.59 | 0.94 | 0.43 | 0.127 | 0.424 | |
| 5alpha-androstan-3alpha,17beta-diol monosulfate (1) | 0.99 | 0.829 | 0.455 | 1.00 | 1.000 | 0.600 | 1.00 | 0.96 | 0.86 | 1.09 | 0.16 | 0.72 | 1.10 | 0.187 | 0.488 | |
| 5alpha-androstan-3beta,17beta-diol disulfate | 0.78 | 0.131 | 0.151 | 0.76 | 0.048 | 0.116 | 0.99 | 0.96 | 0.86 | 1.12 | 0.43 | 0.90 | 0.86 | 0.367 | 0.655 | |
| andro steroid monosulfate C19H28O6S (1)* | 0.32 | 0.004 | 0.028 | 0.33 | 0.001 | 0.036 | 0.89 | 0.79 | 0.80 | 1.03 | 0.93 | 0.99 | 0.38 | 0.017 | 0.242 | |
The table shows the main analysis results from comparing provoked vestibulodynia (PVD) and healthy controls (HC) subjects in all subjects (Fold Change, p, q) as well as the false discovery rate corrected p values (q) associated with the hormone subgroup sensitivity analyses. Abbreviations: Fold Change: ratio of means, p =probability, q = 5% false discovery rate corrected p value.
In plasma samples, few differences in steroid metabolites were observed. Women with PVD showed significantly lower levels of pregnenolone (pregnenolone sulfate, pregnenediol disulfate) and progestin (5-alpha-pregnan-diol disulfate).
Subgroup analyses
To investigate the potential influence of systemic hormonal contraceptive use on differences in the steroid hormone metabolites observed in the whole sample, we examined differences in the steroid metabolites in the subgroups of women with PVD and HC women not using hormonal contraceptives (i.e., PVD NH, HC NH) and using systemic hormone contraceptives (i.e., PVD SH, HC SH)
PVD NH compared to HC NH. As shown, in Table 3 (No hormone subgroup) we observed significantly lower levels of several metabolites in vaginal fluid samples from PVD NH compared to HC NH, related to pathways involving pregnenolone (e.g., 17alpha-hydroxypregnenolone 3-sulfate, 21-hydroxypregnenolone disulfate, pregnenediol disulfate), progestin (5alpha-pregnan-3beta, 20beta-diol monosulfate(1), 5alpha-pregnan-3beta, 20alpha-diol disulfate, pregnanediol-3-glucuronide), and androgens (dehydroisoandrosterone sulfate (DHEA-S), 16a-hydroxy DHEA 3-sulfate, androstenediol (3beta,17beta) disulfate (1), androstenediol (3beta,17beta) disulfate (2), andro steroid monosulfate). In plasma samples, no differences below q < .10 were observed.
PVD SH compared HC SH. In PVD SH compared to HC SH, no significant differences in vaginal or plasma concentrations of steroid hormones were detected (see Tables 3 and 4, Systemic hormone subgroup). Interestingly, within group (i.e., diagnosis) comparisons (described below) indicated that that use of systemic contraceptives significantly reduced plasma metabolite levels in both PVD and HC and there was a trend for a reduction of vaginal steroid hormone metabolite levels in HCs but not PVD.
Table 4.
Plasma concentrations of steroid pathway metabolites.
| All subjects |
Subgroup analysis |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PVD (n = 54)/HC(n = 49) |
No hormone subgroup:PVD(n = 25)/HC(n = 29) |
Systemic hormone SubgroupPVD (N = 22)/HC(n = 14) |
PVD Systemic/PVD No hormone |
HC Systemic/HC No hormone |
||||||||||||
| Subpathways | Biochemical | Fold Change | p | q | Fold Change | p-value | q-value | Fold Change | p-value | q-value | Fold Change | p-value | q-value | Fold Change | p-value | q-value |
| Pregnenolone steroids | pregnenolone sulfate | 0.68 | 0.001 | 0.065 | 0.77 | 0.063 | 0.562 | 0.77 | 0.147 | 0.666 | 0.70 | 0.019 | 0.076 | 0.70 | 0.044 | 0.196 |
| 17alpha-hydroxypregnenolone 3-sulfate | 0.56 | 0.002 | 0.112 | 0.54 | 0.003 | 0.229 | 0.88 | 0.632 | 0.838 | 0.83 | 0.392 | 0.482 | 0.51 | 0.009 | 0.086 | |
| 21-hydroxypregnenolone disulfate | 0.83 | 0.019 | 0.248 | 0.80 | 0.092 | 0.606 | 0.87 | 0.432 | 0.787 | 0.65 | 0.002 | 0.017 | 0.59 | 0.002 | 0.031 | |
| pregnenediol disulfate (C21H34O8S2)* | 0.69 | 6.4E-05 | 0.017 | 0.85 | 0.262 | 0.725 | 0.78 | 0.166 | 0.666 | 0.55 | 9.3E-05 | 0.002 | 0.60 | 0.004 | 0.054 | |
| pregnenediol sulfate (C21H34O5S)* | 0.78 | 0.005 | 0.148 | 0.62 | 0.001 | 0.113 | 0.77 | 0.149 | 0.666 | 0.94 | 0.674 | 0.608 | 0.76 | 0.107 | 0.312 | |
| Progestin steroids | 5alpha-pregnan-3beta-ol,20-one sulfate | 0.37 | 0.010 | 0.165 | 0.60 | 0.093 | 0.606 | 0.89 | 0.773 | 0.884 | 0.49 | 0.031 | 0.101 | 0.33 | 0.004 | 0.054 |
| 5alpha-pregnan-3beta,20beta-diol monosulfate (1) | 0.47 | 0.007 | 0.158 | 0.63 | 0.082 | 0.600 | 0.83 | 0.586 | 0.835 | 0.39 | 0.001 | 0.012 | 0.29 | 0.000 | 0.012 | |
| 5alpha-pregnan-3beta,20alpha-diol monosulfate (2) | 0.46 | 0.020 | 0.252 | 0.77 | 0.393 | 0.799 | 0.72 | 0.395 | 0.766 | 0.20 | 3.2E-06 | 1.0E-04 | 0.22 | 0.000 | 0.007 | |
| 5alpha-pregnan-3beta,20alpha-diol disulfate | 0.43 | 0.002 | 0.101 | 0.57 | 0.028 | 0.439 | 0.73 | 0.350 | 0.751 | 0.42 | 0.002 | 0.016 | 0.33 | 0.001 | 0.017 | |
| 5alpha-pregnan-diol disulfate | 0.42 | 0.002 | 0.098 | 0.38 | 0.004 | 0.229 | 0.78 | 0.567 | 0.833 | 0.70 | 0.306 | 0.418 | 0.34 | 0.010 | 0.086 | |
| pregnanediol-3-glucuronide | 0.53 | 0.053 | 0.346 | 0.83 | 0.425 | 0.817 | 0.92 | 0.791 | 0.892 | 0.38 | 2.0E-04 | 0.003 | 0.34 | 0.000 | 0.012 | |
| pregnanolone/allopregnanolone sulfate | 0.36 | 0.002 | 0.115 | 0.52 | 0.040 | 0.499 | 0.69 | 0.371 | 0.760 | 0.55 | 0.083 | 0.195 | 0.41 | 0.027 | 0.154 | |
| Corticosteroids | Cortisol | 1.08 | 0.888 | 0.628 | 0.85 | 0.254 | 0.719 | 1.05 | 0.776 | 0.884 | 2.33 | 2.8E-07 | 2.8E-05 | 1.87 | 0.001 | 0.019 |
| Cortisone | 0.98 | 0.454 | 0.502 | 0.86 | 0.091 | 0.606 | 1.04 | 0.748 | 0.875 | 1.31 | 0.005 | 0.030 | 1.08 | 0.465 | 0.631 | |
| Androgenic steroids | dehydroisoandrosterone sulfate (DHEA-S) | 0.89 | 0.073 | 0.357 | 0.92 | 0.452 | 0.837 | 0.90 | 0.490 | 0.806 | 0.72 | 0.010 | 0.048 | 0.73 | 0.036 | 0.177 |
| 16a-hydroxy DHEA 3-sulfate | 0.67 | 0.013 | 0.214 | 0.67 | 0.041 | 0.499 | 0.71 | 0.180 | 0.666 | 0.63 | 0.028 | 0.097 | 0.59 | 0.032 | 0.167 | |
| androsterone glucuronide | 1.04 | 0.852 | 0.620 | 1.23 | 0.254 | 0.719 | 1.00 | 0.988 | 0.934 | 0.50 | 4.0E-04 | 0.005 | 0.61 | 0.028 | 0.157 | |
| epiandrosterone sulfate | 0.97 | 0.987 | 0.651 | 1.14 | 0.525 | 0.862 | 1.21 | 0.466 | 0.799 | 0.61 | 0.022 | 0.084 | 0.57 | 0.027 | 0.154 | |
| androsterone sulfate | 0.96 | 0.885 | 0.628 | 1.05 | 0.819 | 0.991 | 1.35 | 0.258 | 0.717 | 0.68 | 0.082 | 0.195 | 0.53 | 0.014 | 0.108 | |
| etiocholanolone glucuronide | 1.08 | 0.643 | 0.563 | 1.28 | 0.190 | 0.669 | 0.99 | 0.968 | 0.934 | 0.53 | 0.002 | 0.014 | 0.68 | 0.095 | 0.291 | |
| 5alpha-androstan-3alpha,17alpha-diol monosulfate | 1.06 | 0.773 | 0.596 | 1.11 | 0.637 | 0.913 | 1.03 | 0.923 | 0.934 | 0.47 | 0.002 | 0.014 | 0.51 | 0.014 | 0.109 | |
| androstenediol (3beta,17beta) monosulfate (1) | 0.92 | 0.336 | 0.460 | 0.92 | 0.586 | 0.887 | 0.94 | 0.779 | 0.884 | 0.68 | 0.023 | 0.087 | 0.66 | 0.036 | 0.177 | |
| androstenediol (3beta,17beta) monosulfate (2) | 1.04 | 0.747 | 0.594 | 0.98 | 0.873 | 1.000 | 1.11 | 0.614 | 0.836 | 0.88 | 0.420 | 0.491 | 0.77 | 0.180 | 0.415 | |
| androstenediol (3beta,17beta) disulfate (1) | 0.91 | 0.087 | 0.358 | 0.67 | 0.008 | 0.273 | 1.08 | 0.669 | 0.856 | 1.25 | 0.162 | 0.285 | 0.77 | 0.159 | 0.386 | |
| androstenediol (3beta,17beta) disulfate (2) | 0.89 | 0.031 | 0.321 | 0.83 | 0.049 | 0.511 | 0.95 | 0.669 | 0.856 | 0.82 | 0.055 | 0.148 | 0.71 | 0.006 | 0.069 | |
| androstenediol (3alpha, 17alpha) monosulfate (2) | 0.92 | 0.383 | 0.468 | 1.01 | 0.938 | 1.000 | 1.02 | 0.917 | 0.934 | 0.43 | 5.1E-06 | 2.0E-04 | 0.42 | 0.000 | 0.005 | |
| androstenediol (3alpha, 17alpha) monosulfate (3) | 0.83 | 0.113 | 0.385 | 0.82 | 0.212 | 0.685 | 0.97 | 0.887 | 0.934 | 0.69 | 0.033 | 0.102 | 0.58 | 0.008 | 0.082 | |
| 5alpha-androstan-3alpha,17beta-diol monosulfate (1) | 0.86 | 0.957 | 0.644 | 0.98 | 0.941 | 1.000 | 1.55 | 0.183 | 0.666 | 0.51 | 0.013 | 0.058 | 0.32 | 0.001 | 0.013 | |
| 5alpha-androstan-3alpha,17beta-diol disulfate | 1.06 | 0.794 | 0.601 | 0.80 | 0.553 | 0.872 | 1.15 | 0.772 | 0.884 | 1.46 | 0.338 | 0.441 | 1.02 | 0.965 | 0.810 | |
| 5alpha-androstan-3beta,17beta-diol monosulfate (2) | 0.97 | 0.910 | 0.634 | 1.07 | 0.778 | 0.980 | 1.32 | 0.353 | 0.751 | 0.55 | 0.016 | 0.068 | 0.44 | 0.005 | 0.067 | |
| 5alpha-androstan-3beta,17beta-diol disulfate | 0.90 | 0.875 | 0.627 | 0.79 | 0.286 | 0.743 | 1.51 | 0.156 | 0.666 | 1.10 | 0.678 | 0.609 | 0.58 | 0.049 | 0.205 | |
| 5alpha-androstan-3beta,17alpha-diol disulfate | 0.95 | 0.539 | 0.532 | 1.52 | 0.311 | 0.761 | 1.50 | 0.447 | 0.793 | 0.68 | 0.382 | 0.474 | 0.69 | 0.470 | 0.632 | |
| andro steroid monosulfate C19H28O6S (1)* | 0.68 | 0.038 | 0.342 | 0.76 | 0.214 | 0.685 | 0.69 | 0.198 | 0.666 | 0.51 | 0.005 | 0.028 | 0.56 | 0.034 | 0.175 | |
The table shows the main analysis results from comparing provoked vestibulodynia (PVD) and healthy controls (HC) subjects in all subjects (Fold Change, p, q) as well as the false discovery rate corrected p values (q) associated with the hormone subgroup sensitivity analyses. Abbreviations: Fold Change: ratio of means, p =probability, q = 5% false discovery rate corrected p value.
PVD SH compared to PVD NH. No significant differences in steroid hormones were detected between PVD NH and PVD SH in vaginal swab samples. However, numerous within group differences were detected in plasma levels of steroids in PVD SH compared to PVD NH (see Table 4, PVD Systemic/PVD No hormone). As expected, PVD SH had significantly lower levels of pregnenolone (e.g., pregnenediol sulfate progestin (e.g., 5alpha-pregnan-3beta,20alpha-diol disulfate, pregnanediol-3-glucuronide,) and androgens (e.g., 16a-hydroxy DHEA 3-sulfate, 5alpha-androstan-3alpha,17alpha-diol monosulfate(1)). Plasma levels of corticosteroids (i.e., cortisol, cortisone) were elevated in PVD SH compared to PVD NH.
HC SH compared to HC NH. As observed in PVD, HC SH compared to HC NH also had significant reductions in plasma levels of hormone metabolites comprising pregnenolone, progestin and androgen pathways (see Table 4, HC Systemic/HC No hormone). Additionally, plasma cortisol but not cortisone was also elevated in HC SH compared to HC NH. Reductions in vaginal levels of hormones metabolite did not survive conservative FDR correction (q < .05). However, at uncorrected significance levels (p uncorrected <.05), HC SH compared HC NH did show reductions metabolite concentrations in each pregnenolone, progestin and androgen pathways.
Association between vulvar vestibular pain and vaginal muscle tenderness and vaginal levels of dysregulated steroid hormones in PVD NH. As can be seen in Table 5, the observed decreases in vaginal levels of the androgens, androstenediol (3beta,17beta) disulfate (1), androstenediol (3beta,17beta) disulfate (2), dehydroepiandrosterone sulfate (DHEA-S), and androsterone sulfate were associated with moderate to large effect size increases in total vulvar vestibular pain NRS scores. Reduced vaginal levels of dehydroepiandrosterone sulfate (DHEA-S), and androsterone sulfate were also correlated with increased total muscle tenderness NRS scores.
Table 5.
Symptom correlations with vaginal metabolite concentrations showing differences in PVD NH compared to HC NH.
|
Vulvar vestibular pain total |
Total vaginal muscle tenderness |
||||
|---|---|---|---|---|---|
| Metabolites | Subpathway | R | P | r | p |
| Androstenediol (3beta,17beta) disulfate (1) | Androgenic Steroids | −0.50 | 0.01 | ||
| Androstenediol (3beta,17beta) disulfate (2) | Androgenic Steroids | −0.41 | 0.04 | ||
| Dehydroepiandrosterone sulfate (DHEA-S) | Androgenic Steroids | −0.64 | 0.0005 | −0.51 | 0.009 |
| Androsterone sulfate | Androgenic Steroids | −0.71 | 0.00007 | −0.48 | 0.02 |
Correlations were compute in PVD NH (N = 25). r: spearman’s rank correlation; p: probability value.
Link between vaginal steroid metabolites and functional connectivity in pain processing regions in PVD NH
The associations between the vaginal concentrations of steroid hormones that were reduced in PVD NH compared to HC NH (Table 3) with global connectivity of pain processing regions was examined. The decreases in vaginal concentration of three of the androgenic steroids and one progestin were associated with decreased global connectivity of sensory and motor cortices (Table 6). No significant associations were observed with insula, subcortical regions or brainstem.
Table 6.
Association between the functional connectivity strength of pain processing regions and vaginal metabolite concentrations showing differences in PVD NH compared to HC.
| Biochemical name | Subpathway | Hemisphere | Brain region | r | p |
|---|---|---|---|---|---|
| 16a-Hydroxy DHEA 3-sulfate | Androgenic Steroid | L | Postcentral sulcus (S1) | 0.41 | 0.05 |
| R | Precentral gyrus (M1) | 0.44 | 0.03 | ||
| L | Precentral gyrus (M1) | 0.45 | 0.03 | ||
| R | Subcentral gyrus (central operculum) and sulci | 0.48 | 0.02 | ||
| R | Superior part of the precentral sulcus | 0.44 | 0.04 | ||
| Androstenediol (3beta,17beta) disulfate (2) | R | Superior part of the precentral sulcus | 0.42 | 0.049 | |
| Androsterone sulfate | R | paracentral lobule | 0.43 | 0.04 | |
| L | Postcentral gyrus (S1) | 0.44 | 0.04 | ||
| Progestin Steroid | R | paracentral lobule | 0.44 | 0.03 | |
| Pregnanediol-3-glucuronide | L | Postcentral gyrus (S1) | 0.45 | 0.03 | |
| R | Subcentral gyrus (central operculum) and sulci | 0.41 | 0.049 |
Correlations were computed in PVD NH (N = 25). HC: healthy control; M1: primary motor cortex; PVD: provoked vestibulodynia; NH: no hormones; r: Spearman rank correlation; p; probability; S1: primary sensory cortex.
Discussion
Women with PVD compared to HC had reduced concentrations of pregnenolone, progestin and androgen metabolites involved in the steroid hormone biosynthesis. These reductions were observed primarily in vaginal fluid not plasma suggesting localized effects in the vagina and vestibule rather than systemic alterations. The observed reductions in vaginal levels of several androgenic metabolites were associated with increased total vulvar vestibular pain and vaginal muscle tenderness and decreased functional connectivity in pain processing brain regions, the primary sensory and motor cortices. We observed that use of systemic hormonal contraceptives resulted reduced plasma concentrations of the metabolites involved in steroidogenesis in both PVD and HC. There was also some evidence that use of systemic hormones reduced vaginal levels of these metabolites in HCs but not PVD. Finally, pain duration, total vulvar vestibule pain and total vaginal muscle tenderness did not differ in women with PVD using systemic hormonal contraceptives compared to women not using hormones or those using local hormones.
The lower vaginal levels of steroidogenic metabolites in women with PVD, including androgenic steroids (e.g. 16a-hydroxy DHEA 3-sulfate and androstenediol disulfate, a metabolite of DHEA) and pregnenolone, a precursor to both progesterone and DHEA synthesis, could lead to alterations in mucosal structure and thinning of the mucosa of the vestibule rendering the tissue more vulnerable to injury and inflammation.4,23 The vaginal and vulvar epithelium express high levels of androgen receptors.27 Mucin-secreting vestibular glands also express high levels of androgen receptors; thus, changes in steroids observed in PVD group could ultimately contribute to decreased mucin production, vulnerability to chemical, physical or microbial damage and hypersensitivity. Androgens are aromatized to estrogens (not detected in the current dataset) therefore it is difficult to affirm that their final peripheral effect is caused by the androgens or by conversion to estrogens or by combination of both. Lower local concentrations of androgen could result in diminished estrogen binding in vulvar and vaginal tissues.
Because progesterone and estrogen can modulate pain perception, the changes detected in vaginal fluid could also contribute to mechanical allodynia in vestibule and vaginal muscles. The progestin, 5 alpha-pregnan3beta, 20 beta diol, was lower in PVD vaginal fluid and is a neurosteroid capable of binding steroid membrane GABA A receptors with inhibitory, excitatory and neurotrophic effects.47 Androgens have broad anti-inflammatory effects on cellular and molecular levels and can regulate the expression of inflammatory cytokines, known to sensitize nociceptors, such as TNF-alpha, interleukin-6, and interleukin-1beta.48 Taken together, alterations in steroid hormones (as observed in PVD) could directly affect the vaginal mucosa (e.g., resulting vulnerability to irritation and inflammation) and modulate nociception through changes in innervation.
The failure to detect estrogen hormone metabolites in our samples is a limitation of the Metabolon global untargeted metabolomic profiling platform. The library does include estriol and estrone however over 95% of the untargeted studies run by Metabolon fail to detect any estrogen hormones. This could be due to a combination of factors, including highly hydrophobic nature of estrogen hormones that may result in suboptimal methanol extraction used for the untargeted approach. This could have resulted in estrogen levels being below the threshold of detection of the employed methodology.
The use of systemic hormonal contraceptives increased plasma concentrations of corticosteroid hormones (i.e., cortisol, cortisone) levels in PVD SH compared to PVD NH, but could not account for the vaginal metabolite findings. Systemic oral hormonal contraceptives increase free circulating cortisol in the blood.49 These findings warrant further investigation but may also be consistent with research demonstrating systemic hormonal contraceptive usage is associated with increased cortisol and dysregulated stress-response in the hypothalamic-pituitary-adrenal (HPA) axis.49
In HCs but not PVD, weak evidence (p uncorrected <.05) suggested that the use of systemic hormonal contraceptives lowered vaginal concentrations of pregnenolone, progestin and androgen metabolites. As such, the differences in vaginal levels of steroid hormone metabolites observed between PVD and HC that were not using hormone contraceptives were no longer observed when comparing PVD SH to HC SH,
Additionally, within diagnosis comparisons revealed that use of systemic hormone lowered plasma levels of pregnenolone, progestin and androgen metabolites in both women with PVD and HCs. Systemic oral hormonal contraceptives increase sex hormone binding globulin (SHBG) and decrease ovarian estrogen and testosterone production resulting in lower free plasma testosterone. Combined hormonal contraceptives also inhibit the enzyme 5-alpha reductase, which converts testosterone into dihydrotesterone, the compound that binds to cellular androgen receptors. Additionally, combined hormonal contraceptives can have different progestins of varying androgenicity. Only two women using SH in each group (PVD and HC) were using the subdermal progestin implant that is unlikely to significantly affect hepatic binding proteins; the remainder took combined estrogen progestinal agents. Unfortunately, plasma estradiol and free testosterone were not measured in this initial pilot study. The lack of difference in steroid metabolites between women with PVD and HC using systemic contraceptives is of interest and could represent a risk factor for future development of symptoms in some healthy individuals. This study highlights the potential impact of steroid hormones and the need to consider this factor when studying PVD patients.
In the present study, decreases in androgenic and progestin metabolites in vaginal fluid of women with PVD were association with decreased connectivity strength of primary sensorimotor cortices. The connectivity strength of brain region reflects its global influence on brain network functioning. Symptom associated alterations in the gray matter density, activity (during evoked pain studies), and intrinsic resting state connectivity of sensorimotor cortices have been reported in women with PVD compared to HCs.5,6,8,10 We have also shown women with PVD showed microstructural alterations in cortico-thalamic-basal ganglia white matter tracts associated with ascending nociceptive processing (i.e, primary sensory cortex, thalamus, basal ganglia)5. The observed decreases in functional connectivity strength of these key pain processing regions (with decreased concentrations of androgenic and progestin metabolites) may reflect central sensitization and lead to biased sensory experiences.
The current study examined whether metabolites involved in the biosynthesis of steroid hormones linked to development of neuroinflammation and/or hyperalgesia, or maintenance of genital tissue structure were altered in women with PVD, but the study was not designed to test the specific nature or direction of these associations. Specifically, while these alterations could lead to increased ascending transmission and modulation of signals from the vagina and vulvar vestibule and long lasting allodynia and hyperalgesia, future longitudinal studies are needed to determine where the causal association lies.
A substantial number of women were using various types of hormonal contraceptives Although subgroup analyses revealed that use of systemic hormones is linked to metabolite levels, the women comprising this subgroup were using different types and dosages of systemic hormone contraceptives. Although in this study, the sample size limited the ability to determine whether specific types of hormone contraceptives which work via different mechanisms may have potentially different influences on the observed reductions in metabolites, this is an important line of research for future studies. Additionally, larger sample sizes will be needed for adequately powered subgroup analyses.
By definition untargeted metabolomic profiling is discovery-based and the findings from this cross-sectional study will require replication in a larger independent data set. Additionally, targeted metabolomic studies of androgen, pregnenolone and progestin steroid biosynthesis and signaling pathways in vaginal fluid as well as tissue will be important for validation of these candidate biomarkers. The inclusion of estrogen pathways that is possible in targeted metabolomic profiling is also recommended given the known role in maintaining the structural integrity of the vaginal tissue and pain perception. Ultimately, longitudinal studies comprising concurrent measurement of systemic steroid hormones such as estradiol and free testosterone, pro-and anti-inflammatory cytokines and micro-RNA expression associated with inflammation and pain will help illuminate the relationship between these distinct but interacting mechanisms contributing to peripheral and central sensitization and symptoms in PVD.
In conclusion, women with PVD have significant reductions in vaginal fluid concentrations of metabolites involved in the biosynthesis of steroid hormones previously shown to be involved in maintaining tissue integrity and nociceptive processing. Reductions in vaginal levels of androgenic hormone metabolites showed strong associations with increased vulvar vestibule pain on exam and vaginal muscle tenderness. Ultimately, certain steroids may be important peripheral biological markers for understanding the pathophysiology of symptoms in PVD, may provide potential diagnostic markers, and could lead to new targets for therapeutic intervention.
Supplemental Material
Supplemental material, sj-pdf-1-mpx-10.1177_17448069211041853 for Reduced concentrations of vaginal metabolites involved in steroid hormone biosynthesis are associated with increased vulvar vestibular pain and vaginal muscle tenderness in provoked vestibulodynia: An exploratory metabolomics study by Jennifer S Labus, Emeran A Mayer, Kjersti Aagaard, Jean Stains RN Katarzyna Broniowska and Andrea Rapkin in Molecular Pain
Acknowledgments
All scans were performed at the Ahmanson Lovelace Brain Mapping Center, UCLA. Some of the findings included in this manuscript have been presented at the following meetings: Annual Scientific Meeting on Pelvic Pain, International Pelvic Pain Society, Chicago, Illinois, 19–20 October 2018.
Footnotes
Author contributions: All authors have read and approved the paper. Each author made the following contributions: 1. Funding (AR., JSL). 2. Study conceptualization and design (A.R., J.S.L.). 3. Data acquisition (JS, JSL, AR).4. Data analysis (JSL, KB, CR.). 5. Data interpretation (AR., EM, JSL, KA, KB). 6. Manuscript preparation and critical revisions (AR, EM., JS, JSL, KA, KB).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from National Institutes of Health: R01 NICHD076756 (JSL/AR), R21 NICHD086737 (JSL/AR); NIH R01 DK048351 (EAM); R01-HD091731, R21-ES029462, R01-DK089201 (KMA)
ORCID iD: Andrea Rapkin https://orcid.org/0000-0003-3254-8671
Supplemental material: Supplemental material for this article is available online.
References
- 1.Bornstein J, Goldstein AT, Stockdale CK, Bergeron S, Pukall C, Zolnoun D, Coady D, consensus vulvar pain terminology committee of the International Society for the Study of Vulvovaginal Disease (ISSVD), the International Society for the Study of Women's Sexual Health (ISSWSH), the International Pelvic Pain Society (IPPS). Consensus vulvar pain terminology committee of the international society for the study of vulvovaginal disease tISftSoWsSH and the international pelvic pain S. 2015 ISSVD, ISSWSH and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. Obstet Gynecol 2016; 127: 745–751. [DOI] [PubMed] [Google Scholar]
- 2.Lev-Sagie A, Witkin SS.Recent advances in understanding provoked vestibulodynia. F1000Res 2016; 5: 2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pukall CF, Goldstein AT, Bergeron S, Foster D, Stein A, Kellogg-Spadt S, Bachmann G.Vulvodynia: definition, prevalence, impact, and pathophysiological factors. J Sex Med 2016; 13: 291–304. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein AT, Belkin ZR, Krapf JM, Song WT, Khera M, Jutrzonka SL, Kim NN, Burrows LJ, Goldstein I.Polymorphisms of the androgen receptor gene and hormonal contraceptive induced provoked vestibulodynia. J Sex Med 2014; 11: 2764–2771. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt R, Gupta A, Rapkin AJ, Kilpatrick L, Hamadami K, Pazmany E, Van Oudenhove L, Stains J, Aerts L, Enzli P, Tillisch K, Mayer EA, Labus JS.Altered gray matter volume in sensorimotor and thalamic regions associated with pain in localized provoked vestibulodynia: a voxel-based morphometry study. J Pain 2019; 160: 1529–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, Rapkin AJ, Gill Z, Kilpatrick L, Fling C, Stains J, Masghati S, Tillisch K, Mayer EA, Labus JS.Disease-related differences in resting-state networks: a comparison between localized provoked vulvodynia, irritable bowel syndrome, and healthy control subjects. Pain 2015; 156: 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Woodworth DC, Ellingson BM, Rapkin AJ, Naliboff B, Kilpatrick LA, Stains J, Masghati S, Tillisch K, Mayer EA, Labus JS.Disease-related microstructural differences in the brain in women with provoked vestibulodynia. J Pain 2018; 19: 528.e1–e528.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampson JP, Reed BD, Clauw DJ, Bhavsar R, Gracely RH, Haefner HK, Harris RE.Augmented central pain processing in vulvodynia. J Pain 2013; 14: 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pukall CF, Strigo IA, Binik YM, Amsel R, Khalife S, Bushnell MC.Neural correlates of painful genital touch in women with vulvar vestibulitis syndrome. Pain 2005; 115: 118–127. [DOI] [PubMed] [Google Scholar]
- 10.Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC.Increased gray matter density in young women with chronic vulvar pain. Pain 2008; 140: 411–419. [DOI] [PubMed] [Google Scholar]
- 11.Pazmany E, Ly HG, Aerts L, Kano M, Bergeron S, Verhaeghe J, Peeters R, Tack J, Dupont P, Enzlin P, Van Oudenhove L.Brain responses to vestibular pain and its anticipation in women with genito-pelvic pain/penetration disorder. Neuroimage Clin 2017; 16: 477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciszek BP, Khan AA, Dang H, Slade GD, Smith S, Bair E, Maixner W, Zolnoun D, Nackley AG.MicroRNA expression profiles differentiate chronic pain condition subtypes. Transl Res 2015; 166: 706–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aalto A, Huotari-Orava R, Luhtala S, Mäenpää J, Staff S.Expression of estrogen-related receptors in localized provoked vulvodynia. Biores Open Access 2020; 9: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eva LJ, MacLean AB, Reid WM, Rolfe KJ, Perrett CW.Estrogen receptor expression in vulvar vestibulitis syndrome. Am J Obstet Gynecol 2003; 189: 458–461. [DOI] [PubMed] [Google Scholar]
- 15.Johannesson U, Sahlin L, Masironi B, Hilliges M, Blomgren B, Rylander E, Bohm-Starke N.Steroid receptor expression and morphology in provoked vestibulodynia. Am J Obstet Gynecol 2008; 198: 311 e311–e316. [DOI] [PubMed] [Google Scholar]
- 16.Bohm-Starke N, Johannesson U, Hilliges M, Rylander E, Torebjork E.Decreased mechanical pain threshold in the vestibu'lair mucosa of women using oral contraceptives – a contributing factor in vulvar vestibulitis? J Reprod Med 2004; 49: 888–892. [PubMed] [Google Scholar]
- 17.Bouchard C, Brisson J, Fortier M, Morin C, Blanchette C.Use of oral contraceptive pills and vulvar vestibulitis: a case-control study. Am J Epidemiol 2002; 156: 254–261. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein A, Burrows L, Goldstein I.Can oral contraceptives cause vestibulodynia? J Sex Med 2010; 7: 1585–1587. [DOI] [PubMed] [Google Scholar]
- 19.Harlow BL, Vitonis AE, Stewart EG.Influence of oral contraceptive use on the risk of adult-onset vulvodynia. J Reprod Med 2008; 53: 102–110. [PubMed] [Google Scholar]
- 20.Griebling TL, Liao ZH, Smith PG.Systemic and topical hormone therapies reduce vaginal innervation density in postmenopausal women. Menopause 2012; 19: 630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornung R, Benton W, Tongkhuya S, Uphouse L, Averitt D.Protective role of progesterone against allodynia in rats with persistent temporomandibular joint inflammation. J Pain 2017; 18: S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labrie F, Martel C, Pelletier G.Is vulvovaginal atrophy due to a lack of both estrogens and androgens? Menopause 2017; 24: 452–461. [DOI] [PubMed] [Google Scholar]
- 23.Murina F, Barbieri S, Lubrano C, Cetin I.Vestibular mucosa thickness measured by ultrasound in patients affected by vestibulodynia: a case-control study. Sex Med 2021; 9: 100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coronel MF, Labombarda F, De Nicola AF, González SL.Progesterone reduces the expression of spinal cyclooxygenase-2 and inducible nitric oxide synthase and prevents allodynia in a rat model of central neuropathic pain. Eur J Pain 2014; 18: 348–359. [DOI] [PubMed] [Google Scholar]
- 25.Coronel MF, Labombarda F, Roig P, Villar MJ, De Nicola AF, González SL.Progesterone prevents nerve injury-induced allodynia and spinal NMDA receptor upregulation in rats. Pain Med 2011; 12: 1249–1261. [DOI] [PubMed] [Google Scholar]
- 26.Jarahi M, Sheibani V, Safakhah HA, Torkmandi H, Rashidy-Pour A.Effects of progesterone on neuropathic pain responses in an experimental animal model for peripheral neuropathy in the rat: a behavioral and electrophysiological study. Neuroscience 2014; 256: 403–411. [DOI] [PubMed] [Google Scholar]
- 27.Traish AM, Vignozzi L, Simon JA, Goldstein I, Kim NN.Role of androgens in female genitourinary tissue structure and function: implications in the genitourinary syndrome of menopause. Sex Med Rev 2018; 6: 558–571. [DOI] [PubMed] [Google Scholar]
- 28.Ivanisevic J, Thomas A.Metabolomics as a tool to understand pathophysiological processes. Methods Mol Biol 2018; 1730: 3–28. [DOI] [PubMed] [Google Scholar]
- 29.Kaddurah-Daouk R, Weinshilboum R, Pharmacometabolomics Research Network. Metabolomic signatures for drug response phenotypes: pharmacometabolomics enables precision medicine. Clin Pharmacol Ther 2015; 98: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wishart DS.Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov 2016; 15: 473–484. [DOI] [PubMed] [Google Scholar]
- 31.Wishart DS.Metabolomics for investigating physiological and pathophysiological processes. Physiol Rev 2019; 99: 1819–1875. [DOI] [PubMed] [Google Scholar]
- 32.Handelman SK, Romero R, Tarca AL, Pacora P, Ingram B, Maymon E, Chaiworapongsa T, Hassan SS, Erez O.The plasma metabolome of women in early pregnancy differs from that of non-pregnant women. Plos One 2019; 14: e0224682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson E, Reid G.Metabolomics as a clinical testing method for the diagnosis of vaginal dysbiosis. Am J Reprod Immunol 2018; 80: e12979. [DOI] [PubMed] [Google Scholar]
- 34.Borgogna JC, Shardell MD, Santori EK, Nelson TM, Rath JM, Glover ED, Ravel J, Gravitt PE, Yeoman CJ, Brotman RM.The vaginal metabolome and microbiota of cervical HPV-positive and HPV-negative women: a cross-sectional analysis. BJOG 2020; 127: 182–192. 2019/11/22. DOI: 10.1111/1471-0528.15981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Chaiworapongsa T, Gomez R, Nien JK, Yoon BH, Mazor M, Luo J, Banks D, Ryals J, Beecher C.Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J Matern-Fetal Neonatal Med 2010; 23: 1344–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polat IH, Marin S, Rios J, Larroya M, Sanchez-Garcia AB, Murillo C, Rueda C, Cascante M, Gratacos E, Cobo T.Exploratory and confirmatory analysis to investigate the presence of vaginal metabolome expression of microbial invasion of the amniotic cavity in women with preterm labor using high-performance liquid chromatography. Am J Obstet Gynecol 2021; 224: 90.e1–90.e9. [DOI] [PubMed] [Google Scholar]
- 37.Miller JS, Rodriguez-Saona L, Hackshaw KV.Metabolomics in central sensitivity syndromes. Metabolites 2020; 10: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teckchandani S, Nagana Gowda GA, Raftery D, Curatolo M.Metabolomics in chronic pain research. Eur J Pain 2021; 25: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tokarz J, Haid M, Cecil A, Prehn C, Artati A, Moller G, Adamski J.Endocrinology meets metabolomics: achievements, pitfalls, and challenges. Trends Endocrinol Metab 2017; 28: 705–721. [DOI] [PubMed] [Google Scholar]
- 40.Alappattu M, Robinson M, Lamvu G.Vulvodynia is not created equally: pain-related distress subgroups in vulvodynia. J Pain 2016; 17: S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen R, Brown C, Heiman I, Leiblum, Meston C, Shabsigh R, Ferguson D, D'Agostin Jr, R. The Femail Function Index (FSFI): a multidimensional self-report intrument for the assessment of female sexual funtion. J Sex Marital Therapy 2000; 26: 191–208. [DOI] [PubMed] [Google Scholar]
- 42.Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 2010; 53: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–980. [DOI] [PubMed] [Google Scholar]
- 44.Hancock GR, Klockars AJ. The quest for alpha: developments in multiple comparison procedures in The Quarter century since games (1971). Rev Educ Res 1996; 66: 269–306. [Google Scholar]
- 45.Rosenthal R, Rosnow RL, Rubin DB. Contrasts and effect sizes in behavioral research: a correlational approach. Cambridge, UK: Cambridge University Press, 2000. p. 212. [Google Scholar]
- 46.Stockstill K, Doyle TM, Yan X, Chen Z, Janes K, Little JW, Braden K, Lauro F, Giancotti LA, Harada CM, Yadav R, Xiao WH, Lionberger JM, Neumann WL, Bennett GJ, Weng HR, Spiegel S, Salvemini D. Dysregulation of sphingolipid metabolism contributes to bortezomib-induced neuropathic pain. J Exp Med 2018; 215: 1301–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang MD, Rahman M, Zhu D, Johansson IM, Backstrom T. 3Beta-hydroxysteroids and pregnenolone sulfate inhibit recombinant rat GABA(A) receptor through different channel property. Eur J Pharmacol 2007; 557: 124–131. [DOI] [PubMed] [Google Scholar]
- 48.Traish A, Bolanos J, Nair S, Saad F, Morgentaler A. Do androgens modulate the pathophysiological pathways of inflammation? Appraising the contemporary evidence. JCM 2018; 7: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hertel J, Konig J, Homuth G, Van der Auwera S, Wittfeld K, Pietzner M, Kacprowski T, Pfeiffer L, Kretschmer A, Waldenberger M, Kastenmller G, Artati A, Suhre K, Adamski J, Langner S, Volker U, Volzke H, Nauck M, Friedrich N, Grabe HJ. Evidence for stress-like alterations in the HPA-Axis in women taking oral contraceptives. Sci Rep-Uk 2017; 7: 14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-mpx-10.1177_17448069211041853 for Reduced concentrations of vaginal metabolites involved in steroid hormone biosynthesis are associated with increased vulvar vestibular pain and vaginal muscle tenderness in provoked vestibulodynia: An exploratory metabolomics study by Jennifer S Labus, Emeran A Mayer, Kjersti Aagaard, Jean Stains RN Katarzyna Broniowska and Andrea Rapkin in Molecular Pain