Abstract
Objective:
This study was conducted to evaluate the persisting Covid-19-related symptoms of the cases included in our study and to assess their cardiac findings to determine the impact of Covid-19 on children’s cardiovascular health.
Methods:
In this study, 121 children between the ages of 0– and 18 with Covid-19 were evaluated based on their history, blood pressure values, and electrocardiography and echocardiography results. These findings were compared with the findings of the control group which consisted of 95 healthy cases who were in the same age range as the study group and did not have Covid-19. The results were evaluated using the statistics program, SPSS 21.
Results:
There was no significant difference between the study group and the control group in terms of age, weight, and body mass index. The clinical symptoms (chest and back pain, dizziness, headache, palpitation, fatigue, shortness of breath, loss of balance, coughing) of 37.2% of the cases persisted for at least 1 month after Covid-19 recovery. Statistically significant differences were found in systolic blood pressure, left ventricular ejection fraction, relative wall thickness, and tricuspid annular plane systolic excursion.
Conclusion:
The continuation of some cases’ clinical symptoms post-recovery indicates that long Covid infection can be observed in children. The fact that statistically significant differences were observed between the echocardiographic parameters of the study and control groups suggests that Covid-19 may have effects on the cardiovascular system. To shed light on the long Covid cases among children and the infection’s cardiac impacts, it would be beneficial to conduct more comprehensive studies on this matter.
Keywords: Covid-19, long Covid, echocardiography, myocarditis, cardiac involvement, systolic blood pressure
The Covid-19 outbreak, caused by the SARS-CoV-2 virus, has been on the world agenda ever since the first infection case was reported in Wuhan, China, in December, 2019 and then declared a pandemic by the WHO in March, 2020.1–3 Initially, it was thought that children were in the lower risk category for Covid-19 and experienced milder symptoms than adults.4 However, this was soon proven to be inaccurate as severe cases were observed in children as well.5 Although Covid-19 in children mostly affects the upper respiratory tract and the gastrointestinal system, it was also observed to have an impact on various other organs and systems, such as in the case of multisystemic inflammatory syndrome related Covid (MIS-C) which presents itself with Kawasaki-like symptoms and affects the entire body.6,7 Another interesting aspect of the infection is that in some cases the clinical symptoms may persist even months after recovery.8–10 This form of the infection, defined either as long Covid or post-Covid-19 syndrome,9 is known to affect children11 as well as adults, and has only recently begun to be understood. Given this, there currently is not much research on this subject. Furthermore, Covid-19 is also known to affect the cardiovascular system.12–16 In cases with Covid-19, myocarditis may occur, either directly as a result of the virus itself or due to the cytokines that are released during the infection; cardiac problems such as heart failure and arrhythmia may occur as a result of hypoxia due to severe lung infection or as a side effect of the medication used.12,15 Although it is reported that most of these effects are temporary, it is not known for certain whether any cardiac pathologies might develop during the follow-up period of Covid-19 cases. Although many studies have been done on the cardiac effects of Covid-19 in adults there are quite few studies on children. Hence, this study was conducted to evaluate the clinical picture of the long Covid cases in our study group and to assess the cardiac findings of children with Covid-19 to determine the impact of this infection on children’s cardiovascular health.
Methods
This study was conducted in one center, between 17 March, 2021, and 10 June, 2021, on children who had Covid-19. The study group consisted of 121 cases between the ages of 0 and 18, who tested positive for Covid-19. In addition, cases who had a history of Covid-19 but applied to our hospital’s ward for different reasons during the time in which this study was conducted were also included in the study group. All the cases included in the study group have had the infection for at least 1 month and at most 1 year ago. Cases that were in the acute period of the infection were not included in the study. The control group was randomly selected among the cases who applied to our ward with various complaints such as innocent murmur, non-specific chest pain, and for sports examinations and had an age and sex distribution similar to the study group.
The cases were questioned on whether they had any contact with Covid-19 and any diseases that might cause cardiac pathologies. Cases that did not have any Covid-19 positive family members or acquaintances and any contact with Covid-19 were selected for the control group.
All cases in both the study and the control group were examined based on height, weight, body mass index, blood pressure values, and electrocardiography and echocardiography findings. Medical history was taken from both the cases themselves and their families. Among the cases in the study group, 11 were asymptomatic, 83 received out-patient treatment, and 27 received in-patient treatment.
The blood pressures of the cases were taken with an oscillometric blood pressure device (Mindray, PM 9000) using a cuff suitable for their age, and their electrocardiograms were taken with the Cardiovit AT-102 plus (Schiller) device. Because infants mostly do not stay calm during these measurements, only the blood pressure values of cases over the age of 2 were taken into consideration to prevent any inaccurate measurements.
The cases’ echocardiograms were taken by two paediatric cardiologists using the Vivid S6 (GE Healthcare Systems) and Philips Affiniti 50 echocardiography device (Philips Medical Systems Andover, USA). The echocardiography findings were evaluated as structural and functional cardiac pathologies. The evaluations were done according to the guidelines of the American Society of Echocardiography. The conventional measurements and tricuspid annular plane systolic excursion were taken with M mode, mitral inflow peak velocities were taken with spectral Doppler, and early diastolic septal and lateral mitral annular peak velocities were taken with tissue Doppler.
All statistical analyses and tests were performed using SPSS version 21 (IBM SPSS Statistics, IBM Corporation, Armonk, NY, USA) on a personal computer. Normality of variable distribution was assessed with the Kolmogorov–Smirnov test, histogram, coefficient of variation, Detrended Q-Q Plot, and by analysis of skewness and kurtosis. p values <0.05 were considered statistically significant. Group comparisons were performed with the Independent-Samples T-test and Mann–Whitney U-test (distribution of data was non-Gaussian). A Chi-squared test was used to analyse the frequency data. The charts were produced using MS Excel 2016.
A report was taken from the Ethics Committee for our Hospital (17.03.2021/N0:74) and Ministry of Health, General Directorate of Health Services Scientific Research Platform (2021-04-20T18_01_49). During the study, the Helsinki Declaration was adhered to.
Results
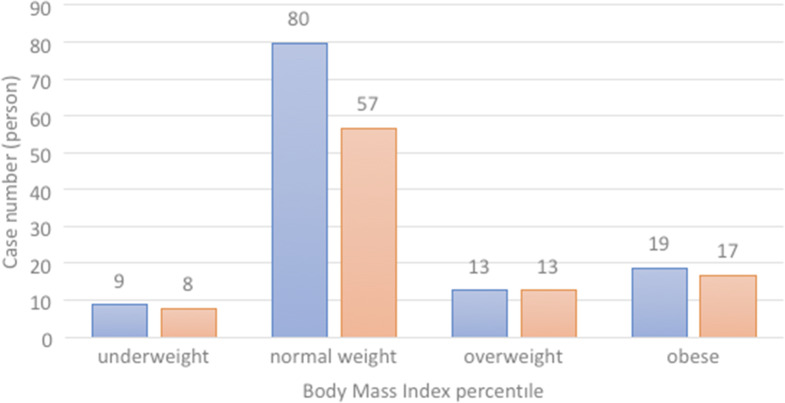
In this study, 216 cases were evaluated. In total, 121 of these cases had Covid-19, whereas 95 were in the control group. The distribution of the cases by age and sex is presented in Table 1, and the distribution according to BMI percentiles is given in Figure 1.
Table 1.
The distribution of cases by age (month) and sex
| Group | Sex | 0–12 | 13–60 | 61–120 | 121–180 | 181–240 | Total |
|---|---|---|---|---|---|---|---|
| Covıd 19 | Male | 9 | 14 | 9 | 23 | 10 | 65 |
| Female | 6 | 11 | 13 | 14 | 12 | 56 | |
| Control | Male | 4 | 10 | 19 | 12 | 5 | 50 |
| Female | 5 | 9 | 10 | 10 | 11 | 45 | |
| Total | Male | 13 | 24 | 28 | 35 | 15 | 115 |
| Female | 11 | 20 | 23 | 24 | 23 | 101 |
Figure 1.
The distribution of cases according to BMI percentiles.
The statistical data of the cases that had Covid-19 and the control group is presented in Table 2.
Table 2.
The distribution of the cases by age, weight, and BMI
| Covıd-19 (n = 121) | Control (n = 95) | Control versus Covıd-19 | |
|---|---|---|---|
| Median (interquartile range–range) | Median (interquartile range–range) | p value* | |
| Age | 9.16 (10.88–17.92) | 8.42 (8.67–17.42) | > 0.05 (0.704) |
| Weıght | 35.6 (38.2–85) | 31 (31–75.5) | > 0.05 (0.694) |
| BMI | 18.38 (5.12–20.31) | 18.05 (5.03–15.56) | > 0.05 (0.377) |
BMI = body mass index.
Mann–Whitney U test.
There was no statistically significant difference between the groups in terms of age, weight, and BMI.
All the cases in the study group had at least one PCR positive individual in their family and contact with Covid-19. In all 27 of the cases had received in-patient treatment. There were approximately 5.6 months (min. 1, max. 12) between the time when the cases had Covid-19 and the time the study was conducted. The clinical findings of the cases related to Covid-19 that were reported by their families are presented in Table 3.
Table 3.
Covid-19-related clinical findings of the cases
| Finding | Number of cases | (%) |
|---|---|---|
| Fever | 73 | 63.33 |
| Fatigue | 72 | 59.50 |
| Headache, myalgia | 61 | 52.24 |
| Sore throat | 31 | 25.62 |
| Cough | 33 | 27.27 |
| Shortness of breath | 10 | 8.26 |
| Stomach ache, vomiting | 27 | 22.31 |
| Diarrhoea | 22 | 18.18 |
| Ocular findings | 11 | 9.09 |
| Skin rash | 14 | 11.57 |
| Taste and smell disorders | 13 | 10.74 |
| Neurological | 7 | 5,79 |
As can be seen in Table 3, fever, fatigue, headache, myalgia, sore throat, coughing, shortness of breath, stomach ache, vomiting, diarrhoea, and taste and smell disorders were the symptoms that were observed in the cases in the study group based on what their families have reported. The number and percentage of each symptom are given in Table 3.
In addition to the findings of the cases given in Table 3, the distribution of asymptomatic cases, cases who received in-patient and out-patient treatment according to their age and sex is presented in Table 4.
Table 4.
The distribution of asymptomatic, out-patient, and in-patient cases with Covid-19 according to age and sex
| Age (Month) | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Asymptomatic | Out-patient | In-patient | Total | Asymptomatic | Out-patient | In-patient | Total | |
| 0–12 | 0 | 2 | 7 | 9 | 1 | 1 | 4 | 6 |
| 13–60 | 1 | 12 | 1 | 14 | 0 | 7 | 4 | 11 |
| 61–120 | 2 | 6 | 1 | 9 | 2 | 9 | 2 | 13 |
| 121–180 | 3 | 19 | 1 | 23 | 1 | 11 | 2 | 14 |
| 181–240 | 0 | 7 | 3 | 10 | 1 | 9 | 2 | 12 |
| Total | 6 | 46 | 13 | 65 | 5 | 37 | 14 | 56 |
In all, 45 of the patients (37.19%) with Covid-19 had the symptoms given in Table 5 when the study was done. Among these symptoms were headache, dizziness, syncope, loss of balance, palpitation, chest and backache, shortness of breath, coughing, and fatigue. The number and percentage of the cases that had these symptoms are presented in Table 5. There was no correlation between the severity of Covid-19 and the infection’s persisting symptoms. The rest of the patients did not have any symptoms at the time of the study.
Table 5.
The number and percentage of the cases with Covid-19 symptoms at the time of the study distributed according to the severity of infection
| Symptom | Number of cases | % | Asymptomatic | Out-patient | In-patient |
|---|---|---|---|---|---|
| Chest and backache | 23 | 51.11 | 3 | 20 | 0 |
| Dizziness ± syncope | 7 | 15.56 | 1 | 6 | 0 |
| Headache, dizziness | 3 | 6.67 | 0 | 3 | 0 |
| Palpitation±chest pain | 5 | 11.11 | 1 | 4 | 0 |
| Shortness of breath | 4 | 8.89 | 1 | 2 | 1 |
| Fatigue | 1 | 2.22 | 0 | 1 | 0 |
| Loss of balance | 1 | 2.22 | 0 | 1 | 0 |
| Coughing | 1 | 2.22 | 0 | 1 | 0 |
| Total | 45 | 100 | 6 | 38 | 1 |
The distribution of the cases that had symptoms at the time of the study and the cases that did not have any symptoms is presented in Table 6.
Table 6.
The distribution of the cases that had symptoms at the time of the study and the cases with no symptoms present
| No symptoms | Symptoms present | Total | |
|---|---|---|---|
| Asymptomatic | 5 | 6 | 11 |
| Out-patient | 47 | 36 | 83 |
| In-patient | 24 | 3 | 27 |
| Total | 76 | 45 | 121 |
A statistically significant difference (p = 0.005 < 0.05) was found between the cases who had and did not have Covid-19 symptoms at the time of the study in terms of being asymptomatic or receiving either out-patient or in-patient treatment.
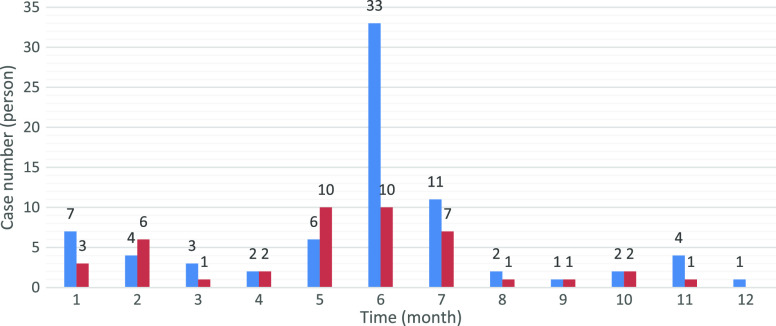
The distribution of the number of months between the time at which the cases had Covid-19 and when the study was conducted is presented in Figure 2.
Figure 2.
The distribution of the number of months between the time at which the cases had Covid-19 and when the study was conducted according to the presence of symptoms at the time of the study.
The basal rhythm in the electrocardiographic examinations of the cases was sinus rhythm. Arrhythmia was not observed. There was no significant difference in terms of heart rates in ECG, PR intervals, and Qtc values between the study and control groups (Table 7). Although there was no significant difference in terms of diastolic blood pressure, a statistically significant difference was found in terms of systolic blood pressure values (Table 7).
Table 7.
Blood pressure and ECG values
| Covid-19 | Control | Control versus Covid -19 | |||
|---|---|---|---|---|---|
| Mean (Min–Max) | n | p value* | n | p value | |
| Systolic BP | 111.51 (92–139) | 91 | 106.64 (85–137) | 78 | <0.05 (0.005)* |
| Diastolic BP | 70.75 (47–89) | 91 | 68.58 (39–98) | 78 | >0.05*(0.161)* |
| PR | 119.18 (68–172) | 108 | 119.09 (75–160) | 87 | >0.05 (0.972)* |
| QTC | 415.94 (349–460) | 119 | 417.16 (380–451) | 95 | >0.05 (0.616)* |
| HR | 97 (29–98)*** | 113 | 97 (24.75–92)*** | 92 | >0.05 0.895** |
BP = blood pressure; HR = Heart Rate; PR = Interval of PR; QTC = Corrected QT Interval.
: Independent t-test.
: Mann-Whitney U test.
: Values are median (interquartile range).
Both the study and control groups were similar in echocardiographical examinations (Table 8). In all, 99 of the 121 cases that had Covid-19 (81.82%) did not have any structural cardiac pathologies. Six of the cases had patent foramen ovale (PFO), seven cases had trivial mitral valve insufficiency, one case had mitral valve prolapse (MVP), three cases had bicuspid aortic valve (BAV), one case had a small ventricular septal defect (VSD) and one case had a spontaneously closed VSD, and one case had thin patent ductus arteriosus (PDA), two cases had persistent left superior vena cava. A total of 80 cases in the control group (84.21%) had no structural cardiac pathologies. Seven cases had patent foramen ovale, two cases had trivial aortic valve insufficiency, three cases had trivial mitral valve insufficiency, one case had mitral valve prolapse, one case had thin patent ductus arteriosus, and one case had a small ventricular septal defect.
Table 8.
The statistical evaluation of the echocardiographic parameters of the study and control groups
| Covid-19 (n = 121) | Control (n = 95) | Control versus Covid-19 | |
|---|---|---|---|
| Mean (Min–Max) | Mean (Min–Max) | p-value | |
| LVEDD (MM) | 37.35 (20.5–52.5) | 36.8 (21–52) | >0.05 (0.608)* |
| IVSD (MM) | 7.55 (4–12.4) | 7.27 (4.5–12) | >0.05 (0.231)* |
| LVPWD (MM) | 7.22 (4.1–12.1) | 6.74 (3.5–11) | <0.05 (0.038)* |
| RWT | 0.38 (0.27–0.53) | 0.37 (0.26–0.54) | <0.05 (0.04)* |
| LVEF (%) | 66.99 (36–80) | 68.25 (60–76) | >0.05 (0.07)* |
| LVSF (%) | 36 (5.75–36)*** | 38 (6–13)*** | >0.05 (0.085)** |
| LA /AO | 1.17 (1–1.68) | 1.19 (0.96–1.40) | >0.05 (0.538) * |
| TAPSE (mm) | 22 (4.9–23.2)*** | 20 (4–17) | <0.05 (0.004)** |
| E/A ratio | 1.6 (0.4–2)*** | 1.6 (0.5–1.9) *** | >0.05 (0.527)** |
| Mitral septal E/E‘, M/S ratio | 8.3 (2.55–25.6)*** | 8.6 (2.1–9.9) *** | >0.05 (0.625)** |
| LateraL E/E‘ ratio | 6.6 (2.05–9.3) *** | 6.1 (1.9–7.7)*** | >0.05 (0.052)** |
IVSD = Interventricular septal diameter; LVEDD = left ventricular end diastolic diameter; LVPWD = left ventricular posterior wall diameter; LVEF = left ventricular ejection fraction; LVFS = left ventricular fractional shortening; LA/Ao = left atrial to aortic ratio; RWT = Relative Wall thickness; TAPSE = tricuspid annular plane systolic excursion.
: Independent t-test.
: Mann–Whitney U test.
: Values are median (interquartile range).
When the echocardiographical M mode and tissue Doppler results were compared, a statistically significant difference was found in terms of left ventricular posterior wall diameter, relative wall thickness, and tricuspid annular plane systolic excursion values between the control and study groups. No significant differences were observed in the other parameters given in Table 8.
There was no significant difference between the groups based on sex in any of the parameters that were evaluated in this study (p = 0.874 – Pearson Chi-Square).
Discussion
During the course of the past one and a half years since the Covid-19 pandemic began many studies have been done on the different effects of the virus and our understanding of the virus has improved significantly, various strains and forms of the virus have been identified, and various methods of controlling the virus have been devised.2 With these improvements, our understanding of Covid-19’s impact on children has progressed. Although it was initially thought that the infection was mostly milder in children than in adults,4 this was soon seen to be inaccurate in many cases. In the studies conducted in the first days of the Covid-19 pandemic, it was stated that the rate of Covid-19 infection in children was only 1–5% and that it caused less severe symptoms than in adults.4,17 Despite this common belief, in later studies a similar rate of seroconversion was found in adults and children, suggesting that both groups had similar levels of sensitivity for the infection.18
In addition, as more patients were examined, it was observed that in some cases symptoms such as fatigue, joint and muscle pain and weakness, respiratory problems, heart palpitations, sleep disorders, anxiety, depression, and lung problems persisted in the long-term even after recovery,8 bringing up a type of Covid-19 infection referred to as long Covid or post-Covid-19 syndrome.9 This case can also be seen among children,10 as supported by a paediatric cohort study done in Russia, in which fatigue, sleep disturbance, sensory, gastrointestinal, dermatological, neurological, respiratory, cardiovascular, and musculoskeletal problems were observed in children with Covid-19 in the long term.19 Our findings were also in line with these, as shown in Figure 2, evoking long Covid in our study group. Though it is not known for certain, the continuation of such symptoms in the long term raises the question of whether the Covid-19 virus can cause chronic disease or not.
Besides the aforementioned symptoms, in a study, it has been observed that Covid-19 related cardiac symptoms such as chest pain, heart palpitation, and tachycardia have continued up to 6 months among Covid-19 survivors,8–10 indicating that long-term cardiac symptoms may develop in long Covid cases. In another study done by Huang et al, the cardiac MR scans of more than 50% of Covid-19 survivors in recovery after the acute period have shown myocardial oedema and late gadolinium enhancement and disorders in the right ventricle functions in 31%.20 In yet another study done by Moody et al, on hospitalised children with pneumonia due to Covid-19, it was observed in 79 cases that although the acute anomalies and function and size changes in the ventricles have improved throughout the 3-month ECHO follow-up period, adverse ventricular remodeling persisted in 29% of the cases,21 once again indicating the long-term cardiac effects of Covid-19. Likewise, in our study group, at the time of the study – at least 1 month after the patients recovered from Covid-19 – chest pain persisted in 23 of the cases, whereas heart palpitations along with chest pain persisted in 5, as shown in Table 5. Similarly, in a recent case report done in Italy, it was observed that a 14-year-old girl who previously did not have any cardiorespiratory problems developed persistent chest pain and tachycardia after Covid-19’s acute phase.22 For this reason, with this study, we have examined whether cardiac symptoms developed as a result of Covid-19 both in cases whose symptoms disappeared after recovery and long Covid cases whose symptoms persisted.
The studies done on Covid-19’s cardiovascular effects and the cardiac pathologies that might develop as a result of the infection are mostly focused on adults, severe cases in the acute period, and cardiac-related morbidities due to Covid-19. In the studies done on adults with Covid-19, arrhythmia was found in 16.7% of the cases, whereas myocardial damage was found in 7.2%;23 when troponin levels, ECHO, and ECG findings were examined, cardiac involvement was observed in 12.5% of the cases;24 and it was even stated that 40% of the Covid-19 related deaths in Wuhan were caused by myocardial damage.25 The studies done on the infection’s cardiac effects on children are mostly centred on severe cases such as patients with MIS-C.26 In such studies, it was stated that cardiac involvement develops in 53% of children with MIS-C27 and that an increase in troponin levels and disorders in systolic functions tend to occur in most MIS-C cases during the infection’s acute period.23
Contrarily, our current understanding of the cardiovascular impact of Covid-19 on asymptomatic or mild cases – which make up a majority of all Covid-19 cases – is quite limited as there are not nearly enough studies done on this group. Another factor that contributes to this uncertainty is the fact that echocardiographic measurements and cardiac enzyme tests are not routinely checked if there are no symptoms of cardiac disorders, making it difficult to assess the infection’s cardiac effects. Considering this, clinical and echocardiographic examinations would be beneficial to follow the long-term cardiac effects of Covid-19 and hence to shed light on this matter.12 Accordingly, in this study, we have used clinical measurements such as height, weight, BMI, and blood pressure values, as well as echocardiographic measurements such as M-mode, 2D, tissue Doppler, and spectral Doppler to evaluate asymptomatic and mild Covid-19 cases among children.
In our study, neither the echocardiographic measurements of both the study and control groups have revealed any structural or functional anomalies nor has a comparison between the echocardiograms of the patients with mild structural pathologies in both groups and their echocardiograms prior to getting Covid-19 shown any significant difference. However, once the results of the study and control groups were compared with each other, a statistically significant difference was detected in the left ventricular posterior wall diameter, relative wall thickness, and tricuspid annular plane systolic excursion values, as presented in Table 8. We could not compare the echocardiographic measurements of the cases in the study group taken at the time of the study with their previous echocardiographic findings from the time when they had Covid-19 since there were no records. Nevertheless, the fact that right ventricle hypertrophy and remodelling were observed due to Covid-19 in the previously mentioned study done by Moody et al21 supports this statistical difference between our study and control groups. In addition, myocardial oedema may have contributed to this statistical difference, as observed in the post-Covid-19 MR imaging findings in the study done by Huang et al20 These possible changes in the myocardial tissue may also be factors that might cause the persisting clinical symptoms in long Covid cases. On the other hand, the statistical difference in the systolic blood pressure findings of the study and control groups may be associated with autonomous nervous system dysfunction,28 an increase in sympathetic activity, and left or right ventricle hypertrophy and remodelling that might develop consequently.
Though it is also possible that these findings may be the result of different factors besides Covid-19. To clear any uncertainties on this topic, more extensive and multi-centred studies should be conducted on Covid-19’s cardiac effects and the cases where the infection’s symptoms persist in the long term.
Conclusion
Covid-19 is an infection that has reached a pandemic status and has taken the entire world by storm over the past one and a half years. New aspects of the infection are unravelled each day as more cases are examined and more studies are conducted. Some of these newly understood aspects are the infection’s impact on the cardiovascular system and long Covid that manifests itself with persisting clinical symptoms. Currently, there are not many on the cardiac effects of Covid-19 and long Covid, especially in children. Hence, it is necessary to conduct more numerous and extensive studies on this matter.
Limitations
This study encompasses electrocardiography, conventional echocardiography, and tissue Doppler findings for the evaluation of myocardial functions, yet does not include echocardiographic modalities such as strain and speckle, and further examinations such as cardiac MR imaging. We could not compare the echocardiographic measurements of the cases in the study group taken at the time of the study with their previous echocardiographic findings from the time when they had Covid-19 since there were no records, as most of the cases were either asymptomatic or mild.
Acknowledgements
This study was dedicated to all the brave and altruistic healthcare professionals who lost their lives in the fight with Covid-19. I express my sincere thanks to Zeynep Eylül Erol for her support in the preparation and translation process of this article and Birsen Karakutuk for her contributions in the recording of the cases and data.
Authors’ contributions
Nurdan Erol planned the study, applied to the Ethics Committee for approval, examined the cases, carried out the echocardiographic measurements, and wrote the manuscript. Abdullah Alpinar helped with the echocardiographic measurements and contributed to the discussion section of the manuscript. Cigdem Erol evaluated the data and analysed the statistics. Erdal Sari did the follow-up of the cases in the Covid-19 ward of our hospital. Kübra Alkan reached out to the cases and invited them to be part of the study. All named authors read and approved the final manuscript.
Financial support
No sources of funding were used in the preparation of this study.
Conflict of interest
None.
Ethics standards
This study was performed in accordance with the standards of ethics outlined in the Declaration of Helsinki, 2013. All patients provided informed written consent (or written assent with parentalconsent, for minors) prior to participation in this study.
References
- 1.Badal S, Bajgain KT, Badal S, et al. Prevalence, clinical characteristics, and outcomes of pediatric COVID-19: a systematic review and meta-analysis. J Clin Virol 2020; 135: 104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohamadian M, Chiti H, Shoghli A, et al. COVID-19: virology, biology and novel laboratory diagnosis. J Gene Med 2021; 23: e3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pousa PA, Mendonc TSC, Oliveira EA, et al. Extrapulmonary manifestations of COVID-19 in children: a comprehensive review and pathophysiological considerations. J Pediat 2021; 97: 116–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludvigsson JF.Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatrica 2020; 109: 1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi K, Kaplan D, Bakar A, et al. Cardiac dysfunction and shock in pediatric patients with COVID-19. Am Coll Cardiol Case Rep 2020; 2: 1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperotto F, Friedman KG, Son MBF, et al. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr 2021; 180: 307–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alsohimea F, Temsaha MH, Al-Nemri AM, et al. COVID-19 infection prevalence in pediatric population: etiology,clinical presentation, and outcome. J Infect Public Heal 2020; 13: 1791–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study lancet 2021, 397:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong SJ.Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors,and treatments. Infect Dis 2021, 1–18. DOI 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carfì A, Bernabei R, Landi F.Persistent symptoms in patients after acuteCOVID-19. JAMA 2020; 324: 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on long COVID in children acta paediatrica 2021, 1–4. [DOI] [PMC free article] [PubMed]
- 12.Harikrishnan S, Mohanan PP, Chopra VK, et al. Cardiological society of India position statement on COVID-19 and heart failure. Indian Heart J 2020; 72: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwenandar F, Japar KV, Damay V, et al. Coronavirus disease 2019 and cardiovascular system: a narrative review. IJC HeartVasculature 2020; 29, 100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh MN, Sorgente A, Fischman DL, et al. The COVID-19 pandemic and cardiovascular complications. What have we learned so far? J am Coll Cardiol Case Rep 2020; 2: 1235–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.. Coronavirus disease 2019 and the cardiovascular system: impacts and implications. Editorial. Indian Heart J 2020; 72: 1–6. [DOI] [PMC free article] [PubMed]
- 16.Sanna G, Serrau G, Bassareo PP, et al. Children’s heart and COVID-19: up-to-date evidence in the form of a systematic review. Eur J Pediatr 2020; 179: 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID‐19 patients in the United States. Nature Sci Rep 2021; 11: 10231. DOI 10.1038/s41598-021-89553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyde Z.COVID-19,children and schools: over looked and at risk. Med J Australia 2020; 213: 444–446. [DOI] [PubMed] [Google Scholar]
- 19.Osmanov IM, Spiridonova E, Bobkova P, et al. Risk factors for long covid in previously hospitalised children using the ISARIC global follow-up protocol: a prospective cohort study. Eur Respir J in press. DOI: 10.1183/13993003.01341-2021. https://erj.ersjournals.com/content/erj/early/2021/06/10/13993003.01341-2021.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Zhao P, Tang D, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. J Am Coll Cardiol Img 2020; 13: 2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moody WE, Liu B, Mahmoud-Elsayed HM, et al. Persisting adverse ventricular remodeling in COVID-19 survivors: a longitudinal echocardiographic study. J Am Soc Echocardiog 2021; 34: 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buonsenso D, Di Giuda D, Sigfrid L, et al. Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post-acute sequelae of SARS-CoV-2 infection. Lancet Child Adolesc Health 2021; 5: 677–680. DOI 10.1016/S2352-4642(21)00196-6. Retrieved July 30, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsubara D, Kauffman HL, Wang Y, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol 2020; 76: 1947–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan. China Int J Cardiol 2020; 311: 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C, WangY Li, etal X.Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020; 383: 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein DS.The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm 2021; 18: 508–509. [DOI] [PMC free article] [PubMed] [Google Scholar]