Abstract
Rice black-streaked dwarf virus (RBSDV), a member of genus Fijivirus in the family Reoviridae, infects rice, maize, barley and wheat, and can seriously affect crop yields. RBSDV is transmitted by the small brown planthopper (Laodelphax striatellus, SBPH) in a persistent manner. RBSDV has 10 linear dsRNA genomic segments, making it difficult to construct infectious clones for functional studies in plants. Here we describe a method for inoculating and maintaining RBSDV on rice in a greenhouse for use in laboratory research. The protocol uses SBPHs mass reared in the laboratory. We also describe in detail the propagation of a healthy planthopper population, the preparation of plant material, RBSDV inoculation and the evaluation of the rice after inoculation.
Keywords: Rice black-streaked dwarf virus, Small brown planthopper, Virus inoculation, RT-PCR, DIBA detection
Background
Rice black-streaked dwarf virus (RBSDV), a recognized member of the second group within the genus Fijivirus, family Reoviridae, seriously affects the production of rice and maize in East Asia (Shikata and Kitagawa, 1977; Zhang et al., 2001a ). RBSDV is propagatively transmitted to rice, maize, barley and wheat by the small brown planthopper (Laodelphax striatellus, SBPH) in a persistent manner (Shikata and Kitagawa, 1977). SBPH can acquire RBSDV from fresh or frozen infected rice leaves and transmit it to healthy plants (Shikata and Kitagawa, 1977; Li et al., 2010 ). Once SBPH has acquired the virus, the virus can multiply in the insect vector and can be transmitted to host plants throughout the life of the insect. However, SBPH cannot transmit the virus transovarially (Boccardo and Milne, 1984). RBSDV has icosahedral, double-layered particles about 70-75 nm in diameter and 10 linear genomic segments of double-stranded RNA (dsRNA), ranging in size from approximately 1.8 to 4.5 kb ( Marzachi et al., 1995 ; Zhang et al., 2001b ). Our previous studies have shown the complexity of the interaction between RBSDV and host plants ( Sun et al., 2015 ; He et al., 2017 ). Unfortunately, the numerous genomic segments make it difficult to construct infectious cDNA clones for reverse genetics studies. To facilitate the investigation of the interaction between RBSDV and its host plants, we describe a simple and efficient method for maintaining RBSDV infection in a greenhouse, including the propagation of the healthy planthopper population, preparation of rice plants, RBSDV inoculations and finally the evaluation of the rice disease symptom after inoculation.
Materials and Reagents
Filter paper (Sangon Biotech, catalog number: F503311)
Fine soil
Gauze and rubber band
RNase-free tube (Thermo Fisher Scientific, InvitrogenTM, catalog number: AM12400)
RNase-free tip of pipettes (Sangon Biotech, catalog number: F611216)
Brush
Washing bottle (Sangon Biotech, catalog number: F505001-001)
Pooter
Black cloth
Toothpick
Fresh leaves (collected from 30-day-old rice)
-
RNA extraction reagent
Trizol reagent kit (Thermo Fisher Scientific, InvitrogenTM, catalog number: 15596018)
Chloroform (ShangHai LingFeng Chemical Reagent, catalog number: 1519021)
75% ethanol (Singpharm Chemical Chemical Reagent, catalog number: 10009257)
-
RNase-free double distilled H2O
Note: All stored at 4 °C.
Reverse transcription kit (Vazyme Biotech, catalog number: R223-01, stored at -20 °C)
Sterilized beads (Sangon Biotech, catalog number: B529319-0025)
TAE buffer (Sangon Biotech, catalog number: B548101-0500)
Agarose (YEASEN, catalog number: 10208ES60)
DNA maker 2,000 bp (Takara Bio, catalog number: 3427A)
Ethidium bromide (Sangon Biotech, catalog number: A600195)
Anti-P10
Horseradish peroxidase-labeled goat antimouse immunoglobulin G (YEASEN, catalog number: 33201ES60)
TMB color-substrate solution (Sangon Biotech, catalog number: C510025-005)
Sodium carbonate (Na2CO3)
Sodium bicarbonate (NaHCO3)
Sodium chloride (NaCl)
Potassium chloride (KCl)
Potassium phosphate monobasic (KH2PO4)
Sodium phosphate dibasic dodecahydrate (Na2HPO4·12H2O)
5% skimmed milk powder (Sangon Biotech, catalog number: A600669)
4-Chloro-1-naphthol
30% H2O2
Fertilizer solution (containing 5% carbamide)
Carbonate coating solution (see Recipes)
10x phosphate-buffered saline (PBS) (see Recipes)
PBS-T (see Recipes)
Blocking solution (see Recipes)
TMB color-substrate solution (see Recipes)
Equipment
1 L beakers (Sangon Biotech, catalog number: F606544-0001)
PCR machine (Thermo Fisher Scientific, Applied BiosystemsTM, model: VeritiTM 96-Well Thermal Cycler)
Pipettes
Glass bottle with blue cap (Sangon Biotech, catalog number: F505041)
Microwave oven (Sangon Biotech, catalog number: G003408-0001)
Electrophoresis tank (Sangon Biotech, catalog number: G500310-0001)
Gel imaging system
Eppendorf centrifuge (Thermo Fisher Scientific, for use at room temperature and 4 °C)
Micro vacuum concentrator (Gene Company Limited)
Automatic sample grinding machine (Shanghai Jingxin Industrial Development, model: Tissuelyser-96)
Software
Gel imaging software
Procedure
-
Preparation of seedlings for raising insects
Prior to inoculation of RBSDV, 80-100 rice seedlings are needed to obtain a big population of the small brown planthoppers. Plump seeds of the rice variety ‘Wuyujing 3’ are pre-germinated by immersing them in water for 2 days, rinsing thoroughly with water and then allowing them to germinate on water-soaked filter paper for 1 day.
150-200 ml of fine soil is added to clean 1 L beakers and moistened with 100 ml water added along the beaker wall.
About 100 pre-germinated seeds are then scattered on the soil and sprinkled with a thin layer of soil. The beakers are covered with gauze (Figure 1) and placed on the shelf in a growth room at 25-27 °C, relative humidity 50%-70%, 14/24 h light with intensity 5,000-7,000 lux until the rice seedlings have grown to 6 cm (about 10-day-old seedlings), when they can be used for feeding the small brown planthoppers (Figure 2).
-
The source of RBSDV infected rice plants
RBSDV-infected rice plants (Figure 3) are collected from the field (Jining city, Shandong Province) and the presence of RBSDV is confirmed by RT-PCR. Total RNA is extracted from leaves with symptoms using Trizol Reagent. Reverse transcription is performed using the reverse transcription kit with random primers, following the instructions supplied (Vazyme). The primers used were designed from the published sequence of S10 of RBSDV (S10-Forward: AAC AAC CGA CCA ACA ATC AC and S10-Reverse: GAG CAG GAA CTT CAC GAC AG). The detailed steps are as follows:
-
Reverse transcription (10 μl), the aim of this step is to reverse transcribe the RNA into cDNA.
-
Remove Genomic DNA (the reaction in the RNase-free tube) using the following reaction:
RNase-free ddH2O to 8 μl
4x gDNA mix (containing DNase) 2 μl
Total RNA 1-500 ng
Mix the solution using pipettes and then heat at 42 °C for 2 min.
-
Conduct the reverse transcription reaction as follows:
5x HiScript II Q Select RT SuperMix 2 μl
Mixed solution from step B1a 8 μl
Mix the solution with pipettes and then place in the PCR machine for the following procedure:
25 °C 10 min
50 °C 30 min
85 °C 5 min
-
-
PCR amplification
-
Prepare a mixture as follows:
2x HieffTM PCR Master Mix 5 μl
Forward primer 0.3 μl
Reverse primer 0.3 μl
cDNA from reaction above 0.5 μl
RNase-free ddH2O To 10 μl
Mix the solution fully, put it into the PCR machine and set up the following amplification reaction:
94 °C 3 min
94 °C 30 sec
58 °C 30 sec 35 cycles
72 °C 30 sec
72 °C 10 min
4 °C End
-
Agarose gel electrophoresis
Preparation of gels: Firstly, weigh 1 g of agarose into a glass bottle with blue cap. Secondly, add 100 ml TAE buffer, and heat in a microwave oven until completely dissolved.
Production of plastic sheet: After the above-mentioned agarose gel has cooled to about 65 °C, add the right amount of ethidium bromide dye and then pour into a transparent plate with a pre-inserted comb.
Add sample: Firstly, put the agarose gel plate into an electrophoresis tank and gently move the plate to squeeze out the bubbles within the discharge hole. Then, add 5 μl of DNA marker to the first well followed by the PCR products in turn to the following wells. To avoid cross-contamination between samples, use a new pipette tip for each sample. Finally, connect to the power supply and adjust the voltage to 150-180 V.
Observation: Turn off the power when the front of the band has reached about halfway down the gel, remove the plate and gently dry the electrophoresis buffer. Then put the gel into a gel imaging system to observe.
-
-
-
Propagation of the healthy planthopper population
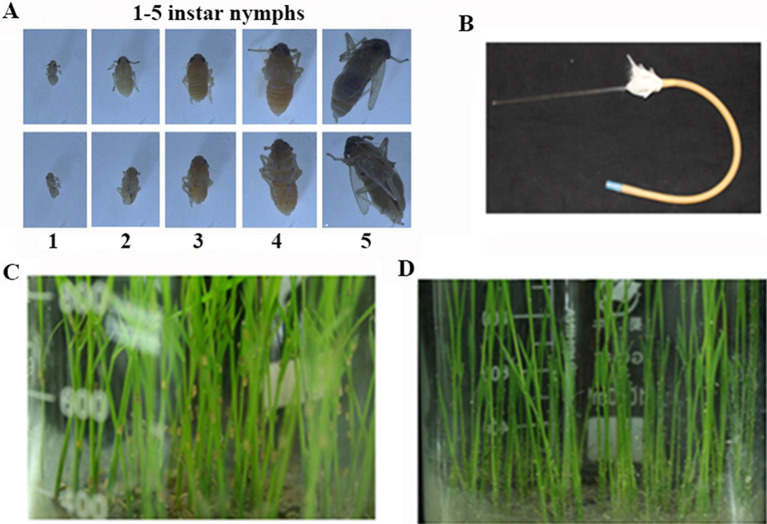
It takes about a month for planthoppers to complete their life cycle which consists of 5 instar nymph stages (Figure 4A), and 1 adult stage. The fifth instar nymphs (recognized through the short black line on the abdomen of the female) have the ability to spawn. The adults are transferred to fresh ‘Wuyujing 3’ rice seedlings (100-150 planthoppers/beaker) with the pooter (Figure 4B), allowed to spawn for 48 h (Figure 4C), and the adults are then removed with a brush. Generally, small white hatched nymphs will be seen on the rice seedlings 7-10 days later (Figure 4D). The nymphs on the seedlings can be used to acquire RBSDV. It is advised to restrict the watering of rice seedlings before collecting nymphs.
-
Rice black-streaked dwarf virus inoculations
Several RBSDV-infected rice plants prepared as described in Procedure B are washed with water and planted in a 3 L beaker.
First to second instar nymphs prepared as described in Procedure C are collected from the rice plants on which they have been reared, and released onto the RBSDV-infected rice plants (about 150-200 planthoppers/plant) for 3-5 days to acquire virus (Figure 5).
Subsequently, the planthoppers are transferred to healthy ‘Wuyujing 3’ rice seedlings for an incubation period of 10-12 days. The proportion of viruliferous planthoppers is then assessed by using a dot immunobinding assay (DIBA) with antibodies against the viral structural proteins (Zhou and Liu, 2004; Yang et al., 2007 ) (see Procedure E).
For RBSDV inoculation, planthoppers are transferred by pooter to test seedlings at the 2-3 leaf stage and allowed to feed for 3 days. The numbers transferred are calculated to ensure that three viruliferous adult planthoppers are present on each seedling. The planthoppers are then removed and the seedlings are grown in the greenhouse for symptom observation.
-
Dot Immunobinding Assay (DIBA)
To determine the viruliferous rate of the SBPH population, the method for DIBA is used for the detection of RBSDV in single SBPH. The detailed steps are as follows:
Put a single SBPH into a fresh centrifuge tube (500 μl) and add 80 μl carbonate coating solution. Then use a toothpick to mash the insect and pipette 2 μl supernatant onto the NC membrane. Dry it at room temperature.
Immerse the NC membrane into PBS-T blocking solution containing 5% skimmed milk in the condition (37 °C, 50 rpm, shake 1 h).
Transfer the NC membrane into blocking solution containing anti-P10 (1:1,000) at the condition (37 °C, 50 rpm, shake 2 h). Wash the membrane 3 times with PBS-T for 10 min each time.
Transfer the NC membrane into blocking solution containing horseradish peroxidase-labeled goat antimouse immunoglobulin G (1:5,000) at the condition (37 °C, 50 rpm, shake 1.5 h). Wash the membrane 3 times with PBS-T for 10 min each time.
Remove the PBS-T solution from the NC membrane with filter paper and cover the NC membrane with TMB color-substrate solution. React for 5-15 min in the dark until the positive control is conspicuous.
-
Symptom evaluation after RBSDV inoculation
Symptoms on the inoculated rice seedlings (Procedure D) are assessed 30 days after transfer to the glasshouse by measuring the plant height and by RT-PCR (see Procedure B).
Figure 1. Cultivating rice seedlings in beakers.

Figure 2. Planted rice seedlings indoors.

Figure 3. RBSDV infected rice plants.

Figure 4. The adult planthoppers spawned on ‘Wuyujing 3’ rice seedlings.
A. The different stage of planthoppers; B. The pooter used in this study; C. The adult planthoppers were spawning; D. Hatched out the first and second instar nymphs.
Figure 5. The instar nymphs acquire virus on RBSDV infected rice plants.

Data analysis
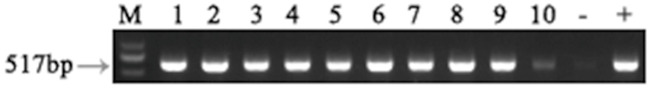
The RBSDV infected rice plants used as source plants are identified by RT-PCR. Figure 6 shows an example of the 517 bp PCR products amplified by the S10 primers. These results suggest that most plants collected from fields were infected by RBSDV.
After a 10-12 days incubation period, DIBA is used to assess the proportion of viruliferous planthoppers (Figure 7). In this example, 60 planthoppers were tested, of which 21 (35%) were infected with RBSDV. Based on these results, eight planthoppers were used to inoculate each of the test rice seedlings.
RBSDV infected rice plants show significant dwarfing symptoms compared with the control (Figure 8A). RT-PCR is used to confirm whether the dwarf rice is infected by RBSDV (Figure 8B). In this example, all 22 inoculated rice seedlings were tested and the 17 dwarfed plants were all shown to be infected by RBSDV, while the other plants were healthy. This infection rate of 77% indicates that the inoculation of RBSDV on rice through planthoppers in the greenhouse is feasible and efficient. The overall process of RBSDV inoculation by SBPH is illustrated in Figure 9.
Figure 6. Bands amplified by RT-PCR from ten separate rice plants collected from the field as potential sources of RBSDV for inoculation experiments.
M: Marker; 1-9: RBSDV infected rice plants; +: Positive control; -: Negative control.
Figure 7. DIBA assay to determine the proportion of viruliferous planthoppers.
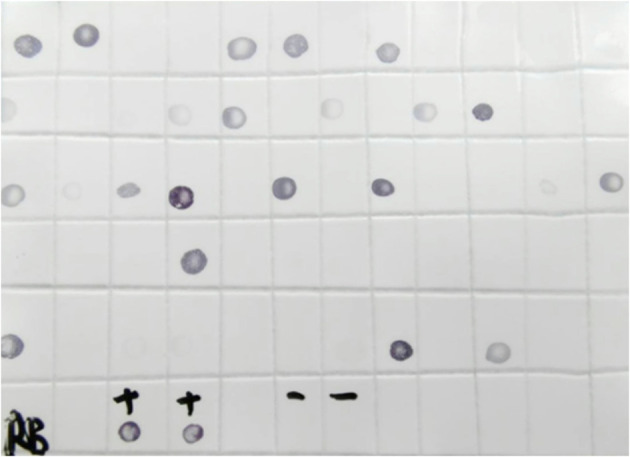
+: Positive control; -: Negative control; Black spots, viruliferous planthoppers.
Figure 8. The symptoms and detection of RBSDV 30 days after inoculation using viruliferous insect vectors.
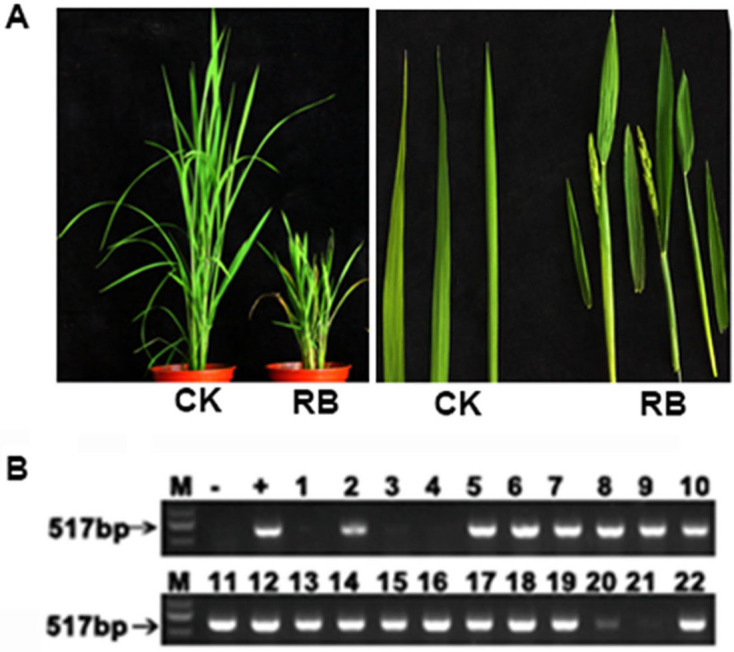
A. Healthy (CK) and RBSDV-infected (RB) plants; B. RT-PCR results showing the detection of RBSDV in the 22 inoculated rice seedlings. M: Marker; +: Positive control; -: Negative control.
Figure 9. The overall process of RBSDV inoculation by SBPH.
Notes
Keep the soil of growing rice seedlings dry when collecting the nymphs.
Recipes
-
Carbonate coating solution
1.59 g Na2CO3
2.93 g NaHCO3
Dissolve in 900 ml of distilled water, make the constant volume to 1,000 ml (pH = 9.6)
-
10x phosphate-buffered saline (PBS)
16 g NaCl
4 g KCl
4 g KH2PO4
6 g Na2HPO4·12H2O
Dissolve in 900 ml of distilled water, make the constant volume to 1,000 ml (pH = 7.5)
-
PBS-T
10x PBS diluted by 1:9 volume ratio, added 0.1% Tween 20
-
Blocking solution
5% (w/v) skimmed milk powder in PBS-T
-
TMB color-substrate solution
3 mg 4-chloro-1-naphthol
2 ml ethanol
7 μl 30% H2O2
10 ml PBS
Adjust pH to 7.5
Acknowledgments
We thank Professor Yijun Zhou (Jiangsu Academy of Agricultural Sciences, Nanjing, China) and his team for providing us with the healthy planthoppers, and Jianxiang Wu (Zhejiang University) for providing antibody. We thank Professor M. J. Adams, Stevenage, UK for the help in correcting the English of the manuscript. The protocol was modified from previous works, including Zhang et al. (2001a), Sun et al. (2015), and He et al. (2017) and has been successfully used for studies on viral characterization and virus-plant interaction. All authors declare no any conflicts of interest.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Boccardo G. and Milne R. G.(1984). Plant reovirus group. CMI/AAB, Descriptions of Plant Viruses No.294, Commonwealth Microbiology Institute and Association of Applied Biology. United Kingdom.
- 2.He Y., Zhang H., Sun Z., Li J., Hong G., Zhu Q., Zhou X., MacFarlane S., Yan F. and Chen J.(2017). Jasmonic acid-mediated defense suppresses brassinosteroid-mediated susceptibility to rice black streaked dwarf virus infection in rice. New Phytol 214(1): 388-399. [DOI] [PubMed] [Google Scholar]
- 3.Li L., Li H. W., Dong H. B., Wang X. F. and Zhou G. H.(2010). Transmission by Laodelphax striatellus fallen of rice black streaked dwarf virus from frozen infected rice leaves to healthy plants of rice and maize . J Phytopathol 159: 1-5. [Google Scholar]
- 4.Marzachi C., Boccardo G., Milne R., Isogai M. and Uyeda I.(1995). Genome structure and variability of fijiviruses. Seminars in Virology 6: 103-108. [Google Scholar]
- 5.Shikata E. and Kitagawa Y.(1977). Rice black-streaked dwarf virus: its properties, morphology and intracellular localization. Virology 77(2): 826-842. [DOI] [PubMed] [Google Scholar]
- 6.Sun Z., He Y., Li J., Wang X. and Chen J.(2015). Genome-wide characterization of rice black streaked dwarf virus-responsive microRNAs in rice leaves and roots by small RNA and degradome sequencing. Plant Cell Physiol 56(4): 688-699. [DOI] [PubMed] [Google Scholar]
- 7.Yang J., Zhang H. M., Chen J. P. and Dai L. Y.(2007). Prekaryotic expression antiserum preparation and some properties of p8 protein of rice black-streaked dwarf fijivirus. Acta Phytophylacica Sinica 34(3): 252-256. [Google Scholar]
- 8.Zhang H. M., Chen J. P. and Adams M. J.(2001). Molecular characterization of segments 1 to 6 of rice black-streaked dwarf virus from China provides the complete genome. Arch Virol 146: 2331-2339. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H. M., Chen J. P., Lei J. L. and Adams M. J.(2001). Sequence analysis shows that a dwarfing disease on rice, wheat and maize in china is caused by rice black-streaked dwarf virus. Eur J Plant Pathol 107: 563-567. [Google Scholar]
- 10.Zhou Y. J. and Liu H. J.(2004). Immunoassay of rice stripe virus carried by Laodelphax striatellus. Jiangsu Agricultural Sciences 1: 50-51. [Google Scholar]