Abstract
Collagen is one of the foremost components of tissue extracellular matrix (ECM). It provides strength, elasticity and architecture to the tissue enabling it to bear the wear and tear from external factors like physical stress as well as internal stress factors like inflammation or other pathological conditions. During normal pregnancy or pregnancy related pathological conditions like preterm premature rupture of membranes (PPROM), collagen of the fetal membrane undergoes dynamic remodeling defining biochemical properties of the fetal membrane. The protocol in this article describes the histochemical method to stain total collagen by Picrosirius red stain which is a simple, quick and reliable method. This protocol can be used on paraformaldehyde (PFA) and formaldehyde fixed paraffin embedded tissue sections. We further describe the staining and distribution of collagen in different mouse reproductive tissues and also demonstrate how this technique in combination with polarization microscopy is useful to detect the distribution of different subtypes of collagen.
Keywords: Collagen, Histochemical staining, Picrosirius red, Reproductive tissues, Birefringence, Polarization
Background
Collagen is the principal load-bearing polymer in all connective tissues ranging from skin to bone. Collagen networks strongly stiffen when a mechanical force is applied, thus preventing excessive deformation of the tissue. There are 16 types of collagen, of which the type I, II, and III nearly comprise the 80% of the collagen in the body that are packed together to form long thin fibrils. Collagen type IV forms a two-dimensional reticulum; while several other collagen types are associated with fibril-type collagen, linking them to each other or to other matrix components. These collagens along with the other components of the extracellular matrix (ECM) undergo constant remodeling to provide required biochemical properties like tensile strength and elasticity. This unique attribute of collagen is one of the influencing factors of the stability of reproductive tissues and its dysregulation can lead to adverse events such as abnormal placentation, rupture of membranes ( Hampson et al., 1997 ; Marpaung, 2016) and pathological conditions of the reproductive tract, such as endometriosis (Shimizu and Hokano, 1990) etc.
In tissues, the basement membrane is rich in collagen, in addition it is found in the stroma and lining the connective tissues. Physical, mechanical or chemical damage of a tissue or organ would lead to disruption of collagen deposition, and organization. Hence, assessing the patterns of collagen distribution would provide us an idea about the tensile strength of the tissue/organs. Any alterations from normal patterns of collagen distribution would imply tissue damage. In this study, chorio-decidual tissue has been used as a model basement membrane. Changes in the biochemical properties of this feto-maternal membrane during pregnancy and various pathological conditions lead to its preterm rupture (Sebire, 2001; Fujimoto et al., 2002 ; Wang et al., 2004 ; Vega Sánchez et al., 2004 ; Surve et al., 2016 ).
Sirius red is a histology stain used to mark total collagen as well as differentiate between varying collagen types for evaluation of collagen distribution in tissues. The sulphonic acid group of Sirius red reacts with basic amino groups of lysine and hydroxylysine and guanidine group of arginine (present in the collagen molecule ( Junqueira et al., 1979 )). Thereby, being an anionic dye, it attaches to all the varying types of collagen isoforms. In bright field, collagen appears as bundles of pink to red fibers which get disturbed in pathological conditions. The same larger collagen fibers under polarized light appear bright yellow to orange and the thinner ones, including reticular fibers, look green. This birefringence or double refraction, whereby incident light is split by polarization into two different paths, is highly specific for collagen. The amount of polarized light absorbed by the Sirius red dye stringently depends on the orientation of the collagen bundles enabling to differentiate different collagen types ( Junqueira et al., 1979 ; Lattouf et al., 2014 ). This method is very simple, quick, economic and reliable in comparison with other commonly used staining methods for collagen.
Materials and Reagents
Glass coverslips (HiMedia Laboratories, catalog number: CG108)
Glass slides (size, 76 x 26 mm) (HiMedia Laboratories, catalog number: CG029)
Paraffin mold and embedding cassette (Simport, catalog number: M490-2)
Mice strain: C57BL/6 Black (Experimental Animal Facility, ICMR-National Institute of Research in Reproductive Health)
Distilled water (D/W)
Formaldehyde (Sigma-Aldrich, catalog number: F8775)
Xylene (Merck, catalog number: 1086342500)
Methanol (Merck, catalog number: 1070182521)
Ethanol (Merck, catalog number: 1085430250)
Paraformaldehyde (Sigma-Aldrich, catalog number: P6148)
Sodium phosphate monobasic (NaH2PO4)
Sodium phosphate dibasic (Na2HPO4)
Sodium chloride (NaCl)
Potassium chloride (KCl)
Potassium phosphate monobasic (KH2PO4)
Paraffin wax (Merck, catalog number: 1073371000)
Poly-lysine (Sigma-Aldrich, catalog number: P8920)
Picric acid (Fisher Scientific, catalog number: 13205)
Direct Red 80 (Sigma-Aldrich, catalog number: 365548)
Glacial acetic acid (CH3COOH) (Merck, catalog number: 1.93002)
Haematoxylin (C.I. 75290) (EMD Millipore, catalog number: 104302)
Iron(III) chloride (ferric chloride) (Merck, catalog number: 803945)
Sodium bicarbonate (Merck, catalog number: 106329)
Hydrochloric acid (37%) (Merck, catalog number: 1.93001)
D.P.X. mountant liquid (HiMedia Laboratories, catalog number: GRM655)
Weigert’s haematoxylin solution (see Recipes)
4% paraformaldehyde (PFA) (see Recipes)
10% formaldehyde (see Recipes)
Phosphate buffered saline (PBS) (see Recipes)
Poly-lysine coated glass slides (see Recipes)
Picrosirius red solution (see Recipes)
Acidified water (see Recipes)
Equipment
Coplin jar (Thermo Scientific, catalog number: 107)
Bright field microscope (Leica, model: Leica DMi8)
-
Polarization microscope (Leica, model: Leica DMi8)
Note: Items 2 and 3 are the same microscope. For polarization applications, a polarizer (Leica Microsystems, Germany) is placed in the light path of the bright field microscope.
Hot plate (LED Digital, Lab Depot, model: MS7-H550-S)
Microtome (Leica, model: Leica RM2255)
Procedure
Sacrifice mice by cervical dislocation as per the institute ethical guidelines and dissect it to collect the respective tissues. Briefly, following euthanization, make a 1.5-cm midline incision in the lower abdomen and collect different reproductive tissues. To obtain chorio-decidua, incise the bicornuate uterine horns longitudinally along the anti-mesenteric border and collect the fetal membrane.
Fix the tissue overnight at 4 °C in 10% buffered formaldehyde or 4% PFA.
Wash 5 times in PBS (see Recipes) and dehydrate in methanol gradient series (30%, 50%, 70%, 90%, 100% 30 min each) and xylene till tissue gets translucent.
Add molten paraffin (heated at 60 °C on a hot plate) in xylene (v/v, 1:1) and incubate at room temperature for 15 min.
Add molten wax to the tissues and incubate at 60 °C for 15 min. Change the wax and leave the tissues in wax at room temperature.
Next day melt the wax containing the tissue and give one more change of wax and the final incubation lasts for 15 min. For large tissue, the incubation time in wax may be increased to 30-45 min. The timing has to be empirically determined to suit the tissue type.
Pour molten paraffin in the mold and place the tissue in the desired orientation (placing it vertically) and overlay with embedding cassette on it. Leave it overnight at room temperature to completely solidify.
De-mold paraffin embedded tissue along with embedding cassette and section according to standard histopathology protocols using a microtome.
Collect the sections on a poly-lysine coated slide (see Recipes) and let it dry overnight at 37 °C.
Warm the sections on a hot plate (at 60 °C) and quickly dip in xylene containing Coplin jar.
De-paraffinize the sections in xylene twice for 30 min each. Ensure that the sections are nearly translucent.
Dip the slides through either methanol or ethanol gradient series (100%, 90%, 70%, 50%, 30%, D/W 5 min each).
Stain nuclei with haematoxylin (see Recipes) for 5-10 min.
Wash the slides in running tap water for 2 min.
Put the slides in Picrosirius red (see Recipes) containing Coplin jar for 1 h.
Wash the slides twice with fresh acidified water (see Recipes) (5 min each).
Wash the slides in D/W and dehydrate the sections through either methanol or ethanol gradients (50%, 70%, 90% for 5 min each).
Give three changes of 100% ethanol (5 min each).
Incubate in xylene for 30 min (two changes).
Mount in a D.P.X. and let it dry overnight.
Observe under a bright field or polarized microscope. Images can be captured using a routine digital camera or a CCD.
Data analysis
Haematoxylin stains cell nucleus violet in color. However, cell nuclei may appear black or brown or grey. Under a bright-field microscope, collagen appears pink to red in color on a pale yellow background of cytoplasm as a result of staining with Sirius red dye. The Picrosirius red staining not only provides information about collagen distribution in a tissue sample, but also detects bundles of collagen fibers in tissue sections. If one is studying disruption of collagen fibers leading to interruption of bundle formation, staining of collagen by this method can be of great value.
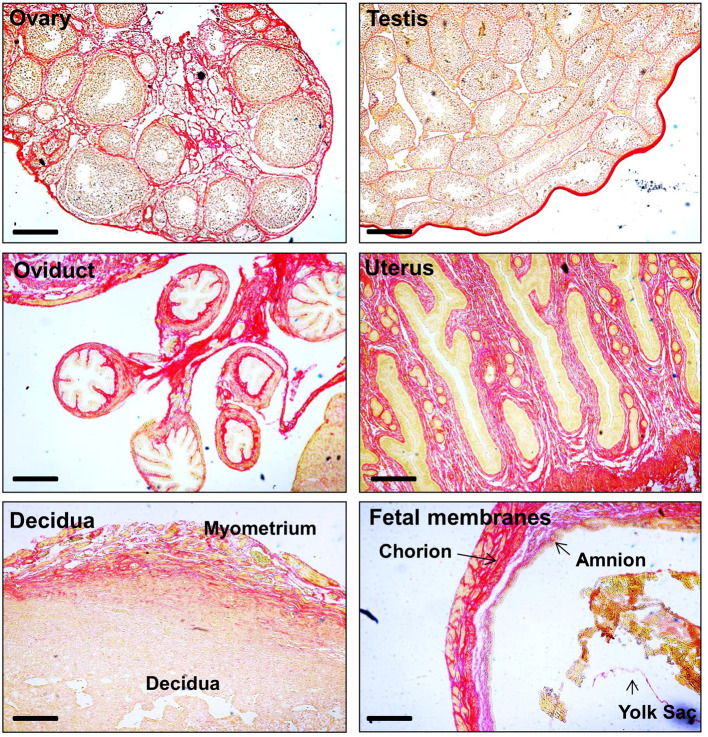
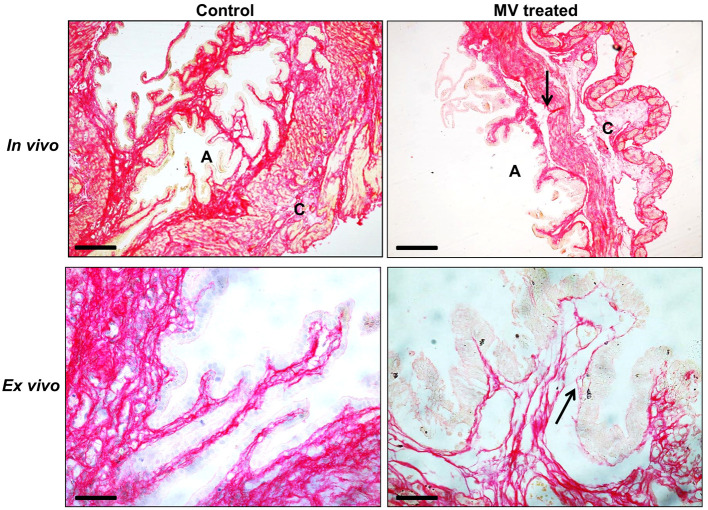
Using Picrosirius red staining, we could demonstrate varying distribution of collagen in mouse reproductive tissues (Figure 1). To demonstrate how this technique is useful to detect collagen degradation, mouse chorio-decidua/amnion was treated with membrane vesicles (MVs) from group B Streptococcus (GBS) which has collagenase activity ( Surve et al., 2016 ). In the normal tissues, bundles of collagen are seen in the chorion and amnion. However, this is fragmented upon treatment with GBS MV. The fragmentation and degradation are clearly visible in the GBS MV treated tissues (Figure 2).
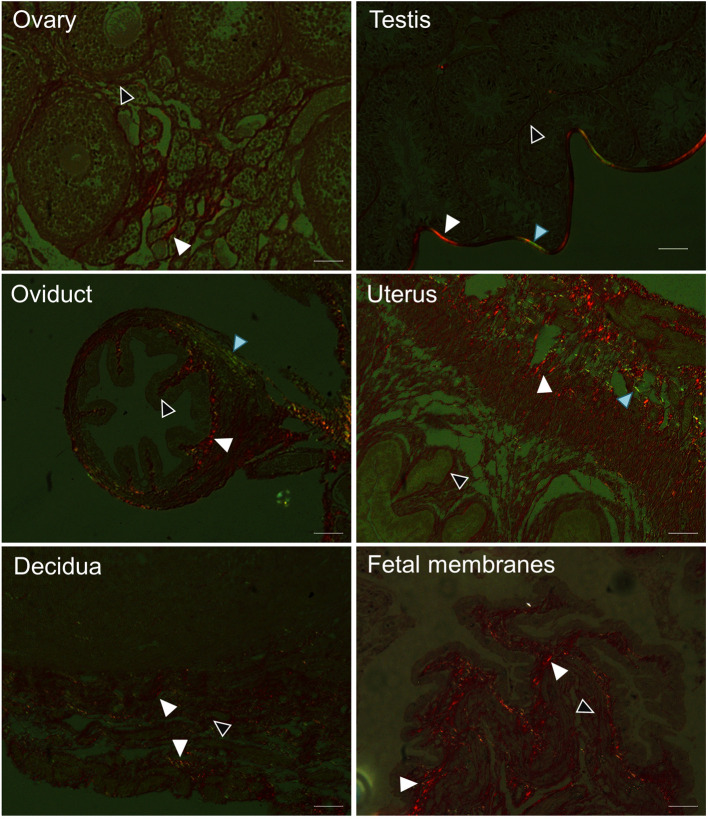
We utilized the birefringence property of the different forms of collagen to demonstrate their distribution in various mouse reproductive tissues by polarization microscopy (Figure 3). Under polarization microscope, collagen I appears yellow-red (white arrowhead), collagen III appears green (blue arrowhead) and collagen IV shows weak to no birefringence (white outlined black arrow head) (Montes and Junqueira, 1991). A summary of these findings is shown in Table 1.
Figure 1. Collagen staining by Picrosirius red dye in various mouse reproductive tissues and fetal membrane.
Red to pink is collagen stain. Yellow is cytoplasm. Scale bars = 50 µm.
Figure 2. Collagen degradation in the mouse amnion (A) and chorion (C) in response to membrane vesicles (MVs) from GBS.
In vivo data are from mouse injected with GBS MVs in the amniotic sacs on E16.5. Ex vivo are chorio-amnion incubated with GBS MVs for 16h. Arrows show collagen degradation. Scale bars = 50 µm.
Figure 3. Detection of collagen subtypes by polarization microscopy of Picrosirius red dye stained mouse tissues.
Collagen I is yellow-red (white arrow head), collagen III is green (Blue arrow head), collagen IV is weak to no birefringence (white outlined black arrow head). Scale bars = 50 µm.
Table 1. Distribution of collagen in mouse reproductive tissues and in pregnancy as detected by Picrosirius red dye staining coupled with polarization microscopy.
| Collagen distribution as judged by polarization microscopy | |||
|---|---|---|---|
| Birefringence | Yellow-red | Green | Weak to none |
| Collagen I | Collagen III | Collagen IV | |
| Testis | Present | Present | Present |
| Ovary | Weakly Present | Absent | Present |
| Endometrium | Present | Present | Present |
| Oviduct | Present | Present | Present |
| Decidua (E8.5) | Absent | Absent | Weakly Present |
| Fetal membranes | Present | Present | Present |
Notes
The staining of collagen fibers and its distribution are fairly constant from day to day basis. The staining is reproducible with varying tissues with the same protocol.
Even if the tissue looks stained in shorter time, it should not be taken out before 1 h.
No differences in collagen staining were observed in fresh tissue sections vs. those that were stored at room temperature for more than 2-3 months. Sections that were stained earlier (2 months) with Picrosirirus red demonstrated similar colors and intensity.
Birefringence did not alter upon storage of stained sections. The intensity of birefringence was similar in fresh sections vs. those that were stained and stored at room temperature for more than 2-3 months.
Recipes
-
Weigert’s haematoxylin solution
-
Prepare Haematoxylin stock solution
1.0 g Haematoxylin
100.0 ml 96% ethanol
Mix it properly and keep mixture for 1 week for maturation at room temperature
-
Prepare iron(III) chloride stock solution
1.16 g iron(III) chloride
1.0 ml HCl (25%)
99.0 ml distilled water
Dissolve it thoroughly
Combine haematoxylin stock solution with iron(III) chloride stock solution in 1:1 ratio to obtain Weigert’s haematoxylin dye solution. Mix it thoroughly prior to use
-
-
10% neutral buffered formalin
0.4 g NaH2PO4
0.65 g Na2HPO4
90 ml distilled water
10 ml formaldehyde
-
4% paraformaldehyde
4 g paraformaldehyde
100 ml 1x PBS
Heat at 65 °C for 5 min till the PFA dissolves
-
Composition of PBS
0.8 g NaCl
0.144 g Na2HPO4
200 mg KCl
240 mg KH2PO4
Add 100 ml D/W
-
Poly-lysine coated glass slides
Poly-lysine: 10 µl/coverslip
Apply on upper surface of clean glass slide evenly
Allow it to air dry at RT and store it at RT for further use. No need to wash
-
Picrosirius red solution
0.1 g Direct red 80
Saturated aqueous solution of picric acid (1.3 g in 100 ml distilled water)
Mix it gently till it gets completely dissolved
-
Acidified water
1 ml glacial acetic acid in 200 ml distilled water
Acknowledgments
MV Surve and S Bhutda acknowledge fellowship from UGC, Govt. of India, and A Anil acknowledges the same from CSIR, Govt. of India. N Singh acknowledges Junior Research Fellowship from Department of Biotechnology, Govt. of India. Financial assistance from Indian Council of Medical Research (ICMR) and IIT Bombay seed grant to D Modi and A Banerjee, respectively, are also acknowledged. The authors have no conflicts of interest.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Fujimoto T., Parry S., Urbanek M., Sammel M., Macones G., Kuivaniemi H., Romero R. and Strauss J. F. 3rd(2002). A single nucleotide polymorphism in the matrix metalloproteinase-1(MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem 277(8): 6296-6302. [DOI] [PubMed] [Google Scholar]
- 2.Hampson V., Liu D., Billett E. and Kirk S.(1997). Amniotic membrane collagen content and type distribution in women with preterm premature rupture of the membranes in pregnancy. Br J Obstet Gynaecol 104(9): 1087-91. [DOI] [PubMed] [Google Scholar]
- 3.Junqueira L. C., Bignolas G. and Brentani R. R.(1979). Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11(4): 447-455. [DOI] [PubMed] [Google Scholar]
- 4.Lattouf R., Younes R., Lutomski D., Naaman N., Godeau G., Senni K. and Changotade S.(2014). Picrosirius red staining: a useful tool to appraise collagen networks in normal and pathological tissues. J Histochem Cytochem 62(10): 751-758. [DOI] [PubMed] [Google Scholar]
- 5.Marpaung J.(2016). Association between the thickness of the collagen in the amniotic membrane with the incidence of premature rupture of membranes. Int J Reprod Contracept Obstet Gynecol 5(2):296-299. [Google Scholar]
- 6.Montes G. S. and Junqueira L. C. U.(1991). The use of the picrosirius polarization method for the study of the biopathology of the collagen. Mem Inst Oswaldo Cruz 86(III): 1-11. [DOI] [PubMed] [Google Scholar]
- 7.Sebire N. J.(2001). Choriodecidual inflammatory syndrome(CoDIS) is the leading, and under recognised, cause of early preterm delivery and second trimester miscarriage. Med Hypotheses 56(4): 497-500. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu K. and Hokano M.(1990). Effect of loss of mechanical distension on collagen degradation in the mouse uterus. J Anatomy 173: 161-167. [PMC free article] [PubMed] [Google Scholar]
- 9.Surve M. V., Anil A., Kamath K. G., Bhutda S., Sthanam L. K., Pradhan A., Srivastava R., Basu B., Dutta S., Sen S., Modi D. and Banerjee A.(2016). Membrane vesicles of group B Streptococcus disrupt feto-maternal barrier leading to preterm Birth . PLoS Pathog 12(9): e1005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vega Sánchez R., Estrada Gutierrez G., Cerbulo Vazquez A., Beltran Montoya J. and Vadillo Ortega F.(2004). Characterization of chorioecidual space as an effector molecule-rich environment that induces rupture of fetal membranes during labor. Ginecol Obstet Mex 72: 593-601. [PubMed] [Google Scholar]
- 11.Wang H., Parry S., Macones G., Sammel M. D., Ferrand P. E., Kuivaniemi H., Tromp G., Halder I., Shriver M. D., Romero R. and Strauss J. F. 3rd. (2004). Functionally significant SNP MMP8 promoter haplotypes and preterm premature rupture of membranes(PPROM). Hum Mol Genet 13(21): 2659-2669. [DOI] [PubMed] [Google Scholar]