Abstract
Glaucoma is a disease involving impaired visual function accompanied by degeneration and necrosis of the optic nerve. Epigallocatechin-3-gallate (EGCG) exerts a neuroprotective effect against the degeneration of retinal ganglion cells. However, whether EGCG can relieve glaucoma and the possible mechanisms remain unclear. In order to determine the function of EGCG in glaucoma, an acute glaucoma rat model was established. Optic neuropathology was examined by haematoxylin-eosin staining and immunofluorescence staining for class III-β tubulin. The levels of inflammation-associated cytokines, such as interleukin (IL)-4, IL-6, TNF-α, IL-1β, IL-13 and IFN-γ were measured by flow cytometry. T cell proliferation was assessed by the carboxyfluorescein diacetate succinimidyl ester method. Finally, the functional role of EGCG in glaucoma was explored. The levels of the inflammation-associated proteins p-IκBα and p-p65 were measured by western blot analysis. The results showed that optic nerve injury occurred, and elevated levels of the inflammatory cytokines IL-4, IL-6, TNF-α, IL-1β, IL-13 and IFN-γ were observed in the rat model of acute glaucoma. In addition, an increased T lymphocyte proliferation rate and imbalance of Th1/Th2 cytokines were present in the models. Importantly, treatment with EGCG significantly alleviated optic nerve injury. At the molecular level, EGCG decreased the levels of inflammation-associated cytokines, decreased the proliferation rate of T lymphocyte cells, and repaired the imbalance of Th1/Th2 cytokines. Moreover, EGCG inhibited the increase in the phosphorylation of IκBα and p65 caused by modelling and thus suppressed the activation of the nuclear factor (NF)-κB signalling pathway. The findings of the present study indicate that EGCG could attenuate the symptoms of glaucoma and inhibit inflammatory responses by suppressing the NF-κB signalling pathway in a rat glaucoma model.
Keywords: EGCG, glaucoma, inflammation, NF-κB signalling pathway, Th1/Th2
Introduction
Glaucoma is an irreversible ophthalmic disease leading to blindness, and there are several types of glaucoma, including congenital glaucoma, primary glaucoma, secondary glaucoma and mixed glaucoma. High intraocular pressure (IOP) is the primary cause of acute glaucoma and is accompanied by optic atrophy, degeneration and necrosis of retinal nerve layers (1-3). While decreasing the IOP is a key treatment for glaucoma, it is difficult to stop the progression of glaucoma (4). Several factors are implicated in the pathogenesis of glaucoma, such as impaired blood circulation, excitotoxic reactions caused by excessive accumulation of glutamate, free radical production, oxidative stress and immunological factors (5). Therefore, lowering the IOP is insufficient. Emerging evidence has indicated that proinflammatory factors play a key role in glaucoma development and progression. Identifying novel and effective therapeutic interventions to decrease inflammatory responses might be helpful for treating this disease and ultimately decrease the rate of blindness.
The nuclear factor κB (NF-κB) signalling pathway is critical for inflammation and is involved in various autoimmune diseases (6). The activation of NF-κB by different stimuli causes the phosphorylation and ubiquitination of IκB and subsequently activates the transcription and expression of several downstream genes. By this mechanism, the NF-κB signalling pathway directly regulates the expression of Th1- and Th2-type cytokines and the inflammatory response (7,8). In contrast, inhibition of the NF-κB signalling pathway by Pueraria and arylsulfonyl indoline-benzamide greatly suppresses the release of proinflammatory cytokines in mice with glaucoma and thus attenuates injury (1,9). Accordingly, the NF-κB signalling pathway plays an important role in glaucoma and may be a potential target for glaucoma therapy.
Epigallocatechin-3-gallate (EGCG), a major antioxidant catechin found in green tea (Camellia sinensis), has key anti-inflammatory (10,11), antioxidative (12,13), and anticancer (14-16) effects. It has been reported that EGCG attenuates hypothalamic inflammation by inhibiting the JAK2/STAT3 signalling pathways in microglia (17). Additionally, EGCG has been shown to exert beneficial effects against conditions such as liver inflammation (18), arthritis (19) and nephritis (20). It has been reported that EGCG can inhibit the phosphorylation and ubiquitination of IκB, thereby suppressing the activation of the NF-κB signalling pathway and decreasing the production of inflammatory cytokines (21). However, there have been few studies on whether EGCG can be used for glaucoma treatment.
The present study investigated the potential therapeutic effect of EGCG in glaucoma by using a rat glaucoma model established by high-pressure perfusion. Optic neuropathy was observed, and the levels of inflammatory-associated factors were measured in the glaucoma model rats treated with or without EGCG. Importantly, the mechanism by which EGCG relieves glaucoma was further explored. Overall, the present study provides a theoretical basis for glaucoma therapy.
Materials and methods
Establishment of a rat glaucoma model and drug treatment
Fifteen male Sprague-Dawley rats and 15 female Sprague-Dawley rats from Hunan SJA Laboratory Animal Co., Ltd. (6-8 weeks old; weight, 220-250 g) were used to establish the glaucoma model. The animals were housed in the standard animal facility with 12:12 h (light/dark cycle), 40-50% humidity, and 22˚C temperature. The rats had free access to food and water. No obvious eye disease was observed before surgery. The rats were randomly divided into six groups (n=5 in each group): Control group, 1-day group, 3-day group, 7-day group, 14-day group, and 28-day group. The rats were anaesthetized via intraperitoneal injection of 2% pentobarbital sodium (50 mg/kg). Following anaesthesia, a needle was injected into the corneoscleral margin of the rat eyeball without touching the lens to prevent cataract formation. Normal saline was infused into the centre of the anterior chamber for 60 min. No iris or lens injury was induced during the operation (22). At the indicated time point (day 1, 3, 7, 14 or 28), all the rats in each group were anesthetized with 3% sodium pentobarbital (30 mg/kg) and 3-5 ml of abdominal aortic blood was collected. Then, eyeballs samples were collected after rats were sacrificed by cervical dislocation. The samples were used for assessment of pathological changes and inflammatory responses.
In order to evaluate the treatment effect of EGCG (Aladdin; cat. no. E107404-500 mg), 20 Sprague-Dawley rats (10 males and 10 females) were randomly divided into four groups (n=5 in each group): Control group (sham surgery without saline injection), glaucoma model group (no EGCG in drinking water), 14-day EGCG group (each rat was given 50 mg/kg/day EGCG by gavage for 14 continuous days immediately after modelling) (4), and 28-day EGCG group (each rat was given 50 mg/kg/day EGCG by gavage for 28 continuous days immediately after modelling). All animals in each group were sacrificed at the indicated time point (day 14 or 28), and samples were collected and used for assessment of pathological changes and inflammatory responses.
The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Please change it to The Third Xiangya Hospital of Central South University.
Haematoxylin-eosin (HE) staining
Rat eyeballs were collected from each group at specific time points and fixed in FAS fixation solution (Wuhan Servicebio Technology Co., Ltd.; cat. no. G1109) for >24 h at room temperature. PBS was used to rinse off the fixed solution and the tissues were embedded in paraffin. Embedded tissues were cut into 5-µm slices. The sections were stained with haematoxylin for 10 min at room temperature and rinsed by water, followed by eosin staining for 10 min at room temperature. The stained slices were washed once with 85% ethanol for 5 min at room temperature, and then washed once with 95% ethanol for 5 min at room temperature. Finally, the slices washed twice with 100% ethanol for 10 min each at room temperature, followed by imaging with light microscopy (BA400; McAudi Industrial Group Co., Ltd; magnification, x40).
Assessment of T cell proliferation by the carboxyfluorescein diacetate succinimidyl ester (CFSE) method
Rats in each group were sacrificed at specific time points, and the blood was collected from each rat to assess T lymphocyte proliferation. The procedure was performed as previously described (23). Briefly, cells were isolated by gradient centrifugation and counted with a cell counter. Cell suspension (1 ml) was added to the bottom of the tube. CFSE (cat. no. 423801) diluted with PBS was added (final CFSE concentration, 5 µmol/l), and the tube was rapidly vortexed to ensure homogeneous dispersal. After labelling with CFSE, the cells were treated with RPMI-1640 (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 5% foetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and cultured at 37˚C in a 5% CO2 incubator for 72 h. After that, the cells were incubated for 20 min at room temperature with an APC-conjugated anti-rat CD3 antibody (1 µg per million cells; cat. no. 201413; BioLegend, Inc.) and analysed within 1 h on a FACSCalibur flow cytometer (BD Biosciences). Acquired data were analyzed using FlowJo software (v7.6; FlowJo LLC).
Measurement of intracellular cytokine levels by flow cytometry
Rats in the control, 1-day, 3-day, 7-day, 14-day and 28-day groups were sacrificed on days 1, 3, 7, 14 and 28, respectively, and rats from the 14- and 28-day EGCG groups were sacrificed on days 14 and 28, respectively. Blood was collected from each rat and used to measure the levels of intracellular IL-4, IL-6, TNF-α, IL-1β, IL-13 and IFN-γ (custom panel; BioLegend, Inc.) and the levels of Th1- and Th2-type cytokines (cat. no. 740405; BioLegend, Inc.) were measured by flow cytometry. The protocol was performed as previously described (24,25).
Western blot analysis of inflammation-associated protein expression
The levels of the inflammation-associated proteins phosphorylated (p)-p65 (cat. no. 3033T), p65 (cat. no. 8242T), p-IκBα (cat. no. 9246S) and IκBα (cat. no. 4814T) (all from Cell Signaling Technology, Inc) were assessed by western blotting. The tissues were lysed in RIPA lysis buffer (Abcam) supplemented with protease inhibitor. The protein concentration of each sample was quantified by BCA assay with the BCA assay kit (Bio-Rad Laboratories, Inc.). Equal amounts of proteins from each sample (20 mg) were loaded to 12% SDS-PAGE gels for electrophoresis and then transferred onto polyvinylidene difluoride (EMD Millipore) membranes. The membranes were blocked in 5% dry skimmed milk in TBST buffer (0.02 M Tris-base, pH 7.6, 0.8% NaCl and 0.1% Tween-20) for 1 h at room temperature. Specific primary antibodies (1:1,000) or a β-actin antibody (1:1,000; Cell Signaling Technology, Inc.) was added, and the membranes were incubated at 4˚C overnight. After washing three times, the membranes were incubated with the appropriate HRP-conjugated secondary antibodies (1:3,000; cat. nos. BA1075 and BA1054; Boster Biological Technology) at room temperature for 1 h. Finally, the protein bands were detected by an enhanced chemiluminescence kit (cat. no. 32209; Thermo Fisher Scientific, Inc.) and then semi-quantified with ImageJ software (v1.8.0; National Institutes of Health).
Immunofluorescence staining
Rat eyeballs were fixed in FAS fixation solution for >24 h at room temperature, embedded in paraffin and sectioned into 5-µm slices. The sections were blocked in blocking buffer containing 5% BSA in PBS for 30 min at 37˚C. The sections were then incubated with an anti-class III β-tubulin primary antibody (1:1,000; cat. no. ab18207; Abcam) overnight at 4˚C (26). After washing three times, the sections were incubated with secondary antibody (1:2,000; cat. no. ab150077; Abcam) labelled with fluorescein at room temperature for 1 h in the dark. After washing, anti-fluorescence quenching agent (cat. no. G1401; Wuhan Servicebio Technology Co., Ltd.) was applied, and the sections were mounted on glass slides. Finally, the samples were imaged with a fluorescence microscope (magnification, x40; TE2000-U; Nikon Corporation).
Statistical analysis
For statistical analyses, one-way ANOVA with Tukey's post hoc test was performed using SPSS software (v12.0; SPSS Inc.). The results are presented as the means ± standard deviations of three independent experiments. P<0.05 was considered to indicate a statistically significant difference.
Results
Successful establishment of an acute glaucoma rat model
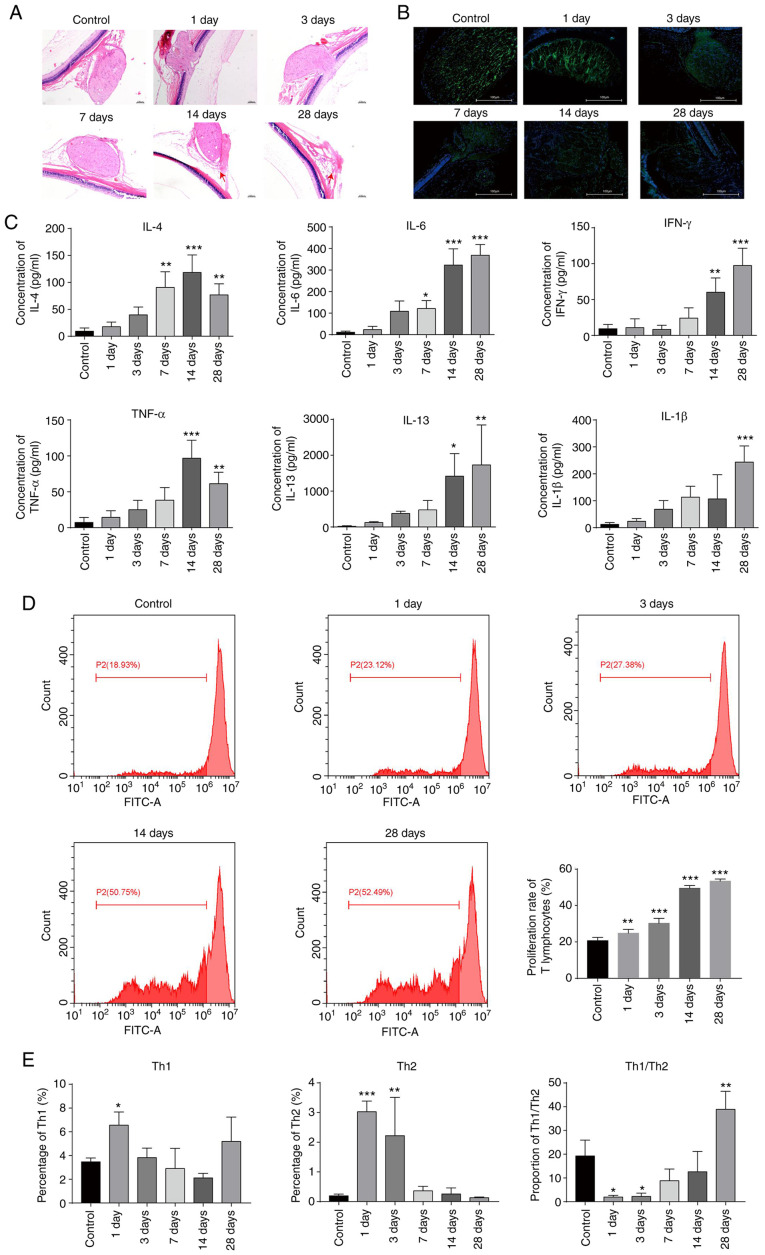
The eyeballs of the animals were subjected to high-pressure perfusion to establish an acute glaucoma model (27). Rat eyeballs were removed for HE staining on days 1, 3, 7, 14 and 28 after modelling to observe the neuropathological changes. The results showed that gradual injury of the optic nerve, manifested as optic lesions and atrophy, occurred over time after modelling and that degeneration and necrosis of the retinal nerve layer also occurred (Fig. 1A). In addition, the fluorescence intensity of the class III-β tubulin protein in the 28-day group was lower compared with that in the control group (Fig. 1B), indicating that neuronal cells were damaged in the 28-day group. Flow cytometry revealed that the levels of intracellular cytokines, including IL-4, IL-6, TNF-α, IL-1β, IL-13 and IFN-γ, were gradually elevated following modelling, with the levels of IL-4 and TNF-α reaching the peak at day 14 and the rest peaking at day 28 (Fig. 1C). Furthermore, the proliferation rate of T lymphocytes was measured by the CFSE method and found that the proliferation rate gradually increased with time after modelling (Fig. 1D). Total Th1 and Th2 cytokine levels were also assessed by flow cytometry. The results indicated that the levels of both Th1 and Th2 cytokines were increased during the early days after remodelling (Fig. 1E). However, the levels returned to baseline levels by the end of the experiment (Fig. 1E). The ratio of Th1 to Th2 cytokines was greatly diminished on day 1 and day 3 after remodelling but gradually recovered to a normal level and was increased by day 28, indicating an imbalance of Th1/Th2 cytokines developed during the progression of glaucoma (Fig. 1E). These results provide evidence that our animal model of acute glaucoma was successfully established.
Figure 1.
Successful establishment of an acute glaucoma rat model. (A) Observation of optic neuropathy by haematoxylin-eosin staining after modelling. Magnification, x40. (B) Measurement of class III β-tubulin levels by immunofluorescence staining after modelling. Magnification, x40 (C) Measurement of the levels of cytokines, including IL-4, IL-6, TNF-α, IL-1β, IL-13 and IFN-γ, by flow cytometry after modelling. (D) Assessment of T lymphocyte proliferation by the CFSE method on days 1, 3, 14, and 28 after modelling. The cells were treated with CFSE and analysed by flow cytometry. (E) The expression levels of Th1 and Th2 cytokines determined by flow cytometry on days 1, 3, 7, 14, and 28 after modelling. *P<0.05; **P<0.01; ***P<0.001. IL, interleukin; TNF, tumour necrosis factor; IFN, interferon; CFSE, carboxyfluorescein diacetate succinimidyl ester.
EGCG suppressed the expression of inflammatory cytokines in rats with acute glaucoma
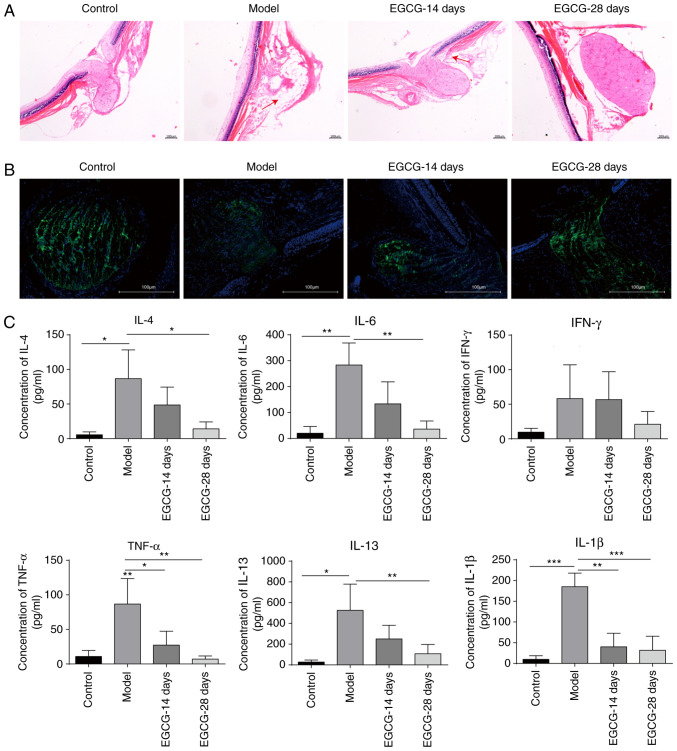
In order to study the role of EGCG in acute glaucoma, animals were fed with EGCG via gavage following modelling. The HE staining results showed that EGCG greatly attenuated the optical injury caused by high pressure, with better effects being observed following 28 days of treatment compared with after 14 days of treatment (Fig. 2A). In addition, the protein expression of class III-β tubulin was upregulated in the 28-day EGCG group compared with the model group, indicating that EGCG could repair the damage to the optic nerve induced by acute glaucoma (Fig. 2B). The cytokine levels were measured after EGCG treatment. The levels of cytokines, including IL-4, IL-6, TNF-α, IL-1β, IL-13 and IFN-γ, were significantly increased in the model group compared with the control group (Fig. 2C). However, these increases were significantly suppressed following treatment with EGCG for 14 days, and the levels of these cytokines returned to baseline levels after 28 days of EGCG treatment (Fig. 2C). The aforementioned results indicate that EGCG can suppress inflammatory responses in glaucoma and thus attenuate optical injury.
Figure 2.
EGCG suppresses the expression of inflammatory cytokines in rats with acute glaucoma. (A) Observation of optic neuropathy by haematoxylin-eosin staining after treatment with EGCG. Magnification, x40. (B) Analysis of class III β-tubulin levels by immunofluorescence staining in the control group, model group and groups treated with EGCG for 14 and 28 days. Magnification, x40. (C) Measurement of the levels of cytokines, including IL-4, IL-6, TNF-α, IL-1β, IL-13 and IFN-γ, by flow cytometry after treatment with EGCG. *P<0.05; **P<0.01; ***P<0.001. EGCG, epigallocatechin-3-gallate; IL, interleukin; TNF, tumour necrosis factor; IFN, interferon.
EGCG decreased T cell proliferation and the Th1/Th2 ratio in the peripheral blood of rats with glaucoma
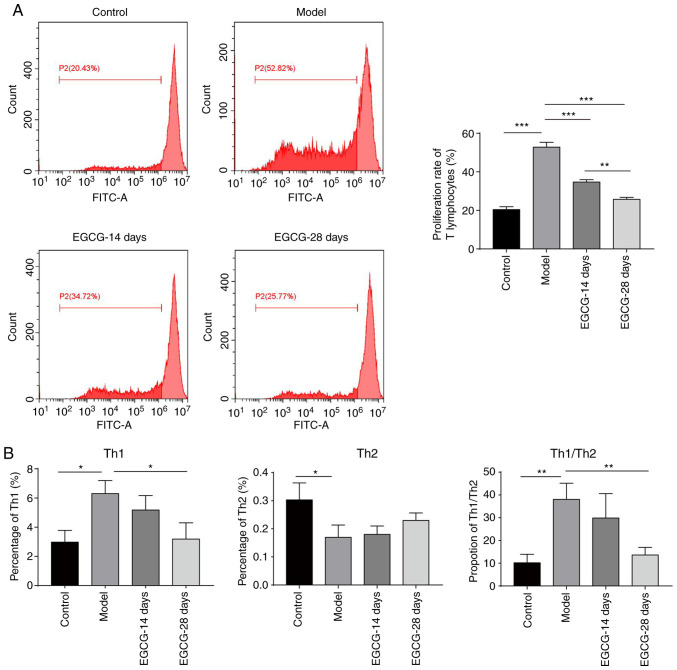
The aforementioned results indicate that the proliferation rate of T lymphocytes was increased and that an imbalance of Th1/Th2 cells occurred in the glaucoma model group; it was then assessed whether EGCG regulated this process. As shown in Fig. 3A, treatment with EGCG for 14 days significantly decreased the T lymphocyte proliferation rate, and 28 days of treatment further decreased the rate (Fig. 3A). Furthermore, the increase in the levels of Th1-type and Th2-type cytokines following modelling were suppressed by EGCG treatment (Fig. 3B). The Th1/Th2 ratio was also restored by EGCG treatment (Fig. 3B). Altogether, these data demonstrate that EGCG can suppress T cell proliferation and restore the imbalance of Th1/Th2 cytokines during glaucoma.
Figure 3.
EGCG decreases T cell proliferation and the Th1/Th2 ratio in the peripheral blood of rats with glaucoma. (A) Analysis of T lymphocyte proliferation by the CFSE method in the control group, model group and groups treated with EGCG for 14 and 28 days. The cells were treated with CFSE and analysed by flow cytometry. (B) The expression levels of Th1 and Th2 cytokines determined by flow cytometry in the control group, model group and groups treated with EGCG for 14 and 28 days. *P<0.05; **P<0.01; ***P<0.001. CFSE, carboxyfluorescein diacetate succinimidyl ester; EGCG, epigallocatechin-3-gallate.
EGCG inhibits the NF-κB signalling pathway
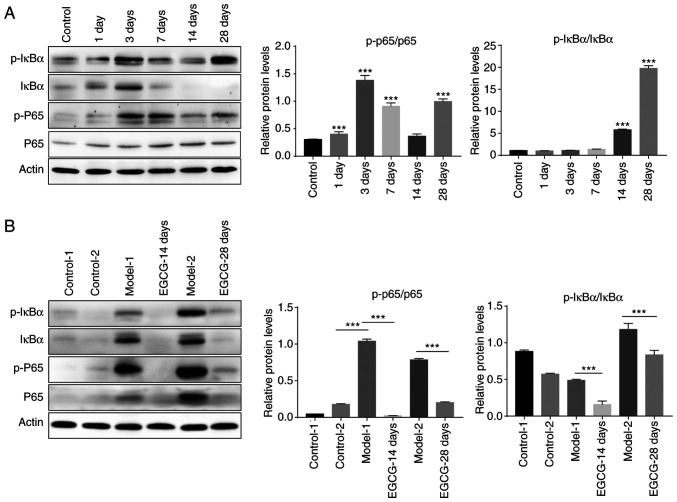
In order to study the mechanism by which EGCG suppressed inflammatory responses, the expression levels of inflammation-associated proteins were measured by western blot analysis. The results showed that p-IκBα/IκBα and p-p65/p65 levels gradually increased with time after the establishment of the glaucoma model (Fig. 4A). Notably, after treatment with EGCG, the increase in both p-IκBα/IκBα and p-p65/p65 levels induced by modelling were significantly inhibited (Fig. 4B). The aforementioned results indicated that the NF-κB signalling pathway is markedly activated in the course of glaucoma and that this activation can be inhibited by EGCG treatment.
Figure 4.
EGCG inhibits the nuclear factor-κB signalling pathway. (A) Expression levels of phosphorylated and unphosphorylated IκBα and p65 determined by western blot analysis on days 1, 3, 7, 14 and 28 after modelling. (B) Expression levels of phosphorylated and unphosphorylated IκBα and p65 determined by western blot analysis in the control group, model group, and groups treated with EGCG for 14 and 28 days. ***P<0.001 EGCG, epigallocatechin-3-gallate; p-, phosphorylated-.
Discussion
Glaucoma caused by high IOP is an optic nerve disease that can ultimately result in blindness. Currently, treatments for this disease include drugs (28) and surgery (29,30) to control IOP and limit optic nerve damage. However, prognosis remains to be improved, due to the various unwanted side effects of these treatments (31). In the present study, through the use of an animal model, it was demonstrated that EGCG significantly ameliorates optic injury in the course of glaucoma by suppressing inflammatory responses and restoring the balance of Th1 and Th2 cytokines via the NF-κB pathway. The present study provides an avenue for the development of therapeutic strategies for glaucoma.
High IOP is a key risk factor for glaucoma. Thus, many studies have established animal models of glaucoma by increasing the IOP (32,33). It has been reported that IOP can be increased by injecting saline into the eyes. For example, Zhong (34) successfully generated an ocular hypertension rat model by injecting hypertonic saline into the episcleral veins once weekly for two weeks. The success rate of this method was >80%, and IOP was elevated by 81% for 24 weeks. Similarly, Morrison et al (35) established an experimental rat model of acute glaucoma through injecting hypertonic saline into the aqueous outflow pathway, and the resulting inflammatory response led to increased IOP one week later. In the present study, acute glaucoma was modelled in rats by high-pressure infusion of saline. After infusion, optic nerve damage begins to develop, such as optic atrophy, degeneration and necrosis of the retinal nerve layer. In addition, a decrease in class III-β tubulin protein expression was observed, further indicating damage to optic nerves. Accompanying these pathological changes were marked increases in the levels of proinflammatory Th1/Th2 cytokines. These results indicate that the animal model was successfully established; thus, this approach for increasing IOP could be used as an alternative method for modelling acute glaucoma in animals.
EGCG is the main ingredient of green tea and has been shown to have a neuroprotective effect (36,37). For instance, Kian et al (38) found that EGCG may be effective in protecting neuronal cells against apoptosis and may increase neuronal survival time following nerve transection. Furthermore, Shen et al (4) observed that EGCG exerts a neuroprotective effect on retinal ganglion cells (RGCs) in chronic glaucoma. Consistently, the present study indicated that EGCG attenuates optic nerve injury induced by high IOP, as indicated by decreased optic atrophy, optic nerve degeneration and necrosis. Taken together, the present study results and those of previous studies indicate that EGCG has a conserved neuroprotective role. It might be interesting to examine whether EGCG can be applied for the treatment of other neurological diseases, such as Alzheimer's disease and Parkinson's disease.
Inflammation significantly contributes to the development and progression of glaucoma, and substantial changes in the levels of cytokines, such as IFN-γ, TNF-α, IL-1 and IL-6, have been reported (39,40). In patients with glaucoma, the expression of IL-4, IL-6, TNF-α, IL-1β, IL-13 and IFN-γ is upregulated, and this upregulation exacerbates the disease. The present study found that EGCG inhibits the increase in IL-4, IL-6, TNF-α, IL-1β, IL-13 and IFN-γ expression levels in vivo, suggesting that it suppresses inflammatory responses. In addition, other studies have shown that the T cell response plays a role in neurodegeneration (41,42). Indeed, T cell proliferation has been observed in glaucoma. T cell proliferation in the context of glaucoma could induce the production of proinflammatory cytokines and thus promote inflammation. The present study data showed that EGCG greatly decreases the proliferation rate of T cells. This might be one of the mechanisms by which the inflammatory responses are inhibited by EGCG in glaucoma. In addition, the Th1/Th2 ratio reflects inflammation in vivo and is associated with the progression of glaucoma. The finding that the Th1/Th2 ratio was restored by EGCG in the present glaucoma model further suggests that EGCG serves a neuroprotective role in glaucoma. This finding is consistent with growing evidence indicating that an imbalance in Th1/Th2 cytokine production contributes to glaucomatous optic neuropathy (43).
Activation of the NF-κB pathway plays a crucial role in inflammation, as it can promote the transcription and expression of several proinflammatory cytokines, which may further facilitate T-cell communication (44). The present study confirmed that the NF-κB pathway was activated in a rat model. Notably, EGCG treatment remarkably suppressed NF-κB pathway activation, indicating that EGCG inhibits inflammation by inhibiting the NF-κB signalling pathway. Future studies are required to determine whether other mechanisms are involved in the neuroprotective role of EGCG in glaucoma. Additionally, it remains to be further explored whether the neuroprotective effect of EGCG is dose-dependent.
In conclusion, the present study results demonstrate that EGCG attenuates optic nerve injury during glaucoma by suppressing the activation of the NF-κB signalling pathway and inflammatory responses. This study indicates that EGCG could serve as a promising adjuvant intervention for glaucoma treatment in the future.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
WHZ and YNC were responsible for the conception and design, and revision of the manuscript. WHZ prepared the draft of the manuscript and performed the major experiments that contributed to the acquisition, analysis and interpretation of data. YC and LMG assisted in the experiments and analyzed the data. WHZ and YNC confirmed the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), The Third Xiangya Hospital of Central South University [approval no. LLSC (LA) 2018-038].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lei X, Zhao Y. Neovascular glaucoma regulation by arylsulfonyl indoline-benzamide (ASIB) through targeting NF-κB signalling pathway. 3 Biotech. 2019;9(211) doi: 10.1007/s13205-019-1730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 3.Foster PJ. The epidemiology of primary angle closure and associated glaucomatous optic neuropathy. Semin Ophthalmol. 2002;17:50–58. doi: 10.1076/soph.17.2.50.14718. [DOI] [PubMed] [Google Scholar]
- 4.Shen C, Chen L, Jiang L, Lai TY. Neuroprotective effect of epigallocatechin-3-gallate in a mouse model of chronic glaucoma. Neurosci Lett. 2015;600:132–136. doi: 10.1016/j.neulet.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Wong M, Huang P, Li W, Li Y, Zhang SS, Zhang C. T-helper1/T-helper2 cytokine imbalance in the iris of patients with glaucoma. PLoS One. 2015;10(e0122184) doi: 10.1371/journal.pone.0122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Zhao Q. Kaempferitrin inhibits proliferation, induces apoptosis, and ameliorates inflammation in human rheumatoid arthritis fibroblast-like synoviocytes. Phytother Res. 2019;33:1726–1735. doi: 10.1002/ptr.6364. [DOI] [PubMed] [Google Scholar]
- 7.Niu Y, Dong Q, Li R. Matrine regulates Th1/Th2 cytokine responses in rheumatoid arthritis by attenuating the NF-kappaB signaling. Cell Biol Int. 2017;41:611–621. doi: 10.1002/cbin.10763. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Lu R, Li H, Zhou Y, Zhang P, Bai L, Chen D, Chen J, Li J, Yu P, et al. Zhen-wu-tang ameliorates membranous nephropathy rats through inhibiting NF-κB pathway and NLRP3 inflammasome. Phytomedicine. 2019;59(152913) doi: 10.1016/j.phymed.2019.152913. [DOI] [PubMed] [Google Scholar]
- 9.Wei HY, Zhang YJ, Zhao SZ. Puerarin regulates neovascular glaucoma through pigment epitheliumderived growth factorinduced NF-κB signaling pathway. Mol Med Rep. 2018;17:7866–7874. doi: 10.3892/mmr.2018.8800. [DOI] [PubMed] [Google Scholar]
- 10.Kar AK, Singh A, Dhiman N, Purohit MP, Jagdale P, Kamthan M, Singh D, Kumar M, Ghosh D, Patnaik S. Polymer-assisted in situ synthesis of silver nanoparticles with epigallocatechin gallate (EGCG) impregnated wound patch potentiate controlled inflammatory responses for brisk wound healing. Int J Nanomedicine. 2019;14:9837–9854. doi: 10.2147/IJN.S228462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen T, Payan B, Zambrano A, Du Y, Bondesson M, Mohan C. Epigallocatechin-3-gallate suppresses neutrophil migration speed in a transgenic zebrafish model accompanied by reduced inflammatory mediators. J Inflamm Res. 2019;12:231–239. doi: 10.2147/JIR.S224834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yelins'ka AM, Liashenko LI, Kostenko VO. Quercetin potentiates antiradical properties of epigallocatechin-3-gallate in periodontium of rats under systemic and local administration of lipopolisaccharide of salmonella typhi. Wiad Lek. 2019;72:1499–1503. [PubMed] [Google Scholar]
- 13.Wang J, Jia R, Celi P, Ding X, Bai S, Zeng Q, Mao X, Xu S, Zhang K. Green tea polyphenol epigallocatechin-3-gallate improves the antioxidant capacity of eggs. Food Funct. 2020;11:534–543. doi: 10.1039/c9fo02157d. [DOI] [PubMed] [Google Scholar]
- 14.Sharifi-Rad M, Pezzani R, Redaelli M, Zorzan M, Imran M, Ahmed Khalil A, Salehi B, Sharopov F, Cho WC, Sharifi-Rad J. preclinical pharmacological activities of epigallocatechin-3-gallate in signaling pathways: An update on cancer. Molecules. 2020;25(467) doi: 10.3390/molecules25030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Md Nesran ZN, Shafie NH, Ishak AH, Mohd Esa N, Ismail A, Md Tohid SF. Induction of endoplasmic reticulum stress pathway by green tea epigallocatechin-3-Gallate (EGCG) in colorectal cancer cells: Activation of PERK/p-eIF2α/ATF4 and IRE1α. Biomed Res Int. 2019;2019(3480569) doi: 10.1155/2019/3480569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Chen L, Lu T, Xie Y, Li C, Jia Z, Cao J. ERα36 is an effective target of epigallocatechin-3-gallate in hepatocellular carcinoma. Int J Clin Exp Pathol. 2019;12:3222–3234. [PMC free article] [PubMed] [Google Scholar]
- 17.Mao L, Hochstetter D, Yao L, Zhao Y, Zhou J, Wang Y, Xu P. Green Tea Polyphenol (-)-Epigallocatechin Gallate (EGCG) attenuates neuroinflammation in palmitic acid-stimulated BV-2 microglia and high-fat diet-induced obese mice. Int J Mol Sci. 2019;20(5081) doi: 10.3390/ijms20205081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Gao Q, Wang T, Kan Z, Li X, Hu L, Peng CY, Qian F, Wang Y, Granato D. Green tea polyphenols and epigallocatechin-3-gallate protect against perfluorodecanoic acid induced liver damage and inflammation in mice by inhibiting NLRP3 inflammasome activation. Food Res Int. 2020;127(108628) doi: 10.1016/j.foodres.2019.108628. [DOI] [PubMed] [Google Scholar]
- 19.Karatas A, Dagli AF, Orhan C, Gencoglu H, Ozgen M, Sahin N, Sahin K, Koca SS. Epigallocatechin 3-gallate attenuates arthritis by regulating Nrf2, HO-1, and cytokine levels in an experimental arthritis model. Biotechnol Appl Biochem. 2020;67:317–322. doi: 10.1002/bab.1860. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Liu J, Lei Y, Liu M. Potential ameliorative effects of epigallocatechin-3-gallate against cigarette smoke exposure induced renal and hepatic deficits. Ecotoxicol Environ Saf. 2020;191(110202) doi: 10.1016/j.ecoenv.2020.110202. [DOI] [PubMed] [Google Scholar]
- 21.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang L, He L, Zhu M, Chen B, Xiao C. Protective effects of carnosic acid on retinal ganglion cells in acute ocular hypertension rats. Int Ophthalmol. 2020;40:1869–1878. doi: 10.1007/s10792-020-01359-8. [DOI] [PubMed] [Google Scholar]
- 23.Azarsiz E, Karaca N, Ergun B, Durmuscan M, Kutukculer N, Aksu G. In vitro T lymphocyte proliferation by carboxyfluorescein diacetate succinimidyl ester method is helpful in diagnosing and managing primary immunodeficiencies. J Clin Lab Anal. 2018;32(e22216) doi: 10.1002/jcla.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin Y, Mitson-Salazar A, Prussin C. Detection of intracellular cytokines by flow cytometry. Curr Protoc Immunol. 2015;110:6.24.1–6.24.18. doi: 10.1002/0471142735.im0624s110. [DOI] [PubMed] [Google Scholar]
- 25.Foster B, Prussin C, Liu F, Whitmire JK, Whitton JL. Detection of intracellular cytokines by flow cytometry. Curr Protoc Immunol Chapter. 2007;6(Unit 6 24) doi: 10.1002/0471142735.im0624s78. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Ma N, Liu MX, Ye BJ, Li YJ, Hu HY, Tang YH. C1q/TNF-related protein-9 attenuates retinal inflammation and protects blood-retinal barrier in db/db mice. Eur J Pharmacol. 2019;853:289–298. doi: 10.1016/j.ejphar.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Paula AP, Paula JS, Silva MJ, Rocha EM, De Moraes CG, Rodrigues ML. Effects of swimming goggles wearing on intraocular pressure, ocular perfusion pressure, and ocular pulse amplitude. J Glaucoma. 2016;25:860–864. doi: 10.1097/IJG.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Sanchez C, Puerto B, Lopez-Caballero C, Contreras I. Unilateral acute iris depigmentation and transillumination after glaucoma surgery with mitomycin application and intracameral moxifloxacin. Am J Ophthalmol Case Rep. 2020;18(100639) doi: 10.1016/j.ajoc.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otarola F, Virgili G, Shah A, Hu K, Bunce C, Gazzard G. Ab interno trabecular bypass surgery with Schlemm s canal microstent (Hydrus) for open angle glaucoma. Cochrane Database Syst Rev. 2020;3(CD012740) doi: 10.1002/14651858.CD012740.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dada T, Midha N, Shah P, Sidhu T, Angmo D, Sihota R. Innovations in glaucoma surgery from Dr. Rajendra Prasad Centre for Ophthalmic Sciences. Indian J Ophthalmol. 2017;65:103–108. doi: 10.4103/ijo.IJO_865_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: A review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong S, Kim CY, Lee WS, Shim J, Yeom HY, Seong GJ. Ocular hypotensive effects of topically administered agmatine in a chronic ocular hypertensive rat model. Exp Eye Res. 2010;90:97–103. doi: 10.1016/j.exer.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Yu S, Tanabe T, Yoshimura N. A rat model of glaucoma induced by episcleral vein ligation. Exp Eye Res. 2006;83:758–770. doi: 10.1016/j.exer.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Zhong L. A modified chronic ocular hypertension rat model for retinal ganglion cell neuroprotection. Front Med. 2013;7:367–377. doi: 10.1007/s11684-013-0266-2. [DOI] [PubMed] [Google Scholar]
- 35.Morrison JC, Johnson EC, Cepurna WO. Hypertonic saline injection model of experimental glaucoma in rats. Methods Mol Biol. 2018;1695:11–21. doi: 10.1007/978-1-4939-7407-8_2. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Mao L, Xu P, Wang Y. Effects of (-)-Epigallocatechin Gallate (EGCG) on energy expenditure and microglia-mediated hypothalamic inflammation in mice fed a high-fat diet. Nutrients. 2018;10(1681) doi: 10.3390/nu10111681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu D, Perkins JT, Hennig B. EGCG prevents PCB-126-induced endothelial cell inflammation via epigenetic modifications of NF-κB target genes in human endothelial cells. J Nutr Biochem. 2016;28:164–170. doi: 10.1016/j.jnutbio.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kian K, Khalatbary AR, Ahmadvand H, Karimpour Malekshah A, Shams Z. Neuroprotective effects of (-)-epigallocatechin-3-gallate (EGCG) against peripheral nerve transection-induced apoptosis. Nutr Neurosci. 2019;22:578–586. doi: 10.1080/1028415X.2017.1419542. [DOI] [PubMed] [Google Scholar]
- 39.Husain S, Abdul Y, Webster C, Chatterjee S, Kesarwani P, Mehrotra S. Interferon-gamma (IFN-gamma)-mediated retinal ganglion cell death in human tyrosinase T cell receptor transgenic mouse. PLoS One. 2014;9(e89392) doi: 10.1371/journal.pone.0089392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borkenstein A, Faschinger C, Maier R, Weger M, Theisl A, Demel U, Graninger W, Irene H, Mossböck G. Measurement of tumor necrosis factor-alpha, interleukin-6, Fas ligand, interleukin-1a, and interleukin-1β in the aqueous humor of patients with open angle glaucoma using multiplex bead analysis. Mol Vis. 2013;19:2306–2311. [PMC free article] [PubMed] [Google Scholar]
- 41.Gramlich OW, Ding QJ, Zhu W, Cook A, Anderson MG, Kuehn MH. Adoptive transfer of immune cells from glaucomatous mice provokes retinal ganglion cell loss in recipients. Acta Neuropathol Commun. 2015;3(56) doi: 10.1186/s40478-015-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wax MB, Tezel G, Yang J, Peng G, Patil RV, Agarwal N, Sappington RM, Calkins DJ. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand. J Neurosci. 2008;28:12085–12096. doi: 10.1523/JNEUROSCI.3200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang P, Zhang SS, Zhang C. The two sides of cytokine signaling and glaucomatous optic neuropathy. J Ocul Biol Dis Infor. 2009;2:78–83. doi: 10.1007/s12177-009-9026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H, Cho KS, Vu THK, Shen CH, Kaur M, Chen G, Mathew R, McHam ML, Fazelat A, Lashkari K, et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat Commun. 2018;9(3209) doi: 10.1038/s41467-018-05681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.