Abstract
Identifying subjects with progressive chronic kidney disease will be important both in clinical practice and in conducting clinical trials. Pruijm et al. (in this issue) demonstrate for the first time that cortical oxygenation as evaluated by blood oxygenation level–dependent magnetic resonance imaging can predict future loss of renal function. These observations provide the necessary stimulus to continue the development of renal blood oxygenation level–dependent magnetic resonance imaging to further improve the sensitivity and specificity to renal oxygenation and hence the predictive power.
Chronic kidney disease (CKD) is considered to be an irreversible and usually progressive reduction in kidney function. However, only about one-half of individuals with CKD stage 3 will progress to end-stage kidney disease over 10 years.1 The ability to identify subjects with progressive CKD is expected to have a major impact on clinical management and in enriching study populations for clinical trials. This has sparked interest in the discovery and development of novel biomarkers that may identify subjects with early CKD who are at risk of progression.
Although there are multiple causes for the development of CKD, the chronic hypoxia hypothesis was proposed as a common pathway for progressive CKD.2 This hypothesis suggests that glomerular injury promotes microvascular obliteration with the loss of peritubular capillaries leading to hypoperfusion and reduced oxygen delivery. While the evidence of this hypothesis is strong in animal models, translation to humans has not been easy. A primary challenge has been the lack of a noninvasive method to monitor renal oxygenation in humans.
Blood oxygenation level–dependent magnetic resonance imaging (BOLD-MRI) is the only known noninvasive method to be sensitive to the oxygenation status of the kidneys that can be readily used in humans. It is based on the change in the magnetic property of hemoglobin based on its oxygenation status with deoxygenated hemoglobin being weakly paramagnetic. In the presence of deoxygenated hemoglobin, the random vascular distribution in tissue results in an effective magnetic field inhomogeneity leading to enhanced apparent T2 relaxation characterized by T2*. Earlier interest in the method was primarily directed at the renal medulla, which is known to naturally exist in a hypoxic milieu and BOLD-MRI is inherently more sensitive to hypoxic tissue.3 Renal BOLD-MRI signals are also influenced by hydration status, sodium avidity, vascular volume, vascular geometry, hematocrit, and factors affecting the oxygen-hemoglobin disassociation curve such as temperature and pH. These may be controlled or accounted for in acute experiments in which the effects of physiological and pharmacologic maneuvers are being studied. Many of the earlier studies were geared toward such acute experiments, and several of them were compared with invasive microprobe measurements of pO2 in animal models.
The application of BOLD-MRI to patients with CKD has resulted in apparent inconsistent findings. Inoue et al.4 showed cortical T2* measures were correlated with the estimated glomerular filtration rate (eGFR), at least in nondiabetic subjects. Yin et al.5 showed a negative correlation in the cortex and a positive correlation in the medulla in terms of R2* (= 1/T2*) versus eGFR in patients with diabetic nephropathy. However, a study with a large number of subjects (N = 280) showed that BOLD-MRI was not correlated with eGFR.6 It should be noted that this study was not designed as a study of CKD. BOLD-MRI data were acquired for subjects already undergoing an abdominal MRI scan, for example, for staging of cancer in abdominal organs or MR angiography of renal or mesenteric arteries. The BOLD-MRI data were then correlated with a recent (within 14 days of the MRI scan) eGFR measurement obtained from the medical records. Clinical CKD classification is based on a persistent reduction in eGFR. A single eGFR estimate may not necessarily indicate CKD. There was no subject preparation or control for concomitant medications; also no control group was included.
The group of Pruijm et al. conducted an initial BOLD-MRI study with a standardized protocol for food and water intake in a sufficiently large number of subjects (N = 95) and still found no significant difference compared with controls. However, when the data were analyzed again with a more objective and semiautomated method, the renal cortex was found to be less well oxygenated in CKD compared with controls. The analysis also afforded a depth profile in the form of an R2* slope, which is a surrogate for the corticomedullary difference in oxygenation. While providing preliminary evidence of reduced oxygenation status in CKD, it is important to note that these observations did not fully validate the chronic hypoxia hypothesis. First, there is no way to appreciate whether tissue is actually “hypoxic” based on the current implementation of BOLD-MRI. Second, the theory actually suggested that the increased hypoxia was associated with progressive CKD.
As an extension of their previous studies, the new report by Pruijm et al.7 provides the first indication that the BOLD-MRI measurements may be predictive of progressive CKD. A longitudinal study was performed over 3 years. The baseline BOLD-MRI measures (cortical R2* and corticomedullary difference in R2*) were found to be significantly correlated with changes in eGFR over the next 3 years. High R2* values (>90th percentile) showed the highest eGFR slopes and were associated with 3 times greater adverse outcomes. Receiver operating characteristic curve analysis of adverse outcomes showed an area under the curve of 0.71 for cortical R2* and 0.80 for R2* slope. At the same time, proteinuria was found to be more strongly correlated with a change in eGFR and had an area under the curve of 0.83. So the BOLD-MRI findings have to be put in perspective. While promising, these data should be only considered as a starting point that stimulates further studies to improve the sensitivity of BOLD-MRI measurements. Further, it is possible that BOLD-MRI measurements can be combined with other imaging (e.g., the presence of fibrosis by diffusion MRI) and conventional measures to improve the predictive power.
Despite its merits, the study by Pruijm et al.7 did have limitations. The observed changes in R2* were small. Whether this is due to confounding effects of lower blood volume and hematocrit deserves further investigation. Recent data with arterial spin labeling perfusion MRI show large reductions in perfusion, even in moderate CKD.8 This could indicate a reduced blood volume (or vascular density). Anemia is also common in CKD, specifically in CKD stages 3b through 5. Although the 12-layer concentric objects analysis method is highly objective, it does compromise the ability to study the medulla. Because the changes in the cortex and medulla in advanced stages of CKD can be different,5 the net effect of averaging over both of these regions can reduce the sensitivity of the method in the inner layers. Last, even though the etiology of CKD seemed to show differences in the rate of change in eGFR, the BOLD-MRI measurements did not show differences to the same extent. This may again point to the limited sensitivity of the technique as currently implemented.
The limitations of renal BOLD-MRI have been discussed in the literature. BOLD-MRI as currently implemented does not directly measure po2 and so cannot characterize tissue as being hypoxic. The multiparametric dependence of the BOLD-MRI signal leads to confounding effects, especially when comparing cohorts such as CKD versus healthy controls. Furthermore, BOLD-MRI inherently assumes a fast equilibrium between blood and tissue compartments, which may not hold in disease. Despite these known limitations, a role for BOLD-MRI still exists given the fact that there is no other noninvasive method to evaluate renal oxygenation in vivo.
In light of the above, a reasonable question to ask is “Where do we go from here?” To start, we may need to determine whether cortical or medullary changes are more important in predicting the progression of CKD. There is limited evidence, at least in diabetic nephropathy, that the functional dependence is opposite in the 2 regions,5 that is, cortex may be less oxygenated while the medulla may be better oxygenated in advanced stages of CKD. Also, it is important to determine whether the sensitivity of BOLD-MRI can be improved by accounting for confounding effects. Given the efficacy of BOLD-MRI to assess acute changes, other “stress” tests similar to the response to furosemide should be considered. Water loading has been considered, but not extensively studied. Multiparametric approaches such as BOLD-MRI combined with perfusion and fibrosis imaging could provide additional predictive power.
A few years ago, there was a commentary and letters to the editor following the report by Michaely et al.6 with one asking “Is there no future for renal BOLD-MRI?” While the studies by Pruijm et al.7 did address many of the technical limitations of the previous study,6 there remains a need to address the fundamental issue of concomitant effects when applying renal BOLD-MRI to compare cohorts. Changes in renal oxygenation may be associated with concomitant changes in vascular density and hematocrit whose contributions to the BOLD-MRI signal may not be additive, but actually competing (as depicted in Figure 1).
Figure 1 |. Schematic overview of the key factors that govern renal R2*.
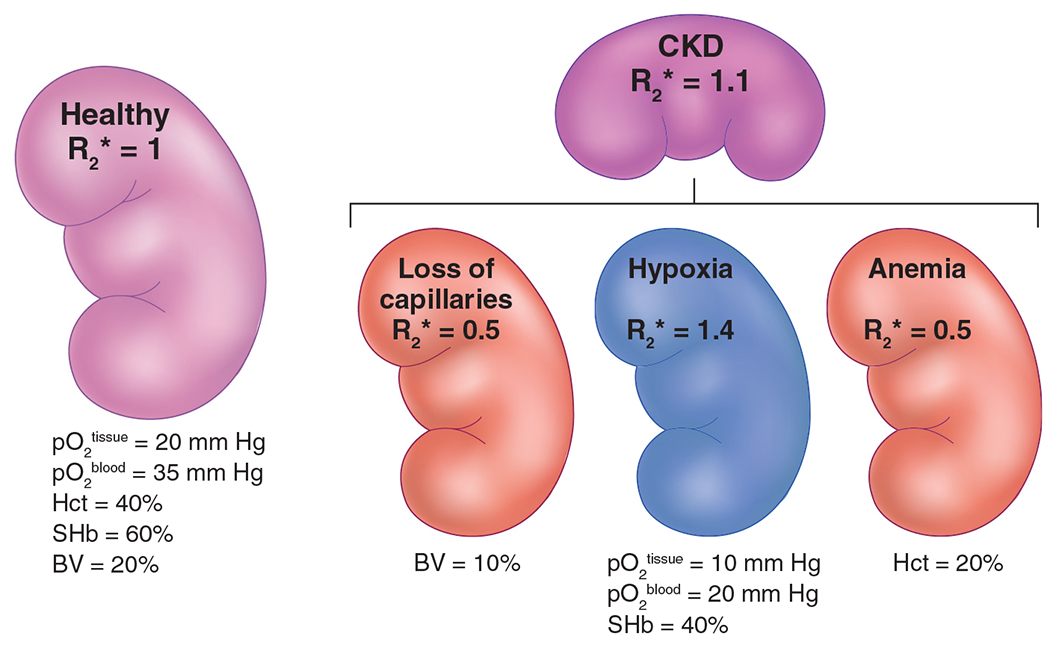
This includes changes in renal blood pO2, hematocrit (Hct), O2 saturation of Hb (SHb), and fractional blood volume (BV). Please note that all values given here are only for illustrative purposes. R2* values were also chosen for illustrative purposes, with healthy renal R2* set at 1.0. In chronic kidney disease (CKD), along with reduced tissue and blood oxygenation, there is loss of microvasculature and anemia. The net contribution makes the R2* in CKD only slightly higher compared with healthy controls.
This potentially could explain the limited sensitivity of renal BOLD-MRI observed to date. A new model has been proposed that allows one to map R2* measurements to blood and tissue pO2 with the knowledge of regional vascular density and hematocrit.9 Incorporating independent measures of vascular density may therefore allow for a more accurate evaluation of renal oxygenation. So, yes there is still a crucial role for renal BOLD-MRI to play in the evaluation of intrarenal oxygenation in CKD.
ACKNOWLEDGMENTS
The work was supported in part by a grant from the National Institutes of Health (R01DK093793).
Footnotes
DISCLOSURE
The author declared no competing interests.
REFERENCES
- 1.Baek SD, Baek CH, Kim JS, et al. Does stage III chronic kidney disease always progress to end-stage renal disease? A ten-year follow-up study. Scand J Urol Nephrol. 2012;46:232–238 [DOI] [PubMed] [Google Scholar]
- 2.Fine LG, Orphanides C, Norman JT. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int Suppl. 1998;65:S74–S78. [PubMed] [Google Scholar]
- 3.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94:3271−3275. [DOI] [PubMed] [Google Scholar]
- 4.Inoue T, Kozawa E, Okada H, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22:1429–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin WJ, Liu F, Li XM, et al. Noninvasive evaluation of renal oxygenation in diabetic nephropathy by BOLD-MRI. Eur J Radiol. 2012;81:1426–1431 [DOI] [PubMed] [Google Scholar]
- 6.Michaely HJ, Metzger L, Haneder S, et al. Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int. 2012;81:684–689 [DOI] [PubMed] [Google Scholar]
- 7.Pruijm M, Milani B, Pivin E, et al. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int. 2018;93:932–940 [DOI] [PubMed] [Google Scholar]
- 8.Li LP, Tan H, Thacker JM, et al. Evaluation of Renal Blood Flow in Chronic Kidney Disease Using Arterial Spin Labeling Perfusion Magnetic Resonance Imaging. Kidney Int Rep. 2017;2:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang JL, Morrell G, Rusinek H, et al. Measurement of renal tissue oxygenation with blood oxygen level-dependent MRI and oxygen transit modeling. Am J Physiol Renal Physiol. 2014;306:F579–F587 [DOI] [PMC free article] [PubMed] [Google Scholar]


