Abstract
Hearing is one of our most important means of communication. Disabling hearing loss (DHL) is a long-standing, unmet problem in medicine, and in many elderly people, it leads to social isolation, depression, and even dementia. Traditionally, major efforts to cure DHL have focused on hair cells (HCs). However, the auditory nerve is also important because it transmits electrical signals generated by HCs to the brainstem. Its function is critical for the success of cochlear implants as well as for future therapies for HC regeneration. Over the past two decades, cell transplantation has emerged as a promising therapeutic option for restoring lost auditory nerve function, and two independent studies on animal models show that cell transplantation can lead to functional recovery. In this article, we consider the approaches most likely to achieve success in the clinic. We conclude that the structure and biochemical integrity of the auditory nerve is critical and that it is important to preserve the remaining neural scaffold, and in particular the glial scar, for the functional integration of donor cells. To exploit the natural, autologous cell scaffold and to minimize the deleterious effects of surgery, donor cells can be placed relatively easily on the surface of the nerve endoscopically. In this context, the selection of donor cells is a critical issue. Nevertheless, there is now a very realistic possibility for clinical application of cell transplantation for several different types of hearing loss.
Keywords: auditory nerve, cell transplantation, glial scar, nerve regeneration, scaffold
Introduction
Over 450 million people suffer disabling hearing loss (DHL), equivalent to 6.1% of the world’s population (https://www.who.int/deafness/estimates/en/). Hearing loss affects our most important means of communication, and it may lead to social isolation, depression, and even dementia in the elderly1.
Traditionally, significant efforts to cure DHL have focused on hair cells (HCs). No less important, however, is the auditory nerve, which contains the sensory neurons that transmit electrical signals generated by HCs to the brainstem2,3.
Auditory nerve damage may occur as a result of various types of insult. These include internal causes, such as neuropathies and intracranial mass lesion, and head trauma, which is a representative external cause3. Over several decades, cell transplantation has emerged as a promising therapeutic option to rebuild lost auditory nerve function. Numerous studies in vitro and in vivo have explored different combinations of cells and delivery methods, and two successful studies have provided proof of principle that cell transplantation can lead to functional recovery. The challenge now is to focus on how the human auditory system can be approached in the clinic, including the selection of donor cells and how auditory nerve function can be restored with surgically acceptable techniques involving minimal intervention.
Degeneration Pattern of Auditory Neurons Following Insult
Insight into why cell transplantation works comes from the nature of tissue degeneration (Fig. 1). When auditory nerve axons are compromised, for example, in neuropathies, closed head injury, microsurgery (MiS), or radiation exposure in radiotherapy (RT) (see the following section), degeneration proceeds centripetally away from the soma, as in Wallerian or anterograde degeneration (Fig. 1B, ). Cochlear nucleus cells and upper neurons up to the cerebrum subsequently degenerate transneuronally4 (Fig. 1B, ). At the same time, retrograde axon degeneration proceeds toward the soma of spiral ganglion cells (SGCs) within the cochlea, leading eventually to the death of the neurons and loss of the peripheral processes (Fig. 1B, ). If auditory neurons degenerate, the HCs that they innervate can still survive. Auditory neurons express the tyrosine receptor kinase B (TrkB) and tyrosine kinase receptor C (TrkC) (Fig. 1A) for brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT-3), which are produced mainly by HCs3,5. Thus, damage to HCs can lead to degeneration of auditory neurons and transneuronal degeneration of the cochlear nucleus cells and upper relay neurons (Fig. 1, , )3. It is well known clinically that degeneration of the HCs is triggered by systemic use of pharmacological agents such as aminoglycoside antibiotics and platinum-based drugs6 and also exposure to intense noise7.
Figure 1.
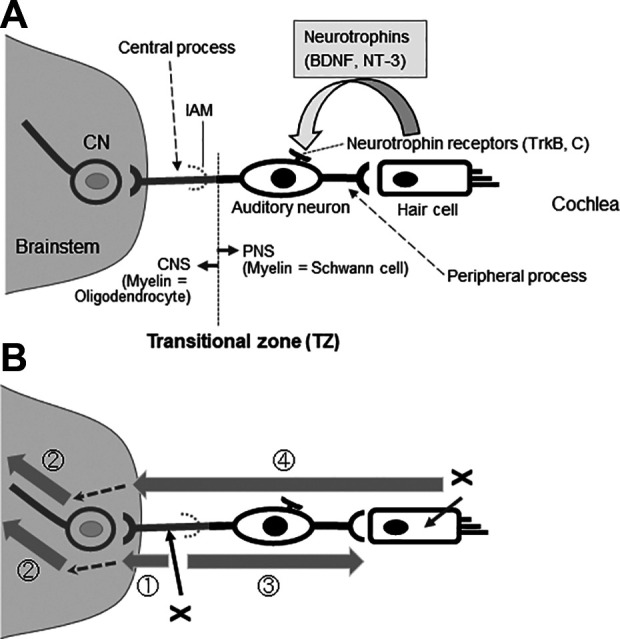
Auditory neurons and their degeneration patterns. (A) The auditory nerve is a bundle of bipolar auditory neurons. The peripheral processes of auditory neurons form synapses with HCs and the central processes with CNs in the brainstem. HCs provide much of the trophic support required for the maintenance and survival of auditory neurons, including BDNF and NT-3. Auditory neurons synthesize the high-affinity tyrosine receptor kinases, TrkB and TrkC. The interface between the PNS and CNS is called the TZ, which is distal to the IAM. Myelin sheaths are formed by oligodendrocytes centrally from the TZ, and the surrounding milieu is astrocytic. Peripheral to the TZ, the myelin sheaths are formed by Schwann cells that are enveloped in endoneurium. The interface is penetrated only by axons. (B) The onset of anterograde (Wallerian) (), trans-neuronal (), and retrograde degeneration () of the auditory nerve depends on the initial site of injury (x). In HC damage, neurodegeneration involves the auditory neuron entirely () and neurodegeneration proceeds to higher-level neurons including the CNs (). Shaded arrows indicate the progression of degeneration, and dotted arrows indicate transneuronal degeneration. BDNF, brain-derived neurotrophic factor; CNS, central nervous system; CNs, cochlear nucleus cells; HC, hair cells; IAM, internal auditory meatus; NT-3, neurotrophin 3; PNS, peripheral nervous system; TZ, transitional zone.
Hearing levels can deteriorate progressively in closed head injury patients8. Similarly, in MiS and RT for vestibular schwannoma (VS), the hearing preservation rates measured within a few years of treatment can be misleading because hearing loss that is unrelated to tumor recurrence continues to progress even after 7 to 8 years9–14 . Various mechanisms are responsible for such delayed hearing loss, but one contributing factor is likely to be the unusually slow speed of auditory nerve degeneration, which can be protracted for years15. There are several reasons for the slow degeneration of the auditory nerve. First, the soma of human SGCs contact each other and can provide mutual trophic support16. Second, non-myelinated Schwann cells (SCs) and satellite glial cells surrounding the soma prevent the SGCs from dying even after HCs are damaged17. Third, SGCs depend on neurotrophins provided mainly by HCs but supporting cells are also a source of neurotrophins18 even after the HCs degenerate. Cochlear implants (CIs) stimulate auditory neurons directly and they exploit the protracted course of auditory nerve degeneration15. Cell transplantation is more likely to succeed for the same reason because degenerated auditory neurons can be replenished progressively by donor cell-derived neurites that seem to regenerate over several months2.
Causes of Auditory Nerve Degeneration and Related Clinical Issues
Neuropathies
The classical description of auditory neuropathy (AN) is that auditory nerve function is impaired but outer HCs in the cochlea are functional19. In AN, speech comprehension is compromised although pure tone audiograms are disproportionately well maintained so patients can hear but cannot understand19. This type of hearing loss is observed in various diseases including a subset of neuropathic and presbycusis patients20,21. Nowadays, the causative sites for AN include not only the auditory nerve and outer HCs but also the inner HCs and inner HCs ribbon synapses (auditory synaptopathy)19. Nevertheless, AN due to auditory nerve dysfunction and auditory neuropathic hearing loss is a potential candidate for cell transplantation2,3,22. Some patients with genetic disorders have polyneuropathic disorders, such as auditory neuropathic hearing loss and optic neuropathy with bilateral blindness20, and their anguish would be alleviated remarkably even if only their hearing was restored. For patients with pathologies in both the auditory nerve and HCs, auditory nerve regeneration would most effectively be coupled with HC regeneration, should that eventually prove to be successful in mammals.
Tumors
VS develops from the vestibular nerve, but the surgical removal of VS inevitably imposes direct mechanical stress to the auditory nerve, potentially leading to the severance of continuity of auditory neurons or to the initiation of auditory nerve degeneration. VS surgery can also have far-reaching effects on the cochlea through the vasculature (the internal auditory artery or labyrinthine artery), leading to cochlear ischemia and reflow phenomena that are inevitably repeated during surgery, eventually leading to HC death. The latter presumption is supported by recordings of distortion product otoacoustic emissions (DPOAEs), which are sounds generated within the cochlea recorded by a microphone fitted into the ear canal23. The amplitude of DPOAEs reflects the blood flow to the cochlea24, and an intraoperative decrease in DPOAEs indicates cochlear ischemia due to mechanical pressure upon the vasculature25,26. Several minutes of cochlear ischemia are sufficient to cause morphological changes of the distal ends of the auditory neurons, and longer periods can cause cessation of internal auditory artery blood flow leading to HC death27,28. Postmortem histological examinations of VS patients without surgery reveal structural changes within the cochlea, including degeneration of HCs and the stria vascularis in the outer wall of the scala media29.
Radiation
Radiotherapy (RT) for the central nervous system (CNS) and peripheral nervous system (PNS) lesions incur multiple pathological processes, including vascular endothelial damage, neuroinflammation, genetic/epigenetic alterations, apoptosis/necrosis of neurons and glial cells, reactive gliosis, and demyelination and deterioration of stem cell and progenitor cell proliferation30. It is extremely difficult to avoid radiation injury to the auditory nerve in RT for VS31. To make matters worse, not only the cochleovestibular nerve but also the facial nerve and other vital structures, such as HCs and the stria vascularis32, are packed in a confined space of the cochlea (Fig. 2). The horizontal diameter of the internal auditory canal is only about 4.5 mm33. Within this narrow canal, the cochleovestibular and facial nerves are compressed by the tumor and take a tortuous course. Reports revealed that radiation doses in the cochlea and cochlear nucleus during RT are correlated with patients’ hearing outcome31,34, implying radiation injury to auditory neurons is responsible for hearing deterioration after RT in addition to that to HCs and the stria vascularis, both vital to hearing32. Patients with small VS in which auditory neurons degenerate but HCs are still functional35 are an ideal candidate for auditory nerve replacement3, and this is the case in a subset of presbycusis or auditory neuropathic patients as mentioned above20,21.
Figure 2.
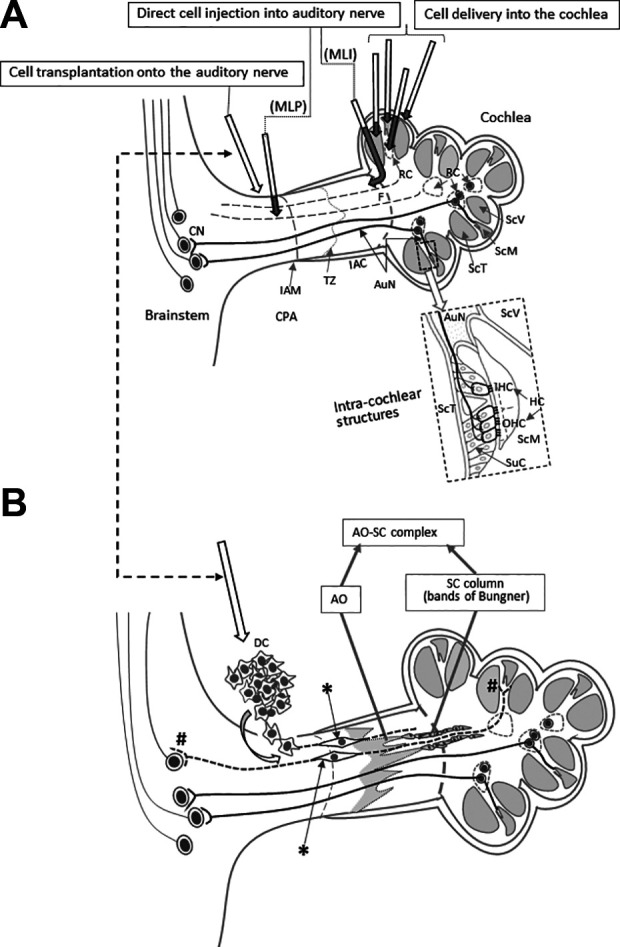
Reported cell delivery methods to restore AuN function. (A) Reported cell delivery methods in Table 1 are shown with arrows in the upper panel. Dark shaded parts of each arrow indicate intracochlear or intraneural portions of each route. Arrows show each route conceptually and do not trace each anatomical route precisely. The dotted rectangle is enlarged to illustrate intracochlear structures in detail. (B) Surface transplantation of DCs on degenerated AuN. DCs transplanted onto the surface of degenerated AuN autonomously enter the nerve, differentiate (*) and form functional synapses with HCs and CNs (#). In degenerated AuN, the AO and SC columns form a continuous, “naturally occurring autologous cell bridge”, the AO–SC complex (a part is shown here), which acts as an anatomical scaffold for DC migration to connect between the PNS and the CNS (see the text). Note regenerating axons run parallel with the AO–SC complex. Studies using systemic delivery of donor cells are not shown here. AO, astrocyte outgrowth; AuN, auditory nerve; CNS, central nervous system; CN, cochlear nucleus cell; CPA, cerebellopontine angle; DC, donor cells; HC, hair cell; IAC, internal auditory canal; IAM, internal auditory meatus; IHC, inner hair cell; OHC, outer hair cell; MLI, membranous labyrinth injured; MLP, membranous labyrinth preserved; PNS, peripheral nervous system; RC, Rosenthal’s canal; ScM, the scala media; SC, Schwann cell; ScT, the scala tympani; ScV, the scala vestibuli; SuC, supporting cell; TZ, the transitional zone.
Head Injury
In patients with a closed head injury even without temporal bone fractures, damage to auditory neurons is observed primarily and/or secondarily following HC damage36–42 . Clinically, cases in which the auditory nerve is damaged without damage to HCs are most suitable for cell transplantation because the HCs can provide trophic support, as mentioned above (Fig. 1). The auditory nerve is particularly vulnerable to external force in the regions of the fundus of the internal auditory canal and the transitional zone (TZ)36–41 . When the medial displacement of the brainstem is greater than that of the cochlea in the temporal bone, the resultant force on the auditory nerve may avulse the auditory neurons from the fundus of the internal auditory canal36–41 . The TZ is the interface between the CNS and PNS43, and it can be highlighted by immunostaining for glial fibrillary acidic protein (GFAP), which is expressed by astrocytes only in the CNS2 and it lies within the internal auditory canal44 (Fig. 3). The TZ is vulnerable to external stretch and shear forces probably because there is an abrupt anatomical change at this point; central to the TZ, myelin sheaths are formed by oligodendrocytes, and the supporting tissue is astrocytic, whilst peripheral to the TZ, the sheaths are SCs enveloped in endoneurium, although the axons are continuous3,39. HC damage in closed head injury may occur due to a breach of the sealing of the membranous labyrinth and/or impairment of blood supply to the cochlea42.
Figure 3.
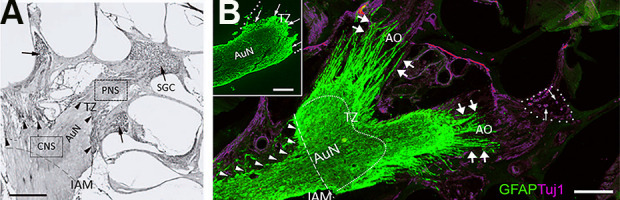
Transitional zone and the astrocyte outgrowth following auditory nerve mechanical compression. (A) Normal TZ. The CNS portion of the AuN extends peripherally, with a dome-like shape (arrowheads with dotted line). Rosenthal’s canals are densely packed with auditory SGC (arrows). Rat, Hematoxylin and Eosin stain, Scale bar, 200 µm. Cited from Sekiya et al. (2007) with publisher’s permission. (B) Gliotic AuN after compression. A glial scar is induced following mechanical compression applied to the CNS portion of the auditory nerve in the cerebellopontine angle (arrowheads). Marked AO is indicated by double arrows. Most auditory SGCs degenerate following sustained compression (single arrows in dotted circle, Rosenthal’s canal). With GFAP antibody, an astrocyte marker, the glial scar is also stained because it contains many reactive astrocytes. An antibody Tuj1 against beta-tubulin stains neurons and neurites, including SGCs. The curved dotted line indicates the default position of the TZ. (Inset) Normal rat AuN. The TZ (arrows) is clearly observed as a peripherally convex, dome-like shape. The PNS portion of the nerve (outlined by dotted line) is GFAP-negative because astrocytes exist only in the CNS. Scale bars, 200 μm. AO, astrocyte outgrowth; AuN, auditory nerve; CNS, central nervous system; GFAP, glial fibrillary acidic protein; IAM, internal auditory meatus; PNS, peripheral nervous system; SGCs, spiral ganglion cells; TZ, transitional zone.
Cochlear Implants (CIs)
Cell transplantation could potentially enhance the performance and candidature for CI in patients, who generally have too few remaining functional auditory neurons45,46. In fact, the minimum number of functional auditory neurons needed for the successful performance of a CI is astonishingly few and estimated to be 5% to 10% of the normal number15. CIs are beneficial to neurofibromatosis type 2 (NF2) patients with bilateral VS47, but a significant number of patients experience a decline in performance as the VS grows48. Hence, replenishing auditory neurons would potentially benefit NF2 patients.
Surgical Options for Cell Delivery
There is extensive literature on cell transplantation to the auditory system, but in this context, the relevant studies are those done in vivo on deafened animals and on deaf humans (Table 1). To establish proof of principle, two main conditions must be met in the analysis of cell integration and functional recovery49. First, there must be an electrophysiological analysis of the restoration of nerve function with an objective method such as the auditory brainstem response (ABR). Second, to link recovery to the transplanted cells, it is important to provide morphological evidence for synaptic connections, not only with HCs in the cochlea but also with neurons of the cochlear nuclei within the brainstem. In other parts of the nervous system, functional improvements have been recorded without morphological integration of the donor cells50–52 by indirect mechanisms, including trophic effects, immunomodulation, and other bystander effects53–55 .
Table 1.
In vivo studies to restore auditory nerve function.
| Study | Site of donor cell delivery | Host animal Deafening procedure |
Verification of functional restoration and synaptogenesis | Donor cell* |
|---|---|---|---|---|
| Cell delivery into the cochlea | ||||
| Hu et al. (2004)79 | ScT (MLI) | Rat Pharmacol, local# |
No | Mouse DRGC |
| Hu et al. (2005)78 | ditto | Guinea pig Pharmacol, local |
No | Mouse ESC and DRGC |
| Hu et al. (2005)80 | ditto | Guinea pig Pharmacol, local |
No | Mouse NSC |
| Coleman et al. (2006)71 | ditto | Guinea pig Pharmacol, systemic** |
No | Mouse ESC |
| Matsuoka et al. (2007)86 | ditto | Gerbil Pharmacol, local |
No | Mouse MSC |
| Parker et al. (2007)87 | ditto | Mouse/guinea pig Sound exposure |
No | Mouse NSC |
| Altschuler et al. (2008)68 | ditto | Guinea pig Pharmacol, systemic |
No | Mouse ESC |
| Lang et al. (2008)85 | ditto | Gerbil Pharmacol, local |
No | Mouse ESC |
| Hu et al. (2009)81 | ditto | Guinea pig Pharmacol, systemic |
No | Mouse DRGC |
| Cho et al. (2011)70 | ditto | Guinea pig Pharmacol, local |
No | Human MSC |
| Pettingill et al. (2011)88 | ditto | Guinea pig Pharmacol, systemic |
No | Schwann cells |
| Warnecke et al. (2012)90 | ditto | Guinea pig Pharmacol, systemic |
No | BDNF-secreting cells |
| He et al. (2014)76 | ditto | Guinea pig Pharmacol, local |
No | Mouse NSC |
| Jang et al. (2015)84 | ditto | Guinea pig Pharmacol, local |
No | Human MSC |
| Fetoni et al. (2014)73 | ditto | Guinea pig Noise exposure |
No | Guinea pig ADSC |
| Gillespie et al. (2015)74 | ditto | Guinea pig Pharmacol, systemic |
No | BDNF-expressing fibroblast |
| Jang et al. (2016)83 | ditto | Guinea pig Pharmacol, local |
No | Human ADSC |
| Xu et al. (2016)92 | ditto | Rat Noise exposure |
No | olfactory epithelium NSC |
| Dai et al. (2016)72 | ditto | Rat Pharmacol, systemic |
No | Rat OEC |
| Wise et al. (2016)91 | ditto | Guinea pig Pharmacol, systemic |
No | Human ESC |
| Chen et al. (2017)69 | ditto | Mouse Pharmacol, systemic |
No | Mouse iPSC |
| Schendzielorz et al. (2017)89 | ditto | Guinea pig Pharmacol, local |
No | Guinea pig ADSC |
| Huang et al. (2019)82 | ditto | Gerbil Pharmacol, local |
No | Mouse NSC |
| Hildebrand et al. (2005)77 | ScM (MLI) | Guinea pig Pharmacol, systemic |
No | Mouse ESC |
| Hu et al. (2005)80 | ditto | Guinea pig Pharmacol, local |
No | Mouse ESC mouse DRGC |
| Lang et al. (2008)85 | ScM, RC (MLI) | Gerbil Pharmacol, local |
No | Mouse ESC |
| Ahn et al. (2008)67 | PSCC (MLI) | Mouse Pharmacol, systemic |
No | Mouse ESC |
| Zhang et al. (2013)65,66 | Cochlea wall (MLP) | Rat Pharmacol, local |
No | Mouse NSC |
| Hackelberg et al. (2017)75 | Scaffold in IAC (MLI) | Guinea pit Pharmacol, local |
No | Human ESC |
| Direct cell injection into auditory nerve (MLI) | ||||
| Tamura et al. (2004)101 | AuN | Mouse Pharmacol, local |
No | Mouse NSC |
| Naito et al. (2004)95 | ditto | Chinchilla Pharmacol, systemic |
No | Bone marrow cell |
| Hu et al. (2004)94 | ditto | Rat Transected AuN |
No | Mouse DRGC, ESC |
| Okano et al. (2005)97 | ditto | Guinea pig Pharmacol, systemic |
No | Mouse ESC |
| Regala et al. (2005)98 | ditto | Guinea pig, rat Pharmacol, systemic |
No | Mouse DRGC |
| Corrales et al. (2006)93 | ditto | Gerbil Pharmacol, local |
No | Mouse ESC |
| Matsuoka et al. (2007)86 | ditto | Gerbil Pharmacol, local |
No | Mouse MSC |
| Shi et al. (2007)100 | ditto | Gerbil Pharmacol, local |
No | Human ESC |
| Altschuler et al. (2008)68 | ditto | Guinea pig Pharmacol, systemic |
No | Mouse ESC |
| Reyes et al. (2008)99 | ditto | Guinea pig Pharmacol, systemic |
No | Mouse ESC |
| Ogita et al. (2010)96 | ditto | Guinea pig Pharmacol, local |
No | Guinea pig MSC-derived spheres |
| Chen et al. (2012)22 | ditto | Gerbil Pharmacol, local |
Yes | Human ESC |
| Direct cell injection into the auditory nerve (MLP) | ||||
| Sekiya et al. (2006)107 | AuN | Rat Compression of AuN | No | Mouse ESC |
| Sekiya et al. (2007)106 | ditto | ditto | No | Mouse auditory neuroblast |
| Palmgren et al. (2012)105 | ditto | Rat Pharmacol, local |
No | Mouse ESC |
| Jiao et al. (2014)104 | ditto | Rat Pharmacol, local |
No | Human neural precursors |
| Chen et al. (2019)103 | ditto | Mouse Pharmacol, local |
No | Human limbus-derived MSC |
| Cell transplantation onto the auditory nerve (MLP) | ||||
| Sekiya et al. (2015)2 | AuN |
Rat Compression of AuN |
Yes | Mouse auditory neuroblast |
| Systemic delivery (MLP) | ||||
| Revoltella et al. (2008)63 | i.v. | Mouse Pharmacol, systemic Noise |
No | Human cord blood stem cells |
| Choi et al. (2012)58 | i.v. | Rat Noise Pharmacol, local |
No | Human MSC |
| Choi et al. (2012)57 | i.v. | Guinea pig Pharmacol, local |
No | Human blood MSC |
| Yoo et al. (2015)64 | i.v. | Autoimmune hearing loss mouse | No | Human ADSC |
| Lang et al. (2016)60 | i.v. | Mouse Pharmacol, local |
No | Mouse and human blood cell |
| Kil et al. (2016)59 | i.v. | Guinea pig Pharmacol, local |
No | MSC from human placenta |
| Ma et al. (2016)62 | i.t. | Congenital deaf albino pig | No | Human umbilical cord MSC |
| Lee et al. (2018)61 | i.v. | Human cases | No | MSC |
| Abd El Raouf et al. (2019)56 | i.v. | Guinea pig Pharmacol, systemic |
No | Guinea pig Harderian gland stem cells |
ABR, auditory brainstem responses; ADSC, adipose tissue-derived stem cell; AuN, auditory nerve; BDNF, brain-derived neurotrophic factor; CPA, cerebellopontine angle; DPOAE, distortion product otoacoustic emissions; DRGC, dorsal root ganglion cell; ESC, embryonic stem cell; IAM, internal auditory meatus; iPSC, induced pluripotent stem cell; i.t., intrathecal injection; i.v., intravenous injection; MLI, membranous labyrinth injured; MLP, membranous labyrinth preserved; MSC, mesenchymal stem cell; NSC, neural stem cell; OEC, olfactory ensheathing cell; RC, Rosenthal’s canal; Ref, reference number; ScM, the scala media; ScT, the scala tympani; ScV, the scala vestibuli.
* “Donor cell” indicates the provenance of donor cell. Donor cells may have been preconditioned in vitro before transplantation. For example, application of neural induction for ESC.
# “Pharmacol, local” indicates that pharmacological agents were applied locally to the auditory system. For example, ouabain applied to the round window of the cochlea.
** “Pharmacol, systemic” indicates application intravenously. For example, ototoxic antibiotics such as kanamycin injected in the tail vein of the host.
Note: Studies using more than one cell delivery routes are repeatedly listed in Table 1. The references in the text and table are listed basically in chronological order.
Equally important from the experimental aspect are clinical relevance, which is reflected in the animal model used to replicate human clinical pathology, and clinical feasibility, which relates to whether or not the surgical techniques can be used in the clinic. In the following sections, we consider a number of in vivo studies in these terms. We focus on local delivery of cells to parts of the inner ear because trials with systemic cell delivery have not led to successful migration of donor cells to the auditory system56–64 (Table 1).
Cell Delivery into the Cochlea with Injury to the Membranous Labyrinth
The soma of spiral ganglion neurons (SGNs) are located within the cochlea, and it is worth knowing whether or not cells delivered into the cochlear fluid spaces or cochlear wall are able to find their way into Rosenthal’s canal in which the SGN soma are housed (Fig. 2A). This does not seem to be the case, and none of the relevant studies have led to functional recovery (Table 1). With the exception of two studies65,66, the membrane that seals intracochlear fluid-containing spaces (the membranous labyrinth = the scala tympani, scala vestibuli, the scala media, and posterior semicircular canal) was breached and/or trespassed (membranous labyrinth injured [MLI])67–92 (Table 1). Importantly, invasion into the membranous labyrinth is clinically unacceptable because it leads to hearing loss3. Furthermore, the cochlea in small experimental animals is easily accessible as it is conspicuous within the hollow dome-like bulla, but it is not as accessible in humans as it is deeply buried in the temporal bone. Thus, this method is not suitable for human patients.
Direct Cell Injection Into Auditory Nerve With Injury to the Membranous Labyrinth
For targeting donor cells to the auditory nerve, this method seems to be more dependable22,68,86,93–101 . Unlike injection into the cochlear fluids, the cells are located into the appropriate neural tract with morphological continuity with the relevant target cells. Nevertheless, the membranous labyrinth is injured as with direct injection into the cochlea. Moreover, intraneural injection with a syringe needle can damage the morbid, fragile auditory nerve and trigger an inflammatory reaction along with reactive gliosis around the needle and transplant102 (see the following sections for further discussions). This method has proved successful in one study22 and offers important proof of principle for clinical translation, especially from the viewpoint of donor cell selection (Table 1; Fig. 2A). However, it remains possible that leakage of the donor cells outside the cochlea might have played a predominant role (see below for the details) in addition to indirect bystander effects that could account for the observed improvement of the ABRs.
Direct Cell Injection Into or Onto Auditory Nerve With Preservation of the Membranous Labyrinth
Even without damaging cochlear structures, cell injection into the cerebellopontine angle portion of the auditory nerve trunk through a hole posterior to the mastoid process (the retromastoid region) has not restored auditory nerve function103–107 .
Surprisingly, functional restoration was observed if donor cells were simply placed onto the surface of the auditory nerve via the retromastoid route, thus preserving the integrity of both the nerve and the membranous labyrinth (membranous labyrinth preserved [MLP])2 (Fig. 2A, B) (Table 1). This “surface transplantation” method can be regarded as a more promising option for cell transplantation, and it is, thus, considered in more detail in the following sections.
What is the Nature of Nerve injury and Degeneration and How Could Cell Transplantation Work for Specific Clinical Conditions?
The success of in vivo experiments with animal models is encouraging, but it is important to understand the biology that underlies the pathology of nerve degeneration and the subsequent structural and biochemical environment that underlies the successful integration of transplanted cells. This not only informs the optimal technique for cell delivery but also the selection and possibly the conditioning of donor cells.
Structural and Biochemical Cues for Cell Transplantation
Protective Addition
In principle, regenerative medicine should add new functional elements without causing further damage, following the principle of “protective addition”. This principle is most effectively met for the auditory nerve by placing donor cells on the tissue surface2. As discussed above, all other delivery techniques involve significant tissue damage.
The Scaffold
The scaffold is an indispensable element for the formation of the nervous system. For example, radial glia plays a crucial role as the scaffold for cell migration from the ventricular zone toward the brain surface108,109. Hence, various artificial scaffolds such as collagen-rich acellular matrices and matrices such as hydrogel with in vitro expanded donor cells attached have been intensively investigated in many neurodegenerative disorders, including spinal cord injury (SCI) with efforts to overcome various obstacles including provocation of host immune responses110–114 . Currently, another practical issue to be solved aiming at clinical translation is the surgical maneuverability of artificial materials in the delicate and confined space of the CNS.
The Scaffold Within: A Natural Autologous cell Scaffold
One of two successful studies was serendipitous but demonstrated that an autologous cell scaffold had been spontaneously formed in collaboration with SCs during the progression of auditory nerve degeneration. This naturally occurring autologous cell scaffold plays key roles in cell integration as described below. A number of donor cells incidentally spilled onto the nerve surface from a hole through which a syringe needle had been inserted for traditional intra-neural injection of donor cells. These “leaked” donor cells autonomously entered the nerve, gradually transformed into the bipolar shape characteristic of auditory SGCs in the nerve, and finally formed synapses with target HCs and cochlear nucleus cells2 (Fig. 2B and 4). Intriguingly, donor cell migration and axon elongation apparently recapitulated processes observed during development. These processes include glia-guided migration115 and migration within GFAP-positive astrocytic, tube-like structures in the rostral migratory stream116,117. Even residual neurons appeared to be used as a migration guide118 (Fig. 4D).
Figure 4.
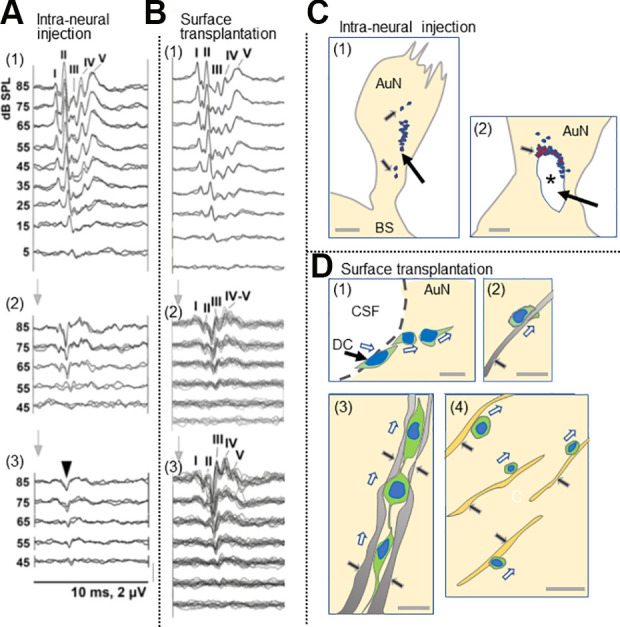
Comparison between intraneural and surface transplantation of cells. (A) Intraneural transplantation of DCs. ABRs before compression (1), 5 weeks after compression before cell transplantation (2), and 3 months after (3). Arrowhead in panel 3, monophasic positive potential indicating electrical failure of nerve impulse transmission. I–V, ABR wave I–V. (B) Surface transplantation of donor cells. ABRs before compression (1), 5 weeks after compression (2), and 3 months after surface transplantation (3). Note a significant improvement of ABRs 3 months after surface transplantation (see Sekiya et al., 2015 for more details). (C) Schematic drawing of fate of intraneurally injected cells. (1) Cell debris mainly in the site of cell transplantation (large arrow), and a few cells are seemingly stuck in the gliotic auditory nerve tissue (small arrows). (2) Large arrow indicates cavity formation (asterisk) in the nerve due to infusion pressure during injection and the infused cell mass. Small arrow indicates cell debris around the cavity. (see Sekiya et al., 2015 for original images). Scale bars: (1), 200 μm; (2) 50 μm. (D) Schematic drawing of various modes of cell migration of donor cells transplanted on the surface of the auditory nerve (see Sekiya et al., 2015 for original images). (1) The DCs autonomously enter the AuN in a chain formation (hollow arrows). CSF, cerebrospinal fluid in the cerebellopontine angle subarachnoid space. (2) Within a gliotic auditory nerve, a transplanted cell is intimately associated with a GFAP+ process (black arrow) derived from the glial scar and migrated (hollow arrow). (3) three migrating donor cells (hollow arrows) form chains within GFAP+ sheaths (2 pairs of black arrows). (4) Migrating transplanted cells (hollow arrows) associated with neurons (black arrow), possibly for guidance. Scale bars: (1, 2, 4), 20 μm; (3) 10 μm. Cited from Sekiya et al. (2015) with publisher’s permission. ABR, auditory evoked brainstem responses; AuN, auditory nerve; BS, brainstem; CSF, cerebrospinal fluid; DCs, donor cells; GFAP, glial fibrillary acidic protein.
Natural Autologous Cell Scaffold—the Astrocyte Outgrowth and SCs Form a Bridge Between the CNS and PNS
When neurons in the CNS die, astrocytes react to form the glial scar (the astrocyte scar), irrespective of the cause, which may be ischemia, mechanical trauma, irradiation, or genetic disorder119–122 . In mouse SCI, reactive astrocytes of the glial scar form characteristic elongated and overlapping processes at the periphery of the lesion core about a week after the insult123. In a rat stroke model, reactive astrocyte processes were apparently longer than in sham rats in the penumbra even 30 days after ischemic and hemorrhagic stroke124. After a stab lesion in the cerebral cortex of mice, one subset of astrocytes directed their processes toward the lesion125. After injection of iron into mice, reactive astrocytes around the lesion core extended long and overlapped processes126.
Similarly, at the cranial and peripheral nerve roots, such elongated processes of reactive astrocytes are observed as a conspicuous tongue-like protrusion toward the periphery, the astrocyte outgrowth (the AO) (Fig. 3). One clinical study demonstrated that auditory nerve specimens taken during VS surgery were gliotic, indicating that reactive astrocytes had invaded the auditory nerve127. Other than damaged auditory nerve, the AO has been reported in a plethora of diseases in which motor and sensory neurons die, including amyotrophic lateral sclerosis128–133 . Electron microscopy shows that the AO comprises processes of reactive astrocytes of the glial scar, which have been known as “glial bundles”, extending from the spinal cord/brainstem128,130,133. It should be noted that the polarity of the AO plays pivotal roles in cell migration and axon elongation134.
Normally, astrocytes in the CNS and SCs in the PNS are apart and mutually exclusive but their mutual repulsion decreases following motor and sensory neuron death in the brainstem/spinal cord135. As a result, the distal tip of the AO extensively apposes with SCs or is directly wrapped by SC cytoplasm within a common basal lamina133,135. Distally, SCs form structures called SC columns or bands of Bungner that can guide regenerating axons back to their targets136. Thus, a continuous structure, the AO–SC complex, forms autonomously and can act as an anatomical bridging scaffold connecting the CNS and the PNS137 (Fig. 2B). In fact, in one study on the auditory nerve, the AO–SC complex appeared to be the only continuous scaffold between the PNS and CNS2.
Furthermore, upon injury, both astrocytes and SCs become rich sources of pro-regenerative molecules, including laminin, N-cadherin neural cell adhesion molecule, nerve growth factor, BDNF, NT-3, and fibroblast growth factor, glial cell line-derived neurotrophic factor, artemin, and vascular endothelial growth factor136,138,139.
Intraneural Injection and Surface Transplantation
It is difficult to compare the different cell transplantation experiments in the auditory system because the donor cells, surgical techniques, and animal models are so varied. However, when intraneural injection and surface transplantation were compared under the same parameters2, surface transplantation was clearly more successful. There was no ABR improvement with intraneural transplantation, and the transmission of electrical activity failed to pass the transplantation site140 (Fig. 4A). Morphological examination revealed a failure of cell migration with cell debris mainly at the site of cell transplantation with a few cells stuck in the midst of the gliotic auditory nerve (Fig. 4C,1). Another finding was cavity formation in the nerve, apparently due to infusion pressure during injection and the large volume of the infused cell mass that might also have damaged residual host neurons and vascular networks (Fig. 4C, 2). In contrast, the animals in which cells were delivered by surface transplantation demonstrated statistically significant improvement of the ABRs measured 3 months after cell transplantation (Fig. 4B). Morphologically, various modes of cell migration were observed as mentioned above (Fig. 4D, 1–4), and synaptic connections with HCs and the cochlear nucleus cells were morphologically confirmed (refer to ref. 2 for the original images).
The Glial Scar, is it Friend or foe?
Emerging evidence challenges the traditional belief that the glial scar is a physical and molecular barrier to neural regeneration141–143 . An in vivo experimental study on SCI showed that regenerating axons skirted around the surface of the glial scar144, indicating that they can negotiate its surface and benefit from the structural and chemical cues that it contains. In unilateral cerebral stroke of the motor cortex in mouse, axons of the contralesional corticospinal tract normally sprout into the denervated spinal cord and contribute to motor functional recovery. In a double knockout of GFAP and vimentin (the principal genes responsible for glial scar formation), corticospinal axons only rarely crossed the midline and the reduced astrocytic reactivity led to impaired neurological recovery142. Another study showed that scar-forming reactive astrocytes do not only have a protective function but also promote axonal regrowth after SCI. In two different transgenic mouse models to either prevent or inhibit glial scar formation, the study showed that there is a failure in axonal regrowth following removal of reactive astrocytes in both acute and chronic glial scars141. Reactive astrocytes in cerebral infarct play a crucial source of a pro-regenerative molecule, the stromal cell-derived factor 1 (SDF-1)145. Blocking SDF-1 action with a neutralizing antibody against a receptor for SDF-1, CXC chemokine receptor 4 (CXCR4), strongly attenuated progenitor migration146, indicating that SDF-1/CXCR4 promotes migration of stem/progenitor cells toward the lesion147.
Pro- and Anti-Regenerative Astrocytes
Astrocytes are not homogenous and are composed of at least five distinct subpopulations, although it is not clear how each subpopulation responds to different insults in different locations148–150 . Astrocytes not only conform to different environmental niches but also show different transcriptional changes induced by different types of injuries149,151. In a non-penetrating lateral fluid percussion brain injury model in adult rats, the morphology of reactive astrocytes is regionally distinct; those in the injured cortex, subcortical white matter tracts, and CA3 region of the hippocampus show a distinct morphology with an enlarged cell body and long intertwined processes, but those in the thalamic nuclei have thicker shorter processes152. Following experimental occlusion of the middle cerebral artery, reactive “A2” astrocytes are likely to be protective as they lead to increased expression of neurotrophic factors and cytokines, transferring mitochondria to injured neurons143,148,153. In contrast, neuroinflammation with systemic endotoxin lipopolysaccharide injection induces neurotoxic “A1” astrocytes143,148,153. A recent study reported that such molecular and functional diversity of astrocytes in the healthy adult brain depends on cues from neurons through neuron-derived sonic hedgehog (Shh)154. This is also another example of glia–neuron interaction (see above).
Thus, it is more likely that there are pro- and anti-regenerative reactive astrocytes, and further research is required to identify those subsets of reactive astrocytes that can aid and contribute to axon elongation efficiently for auditory nerve regeneration.
What is the Ideal Animal Experimental Model?
Studies of the auditory nerve require animal models in which the auditory nerve is selectively, quantifiably, and reproducibly damaged without confounding factors such as concomitant HC damage.
In pharmacological models, to induce auditory nerve degeneration, ouabain is most commonly used22,57,59,60,65,66,70,75,76,82,85,86,93,96,100,103. However, with this approach, SGNs are hard to damage reproducibly to avoiding “sudden and all-or-none type cell death”. It is technically difficult to titrate the dose, so ouabain treatments destroy nearly all SGNs in most of the studies60,85,86,93,96,155–158 . This makes it hard to assess any further damage that may occur through surgical intervention.
Instead, clinically relevant animal experimental models of neurodegenerative disorders, including hearing loss, should ideally involve a reproducible, “intermediate degree” of stable injury to reflect the gradual progression of tissue degeneration and a suitable opportunity to systematically test potential therapies. In fact, this critical issue has long been discussed when creating animal models of SCI159.
Ouabain is usually applied to the round window in the middle ear. It enters the cochlea across the round window membrane and is diluted in the perilymph of the scala tympani before reaching the SGN through Schuknecht’s canaliculae perforantes3,160. Pharmacological agents, including ouabain, that are applied even locally to the cochlea generally diffuse throughout the cochlear fluid space in an uncontrolled manner and tend to affect not only auditory neurons but also HCs158,161. Moreover, the effect of ouabain is different between species; ouabain selectively destroys SGNs in gerbils and mice, whereas in guinea pigs, it preferentially damages HCs158. In rats, if high doses applied to the round window are not sufficiently diluted, then both HCs and SGN can be damaged158.
Ouabain is a potent inhibitor of the ubiquitous Na+-K+ pump162, which maintains a low Na+ and high K+ concentration within most cells to ensure their excitability and to provide the driving force for the transport of glucose, amino acids, and other nutrients into the cell162,163. Thus, a caveat with ouabain is that it may affect not only neurons but also cells in the surrounding epithelial, connective, and muscle tissues. This also applies in systemic administration of pharmacological agents164. Thus, the majority of animal models are not ideal for clinical translation.
In contrast, if mechanical compression is applied to the CNS portion of the auditory nerve, it can produce selective, “intermediate” degree of degeneration of auditory neurons with HCs preserved2,165,166 (Fig. 1B, , 4A, B). This leads to transneuronal death of CNS cells (cochlear nucleus cells) and formation of a protruded bundle of reactive astrocytes of the glial scar (the AO), which plays a crucial role with distal Schwann cell columns for auditory nerve regeneration as elucidated above (Figs. 2B, 3B, 4). Unique to this model, the transneuronal degeneration of cochlear nucleus cells (Fig. 1B, ) can be quantitatively analyzed165,167,168. Mechanical compression is thus likely to be the most realistic model for the clinical conditions that lead to auditory nerve degeneration.
Donor Cells for Auditory Nerve Regeneration
Cell Source
Selection and preparation of donor cells are not the focus of this review, but they are critical issues because the cells must be competent to respond to regenerative cues within the damaged tissue.
As depicted in Table 1, embryonic stem cells (ESC) and neural stem cells (NSC) were most frequently used as xenografts or allografts after preconditioning in vitro with diverse factors such as bFGF, BMP4, and the bHLH transcription factor neurogenin 268,80,93,100,169. These approaches carry a greater risk of immune rejection compared with autologous transplantation170. Even using autologous-induced pluripotent stem cells (iPSCs) as donor cells, immune rejection can be an issue170–172 , despite major histocompatibility complex matching173. Furthermore, the phenotypes of individual iPSCs are not entirely predictable, and preconditioning can be complex, time-consuming, and expensive174–177 .
Human cells, particularly autologous human cells, are the most likely candidates for clinical translation and those derived from mesenchymal stem cells and adipose tissue-derived stem cells are being studied intensively as donor cells in human disease178–184 . Tissue-specific autologous stem cells are naturally strong candidates because they are more closely adapted to the host environment185,186. The human inner ear contains endogenous adult stem cells, as has been shown in other organs187,188,189, although their potential at the clinical level is not yet clear.
Bipolarity, a Key Requisite as Donor Cells for Auditory Nerve Regeneration
Auditory neurons are bipolar, and donor cells must connect both peripherally with HCs and centrally with cochlear nucleus cells. Table 1 shows that functional recovery of the auditory nerve has been achieved only in two studies, one with human ESCs22 and the other with a mouse cell line2. In both cases, the donor cells adopted a bipolar phenotype2,22. The ESCs were conditioned as otic progenitors by simulating the initial, developmental specification of the otic placode with sequential application of selected factors, including NT-3, BDNF, bFGF, and Shh22. The mouse cells were from a conditionally immortal mouse otic neuroblast cell line, US/VOT-N33, derived from a mouse otocyst (inner ear anlage)190. They show that ontogenetic-stage/region-restricted precursors can be successfully integrated into the host tissue, which has also been shown in a study of retinal regeneration191.
Ultimately, the selection of appropriate donor cells must be made in the context of the animal model most closely allied to the clinical application. It cannot be assumed that a given cell type would be equally effective with both intraneural injection and cell surface delivery because the biochemical cues encountered from the damaged tissue may be different. Thus, there is a need for more systematic research with a number of potential donor cell types in carefully controlled animal models. This is recognized more generally in cell transplantation to address a number of technical hurdles, not least those of phenotype instability, cost versus benefit, and ethical issues170,183.
A Minimally Invasive Technique for Cell Transplantation
Endoscopic surface transplantation
Minimal invasiveness of cell delivery is an indispensable requisite for clinical translation192. Endoscopy may fulfill this requirement most efficiently (Fig. 5). It has long been used in clinical otorhinolaryngology, has been reported in neurosurgical procedures since the 1970s193,194, and its safe maneuverability in the CPA has been established195,196.
Figure 5.
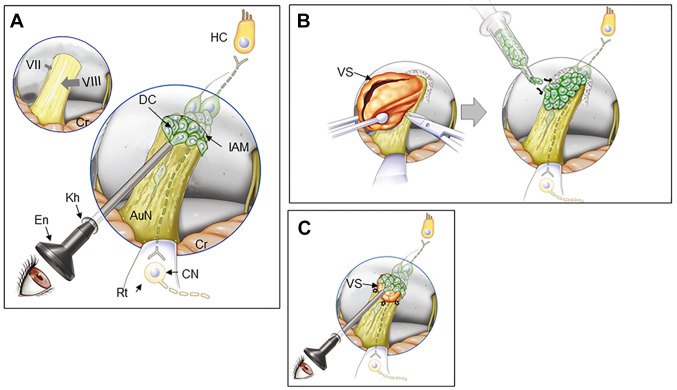
Endoscopic surface transplantation of donor cells. (A) In patients with non-tumorous auditory neuropathic hearing loss, an endoscope can be introduced in the cerebellopontine angle cistern and DCs placed onto the AuN. Normal facial nerve (VII) and vestibulocochlear nerve (VIII) are shown in the left upper corner of the panel. (B) In open surgery for larger VS, following tumor removal (left), donor cells can be placed on the surface of the AuN. The posterior wall of the internal auditory meatus is drilled to expose the tumor entirely. (C) In radiotherapy for small to medium-sized VS (arrows), a similar approach shown in A can be undertaken immediately after treatment. DCs are placed both on the distal side of the tumor through the internal auditory meatus (shown in this figure) and on its medial side if possible (not shown here). The dotted line indicates tumor shrinkage after radiotherapy. Regenerated bipolar neurons (dotted line) are shown in the nerve in each panel. AuN, auditory nerve; CN, cochlear nucleus cells; Cr, cerebellum; DC, donor cell; En, endoscope; HC, hair cell; IAM, internal auditory meatus; kh, keyhole; Rt, retractor; VS, vestibular schwannoma.
Surface transplantation of donor cells to the auditory nerve can be done with an endoscope introduced intracranially through a single keyhole in the retromastoid area. It could be applied to diseases such as auditory neuropathic hearing loss in neuropathies and head trauma (Fig. 5A), VS immediately after tumor removal (Fig. 5B), and VS following RT (Fig. 5C). This simple procedure requires only local anesthesia under sedation, and it is, thus, applicable to physically more sensitive patients, including the elderly.
Surface transplantation has several other advantages. Excessive numbers of cells are not required to compensate for the very high rates of donor cell death as observed in intraparenchymal injection197 because donor cells apparently autonomously enter the host tissue in proportion to the demand and capacity of the host environment2. Moreover, in contrast to intraneural injection, donor cells transplanted onto the nerve are immediately nourished by cerebrospinal fluid, which is a very rich source of nutrients including proteins, ions, lipids, hormones, cholesterol, glucose and metabolites, and pro-regenerative molecules such as BDNF and IGF-2198,199, before they establish a link to the blood supply. In transplantation experiments of Parkinson’s disease, most dopamine neurons injected into the brain died due to apoptosis within the first 24 h of transplantation200, and subsequently, more than 90% of transplanted neurons died by the end of a typical several week transplantation study201,202. In rats, more than 1 week is required after transplantation before sufficient neovascularization is established between the host and transplants203,204. Until then, the intraparenchymally transplanted cells suffer insufficient nutrients diffusing from host vessels located outside the graft perimeter, resulting in apoptotic cell death (see the sections above).
It is worth noting that “surface transplantation” of donor cells is distinct from “stem cell sheet technology” such as that explored in heart, kidney, and liver disorders205,206. In surface transplantation for auditory nerve regeneration, a uniformly molded cell sheet manufactured before cell transplantation cannot be applied to the target area. On the contrary, to facilitate the integration of donor cells into the host, it is important to drop them freely into the narrow spaces within the irregular and complex contours of the tissue surface.
Conclusion
We conclude that there is great potential for clinical translation of cell transplantation in the auditory nerve. Proof of principle has been established; appropriate clinical techniques are available, and there is considerable theoretical support from wide-ranging studies on neurodegeneration and tissue repair. Auditory nerve damage may occur as a result of neuropathies, intracranial mass lesions, head trauma, and even therapeutic intervention. The finding that donor cells placed on the surface of the gliotic auditory nerve autonomously entered the nerve tissue, migrated, and functionally integrated into the host neural circuit makes clinical surgery much more realistic2. For clinical translation, endoscopy provides the best way to deliver viable cells to the tissue surface with minimal damage to residual functional elements in the nerve.
Whilst proof of principle is an important step, there is clearly a need for focused animal experiments that recreate the combination of approaches necessary for clinical application. “From the bench to the clinic” is a slogan that has been repeated also in regenerative medicine. Now it can be accomplished if an appropriate cell transplantation method is applied to humans, choosing potent human stem cells or human cochlear precursors207,208 that may or may not be conditioned to achieve integration.
Notably, the auditory nerve holds an advantageous and suitable position for cell transplantation therapy because HCs, auditory neurons, and cochlear nucleus cells are aligned over a relatively short distance209,210. This contrasts with the recovery of the injured pyramidal tract in SCI, which involves not only axon sprouting but also recruiting endogenous relay neurons211,212.
Finally, transdisciplinary combinations with regenerative studies for both auditory nerve and HCs would pave a new path for more widespread treatment of DHL and even for a number of other neurodegenerative conditions.
Acknowledgment
We appreciate Emeritus Professor Juichi Ito and Professor Koichi Omori, Department of Otolaryngology, Head and Neck Surgery, Kyoto University Graduate School of Medicine for their support.
Footnotes
Author Contributions: TS and MCH conceived the original idea. TS curated and analyzed the data, obtained the funding and wrote the original draft of the article. MCH also contributed to the writing and editing of the article.
Ethical Approval: Our university and hospital do not require ethical approval for any papers based on article review.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the Japan Society for the Promotion of Science (MEXT) (#26931051, 24931046, 13557112, 1559150, 12470281, 09557113-3, 10877209, 08457356-3, 09557113, 08457356, 07457303, 06454407, 03454341, 58770916), Univers Foundation, the General Insurance Association of Japan, the Japan Health Foundation, Osaka Gas Group Welfare Foundation, Zenkyoren and Mitsui Sumitomo Insurance Welfare Foundation.
ORCID iD: Tetsuji Sekiya  https://orcid.org/0000-0002-1325-5331
https://orcid.org/0000-0002-1325-5331
References
- 1.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, et al. Dementia prevention, intervention, and care. Lancet 2017;390(10113):2673–2734. [DOI] [PubMed] [Google Scholar]
- 2.Sekiya T, Holley MC, Hashido K, Ono K, Shimomura K, Horie RT, Hamaguchi K, Yoshida A, Sakamoto T, Ito J. Cells transplanted onto the surface of the glial scar reveal hidden potential for functional neural regeneration. Proc Natl Acad Sci U S A. 2015;112(26):E3431–E3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekiya T, Kojima K, Matsumoto M, Holley MC, Ito J. Rebuilding lost hearing using cell transplantation. Neurosurgery. 2007;60(3):417–433; discussion 433. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd RK, Hardie NA. Deafness-induced changes in the auditory pathway: implications for cochlear implants. Audiol Neurootol. 2001;6(6):305–318. [DOI] [PubMed] [Google Scholar]
- 5.Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci 2002;25(1):51–101. [DOI] [PubMed] [Google Scholar]
- 6.DiSogra RM. Common aminoglycosides and platinum-based ototoxic drugs: cochlear/vestibular side effects and incidence. Semin Hear. 2019;40(2):104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lie A, Skogstad M, Johannessen HA, Tynes T, Mehlum IS, Nordby KC, Engdahl B, Tambs K. Occupational noise exposure and hearing: a systematic review. Int Arch Occup Environ Health. 2016;89(3):351–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alves M, Martins JH, Moura JE, Ramos D, Alves H, Oliveira G, Magalhães I, Silva L, Ribeiro C, Paiva AD. Auditory rehabilitation after cochlear implantation in adults with hearing impairment after head trauma. Cochlear Implants Int. 2014;15(6):312–317. [DOI] [PubMed] [Google Scholar]
- 9.Betchen SA, Walsh J, Post KD. Long-term hearing preservation after surgery for vestibular schwannoma. J Neurosurg. 2005;102(1):6–9. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen R, Claesson M, Stangerup SE, Roed H, Christensen IJ, Caye-Thomasen P, Juhler M. Fractionated stereotactic radiotherapy of vestibular schwannomas accelerates hearing loss. Int J Radiat Oncol Biol Phys. 2012;83(5):e607–e611. [DOI] [PubMed] [Google Scholar]
- 11.Tveiten OV, Carlson ML, Goplen F, Vassbotn F, Link MJ, Lund-Johansen M.Long-term auditory symptoms in patients with sporadic vestibular schwannoma: An international cross-sectional study. Neurosurgery. 2015;77(2):218–227. [DOI] [PubMed] [Google Scholar]
- 12.Carlson ML, Jacob JT, Pollock BE, Neff BA, Tombers NM, Driscoll CL, Link MJ. Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg. 2013;118(3):579–87. [DOI] [PubMed] [Google Scholar]
- 13.Combs SE, Engelhard C, Kopp C, Wiedenmann N, Schramm O, Prokic V, Debus J, Molls M, Grosu AL. Long-term outcome after highly advanced single-dose or fractionated radiotherapy in patients with vestibular schwannomas - pooled results from 3 large German centers. Radiother Oncol. 2015;114(3):378–383. [DOI] [PubMed] [Google Scholar]
- 14.Kim KM, Park CK, Chung HT, Paek SH, Jung HW, Kim DG. Long-term outcomes of gamma knife stereotactic radiosurgery of vestibular schwannomas. J Korean Neurosurg Soc. 2007;42(4):286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rask-Andersen H, Liu W, Erixon E, Kinnefors A, Pfaller K, Schrott-Fischer A, Glueckert R. Human cochlea: anatomical characteristics and their relevance for cochlear implantation. Anat Rec (Hoboken). 2012;295(11):1791–811. [DOI] [PubMed] [Google Scholar]
- 16.Felder E, Kanonier G, Scholtz A, Rask-Andersen H, Schrott-Fischer A. Quantitative evaluation of cochlear neurons and computer-aided three-dimensional reconstruction of spiral ganglion cells in humans with a peripheral loss of nerve fibres. Hear Res 1997;105(1-2):183–190. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Edin F, Atturo F, Rieger G, Lowenheim H, Senn P, Blumer M, Schrott-Fischer A, Rask-Andersen H, Glueckert R. The pre- and post-somatic segments of the human type I spiral ganglion neurons--structural and functional considerations related to cochlear implantation. Neuroscience. 2015;284:470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6(2):136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moser T, Starr A. Auditory neuropathy--neural and synaptic mechanisms. Nat Rev Neurol. 2016;12(3):135–149. [DOI] [PubMed] [Google Scholar]
- 20.Santarelli R, Rossi R, Scimemi P, Cama E, Valentino ML, La Morgia C, Caporali L, Liguori R, Magnavita V, Monteleone A, Biscaro A. OPA1-related auditory neuropathy: site of lesion and outcome of cochlear implantation. Brain. 2015;138(Pt 3):563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res 2015;330:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, Kuhn S, Milo M, Thurlow JK, Andrews PW, Marcotti W, Moore HD, et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012;490(7419):278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemp DT. Otoacoustic emissions, their origin in cochlear function, and use. Br Med Bull. 2002;63:223–41. [DOI] [PubMed] [Google Scholar]
- 24.Telischi FF, Widick MP, Lonsbury-Martin BL, McCoy MJ. Monitoring cochlear function intraoperatively using distortion product otoacoustic emissions. Am J Otol. 1995;16(5):597–608. [PubMed] [Google Scholar]
- 25.Gouveris HT, Victor A, Mann WJ. Cochlear origin of early hearing loss in vestibular schwannoma. Laryngoscope. 2007;117(4):680–683. [DOI] [PubMed] [Google Scholar]
- 26.Kagoya R, Shinogami M, Kohno M, Yamasoba T. Distortion-product otoacoustic emission tests evaluate cochlear function and differentiate cochlear and vestibular schwannoma. Otolaryngol Head Neck Surg 2013;148(2):267–271. [DOI] [PubMed] [Google Scholar]
- 27.Hakuba N, Koga K, Shudou M, Watanabe F, Mitani A, Gyo K. Hearing loss and glutamate efflux in the perilymph following transient hindbrain ischemia in gerbils. J Comp Neurol. 2000;418(2):217–26. [DOI] [PubMed] [Google Scholar]
- 28.Puel JL, Pujol R, Tribillac F, Ladrech S, Eybalin M. Excitatory amino acid antagonists protect cochlear auditory neurons from excitotoxicity. J Comp Neurol. 1994;341(2):241–256. [DOI] [PubMed] [Google Scholar]
- 29.Roosli C, Linthicum FH, Cureoglu S, Merchant SN. Dysfunction of the cochlea contributing to hearing loss in acoustic neuromas: An underappreciated entity. Otol Neurotol. 2012;33(3):473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Yang J, Li G, Li Y, Wu R, Cheng J, Tang Y. Pathophysiological responses in rat and mouse models of radiation-induced brain injury. Mol Neurobiol. 2017;54(2):1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linskey ME. Hearing preservation in vestibular schwannoma stereotactic radiosurgery: what really matters? J Neurosurg. 2008;109(Suppl):129–136. [DOI] [PubMed] [Google Scholar]
- 32.Yamasoba T, Lin FR, Someya S, Kashio A, Sakamoto T, Kondo K. Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear Res. 2013;303:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proctor B.Accessory conduits. Surgical anatomy of the ear and temporal bone. New York: Thieme Medical Publishers; 1989. p 197–206. [Google Scholar]
- 34.Carlstrom LP, Jacob JT, Graffeo CS, Perry A, Oldenburg MS, Foote RL, Pollock BE, Driscoll CL, Carlson ML, Link MJ. Impact of cochlear modiolus dose on hearing preservation following stereotactic radiosurgery for non-vestibular schwannoma neoplasms of the lateral skull base: a cohort study. J Neurosurg 2019:33(3):736–741. [DOI] [PubMed] [Google Scholar]
- 35.Perez de Moura LF. Inner ear pathology in acoustic neurinoma. Arch Otolarngol. 1967;85(2):125–133. [DOI] [PubMed] [Google Scholar]
- 36.Bartholomew RA, Lubner RJ, Knoll RM, Ghanad I, Jung D, Nadol JB, Jr, Alvarez VE, Remenschneider A, Kozin ED. Labyrinthine concussion: Historic otopathologic antecedents of a challenging diagnosis. Laryngoscope Investig Otolaryngol. 2020;5(2):267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corrales CE, Monfared A, Jackler RK. Facial and vestibulocochlear nerve avulsion at the fundus of the internal auditory canal in a child without a temporal bone fracture. Otol Neurotol. 2010;31(9):1508–1510. [DOI] [PubMed] [Google Scholar]
- 38.Makishima K, Snow JB. Pathogenesis of hearing loss in head injury: studies in man and experimental animals. Arch Otolaryngol. 1975;101(7):426–432. [DOI] [PubMed] [Google Scholar]
- 39.Osen KK, Furness DN, Hackney CM. The border between the central and the peripheral nervous system in the cat cochlear nerve: a light and scanning electron microscopical study. Hear Res. 2011;277(1-2):44–53. [DOI] [PubMed] [Google Scholar]
- 40.Pamuk AE, Pamuk G, Bajin MD, Yildiz FG, Sennaroglu L. Traumatic facial and vestibulocochlear nerve injury in the internal acoustic canal in the absence of a temporal bone fracture. J Int Adv Otol. 2018;14(2):330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tokui N, Suzuki H, Udaka T, Hiraki N, Fujimura T, Fujimura K, Makishima K. Delayed-onset temporary auditory threshold shift following head blow in guinea pigs. Hear Res. 2005;199(1-2):111–116. [DOI] [PubMed] [Google Scholar]
- 42.Ishai R, Knoll RM, Chen JX, Wong K, Reinshagen KL, Nadol JB, Jr, Remenschneider AK, Jung DH, Kozin ED. Otopathologic changes in the cochlea following head injury without temporal bone fracture. Otolaryngol Head Neck Surg (United States). 2018;159(3):526–534. [DOI] [PubMed] [Google Scholar]
- 43.Berthold CH, Carlstedt T. Observations on the morphology at the transition between the peripheral and the central nervous system in the cat. II. General organization of the transitional region in S1 dorsal rootlets. Acta Physiol Scand Suppl. 1977;446:23–42. [PubMed] [Google Scholar]
- 44.Tarlov I.Structure of the nerve root. II. Differentiation of sensory from motor roots; observations on identification of function in roots of mixed cranial nerves. Arch Neurol Psychiatry. 1937;37(6):1338–1355. [Google Scholar]
- 45.Lenarz T. Cochlear implant - state of the art. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2017;16:Doc04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong DJ, Moran M, O’Leary SJ. Outcomes after cochlear implantation in the very elderly. Otol Neurotol. 2016;37(1):46–51. [DOI] [PubMed] [Google Scholar]
- 47.Carlson ML. Cochlear implantation in adults. N Engl J Med. 2020;382(16):1531–1542. [DOI] [PubMed] [Google Scholar]
- 48.Peng KA, Lorenz MB, Otto SR, Brackmann DE, Wilkinson EP. Cochlear implantation and auditory brainstem implantation in neurofibromatosis type 2. Laryngoscope. 2018;128(9):2163–2169. [DOI] [PubMed] [Google Scholar]
- 49.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freeman TB, Cicchetti F, Hauser RA, Deacon TW, Li XJ, Hersch SM, Nauert GM, Sanberg PR, Kordower JH, Saporta S, Isacson O. Transplanted fetal striatum in Huntington’s disease: phenotypic development and lack of pathology. Proc Natl Acad Sci U S A. 2000;97(25):13877–13882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.González C, Bonilla S, Flores AI, Cano E, Liste I. An update on human stem cell-based therapy in Parkinson’s disease. Curr Stem Cell Res Ther. 2016;11(7):561–568. [DOI] [PubMed] [Google Scholar]
- 52.Ng TK, Fortino VR, Pelaez D, Cheung HS. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J Stem Cells. 2014;6(2):111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adami R, Scesa G, Bottai D. Stem cell transplantation in neurological diseases: improving effectiveness in animal models. Front Cell Dev Biol. 2014;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitsumoto H, Brooks BR, Silani V. Clinical trials in amyotrophic lateral sclerosis: why so many negative trials and how can trials be improved? Lancet Neurol. 2014;13(11):1127–1138. [DOI] [PubMed] [Google Scholar]
- 55.Redmond DE, Jr, Bjugstad KB, Teng YD, Ourednik V, Ourednik J, Wakeman DR, Parsons XH, Gonzalez R, Blanchard BC, Kim SU, Gu Z, et al. Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci U S A 2007;104(29):12175–12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abd El Raouf HHH, Galhom RA, Ali MHM, Nasr El-Din WA. Harderian gland-derived stem cells as a cytotherapy in a guinea pig model of carboplatin-induced hearing loss. J Chem Neuroanat. 2019;98:139–152. [DOI] [PubMed] [Google Scholar]
- 57.Choi BY, Song JJ, Chang SO, Kim SU, Oh SH. Intravenous administration of human mesenchymal stem cells after noise- or drug-induced hearing loss in rats. Acta Otolaryngol. 2012;132(Suppl 1):S94–S102. [DOI] [PubMed] [Google Scholar]
- 58.Choi MY, Yeo SW, Park KH. Hearing restoration in a deaf animal model with intravenous transplantation of mesenchymal stem cells derived from human umbilical cord blood. Biochem Biophys Res Commun. 2012;427(3):629–636. [DOI] [PubMed] [Google Scholar]
- 59.Kil K, Choi MY, Kong JS, Kim WJ, Park KH. Regenerative efficacy of mesenchymal stromal cells from human placenta in sensorineural hearing loss. Int J Pediatr Otorhinolaryngol. 2016;91:72–81. [DOI] [PubMed] [Google Scholar]
- 60.Lang H, Nishimoto E, Xing Y, Brown LN, Noble KV, Barth JL, LaRue AC, Ando K, Schulte BA. Contributions of mouse and human hematopoietic cells to remodeling of the adult auditory nerve after neuron loss. Mol Ther. 2016;24(11):2000–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee HS, Kim WJ, Gong JS, Park KH. Clinical safety and efficacy of autologous bone marrow-derived mesenchymal stem cell transplantation in sensorineural hearing loss patients. J Audiol Otol. 2018;22(2):105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma Y, Guo W, Yi H, Ren L, Zhao L, Zhang Y, Yuan S, Liu R, Xu L, Cong T, Ek O, et al. Transplantation of human umbilical cord mesenchymal stem cells in cochlea to repair sensorineural hearing. Am J Transl Res. 2016;8(12):5235–5245. [PMC free article] [PubMed] [Google Scholar]
- 63.Revoltella RP, Papini S, Rosellini A, Michelini M, Franceschini V, Ciorba A, Bertolaso L, Magosso S, Hatzopoulos S, Lorito G, Giordano P, et al. Cochlear repair by transplantation of human cord blood CD133+ cells to nod-scid mice made deaf with kanamycin and noise. Cell Transplant. 2008;17(6):665–678. [DOI] [PubMed] [Google Scholar]
- 64.Yoo TJ, Du X, Zhou B. The paracrine effect of mesenchymal human stem cells restored hearing in β-tubulin induced autoimmune sensorineural hearing loss. Hea Res. 2015;330(Pt A):57–61. [DOI] [PubMed] [Google Scholar]
- 65.Zhang PZ, He Y, Jiang XW, Chen FQ, Chen Y, Shi L, Chen J, Chen X, Li X, Xue T, Wang Y, et al. Stem cell transplantation via the cochlear lateral wall for replacement of degenerated spiral ganglion neurons. Hear Res 2013;298:1–9. [DOI] [PubMed] [Google Scholar]
- 66.Zhang PZ, He Y, Jiang XW, Chen FQ, Chen Y, Xue T, Zhou K, Li X, Wang Y, Wu YX, Mi WJ, et al. Up-regulation of stromal cell-derived factor-1 enhances migration of transplanted neural stem cells to injury region following degeneration of spiral ganglion neurons in the adult rat inner ear. Neurosci Lett. 2013;534:101–106. [DOI] [PubMed] [Google Scholar]
- 67.Ahn KS, Jeon SJ, Jung JY, Kim YS, Kang JH, Shin S, Choi T, Choi SJ, Chung P, Shim H. Isolation of embryonic stem cells from enhanced green fluorescent protein-transgenic mouse and their survival in the cochlea after allotransplantation. Cytotherapy. 2008;10(7):759–769. [DOI] [PubMed] [Google Scholar]
- 68.Altschuler RA, O’Shea KS, Miller JM. Stem cell transplantation for auditory nerve replacement. Hear Res. 2008;242(1-2):110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J, Guan L, Zhu H, Xiong S, Zeng L, Jiang H. Transplantation of mouse-induced pluripotent stem cells into the cochlea for the treatment of sensorineural hearing loss. Acta Otolaryngol. 2017:137(11):1136–1142. [DOI] [PubMed] [Google Scholar]
- 70.Cho YB, Cho HH, Jang S, Jeong HS, Park JS. Transplantation of neural differentiated human mesenchymal stem cells into the cochlea of an auditory-neuropathy guinea pig model. J Korean Med Sci. 2011;26(4):492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coleman B, Hardman J, Coco A, Epp S, de Silva M, Crook J, Shepherd R. Fate of embryonic stem cells transplanted into the deafened mammalian cochlea. Cell Trans. 2006;15(5):369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dai Q, Zhang Z, Liu Q, Yu H. The protective effect of olfactory ensheathing cells on post-injury spiral ganglion cells. Acta Otolaryngol. 2016;136(11):1115–1120. [DOI] [PubMed] [Google Scholar]
- 73.Fetoni AR, Lattanzi W, Eramo SLM, Barba M, Paciello F, Moriconi C, Rolesi R, Michetti F, Troiani D, Paludetti G. Grafting and early expression of growth factors from adipose-derived stem cells transplanted into the cochlea, in a guinea pig model of acoustic trauma. Front Cell Neurosci. 2014;8:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gillespie LN, Zanin MP, Shepherd RK. Cell-based neurotrophin treatment supports long-term auditory neuron survival in the deaf guinea pig. J Control Release 2015;198:26–34. [DOI] [PubMed] [Google Scholar]
- 75.Hackelberg S, Tuck SJ, He L, Rastogi A, White C, Liu L, Prieskorn DM, Miller RJ, Chan C, Loomis BR, Corey JM, et al. Nanofibrous scaffolds for the guidance of stem cell-derived neurons for auditory nerve regeneration. PLoS One. 2017;12(7):e0180427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He Y, Zhang PZ, Sun D, Mi WJ, Zhang XY, Cui Y, Jiang XW, Mao XB, Qiu JH. Wnt1 from cochlear schwann cells enhances neuronal differentiation of transplanted neural stem cells in a rat spiral ganglion neuron degeneration model. Cell Trans. 2014;23(6):747–760. [DOI] [PubMed] [Google Scholar]
- 77.Hildebrand MS, Dahl HH, Hardman J, Coleman B, Shepherd RK, de Silva MG. Survival of partially differentiated mouse embryonic stem cells in the scala media of the guinea pig cochlea. J Assoc Res Otolaryngol. 2005;6(4):341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu Z, Andang M, Ni D, Ulfendahl M. Neural cograft stimulates the survival and differentiation of embryonic stem cells in the adult mammalian auditory system. Brain Res. 2005;1051(1-2):137–144. [DOI] [PubMed] [Google Scholar]
- 79.Hu Z, Ulfendahl M, Olivius NP. Survival of neuronal tissue following xenograft implantation into the adult rat inner ear. Exp Neurol. 2004;185(1):7–14. [DOI] [PubMed] [Google Scholar]
- 80.Hu Z, Wei D, Johansson CB, Holmstrom N, Duan M, Frisen J, Ulfendahl M. Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res. 2005;302(1):40–47. [DOI] [PubMed] [Google Scholar]
- 81.Hu Z, Ulfendahl M, Prieskorn DM, Olivius P, Miller JM. Functional evaluation of a cell replacement therapy in the inner ear. Otol Neurotol. 2009;30(4):551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang X, Liu J, Wu W, Hu P, Wang Q. Taurine enhances mouse cochlear neural stem cell transplantation via the cochlear lateral wall for replacement of degenerated spiral ganglion neurons via sonic hedgehog signaling pathway. Cell Tissue Res. 2019;378(1):49–57. [DOI] [PubMed] [Google Scholar]
- 83.Jang S, Cho HH, Kim SH, Lee KH, Cho YB, Park JS, Jeong HS. Transplantation of human adipose tissue-derived stem cells for repair of injured spiral ganglion neurons in deaf guinea pigs. Neural Regen Res. 2016;11(6):994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jang S, Cho HH, Kim SH, Lee KH, Jun JY, Park JS, Jeong HS, Cho YB. Neural-induced human mesenchymal stem cells promote cochlear cell regeneration in deaf Guinea pigs. Clin Exp Otorhinolaryngol. 2015;8(2):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lang H, Schulte BA, Goddard JC, Hedrick M, Schulte JB, Wei L, Schmiedt RA. Transplantation of mouse embryonic stem cells into the cochlea of an auditory-neuropathy animal model: effects of timing after injury. J Assoc Res Otolaryngol. 2008;9(2):225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matsuoka AJ, Kondo T, Miyamoto RT, Hashino E. Enhanced survival of bone-marrow-derived pluripotent stem cells in an animal model of auditory neuropathy. Laryngoscope. 2007;117(9):1629–1635. [DOI] [PubMed] [Google Scholar]
- 87.Parker MA, Corliss DA, Gray B, Anderson JK, Bobbin RP, Snyder EY, Cotanche DA. Neural stem cells injected into the sound-damaged cochlea migrate throughout the cochlea and express markers of hair cells, supporting cells, and spiral ganglion cells. Hear Res. 2007;232(1-2):29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pettingill LN, Wise AK, Geaney MS, Shepherd RK. Enhanced auditory neuron survival following cell-based BDNF treatment in the deaf guinea pig. Plos One. 2011;6(4):e18733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schendzielorz P, Vollmer M, Rak K, Wiegner A, Nada N, Radeloff K, Hagen R, Radeloff A. Adipose-derived stromal cells enhance auditory neuron survival in an animal model of sensory hearing loss. Cytotherapy. 2017;19(10):1197–1207. [DOI] [PubMed] [Google Scholar]
- 90.Warnecke A, Sasse S, Wenzel GI, Hoffmann A, Gross G, Paasche G, Scheper V, Reich U, Esser KH, Lenarz T, Stöver T, et al. Stable release of BDNF from the fibroblast cell line NIH3T3 grown on silicone elastomers enhances survival of spiral ganglion cells in vitro and in vivo. Hear Res. 2012;289(1-2):86–97. [DOI] [PubMed] [Google Scholar]
- 91.Wise AK, Tan J, Wang Y, Caruso F, Shepherd RK. Improved auditory nerve survival with nanoengineered supraparticles for neurotrophin delivery into the deafened cochlea. PLoS One. 2016;11(10):e0164867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu YP, Shan XD, Liu YY, Pu Y, Wang CY, Tao QL, Deng Y, Cheng Y, Fan JP. Olfactory epithelium neural stem cell implantation restores noise-induced hearing loss in rats. Neurosci Lett. 2016;616:19–25. [DOI] [PubMed] [Google Scholar]
- 93.Corrales CE, Pan L, Li H, Liberman MC, Heller S, Edge AS. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: growth of processes into the organ of Corti. J Neurobiol. 2006;66(13):1489–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu Z, Ulfendahl M, Olivius NP. Central migration of neuronal tissue and embryonic stem cells following transplantation along the adult auditory nerve. Brain Res. 2004;1026(1):68–73. [DOI] [PubMed] [Google Scholar]
- 95.Naito Y, Nakamura T, Nakagawa T, Iguchi F, Endo T, Fujino K, Kim TS, Hiratsuka Y, Tamura T, Kanemaru S, Shimizu Y, et al. Transplantation of bone marrow stromal cells into the cochlea of chinchillas. Neuroreport. 2004;15(1):1–4. [DOI] [PubMed] [Google Scholar]
- 96.Ogita H, Nakagawa T, Sakamoto T, Inaoka T, Ito J. Transplantation of bone marrow-derived neurospheres into guinea pig cochlea. Laryngoscope. 2010;120(3):576–581. [DOI] [PubMed] [Google Scholar]
- 97.Okano T, Nakagawa T, Endo T, Kim TS, Kita T, Tamura T, Matsumoto M, Ohno T, Sakamoto T, Iguchi F, Ito J. Engraftment of embryonic stem cell-derived neurons into the cochlear modiolus. Neuroreport. 2005;16(17):1919–1922. [DOI] [PubMed] [Google Scholar]
- 98.Regala C, Duan M, Zou J, Salminen M, Olivius P. Xenografted fetal dorsal root ganglion, embryonic stem cell and adult neural stem cell survival following implantation into the adult vestibulocochlear nerve. Exp Neurol. 2005;193(2):326–333. [DOI] [PubMed] [Google Scholar]
- 99.Reyes JH, O’Shea KS, Wys NL, Velkey JM, Prieskorn DM, Wesolowski K, Miller JM, Altschuler RA. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J Neurosci. 2008;28(48):12622–12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shi F, Corrales CE, Liberman MC, Edge AS. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur J Neurosci. 2007;26(11):3016–3023. [DOI] [PubMed] [Google Scholar]
- 101.Tamura T, Nakagawa T, Iguchi F, Tateya I, Endo T, Kim T-S, Dong Y, Kita T, Kojima K, Naito Y, Omori K, et al. Transplantation of neural stem cells into the modiolus of Mouse cochleae injured by cisplatin. Acta Otolyngol. 2004;124(0):65–68. [DOI] [PubMed] [Google Scholar]
- 102.Cisbani G, Freeman TB, Soulet D, Saint-Pierre M, Gagnon D, Parent M, Hauser RA, Barker RA, Cicchetti F. Striatal allografts in patients with Huntington’s disease: impact of diminished astrocytes and vascularization on graft viability. Brain. 2013;136(Pt 2):433–43. [DOI] [PubMed] [Google Scholar]
- 103.Chen HC, Liang CM, Wang CH, Huang MY, Lin YY, Shih CP, Kuo CY, Lin YC, Chen HK. Transplantation of human limbus–derived mesenchymal stromal cells via occipital approach improves hearing in animal auditory neuropathy. Int J Ped Otorhinolaryngol. 2019;117:67–72. [DOI] [PubMed] [Google Scholar]
- 104.Jiao Y, Palmgren B, Novozhilova E, Englund Johansson U, Spieles-Engemann AL, Kale A, Stupp SI, Olivius P. BDNF increases survival and neuronal differentiation of human neural precursor cells cotransplanted with a nanofiber gel to the auditory nerve in a rat model of neuronal damage. Biomed Res Int. 2014;2014:356415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Palmgren B, Jiao Y, Novozhilova E, Stupp SI, Olivius P. Survival, migration and differentiation of mouse tau-GFP embryonic stem cells transplanted into the rat auditory nerve. Exp Neurol. 2012;235(2):599–609. [DOI] [PubMed] [Google Scholar]
- 106.Sekiya T, Holley MC, Kojima K, Matsumoto M, Helyer R, Ito J. Transplantation of conditionally immortal auditory neuroblasts to the auditory nerve. Eur J Neurosci. 2007;25(8):2307–2318. [DOI] [PubMed] [Google Scholar]
- 107.Sekiya T, Kojima K, Matsumoto M, Kim TS, Tamura T, Ito J. Cell transplantation to the auditory nerve and cochlear duct. Exp Neurol. 2006;198(1):12–24. [DOI] [PubMed] [Google Scholar]
- 108.Castello MA, Gleeson JG. Insight into developmental mechanisms of global and focal migration disorders of cortical development. Curr Opin Neurobiol. 2021;66:77–84. [DOI] [PubMed] [Google Scholar]
- 109.Suter TACS, Jaworski A. Cell migration and axon guidance at the border between central and peripheral nervous system. Science. 2019;365(6456):eaaw8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen YS, Harn HJ, Chiou TW. The role of biomaterials in implantation for central nervous system injury. Cell Transplant. 2018;27(3):407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fu RH, Wang YC, Liu SP, Shih TR, Lin HL, Chen YM, Sung JH, Lu CH, Wei JR, Wang ZW, Huang SJ, et al. Decellularization and recellularization technologies in tissue engineering. Cell Transplant. 2014;23(4-5):621–630. [DOI] [PubMed] [Google Scholar]
- 112.Johnson PJ, Tatara A, Shiu A, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 and platelet-derived growth factor from fibrin scaffolds containing neural progenitor cells enhances survival and differentiation into neurons in a subacute model of SCI. Cell Transplant. 2010;19(1):89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eberli D, Atala A. Tissue engineering using adult stem cells. Elsevier; 2006;420:287–302. [DOI] [PubMed] [Google Scholar]
- 114.Struzyna LA, Katiyar K, Cullen DK. Living scaffolds for neuroregeneration. Curr Opin Solid State Mat Sci. 2014;18(6):308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Silva CG, Peyre E, Nguyen L. Cell migration promotes dynamic cellular interactions to control cerebral cortex morphogenesis. Nat Rev Neurosci. 2019;20(6):318–329. [DOI] [PubMed] [Google Scholar]
- 116.Ghashghaei HT, Lai C, Anton ES. Neuronal migration in the adult brain: are we there yet? Nat Rev Neurosci. 2007;8(2):141–151. [DOI] [PubMed] [Google Scholar]
- 117.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271(5251):978–981. [DOI] [PubMed] [Google Scholar]
- 118.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338(6211):161–164. [DOI] [PubMed] [Google Scholar]
- 119.Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M.Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105(9):3581–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pekny M, Wilhelmsson U, Bogestal YR, Pekna M. The role of astrocytes and complement system in neural plasticity. Int Rev Neurobiol. 2007;82:95–111. [DOI] [PubMed] [Google Scholar]
- 121.Silver J, Schwab ME, Popovich PG. Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb Perspect Biol. 2015;7(3):a020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33(31):12870–12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Choudhury GR, Ding S. Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol Dis. 2016;85:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16(5):580–586. [DOI] [PubMed] [Google Scholar]
- 126.Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28(13):3264–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Matsunaga T, Kanzaki J, Hosoda Y. Gliosis of the eighth nerve transitional region in patients with cerebellopontine angle schwannoma. Acta Otolaryngol. 1994;114(4):393–398. [DOI] [PubMed] [Google Scholar]
- 128.Ghatak NR, Nochlin D. Glial outgrowth along spinal nerve roots in amyotrophic lateral sclerosis. Ann Neurol. 1982;11(2):203–206. [DOI] [PubMed] [Google Scholar]
- 129.Ince PG, Highley JR, Wharton SB. Motor neuron disorders. In: Love S, editor. Greenfield’s neuropathology. 9th ed. 1. Boca Raton, FL, U. S. A.: CRC Press; 2015. p 817–848. [Google Scholar]
- 130.Iwata M, Hirano A. “Glial bundles” in the spinal cord late after paralytic anterior poliomyelitis. Ann Neurol. 1978;4(6):562–563. [DOI] [PubMed] [Google Scholar]
- 131.Kimura T, Budka H. Glial bundles in spinal nerve roots. An immunocytochemical study stressing their nonspecificity in various spinal cord and peripheral nerve diseases. Acta Neuropathol. 1984;65(1):46–52. [DOI] [PubMed] [Google Scholar]
- 132.Simone C, Ramirez A, Bucchia M, Rinchetti P, Rideout H, Papadimitriou D, Re DB, Corti S.Is spinal muscular atrophy a disease of the motor neurons only: Pathogenesis and therapeutic implications? Cell Mol Life Sci. 2016;73(5):1003–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yamamoto T, Iwasaki Y, Konno H, Kudo H. Glial bundle formation in spinal roots following experimental neuronopathy. Ann Neurol. 1986;20(2):267–271. [DOI] [PubMed] [Google Scholar]
- 134.Ayala R, Shu T, Tsai L-H.Trekking across the brain: The journey of neuronal migration. Cell. 2007;128(1):29–43. [DOI] [PubMed] [Google Scholar]
- 135.Fraher J, Dockery P. Injury-induced changes in spinal root transitional zones. Morphometric ultrastructural studies. In: Aldskogius H, Fraher J, eds. Glial interfaces in the nervous system. Role in repair and plasticity. Amsterdam: IOS Press; 2002. p 41–59. [Google Scholar]
- 136.Jessen KR, Mirsky R, Lloyd AC. Schwann cells: Development and role in nerve repair. Cold Spring Harb Perspect Biol. 2015;7(7):a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sekiya T, Holley MC. ‘Surface transplantation’ for nerve injury and repair: The quest for minimally invasive cell delivery. Trends Neurosci. 2018;41(7):429–441. [DOI] [PubMed] [Google Scholar]
- 138.Fan B, Wei Z, Yao X, Shi G, Cheng X, Zhou X, Zhou H, Ning G, Kong X, Feng S. Microenvironment imbalance of spinal cord injury. Cell Transplant. 2018;27(6):853–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol 2008;86(4):342–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moller AR.Generation of electrical activity in the nervous system and muscles. Intraoperative neurophysiological monitoring. 3 ed. New York: Springer; 2011. p 23–41. [Google Scholar]
- 141.Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu Z, Li Y, Cui Y, Roberts C, Lu M, Wilhelmsson U, Pekny M, Chopp M. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia. 2014;62(12):2022–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32(18):6391–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19(14):5810–5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63(1):84–96. [DOI] [PubMed] [Google Scholar]
- 146.Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26(1):125–134. [DOI] [PubMed] [Google Scholar]
- 147.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101(52):18117–18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Adams KL, Gallo V. The diversity and disparity of the glial scar. Nat Neurosci. 2018;21(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, Mohila CA, et al. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci. 2017;20(3):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Westergard T, Rothstein JD. Astrocyte diversity: Current insights and future directions. Neurochem Res. 2020;45(6):1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhäuser C, Volterra A, Carmignoto G, Agarwal A, Allen NJ, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nature Neurosci. 2021;24(3):312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hill SJ, Barbarese E, McIntosh TK. Regional heterogeneity in the response of astrocytes following traumatic brain injury in the adult rat. J Neuropathol Exp Neurol. 1996;55(12):1221–1229. [DOI] [PubMed] [Google Scholar]
- 153.Hilton BJ, Bradke F. Can injured adult CNS axons regenerate by recapitulating development? Dev (Cambridge). 2017;144(19):3417–3429. [DOI] [PubMed] [Google Scholar]
- 154.Farmer WT, Abrahamsson T, Chierzi S, Lui C, Zaelzer C, Jones EV, Bally BP, Chen GG, Theroux JF, Peng J, Bourque CW, et al. Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science. 2016;351(6275):849–8454. [DOI] [PubMed] [Google Scholar]
- 155.Lang H, Li M, Kilpatrick LA, Zhu J, Samuvel DJ, Krug EL, Goddard JC. Sox2 up-regulation and glial cell proliferation following degeneration of spiral ganglion neurons in the adult mouse inner ear. J Assoc Res Otolaryngol. 2011;12(2):151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lang H, Schulte BA, Schmiedt RA. Ouabain induces apoptotic cell death in type I spiral ganglion neurons, but not type II neurons. J Assoc Res Otolaryngol. 2005;6(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang ZJ, Guan HX, Yang K, Xiao BK, Liao H, Jiang Y, Zhou T, Hua QQ. Dose-dependent effects of ouabain on spiral ganglion neurons and Schwann cells in mouse cochlea. Acta Otolaryngol. 2017;137(10):1017–1023. [DOI] [PubMed] [Google Scholar]
- 158.Fu Y, Ding D, Jiang H, Salvi R. Ouabain-induced cochlear degeneration in rat. Neurotox Res. 2012;22(2):158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lighthall JW, Anderson TE. In vivo models of experimental and spinal cord trauma. In: Salzman SK, Faden AI, editors. The Neurobiology of central nervous system trauma. Oxford: Oxford University Press; 1994. p 3–11. [Google Scholar]
- 160.Lim DJ.Functional structure of the organ of Corti: A review. Hear Res. 1986;22:117–46. [DOI] [PubMed] [Google Scholar]
- 161.Schomann T, Ramekers D, de Groot J, van der Ploeg CH, Hendriksen FGJ, Bohringer S, Klis SFL, Frijns JHM, Huisman MA. Ouabain does not induce selective spiral ganglion cell degeneration in guinea pigs. Biomed Res Int. 2018;2018:1568414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lingrel JB, Kuntzweiler T.Na+, K(+)-ATPase. J Biol Chem. 1994;269(31):19659–19662. [PubMed] [Google Scholar]
- 163.Luan Z, Reddig K, Li HS. Loss of Na(+)/K(+)-ATPase in drosophila photoreceptors leads to blindness and age-dependent neurodegeneration. Exp Neurol. 2014;261:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ding D, Allman BL, Salvi R.Review: Ototoxic characteristics of platinum antitumor drugs. Anat Rec (Hoboken). 2012;295(11):1851–1867. [DOI] [PubMed] [Google Scholar]
- 165.Matsumoto M, Sekiya T, Kojima K, Ito J. An animal experimental model of auditory neuropathy induced in rats by auditory nerve compression. Exp Neurol. 2008;210(1):248–56. [DOI] [PubMed] [Google Scholar]
- 166.Sekiya T, Matsumoto M, Kojima K, Ono K, Kikkawa YS, Kada S, Ogita H, Horie RT, Viola A, Holley MC, Ito J. Mechanical stress-induced reactive gliosis in the auditory nerve and cochlear nucleus. J Neurosurg. 2011;114(2):414–425. [DOI] [PubMed] [Google Scholar]
- 167.Sekiya T, Canlon B, Viberg A, Matsumoto M, Kojima K, Ono K, Yoshida A, Kikkawa YS, Nakagawa T, Ito J. Selective vulnerability of adult cochlear nucleus neurons to de-afferentation by mechanical compression. Exp Neurol. 2009;218(1):117–123. [DOI] [PubMed] [Google Scholar]
- 168.Sekiya T, Viberg A, Kojima K, Sakamoto T, Nakagawa T, Ito J, Canlon B. Trauma-specific insults to the cochlear nucleus in the rat. J Neurosci Res. 2012;90(10):1924–1931. [DOI] [PubMed] [Google Scholar]
- 169.Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, Kuhn S, Milo M, Thurlow JK, Andrews PW, Marcotti W, Moore HD, et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012;490(7419):278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zheng D, Wang X, Xu RH. Concise Review: One stone for multiple birds: Generating universally compatible human embryonic stem cells. Stem Cells. 2016;34(9):2269–2275. [DOI] [PubMed] [Google Scholar]
- 171.Boyd AS, Rodrigues NP, Lui KO, Fu X, Xu Y.Concise review: Immune recognition of induced pluripotent stem cells. Stem Cells. 2012;30(5):797–803. [DOI] [PubMed] [Google Scholar]
- 172.de Almeida PE, Ransohoff JD, Nahid A, Wu JC. Immunogenicity of pluripotent stem cells and their derivatives. Circ Res. 2013;112(3):549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Aron Badin R, Bugi A, Williams S, Vadori M, Michael M, Jan C, Nassi A, Lecourtois S, Blancher A, Cozzi E, Hantraye P, et al. MHC matching fails to prevent long-term rejection of iPSC-derived neurons in non-human primates. Nat Commun. 2019;10(1):4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tokumoto Y, Ogawa S, Nagamune T, Miyake J. Comparison of efficiency of terminal differentiation of oligodendrocytes from induced pluripotent stem cells versus embryonic stem cells in vitro. J Biosci Bioeng. 2010;109(6):622–628. [DOI] [PubMed] [Google Scholar]
- 175.Feraud O, Valogne Y, Melkus MW, Zhang Y, Oudrhiri N, Haddad R, Daury A, Rocher C, Larbi A, Duquesnoy P, Divers D, et al. Donor dependent variations in hematopoietic differentiation among embryonic and induced pluripotent stem cell lines. PLoS One. 2016;11(3): e0149291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lauss M, Stary M, Tischler J, Egger G, Puz S, Bader-Allmer A, Seiser C, Weitzer G. Single inner cell masses yield embryonic stem cell lines differing in lifr expression and their developmental potential. Biochem Biophys Res Commun. 2005;331(4):1577–1586. [DOI] [PubMed] [Google Scholar]
- 177.Stewart MH, Bosse M, Chadwick K, Menendez P, Bendall SC, Bhatia M. Clonal isolation of hESCs reveals heterogeneity within the pluripotent stem cell compartment. Nat Methods. 2006;3(10):807–815. [DOI] [PubMed] [Google Scholar]
- 178.Roemer A, Köhl U, Majdani O, Klöß S, Falk C, Haumann S, Lenarz T, Kral A, Warnecke A. Biohybrid cochlear implants in human neurosensory restoration. Stem Cell Res Ther. 2016;7(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Warnecke A, Mellott AJ, Römer A, Lenarz T, Staecker H. Advances in translational inner ear stem cell research. Hear Res. 2017;353:76–86. [DOI] [PubMed] [Google Scholar]
- 180.Duma C, Kopyov O, Kopyov A, Berman M, Lander E, Elam M, Arata M, Weiland D, Cannell R, Caraway C, Berman S, et al. Human intracerebroventricular (ICV) injection of autologous, non-engineered, adipose-derived stromal vascular fraction (ADSVF) for neurodegenerative disorders: results of a 3-year phase 1 study of 113 injections in 31 patients. Mol Biol Rep. 2019;46(5):5257–5272. [DOI] [PubMed] [Google Scholar]
- 181.Kuzma-Kozakiewicz M, Marchel A, Kaminska A, Gawel M, Sznajder J, Figiel-Dabrowska A, Nowak A, Maj E, Krzesniak NE, Noszczyk BH, Domanska-Janik K, et al. Intraspinal transplantation of the adipose tissue-derived regenerative cells in amyotrophic lateral sclerosis in accordance with the current experts’ recommendations: Choosing optimal monitoring tools. Stem Cells Int. 2018;2018:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Planchon SM, Lingas KT, Reese Koç J, Hooper BM, Maitra B, Fox RM, Imrey PB, Drake KM, Aldred MA, Lazarus HM, Cohen JA. Feasibility of mesenchymal stem cell culture expansion for a phase I clinical trial in multiple sclerosis. Mult Scler J Exp Transl Clin. 2018;4(1):205521731876528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Atoui R, Chiu RC. Mesenchymal stromal cells as universal donor cells. Expert Opin Biol Ther. 2012;12(10):1293–1297. [DOI] [PubMed] [Google Scholar]
- 184.Zheng H, Zhang B, Chhatbar PY, Dong Y, Alawieh A, Lowe F, Hu X, Feng W.Mesenchymal stem cell therapy in stroke: a systematic review of literature in pre-clinical and clinical research. Cell Transplant. 2018;27(12):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Worku MG.Pluripotent and multipotent stem cells and current therapeutic applications: Review. Stem Cells Cloning. 2021;14:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mimeault M, Hauke R, Batra SK. Stem Cells: A revolution in therapeutics — Recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther. 2007;82(3):252–264. [DOI] [PubMed] [Google Scholar]
- 187.Wang T, Chai R, Kim GS, Pham N, Jansson L, Nguyen D-H, Kuo B, May LA, Zuo J, Cunningham LL, Cheng AG. Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nat Commun. 2015;6(1):6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Coleman B, De Silva MG, Shepherd RK. Concise review: the potential of stem cells for auditory neuron generation and replacement. Stem Cells. 2007;25(11):2685–2694. [DOI] [PubMed] [Google Scholar]
- 189.Rask-Andersen H, Bostrom M, Gerdin B, Kinnefors A, Nyberg G, Engstrand T, Miller JM, Lindholm D.Regeneration of human auditory nerve. In vitro/in video demonstration of neural progenitor cells in adult human and guinea pig spiral ganglion. Hear Res. 2005;203(1-2):180–191. [DOI] [PubMed] [Google Scholar]
- 190.Nicholl AJ, Kneebone A, Davies D, Cacciabue-Rivolta DI, Rivolta MN, Coffey P, Holley MC. Differentiation of an auditory neuronal cell line suitable for cell transplantation. Eur J Neurosci. 2005;22(2):343–353. [DOI] [PubMed] [Google Scholar]
- 191.MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–207. [DOI] [PubMed] [Google Scholar]
- 192.Sanberg PR, Eve DJ, Cruz LE, Borlongan CV. Neurological disorders and the potential role for stem cells as a therapy. Br Med Bull. 2012;101(1):163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Apuzzo ML, Heifetz MD, Weiss MH, Kurze T. Neurosurgical endoscopy using the side-viewing telescope. J Neurosurg. 1977;46(3):398–400. [DOI] [PubMed] [Google Scholar]
- 194.Fukushima T.Endoscopy of Meckel’s cave, cisterna magna, and cerebellopontine angle. Technical note. J Neurosurg. 1978;48(2):302–306. [DOI] [PubMed] [Google Scholar]
- 195.Artz GJ, Hux FJ, LaRouere MJ, Bojrab DI, Babu S, Pieper DR. Endoscopic vascular decompression. Otol Neurotol. 2008;29(7):995–1000. [DOI] [PubMed] [Google Scholar]
- 196.Miyazaki H, Deveze A, Magnan J.Neuro-otologic surgery through minimally invasive retrosigmoid approach: Endoscope assisted microvascular decompression, vestibular neurotomy, and tumor removal. Laryngoscope. 2005;115(9):1612–1617. [DOI] [PubMed] [Google Scholar]
- 197.Abati E, Bresolin N, Comi GP, Corti S. Preconditioning and cellular engineering to increase the survival of transplanted neural stem cells for motor neuron disease therapy. Mol Neurobiol 2018;56(5):3356–3367. [DOI] [PubMed] [Google Scholar]
- 198.Spector R, Robert Snodgrass S, Johanson CE. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp Neurol. 2015;273:57–68. [DOI] [PubMed] [Google Scholar]
- 199.Zappaterra MW, Lehtinen MK. The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond. Cell Mol Life Sci. 2012;69(17):2863–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zawada WM, Zastrow DJ, Clarkson ED, Adams FS, Bell KP, Freed CR. Growth factors improve immediate survival of embryonic dopamine neurons after transplantation into rats. Brain Res. 1998;786(1-2):96–103. [DOI] [PubMed] [Google Scholar]
- 201.Bjorklund A, Stenevi U, Schmidt RH, Dunnett SB, Gage FH. Intracerebral grafting of neuronal cell suspensions. II. Survival and growth of nigral cell suspensions implanted in different brain sites. Acta Physiol Scand Suppl. 1983;522:9–18. [PubMed] [Google Scholar]
- 202.Boonman Z, Isacson O. Apoptosis in neuronal development and transplantation: role of caspases and trophic factors. Exp Neurol. 1999;156(1):1–15. [DOI] [PubMed] [Google Scholar]
- 203.Broadwell RD, Charlton HM, Ebert PS, Hickey WF, Shirazi Y, Villegas J, Wolf AL. Allografts of CNS tissue possess a blood-brain barrier. II. Angiogenesis in solid tissue and cell suspension grafts. Exp Neurol. 1991;112(1):1–28. [DOI] [PubMed] [Google Scholar]
- 204.Dusart I, Nothias F, Roudier F, Besson JM, Peschanski M. Vascularization of fetal cell suspension grafts in the excitotoxically lesioned adult rat thalamus. Dev Brain Res. 1989;48(2):215–228. [DOI] [PubMed] [Google Scholar]
- 205.Guo R, Morimatsu M, Feng T, Lan F, Chang D, Wan F, Ling Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res Ther. 2020;11(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kim K, Bou-Ghannam S, Kameishi S, Oka M, Grainger DW, Okano T.Allogeneic mesenchymal stem cell sheet therapy: A new frontier in drug delivery systems. J Control Release. 2021;330:696–704. [DOI] [PubMed] [Google Scholar]
- 207.Lopez-Juarez A, Lahlou H, Ripoll C, Cazals Y, Brezun JM, Wang Q, Edge A, Zine A. Engraftment of human stem cell-derived otic progenitors in the damaged cochlea. Mol Ther. 2019;27(6):1101–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rivolta MN. Stem cells and cell lines from the human auditory organ: applications, hurdles and bottlenecks in the development of regenerative therapies for deafness. Drug Discov Today. 2010;15(7-8):283–286. [DOI] [PubMed] [Google Scholar]
- 209.Krombach GA, van den Boom M, Di Martino E, Schmitz-Rode T, Westhofen M, Prescher A, Gunther RW, Wildberger JE. Computed tomography of the inner ear: size of anatomical structures in the normal temporal bone and in the temporal bone of patients with Meniere’s disease. Eur Radiol. 2005;15(8):1505–1513. [DOI] [PubMed] [Google Scholar]
- 210.Lang J. Anatomy of the brainstem and the lower cranial nerves, vessels, and surrounding structures. Am J Otol. 1985;6(SUPPL.):1–19. [PubMed] [Google Scholar]
- 211.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7(3):269–277. [DOI] [PubMed] [Google Scholar]
- 212.Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7(8):644–53. [DOI] [PubMed] [Google Scholar]