Abstract
Human induced pluripotent stem cells (hiPSCs) are a promising tool in cell-based therapies for degenerative diseases. A safe application of hiPSCs in vivo, requires the detection of the presence of residual undifferentiated pluripotent cells that can potentially cause the insurgence of teratomas. Several studies point out that metabolic products may provide an alternative method to identify the different steps of cells differentiation. In particular, the analysis of volatile organic compounds (VOCs) is gaining a growing interest in this context, thanks to its inherent noninvasiveness. Here, a protocol for VOCs analysis from human induced pluripotent stem cells (hiPSCs) is illustrated. It is based on Solid-Phase Microextraction (SPME) technique coupled with gas chromatography-mass spectrometry (GC/MS). The method is applied to measure the volatile metabolite modifications in cells headspace during cell reprogramming from chorionic villus samples (CVS) to hiPSCs, and along hiPSCs in vitro differentiation into early neural progenitors (NPs), passing through embryoid bodies (EBs) formation.
Keywords: Gas chromatography-mass spectrometry, GC/MS, Human induced pluripotent stem cell, hiPSC, Solid phase microextraction, SPME, Volatile organic compounds, VOCs, Metabolic profile
Background
Cellular metabolism is proposed as an alternative to studying stem cells during the various steps of differentiation. Indeed, it is reasonable to suppose that the transition of stem cells from pluripotency to the complete differentiation, might give rise to a dramatic change of metabolic products. First evidence of this assumption was observed between induced pluripotent stem cells, parental fibroblasts, and embryonic stem cells ( Meissen et al., 2012 ).
Within the metabolic products, the volatile organic compounds (VOCs) are attracting interest for the supposed simplicity of their collection, the intrinsic non-invasiveness and the wide availability of the analysis methods ( Boots et al., 2015 ). To this regard, several studies show that the headspace of cancer cells exhibit a VOCs profile which is altered as compared to that of normal cells ( Sponring et al., 2010 ; Peled et al., 2013 ; Filipiak et al., 2016 ).
Recently, we investigated the VOCs profiles of hiPSCs along the successive steps of differentiation ( Capuano et al., 2017 ). Results support the hypothesis that the volatile fraction of the metabolic profile changes along the differentiation process as a reflection of the dramatic variations occurring in the cells.
GC/MS analysis evidences a number of compounds whose relative abundance can signal the difference between the various phases of the differentiation. Most of these compounds are aldehydes, alcohols, and alkanes. It is worth to remark that these compounds are detected because of their affinity with the chosen SPME and the GC/MS column. As a consequence, at this stage, it cannot be excluded that additional and even more discriminating volatile compounds might be found using different experimental setups. However, the outlined protocol is thoroughly valid even when different materials for SPME and GC/MS columns are selected. In other words, the protocol to sample volatile compounds from cell cultures and to analyze them with GC/MS is valid in general. Albeit, changes in the materials of the SPME fiber and the column modify the sensitivity respect to volatile compounds, then the use of different materials may highlight the presence of some classes of compounds and hinder the detection of others.
Materials and Reagents
60 mm tissue-culture sterile dish (Corning, Falcon®, catalog number: 353004)
25-cm2 tissue-culture sterile flask (Corning, catalog number: 430639)
35 mm tissue-culture sterile dish (Corning, Falcon®, catalog number: 353001)
Sterile cell scraper (Corning, Falcon®, catalog number: 353086)
15-ml conical sterile tubes (Corning, Falcon, catalog number: 352096)
50-ml conical sterile tubes (Corning, Falcon, catalog number: 352070)
4-well tissue-culture sterile plates (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 176740)
6-well tissue-culture sterile plates (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 140675)
100 mm tissue-culture sterile dish (Corning, Falcon®, catalog number: 353003)
60 mm ultra-low attachment culture sterile dishes (Corning, catalog number: 3261)
5-ml plastic disposable sterile pipette (SARSTEDT, catalog number: 86.1253.001)
10-ml plastic disposable sterile pipette (SARSTEDT, catalog number: 86.1254.001)
25-ml plastic disposable sterile pipette (SARSTEDT, catalog number: 86.1685.001)
0.22 µm sterile filter system (Corning, catalog number: 431097)
-
Sterile glass Pasteur pipettes (VWR, catalog number: HECH40567001)
Manufacturer: Glaswarenfabrik Karl Hecht, catalog number: 40567001.
Sterile CryoTube Vials (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 375418)
50/30 μm Divinylbenzene/Carboxen/PDMS (DVB/CAR/PDMS) SPME fiber assembly, needle size 24 ga, for use with manual holder (Sigma-Aldrich, catalog number: 57328-U)
SPME Fiber Holder for use with manual sampling (Sigma-Aldrich, catalog number: 57330-U)
-
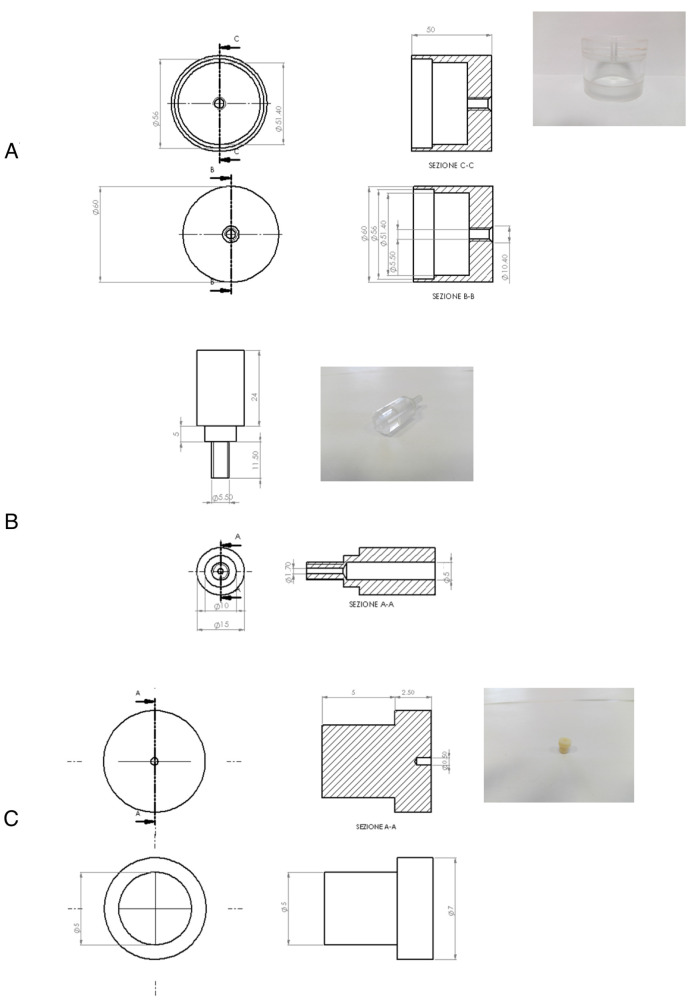
Customized lids in polymethylmethacrylate (PMMA) for 60 mm tissue-culture dish (50 mm i.d x 60 mm h) having a suitable support for Solid Phase Micro-Extraction fiber insertion (1 mm i.d. x 40 mm h) (Figure 1). This system is made of three parts: a) the lid; b) the SPME fiber support and c) Shimadzu GC septa (Shimadzu, catalog number: 201-35584). Details are shown in Figure 2
Figure 2. Details of the PMMA customized lid.
A. The lid; B. The SPME fiber support; C. the Shimadzu GC septa (Shimadzu). Gelatin from porcine skin, Type A (Sigma-Aldrich, catalog number: G1890)
Dulbecco’s phosphate buffered saline (D-PBS) (Mediatech, catalog number: 21-031-CV)
Collagenase type XI (Sigma-Aldrich, catalog number: C7657)
0.25% trypsin-EDTA (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056)
Trypsin-EDTA (CARLO ERBA Reagents, catalog number: FA30WL0940100)
Collagenase type IV (Sigma-Aldrich, catalog number: C5138)
Accutase (Merck, catalog number: SCR005)
Poly-L-ornithine solution (Sigma-Aldrich, catalog number: P4957)
2-Ethyl-1-Hexanol (Sigma-Aldrich, catalog number: 08607)
Styrene (Sigma-Aldrich, catalog number: 45993)
Hexanal (Sigma-Aldrich, catalog number: 18109)
3-Hexen-1-ol, propanoate, (Z)- (Sigma-Aldrich, catalog number: W393304)
Acetone (Sigma-Aldrich, catalog number: 48358)
o-Cymene (In NIST 127 and NIST 147 mass spectral libraries it appears with the alternative name: Benzene, 1-methyl-2-(1-methylethyl)-) (Sigma-Aldrich, catalog number: 255270)
1,4-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- (Sigma-Aldrich, catalog number: 86476)
Propanoic acid, 2-hydroxy-2-methyl-, ethyl ester (Sigma-Aldrich, catalog number: E31200)
Nonanal (Sigma-Aldrich, catalog number: 442719)
Vinyl butyrate (Sigma-Aldrich, catalog number: 19390)
Tridecane (Sigma-Aldrich, catalog number: 442713)
Decanal (Sigma-Aldrich, catalog number: 59581)
Propanoic acid, 2-methyl-, anhydride (Sigma-Aldrich, catalog number: 245771)
Butanoic acid, 2-methylpropyl ester (Sigma-Aldrich, catalog number: 94888)
Hexadecane (Sigma-Aldrich, catalog number: 442679)
Methanone, (4-aminophenyl)phenyl- (Sigma-Aldrich, catalog number: A41402)
Heptadecane (Sigma-Aldrich, catalog number: 51578)
3,5-Dimethyl-4-octanone (Sigma-Aldrich, catalog number: S504696)
Butanoic acid, anhydride (Sigma-Aldrich, catalog number: 19270)
Mouse Laminin (Merck, catalog number: CC095)
CHANG MEDIUM C Lyophilized (Irvine Scientific, catalog number: T101-019)
Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10270)
Penicillin-streptomycin solution (CARLO ERBA Reagents, catalog number: FA30WL0022100)
L-Glutamine (CARLO ERBA Reagents, catalog number: FA30WX0550100)
Dulbecco’s modified Eagle’s medium–high glucose (DMEM) (Sigma-Aldrich, catalog number: D5671)
DMEM/F-12 1:1 (Sigma-Aldrich, catalog number: D6421)
Knockout SR Serum replacement (KOSR) (Thermo Fisher Scientific, GibcoTM, catalog number: 10828028)
MEM non essential amino acids (Thermo Fisher Scientific, GibcoTM, catalog number: 11140050)
2-Mercaptoethanol (Thermo Fisher Scientific, GibcoTM, catalog number: 31350010)
Basic fibroblast growth factor (bFGF) (Thermo Fisher Scientific, GibcoTM, catalog number: PHG6015)
Retinoic acid (Sigma-Aldrich, catalog number: R2625)
Hedgehog pathway activator (Hh-Ag1.3) (Curis, Lexington, MA)
Human BDNF (PeproTech, catalog number: 450-02)
Recombinant Human CNTF (PeproTech, catalog number: 450-13)
Human GDNF (PeproTech, catalog number: 450-10)
Chang medium (CVS medium) (see Recipes)
MEF medium (see Recipes)
hiPS cell medium (see Recipes)
EB medium 1 (see Recipes)
EB medium 2 (see Recipes)
Figure 1. Customized lid in polymethylmethacrylate (PMMA).
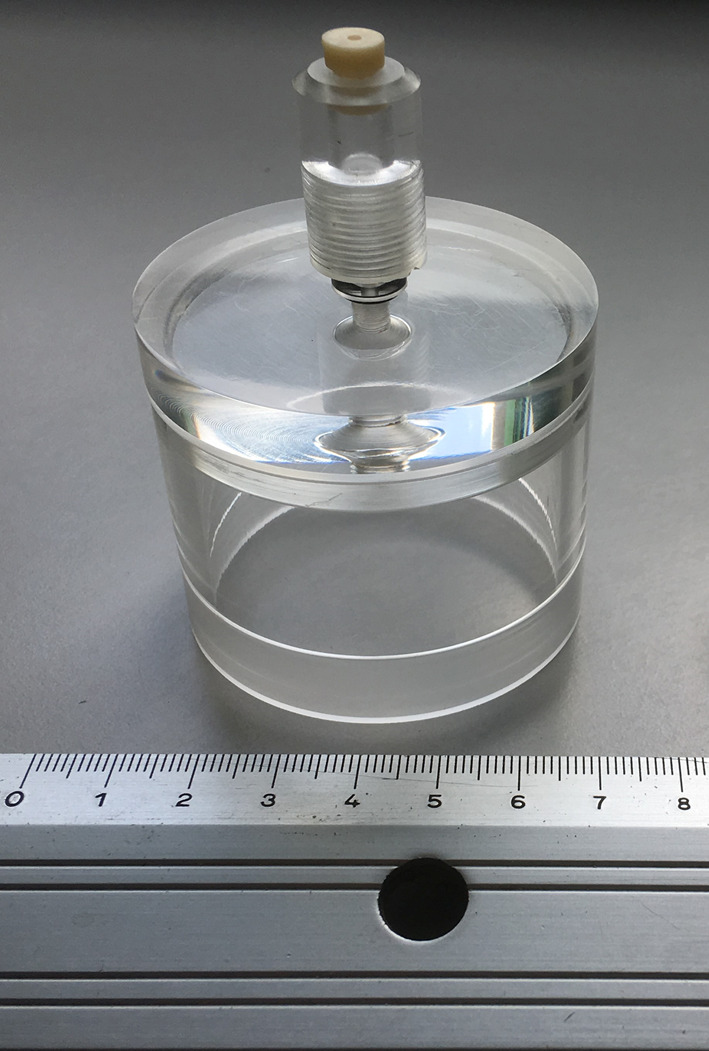
This system is suitable for headspace creation above 60 mm tissue-culture dishes and it is equipped with a support for Solid Phase Micro-Extraction fiber insertion to allow headspace VOC sampling.
Equipment
CO2 incubator (37 °C) (Thermo Fisher Scientific, Thermo ScientificTM, model: HeracellTM 150i)
Inverted microscope (Nikon Instruments, model: Eclipse TE2000-S)
Pipettes (20 µl, 200 µl and 1000 µl) (Gilson, catalog number: F167300)
Centrifuge (provided with TX-200 swinging bucket rotor (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 75003658) containing round buckets (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 75003659) suitable to process up to 20 x 15 ml conical tubes. Flexible capacity with adapters ranging from 15 to 50 ml. Maximum speed 5,500 rpm) (Thermo Fisher Scientific, Thermo ScientificTM, model: HeraeusTM MultifugeTM X1, catalog number: 75004210)
Herasafe KS, Class II biological safety cabinet with UV surface disinfection irradiator (Thermo Fisher Scientific, Thermo ScientificTM, model: HerasafeTM KS, Class II, catalog number: 51022481)
Zoom stereomicroscope (Nikon Instruments, model: SMZ100)
Gas chromatography-mass spectrometry (GC/MS) system (Shimadzu, model: GCMS-QP2010)
SPME liner for SPL injector, special SPME liner, without filling, Phenylmethyl-deactivated deactivated (Shimadzu, catalog number: 961-01482-01)
Equity-5 capillary column (poly(5% diphenyl/95% dimethyl siloxane) phase; 30 m length x 0.25 mm i.d.; film thickness: 0.25 μm) (Sigma-Aldrich, catalog number: 28089-U)
Ultra-high purity helium (99.999%)
Software
GCMS solution (version 2.4, Shimadzu Corporation)
NIST 127 and NIST 147 mass spectral libraries (National Institute of Standards and Technology-http://www.nist.gov/)
MATLAB® (version R2016 b, MathWorks)
Procedure
-
Preparation of cell culture sample for volatile metabolite analysis
-
1
Chorionic villus samples (CVS) from three independent samples
60 mm culture dishes are coated with 2 ml of 0.1% gelatin and incubated at 37 °C for at least 20 min.
CVS cells are disaggregated after several PBS washes by serial incubation with collagenase XI at 37 °C for 30 min, followed by 0.25% trypsin-EDTA at 37 °C for 10 min.
Aggregates are dissociated using gentle pipetting, centrifuged at 266 × g for 10 min and seeded on three gelatin-coated 60 mm culture dishes with 2 ml Chang medium (see Recipes) as previously described in Spitalieri et al., 2009 .
CVS cells are detached from 25 cm2 culture flasks using trypsin and seeded at 2.5 x 106 with 2 ml Chang medium (see Recipes) on three gelatin-coated 60 mm culture dishes.
Cell culture dishes are incubated at 37 °C in an incubator with 5% CO2 for 24 h.
-
2
Human induced pluripotent stem cells (hiPSCs)
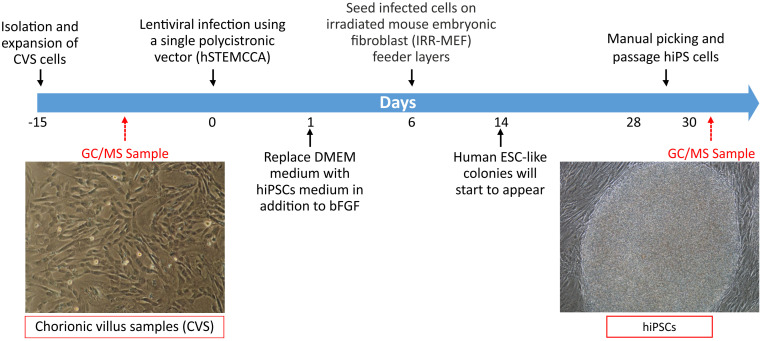
CVS cells are reprogrammed in human pluripotent stem cells (hiPSCs) using a single lentiviral ‘stem cell cassette’, flanked by loxP sites (hSTEMCCA-loxP), encoding for reprogramming factors (OCT4, SOX2, KLF4, and c-MYC) in a single polycistronic vector ( Spitalieri et al., 2015 ). Details are reported in Figure 3.
Once derived, hiPS cells are expanded, using mechanical passage under the stereomicroscope when reach confluence (Please see Note 1) and placed on irradiated mouse embryonic fibroblast (MEF) feeder layers on until line is well established in 35 mm culture plates for at least five passages.
hiPSC medium (see Recipes) is changed every day. Please see Note 2.
Three independent hiPS cell lines with different genotype are passaged on irradiated mouse embryonic fibroblast (MEF) feeder layers with 2 ml hiPSC medium on 60 mm culture dish (4 x 106 cells/dish). Please see Note 2.
Cell culture dishes are incubated at 37 °C in an incubator with 5% CO2 for 24 h.
-
3
Floating EBs
Days 1 to 6
-
a
Neural induction is performed after the formation of cell aggregates called embryoid bodies (EBs) as reported, with some modifications, by Nakahama and Di Pasquale, 2016.
-
b
hiPS cell lines are treated with collagenase IV/Accutase solution at 37 °C for 8 min and detached from the plate bottom using a cell scraper.
-
c
The colonies are collected in a 15 ml conical tube, centrifuged at 17 x g for 5 min and plated in hiPSC medium without basic fibroblast growth factor (bFGF) on ultra-low attachment plates in 2:1 ratio (e.g., for two 35 mm dish of hiPSCs, use 1 dish of 60 mm ultra-low attachment dish). The cell pellet is gently resuspended, avoiding to break up clumps too much. Please see Note 3.
-
d
hiPSC medium without bFGF is daily changed for the first 6 days, except for the day after passage. Please see Note 4.
-
e
To change medium EBs are collected in a 15 ml conical tube, centrifuged at 17 × g for 5 min and gently resuspended in fresh medium.
-
f
Three independent floating EBs samples (2 x 106 samples/dish) are placed in 2 ml hiPSC medium without basic fibroblast growth factor (bFGF) on 60 mm ultra-low attachment culture dishes.
-
g
Cell culture dishes are incubated at 37 °C in an incubator with 5% CO2 for 24 h.
Days 7 to 13
-
h
On day 7, EBs are collected using a 10 ml pipette into a 15 ml conical tube under the stereomicroscope, centrifuged at 17 × g for 5 min and gently resuspended in EB medium 1 (see Recipe 4).
-
i
Cell culture dishes are incubated at 37 °C in an incubator with 5% CO2 and EB medium 1 is daily changed for another week.
Days 14 to 20
-
j
On day 14, EB medium 1 is removed and replaced with EB medium 2 (see Recipe 5), following the same procedure reported above at step A3e.
-
k
Cell culture dishes are incubated at 37 °C in an incubator with 5% CO2 and EB medium 2 is daily changed for another 7 days.
-
a
Day 21
-
4
Early Neural Progenitors (NPs)
60 mm Petri dishes are coated with 0.01% poly-L-ornithine overnight at 37 °C and then with 20 µg/ml laminin for 2 h at 37 °C. The plates are washed with PBS three times and left in a laminar flow hood to dry ( Varga et al., 2014 ).
Floating EBs are collected into a 15 ml conical tube, centrifuged at 17 × g for 5 min and gently resuspended and transferred onto 60 mm poly-L-ornithine/laminin-coated-plates ( Varga et al., 2014 ) in 2 ml EB medium 2.
-
Cell culture dishes are incubated at 37 °C in an incubator with 5% for 24 h. All samples preparation are performed as previously described in Capuano et al., 2017 .
Differentiation steps of hiPSCs into NPs and timeline of analysis are shown in Figure 4.
-
1
-
Analysis of cell culture volatile metabolite by SPME linked to GC/MS
-
This protocol is applied to the analysis of the following samples:
CVS and hiPSCs after an incubation time of 24 h (after steps A1d and A2d respectively);
Floating EBs at different differentiation steps on days 1 and 4, 9 and 11, 15 and 18 (during phases A3e, A3g, A3i respectively);
Early Neural Progenitors (NPs) 24 h after plating (after step A4b);
Chang medium;
hiPSC medium;
hiPSC medium without bFGF;
EB medium 1;
EB medium 2.
Note: Cell culture media are always previously incubated for 24 h before VOC sampling. These are the ‘sample zero’ for the analysis of each cell line.
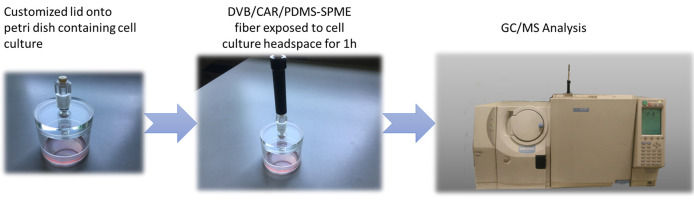
The customized lid is sterilized under UV light in a biological hood for 20 min before any sampling procedure, in order to prevent culture contamination.
Immediately after sterilization, the lid is put on the Petri dish containing the cell culture of interest cultivated in the incubator.
-
SPME fiber holder is assembled (Figure 5).
To ensure the reproducibility of the volatile compounds sampling, it is recommended to set the depth of the SPME needle at the same value. In this experiment, the sampling system required to set it to the 4-depth gauge setting on the plunger.
Note: In order to prevent SPME fiber contamination, it is better to assemble this component immediately before its use. Twelve hours before any sampling procedure, SPME fibers have been conditioned following SUPELCO guidelines: DVB/CAR/PDMS fiber was baked at 270 °C for 1 h and then stored in its own box until use.
The needle protecting SPME fiber is inserted in the appropriate lid support and then the fiber is exposed to the cell culture headspace.
Volatile metabolites are preconcentrated using Solid-Phase Microextraction (SPME) technique ( Capuano et al., 2017 ). A 50/30 μm Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PSMS) fiber (SUPELCO) is inserted for 1 h to cell culture headspace. Samples are maintained in the incubator at a temperature of 37 °C and 5% CO2 during fiber exposure, in order to avoid any stress effect on cell growth ( Capuano et al., 2017 ).
After VOC adsorption, fiber is pulled inside the needle and removed from the holder to be placed in its box; fiber is stored at 4 °C until GC/MS analysis, performed within three hours.
-
GC/MS analysis is carried out using a GCMS-QP2010 (Shimadzu) equipped with an Equity-5 (or equivalent) capillary column and a proper liner for SPME (for details see Equipment section). The volatile compounds adsorbed onto DVB/CAR/PDMS fiber are desorbed in splitless mode at 250 °C for 3 min in the GC injection port. High-purity helium is used as a carrier gas at 0.7 ml/min of column flow, total flow of 5.9 ml/min and pressure of 24.9 kPa. Analysis is performed working in linear velocity constant mode at 30.2 cm/sec. Inlet, ion source and transfer line temperatures are maintained at 250 °C. Initial oven temperature of 40 °C is maintained for 5 min, followed by temperature gradient to 220 °C at 7 °C/min, then increased by 15 °C/min to a final temperature of 300 °C, held for 3 min (total runtime: 39 min). The mass spectrometer is a single quadrupole analyzer operating in electron ionization mode, with electrons energy of 70 eV. Mass spectra are recorded from 40 to 450 m/z in full scan mode.
Steps from B2 to B8 are summarized in Figure 6.
Chromatogram acquisition and data elaboration are made with Shimadzu workstation software GCMS solution (version 2.4). Chemical identification of detected peaks is performed using both NIST 127 and NIST 147 libraries. Examples of GC/MS spectra are shown in Figures 7 and 8.
The identity of those compounds in Table 1 which are commercially available was validated comparing the retention times and the mass spectra registered in sample chromatograms with those obtained from the analysis of a solution composed by standard compounds and culture media. The VOC collection and the analysis have been performed following the same procedure used for the samples (see steps B6-B8). The abundance of a subset of these compounds was found statistically different among the cells lines ( Capuano et al., 2017 ).
-
Styrene and 2-ethyl-1-hexanol are identified and quantified by comparing the retention times and the mass spectra with those obtained with analytical standards. Three concentrations of Styrene and 1-hexenol-2-ethyl are prepared diluting 1, 10, 100 times the pure compounds in 2 ml of different culture media in 60 mm Petri dishes. VOCs in the headspace are collected by DVB/CAR/PDMS-SPME fiber exposed for 1 h to the sample. The concentration (Cx) of Styrene and 2-ethyl-1-hexanol in the headspace of standard solutions is estimated by Antoine’s law (considered in parts per billion by volume (ppbv)):
where, P is the vapor pressure (bar) and T the working temperature (K). Parameters A, B and C are empirical constants depending on the nature of the substance using the parameters available, in NIST database (http://webbook.nist.gov/chemistry). The amount (Ax) of styrene and 2-ethyl-1-hexanol (in ppbv) in the cells culture headspace (CVS, hiPSCs, EBs at different stage, and early NPs) and their culture media is calculated from the peak area in the sample (PAx) and the peak area of the standard (PSx), as follows:
Note: This procedure is valid for any compound identified by an analytical standard.
-
Figure 3. Timeline of GC/MS analysis in CVS cells and hiPSCs.
CVS cells are isolated from human samples, analyzed for volatile metabolite by SPME linked to GC/MS and then reprogrammed in hiPSCs. Embryonic stem cell-like colonies are observed on feeder layers after 14 days of infection and after expansion using mechanical passage are subjected to VOCs analysis.
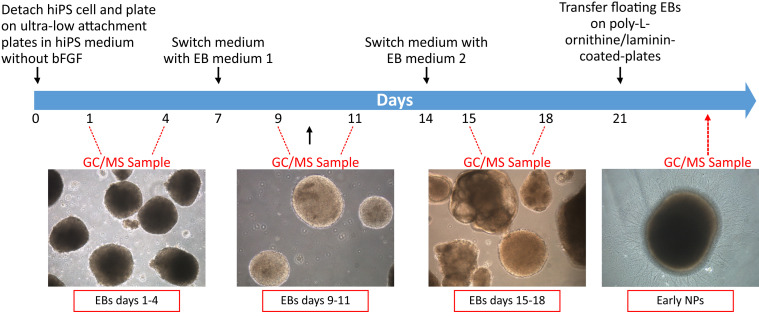
Figure 4. Timeline of GC/MS sampling during NPs differentiation.
Human induced pluripotent stem cells (hiPSCs) are differentiated into early neural progenitors (NPs) through the formation of floating embryoid bodies (EBs). VOCs analysis is carried out at different days of differentiation (floating EBs days 1-4, floating EBs days 9-11, floating EBs days 15-18 and early NPs 24 h after plating).
Figure 5. SPME fiber equipment.
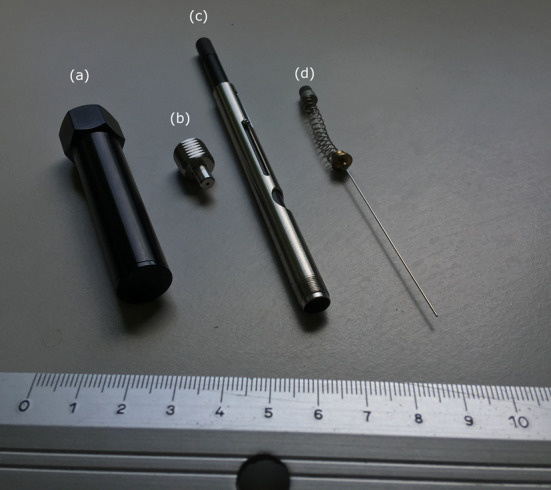
Components (a), (b) and (c) constitute the SPME Fiber Holder for use with manual sampling; the element (d) is the needle system that contains and protects DVB/CAR/PDMS fiber.
Figure 6. Workflow of VOC analysis on cell culture by SPME coupled to gas chromatography-mass spectrometry.
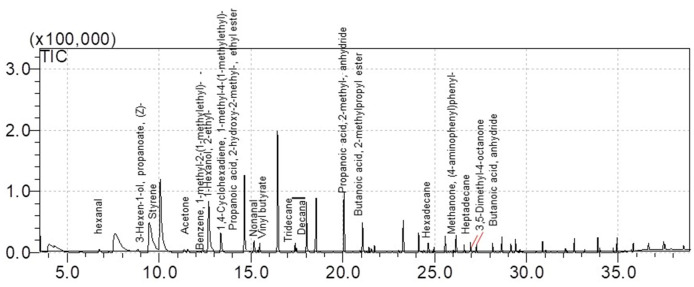
Figure 7. GC/MS spectrum of the volatile metabolites of floating EBs cells culture at day 9, derived from total ion chromatograms.
. Data elaborated with the GCMS solution software. Volatile compound profile may differ from those shown here, in terms of composition and concentration, because of altered cells culturing conditions and/or different considered cell lines.
Figure 8. Comparison of GC/MS spectra derived from total ion chromatograms of the volatile metabolic profiles of Chorionic villus (CVS) (red line), floating embryoid bodies (EBs) at day 9 (black line), human induced pluripotent stem cells (hiPSCs) (blue line) and early neural progenitors (NPs) (pink line).
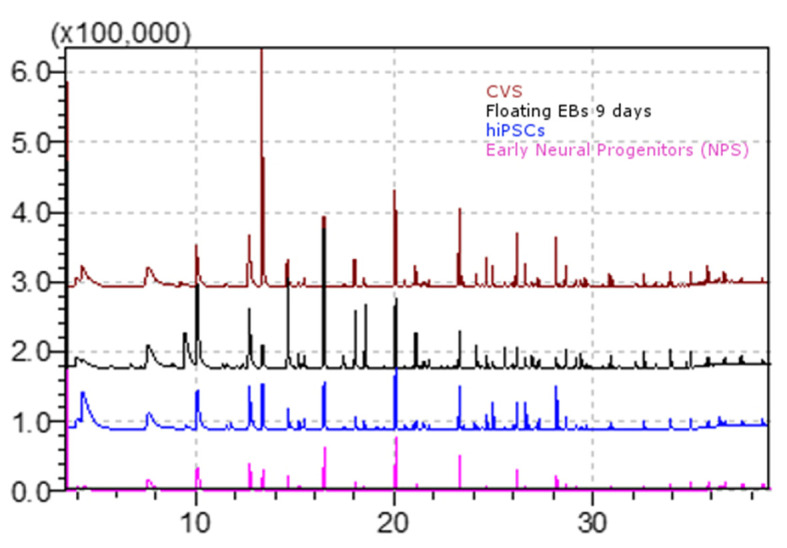
Data elaborated with the GCMS solution software. Volatile compounds profiles may differ from those shown here, in terms of composition and concentration, as a result of altered cell culturing conditions and/or different considered cell lines.
Table 1. List of compounds found in the chromatogram of all samples and for which identification has been validated with an analytical standard.
| Retention time (min) | Compound name | Main peaks (m/z) | CAS # |
| 6.725 | Hexanal | 44 | 66-25-1 |
| 9.270 | 3-Hexen-1-ol, propanoate, (Z)- | 57 67 82 | 33467-74-2 |
| 9.486 | Styrene | 104 | 629-20-9 |
| 11.342 | Acetone | 43 | 67-64-1 |
| 13.204 | Benzene, 1-methyl-2-(1-methylethyl)- | 119 | 527-84-4 |
| 13.345 | 1-Hexanol, 2-ethyl- | 41 43 57 | 104-76-7 |
| 14.098 | 1,4-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- | 93 | 99-85-4 |
| 14.424 | Propanoic acid, 2-hydroxy-2-methyl-, ethyl ester | 59 | 80-55-7 |
| 15.178 | Nonanal | 41 43 57 | 124-19-6 |
| 15.250 | Vinyl butyrate | 43 71 | 123-20-6 |
| 17.404 | Tridecane | 43 57 71 | 629-50-5 |
| 17.469 | Decanal | 41 43 55 57 | 112-31-2 |
| 20.553 | Propanoic acid, 2-methyl-, anhydride | 43 71 | 97-72-3 |
| 20.958 | Butanoic acid, 2-methylpropyl ester | 43 56 71 | 539-90-2 |
| 24.967 | Hexadecane | 43 57 71 85 | 17301-25-6 |
| 26.324 | Methanone, (4-aminophenyl)phenyl- | 120 197 | 1137-41-3 |
| 26.609 | Heptadecane | 43 57 71 85 | 629-78-7 |
| 26.825 | 3,5-Dimethyl-4-octanone | 43 57 71 | 7335-17-3 |
| 27.500 | Butanoic acid, anhydride | 43 71 | 106-31-0 |
Data analysis
Each kind of cell line and culture medium is replicated three times in independent samples.
Measured chromatograms are integated and the absolute area of each peak is considered to estimate VOC abundance.
Chromatographic peak alignment is performed manually, considering retention time and mass spectra of peaks in the several chromatograms.
GC/MS VOC abundance data are arranged in matrices where each row corresponds to a sample and columns contain area under the curve of each integrated peaks. Data have been analyzed with multivariate techniques such as Principal Component Analysis (PCA) ( Capuano et al., 2017 ).
The statistical significance of VOC abundances among different cell lines are evaluated by a parametric Kruskal-Wallis rank sum test applied for binary comparison among different cell lines (Table 2). The same test is applied to culture media related data, in order to eliminate VOCs characterizing different media rather than cell metabolism.
Significant compounds in culture media discrimination, resulting from Kruskal-Wallis rank sum test, are not considered for multivariate data analysis.
The abundance of all compounds selected by the Kruskal-Wallis rank sum test (P < 0.05) forms the pattern of cell headspace. Not normalized data are analyzed using the principal component analysis (PCA). This multivariate method allows comparing the different cell line data in order to represent differences and similarities between them graphically. Results of PCA are thoroughly discussed in Capuano et al., 2017 .
All data analysis is performed using MATLAB.
Table 2. Cell culture couples considered for binary comparison using Kruskal-Wallis rank sum test.
|
Notes
Mechanical passage of hiPS cells has to be performed using a stereomicroscope under a regular laminar flow hood. Cells should be passaged approximately every 5 days, at around 60-75% confluence, without the colony of cells touching each other, removing differentiating areas with pulled glass Pasteur pipettes.
hiPS cell medium must be renewed every day for optimal growth, however, occasional double feeding (adding twice the required volume of medium during one feed) is possible without affecting culture quality. For instance, it is possible to perform a double feed on a Friday, with the next medium change on Sunday. hiPS cell medium must be pre-warmed at room temperature and protected from light, suggesting to thaw and add growth factor to the medium just prior to use.
Avoid over-pipetting to break up the colonies too much, checking the size under the stereomicroscope.
Recipes
-
Chang medium
CHANG MEDIUM C Lyophilized containing the following supplements:
10% fetal bovine serum (FBS)
100 U/ml penicillin
100 mg/ml streptomycin
2 mM L-glutamine
-
MEF medium
D-MEM containing the following supplements:
10% fetal bovine serum (FBS)
100 U/ml penicillin
100 mg/ml streptomycin
2 mM L-glutamine
-
hiPS cell medium
DMEM/F-12 containing the following supplements:
20% KnockOut Serum Replacement (KOSR)
100 U/ml penicillin
100 mg/ml streptomycin
2 mM L-glutamine
1% MEM non essential amino acids
0.1 mM β-mercaptoethanol
Finally, the medium is sterilized using a 0.22 μm bottle-top vacuum filter and supplemented with 10 ng/ml basic fibroblast growth factor (bFGF)
-
EB medium 1
hiPS cell medium without bFGF supplemented with:
1 µM RA (retinoic acid) 500 mM Hh-Ag1.3 (sonic-hedgehog pathway agonist)
-
EB medium 2
hiPS cell medium minus bFGF supplemented with:
1 µM RA
500 mM Hh-Ag1.3 (a Sonic Hedgehog pathway agonist)
20 ng/ml Ciliary neurotrophic factor (CNTF)
10 ng/ml brain-derived neurotrophic factor (BDNF)
10 ng/ml glial cell line-derived neurotrophic factor (GDNF)
Acknowledgments
This is a detailed protocol of the analyses reported in Capuano et al. (2017), adapted from previous works ( Spitalieri et al., 2015 ; Murdocca et al., 2016a and 2016b). The authors declare that they have no competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Boots A. W., Bos L. D., van der Schee M. P., van Schooten F. J. and Sterk P. J.(2015). Exhaled molecular fingerprinting in diagnosis and monitoring: validating volatile promises. Trends Mol Med 21(10): 633-644. [DOI] [PubMed] [Google Scholar]
- 2.Capuano R., Spitalieri P., Talarico R. V., Domakoski A. C., Catini A., Paolesse R., Martinelli E., Novelli G., Sangiuolo F. and Di Natale C.(2017). A preliminary analysis of volatile metabolites of human induced pluripotent stem cells along the in vitro differentiation . Sci Rep 7(1): 1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filipiak W., Mochalski P., Filipiak A., Ager C., Cumeras R., Davis C. E., Agapiou A., Unterkofler K. and Troppmair J.(2016). A compendium of volatile organic compounds(VOCs) released by human cell lines. Curr Med Chem 23(20): 2112-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meissen J. K., Yuen B. T., Kind T., Riggs J. W., Barupal D. K., Knoepfler P. S. and Fiehn O.(2012). Induced pluripotent stem cells show metabolomic differences to embryonic stem cells in polyunsaturated phosphatidylcholines and primary metabolism. PLoS One 7(10): e46770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murdocca M., Ciafre S. A., Spitalieri P., Talarico R. V., Sanchez M., Novelli G. and Sangiuolo F.(2016). SMA Human iPSC-derived motor neurons show perturbed differentiation and reduced miR-335-5p expression. Int J Mol Sci 17(8): 1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murdocca M., Mango R., Pucci S., Biocca S., Testa B., Capuano R., Paolesse R., Sanchez M., Orlandi A., di Natale C., Novelli G. and Sangiuolo F.(2016). The lectin-like oxidized LDL receptor-1: a new potential molecular target in colorectal cancer. Oncotarget 7(12): 14765-14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakahama H. and Di Pasquale E.(2016). Generation of cardiomyocytes from pluripotent stem cells. Methods Mol Biol 1353: 181-190. [DOI] [PubMed] [Google Scholar]
- 8.Peled N., Barash O., Tisch U., Ionescu R., Broza Y. Y., Ilouze M., Mattei J., Bunn P. A. Jr Hirsch, Haick F. R., H (2013). Volatile fingerprints of cancer specific genetic mutations. Nanomedicine 9(6): 758-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spitalieri P., Cortese G., Pietropolli A., Filareto A., Dolci S., Klinger F. G., Giardina E., Di Cesare S., Bernardini L., Lauro D., Scaldaferri M. L., Citro G., Novelli G., De Felici M. and Sangiuolo F.(2009). Identification of multipotent cytotrophoblast cells from human first trimester chorionic villi. Cloning Stem Cells 11(4): 535-556. [DOI] [PubMed] [Google Scholar]
- 10.Spitalieri P., Talarico R. V., Botta A., Murdocca M., D'Apice M. R., Orlandi A., Giardina E., Santoro M., Brancati F., Novelli G. and Sangiuolo F.(2015). Generation of human induced pluripotent stem cells from extraembryonic tissues of fetuses affected by monogenic diseases. Cell Reprogram 17(4): 275-287. [DOI] [PubMed] [Google Scholar]
- 11.Sponring A., Filipiak W., Ager C., Schubert J., Miekisch W., Amann A. and Troppmair J.(2010). Analysis of volatile organic compounds(VOCs) in the headspace of NCI-H1666 lung cancer cells. Cancer Biomark 7(3): 153-161. [DOI] [PubMed] [Google Scholar]
- 12.Varga E., Nemes C., Davis R. P., Ujhelly O., Klincumhom N., Polgar Z., Muenthaisong S., Pirity M. K. and Dinnyes A.(2014). Generation of transgene-free mouse induced pluripotent stem cells using an excisable lentiviral system. Exp Cell Res 322(2): 335-44. [DOI] [PubMed] [Google Scholar]