Abstract
Bacterial blight caused by Xanthomonas oryzae pv. oryzae (Xoo) is one of the most serious bacterial diseases and a major impediment to the increase of rice yield. Appropriate methods for inoculation of Xoo and disease scoring are necessary to investigate the nature of the disease and the mechanism of plant resistance to the pathogen. As the most-widely grown crop in the worldwide, rice yield plays an important role in food security. Uncovering mechanisms of plant-pathogen interaction of rice and Xoo will help develop rice plants that are more resistant to disease caused by Xoo. Here we describe our validated and efficient methods for inoculation of Xoo and disease scoring.
Keywords: Xanthomonas oryzae pv. oryzae, Bacterial blight, Leaf clipping method, Inoculation, Growth rate, Plant-pathogen interactions
Background
Bacterial blight is a vascular disease starting with Xanthomonas oryzae pv. oryzae (Xoo) invasion of rice leaves through wounds, opening and hydathodes at the leaf tip and margin (Niño- Liu et al., 2006 ). After multiplying in the intercellular spaces of underlying epitheme, Xoo enters and spreads into the rice plant through the xylem, causing long, grey to white, opaque necrotic lesions. The lesion length and bacterial growth rate can be taken as a measure of the progression of blight disease (Mew, 1984; Niño- Liu et al., 2006 ). Appropriate artificial inoculation methods and assessment of disease occurrence are necessary to investigate the nature of the disease and plant strategies to defend the pathogen. Mew (1984) has briefly summarized several artificial Xoo inoculation methods that include needle-pricking, spraying, and leaf clipping, or dipping of non-leaf parts of rice with bacterial suspension. Leaf clipping method was originally developed by Kauffman et al. (1973) , which enables crosscut veins to be exposed to Xoo suspension by cutting off leaf tips with Xoo suspension infected scissors. Our laboratory has found that leaf clipping method is an effective and simple method, and appropriate to the natural infection of Xoo.
Materials and Reagents
Filter paper (Whatman)
Petri dishes (9 cm diameter) (ASONE)
Cultivated land soil
Compound fertilizer (N: 15%; P: 15%; K: 15%)
Kimwipe (Kimberly-Clark)
1.5 ml Eppendorf tube (Eppendorf)
10 ml and 50 ml tubes (BD Falcon)
Tips (200 μl and 1,000 μl) (Axygen)
-
Xanthomonas oryzae pv. oryzae (Xoo) stocks (provided by International Rice Research Institute/IRRI)
Note: The Xoo strains are maintained as glycerol stock in -80 °C for long term storage. Every strain should be retrieved on a peptone sucrose agar (PSA) plate.
Rice (Oryza sativa subsp. indica) seeds (rice variety IR24, provided by IRRI’s International Rice Genebank)
Sterilized water (Milli-Q)
-
Ethanol (Sigma-Aldrich, catalog number: 24102)
Note: This product has been discontinued.
Glycerol (Sigma-Aldrich, catalog number: G5516)
Peptone (Sigma-Aldrich, catalog number: P4963)
L-Glutamic acid (Sigma-Aldrich, catalog number: G5667)
Sucrose (Sigma-Aldrich, catalog number: V900116)
Agar (Sigma-Aldrich, catalog number: 17209)
Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: P5958)
Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266)
Peptone sucrose agar (PSA) solid media (see Recipes)
1 M MgCl2 stock solution (see Recipes)
Equipment
Ruler, scissors, tweezers, mortar and pestle
Pipette (Eppendorf)
Precision balance (Mettler-Toledo, model: ME303TE)
Growth chamber for rice seed germination (Conviron, model: ACT 26)
Incubation chamber (28 °C) for Xoo growth (Biolab Scientific, model: BIFG-101)
Spectrophotometer (Beckman Coulter, model: DU-640)
Greenhouse capable of temperature and humidity control for growing rice plants
Procedure
-
Preparation of rice materials
Sow rice seeds (at least 20 seeds per genotype) on a piece of filter paper in each Petri dish with appropriate volume of sterile water and place all the Petri dishes in an incubation chamber (14 h light and 10 h dark photoperiod, at 28-36 °C) for one week.
Transplant the one-week-old seedlings into pots (20 cm in diameter and 30 cm in height) containing 80% volume of cultivated land soil (two seedlings per pot). Water the plants every two days and fertilize the plants every two weeks.
-
Preparation of Xoo
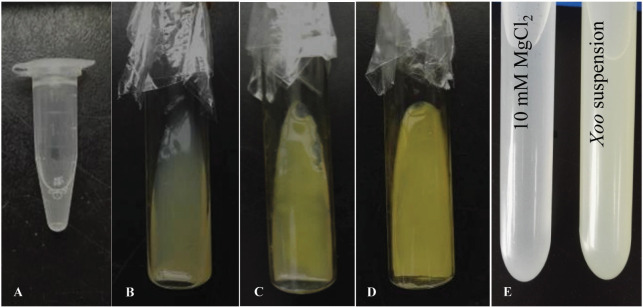
Grow Xoo by transferring 50 μl of Xoo stock from -80 °C (Figure 1A) to a solid PSA media (see Recipes) (Figure 1B) and incubate at 28 °C for three days until a biofilm is formed (Figure 1C) for Xoo activation. The activated Xoo strain can be stored at 4 °C for up to four weeks.
Two days before inoculating rice plants, subculture of Xoo by transferring activated Xoo from the original solid PSA media to a new solid PSA media and incubating at 28 °C for an additional two days (Figure 1D).
At the day of inoculation, suspend Xoo from the most recent solid PSA media in 10 mM sterilized MgCl2 solution (see Recipes) (pH = 7.0) to OD600 = 0.5 (Figure 1E).
-
Inoculation of rice with Xoo and disease scoring
-
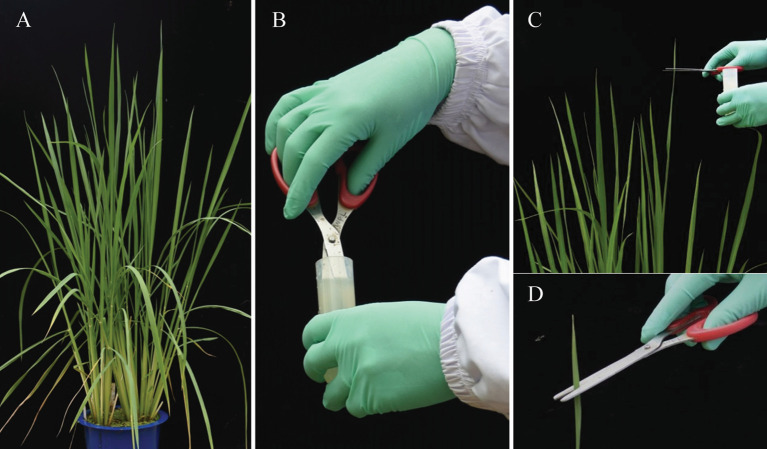
Inoculation of rice leaves by leaf clipping method. Dip scissor tips into the Xoo suspension (Figure 2B) and cut the leaf tip (approximately 2-3 cm for seedling plants and 4-5 cm for adult plants) away from the leaf (Figures 2C and 2D). Plants were grown at 28-32 °C (light, 12 h), 28-32 °C (dark, 12 h), 90% relative humidity.
Notes:
Clip one to two leaves with one pair of dipped scissor tips into the Xoo suspension for one time.
For statistical analyses of infectious disease, inoculate more than ten leaves per plant and obtain at least five data points for the lesion length.
Rice plants can be transferred into a growth chamber for inoculation.
Cut the tip of the fully extended 1st and 2nd leaves, or just 2nd leaf of seedling plants (before tillering stage). Cut the tip of the fully extended 1st and 2nd leaves, or just 1st leaf per tiller for adult plants (tillering stage or heading stage).
-
Xoo growth rate (Figure 3)
-
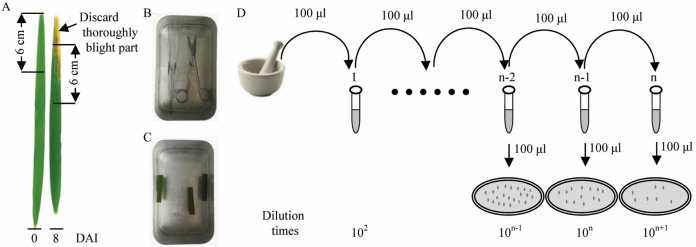
Sample 6 cm-length leaf fragment every two days until to 14 days (Figure 3A).
Note: At day 0, harvest samples at 30 min after inoculation. For later time points, discard the thoroughly blight part close to the inoculated site (Xoo is a biotrophic bacterium and does not exist in the thoroughly blight part where is dead leaf tissue; the discarded part should not contain any green tissue) and harvest 6 cm-length leaf fragment next to the inoculated site.
-
The surface of the leaf fragment are sterilized by immersing in 75% ethanol for 1 min (Figure 3C). Dry the leaf fragment with sterilized Kimwipe.
Note: The scissors and tweezers are sterilized by immersing in 75% ethanol before use (Figure 3B).
Cut the leaf fragment into a mortar and grind the sample to homogenize with 1 ml sterilized water.
Take 100 μl homogenate from the mortar to a 1.5 ml tube (tube 1) containing 900 μl sterilized water and mix thoroughly.
Take 100 μl diluted homogenate from the tube 1 to a new 1.5 ml tube (tube 2) containing 900 μl sterilized water and mix thoroughly. Repeat this step for several times until to tube n with appropriate dilution.
Take 100 μl diluted homogenate from each of the diluted tubes and spread on PSA solid media. Incubated at 28 °C for 2-3 days until obvious colonies formed (Figure 3D).
-
Calculate the number of bacteria on three serial dilution plates which have measurable colonies formation individually. For statistical analysis of bacteria, take tube n as an example. N = (Nn-2 x 10n-1 + Nn-1 x 10n + Nn x 10n+1)/3 (N, colony forming unit on the selected 6 cm-length leaf fragment; Nn-2 to Nn represent bacteria colonies from three serial dilution plates corresponding to tube n-2 to tube n, respectively; 10n-1 to 10n+1 represent dilution times corresponding to tube n-2 to tube n).
Notes:
At least calculate three plates and take an average for the number of bacteria of a selected 6 cm-length leaf fragment from one leaf.
For each time point, at least three leaves from three different plants were collected for biological replicates.
-
Score the disease by measuring the lesion length at one week (for seedling stage) or two weeks (for adult stage) after inoculation (Figure 4).
-
Preparation of Xoo stocks
Pick several colonies from PSA solid media streaked with diluted homogenate to 10 ml tubes containing 3 ml PSA liquid medium (one colony per tube) and incubate at 28 °C for two days.
Transfer 100 μl liquid culture into a new 1.5 ml tube and add the same volume of 50% sterilized glycerol, then mix and store at -80 °C.
-
Figure 1. Xoo preparation.
Spread 50 μl Xoo stock solution (A) to a solid PSA media (B) and incubate at 28 °C for three days until a biofilm is formed (C). Two days before inoculation, transfer Xoo from (B) to a new solid PSA media and incubate at 28 °C for two days (D). At the day of inoculation, make Xoo suspension by diluting Xoo to an OD600 of 0.5 from (D) with 10 mM MgCl2 (E).
Figure 2. Xoo inoculation .
. A. Rice plants grown in green house; B. Dipping scissor tips into the Xoo suspension; C and D. Cutting the leaf tip away from the leaf.
Figure 3. Procedure of Xoo dilution and growth.
A and B. Take a 6 cm-length leaf fragment next to the inoculated site with pretreated scissors and tweezers. C. Immerse the leaf fragment into 75% ethanol for 1 min for surface sterilization. D. After being dried, cut the leaf fragment and grind the sample to homogenize with 1 ml sterilized water, and take 100 μl homogenate to a new 1.5 ml tube containing 900 μl sterilized water and mix thoroughly. Repeat this step for several times until to tube n with appropriate dilution. Take 100 μl diluted homogenate from each of the diluted tubes and spread on PSA solid media. Incubated at 28 °C for 2-3 days until obvious colonies formed.
Figure 4. Disease scoring.
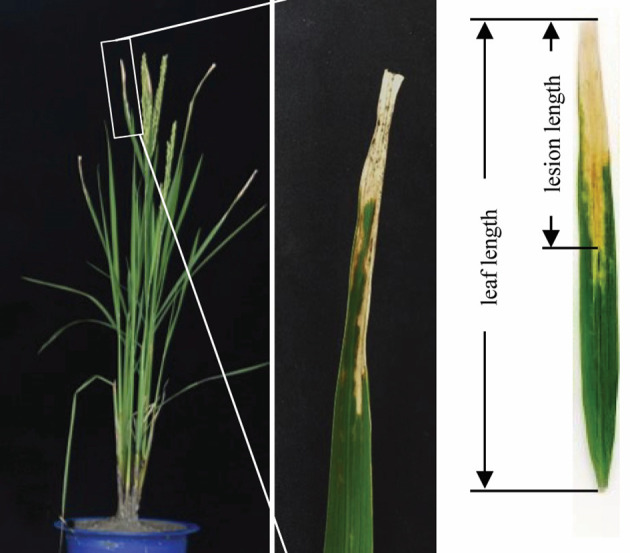
Two weeks after inoculation lesions developed on inoculated leaves. The disease was scored by measuring the lesion length. The lesion length is the length from inoculation site to the edge of thoroughly blight leaf middle veins.
Data analysis
Data analysis see Yuan et al. (2016) .
Recipes
-
Peptone sucrose agar (PSA) solid media (1 L)
10 g peptone
1 g L-glutamic acid
10 g sucrose
Dissolve the above gradients in 900 ml sterile distilled H2O and adjust the pH of the medium to 7.0 using 1 N KOH and bring volume up to 1 L. Add 20 g agar and autoclave at 121 °C for 30 min
-
1 M MgCl2 stock solution
Dissolve 203.3 g MgCl2·6H2O in 800 ml sterile H2O and adjust the volume to 1 L and sterilize by autoclaving for 30 min at 121 °C
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31371926, 31501618). The protocol was adapted from Yuan et al. (2016).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Kauffman H. E., Reddy A. P. K., Hsieh S. P. Y and Merca S. D.(1973). An improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae . Plant Dis Rep 57(6): 537-541. [Google Scholar]
- 2.Mew T. W.(1984). Scanning electron microscopy of virulent and avirulent strains of Xanthomonas campestris pv. oryzae on rice leaves . Phytopathology 74(6):635-641. [Google Scholar]
- 3.Niño-Liu D. O., Ronald P. C. and Bogdanove A. J.(2006). Xanthomonas oryzae pathovars: model pathogens of a model crop . Mol Plant Pathol 7(5): 303-324. [DOI] [PubMed] [Google Scholar]
- 4.Yuan M., Ke Y., Huang R., Ma L., Yang Z., Chu Z., Xiao J., Li X. and Wang S.(2016). A host basal transcription factor is a key component for infection of rice by TALE-carrying bacteria. Elife 5: e19605. [DOI] [PMC free article] [PubMed] [Google Scholar]