Abstract
Background
Despite the rapidly emerging reports of olfactory dysfunction amongst adult patients with coronavirus disease 2019, cases involving children and adolescents are scarcely reported. The literature was reviewed to elucidate olfactory dysfunction amongst children and adolescents with coronavirus disease 2019.
Methods
A search of the literature published from 1 December 2019 to 30 April 2021 was conducted using four databases, based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. The search was performed over one month (May 2021).
Results
Only 9 articles were identified, with a total of 316 laboratory confirmed coronavirus disease 2019 positive children and adolescents, of whom 156 reported olfactory dysfunction. Four studies reported olfactory dysfunction based on subjective tests; four studies carried out objective assessment. Most studies reported on olfaction recovery.
Conclusion
The literature review revealed an olfactory dysfunction rate of 49 per cent amongst children and adolescents with coronavirus disease 2019. Persistence of olfactory dysfunction was reported in 7.1 per cent of the patients. Further studies involving objective measures need to be carried out in children and adolescents with coronavirus disease 2019.
Key words: Coronavirus, COVID-19, Smell, Olfactory Impairment, Children, Adolescents, Pediatrics, Review
Introduction
Since the emergence of coronavirus disease 2019 (Covid-19) in December 2019, various novel presentations have burgeoned. Nevertheless, early detection of Covid-19 patients remains the key to curbing community spread whilst controlling the raging pandemic. Ever since new-onset olfactory dysfunction became one of the primary screening symptoms, awareness of this entity has increased. Yet, most of the reported cases involve adult patients. Additionally, anosmia has been regarded as a highly specific and moderately sensitive screening symptom for Covid-19 infection in adults.1
Children and adolescents are not excluded from being afflicted with Covid-19, although early reports suggest that they have milder symptoms and are less likely to be hospitalised than adults. Yet, children and adolescents who are asymptomatic may play a role in community transmission of the virus. This review aimed to cover the current literature available on the prevalence of olfactory dysfunction amongst children and adolescents with Covid-19.
Materials and methods
We performed a comprehensive search using four databases, namely PubMed®, Embase (Elsevier, Amsterdam, the Netherlands), Scopus (Elsevier) and Google Scholar, of literature published between 1 December 2019 and 30 April 2021. The search was carried out over a one-month period (May 2021) in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines2 and the Cochrane Handbook for Systematic Reviews of Interventions.3 Published or in-press peer-reviewed cross-sectional case series and case reports providing information on olfactory disturbance amongst children who were positive for Covid-19 were included.
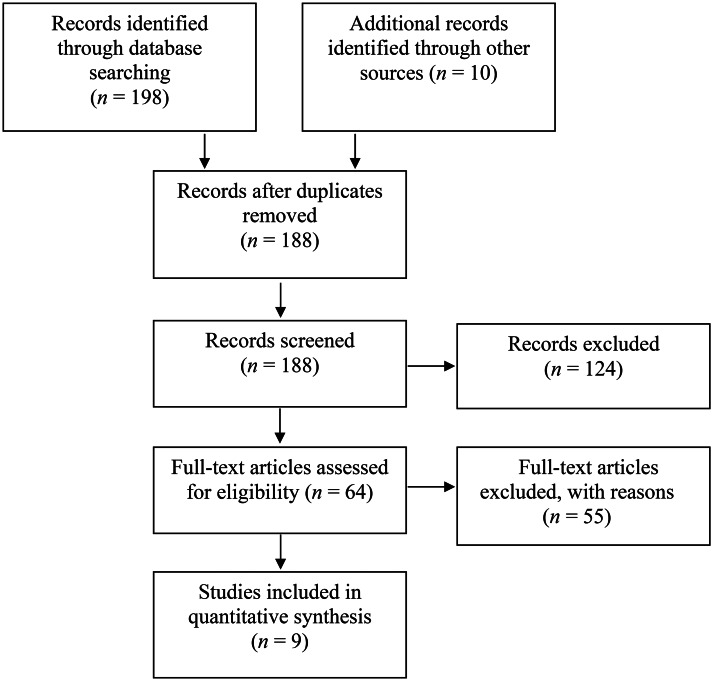
The following search terms (key words) were either used individually or combined: ‘Coronavirus’, ‘COVID-19’, ‘SARS-CoV-2’, ‘olfactory’, ‘children’, ‘adolescents’, ‘paediatric’, ‘pediatric’, ‘smell’, ‘olfaction’, ’anosmia’, ‘hyposmia’, ‘dysosmia’, ‘parosmia’ and ‘phantosmia’. In addition, the reference lists of all included articles were explored to identify articles not found by the electronic searches. Complete details of the search strategy are shown in Figure 1. Duplicate studies were excluded using EndNote™ X10 reference management software.
Fig. 1.
Flow diagram of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (‘PRISMA’) for the systematic literature search.
Studies were considered eligible for inclusion if: (1) they were case reports, case series or observational studies; (2) patients included had laboratory confirmed Covid-19 and were younger than 18 years of age; and (3) they included patients with new-onset olfactory dysfunction related to Covid-19. Additionally, correspondence letters or letters to the editor that fulfilled the mentioned eligibility criteria were included. Studies were excluded if: (1) patients included were aged above 18 years; (2) Covid-19 was not diagnosed via a laboratory confirmed method; (3) patients included did not have olfactory dysfunction; (4) patients included had olfactory dysfunction prior to Covid-19; (5) they were an abstract-only study; and (6) articles were written in languages other than English.
The titles and abstracts of each article identified in the search were assessed for eligibility based on the inclusion criteria by two independent reviewers (JS and JK). Full-text articles were analysed individually. Any discrepancies during the selection process were resolved by discussion and consensus.
Two reviewers (JS and JK) extracted the data independently with a standardised data collection form, which included: (1) basic information; (2) demographic information (age and gender); (3) presence of olfactory and/or gustatory dysfunction; (4) other Covid-19-related symptoms; (5) subjective and/or objective assessment of the olfactory or gustatory dysfunction; and (6) outcome of olfactory and/or gustatory symptoms. If data were missing or reported in an unusable method, the study was excluded. After scrutiny by the panel members, nine articles were selected based on our objective and selection criteria.
Results
A total of nine articles, originally published online between 1 December 2019 and 30 April 2021, that fulfilled the selection criteria were included in the review (Table 1).4–12 The articles included three retrospective studies,4,5,11 three case reports,6,9,10 two prospective studies7,12 and one case series.8 Patients were from Asia in five articles,6–8,10,11 Europe in four articles4,5,9,11 and Russia in one article.12 One study included patients from three countries: China, Germany and France.11 The total number of patients included in this review is 316, of whom a total of 156 had olfactory dysfunction (49 per cent). The total number of patients in each article ranged from 1 to 68. The age and gender of the patients included were not reported in most studies.
Table 1.
Summary of findings for included studies
| Author (country) | Study design | Total children with Covid-19 (n) | Covid-19 confirmation procedure | Children diagnosed with Covid-19 with olfactory dysfunction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total children with olfactory dysfunction (n) | Gender (n) | Age (years) | Olfactory dysfunction assessment type | Investigation of olfactory dysfunction | Specific treatment for olfactory dysfunction | Outcome | MIS-C | |||||
| Bernaola Abraira et al.4 (Spain) | Retrospective study | 30 | RT-PCR | 5 | NR | NR | Subjective | Questionnaire & severity scale by Izquierdo-Dominguez | NR | Complete recovery in 4 months | NR | |
| Concheiro-Guisan et al.5 (Spain) | Retrospective study | 33 | RT-PCR | 5 | 2F, 3M | NR | Objective | Odour ID test | NR | NR | NR | |
| Kasuga et al.6 (Japan) | Case report | 1 | RT-PCR | 1 | F | 13 | Subjective + objective | VAS (smell & taste score = 0%), IV Alinamin® olfaction test & odour ID test | NR | Complete recovery | NR | |
| Kumar et al.7 (India) | Prospective study | 141 | RT-PCR | 62 | NR | 10–19 | NR | NR | NR | Olfactory dysfunction persists in 3 patients | NR | |
| Mak et al.8 (Hong Kong) | Case series | 3 | RT-PCR | 3 | 2F, 1M | 15.7 | NR | NR | NR | Olfactory dysfunction persists in 1 patient | NR | |
| Maniaci et al.9 (Italy) | Case report | 1 | RT-PCR | 1 | M | 15 | NR | NR | NR | Olfactory dysfunction persists in 1 patient | NR | |
| Marhaeni et al.10 (Indonesia) | Case report | 1 | RT-PCR | 1 | NR | NR | NR | NR | NR | NR | NR | |
| Qiu et al. 11 (China, Germany, France) | Retrospective study | 27 | RT-PCR | 10 | 6M, 4F | 15–17 | Subjective + objective (1 child) | Questionnaire, VAS & olfactory stimuli test (China) | NR | Complete recovery in 3/9 patients* | NR | |
| Rusetsky et al.12 (Russia) | Prospective study | 79 | RT-PCR | 68 | NR | NR | Subjective + objective | SNOT-22 & odour ID test | NR | Complete recovery in 2 months | NR | |
*Follow-up data were not obtained for one child in the German cohort. Covid-19 = coronavirus disease 2019; MIS-C = coronavirus disease 2019 related Multisystem Inflammatory Syndrome in Children; RT-PCR = reverse transcription polymerase chain reaction; NR = not reported; F = females; M = males; ID = identification; VAS = visual analogue score; IV = intravenous; SNOT-22 = Sino-Nasal Outcome Test-22
All patients included were diagnosed with Covid-19 on the basis of laboratory based reverse transcription polymerase chain reaction testing. Only five studies reported on the mode of olfactory assessment: objective evaluation was carried out in four studies5,6,11,12 and subjective assessment in four studies.4,6,11,12 The psychophysical assessment was performed using an odour identification test in four articles.5,6,11,12 Kasuga et al. used the Alinamin® test, an intravenous olfaction test.6 Questionnaire evaluation was used in three studies,4,11,12 and a visual analogue scale was used in two studies.6,11
None of the included studies mentioned distortion or alteration of smell such as parosmia or phantosmia. In addition, no specific treatment for olfactory dysfunction was mentioned. The persistence of olfactory dysfunction was reported in 11 patients (7.1 per cent).7–9,11 None of the children included in this review developed Covid-19-associated multisystem inflammatory syndrome.
Discussion
Smell or olfaction is regarded as the primordial organ of sense, and has been traditionally associated with safety, nutrition and the sensation of pleasure, as well as general quality of life.13 Olfaction plays an important role in children, as smell is important in attaining a balanced diet for proper nutrition.14 The prevalence of olfactory dysfunction in the general population has been reported to be nearly 6 per cent,15 and the prevalence in children is believed to be lower,16 although no concrete data exist to date.17 Olfactory dysfunction in children has been linked to a myriad of conditions, including autism,18 attention-deficit disorder,19 head trauma20 and cleft palate.21
Based on a recent meta-analysis of 83 studies, the prevalence of olfactory dysfunction in individuals with Covid-19 was reported to be 47.85 per cent.22 Yet, the prevalence of olfactory dysfunction in children with Covid-19 has been postulated by experts to be much lower.11 This conjecture is based on several factors, including the gene expression of angiotensin-converting enzyme 2 (ACE-2) (responsible for binding with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in the nasal mucosa), which is age-dependent and lower in children.23 In addition, the immaturity of immunological development in children is considered one of the leading factors behind the lower prevalence of olfactory dysfunction in children.9 Interestingly, in our review, approximately 50 per cent of children and adolescents with Covid-19 developed olfactory dysfunction. We believe that the number of children and adolescents with olfactory dysfunction is higher than expected when a proper olfaction assessment is carried out.
Olfactory dysfunction can be detected through various objective assessment tools, or subjectively by questionnaires or history-taking. However, the challenge in detecting olfactory dysfunction in children lies in the inconsistency of assessment tools as well as the limited availability of age-appropriate paediatric reference ranges.24
Objective assessment tools available to assess olfactory dysfunction in children include: odour detection, which requires odour to be detected; odour identification, which requires odour to be recognised; and odour discrimination, which requires the ability to differentiate one odour from another. The odour detection threshold assesses odorants at different concentrations, whereas cortical processing of the olfaction is assessed via electrophysiological measurements and olfactory evoked potentials.24 The odour identification test is the most favoured test for olfactory dysfunction in children. Yet, the reliability of odour identification tasks in children has been challenged by many authors, as identifying the odour has been linked to memory, attention and linguistic processing rather than the odour itself.25 In this review, four studies reported olfactory dysfunction based on objective assessment,5,6,11,12 and all used odour identification tests. As part of their olfactory dysfunction assessment, Kasuga et al. utilised the Alinamin test, which involves the intravenous administration of propyl disulphide; this is followed by determination of latency, the time between the initiation of the injection and recognition of the odour (garlic), as well as duration, and the time between recognition and disappearance of the odour.6
Subjective assessment tools for olfactory dysfunction available for children include questionnaires. However, no validated questionnaires that assess olfactory dysfunction in children are available to date. Additionally, objective or psychophysical testing was found to be more sensitive than subjective surveys when performed in the same study.11,12 This may be attributed to the fact that olfactory dysfunction is only recognised when it results in significant loss of smell, such as anosmia, rather than hyposmia.22
Olfactory dysfunction amongst Covid-19 patients has been reported to be short-lived in children, as previously reported in adults.26 Rapid recovery was reported in most of the included studies. However, olfactory dysfunction persisted in 11 (7.1 per cent) of the patients included in this review. Partial or complete olfaction recovery has been reported in most studies involving adult patients,22 although it may take several months27 because of the longer time needed for regeneration of the damaged olfactory neurons.
None of the studies included in this review reported on specific treatment directed to olfactory dysfunction. Both oral as well as nasal steroids, which are routinely used in treating anosmia, are avoided because of the risk of further suppressing the immune system in Covid-19 patients.
Although no published paediatric olfactory dysfunction screening tools have been validated for use in children with Covid-19, simple questions regarding changes in smell, either a decrease, alteration, distortion, or complete loss of smell, ought to be included during screening. Olfactory dysfunction symptoms reported by children warrant immediate investigation to rule out Covid-19. In parallel, it has been suggested that olfactory education should be included hand-in-hand with other health promotion teaching regarding Covid-19, such as handwashing techniques, within playschools, nurseries and schools.
Olfactory dysfunction has been identified as a notable clinical manifestation of coronavirus disease 2019 (Covid-19) in adults and children
This review indicates that olfactory dysfunction affects 49 per cent of children and adolescents with Covid-19
Prevalence may be higher as there is no definite investigation method for olfactory dysfunction in children, especially toddlers
The recovery rate for olfactory dysfunction is high
Olfactory dysfunction persistence after one month requires follow up and olfactory retraining therapy
Conclusion
Based on our review, the prevalence of olfactory dysfunction was found to be 49 per cent among children and adolescents with Covid-19. Early recovery was documented in most studies, although the persistence of symptoms was noted in 7.1 per cent of patients.
Acknowledgement
We would like to acknowledge all involved in this study.
Competing interests
None declared
References
- 1.Wee LE, Chan YFZ, Teo NWY, Cherng BPZ, Thien SY, Wong HMet al. The role of self-reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID-19. Eur Arch Otorhinolaryngol 2020;277:2389–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moher D, Liberati A, Tetzlaff J, Altman DG;PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011). London: Cochrane Collaboration, 2011 [Google Scholar]
- 4.Bernaola Abraira M, Bartha De Las Peñas I, López-Araujo GA, Escudero Diez C, Rodríguez Del Río P, Morales-Cabeza Cet al. Olfactory and gustatory dysfunction in pediatric patients with coronavirus disease (COVID-19). J Investig Allergol Clin Immunol 2021;31:277–9 [DOI] [PubMed] [Google Scholar]
- 5.Concheiro-Guisan A, Fiel-Ozores A, Novoa-Carballal R, González-Duran ML, Portugués de la Red M, Martínez-Reglero Cet al. Subtle olfactory dysfunction after SARS-CoV-2 virus infection in children. Int J Pediatr Otorhinolaryngol 2021;140:110539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasuga Y, Nishimura K, Go H, Nakazaki K, Shimizu S, Kanezawa Ket al. Severe olfactory and gustatory dysfunctions in a Japanese pediatric patient with coronavirus disease (COVID-19). J Infect Chemother 2021;27:110–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar L, Kahlon N, Jain A, Kaur J, Singh M, Pandey AK. Loss of smell and taste in COVID-19 infection in adolescents. Int J Pediatr Otorhinolaryngol 2021;142:110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mak PQ, Chung KS, Wong JS, Shek CC, Kwan MY. Anosmia and ageusia: not an uncommon presentation of COVID-19 infection in children and adolescents. Pediatr Infect Dis J 2020;39:e199–200 [DOI] [PubMed] [Google Scholar]
- 9.Maniaci A, Iannella G, Vicini C, Pavone P, Nunnari G, Falsaperla Ret al. A case of COVID-19 with late-onset rash and transient loss of taste and smell in a 15-year-old boy. Am J Case Rep 2020;21:e925813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marhaeni W, Wijaya AB, Kusumaningtyas P, Mapianto RS. Thalassemic child presenting with anosmia due to COVID-19. Indian J Pediatr 2020;87:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu C, Cui C, Hautefort C, Haehner A, Zhao J, Yao Qet al. Olfactory and gustatory dysfunction as an early identifier of COVID-19 in adults and children: an international multicenter study. Otolaryngol Head Neck Surg 2020;164:714–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rusetsky Y, Meytel I, Mokoyan Z, Fisenko A, Babayan A, Malyavina U. Smell status in children infected with SARS-CoV-2. Laryngoscope 2021;131:E2475–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto JM. Olfaction. Proc Am Thorac Soc 2011;8:46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruijschop RM, Boelrijk AE, de Graaf C, Westerterp-Plantenga MS. Retronasal aroma release and satiation: a review. J Agric Food Chem 2009;57:9888–94 [DOI] [PubMed] [Google Scholar]
- 15.Brämerson A, Johansson L, Ek L, Nordin S, Bende M. Prevalence of olfactory dysfunction: the Skövde population-based study. Laryngoscope 2004;114:733–7 [DOI] [PubMed] [Google Scholar]
- 16.Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AFet al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg 1991;117:519–28 [DOI] [PubMed] [Google Scholar]
- 17.Schriever VA, Agosin E, Altundag A, Avni H, Cao Van H, Cornejo Cet al. Development of an international odor identification test for children: The Universal Sniff Test. J Pediatr 2018;198:265–72 [DOI] [PubMed] [Google Scholar]
- 18.Bennetto L, Kuschner ES, Hyman SL. Olfaction and taste processing in autism. Biol Psychiatry 2007;62:1015–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karsz FR, Vance A, Anderson VA, Brann PG, Wood SJ, Pantelis Cet al. Olfactory impairments in child attention-deficit/ hyperactivity disorder. J Clin Psychiatry 2008;69:1462–8 [DOI] [PubMed] [Google Scholar]
- 20.Roberts MA, Simcox AF. Assessing olfaction following pediatric traumatic brain injury. Appl Neuropsychol 1996;3:86–8 [DOI] [PubMed] [Google Scholar]
- 21.Richman RA, Sheehe PR, McCanty T, Vespasiano M, Post EM, Guzi Set al. Olfactory deficits in boys with cleft palate. Pediatrics 1998;82:840–4 [PubMed] [Google Scholar]
- 22.Saniasiaya J, Islam MA, Abdullah B. Prevalence of olfactory dysfunction in coronavirus disease 2019 (COVID-19): a meta-analysis of 27,492 patients. Laryngoscope 2021;131:865–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 2020;323:2427–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvo-Henríquez C, Chiesa-Estomba C, Martinez-Capoccioni G, Lechien JR, Mota-Rojas X, Mayo-Yáñez Met al. Methods to assess olfaction in pediatric patients: a systematic review from the International YO-IFOS Study Group. Eur Arch Otorhinolaryngol 2020;277:313–21 [DOI] [PubMed] [Google Scholar]
- 25.Thomas-Danguin T, Rouby C, Sicard G, Vigouroux M, Farget V, Johanson Aet al. Development of the ETOC: a European test of olfactory capabilities. Rhinology 2003;41:142–51 [PubMed] [Google Scholar]
- 26.Ramasamy K, Saniasiaya J, Abdul Gani N. Olfactory and gustatory dysfunctions as a clinical manifestation of coronavirus disease 2019 in a Malaysian tertiary center. Ann Otol Rhinol Laryngol 2021;130:513–19 [DOI] [PubMed] [Google Scholar]
- 27.Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RMet al. Position paper on olfactory dysfunction. Rhinology 2016;56:1–30 [DOI] [PubMed] [Google Scholar]