Key Points
Question
Do individuals with sagittal synostosis experience greater cognitive, behavioral, and/or psychological difficulties, compared with their healthy peers?
Findings
In this meta-analysis, data from 32 independent studies involving a pooled sample of 1422 children and adults and examining 16 domains were analyzed. Overall, results were highly variable, with individual study results ranging from moderately positive findings for global development, where the children with sagittal synostosis were functioning at better levels than their peers, to large negative differences between groups for general cognition.
Meaning
These findings suggest that some children with sagittal synostosis experience negative outcomes; thus, ongoing monitoring and referral to support services as required are critical.
This meta-analysis reviews research on individuals with sagittal synostosis to determine whether, and to what extent, they experience cognitive, behavioral, and psychological difficulties compared with their healthy peers or normative data for each measure.
Abstract
Importance
Findings on the cognitive, behavioral, and psychological functioning of individuals with sagittal synostosis (SS) are highly disparate, limiting their clinical utility.
Objective
To identify and review research on individuals with SS and to determine whether, and to what extent, they experience cognitive, behavioral, and psychological difficulties compared with their healthy peers or normative data for each measure.
Data Sources
PubMed, Scopus, Embase, and PsycINFO were searched through January 2021 with no date restrictions. Scopus citation searches and manual checks of the reference lists of included studies were conducted.
Study Selection
Studies included participants of any age who had received a diagnosis of single-suture (isolated or nonsyndromic) SS or scaphocephaly and who had been assessed on cognitive, behavioral, and psychological outcomes.
Data Extraction and Synthesis
Data were independently extracted by 2 reviewers. Case-control outcomes (individuals with SS vs healthy peers or normative data) were compared using random-effects models with 3 effect sizes calculated: weighted Hedges g (gw), odds ratios (ORs), and mean prevalence rates. This study follows the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines.
Main Outcomes and Measures
Findings were categorized by surgical status (conservatively managed, presurgery, postsurgery, or combined); domain (eg, general cognition); type of cognitive, behavioral, or psychological measure (objective or subjective); and source of comparison data (peers or normative data).
Results
Data from 32 studies, involving a pooled sample of 1422 children and adults with SS (mean [SD] age at assessment, 5.7 [6.6] years; median [interquartile range] age, 3.3 [0.5-10.3] years), were analyzed. Data on sex were available for 824 participants, and 642 (78%) were male. Individual study results varied substantially. Objective tests identified significant moderate group differences on 3 of 16 examined domains: presurgical motor functioning (3 studies; gw = −0.42; 95% CI, −0.67 to −0.18; P < .001), postsurgical short-term memory (2 studies; gw = −0.45; 95% CI, −0.72 to −0.17; P < .001), and postsurgical visuospatial ability (6 studies; gw = 0.31; 95% CI, 0.18 to 0.44; P < .001). Prevalence estimates and ORs varied widely, with 15 studies showing prevalence estimates ranging from 3% to 37%, and 3 studies showing ORs ranging from 0.31 (95% CI, 0.01 to 6.12) for processing speed in the conservatively managed sample to 4.55 (95% CI, 0.21 to 98.63) for postsurgical visuospatial abilities.
Conclusions and Relevance
In this meta-analysis, findings for the functioning of participants with SS were highly disparate and often of low quality, with small samples sizes and control groups rarely recruited. Nonetheless, the findings suggest that some individuals with SS experience negative outcomes, necessitating routine assessment.
Introduction
Sagittal synostosis (SS), also known as scaphocephaly, occurs when the fibrous connective tissue joint that runs along the top of the skull between the 2 parietal bones (sagittal suture) fuses prematurely (ie, before adulthood), thereby restricting normal transverse growth of the skull.1 Instead, as the brain continues to grow, the skull compensates by growing at the remaining open cranial sutures, resulting in a long and narrow head shape with fullness (bossing) of the forehead.2
Of the major cranial sutures (sagittal, metopic, coronal, and lambdoid), SS is the most common craniosynostosis, found in approximately one-half of nonsyndromic (isolated) cases, occurring in approximately 2 to 3 births per 10 000, and diagnosed more frequently in boys than girls.3 SS cases are increasing globally, although the reasons for this increase are unknown. Suggested causal mechanisms4,5 include genetics (eg, AXIN2 gene variation), gestational exposure to environmental factors (eg, maternal substance use), hormonal influences (eg, maternal thyroid dysfunction), mechanical forces (eg, intrauterine constraint), and familial cases (approximately 2% of SS cases).6,7,8,9,10
SS typically requires surgical management to improve the child’s appearance and/or ensure the shape of the cranial vault so that the brain can grow and develop normally. The brain growth curve guides the appropriate choice of surgical technique and timing.11,12 Despite these treatment aims, findings on cognitive functioning in individuals with SS indicate considerable problems. For example, general cognitive problems have been estimated to affect 4% to 37% of children with SS,13,14,15,16 whereas measures of general cognition have shown those with SS to be performing better than,17 worse than,18 and even comparable to19,20 their peers. Results for specific cognitive domains include verbal or language problems reported in 7% to 37%14,21,22,23 of children with SS and visuospatial deficits in 7% of children with SS.21,24 Parental reports indicate that behavioral problems are common, with 26% of children with SS exhibiting externalizing traits (eg, restlessness, temper tantrums) and 14% with internalizing behaviors (eg, fears).25 Moreover, children’s concerns with their appearance may lead to social isolation and anxiety, which can affect their psychological well-being, although research in this area is sparse.26,27,28,29
Interpretation of the aforementioned findings is, however, complicated by the use of diverse outcome measures (eg, Bayley Scales of Infant Development [BSID] and Wechsler Preschool and Primary Scale of Intelligence),30,31,32,33 in addition to differences in the surgical status of samples (eg, conservatively managed vs presurgical vs postsurgical). Moreover, pathological differences in the way that different sutures fuse—with midline sutures (ie, sagittal and metopic) being more vulnerable to some genetic variations (eg, SMAD6) than coronal and lambdoid sutures—could lead to differential outcomes.3,34 These findings highlight a need to consider the effect of distinct methodological and clinical differences when examining cognitive, behavioral, and psychological outcomes in individuals with SS.
Given the aforementioned issues, it is currently difficult to draw conclusions about the associations of SS with performance and functioning. For this reason, the current meta-analysis had 2 primary aims: to provide a comprehensive up-to-date review of this literature by identifying all research that examined cognitive, behavioral, and psychological outcomes of individuals with SS; and to determine whether and to what extent children and adults with SS experience difficulties across these domains.
Methods
Literature Search and Inclusion Criteria
This study follows the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines.35 The review protocol was preregistered with the PROSPERO International Register of Systematic Reviews. With the assistance of an expert research librarian, comprehensive searches of the PubMed, Scopus, Embase, and PsycINFO electronic databases were conducted in August 2020 and again in January 2021, with no date restrictions (eTable 1 in the Supplement). In addition, reference lists were manually examined and Scopus citation searches undertaken for all included studies.
All studies had to meet the following criteria: (1) participants received a diagnosis of single-suture (isolated or nonsyndromic) SS or scaphocephaly; (2) cognitive, behavioral, and psychological outcomes were assessed; (3) quantitative data were suitable for the calculation of effect sizes; (4) details of the specific measure used were provided so that normative data could be obtained (where no comparison group was recruited); (5) the study was published in a peer-reviewed journal (so-called gray literature was excluded) in English; and (6) studies were original research with 2 or more participants (excludes reviews and case studies). Cases of syndromic SS (eg, Apert syndrome), multiple affected sutures, and other conditions known to affect functioning (>10% of the sample) were excluded. Studies that included participants who were identified for inclusion because they had cognitive or behavioral problems were excluded.
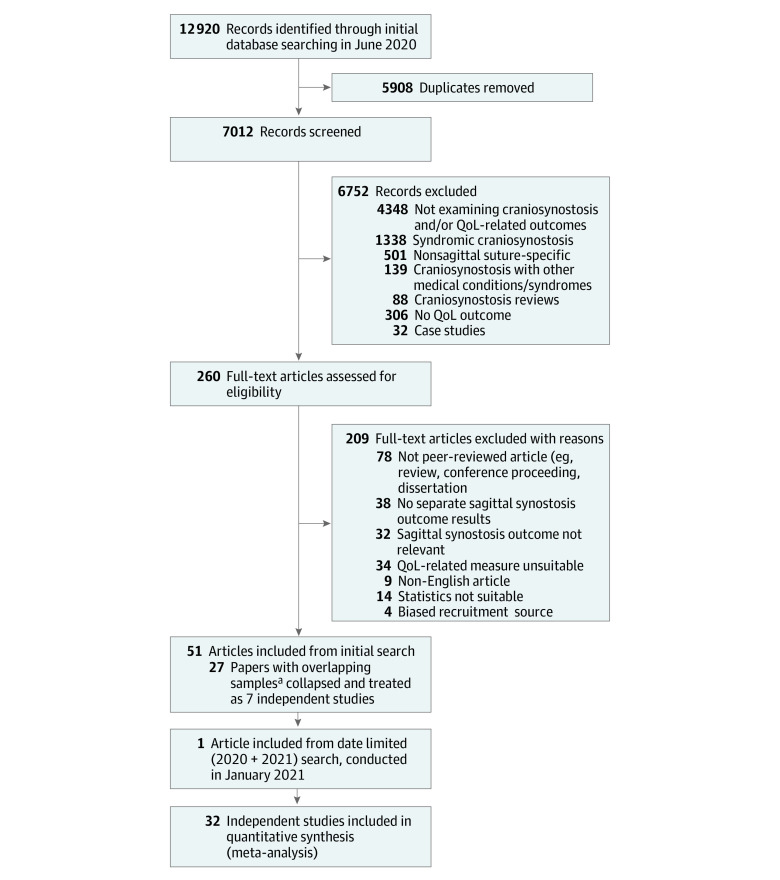
The literature search initially identified 12 920 records, which were imported into Covidence screening software (Veritas Health Innovation). Two authors (A.J.O. and R.M.R.) independently assessed all studies for which eligibility was ambiguous, after which a consensus decision was made. eTable 2 in the Supplement provides details for the final sample of 52 included articles (32 independent studies). Studies using overlapping samples were combined and treated as nonindependent studies (eTable 3 in the Supplement). The Figure details the study selection process.
Figure. Flowchart of the Study Selection Process.
QoL indicates quality of life.
aDetails of articles with overlapping samples that were collapsed and treated as independent studies are provided in eTable 3 in the Supplement.
Data Collection and Preparation
Date extraction was performed by 2 independent reviewers (A.J.O. and a research assistant) using a prepiloted data form, focusing on demographic, clinical, methodological, and effect size data (eTable 4 in the Supplement). Discrepancies were resolved by consensus (91% agreement obtained). A total of 57 individual measures, broadly classified into 16 corresponding domains, were used to assess outcomes (eTable 5 in the Supplement).36,37 Individual study results were also grouped according to surgery status (ie, conservatively managed, presurgery, postsurgery, and mixed), type of measure (objective vs subjective), and comparison group (ie, healthy peers vs normative data). In addition, the impact of mean age at assessment was examined by grouping studies according to the broad developmental stage of their sample. The data were standardized before analysis, and the authors of 4 studies19,38,39,40 were contacted and asked to provide further information, with 1 responding.
Study Reporting Quality
All studies were rated using modified versions of the National Institutes of Health Study Quality Assessment Tool–Observational Cohort and Cross-Sectional Studies (12 criteria) and Case-Control Studies (14 criteria). Each criterion was categorized (met, not met, not reported, or not applicable), and the percentage of included studies that met each criterion was determined. Data extraction was undertaken by 2 independent coders (A.J.O. and a research assistant), and discrepancies were resolved by consensus.
Statistical Analysis
Effect size data were entered into Comprehensive Meta-Analysis statistical software version 3.3 (Biostat, Inc) with conservative random-effects models generated. P values assessed statistical significance (set at 2-sided P < .05), and 95% CIs determined precision.
Three effect sizes were calculated. First, standardized mean differences (Hedges g) compared individuals with SS vs healthy control participants or normative data (24 studies). Effect sizes were weighted (inverse variance method) and pooled (gw). A negative gw indicated poorer functioning in the SS group vs the comparisons, whereas a positive gw indicated better functioning.41 Second, proportions, weighted by sample size, examined the prevalence of cognitive, behavioral, and psychological problems in those with SS (15 studies). Third, odds ratios (ORs) determined the likelihood (increased occurrence, OR > 1; decreased occurrence, OR < 1) of experiencing cognitive, behavioral, or psychological problems after SS compared with healthy peers (3 studies).
Between-study heterogeneity was explored using prediction intervals (pooled analyses with ≥5 included studies), which represent the extent to which the true effect size varies across populations,42 in addition to I2 and τ values (≥2 included studies). Orwin failsafe N (Nfs) values were calculated for pooled analyses with 2 or more studies, given that the small number of studies in each analysis (range, 1-9 studies) rendered more formal statistical tests of publication bias problematic.
Results
Summary details for the 32 independent studies (1422 participants) included in the meta-analysis are shown in Table 1, with study-specific information provided in eTable 2 in the Supplement. The majority of studies included small sample sizes (mean [SD], 44.4 [42.5] participants) and children (mean [SD] age at assessment, 5.7 [6.6] years; median [interquartile range], 3.3 [0.5-10.3] years). Data on sex were available for 824 participants, and 642 (78%) were male. Samples were typically assessed postsurgically (25 studies), and comparison groups were not routinely used (23 studies).
Table 1. Summary Details for the 32 Independent Studies Included in the Meta-analysis.
| Characteristic | Studies, No.a | Participants, No. (%)a | Value |
|---|---|---|---|
| Sample size, mean (SD), No. of participants | 32 | 1422 | 44.4 (42.5) |
| Age at first assessment, y | |||
| Mean (SD) | 24 | 1208 | 5.7 (6.6) |
| Median (interquartile range) | 3.3 (0.5-10.3) | ||
| Surgical status, mean (SD), No. of participantsb | |||
| CM | 4 | 98 | 24.5 (20.7) |
| Presurgery | 13 | 581 | 44.7 (38.7) |
| Postsurgery | 25 | 915 | 36.6 (32.4) |
| Mixedc | 4 | 193 | 48.3 (32.5) |
| Sexd | |||
| Female | 20 | 182 (22.0) | NA |
| Male | 20 | 642 (78.0) | NA |
| Study focus | |||
| Studies examining sagittal synostosis only | 18 | 996 (70.0) | NA |
| Studies examining multiple suture types | 14 | 426 (30.0) | NA |
| Control group used | |||
| Yes | 9 | 262 (18.4) | NA |
| No | 23 | 1160 (81.6) | NA |
| Origin of study | |||
| US | 15 | 469 (32.9) | NA |
| Europe | 10 | 534 (37.6) | NA |
| United Kingdom | 3 | 283 (19.9) | NA |
| Asia | 2 | 104 (7.3) | NA |
| Australia | 2 | 32 (2.3) | NA |
| Surgical technique reported | |||
| Yes | 16 | 801 (56.3) | NA |
| No | 12 | 459 (32.3) | NA |
| CM or presurgery only | 4 | 162 (11.4) | NA |
| Surgical procedures, No. | |||
| 1 | 3 | 48 (3.4) | NA |
| ≥2 | 6 | 216 (15.2) | NA |
| Not reported | 20 | 1042 (73.3) | NA |
| CM or presurgery only | 3 | 116 (8.1) | NA |
| Criteria for surgery reported | |||
| Yes | 6 | 170 (12.0) | NA |
| No | 26 | 1252 (88.0) | NA |
| Family history of craniosynostosis reported | |||
| Yes | 0 | 0 | NA |
| No | 32 | 1422 (100.0) | NA |
| Genetic status examined | |||
| Yes | 5 | 373 (26.2) | NA |
| No | 27 | 1049 (73.8) | NA |
| Socioeconomic status examined | |||
| Yes | 10 | 496 (34.9) | NA |
| No | 22 | 926 (65.1) | NA |
| Neonatal and/or perinatal problems reported | |||
| Yes | 9 | 483 (34.0) | NA |
| No | 23 | 939 (66.0) | NA |
Abbreviations: CM, conservatively managed; NA, not applicable.
Refers to the total number of studies and participants for which data were available.
Some studies reported data for multiple groups.
Refers to CM, presurgery, and postsurgery combined.
Data on sex were available for 824 participants.
Overall reporting quality for each of the included studies was poor. Results for single-group observational studies are presented in eTable 6 and eFigure 1 in the Supplement, whereas findings for case-control studies are reported in eTable 7 and eFigure 2 in the Supplement.
Estimated effect sizes varied considerably across the included studies, as reflected by the heterogeneity indices (Table 2, Table 3, and Table 4 and eTable 8 in the Supplement). The results, for the most part, were also susceptible to publication bias (Nfs). Individual findings, grouped by surgical status (conservatively managed, presurgery, postsurgery, and combined) are discussed in more detail in the following subsections.
Table 2. Outcomes of Children and Adults With Conservatively Managed SS.
| Domain and comparison data | Standardized mean group differences | Prevalence rates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies, No. | Participants with SS, No. | Hedges g (95% CI) | P value | I 2 | τ | N fs | Studies, No. | Participants with SS, No. | Prevalence (95% CI) | Studies, No. | Participants with SS, No. | OR (95% CI)a | |
| Objective measures | |||||||||||||
| General cognition | |||||||||||||
| Peers | 1 | 30 | −0.08 (−0.67 to 0.51) | .79 | NA | NA | NA | 2 | 65 | 0.05 (0.02 to 0.14) | NA | NA | NA |
| Normsb | 2 | 17 | 0.19 (−0.29 to 0.67) | .43 | 0.00 | 0.00 | 0 | ||||||
| Motor functioning, norms | 1 | 13 | −0.15 (−0.70 to 0.39) | .59 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Verbal abilities, norms | 1 | 14 | 0.09 (−0.44 to 0.63) | .74 | NA | NA | NA | 1 | 14 | 0.07 (0.01 to 0.37) | 1 | 13 | 0.33 (0.02 to 6.65) |
| Visuospatial abilities, norms | 1 | 14 | 0.02 (−0.51 to 0.56) | .93 | NA | NA | NA | 1 | 17 | 0.07 (0.01 to 0.36) | 1 | 16 | 0.69 (0.04 to 11.11) |
| Adaptive and social skills, norms | 1 | 13 | −0.14 (−0.69 to 0.41) | .62 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Arithmetic | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 1 | 18 | 0.71 (0.04 to 12.43) |
| Attention | NA | NA | NA | NA | NA | NA | NA | 1 | 19 | 0.13 (0.03 to 0.41) | 1 | 26 | 1.04 (0.48 to 2.27) |
| Executive function | NA | NA | NA | NA | NA | NA | NA | 1 | 12 | 0.08 (0.01 to 0.41) | NA | NA | NA |
| Global development, norms | 1 | 23 | 0.58 (0.17 to 0.99) | .01 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Memory: shorter-term | NA | NA | NA | NA | NA | NA | NA | 1 | 18 | 0.09 (0.03 to 0.23) | 1 | 16 | 0.70 (0.05 to 10.33) |
| Processing speed | NA | NA | NA | NA | NA | NA | NA | 1 | 14 | 0.07 (0.01 to 0.37) | 1 | 14 | 0.31 (0.01 to 6.12) |
| Subjective measures | |||||||||||||
| Behavior | |||||||||||||
| Externalizing, norms | 1 | 24 | −0.25 (−0.65 to 0.15) | .22 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Internalizing, norms | 1 | 24 | −0.30 (−0.70 to 0.10) | .14 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Overall, norms | 1 | 24 | −0.29 (−0.69 to 0.11) | .16 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Quality of life, norms | 1 | 24 | 0.00 (−0.40 to 0.40) | >.99 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Abbreviations: NA, not applicable; Nfs, Orwin failsafe N; OR, odds ratio; SS, sagittal synostosis.
ORs are shown on a logarithmic scale.
Norms refers to comparisons against normative data for each measure.
Table 3. Outcomes of Children With SS Who Had Not Yet Undergone Surgery.
| Domain and comparison data | Standardized mean group differences | Prevalence rates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies, No. | Participants with SS, No. | Hedges g (95% CI) | P value | I 2 | τ | N fs | Studies, No. | Participants with SS, No. | Prevalence (95% CI) | |
| General cognition | ||||||||||
| Peers | 3 | 89 | −0.28 (−0.67 to 0.10) | .15 | 34.48 | 0.21 | 1 | 1 | 27 | 0.37 (0.21 to 0.56) |
| Normsa | 4 | 160 | −0.15 (−0.89 to 0.60) | .70 | 94.99 | 0.74 | 0 | |||
| Motor functioning | ||||||||||
| Peers | 3 | 108 | −0.42 (−0.67 to −0.18) | <.001 | 0.00 | 0.00 | 3 | 2 | 114 | 0.17 (0.07 to 0.36) |
| Norms | 4 | 166 | −0.30 (−10.01 to 0.41) | .41 | 94.80 | 0.71 | 2 | |||
| Verbal abilities | ||||||||||
| Peers | 3 | 99 | 0.01 (−0.24 to 0.26) | .96 | 0.00 | 0.00 | 0 | 2 | 90 | 0.13 (0.03 to 0.40) |
| Norms | 2 | 103 | 0.17 (−0.03 to 0.37) | .09 | 0.00 | 0.00 | 0 | |||
| Visuospatial abilities | ||||||||||
| Peers | 1 | 28 | −0.09 (−0.61 to 0.43) | .73 | NA | NA | NA | 1 | 87 | 0.07 (0.03 to 0.15) |
| Norms | 1 | 26 | 0.44 (0.04 to 0.83) | .03 | NA | NA | NA | |||
| Adaptive and social skills | ||||||||||
| Peers | 1 | 28 | −0.21 (−0.72 to 0.31) | .44 | NA | NA | NA | 1 | 87 | 0.06 (0.02 to 0.13) |
| Norms | 1 | 26 | 0.21 (−0.60 to 0.18) | .30 | NA | NA | NA | |||
| Global development | ||||||||||
| Peers | 1 | 28 | −0.31 (−0.83 to 0.21) | .25 | NA | NA | NA | 5 | 378 | 0.15 (0.09 to 0.24) |
| Norms | 1 | 26 | 0.30 (−0.09 to 0.70) | .13 | NA | NA | NA | |||
Abbreviations: NA, not applicable; Nfs, Orwin failsafe N; SS, sagittal synostosis.
Norms refers to comparisons against normative data for each measure.
Table 4. Outcomes of Children and Adults With SS Who Had Undergone Surgery.
| Domain and comparison data | Standardized mean group differences | Prevalence rates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies, No. | Participants with SS, No. | Hedges g (95% CI) | P value | I 2 | τ | N fs | Studies, No. | Participants with SS, No. | Prevalence (95% CI) | Studies, No. | Participants with SS, No. | OR (95% CI)a | |
| Objective measures | |||||||||||||
| General cognition | |||||||||||||
| Peers | 2 | 113 | −0.12 (−0.57 to 0.33) | .60 | 49.78 | 0.24 | 0 | 3 | 112 | 0.20 (0.11 to 0.34) | NA | NA | NA |
| Normsb | 9 | 299 | 0.01 (−0.26 to 0.27) | .96 | 75.71 | 0.34 | 0 | ||||||
| Motor functioning | |||||||||||||
| Peers | 3 | 131 | 0.18 (−0.41 to 0.05) | .13 | 0.00 | 0.00 | 0 | 2 | 81 | 0.12 (0.03 to 0.36) | NA | NA | NA |
| Norms | 7 | 244 | 0.01 (−0.31 to 0.34) | .93 | 83.10 | 0.40 | 0 | ||||||
| Verbal abilities | |||||||||||||
| Peers | 3 | 122 | −0.65 (−1.34 to 0.03) | .06 | 74.77 | 0.52 | 7 | 3 | 84 | 0.15 (0.08 to 0.27) | NA | NA | NA |
| Norms | 7 | 266 | 0.23 (−0.01 to 0.47) | .06 | 69.71 | 0.26 | 1 | ||||||
| Visuospatial abilities | |||||||||||||
| Peers | 3 | 102 | −0.07 (−0.32 to 0.17) | .56 | 0.00 | 0.00 | 0 | 1 | 63 | 0.05 (0.02 to 0.14) | 1 | 35 | 4.55 (0.21 to 98.63) |
| Norms | 6 | 248 | 0.31 (0.18 to 0.44) | <.001 | 0.00 | 0.00 | 3 | ||||||
| Adaptive and social skills, norms | 2 | 76 | 0.07 (−0.26 to 0.41) | .67 | 48.57 | 0.17 | 0 | 1 | 63 | 0.03 (0.01 to 0.12) | NA | NA | NA |
| Arithmetic | |||||||||||||
| Peers | 1 | 75 | −0.21 (−0.48 to 0.06) | .12 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Norms | 1 | 10 | −0.24 (−0.86 to 0.38) | .45 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Attention | |||||||||||||
| Peers | 2 | 94 | −0.40 (−1.08 to 0.29) | .26 | 73.58 | 0.43 | 2 | NA | NA | NA | NA | NA | NA |
| Norms | 1 | 38 | −0.51 (−0.84 to −0.19) | <.001 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Executive function, peers | 1 | 75 | −0.18 (−0.45 to 0.09) | .19 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Global development, norms | 2 | 76 | 0.34 (−0.09 to 0.76) | .13 | 68.17 | 0.26 | 1 | 1 | 26 | 0.15 (0.06 to 0.35) | NA | NA | NA |
| Learning difficulties | NA | NA | NA | NA | NA | NA | NA | 4 | 143 | 0.23 (0.11 to 0.41) | 1 | 70 | 0.64 (0.35 to 1.17) |
| Memory | |||||||||||||
| Shorter term | |||||||||||||
| Peers | 2 | 92 | −0.20 (−0.45 to 0.05) | .12 | <.001 | 0.00 | 0 | NA | NA | NA | NA | NA | NA |
| Norms | 2 | 51 | −0.45 (−0.72 to −0.17) | <.001 | <.001 | 0.00 | 3 | NA | NA | NA | NA | NA | NA |
| Longer term, peers | 1 | 74 | −0.18 (−0.45 to 0.10) | .20 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Processing speed, norms | 2 | 48 | −0.26 (−1.14 to 0.63) | .57 | 79.89 | 0.58 | 1 | NA | NA | NA | NA | NA | NA |
| Subjective measures | |||||||||||||
| Motor functioning | NA | NA | NA | NA | NA | NA | NA | 1 | 138 | 0.10 | NA | NA | NA |
| Verbal abilities, peers | 1 | 70 | −0.01 (−0.31 to 0.28) | .93 | NA | NA | NA | 1 | 138 | 0.09 | NA | NA | NA |
| Adaptive and social skills, norms | 1 | 75 | 0.15 (−0.08 to 0.38) | .19 | NA | NA | NA | 1 | 140 | 0.12 | NA | NA | NA |
| Attention, peers | 1 | 76 | −0.02 (−0.31 to 0.26) | .87 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Behavior | |||||||||||||
| Externalizing | |||||||||||||
| Peers | 2 | 83 | −0.04 (−0.30 to 0.22) | .76 | 0.00 | 0.00 | 0 | 1 | 94 | 0.26 (0.20 to 0.32) | 1 | 76 | 1.48 (0.79 to 2.74) |
| Norms | 3 | 192 | −0.14 (−0.29 to 0.02) | .08 | 13.45 | 0.05 | 0 | ||||||
| Internalizing | |||||||||||||
| Peers | 2 | 83 | −0.19 (−0.62 to 0.25) | .40 | 24.24 | .20 | 0 | 1 | 94 | 0.14 (0.10 to 0.20) | 1 | 76 | 1.17 (0.65 to 2.09) |
| Norms | 3 | 192 | −0.07 (−0.21 to 0.07) | .32 | 0.00 | 0.00 | 0 | ||||||
| Overall | |||||||||||||
| Peers | 2 | 83 | 0.02 (−0.33 to 0.38) | .91 | 13.13 | 0.14 | 0 | 1 | 94 | 0.22 (0.15 to 0.32) | 1 | 76 | 1.52 (0.83 to 2.81) |
| Norms | 1 | 75 | 0.17 (−0.15 to 0.49) | .30 | NA | NA | NA | ||||||
| Executive function | |||||||||||||
| Peers | 1 | 75 | 0.04 (−0.25 to 0.33) | .79 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Norms | 3 | 192 | −0.40 (−0.66 to −0.14) | <.001 | 68.20 | 0.19 | 3 | NA | NA | NA | NA | NA | NA |
| Memory: shorter term, norms | 3 | 192 | −0.39 (−0.63 to −0.15) | <.001 | 62.22 | 0.17 | 3 | NA | NA | NA | NA | NA | NA |
| Quality of life, norms | 1 | 5 | 1.29 (0.42 to 2.17) | <.001 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Satisfaction with appearance, peers | 1 | 40 | −0.06 (−0.50 to 0.37) | .78 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Self-concept, peers | 1 | 7 | −0.03 (−0.95 to 0.88) | .94 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Abbreviations: NA, not applicable; Nfs, Orwin failsafe N; OR, odds ratio; SS, sagittal synostosis.
ORs are shown on a logarithmic scale.
Norms refers to comparisons against normative data for each measure.
Conservatively Managed Samples
Four independent studies (9 articles)16,21,43,44,45,46,47,48 examined 13 domains (Table 2 and eFigure 3 in the Supplement). Only 1 domain, global development, reached significance: the SS group functioned better than published normative data in 1 study (g = 0.58; 95% CI, 0.17 to 0.99; P = .01).43 Estimates provided by individual studies in this domain were, however, imprecise, as reflected by the wide 95% CIs. The single study that recruited healthy peers found a small but nonsignificant negative effect size for general cognition (g = −0.08; 95% CI, −0.67 to 0.51; P = .79),21 whereas the remaining 2 studies43,47 contributing to this domain reported small positive effect sizes. Similarly, the single study that used parent ratings of child behavior and quality of life reported no significant group differences, despite parents rating the behavior of their child with SS more poorly than their sibling.21
Prevalence estimates of cognitive difficulties among SS groups were modest, ranging from 5% (general cognition) to 13% (attention). With regard to ORs, no domains reached significance, but the SS group functioned better across some domains, as suggested by OR values ranging from 0.31 (95% CI, 0.01-6.12) for processing speed to 0.71 (95% CI, 0.04-12.43) for arithmetic to 4.55 (95% CI, 0.21-98.63) for visuospatial abilities. The nonsignificant findings may reflect the small SS sample sizes (<26 participants) used in these studies.
Presurgical Samples
Presurgical outcomes are based on 13 independent studies (21 articles)13,15,16,18,20,23,24,43,44,45,46,47,48,49,50,51,52,53,54,55,56 (Table 3 and eFigure 4 in the Supplement). Mean pooled group differences were typically larger compared with healthy peers, although the differences were not significant (eg, general cognition: gw for peers = −0.28; 95% CI, −0.67 to 0.10; P = .15; gw for normative data = −0.15; 95% CI, −0.89 to 0.60; P = .70). Only tests of motor functioning reached significance (gw = −0.42; 95% CI, −0.67 to −0.18; P < .001). One notable individual finding (eFigure 4 in the Supplement) was a study demonstrating very large and significant mean differences in both general cognition (gw = −1.03; 95% CI, −1.36 to −0.71; P < .001) and motor functioning (gw = −1.04; 95% CI, −1.37 to −0.71; P < .001) indicating that, before surgery, these children were functioning a full SD below BSID second version norms.15,54
Up to 37% of children (10 of 27 children) were identified as experiencing cognitive impairment, with 6% (5 of 87 children) demonstrating problems with adaptive and social functioning (eg, waving bye-bye). Despite individual study differences, with small sample size studies likely providing less reliable data, pooled and weighted prevalence estimates were comparable across domains (range, 13% to 17%).
Postsurgical Samples
Among the 25 independent studies (42 articles)15,16,18,19,20,22,23,24,25,38,43,44,45,46,47,48,49,54,55,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79 examining postsurgical results, the largest effect sizes were seen among those that compared test scores of SS groups with those of healthy peers, indicating poorer performances among the former (Table 4 and eFigure 5 in the Supplement). However, only studies that involved normative data comparisons reached significance: moderate group differences were noted for visuospatial abilities (6 studies; gw = 0.31; 95% CI, 0.18 to 0.44; P < .001), attention (1 study; gw = −0.51; 95% CI, −0.84 to −0.19; P < .001), and shorter-term memory (2 studies; gw = −0.45; 95% CI, −0.72 to −0.17; P < .001). The diversity of results is reflected among studies that assessed verbal abilities, specifically, with individual studies (eFigure 5 in the Supplement) in this domain reporting moderate-to-large and negative effects (peer comparison) but also a small positive effect (normative data comparison).
Where subjective measures were used (8 studies), significant group differences in executive functioning (3 studies; gw = −0.40; 95% CI, −0.66 to −0.14; P < .001), short-term memory (3 studies gw = −0.39; 95% CI, −0.63 to −0.15; P < .001), and quality of life (1 study; gw = 1.29; 95% CI, 0.42 to 2.17; P < .001) were noted: parents reported poorer cognition, but also enhanced quality of life, for their child compared with normative data. These findings should be considered tentative, given the small number of studies that contributed to these data.
Prevalence rates based on objectively assessed problems (9 studies) identified significant learning difficulties (33 of 143 children [23%]) and general cognitive problems (22 of 112 children [20%]) among children with SS, with fewer problems reported in other domains. In addition, parents and teachers reported a higher rate of externalizing behavior problems (24 of 94 children [26%]) compared with internalizing behavior problems (13 of 94 children [14%]), whereas few problems with adaptive and social functioning were reported (2 of 63 children [3%]).
Surgical Status Not Specified
The data for 4 independent studies (7 articles)43,44,45,46,80,81 examining mixed SS samples were compared with normative data (eTable 9 in the Supplement). Of the 4 domains examined, only motor functioning was associated with a significant group difference: the SS group displayed poorer function in comparison to normative data (1 study; g = −0.93; 95% CI, −1.18 to −0.69; P < .001). Moreover, a large percentage of children were found to have verbal (28 of 76 children [37%]) and general cognitive (14 of 71 children [20%]) issues.
Age at Assessment
Findings were analyzed according to mean age at assessment (24 studies). However, there was no clear pattern of findings (eFigure 6 in the Supplement).
Discussion
Data from 32 independent studies, involving a pooled sample of 1422 children and adults, were analyzed. Overall, results were highly variable, ranging from individual study results including moderate positive findings, whereby children with SS were functioning at better levels than their peers, to large negative differences.
Few studies examined the outcomes of children whose SS was conservatively managed. Only 1 study21 recruited controls (siblings), thereby controlling for family-level environmental factors; however, siblings of children with health conditions are also known to experience difficulties, limiting the conclusions that can be drawn.82,83 Global development was the only domain to demonstrate that participants with SS were performing better than the normative data; however, the Griffiths Mental Development Scale (GMDS)44 has been shown to overestimate developmental functioning.84,85,86 Moreover, normative data for the GMDS were not updated for some years; thus, higher test scores may reflect the Flynn effect, whereby raw intelligence quotient scores increase over time.87 Siblings were also used as controls in the single study that assessed behavior and quality of life, using parental reports to compare their children (ie, child with SS vs sibling).21 No differences were found between groups, perhaps highlighting the challenges of using siblings and, moreover, the importance of multi-informant ratings to mitigate differential effects of child and parent ratings.
Findings on cognitive functioning for children in the presurgical sample who later went on to have surgery were also highly variable, indicating that although some children with SS experience cognitive difficulties, this is not always the case. It is noteworthy that, on all assessed domains, the SS group performed more poorly when compared with healthy controls rather than normative data, although few of these group differences were significant. Notably, different versions of the same measure, such as the BSID (first, second, and third versions), generated different effect estimates, ranging from moderate negative to moderate positive group differences. These findings suggest that factors other than surgical status and the measure used (eg, socioeconomic status) need to be considered. Similar to the GMDS, the BSID third version may also overestimate child development88; hence, the findings may reflect an artifact of the actual measure used, rather than an accurate indication of child development.
Findings for children who had undergone surgical treatment for their SS were also highly disparate. Notably, subjective ratings by parents on the Brief Working Memory Index reflected those on objective tests (eg, Children’s Memory Scale), suggesting that parents were accurately observing and reporting behavioral signs of their child’s short-term memory difficulties. No significant group differences in psychological functioning were reported; hence, the experience of having SS and its associated surgical procedures in early childhood may play a lifelong role in increasing people’s resilience and outlook.78 Qualitative studies examining the psychological impact of SS on the individual and their families may provide greater detail about the strengths imparted to all family members during these challenges.
The understanding of the SS disease process has evolved, and, with the advent of surgical innovations, the operative approaches for managing patients have changed considerably, from early suturectomy to more extensive cranial vault remodeling and variants thereof.11 More recently, spring-assisted surgery and endoscopic-assisted craniectomies with or without helmets have been described.1,77 There is a wide disparity of opinions about the appropriate treatment of SS, with extended calvarial vault remodeling being the most commonly performed procedure worldwide.89 This technique is reproducible and adheres to the principle of removing the affected suture, spanning the adjacent functional suture, and, thus, reducing the primary deformity and allowing space for the brain to grow and expand unhindered.11 Similarly, there is no consensus on the optimal age for surgery, with the brain growth curve influencing both the appropriate choice of technique and timing.12 Both these factors are likely be associated with outcomes, with additional research needed to clarify the impact of both the short-term and long-term functioning of children and adults with SS who have undergone surgery.
Limitations
There are a number of limitations that warrant consideration. First, because comparison groups were often not recruited, normative data from the specific measure were used to generate effect sizes. Normative data may not be representative because of changes in population composition (eg, education, age, or economic status) over time,90 highlighting a need for future research to recruit appropriately matched comparison groups. Second, potentially relevant studies were excluded because they did not specify which version of a measure was used, and/or normative data could not be accessed. Similarly, some data could not be used because studies combined their findings for different sutures (eg, sagittal and metopic), despite known pathophysiological differences between sutures.34 Future research should report data for different sutures separately. Third, the exact criteria used to identify children with problems differed between studies and, moreover, the measures used to assess neurodevelopmental functioning in infants (eg, GMDS) may not accurately predict later performance.91,92 Fourth, studies did not consistently report key sample parameters, such as age, sex, and type of surgical treatment, which are variables that should be examined because they have been shown to be associated with cognitive functioning.46,69
Conclusions
The current findings highlight that, on the basis of the reviewed literature, there were no consistent associations between SS and neurocognitive delays. Nonetheless, some children were experiencing difficulties (eg, attention or short-term memory problems), indicating that ongoing monitoring and assessment are important to ensure that children with difficulties are referred to support services as required. Future research should recruit larger samples with well-matched comparison groups and should examine conservatively managed samples more often, in addition to comprehensively reporting both sample characteristics and outcomes, according to important demographic (eg, age and sex) and clinical (eg, surgical technique) variables.
eTable 1. Electronic Search Strategy
eTable 2. Summary Details of the Meta-analyzed Studies
eTable 3. Studies Using Overlapping Samples - Combined and Treated as Non-independent Studies in the Current Meta-analysis
eTable 4. Data Extracted From Studies Included in the Meta-analysis (Where Reported)
eTable 5. Summary of the Cognitive Tests That Were Used by the Studies and Their Corresponding Cognitive Domain
eTable 6. Adapted NIH Quality Assessment of Observational Cohort and Cross-Sectional Studies
eTable 7. NIH Quality Assessment of Case-Control Studies
eTable 8. Prediction Intervals for Pooled Analyses With ≥5 Included Studies
eTable 9. Outcomes of Mixed (Conservatively Managed + Presurgical + Postsurgical) Samples of Children With Sagittal Synostosis
eFigure 1. Percentage of Observational Cross-Sectional and Cohort Studies Meeting Each of the Adapted NIH Study Quality Criteria (Nstudies = 23)
eFigure 2. Percentage of Case-Control Studies Meeting Each of the Adapted NIH Study Quality Criteria (Nstudies = 9)
eFigure 3. Outcomes of Children With Conservatively Managed Sagittal Synostosis
eFigure 4. Presurgical Functioning of Children With Sagittal Synostosis: Had Not Yet Undergone Surgery Prior to Being Assessed
eFigure 5. Outcomes of Children With Operated Sagittal Synostosis: Had Undergone Surgery Prior to Being Assessed
eFigure 6. Pooled Analyses for Each Cognitive, Behavioral and Psychological Domain, Partitioned According to Surgical Status (Conservatively Managed, Presurgical, Postsurgical) and Age at Assessment: A (0 – 0.11); B (1:0 – 2:11); C (3:0 – 6:11); D (7:0 – 17:11); E (>18:0)
eReferences
References
- 1.Proctor MR, Meara JG. A review of the management of single-suture craniosynostosis, past, present, and future. J Neurosurg Pediatr. 2019;24(6):622-631. doi: 10.3171/2019.7.PEDS18585 [DOI] [PubMed] [Google Scholar]
- 2.Garza RM, Khosla RK. Nonsyndromic craniosynostosis. Semin Plast Surg. 2012;26(2):53-63. doi: 10.1055/s-0032-1320063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tønne E, Due-Tønnessen BJ, Wiig U, et al. Epidemiology of craniosynostosis in Norway. J Neurosurg Pediatr. 2020;1-8. [DOI] [PubMed] [Google Scholar]
- 4.Lee HQ, Hutson JM, Wray AC, et al. Changing epidemiology of nonsyndromic craniosynostosis and revisiting the risk factors. J Craniofac Surg. 2012;23(5):1245-1251. doi: 10.1097/SCS.0b013e318252d893 [DOI] [PubMed] [Google Scholar]
- 5.Mathijssen IMJ. Guideline for care of patients with the diagnoses of craniosynostosis: working group on craniosynostosis. J Craniofac Surg. 2015;26(6):1735-1807. doi: 10.1097/SCS.0000000000002016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyadjiev SA; International Craniosynostosis Consortium . Genetic analysis of non-syndromic craniosynostosis. Orthod Craniofac Res. 2007;10(3):129-137. doi: 10.1111/j.1601-6343.2007.00393.x [DOI] [PubMed] [Google Scholar]
- 7.Carmichael SL, Ma C, Rasmussen SA, et al. Craniosynostosis and risk factors related to thyroid dysfunction. Am J Med Genet A. 2015;167A(4):701-707. doi: 10.1002/ajmg.a.36953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirschner RE, Gannon FH, Xu J, et al. Craniosynostosis and altered patterns of fetal TGF-β expression induced by intrauterine constraint. Plast Reconstr Surg. 2002;109(7):2338-2346. doi: 10.1097/00006534-200206000-00028 [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz E, Mihci E, Guzel Nur B, Alper OM. A novel AXIN2 gene mutation in sagittal synostosis. Am J Med Genet A. 2018;176(9):1976-1980. doi: 10.1002/ajmg.a.40373 [DOI] [PubMed] [Google Scholar]
- 10.Zeiger JS, Beaty TH, Hetmanski JB, et al. Genetic and environmental risk factors for sagittal craniosynostosis. J Craniofac Surg. 2002;13(5):602-606. doi: 10.1097/00001665-200209000-00002 [DOI] [PubMed] [Google Scholar]
- 11.Chummun S, McLean NR, Flapper WJ, David DJ. The management of nonsyndromic, isolated sagittal synostosis. J Craniofac Surg. 2016;27(2):299-304. doi: 10.1097/SCS.0000000000002363 [DOI] [PubMed] [Google Scholar]
- 12.David DJ. Advances in the management of the craniosynostoses. ANZ J Surg. 2003;73(11):949-957. doi: 10.1046/j.1445-2197.2003.02825.x [DOI] [PubMed] [Google Scholar]
- 13.Chuang C, Rolison M, Yang JF, et al. Normalization of speech processing after whole-vault cranioplasty in sagittal synostosis. J Craniofac Surg. 2018;29(5):1132-1136. doi: 10.1097/SCS.0000000000004474 [DOI] [PubMed] [Google Scholar]
- 14.Shipster C, Hearst D, Somerville A, Stackhouse J, Hayward R, Wade A. Speech, language, and cognitive development in children with isolated sagittal synostosis. Dev Med Child Neurol. 2003;45(1):34-43. doi: 10.1111/j.1469-8749.2003.tb00857.x [DOI] [PubMed] [Google Scholar]
- 15.Lee MC, Shim KW, Park EK, Yun IS, Kim DS, Kim YO. Expansion and compression distraction osteogenesis based on volumetric and neurodevelopmental analysis in sagittal craniosynostosis. Childs Nerv Syst. 2015;31(11):2081-2089. doi: 10.1007/s00381-015-2843-y [DOI] [PubMed] [Google Scholar]
- 16.Arnaud E, Renier D, Marchac D. Prognosis for mental function in scaphocephaly. J Neurosurg. 1995;83(3):476-479. doi: 10.3171/jns.1995.83.3.0476 [DOI] [PubMed] [Google Scholar]
- 17.Speltz ML, Collett BR, Wallace ER, et al. Intellectual and academic functioning of school-age children with single-suture craniosynostosis. Pediatrics. 2015;135(3):e615-e623. doi: 10.1542/peds.2014-1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Da Costa AC, Anderson VA, Holmes AD, et al. Longitudinal study of the neurodevelopmental characteristics of treated and untreated nonsyndromic craniosynostosis in infancy. Childs Nerv Syst. 2013;29(6):985-995. doi: 10.1007/s00381-012-2017-0 [DOI] [PubMed] [Google Scholar]
- 19.Magge SN, Westerveld M, Pruzinsky T, Persing JA. Long-term neuropsychological effects of sagittal craniosynostosis on child development. J Craniofac Surg. 2002;13(1):99-104. doi: 10.1097/00001665-200201000-00023 [DOI] [PubMed] [Google Scholar]
- 20.Speltz ML, Endriga MC, Mouradian WE. Presurgical and postsurgical mental and psychomotor development of infants with sagittal synostosis. Cleft Palate Craniofac J. 1997;34(5):374-379. doi: 10.1597/1545-1569_1997_034_0374_papmap_2.3.co_2 [DOI] [PubMed] [Google Scholar]
- 21.Boltshauser E, Ludwig S, Dietrich F, Landolt MA. Sagittal craniosynostosis: cognitive development, behaviour, and quality of life in unoperated children. Neuropediatrics. 2003;34(6):293-300. doi: 10.1055/s-2003-44667 [DOI] [PubMed] [Google Scholar]
- 22.Korpilahti P, Saarinen P, Hukki J. Deficient language acquisition in children with single suture craniosynostosis and deformational posterior plagiocephaly. Childs Nerv Syst. 2012;28(3):419-425. doi: 10.1007/s00381-011-1623-6 [DOI] [PubMed] [Google Scholar]
- 23.Scheuerle J, Guilford AM, Habal MB. A report of behavioral data on three groups of patients with craniofacial disorders. J Craniofac Surg. 2004;15(2):200-208. doi: 10.1097/00001665-200403000-00004 [DOI] [PubMed] [Google Scholar]
- 24.Chieffo DPR, Arcangeli V, Bianchi F, et al. Single-suture craniosynostosis: is there a correlation between preoperative ophthalmological, neuroradiological, and neurocognitive findings? Childs Nerv Syst. 2020;36(7):1481-1488. doi: 10.1007/s00381-020-04521-w [DOI] [PubMed] [Google Scholar]
- 25.Care H, Horton J, Kearney A, et al. Introduction to the craniofacial collaboration UK: a developmental screening protocol at the United Kingdom’s four highly specialized craniofacial centers. J Craniofac Surg. 2019;30(1):83-86. doi: 10.1097/SCS.0000000000004846 [DOI] [PubMed] [Google Scholar]
- 26.Cloonan YK, Collett B, Speltz ML, Anderka M, Werler MM. Psychosocial outcomes in children with and without non-syndromic craniosynostosis: findings from two studies. Cleft Palate Craniofac J. 2013;50(4):406-413. doi: 10.1597/11-074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tillman KK, Höijer J, Ramklint M, Ekselius L, Nowinski D, Papadopoulos FC. Nonsyndromic craniosynostosis is associated with increased risk for psychiatric disorders. Plast Reconstr Surg. 2020;146(2):355-365. doi: 10.1097/PRS.0000000000007009 [DOI] [PubMed] [Google Scholar]
- 28.van der Vlugt JJB, van der Meulen JJNM, Creemers HE, Willemse SP, Lequin ML, Okkerse JME. The risk of psychopathology in children with craniosynostosis. Plast Reconstr Surg. 2009;124(6):2054-2060. doi: 10.1097/PRS.0b013e3181bcf2dc [DOI] [PubMed] [Google Scholar]
- 29.Barritt J, Brooksbank M, Simpson D. Scaphocephaly: aesthetic and psychosocial considerations. Dev Med Child Neurol. 1981;23(2):183-191. doi: 10.1111/j.1469-8749.1981.tb02440.x [DOI] [PubMed] [Google Scholar]
- 30.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 4th ed. Pearson; 2012. [Google Scholar]
- 31.Bayley N. Bayley Scales of Infant Development. The Psychological Corporation; 1969. [Google Scholar]
- 32.Bayley N. Manual for the Bayley Scales of Infant Development. 2nd ed. The Psychological Corporation; 1993. [Google Scholar]
- 33.Bayley N. Bayley Scales of Infant and Toddler Development: Administration Manual. 3rd ed. Harcourt; 2006. [Google Scholar]
- 34.Wilkie AOM, Johnson D, Wall SA. Clinical genetics of craniosynostosis. Curr Opin Pediatr. 2017;29(6):622-628. doi: 10.1097/MOP.0000000000000542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 36.Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. 3rd ed. Oxford University Press; 2012. [Google Scholar]
- 37.Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests. Oxford University Press; 2006. [Google Scholar]
- 38.Chieffo D, Tamburrini G, Massimi L, et al. Long-term neuropsychological development in single-suture craniosynostosis treated early. J Neurosurg Pediatr. 2010;5(3):232-237. doi: 10.3171/2009.10.PEDS09231 [DOI] [PubMed] [Google Scholar]
- 39.Liu TJ, Wu SH, Fan SS, Chen ZH, Gu S. A new technique for sagittal synostosis: a plurality of small incisions minimally invasive technique used on infants and young patients. J Craniofac Surg. 2018;29(8):2065-2069. doi: 10.1097/SCS.0000000000004791 [DOI] [PubMed] [Google Scholar]
- 40.Moreno-Villagómez J, Yáñez-Téllez MG, Prieto-Corona B, Seubert-Ravelo AN, García A. Behavioral disorders of preschool children with non-syndromic craniosynostosis. J Craniofac Surg. 2020;31(1):147-149. doi: 10.1097/SCS.0000000000006008 [DOI] [PubMed] [Google Scholar]
- 41.Cohen J. A power primer. Psychol Bull. 1992;112(1):155-159. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 42.Borenstein M. Common Mistakes in Meta-Analysis and How to Avoid Them. Biostat, Inc; 2019. [Google Scholar]
- 43.Bellew M, Chumas P, Mueller R, Liddington M, Russell J. Pre- and postoperative developmental attainment in sagittal synostosis. Arch Dis Child. 2005;90(4):346-350. doi: 10.1136/adc.2003.035824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellew M, Liddington M, Chumas P, Russell J. Preoperative and postoperative developmental attainment in patients with sagittal synostosis: 5-year follow-up. J Neurosurg Pediatr. 2011;7(2):121-126. doi: 10.3171/2010.11.PEDS10216 [DOI] [PubMed] [Google Scholar]
- 45.Bellew M, Chumas P. Long-term developmental follow-up in children with nonsyndromic craniosynostosis. J Neurosurg Pediatr. 2015;16(4):445-451. doi: 10.3171/2015.3.PEDS14567 [DOI] [PubMed] [Google Scholar]
- 46.Bellew M, Mandela RJ, Chumas PD. Impact of age at surgery on neurodevelopmental outcomes in sagittal synostosis. J Neurosurg Pediatr. 2019;23(4):1-8. doi: 10.3171/2018.8.PEDS18186 [DOI] [PubMed] [Google Scholar]
- 47.Kapp-Simon KA, Figueroa A, Jocher CA, Schafer M. Longitudinal assessment of mental development in infants with nonsyndromic craniosynostosis with and without cranial release and reconstruction. Plast Reconstr Surg. 1993;92(5):831-839. doi: 10.1097/00006534-199392050-00008 [DOI] [PubMed] [Google Scholar]
- 48.Kapp-Simon KA. Mental development and learning disorders in children with single suture craniosynostosis. Cleft Palate Craniofac J. 1998;35(3):197-203. doi: 10.1597/1545-1569_1998_035_0197_mdaldi_2.3.co_2 [DOI] [PubMed] [Google Scholar]
- 49.Da Costa AC, Anderson VA, Savarirayan R, et al. Neurodevelopmental functioning of infants with untreated single-suture craniosynostosis during early infancy. Childs Nerv Syst. 2012;28(6):869-877. doi: 10.1007/s00381-011-1660-1 [DOI] [PubMed] [Google Scholar]
- 50.Speltz ML, Kapp-Simon K, Collett B, et al. Neurodevelopment of infants with single-suture craniosynostosis: presurgery comparisons with case-matched controls. Plast Reconstr Surg. 2007;119(6):1874-1881. doi: 10.1097/01.prs.0000259184.88265.3f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kapp-Simon KA, Leroux B, Cunningham M, Speltz ML. Multisite study of infants with single-suture craniosynostosis: preliminary report of presurgery development. Cleft Palate Craniofac J. 2005;42(4):377-384. doi: 10.1597/04-044.1 [DOI] [PubMed] [Google Scholar]
- 52.Ruiz-Correa S, Starr JR, Lin HJ, Kapp-Simon KA, Cunningham ML, Speltz ML. Severity of skull malformation is unrelated to presurgery neurobehavioral status of infants with sagittal synostosis. Cleft Palate Craniofac J. 2007;44(5):548-554. doi: 10.1597/06-190.1 [DOI] [PubMed] [Google Scholar]
- 53.Imahiyerobo TA, Johns AL, Christian EA, et al. Risk factors for preoperative developmental delay in patients with nonsyndromic sagittal craniosynostosis. Plast Reconstr Surg. 2019;143(1):133e-139e. doi: 10.1097/PRS.0000000000005108 [DOI] [PubMed] [Google Scholar]
- 54.Lee MC, Shim KW, Yun IS, Park EK, Kim YO. Correction of sagittal craniosynostosis using distraction osteogenesis based on strategic categorization. Plast Reconstr Surg. 2017;139(1):157-169. doi: 10.1097/PRS.0000000000002899 [DOI] [PubMed] [Google Scholar]
- 55.Gewalli F, Guimarães-Ferreira JP, Sahlin P, et al. Mental development after modified π procedure: dynamic cranioplasty for sagittal synostosis. Ann Plast Surg. 2001;46(4):415-420. doi: 10.1097/00000637-200104000-00011 [DOI] [PubMed] [Google Scholar]
- 56.Engel M, Hoffmann J, Mühling J, Castrillón-Oberndorfer G, Seeberger R, Freudlsperger C. Subtotal cranial vault remodelling in anterior sagittal suture closure: impact of age on surgical outcome. Int J Oral Maxillofac Surg. 2012;41(10):1232-1237. doi: 10.1016/j.ijom.2012.05.026 [DOI] [PubMed] [Google Scholar]
- 57.Chuang C, Chaunzwa TL, Wu R, et al. Long-term neurocognitive outcomes in sagittal synostosis: the impact of reoperation. J Craniofac Surg. 2021;32(1):58-61. doi: 10.1097/SCS.0000000000006909 [DOI] [PubMed] [Google Scholar]
- 58.Da Costa AC, Walters I, Savarirayan R, Anderson VA, Wrennall JA, Meara JG. Intellectual outcomes in children and adolescents with syndromic and nonsyndromic craniosynostosis. Plast Reconstr Surg. 2006;118(1):175-181. doi: 10.1097/01.prs.0000221009.93022.50 [DOI] [PubMed] [Google Scholar]
- 59.Hashim PW, Patel A, Yang JF, et al. The effects of whole-vault cranioplasty versus strip craniectomy on long-term neuropsychological outcomes in sagittal craniosynostosis. Plast Reconstr Surg. 2014;134(3):491-501. doi: 10.1097/PRS.0000000000000420 [DOI] [PubMed] [Google Scholar]
- 60.Patel A, Yang JF, Hashim PW, et al. The impact of age at surgery on long-term neuropsychological outcomes in sagittal craniosynostosis. Plast Reconstr Surg. 2014;134(4):608e-617e. doi: 10.1097/PRS.0000000000000511 [DOI] [PubMed] [Google Scholar]
- 61.Kapp-Simon KA, Wallace E, Collett BR, Cradock MM, Crerand CE, Speltz ML. Language, learning, and memory in children with and without single-suture craniosynostosis. J Neurosurg Pediatr. 2016;17(5):578-588. doi: 10.3171/2015.9.PEDS15238 [DOI] [PubMed] [Google Scholar]
- 62.Kljajić M, Maltese G, Tarnow P, Sand P, Kölby L. Sustained attention and vigilance of children treated for sagittal and metopic craniosynostosis. Child Neuropsychol. 2020;26(4):475-488. doi: 10.1080/09297049.2019.1682130 [DOI] [PubMed] [Google Scholar]
- 63.Kljajić M, Maltese G, Tarnow P, Sand P, Kölby L. The cognitive profile of children with nonsyndromic craniosynostosis. Plast Reconstr Surg. 2019;143(5):1037e-1052e. doi: 10.1097/PRS.0000000000005515 [DOI] [PubMed] [Google Scholar]
- 64.Moreno-Villagómez J, Yáñez-Téllez G, Prieto-Corona B, Seubert-Ravelo AN, García A. Cognitive performance of preschool children with different types of non-syndromic craniosynostosis. Brain Impair. 2020;22(2):125-134. doi: 10.1017/BrImp.2020.7 [DOI] [Google Scholar]
- 65.Speltz ML, Morton K, Goodell EW, Clarren SK. Psychological functioning of children with craniofacial anomalies and their mothers: follow-up from late infancy to school entry. Cleft Palate Craniofac J. 1993;30(5):482-489. doi: 10.1597/1545-1569_1993_030_0482_pfocwc_2.3.co_2 [DOI] [PubMed] [Google Scholar]
- 66.Speltz ML, Birgfeld C, Starr JR, Collett B, Kapp-Simon K. The effects of whole-vault cranioplasty versus strip craniectomy on long-term neuropsychological outcomes in sagittal craniosynostosis. Plast Reconstr Surg. 2015;135(3):646e-647e. doi: 10.1097/PRS.0000000000001067 [DOI] [PubMed] [Google Scholar]
- 67.Speltz ML, Collett BR, Wallace ER, Kapp-Simon K. Behavioral adjustment of school-age children with and without single-suture craniosynostosis. Plast Reconstr Surg. 2016;138(2):435-445. doi: 10.1097/PRS.0000000000002383 [DOI] [PubMed] [Google Scholar]
- 68.Collett BR, Kapp-Simon KA, Wallace E, Cradock MM, Buono L, Speltz ML. Attention and executive function in children with and without single-suture craniosynostosis. Child Neuropsychol. 2017;23(1):83-98. doi: 10.1080/09297049.2015.1085005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cradock MM, Gray KE, Kapp-Simon KA, Collett BR, Buono LA, Speltz ML. Sex differences in the neurodevelopment of school-age children with and without single-suture craniosynostosis. Childs Nerv Syst. 2015;31(7):1103-1111. doi: 10.1007/s00381-015-2671-0 [DOI] [PubMed] [Google Scholar]
- 70.Naumann HL, Haberkern CM, Pietila KE, et al. Duration of exposure to cranial vault surgery: associations with neurodevelopment among children with single-suture craniosynostosis. Paediatr Anaesth. 2012;22(11):1053-1061. doi: 10.1111/j.1460-9592.2012.03843.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Starr JR, Collett BR, Gaither R, et al. Multicenter study of neurodevelopment in 3-year-old children with and without single-suture craniosynostosis. Arch Pediatr Adolesc Med. 2012;166(6):536-542. doi: 10.1001/archpediatrics.2011.1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Starr JR, Kapp-Simon KA, Cloonan YK, et al. Presurgical and postsurgical assessment of the neurodevelopment of infants with single-suture craniosynostosis: comparison with controls. J Neurosurg. 2007;107(2)(suppl):103-110. doi: 10.3171/PED-07/08/103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toth K, Collett B, Kapp-Simon KA, et al. Memory and response inhibition in young children with single-suture craniosynostosis. Child Neuropsychol. 2008;14(4):339-352. doi: 10.1080/09297040701594888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wallace ER, Collett BR, Kapp-Simon K, Starr JR, Birgfeld C, Speltz ML. Visuomotor function in school-age children with single-suture craniosynostosis. J Dev Behav Pediatr. 2016;37(6):483-490. doi: 10.1097/DBP.0000000000000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Virtanen R, Korhonen T, Fagerholm J, Viljanto J. Neurocognitive sequelae of scaphocephaly. Pediatrics. 1999;103(4, pt 1):791-795. doi: 10.1542/peds.103.4.791 [DOI] [PubMed] [Google Scholar]
- 76.Cabrejo R, Lacadie C, Brooks E, et al. Understanding the learning disabilities linked to sagittal craniosynostosis. J Craniofac Surg. 2019;30(2):497-502. doi: 10.1097/SCS.0000000000005194 [DOI] [PubMed] [Google Scholar]
- 77.Chandler L, Allam O, Park KE, et al. Spring-assisted strip craniectomy versus cranial vault remodeling: long-term psychological, behavioral, and executive function outcomes. J Craniofac Surg. 2020;31(7):2101-2105. doi: 10.1097/SCS.0000000000006806 [DOI] [PubMed] [Google Scholar]
- 78.Mazzaferro DM, Naran S, Wes AM, Magee L, Taylor JA, Bartlett SP. Quality of life in adults with nonsyndromic craniosynostosis. Plast Reconstr Surg. 2018;141(6):1474-1482. doi: 10.1097/PRS.0000000000004408 [DOI] [PubMed] [Google Scholar]
- 79.Salokorpi N, Savolainen T, Sinikumpu JJ, et al. Outcomes of 40 nonsyndromic sagittal craniosynostosis patients as adults: a case-control study with 26 years of postoperative follow-up. Oper Neurosurg (Hagerstown). 2019;16(1):1-8. doi: 10.1093/ons/opy047 [DOI] [PubMed] [Google Scholar]
- 80.Byun IH, Hong JW, Hussein MA, Kim YO. Demographic characteristics of craniosynostosis patients in Asia. J Craniomaxillofac Surg. 2018;46(4):674-678. doi: 10.1016/j.jcms.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 81.Noetzel MJ, Marsh JL, Palkes H, Gado M. Hydrocephalus and mental retardation in craniosynostosis. J Pediatr. 1985;107(6):885-892. doi: 10.1016/S0022-3476(85)80181-5 [DOI] [PubMed] [Google Scholar]
- 82.Sharpe D, Rossiter L. Siblings of children with a chronic illness: a meta-analysis. J Pediatr Psychol. 2002;27(8):699-710. doi: 10.1093/jpepsy/27.8.699 [DOI] [PubMed] [Google Scholar]
- 83.Vermaes IPR, van Susante AMJ, van Bakel HJA. Psychological functioning of siblings in families of children with chronic health conditions: a meta-analysis. J Pediatr Psychol. 2012;37(2):166-184. doi: 10.1093/jpepsy/jsr081 [DOI] [PubMed] [Google Scholar]
- 84.Beail N. A comparative study of profoundly multiply handicapped children’s scores on the Bayley and the Griffiths developmental scales. Child Care Health Dev. 1985;11(1):31-36. doi: 10.1111/j.1365-2214.1985.tb00447.x [DOI] [PubMed] [Google Scholar]
- 85.McLean ME, McCormick K, Baird SM. Concurrent validity of the Griffiths' Mental Development Scales with a population of children under 24 months. J Early Interv. 1991;15(4):338-344. doi: 10.1177/105381519101500403 [DOI] [Google Scholar]
- 86.Ramsay M, Piper MC. A comparison of two developmental scales in evaluating infants with Down syndrome. Early Hum Dev. 1980;4(1):89-95. doi: 10.1016/0378-3782(80)90012-2 [DOI] [PubMed] [Google Scholar]
- 87.Lynn R. What has caused the Flynn effect? secular increases in the Development Quotients of infants. Intelligence. 2009;37(1):16-24. doi: 10.1016/j.intell.2008.07.008 [DOI] [Google Scholar]
- 88.Anderson PJ, Burnett A. Assessing developmental delay in early childhood: concerns with the Bayley-III scales. Clin Neuropsychol. 2017;31(2):371-381. doi: 10.1080/13854046.2016.1216518 [DOI] [PubMed] [Google Scholar]
- 89.Doumit GD, Papay FA, Moores N, Zins JE. Management of sagittal synostosis: a solution to equipoise. J Craniofac Surg. 2014;25(4):1260-1265. doi: 10.1097/SCS.0b013e3182a24635 [DOI] [PubMed] [Google Scholar]
- 90.Kendall PC, Marrs-Garcia A, Nath SR, Sheldrick RC. Normative comparisons for the evaluation of clinical significance. J Consult Clin Psychol. 1999;67(3):285-299. doi: 10.1037/0022-006X.67.3.285 [DOI] [PubMed] [Google Scholar]
- 91.Sattler JM. Foundations of Behavioral, Social, and Clinical Assessment of Children. Jerome M. Sattler, Publisher, Inc; 2014. [Google Scholar]
- 92.Sutcliffe AG, Soo A, Barnes J. Predictive value of developmental testing in the second year for cognitive development at five years of age. Pediatr Rep. 2010;2(2):e15. doi: 10.4081/pr.2010.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Electronic Search Strategy
eTable 2. Summary Details of the Meta-analyzed Studies
eTable 3. Studies Using Overlapping Samples - Combined and Treated as Non-independent Studies in the Current Meta-analysis
eTable 4. Data Extracted From Studies Included in the Meta-analysis (Where Reported)
eTable 5. Summary of the Cognitive Tests That Were Used by the Studies and Their Corresponding Cognitive Domain
eTable 6. Adapted NIH Quality Assessment of Observational Cohort and Cross-Sectional Studies
eTable 7. NIH Quality Assessment of Case-Control Studies
eTable 8. Prediction Intervals for Pooled Analyses With ≥5 Included Studies
eTable 9. Outcomes of Mixed (Conservatively Managed + Presurgical + Postsurgical) Samples of Children With Sagittal Synostosis
eFigure 1. Percentage of Observational Cross-Sectional and Cohort Studies Meeting Each of the Adapted NIH Study Quality Criteria (Nstudies = 23)
eFigure 2. Percentage of Case-Control Studies Meeting Each of the Adapted NIH Study Quality Criteria (Nstudies = 9)
eFigure 3. Outcomes of Children With Conservatively Managed Sagittal Synostosis
eFigure 4. Presurgical Functioning of Children With Sagittal Synostosis: Had Not Yet Undergone Surgery Prior to Being Assessed
eFigure 5. Outcomes of Children With Operated Sagittal Synostosis: Had Undergone Surgery Prior to Being Assessed
eFigure 6. Pooled Analyses for Each Cognitive, Behavioral and Psychological Domain, Partitioned According to Surgical Status (Conservatively Managed, Presurgical, Postsurgical) and Age at Assessment: A (0 – 0.11); B (1:0 – 2:11); C (3:0 – 6:11); D (7:0 – 17:11); E (>18:0)
eReferences