Abstract
This protocol was developed to increase the richness of information available from in vivo T cell proliferation studies. DNA labelling techniques such as BrdU incorporation allow precise control of label administration and withdrawal, so that the division history of a population can be tracked in detail over long timeframes (days-weeks). Ki67 is expressed in the nucleus of dividing cells, and is retained for a short time (3-4 days) after division ( Gossel et al., 2017 ); therefore acting as a molecular clock to identify cells that have recently divided. Combining these two techniques allows the integration of current and historical proliferation information from individual cells within a population. This data can subsequently be used to probe population dynamics by fitting mathematical models of proliferation ( Gossel et al., 2017 ).
Keywords: T cells, Ki67, BrdU labelling, T cell proliferation, DNA labelling, Flow cytometry
Background
Quantifying the dynamics of T cell death and division is a significant experimental and computational challenge, yet this information is vital to our understanding of how a healthy immune system develops and is maintained. A variety of techniques exist for in vivo analysis of T cell population dynamics, but the interpretation of data is complicated and results can be model-dependent ( Asquith et al., 2002 ; De Boer and Perelson, 2013). Many of these assays are based on flow cytometric readouts, providing an opportunity to gain novel insights by exploiting the potential for multiparametric analysis. Combining analysis of BrdU uptake alongside Ki67 expression is particularly useful as each represents a distinct parameter of cell division. BrdU is a thymidine analogue that is incorporated into the DNA of dividing cells, and thus permanently marks cells that have divided during the labelling period (Tough and Sprent, 1994; Bonhoeffer et al., 2000 ). In contrast, Ki67 is a transient marker of cell division: the Ki67 protein is expressed during cell cycle and for a short duration (3-4 days) thereafter (De Boer and Perelson, 2013; Gossel et al., 2017 ). Measuring Ki67 expression simultaneously with BrdU uptake therefore provides both recent and historical proliferation information from the same cell.
Materials and Reagents
Petri dishes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 150288)
Nylon mesh (John Staniar & Co., catalog number: NYL9084)
Pipette tips
Insulin syringes (B. Braun Melsungen, catalog number: 9151125)
FACS tubes (Corning, Falcon®, catalog number: 352002)
Mice: adult C57BL/6
-
FITC BrdU Flow Kit (BD, BD Biosciences, catalog number: 559619), containing:
10 mg/ml BrdU
Cytofix/Cytoperm buffer
10x Perm/Wash buffer
Cytoperm permeabilisation buffer plus
1 mg/ml DNase
Anti-BrdU FITC antibody
Phosphate-buffered saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190)
BrdU powder (Sigma-Aldrich, catalog number: B5002)
Sucrose (Sigma-Aldrich, catalog number: S0389)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7906)
Anti-mouse Ki67 eFluor660 antibody (clone SolA15) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 50-5698)
PerCP/Cy5.5 anti-mouse TCR beta chain (clone H57-597) (BioLegend, catalog number: 109228)
Brilliant Violet 711 anti-mouse CD4 (clone RM4-5) (BioLegend, catalog number: 100550)
Brilliant Violet 510 anti-mouse CD25 (clone PC61) (BioLegend, catalog number: 102042)
Brilliant Violet 785 anti-mouse/human CD44 (clone IM7) (BioLegend, catalog number: 103059)
BD Horizon BUV737 rat anti-mouse CD62L (clone MEL-14) (BD, BD Biosciences, catalog number: 565213)
LIVE/DEAD fixable near-IR dead cell stain kit (Thermo Fisher Scientific, InvitrogenTM, catalog number: L10119)
25x BrdU/Sucrose (see Recipes)
PBS/BSA (see Recipes)
Surface Marker Antibody Cocktail (see Recipes)
1x Perm/Wash buffer (see Recipes)
DNase (see Recipes)
Equipment
Pipettes
Dissection tools (sharp scissors and forceps)
Incubator
Refrigerated bench top centrifuge
Cell counter, for example a CASY counter (Omni Life Science, catalog number: 5651697)
Flow cytometer equipped with appropriate lasers and filters for detection of FITC and eFluor660 fluorescence
Procedure
-
In vivo administration of BrdU
Notes:
BrdU is initially administered by intraperitoneal injection to enable precise timing of the start of administration. Following this initial injection, mice are maintained on BrdU administered via the drinking water, to avoid undue stress that might be caused by repeated injections.
A mouse that has not been fed BrdU should also be included, to allow isolation of BrdU-unlabelled cells from the same population to be used as a negative control to determine background levels of staining for the anti-BrdU antibody.
-
Prepare a working solution of 4 mg/ml BrdU by diluting 10 mg/ml BrdU stock solution (provided in BrdU FITC Flow Kit) in sterile PBS.
Note: This can be prepared in advance and aliquots stored at -20 °C for up to two months. Protect from light and avoid repeated freeze-thaw cycles.
Inject 200 μl of BrdU working solution (i.e., 0.8 mg BrdU) per mouse via the intraperitoneal route.
-
Dilute 25x BrdU/Sucrose (see Recipes) to 1x in mouse drinking water. The final concentration in drinking water is 0.8 mg/ml BrdU and 0.5% sucrose.
Note: Water bottles should be protected from light. Sucrose is included here to ensure palatability of the drinking water containing BrdU. BrdU has a limited stability in drinking water and must therefore be refreshed regularly to maintain the dosage level.
Maintain BrdU in drinking water for the desired timeframe, refreshing every 2-3 days.
To withdraw BrdU treatment for a delabelling timecourse, at the required timepoint replace the water bottle containing BrdU with a bottle containing normal drinking water.
-
Co-staining with BrdU and Ki67 for flow cytometry analysis
Note: Refer to the manufacturer’s instructions in the FITC BrdU Flow Kit. Keep samples on ice (except where indicated) and protect from light.
-
Day 1. Staining for cell surface markers, fixation and permeabilisation
Euthanise BrdU-treated mice by cervical dislocation and remove the organs containing the populations of interest for analysis – for example, lymph nodes.
Place the lymph nodes onto a piece of nylon mesh in a Petri dish containing 3 ml of PBS/BSA (see Recipes). Cover with a second piece of nylon mesh and use forceps to apply pressure to this top piece of mesh, gently crushing the organs and releasing the cells into the buffer. Use a pipette to collect the 3 ml of buffer containing the cells in suspension and transfer this cell suspension to a FACS tube.
-
Perform cell counts and transfer 2 million total cells to FACS tubes for staining.
Notes:
CD4 effector memory T cells are approximately 1% of total lymph node cells. Staining 2 million total lymph node cells typically permits analysis of at least 10,000 CD4 effector memory T cells.
It may be possible to stain fewer cells using this protocol, using the same volumes indicated for 2 million cells, but this has not been tested.
Wash cells by adding 1 ml of PBS/BSA to each tube and centrifuge at 300 × g for 5 min at 4 °C. Discard supernatant.
-
Resuspend cells in 100 μl of Surface Marker Antibody Cocktail (see Recipes) and incubate for 1 h on ice.
Notes:
The surface marker staining panel described here was used for the identification of CD4 effector memory cells in mouse lymph nodes, but could be adapted as required to study other populations of interest.
Staining controls using cells from BrdU treated mice should be processed alongside experimental samples. Staining controls should include: single colour controls for each antibody used, an unstained control, and a fluorescence-minus-one control for Ki67-eFluor660. Additionally, cells from a mouse not fed BrdU should be stained with all antibodies as a negative control to determine background levels of staining for the anti-BrdU antibody.
Wash cells by adding 1 ml of PBS/BSA to each tube and centrifuge at 300 × g for 5 min at 4 °C. Discard supernatant.
Fix and permeabilise cells by resuspending in 100 μl Cytofix/Cytoperm buffer (provided in FITC BrdU Flow Kit) and incubate for 30 min on ice.
Wash cells by adding 1 ml freshly prepared 1x Perm/Wash buffer (see Recipes), centrifuge at 300 × g for 5 min at 4 °C and discard supernatant.
-
Resuspend cells in 1 ml PBS/BSA and store at 4 °C overnight.
Note: Overnight storage is not mandatory, but may be preferable when processing large numbers of samples. Proceed directly from step B1f to step B2c if overnight storage is not required.
-
Day 2. Co-staining for BrdU and Ki67
Recover cells from storage and centrifuge at 300 × g for 5 min at 4 °C, discard supernatant.
Wash cells by resuspending pellet in 1 ml of freshly prepared 1x Perm/wash buffer, centrifuge at 300 × g for 5 min at 4 °C and discard supernatant.
Resuspend cells in 100 μl Cytoperm Permeabilisation Buffer Plus (provided in FITC BrdU Flow Kit) and incubate for 10 min on ice.
Wash the Cytoperm Permeabilisation Buffer Plus off the cells by adding 1 ml of freshly prepared 1x Perm/Wash buffer, centrifuge at 300 × g for 5 min at 4 °C and discard supernatant.
Repeat fixation by resuspending cells in 100 μl of Cytofix/Cytoperm buffer and incubate for 5 min at room temperature.
Wash cells by adding 1 ml of freshly prepared 1x Perm/Wash buffer, centrifuge at 300 × g for 5 min at 4 °C and discard supernatant.
Expose incorporated BrdU by resuspending cells in 100 μl of 300 μg/ml DNase (see Recipes) and incubate for 1 h at 37 °C.
Wash cells by adding 1 ml of freshly prepared 1x Perm/Wash buffer, centrifuge at 300 × g for 5 min at 4 °C and discard supernatant.
Co-stain for BrdU and Ki67 by resuspending cells in 50 μl 1x Perm/Wash containing anti-BrdU FITC (provided in FITC BrdU Flow Kit) at a dilution of 1:50 and anti-Ki67 eFluor660 at a dilution of 1:200. Incubate for 1 h at room temperature.
Wash cells by adding 1 ml of freshly prepared 1x Perm/Wash buffer, centrifuge at 300 × g for 5 min at 4 °C and discard supernatant.
Repeat wash to ensure all residual unbound antibody is removed from cells.
Acquire samples on a flow cytometer equipped with appropriate lasers and filters for the detection of FITC and eFluor660 fluorescence.
-
Data analysis
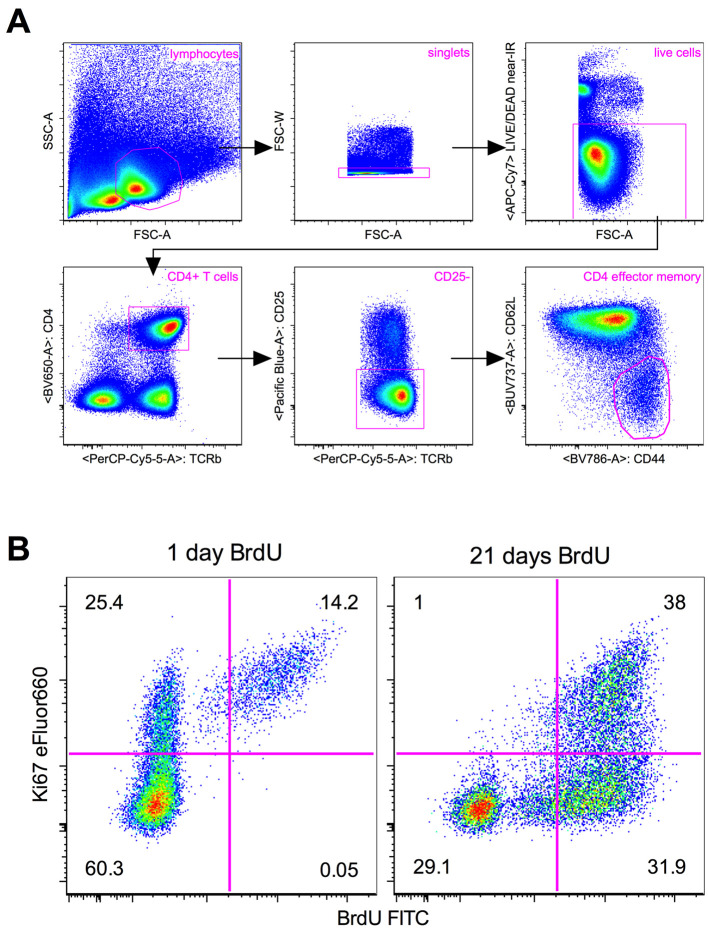
Using appropriate flow cytometry analysis software, gate on population of interest (e.g., TCR+CD4+CD25-CD44hiCD62Llo for CD4 effector memory T cells in Figure 1A) and display a two dimensional dotplot of Ki67 vs. BrdU (Figure 1B).
Apply quadrant gates as shown in Figure 1B, using a BrdU untreated mouse as a negative control for BrdU staining and a fluorescence-minus-one control for Ki67 eFluor660 staining. Cells will fall into a given quadrant depending on their division history, as described in Figure 1B.
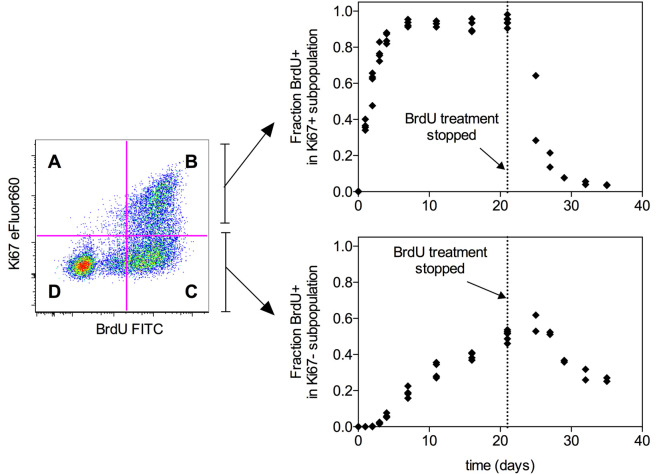
Determine the fraction of cells that have incorporated BrdU within the Ki67+ and Ki67- subpopulations as described in Figure 2, which shows example data for BrdU labelling and delabelling timecourses in the CD4 effector memory population.
The data generated in step 3 can subsequently be used to fit mathematical models of T cell proliferation as described in Gossel et al. (2017) .
Figure 1. Example flow cytometry analysis.
(from Gossel et al., 2017 ). Representative flow cytometry plots showing (A) gating strategy for CD4 effector memory T cells (TCR+CD4+CD25-CD44hiCD62Llo) in lymph nodes, and (B) Ki67 and BrdU in CD4 effector memory T cells from the lymph nodes of mice treated with BrdU for either one day (left) or 21 days (right). Quadrant gates represent the division history of cells as follows: Ki67+BrdU- cells have recently divided (Ki67 lifetime 3-4 days) but have not incorporated BrdU. This may be due to either inefficient uptake of BrdU, or because the cell division did not take place within the BrdU treatment window. Ki67+BrdU+ cells have recently divided (Ki67 lifetime 3-4 days), and this cell division has taken place within the BrdU treatment window. Ki67-BrdU+ cells have divided at some time during the BrdU treatment window, but the lack of Ki67 expression indicates that this cell division was not recent (Ki67 lifetime 3-4 days). Ki67-BrdU- cells have either not divided at all, or have divided but subsequently lost expression of Ki67 (lifetime 3-4 days) and/or BrdU (due to either inefficient uptake of BrdU or label dilution through repeated cell divisions). Numbers indicate the percentage of cells within each quadrant.
Figure 2. Example timecourse data.
(from Gossel et al., 2017 ). Graphs show the fraction of BrdU+ cells in either the Ki67+ or Ki67- subpopulation of CD4 effector memory T cells (TCR+CD4+CD25-CD44hiCD62Llo) in mouse lymph nodes. Each point represents one mouse. Mice were fed BrdU continuously for the indicated time periods up to 21 days. After 21 days, BrdU treatment was withdrawn and the delabelling timecourse was followed for a further 14 days. The fraction of BrdU+ cells within the Ki67+ subpopulation (top) is given by the formula B/(A+B) where A and B refer to the Ki67+BrdU- and Ki67+BrdU+ quadrants. The fraction of BrdU+ cells within the Ki67-+ subpopulation (bottom) is given by the formula C/(C+D) where C and D refer to the Ki67-BrdU+ and Ki67-BrdU- quadrants. These data can subsequently be used to fit mathematical models of T cell proliferation as described in Gossel et al. (2017) .
Notes
BrdU is administered to mice via an initial intraperitoneal injection of 0.8 mg, to ensure precise control of the start time. Thereafter, BrdU is maintained in drinking water at 0.8 mg/ml for the desired time up to 21 days. Sucrose is also added at 0.5% to the drinking water to ensure palatability. For delabelling timecourses, mice are switched back to regular drinking water at the required timepoint.
To eliminate variability caused by processing samples on different days, it is preferable to stagger the start times for different groups so that all mice in an experiment finish their time course on the same day. As an example, for a timecourse of one, two and three days on BrdU, one group of mice would start BrdU treatment on Monday, the next group on Tuesday, and the third group on Wednesday, with all mice in all groups sacrificed together on Thursday.
It should be noted that this protocol is optimized for the concurrent staining of BrdU and Ki67 within the same cell. The fixation protocol and concentration of antibody used for Ki67 eFluor660 differ significantly from the manufacturer’s recommendations for staining with this antibody alone. Any clone and/or fluorochrome substitutions should be thoroughly and carefully tested before use.
Recipes
-
25x BrdU/Sucrose
Add BrdU powder to a concentration of 20 mg/ml in distilled water which has been warmed to 37 °C
Stir gently, protected from light, until dissolved
Add sucrose to 12.5%
Note: This can be prepared in advance and single-use aliquots stored at -20 °C for up to two months, protected from light.
-
PBS/BSA
Supplement PBS with 1% BSA
Filter sterilise and store at 4 °C for up to 1 month
-
Surface Marker Antibody Cocktail–dilute antibodies to required concentration in PBS
PerCP/Cy5.5 anti TCR–dilute to 0.5 μg/ml
BV711 anti CD4–dilute to 0.25 μg/ml
BV510 anti CD25–dilute to 0.25 μg/ml
BV785 anti CD44–dilute to 0.5 μg/ml
BUV737 anti CD62L–dilute to 0.25 μg/ml
Add 1 μl of LIVE/DEAD near-IR dye per 800 μl of surface marker antibody cocktail
-
1x Perm/Wash buffer
Dilute 10x Perm/Wash buffer (provided in FITC BrdU Flow Kit) 1:10 in distilled water
Prepare fresh each time
-
DNase
Dilute 1 mg/ml DNase (provided in FITC BrdU Flow Kit) in PBS to a final concentration of 300 μg/ml
Acknowledgments
This protocol was developed for a published study ( Gossel et al., 2017 ), supported by the National Institutes of Health (R01 AI093870) and the Medical Research Council (MC-PC-13055). The authors declare no conflicts of interest or competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Asquith B., Debacq C., Macallan D. C., Willems L. and Bangham C. R.(2002). Lymphocyte kinetics: the interpretation of labelling data. Trends Immunol 23(12): 596-601. [DOI] [PubMed] [Google Scholar]
- 2.Bonhoeffer S., Mohri H., Ho D. and Perelson A. S.(2000). Quantification of cell turnover kinetics using 5-bromo-2'-deoxyuridine. J Immunol 164(10): 5049-5054. [DOI] [PubMed] [Google Scholar]
- 3.De Boer R. J. and Perelson A. S.(2013). Quantifying T lymphocyte turnover. J Theor Biol 327: 45-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gossel G., Hogan T., Cownden D., Seddon B. and Yates A. J.(2017). Memory CD4 T cell subsets are kinetically heterogeneous and replenished from naive T cells at high levels. Elife 6: e23013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tough D. F. and Sprent J.(1994). Turnover of naive- and memory-phenotype T cells. J Exp Med 179(4): 1127-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]