Abstract
The global society is in a transition, where dealing with climate change and water scarcity are important challenges. More efficient separations of chemical species are essential to reduce energy consumption and to provide more reliable access to clean water. Here, membranes with advanced functionalities that go beyond standard separation properties can play a key role. This includes relevant functionalities, such as stimuli-responsiveness, fouling control, stability, specific selectivity, sustainability, and antimicrobial activity. Polyelectrolytes and their complexes are an especially promising system to provide advanced membrane functionalities. Here, we have reviewed recent work where advanced membrane properties stem directly from the material properties provided by polyelectrolytes. This work highlights the versatility of polyelectrolyte-based membrane modifications, where polyelectrolytes are not only applied as single layers, including brushes, but also as more complex polyelectrolyte multilayers on both porous membrane supports and dense membranes. Moreover, free-standing membranes can also be produced completely from aqueous polyelectrolyte solutions allowing much more sustainable approaches to membrane fabrication. The Review demonstrates the promise that polyelectrolytes and their complexes hold for next-generation membranes with advanced properties, while it also provides a clear outlook on the future of this promising field.
Keywords: review, polyelectrolytes, polyelectrolyte multilayers, membranes, functionality, polyelectrolyte complexes, advanced functionalities, ion selectivity
1. Introduction
Membranes find their place in countless applications from the production of drinking water to the separation of valuable pharmaceuticals, from gas sweetening to hemodialysis.1,2 As the demand for reliable and efficient separations increases, the membrane market will continue to grow. The membrane separation and technology market size was estimated as USD 17.5 billion in 2020 and is expected to grow to USD 25.2 billion by 2027.3 Since the 1960s, when the membrane industry was born, membrane technology has developed rapidly.1 Polymeric membranes have become the norm in sectors, such as desalination processes, and it has been demonstrated that these membranes can be applied for many challenging separations like the separation of azeotropic mixtures,4 the removal of micropollutants from wastewater streams,5 and high-temperature gas separations.6 With such a large range of well-developed membranes now being commercially available, it becomes important to think about the future of this field, how can we still push it forward? We foresee that the next milestone of this technology will be on the development of membranes with functionalities that go beyond standard separation (i.e., advanced functional membranes). Here, we define advanced functionalities as properties that ease the operation, that enhance the separation performance, or any other feature that makes the membrane preferable over others. In this context, they can also be seen as design parameters that follow naturally from the PE material properties. For example, the antifouling behavior of a membrane enables longer-term operations,7 stimuli-responsiveness provides great control over separation performance,8 while more sustainable membrane production is becoming a much more important factor when selecting a membrane for specific applications.9 Moreover, membranes with a highly specific separation behavior, for example ion selectivity, would allow novel membrane processes to recover valuable components,10−13 while membranes with improved stability can allow separations under the extreme conditions (pH, T, salinity etc.) sometimes required for industrial separations.13−17
One group of membrane materials that are especially promising to allow these advanced properties, are polyelectrolytes (PEs), polymers that have charged repeating units. Because of their charge, they are often soluble in water and when oppositely charged PEs interact, they can form insoluble polyelectrolyte complexes (PECs). Their unique properties make PEs very good candidates for building blocks of advanced functional membranes (Figure 1). For example, unlike typical polymers used in membrane production, PEs are hydrophilic due to their charged nature. Hydrophobic membranes suffer from fouling which decreases the production rate and increases the energy cost of the operation. On the other hand, hydrophilic polymers are less prone to foul and easier to clean.18 Hydrophilicity is also required for applications such as pervaporation19 (especially for dehydration of organic/aqueous mixtures) or oil–water separations.20 PEs are highly desired for these kinds of applications, not only because of their hydrophilic nature, but also due to the possibility to tune their features (e.g., swelling and charge density). Especially for weak PEs, where chain conformation can be easily controlled with external stimuli like pH, it is possible to tune the membrane performance by using these features. Moreover, besides other rejection mechanisms, charge exclusion will substantially contribute to the performance of the charged membranes. Indeed, even when the membrane pores are larger than the charged solute, high rejections can be achieved with the help of electrostatic repulsion.21,22 Interactions between the solute and the membrane is a major determining factor for separation properties and it is not necessarily be limited to electrostatic interactions. Certain PE-based membranes exhibit specific selectivities for certain compounds.23,24 For example, PEC multilayers of poly(sodium 4-styrenesulfonate)/(poly(diallyldimethylammonium chloride) (PSS/PDADMAC) are selective for fluoride,25 sulfate,26 and phosphate27 while polyionic liquids (PILs), a subgroup of PEs, show a good selectivity for CO2 depending on the molecular structure of the PILs.28,29 In applications including organic solvents, most of the polymeric membranes need a post-treatment to have good chemical resistance to the solvent. Polyelectrolyte complexes, however, are very stable in solvents because of their ionic cross-linking,30 and as a result, there are already many examples in literature for PEC membranes being used for pervaporation19 and organic solvent filtration.31 The chemical stability of PECs is not restricted to organic solvents, with a good selection of PEs it is possible to obtain membranes with remarkable stability against hypochlorite32 and extreme pH conditions.11,33,34
Figure 1.
PE- and PEC-based membrane systems and their general functionalities.
While some Reviews have focused on the production of PE-based membranes35 or their possible applications like electrolysis, electrodialysis, and fuel cells,36−38 so far there has been a lack of focus on the advanced functionalities that can be obtained by smart use of these promising materials. Therefore, in this review, we take a detailed look at the advanced functionalities that can be obtained using the large variety of PE- and PEC-based systems that have been developed in recent years. The Review brings together the recent literature on advanced functional PE-based membranes with the focus on the last ten years. In general, advanced functionalities stem from the unique features and material properties of PEs, these can also be considered as design parameters and can be utilized to tune membrane properties. In Figure 1, we show an overview of the systems and functionalities discussed in this work. First, we discuss the simplest use of polyelectrolytes as adsorbed monolayers, but in particular as PE brushes. These coatings are mostly used to change the surface properties (e.g., wettability and surface charge) of the coated membrane, leading to excellent examples of antifouling and responsive membranes. After this section, polyelectrolyte multilayer (PEM) coatings are discussed in two parts: PEM-coated porous substrates, where the PEM coating becomes the effective separation layer, and PEM-coated dense membranes, where the PEM coating helps to improve the separation performance. For the PEM coatings applied to dense membranes a large data set is compiled comparing ion selectivities of ion-exchange membranes in literature with an emphasis on the need for consistency in reported values. Subsequently, free-standing, integrally skinned PE-based membranes will be discussed, demonstrating that membranes can now also be prepared completely from polyelectrolytes. Finally, in the outlook we highlight the logical next steps and the significant opportunities that can be pursued for these systems, given their remarkable properties and chemical diversity. We believe that, by the end of this Review, it will be clear how remarkably useful PEs and their complexes are to create the next-generation of advanced functional membranes.
2. Single Polyelectrolyte Layers
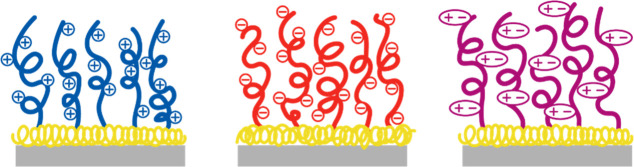
Single polyelectrolyte layers can be composed of either positively or negatively charged polymers. Moreover, the polymers in these single layers can bear both positive and negative charges in their side chains (zwitterionic polymers). In the following, we will consider these three types of polymers (Figure 2). These polymers can be applied via different techniques to the substrate to form single PE layers. They can be physisorbed or covalently bonded to the substrate to form PE films, for example, via catechol groups,39 and they can be prepared by spin coating40 or vapor deposition.41
Figure 2.
Three types of charged brushes are considered: cationic polymers (left), anionic polymers (middle), and zwitterionic polymers (right). The surface anchors (in yellow) to obtain long-term stable polyelectrolyte brushes (PEBs) are often composed of hydrophobic blocks and/or molecular structures that allow for multiple surface bonds. These anchors are not drawn to scale and in reality much thinner than the brush.
When the polyelectrolytes or zwitterionic polymers are attached with one chain-end to the substrate at a density that is high enough for the polymers to stretch away from the grafting plane, so-called polyelectrolyte brushes (PEBs)42−44 are formed. They can be prepared by grafting to45 or grafting from46 techniques. The conformation of PEs in brushes is different from neutral polymers in brushes. For neutral brushes, polymers stretch away from the surface due excluded volume interactions,47,48 while PEBs swell by the high osmotic pressure induced by trapped counterions.49,50 PEBs have gained a lot of attention because of their lubricious,51,52 antifouling53 and stimuli-responsive54,55 properties. For membrane applications, the latter two properties are of particular interest,56 so they will be treated in more detail in the next two sections, before discussing ways to increase the layer stability in section 2.3.
2.1. Fouling Control
Polymers that prefer to interact with the water molecules, instead of foreign elements are good candidates for antifouling brushes, since they will effectively repel all fouling matter.57,58 Many PEBs, such as poly(3-sulfopropyl methacrylate) (PSPMA) brushes and zwitterionic brushes, such as poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) swell strongly in water. Therefore, these brushes are often employed in antifouling coatings.59,60 Other polymers that are often utilized in antifouling coatings are based on sulfobetaines,61,62 carboxybetaines,63 and hydroxyl acrylamides.64 It has been shown that the latter displays the best antifouling performance against nonspecific adsorption of proteins, cells, and microorganisms.65 Antifouling brushes have been applied in different membrane systems, such as on forward osmosis66 and filtration67 membranes for oil–water separations or filtration membranes for (drinking) water treatment.68,69 More details on antifouling solutions on membranes will be discussed in sections 3.2, 4.2, and 5.4. It is, however, important to already mention here that while antifouling coatings can be very effective to reduce membrane fouling, reducing flux decline, they do tend to come with their own penalty to membrane permeance.
Recently, it has been realized that coatings with both antifouling and antimicrobial functions (Figure 3) are needed to effectively prevent biofouling60,70−72 and several strategies have been designed to achieve this. For example, binary brushes can be synthesized that are composed of antifouling polymers, such as PMPC and antimicrobial cationic poly(2-(methacryloyloxy)ethyl trimethylammonium chloride (alkynyl-PMETA) polymers with alkynyl functionalities.71 Often specific types of coatings need to be designed to prevent fouling by specific types of microbes. For instance, low-fouling brushes can be combined with components that kill bacteria, such as Cu ions60 or Ag nanoparticles,73 to prepare antibacterial coatings, while for antiviral coatings a cationic polymer, such as poly(ethylenimine) (PEI), can be utilized directly74 or functionalized with Cu or Ag particles for a better performance.75 The latter two examples have been shown to effective kill and repel viruses in microfiltration membranes for application in drink water with only a small decrease in the transport properties. Moreover, antimicrobial antifouling brush coatings have been applied in reverse osmosis membranes with good permeability.76
Figure 3.
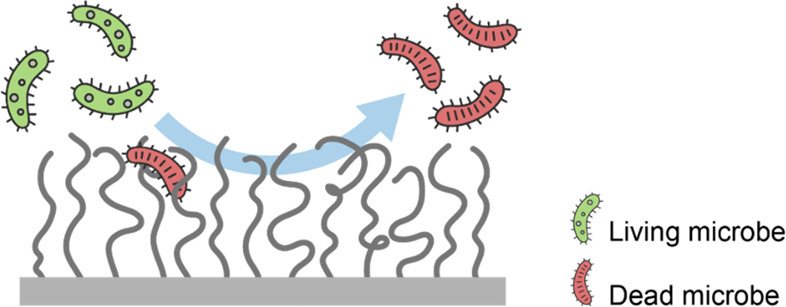
Polyelectrolyte brushes with both antifouling and antimicrobial function. Living microbes will be killed after contact with the brush due to the antimicrobial function, while the dead microbes are repelled by the antifouling function of the coating.
For optimal performance of antifouling PEBs, it is important to consider the design parameters of the brushes. The grafting density has a strong effect on the low-fouling performance of brushes.77,78 A higher grafting density will give rise to a higher polymer density, which increases the osmotic pressure. This makes it more difficult for foreign bodies to penetrate the brush,79−81 though exceptions can be expected for penetrants that are charged.82 In situations where it is difficult to prepare brushes with high grafting densities, branched or comb-polymer brushes can provide an alternative route to obtain high polymer density and, thus, increased effectiveness in preventing fouling.83 Dispersity of the polymers in the brush will alter the polymer density distribution from approximately parabolic to convex.84−86 Since this change reduces the polymer density near the brush surface, the antifouling performance against small particles will be reduced as well.87,88 However, the opposite can occur for large particles, since polydisperse brushes are more difficult to compress.87
2.2. Responsiveness
The conformation of PEs depends strongly on the environment and can be controlled by parameters, such as the ionic concentration or the pH. For example, poly(acrylic acid) (PAA) changes its conformation depending on the pH.89 In basic solutions, the pendant acidic groups deprotonate and charges are created in the side chains. Consequently, the water affinity increases and the polymer swells. In contrast, in an acidic solution, PAA chains collapse because of the protonation of the carboxyl groups. In the form of PEBs, strong interchain effects can enhance the responsiveness and strongly changes the swelling of these brushes. Therefore, the brushes can show a dramatic response to stimuli, such as pH and salt concentration. While PEBs of strongly disassociating PEs, such as PSS tend to be rather insensitive to the pH,42 they respond strongly to the presence of ions.90 Brushes composed of weak PEs, such as PAA, tend to respond to both the pH and the ionic strength of the solution.91 Besides the solvent composition, electric fields can also be utilized to control the conformation of PEBs.92 Since this stimulus can be applied externally, without the need to change the solution conditions, it provides an easy and effective control. The responsiveness of PEBs has made them popular systems for the design of functional surface coatings, because the swelling state of the brushes controls the surface properties of the coating.55 Therefore, they can be used in the development of smart adhesives,93−95 switchable lubricants,96,97 and for wetting control.98,99 Moreover, it allows for the controlled release of fouling components,100 which can be particularly useful in membrane applications. In addition, responsiveness of PEBs to the pH can be employed to tune the oil-adhesiveness of these coatings,101 which is relevant for oil–water separations. More examples of the usage of PE responsiveness in specific membrane applications will be given in sections 3.3 and 5.3.
2.3. Stability
Because of the strong hydration capability of PEBs, the polymers can be strongly stretched, which introduces enhanced tension near the anchor points.102 Moreover, water can reach any hydrolysis-sensitive surface bonds.103,104 This can potentially lead to degrafting of the PEBs, as has been observed for PAA brushes attached to Si wafers kept in 0.1 M ethanolamine buffer (pH 9.0) with 0.5 M NaCl.105 Similar degrafting has been observed for carboxylated poly(oligo(ethylene glycol) methacrylate-random-2-hydroxyethyl methacrylate) (poly(OEGMA-r-HEMA)) brushes grafted from metal surfaces and kept in phosphate buffered saline solutions106 and even for PSPMA brushes attached to Si wafers, exposed to humid air.107 To prevent such degrafting of PEBs, several strategies for strong anchoring of the brushes have been developed (see Figure 2). They are based on the incorporation hydrophobic structures to prevent water from reaching hydrolysis-sensitive bonds or on increasing the number surface bonds. For example, when grafting block copolymers from surfaces that consist of hydrophobic blocks close to the surfaces and hydrophilic blocks exposed to the aqueous liquid,108−111 the hydrophobic blocks will collapse and protect the sensitive surface bonds. Alternatively, enhanced stability can be achieved by utilizing hydrophobic macroinitiators,112,113 tannic acids,72 or mussel-adhesive inspired catechol-based anchoring layers,71,114 that allow for multiple surface bonds. In particular, poly(glycidyl methacrylate) (PGMA) based surface anchors have been shown to be promising solutions for membrane applications.112 PMPC brushes grown from these anchors keep their hydrophilicity even after immersion for 100 000 ppm hours in sodium hypochlorite solution. These strong surface anchors are much thinner (0.5–3 nm) than the PEBs (10–100 nm) and are, therefore, not expected to affect the transport properties of membranes.
3. Polyelectrolyte Multilayers on Porous Substrates
Self-assembly of polyelectrolytes via electrostatic interactions can be used to build up multilayered materials with unique functionalities. Already in 1997, Decher demonstrated that the alternating exposure of a charged surface to positive and negative polyelectrolyte solutions, allows for layer-by-layer (LbL) deposition of thin films of polyelectrolytes, so-called polyelectrolyte multilayers (PEMs).115 The versatility of PEM fabrication via LbL technique on flat surfaces, as well for hollow multilayer capsules, allows for a class of stimuli-responsive materials with a wide range of applications, such as microreactors, microsensors, and drug delivery, for medicine, cosmetics, and pharmaceutics.116 Such stimuli-responsive multilayers, which exhibit specific response to changes in the environmental conditions, like pH, temperature, ionic strength, magnetic field or light,116 are still having a huge impact on today’s chemistry, physics, biology, and materials science.117
Particularly, in the last 10 years, the knowledge on LbL assembly of PEMs on porous supports, as schematically illustrated in Figure 4a for hollow fiber nanofiltration (NF) membranes, has been translated into application35 and production of commercial membranes with advanced separation properties and functionalities.36 PEM coatings allow for a nanometer-control over the membrane active layer thickness and chemistry.132−134 In particular, the availability of different polyelectrolytes as building blocks115,121 as well as coating conditions (e.g., salinity135,136 and pH137,138) allow the production of thin films with engineered functionality for multiple membrane applications, such as ion selectivity,139 fouling control,140 stability against harsh wastewaters,34,141 removal of contaminants from water,142,143 and responsiveness.144
Figure 4.
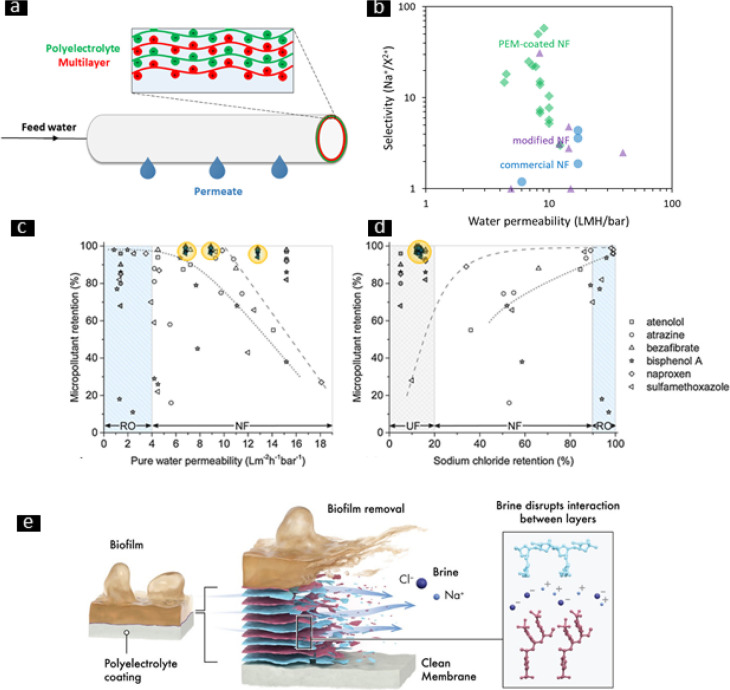
(a) Illustration of a PEM application on hollow fibers for membrane filtration. (b) Comparison of membrane divalent ion-selectivity (Na+/X2+) and water permeability (L·m–2·h–1·bar–1) as a function of different surface modifications from literature. Green diamonds represent single data points for PEM-coated nanofiltration (NF) membranes,118−121 purple triangles modified NF membranes,122−129 and blue circles commercial NF membranes.119,122,123 (c) Permeability vs micropollutant retention and (d) sodium chloride retention vs micropollutant retention for asymmetric and symmetric PEM-coated membranes in comparison with commercial NF and reverse osmosis (RO) membranes. White symbols, commercial membranes; gray symbols, symmetric PEM-coated membranes from Brinke et al.;130 dark symbols inside yellow circles, asymmetric membranes from Brinke et al.;130 dashed lines, best single results obtained with commercial membranes; dotted lines, best single results obtained with commercial membranes for bisphenol A. (e) Sacrificial layer concept applied to control biofilm growth. (c and d) Reproduced with permission from ref (130). Copyright 2020 Elsevier. (e) Reproduced with permission from ref (131). Copyright 2020 Elsevier.
Despite the good separation properties reached by commercial membranes in the past decade, PEM-coated membranes provide a class of advanced functionalities which would benefit the membrane field. Below, we discuss in detail the most relevant ones, with a focus on unique separations, fouling control, responsiveness, and stability.
3.1. Specific Selectivity
A tailored ion selectivity is still believed to be the holy grail of membrane filtration processes. Commercial membranes do exhibit high water-salt selectivity, but their ability to discriminate between different types of ions is still limited.145 Nevertheless, PEM-coated membranes can already tackle challenging separations, including the separation of mono- and divalent ions.121,139,143,146
Cheng et al. demonstrated that a (PDADMAC/PSS)5.5-coated commercial NF membrane at low salinity (50 mM) can selectively remove several divalent cations (X2+), such as Mg2+, Ca2+, Sr2+, and Ba2+, from feedwater with mild salinity, like brackish water.119 The rejection of divalent cations and therefore the Na+/X2+ selectivity of the NF membranes allows for highly selective separations of divalent cations and anions,143,146 which are the main culprits in inorganic scaling.119
In Figure 4b, PEM-coated membranes118−121 are directly compared to commercial NF membranes and to NF membranes prepared by other modification techniques, such as polymer grafting,122 atomic and molecular layer deposition,124,125 graphene oxide,127 and carbon nanotubes incorporation.126 It can be clearly seen that the Na+/X2+ selectivities of PEM-based membranes are higher than those of commercial NF membranes, such as the popular DOW N90122 and NF270,123 while retaining good water permeabilities. Clearly, PEM assembly is an attractive surface modification, which allows for outstanding mono-/divalent ion selectivity also compared to the competing surface modifications.122−129
Specific membrane selectivities are also urgently needed to combat the increasing concentrations of emerging contaminants in the waste and surface waters. Persistent, nonbiodegradable, and bioaccumulative contaminants in surface waters, also known as micropollutants (MPs), pose a severe threat to human health.147 While the densest available reverse osmosis (RO) membranes can remove these contaminants sufficiently, they do so at very low water permeability and by producing a highly saline, difficult to treat, MP waste stream.148
Wang et al. recently fabricated and applied PEM-coated NF membranes with tailored selectivity for the effective removal of MPs from saline wastewaters, allowing a relatively high passage of salt, including for scale-forming divalent cations.143 Brinke et al. succeeded in the preparation of a new class of membranes with unique separation properties, called “Chimera”, via asymmetric assembly of PEMs.130 Coating first an open PSS/poly(allylamine hydrochloride) (PAH)-based multilayer to prevent defects, and second, a thin and dense PAA/PAH-based multilayer for fine-tuned separation properties, makes it possible to design these novel membranes with outstanding retention of micropollutants (98%, Figure 4c) and high water permeability (up to ∼13 L·m–2·h–1·bar–1), outperforming commercial membranes.130 An additional advantage of these asymmetric PEM membranes is their low salt retention (Figure 4d) as salt does not accumulate in the MP-rich stream, facilitating the postfiltration treatment.
We can conclude that coating of porous membranes with PEMs allows for the fabrication of nanofiltration membranes with advanced separation properties compared to traditional membranes. In Table 1, we report various polyelectrolyte systems that, coated on ceramic and polymeric supports, allow for challenging separations of ions, pollutant removal, and water purification. Here, we also report the coating and process conditions to help the reader to evaluate the performance of such PEM-coated membranes. The examples reported here highlight the large variety of possible applications of PEM-based membranes in the field of nanofiltration and, therefore, their significant potential for commercialization.
Table 1. Overview of Coating Conditions and Performance of Commonly Used Polyelectrolyte Systems Successfully Applied on Various Membrane Supports for the Fabrication of PEM-Based NF Membranesa.
| PEs | coating conditions | support | process conditions | performance | ref |
|---|---|---|---|---|---|
| PDADMAC/PSS | (PSS/PDADMAC)4.5 dip coating with 0.02 M polymer and 0.5 M NaCl | PES UF membrane | crossflow filtration 4.8 bar | 13.9 L·m–2·h–1·bar–1 | (149) |
| flow rate: 18 mL/min | 95.9% SO42– rejection | ||||
| 32 chloride/sulfate selectivity | |||||
| (PDADMAC/PSS)6.5 dip coating with 0.1 g/L polymer and 0.5 M NaCl | dense UF HF SPES membrane | crossflow filtration | 10.3 L·m–2·h–1·bar–1 | (135) | |
| Re ∼ 3800 | 79% CaCl2 rejection | ||||
| (PDADMAC/PSS)7 dip coating with 0.1 g/L polymer and 0.05 M NaCl | dense UF HF SPES membrane | crossflow filtration | 15.6 L·m–2·h–1·bar–1 | (135) | |
| Re ∼ 3800 | 71% NaCl and 96% Na2SO4 rejection | ||||
| (PDADMAC/PSS)5.5 dip coating with 0.1 g/L polymer and 0.2 M NaCl | TFC NF membrane | crossflow filtration, 3.45 bar | 8.48 L·m–2·h–1·bar–1 | (119) | |
| crossflow velocity 21.4 cm/s | 97% Mg2+ rejection | ||||
| >30 Na+/Mg2+ selectivity | |||||
| (PDADMAC/PSS)4 dip coating with 0.1 g/L polymer and 0.2 M NaCl | PSf HF UF membrane | crossflow filtration, 6.9 bar | 16.1 L·m–2·h–1·bar–1 | (150) | |
| crossflow velocity 21.4 cm/s | 90% emerging organic contaminants removal | ||||
| <20% scale forming ions passage | |||||
| (PDADMAC/PSS)8 dip coating with 0.1 g/L polymer, 0.05 M NaCl, and pH 6.0 | PES UF membrane | crossflow filtration, 5 bar | excellent stability with 10.7 L·m–2·h–1·bar–1 | (34) | |
| crossflow velocity 1.1 m/s | 95.5% MgSO4 rejection | ||||
| 279 g/mol MWCO after ∼1600 h exposure to both 0.1 M HNO3 and 0.1 M NaOH | |||||
| (PDADMAC/PSS)8 dip coating with 0.02 M polymer, 0.5 M NaCl | porous alumina (UF) | crossflow filtration, 4.8 bar | ∼20.8 L·m–2·h–1·bar–1 | (27) | |
| pH 8.4 | 98% phosphate rejection | ||||
| flow rate: 18 mL/min | 48 chloride/phosphate selectivity | ||||
| PAH/PSS | cross-linked (PAH/PSS)8 dynamic LbL coating with 1 g/L polymer, 0.5 M NaCl, pH ∼ 7 and coating flux 30 L·m–2·h–1; 1 wt % GA solution filtrated at 2.5 bar for 2 h | tubular ceramic α-Al2O3 MF membrane | crossflow filtration, 4 bar | MWCO 170 Da | (151) |
| Re ∼ 6000 | >90% NaCl rejection | ||||
| (PSS/PAH)5 dip coating with 0.02 M PSS at 0.5 M NaCl and pH 2.3, and 0.02 M PAH at 1 M NaCl and pH 2.3 | porous alumina (UF) | crossflow filtration, 4.8 bar | ∼7 L·m–2·h–1·bar–1 | (121) | |
| flow rate: 18 mL/min | 95% MgCl2 rejection | ||||
| 22 Na+/Mg2+ and 50 Na+/Ca2+ selectivity | |||||
| (PSS/PAH)7 dip coating with 0.02 M PSS at 0.5 M NaCl and pH 2.1, and 0.02 M PAH at 0.5 M NaCl and pH 2.3 | porous alumina (UF) | crossflow filtration, 4.8 bar | ∼11.3 L·m–2·h–1·bar–1 | (152) | |
| flow rate: 18 mL/min | 50 glycine/l-glutamine selectivity | ||||
| (PSS/PAH)4.5 dip coating with 0.02 M PSS at 0.5 M MnCl2 and pH 2.1, and 0.02 M PAH at 0.5 M NaBr and pH 2.3 | porous alumina (UF) | crossflow filtration, 4.8 bar, | 99.4% sucrose and >99.9% reactive dyes rejection with small NaCl rejections (∼29%) | (153) | |
| flow rate: 18 mL/min | |||||
| (PSS/PAH)10 dip coating with 0.1 g/L polymer and 0.05 M NaCl; pH 5.5 for PSS and pH 2.0 for PAH | positively charged tight HF UF membrane | crossflow filtration, 6.2 bar | 9 L·m–2·h–1·bar–1 | (154) | |
| crossflow velocity 1 m/s | >95% MgCl2 rejection | ||||
| 267 g/mol MWCO | |||||
| (PAH/PSS)8 dip coating with 0.1 g/L polymer, 0.05 M NaCl, and pH 6.0 | PES HF UF membrane | crossflow filtration, 5 bar | excellent stability with 9.7 L·m–2·h–1·bar–1 | (34) | |
| crossflow velocity 1.1 m/s | 97.5% MgSO4 rejection | ||||
| 249 g/mol MWCO after ∼1600 h exposure to 0.1 M HNO3 | |||||
| (PSS/PAH)8.5 dip coating with 20 mM polymer, 0.5 M NaCl; pH 6.5 for PSS and pH 3.0 for PAH | modified MF alumina | dead-end filtration | 4.8 L·m–2·h–1·bar–1 | (155) | |
| other conditions not available | 50.5 1-butyl-3-methylimidazolium chloride/cellobiose selectivity | ||||
| PAH/PAA | (PAH/PAA)6 dip coating with 0.1 g/L polymer, 0.005 M NaNO3, pH 6 | dense HF SPES membrane | crossflow filtration, 1.5 bar | 48–80% MPs retention with low salt rejection | (156) |
| crossflow velocity 4.5 m/s | (∼17% NaCl) | ||||
| (PAH/PAA)15 dip coating with 0.1 g/L polymer, 0.005 M NaNO3, pH 6; annealed in 100 mM NaNO3 | hydrolyzed PAN membrane | dead-end filtration, 2 bar | 11.8 L·m–2·h–1·bar–1 | (157) | |
| flow rate: 1 mL/min | 69.8% HPO42– rejection | ||||
| (PAH/PAA)10.5 dip coating with 1 g/L polymer, 0.01 M NaCl, pH 6 | PES UF membrane | crossflow filtration, 1.8 bar | 3.6 L·m–2·h–1·bar–1 | (158) | |
| crossflow velocity 0.63 m/s | 44% Cu2+ rejection | ||||
| pH 2 | |||||
| PEI/PSS | (PSS/PEI)10 dip coating with 0.1 g/L polymer and 0.05 M NaCl, and pH 5.5 for PSS and pH 2.0 for PEI | positively charged tight HF UF membrane | crossflow filtration, 6.2 bar | 4 L·m–2·h–1·bar–1 | (154) |
| crossflow velocity 1 m/s | >95% Na2SO4 rejection | ||||
| 239 g/mol MWCO | |||||
| (PSS/PEI)10 spin-coated at 3000 rpm with 0.2 mL/s of 0.02 M polymer, 0.05 M NaCl, and pH 8.0 | PSf UF membrane | crossflow filtration, 10 bar | 0.9 L·m–2·h–1·bar–1 | (159) | |
| crossflow velocity 0.65 m/s | 94% MgCl2 rejection | ||||
| PAS/PSS | (PSS/PAS)10 dip coating with 0.1 g/L polymer and 0.05 M NaCl; pH 5.5 for PSS and pH 2.0 for PAH | positively charged tight HF UF membrane | crossflow filtration, 6.2 bar | 22 L·m–2·h–1·bar–1 | (154) |
| crossflow velocity 1 m/s | >95% Na2SO4 rejection | ||||
| 713 g/mol MWCO | |||||
| PAH/PVSA | (PAH/PVSA)8 dynamic LbL coating with 1 g/L polymer, 0.5 M NaCl, pH ∼ 7 and coating flux 30 L·m–2·h–1 | tubular ceramic α-Al2O3 UF membrane | crossflow filtration, 4 bar | MWCO 115 Da | (151) |
| Re ∼ 6000 | >90% NaCl rejection | ||||
| PVA/PVS | (PAH/PVS)8 dip coating with 0.01 M polymer, 1 M NaCl, pH 1.7 | PAN/PET membrane | dead-end filtration, 5 bar | 84% NaCl and 96% Na2SO4 | (160) |
| PEI/PAA | cross-linked (PEI/PAA)1.5 dynamic LbL coating with 3 g/L PEI and 0.6 g/L PAA; immersion in 3 wt % GA solution for 20 min | hydrolyzed PAN membrane | crossflow filtration, 4 bar | 2.27 L·m–2·h–1·bar–1 | (161) |
| flow rate: 40 L/h | 95% MgCl2 rejection |
HF: Hollow fiber. MF: Microfiltration. NF: Nanofiltration. Re: Reynolds number. TFC: Thin-film composite. UF: Ultrafiltration.
3.2. Fouling Control
One of the major challenges of any membrane filtration is the occurrence of membrane fouling. This phenomenon leads to an increase in operating costs162 and the need for chemical cleaning of the membrane,163 which in turn compromises the membrane stability and permeate quality over time.164 As membrane fouling is a phenomenon that occurs at the water-membrane interface, membrane surface chemistry plays a crucial role in fouling.165
One of the main advantages of PEM coatings is the great deal of control over the membrane surface chemistry, which in turn helps to alleviate membrane fouling.166 For example, Fadhillah et al. produced (PDADMAC/PSS)-based NF membranes with relatively high fouling resistance to protein filtration, presenting only a 10% decrease in permeation flux from its initial value (3.32 L·m–2·h–1·bar–1).167 In addition, Virga et al. recently prepared PEM-based NF membranes with a zwitterionic poly(2-methacryloyloxyethyl phosphorylcholine-co-acrylic acid) (PMPC-co-AA) top layer to filtrate surface water with different contaminants (e.g., proteins, polysaccharides, colloidal nanoparticles, and humic acids).168 These membranes with bioinspired zwitterionic phosphorylcholine coatings exhibit excellent fouling resistance (with a flux decline <5% of its original value, that is, 5.45 L·m–2·h–1·bar–1) and stable selectivity during filtration of surface water.
A different approach used to alleviate fouling focuses on the buildup of “sacrificial” multilayers based on PEs that can be removed, together with the fouling layer, to facilitate the membrane cleaning process,140,169 as illustrated in Figure 4d. The PEM removal is triggered by a quick change in pH,169 surfactant content,140 or ionic strength131 of the feedwater, allowing for organic fouling and biofouling control, both in spiral wound and hollow fiber membrane systems. Finally, the multilayer can be rebuilt on the membrane surface to restore its separation properties.
3.3. Responsiveness
Polyelectrolytes are also well-known to be used as building blocks for responsive116 and self-healing170 materials. Recently, Jiang et al. prepared pH-responsive (poly(methacrylic acid)/poly(alkyl methacrylate))n (PMAA/PAMA) multilayers with tunable interfacial properties,171 while Xu et al. used block copolymer micelles (BCM) and hyaluronic acid (HA) biopolymers to develop temperature-responsive, hydrogen-bonded multilayers.172 However, multilayers can also be salinity-responsive. Irigoyen et al. reported that polyelectrolyte multilayers based on (PDADMAC/PSS)n assembled at 3 M NaCl can reduce their thickness of 46% at low ionic strength, offering interesting applications such as controlled barrier for saline streams.173 Lately, incorporating a zwitterionic polymer via LbL technique, de Grooth et al. prepared (poly(sulfobetaine methacrylate) (PSBMA)/PDADMAC)n electrolyte-responsive membranes.174 They observed an increase in membrane permeability of even more than 100% at 1.5 M NaCl, mostly due to the increased swelling of the zwitterionic layers at high salinity. Such responsivity can potentially be used to facilitate the membrane backwash and, therefore, its cleaning.
3.4. Stability
Membranes are often used to treat challenging wastewaters, where the stability of traditional membranes can become compromised. PEMs allow the production of very stable membranes, which can go beyond that of commercial alternatives. Researchers have investigated different polyelectrolyte chemistries and stabilization strategies, including chemical cross-linking, to prepare stable PEM-coated membranes that can withstand challenging filtration conditions where the presence of organic solvents,31 extreme pH conditions,34 and high salinity175 make difficult the application of commercial membranes.
In particular, Li et al. showed that hydrolyzed polyacrylonitrile supports coated with a sulfonated poly(ether ether ketone) (SPEEK/PDADMAC)n multilayer to be very promising and stable for the filtration of challenging aprotic organic solvents, such as THF and DMF.176 Elshof et al. demonstrated how (PDADMAC/PSS)n-based NF poly(ether sulfone) membranes show excellent and stable performances even under long-term exposure to extreme pH conditions, that is, 1 M HNO3 (pH ≈ 0) and 1 M NaOH (pH ≈ 14).34 Even after more than two months of exposure, the membrane performance was still stable, with a pure water permeability of 10.7 L·m–2·h–1·bar–1, 95.5% MgSO4 retention and molecular weight cutoff of 279 g·mol–1. Furthermore, as cleaning processes can also affect the stability of the membranes, different researchers have demonstrated that PEMs based on strong polyelectrolytes, such as PDADMAC and PSS, can withstand physical (e.g., backwash)32,177 and chemical (via hypochlorite)32 cleaning.
When weak polyelectrolytes are used to build the multilayer131 or when the wastewater to treat contains small, charged molecules (like surfactants),178 PEM-based membranes can suffer from stability issues. In these cases, the stability of PEM membranes can be further increased via covalent cross-linking.151,175,179,180 PAH/PSS-coated poly(ether sulfone) membranes, stabilized via chemical cross-linking, can for example be used to successfully treat challenging wastewaters containing surfactants, such as produced water.181
4. Polyelectrolyte Multilayers on Dense Membranes
Similar to the formation of PEMs on porous supports, PEMs have also been built on dense membranes such as reverse osmosis membranes (ROMs) and various ion-exchange membranes (IEMs), including cation-exchange (CEM), anion-exchange (AEM), Nafion, and bipolar membranes (BPMs). Although such dense membranes are already designed to be selective for a certain type of ions (IEMs) and/or show size-based exclusion properties,182 incorporation of PEMs can further tune their selectivity or impart other desired functionalities.36
4.1. Specific Selectivity
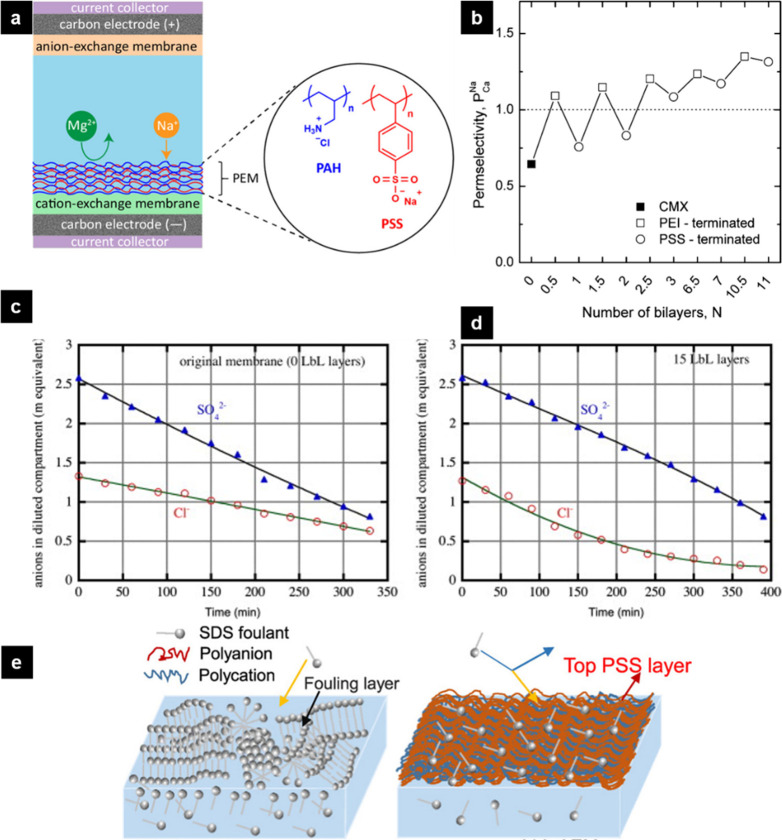
Following the successes of PEM coatings on porous supports, one of the most common applications of PEM-coated dense membranes is tuning the ion selectivity of IEMs in desalination processes. Figure 5a demonstrates the main mechanism to achieve monovalent/divalent cation selectivity via PEM-coated dense membranes. The alternating adsorption of PAH and PSS on a standard cation-exchange membrane can tune the monovalent cation selectivity in desalination. In 2014, Abdu et al. modified a standard-grade CEM (CMX) with an LbL assembly of PEI and PSS and implemented the PEM-coated membrane in an electrodialysis (ED) system.184 The (PEI/PSS)n coating resulted in a higher Ca2+ rejection compared to the pristine CMX and moderate increase in ohmic resistance (PEM-CMX = 60.06 Ω·cm2, CMX = 45.25 Ω·cm2) Figure 5b and 5c depict the change the chloride concentration in the diluted compartments for the bare and PEM-coated anion-exchange membranes. In case of PEM-coated AMX, the chloride concentration is lower than the one with bare AMX, meaning an increase in chloride over sulfate selectivity. Therefore, the addition of a PEM is a feasible way of introducing selectivity in ED. Furthermore, the terminating layer makes a small but distinct difference in selectivity: PEI-terminated layers demonstrated higher Na+/Ca2+ selectivity values compared to the PSS-terminated layers (∼18% increase). The selectivity was rationalized by the charge exclusion of divalent cations and the higher hydrophobicity of the PEI-terminated CMX. 6.5 bilayers of PEI/PSS were enough to reach a permselectivity value (PCaNa) of 1.24, which is comparable with the commercial monovalent cation-selective membrane (CMS) PCa = 1.23). Also, PEM-CMX required ∼50 Wh/mol Na+ while CMS required ∼80 Wh/mol Na+, meaning that similar selectivity values can be achieved with a lower energy consumption value by using PEM-coated CMX. In another ED study, White et al. implemented (PSS/PAH)n layers on a Nafion 115 membrane and obtained K+/Mg2+ selectivity as high as >1000.187 In 2016, White et al. coated (PAH/PSS)5.5 on Nafion 117 to achieve Li+/Co2+ and K+/La3+ selectivities in ED.188 Compared to the mono/divalent cation selectivity of bare Nafion (<2), they reported Li+/Co2+ and K+/La3+ selectivities exceeding 1000. Yang et al. used the same approach in Donnan dialysis with (PAH/PSS)5.5-coated Nafion 115 membrane to differentiate within the monovalent cations and reached K+/Li+ selectivity values between 8 and 60,78 although later the authors reported lower selectivities due to variations in different batches of the Nafion membranes.189 The selectivity was attributed to the larger hydrated radius of Li+ that resulted in a lower diffusion coefficient through the dense PEM layer. Besides, a pH-induced swelling resulted in a further increase of the K+/Li+ selectivity, which was believed to be related to an increased accessibility of cation-exchange sites within the PEM at lower pH. In 2019, Rijnaarts et al. further investigated the mechanism of monovalent cation selectivity in ED and explained that after 8 layers of PAH/PSS the multilayer starts to have excess of PAH in the multilayer.190 The overall positive charge, due to excess PAH, increased with higher number of layers, resulting in increased charge-exclusion toward divalent cations. Sahin et al. reported a similar observation where bare CMX demonstrated selectivity to Mg2+, while the addition of a PEM resulted in a Na+/Mg2+ selectivity of ∼3 in a capacitive deionization (CDI) system due to the charge-exclusion effect of the PEM toward Mg2+ ions.183 The positively charged PEM rejected the Mg2+ more than Na+, resulting in monovalent cation selectivity.
Figure 5.
(a) Illustration of a PEM application on a standard cation-exchange membrane to achieve Na+/Mg2+ selectivity in capacitive deionization. PAH and PSS stand for poly(allylamine hydrochloride) and poly(styrenesulfonate), respectively. Reproduced with permission from ref (183). Copyright 2020 American Chemical Society. (b) Trend in permselectivity (P) as a function of the type of the terminating layer. CMX and PEI stand for standard cation-exchange membrane and poly(ethylenimine), respectively. The empty squares represent the PEI-terminated and the empty circles represent the PSS-terminated multilayers. Reproduced with permission from ref (184). Copyright 2014 American Chemical Society. (c) Change in the anion concentration in time in the diluted compartments of the experiments with the bare membrane and (d) the membrane with 15 layers. (c and d) Reproduced with permission from ref (185). Copyright 2013 Elsevier. (e) Comparison of the bare (left) and the PEM-coated (right) membranes for SDS (sodium dodecyl sulfate) fouling. Reproduced with permission from ref (186). Copyright 2018 Elsevier.
PEMs were also coated on AEMs to achieve mono-/divalent anion selectivity in multiple studies, including ED,185,191 reverse ED,192 dialysis,193 and CDI.194 For instance, Mulyati et al. used (PAH/PSS)7.5 on a standard-grade AEM (Neosepta, AMX) and achieved Cl–/SO42– selectivity in ED185 *Figure 5c and 5d). Recently, Cl–/SO42– selectivity between 7 and 14 was reported by using PDADMAC/PSS-coated AEM in CDI.194 The authors reported that Cl– selectivity was preserved even at low concentrations of Cl– in the solution. Additionally, recent studies reported on the use of biodegradable polyelectrolytes (for example, 2-hydroxypropyltrimethylammonium chloride chitosan, HACC191,195,196 and N–O-sulfonic acid benzyl chitosan, NSBC196) as alternatives for synthetic polyelectrolytes. Upon the addition of the resulting PEMs, the Cl–/SO42– selectivity increased.
So far, multiple ion selectivity definitions have been used by various research groups, which often limits the possibility to directly compare reported selectivity values. The most common selectivity definitions are listed in Table 2.
Table 2. Commonly Used Ion Selectivity Definitions in the Literature.
| symbol | equation | description | |
|---|---|---|---|
| Pji(184) or Sj190 | Ji and Jj are the flux (in mol·m–2·s–1) of the target and competing ions, respectively. ci and cj (in mol/L) are the concentrations of the target and the competing ion on the diluate side, respectively. | ||
| Fji(188),a | Ji and Jj are the flux (in mol·m–2·s–1) of the target and competing ions, respectively, when the source phase contains equal concentrations of the target and the competing ions. | ||
| ρji183 or βj197 | ci,0 and ci,f are initial and final concentrations of the target ion. cj,0 and cj,f are initial and final concentrations of the competing ion. | ||
| Rji(190) | Ri and Rj are the resistance (in Ω·cm2) of the target and the competing ion, respectively. |
In literature, there is no symbol used for this type of selectivity; here, we introduce F for matters of clarity.
There are several factors that may affect the ion selectivity of a PEM-modified membrane during desalination operations. Therefore, we summarized cation and anion selectivity studies in Tables 3 and 4, respectively, to understand the effects of these factors on selectivity. For each selectivity value of a modified or bare membrane, the number of layers in the PEM, the desalination method and the operational conditions, as well as the flux values (if applicable) are listed. Standard-grade AEMs and CEMs (i.e., Fujifilm type 1 AEM and CEM, Neosepta CSE and ASE, and CJMA-2 standard CEM from Hefei Chemjoy Polymer Material) were abbreviated as CMX and AMX in Tables 3 and 4, respectively. Special-grade cation-exchange membranes (CSO (Selemion) and CMS (Neosepta)), as well as anion-exchange membranes (ASV (Selemion) and ACS (Neosepta)), were added in the tables for comparison, as they are commercially available monovalent ion selective membranes.
Table 3. Overview of Selectivity Values and Experimental Details of the Reported Studies Towards Mono/Divalent Cationsa.
| entry | PEM/membrane | no. layers | method | conditions | flux (nmol·cm–2·s–1) | selectivity | ref |
|---|---|---|---|---|---|---|---|
| 1 | PEI/PSS on CMX | 21 | ED | CC, 15 mA/cm2, 0.05 M NaCl and 0.05 M CaCl2 | Ca2+: 44.0 | PCaNa = 1.35 | (184) |
| Na+: 60.1 | |||||||
| 2 | CMX | bare | ED | CC, 15 mA/cm2, 0.05 M NaCl and 0.05 M CaCl2 | Ca2+: 64.5 | PCaNa = 0.64 | (184) |
| Na+: 40.3 | |||||||
| 3 | CSO | bare | ED | CC, 15 mA/cm2, 0.05 M NaCl and 0.05 M CaCl2 | Ca2+: 35.2 | PCaNa = 1.72 | (184) |
| Na+: 60.8 | |||||||
| 4 | CMS | bare | ED | CC, 15 mA/cm2, 0.05 M NaCl and 0.05 M CaCl2 | Ca2+: 41.8 | PCaNa = 1.23 | (184) |
| Na+: 53.1 | |||||||
| 5 | PSS/PAH on Nafion 115 | 11 | ED | CC, 1.27 mA/cm2, 0.01 M KNO3 and 0.01 M Mg(NO3)2 | K+: 6.9 ± 0.2 | FMgK > 1000 | (187) |
| Mg2+: <0.005 | |||||||
| 6 | PSS/PAH on Nafion 115 | 11 | ED | CC, 2.54 mA/cm2, 0.01 M KNO3 and 0.01 M Mg(NO3)2 | K+: 6.28 ± 0.58 | FMgK = 22.1 ± 3.5 | (187) |
| Mg2+: 0.318 | |||||||
| 7 | PSS/PAH on Nafion 115 (1-side) | 11 | ED | CC, 2.54 mA/cm2, 0.01 M KNO3 and 0.01 M Mg(NO3)2 | ND | FMgK = 10.0 ± 3.8 | (187) |
| 8 | PSS/PAH on Nafion 115 | 11 | ED | CC, 2.54 mA/cm2, 0.02 M KNO3 and 0.02 M Mg(NO3)2 | K+: 13.5 ± 0.6 | FMgK = 96 ± 26 | (187) |
| Mg2+: 0.149 | |||||||
| 9 | PSS/PAH on Nafion 115 | 11 | ED | CC, 2.54 mA/cm2, 0.1 M KNO3 and 0.1 M Mg(NO3)2 | K+: 25.2 ± 1.6 | FMgK > 20 000 | (187) |
| Mg2+: < 0.001 | |||||||
| 10 | Nafion 115 | bare | ED | CC, 1.27 mA/cm2, 0.01 M KNO3 and 0.01 M Mg(NO3)2 | K+: 6.4 ± 0.3 | FMgK = 1.8 ± 0.1 | (187) |
| Mg2+: 3.6 ± 0.1 | |||||||
| 11 | PSS/PAH on Nafion 117 | 11 | ED | CC, 0.63 mA/cm2, 0.01 M LiNO3 and 0.01 M Co(NO3)2 | Li+: 2.95 ± 0.2 | FCoLi > 1600 | (188) |
| Co2+: 1.29 ± 0.51 (pmol cm–2 s–1) | |||||||
| 12 | PSS/PAH on Nafion 117 | 11 | ED | CC, 0.63 mA/cm2, 0.02 M LiNO3 and 0.02 M Co(NO3)2 | Li+: 3.18 ± 0.3 | FCoLi > 360 | (188) |
| Co2+: 2.55 ± 1.71 (pmol·cm–2·s–1) | |||||||
| 13 | PSS/PAH on Nafion 117 | 11 | ED | CC, 0.63 mA/cm2, 0.1 M LiNO3 and 0.1 M Co(NO3)2 | Li+: 6.79 ± 0.18 | FCoLi > 6500 | (188) |
| Co2+ < 1 (pmol·cm–2·s–1) | |||||||
| 14 | PSS/PAH on Nafion 117 | 4 | ED | CC, 0.63 mA/cm2, 0.01 M LiNO3 and 0.01 M Co(NO3)2 | Li+: 2.39 ± 0.10 | FCoLi > 430 | (188) |
| Co2+: 3.85 ± 2.49 (pmol·cm–2·s–1) | |||||||
| 15 | PSS/PAH on Nafion 117 | 3 | ED | CC, 0.63 mA/cm2, 0.01 M LiNO3 and 0.01 M Co(NO3)2 | Li+: 1.62 ± 0.10 | FCoLi > 23 | (188) |
| Co2+: 37.3 ± 25.5 (pmol·cm–2·s–1) | |||||||
| 16 | PSS/PAH on Nafion 117 | 11 | ED | CC, 0.63 mA/cm2, 0.01 M K(OAc) and 0.01 M La(OAc)3 | K+: 0.46 ± 0.27 | FLaK > 93 | (188) |
| La3+: 1.58 ± 1.00 (pmol·cm–2·s–1) | |||||||
| 17 | PSS/PAH on Nafion 117 | 11 | ED | CC, 0.63 mA/cm2, 0.02 M K(OAc) and 0.02 M La(OAc)3 | K+: 4.40 ± 0.02 | FLaK > 2400 | (188) |
| La3+: 1.27 ± 0.46 (pmol·cm–2·s–1) | |||||||
| 18 | PSS/PAH on Nafion 117 | 11 | ED | CC, 0.63 mA/cm2, 0.1 M K(OAc) and 0.1 M La(OAc)3 | K+: 7.85 ± 0.69 | FLaK > 7000 | (188) |
| La3+: < 1 (pmol·cm–2·s–1) | |||||||
| 19 | Nafion 117 | bare | ED | CC, 0.63 mA/cm2, 0.01 M LiNO3 and 0.01 M Co(NO3)2 | Li+: 1.9 ± 0.4 | FCoLi = 0.66 ± 0.08 | (188) |
| Co2+: 3.0 ± 0.7 | |||||||
| 20 | Nafion 117 | bare | ED | CC, 0.63 mA/cm2, 0.01 M K(OAc) and 0.01 M La(OAc)3 | ND | FLaK = 1.61 ± 0.26 | (188) |
| 21 | PSS/PAH on Nafion 115 | 11 | DD | 0.01 M KNO3 and 0.01 M LiNO3 + 0.01 M HNO3 in RP | K+: 2.01 ± 0.21 | up to FLiK = 57 ± 22 | (78, 189) |
| Li+: 0.039 ± 0.013 | |||||||
| 22 | PSS/PAH on Nafion 115 | 11 | DD | 0.02 M KNO3 and 0.02 M LiNO3 + 0.01 M HNO3 in RP | K+: 2.83 ± 0.31 | FLiK = 80 ± 9 | (78) |
| Li+: 0.035 ± 0.003 | |||||||
| 23 | PSS/PAH on Nafion 115 | 11 | DD | 0.1 M KNO3 and 0.1 M LiNO3 + 0.01 M HNO3 in RP | K+: 5.28 ± 0.69 | FLiK = 38 ± 13 | (78) |
| Li+: 0.25 ± 0.05 | |||||||
| 24 | PSS/PAH on Nafion 115 | 11 | DD | 0.01 M KNO3 and 0.01 M LiNO3 + 0.02 M HNO3 in RP | K+: 0.35 ± 0.06 | FLiK = 8.3 ± 1.8 | (78) |
| Li+: 0.047 ± 0.010 | |||||||
| 25 | Nafion 115 | bare | DD | 0.01 M KNO3 and 0.01 M LiNO3 + 0.02 M HNO3 in RP | K+: 4.97 ± 0.44 | FLiK = 1.7 ± 0.3 | (78) |
| Li+: 3.03 ± 0.36 | |||||||
| 26 | PSS/PAH on Nafion 115 | 11 | ED | CC, 0.64 mA/cm2, 0.01 M KNO3 and 0.01 M LiNO3 + 0.01 M HNO3 in RP | K+: 2.99 ± 0.13 | FLiK = 2.3 ± 0.1 | (78) |
| Li+: 1.33 ± 0.03 | |||||||
| 27 | Nafion 115 | bare | ED | CC, 0.64 mA/cm2, 0.01 M KNO3 and 0.01 M LiNO3 + 0.01 M HNO3 in RP | K+: 5.56 ± 0.81 | FLiK = 1.3 ± 0.1 | (78) |
| Li+: 4.19 ± 0.38 | |||||||
| 28 | PAH/PSS on CMX (recipe 1) | 11 | RM | 0.5 M NaCl, 0.5 M MgCl2 | ND | RMgNa = 5.7 | (190) |
| 29 | PAH/PSS on CMX (recipe 1) | 21 | RM | 0.5 M NaCl, 0.5 M MgCl2 | ND | RMgNa = 5.8 | (190) |
| 30 | PAH/PSS on CMX (recipe 2) | 11 | RM | 0.5 M NaCl, 0.5 M MgCl2 | ND | RMgNa = 7.8 | (190) |
| 31 | CSO | bare | RM | 0.5 M NaCl, 0.5 M MgCl2 | ND | RMgNa = 6.9 | (190) |
| 32 | CMX | bare | RM | 0.5 M NaCl, 0.5 M MgCl2 | ND | RMgNa = 3.5 | (190) |
| 33 | PAH/PSS on CMX (recipe 2) | 11 | ED | CV, 3.5 V, 25 mM NaCl and 10 mM MgCl2 | Na+≈ 3.5 × 104 | PMgNa = 1.7 | (190) |
| Mg2+≈ 0.5 × 104b | |||||||
| 34 | CMX | bare | ED | CV, 3.5 V, 25 mM NaCl and 10 mM MgCl2 | Na+ ≈ 2 × 104 | PMgNa = 0.5 | (190) |
| Mg2+ ≈ 1.5 × 104b | |||||||
| 35 | PAH/PSS on CMX | 11 | MCDI | CV, 0–1 V, 4 mM NaCl, 4 mM MgCl2 | ND | βMgNa = 2.8 ± 0.2 | (183) |
| 36 | CMX | bare | MCDI | CV, 0–1 V, 4 mM NaCl, 4 mM MgCl2 | ND | βMgNa = 0.5 ± 0.04 | (183) |
| 37 | CMS | bare | MCDI | CV, 0–1 V, 4 mM NaCl, 4 mM MgCl2 | ND | βMgNa = 0.4 ± 0.1 | (183) |
ED: Electrodialysis. DD: Diffusion dialysis. RM: Resistance measurement. MCDI: Membrane capacitive deionization. RP: Receiving phase. CC and CV represent a desalination process with constant current or constant voltage, respectively.
Estimated from the graphs reported in the cited references.
Table 4. Overview of Selectivity Values and Experimental Details of the Reported Studies Towards Mono-/Divalent Anionsa.
| entry | PEM/membrane | no. layers | method | conditions | flux (nmol·cm–2·s–1) | selectivity | ref |
|---|---|---|---|---|---|---|---|
| 1 | PSS/PAH on AMX | 15 | ED | CC, 2 mA/cm2, 0.01 M NaCl, 0.01 M Na2SO4 | ND | PSO4Cl ≈ 2.5b | (185) |
| 2 | AMX | bare | ED | CC, 2 mA/cm2, 0.01 M NaCl, 0.01 M Na2SO4 | ND | PSO4Cl ≈ 0.8b | (185) |
| 3 | PSS-MA and HACC (cross-linked) | 15 | ED | CC 15 mA/cm2, 50 mM NaCl and 50 mM Na2SO4 | ND | PSO4Cl = 4.81 | (191) |
| 4 | AMX | bare | ED | CC 15 mA/cm2, 50 mM NaCl and 50 mM Na2SO4 | ND | PSO4Cl = 0.81 | (191) |
| 5 | PSS/HACC on AMX | 18 | ED | CC, 5 mA/cm2, 0.02 M NaCl and 0.02 M Na2SO4 | ND | PSO4Cl = 2.9 | (200) |
| 6 | PSS/HACC on AMX | 14 | ED | CC, 5 mA/cm2, 0.02 M NaCl and 0.02 M Na2SO4 | ND | PSO4Cl ≈ 2b | (200) |
| 7 | PSS/HACC on AMX | 6 | ED | CC, 5 mA/cm2, 0.02 M NaCl and 0.02 M Na2SO4 | ND | PSO4Cl≈1.5b | (200) |
| 8 | AMX | bare | ED | CC, 5 mA/cm2, 0.02 M NaCl and 0.02 M Na2SO4 | ND | PSO4Cl=0.66 | (200) |
| 9 | PSS/HACC on AMX + cross-linked | 17 | ED | CC, 5 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | ND | PSO4Cl ≈ 3.8b | (195) |
| 10 | PSS/HACC on AMX + cross-linked | 11 | ED | CC, 5 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | ND | PSO4Cl = 4.36 ± 0.13 | (195) |
| 11 | PSS/HACC on AMX + cross-linked | 5 | ED | CC, 5 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | ND | PSO4Cl ≈ 1.5b | (195) |
| 12 | PSS/HACC on AMX | 11 | ED | CC, 5 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | ND | PSO4Cl ≈ 2.1b | (195) |
| 13 | AMX | bare | ED | CC, 5 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | ND | PSO4Cl = 0.39 ± 0.06 | (195) |
| 14 | NSBC/HACC on AMX | 15 | ED | CC, 10 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | ND | PSO4Cl = 47.04 | (196) |
| 15 | AMX | bare | ED | CC, 10 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | ND | PSO4Cl = 0.81 | (196) |
| 16 | ACS | bare | ED | CC, 10 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | ND | PSO4Cl = 13.6 | (196) |
| 17 | ASV | bare | ED | CC, 10 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | ND | PSO4Cl = 22.3 | (196) |
| 18 | PSS/PEI on CMX | 11 | RED | CC, 4.0 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | Cl–: 106.5 | PSO4Cl = 1.67 | (192) |
| SO42–: 50.1 | |||||||
| 19 | PSS/PEI on CMX | 15 | RED | CC, 4.0 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | Cl–: 106.0 | PSO4Cl = 2.44 | (192) |
| SO42–: 43.3 | |||||||
| 20 | PSS/PEI on CMX | 21 | RED | CC, 4.0 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | Cl–: 85.5 | PSO4Cl = 1.89 | (192) |
| SO42–: 42.1 | |||||||
| 21 | CMX | bare | RED | CC, 4.0 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | Cl–: 103.2 | PSO4Cl = 1.1 | (192) |
| SO42–: 95.3 | |||||||
| 22 | ACS | bare | RED | CC, 4.0 mA/cm2, 0.05 M NaCl and 0.05 M Na2SO4 | Cl–: 105.3 | PSO4Cl = 2.7 | (192) |
| SO42–: 35.1 | |||||||
| 23 | PSS/PAH on AMX | 11 | DD | 0.01 M NaCl and 0.01 M Na2SO4 | Cl–: 1.47 ± 0.20 | FSO4Cl = 5.3 ± 1.7 | (193) |
| SO42–: 0.31 ± 0.16 | |||||||
| 24 | PSS/PAH on AMX | 11 | DD | 0.1 M NaCl and 0.1 M Na2SO4 | Cl–: 8.14 ± 0.39 | FSO4Cl = 137 ± 31 | (193) |
| SO42–: 0.06 ± 0.01 | |||||||
| 25 | PSS/PAH on AMX | 11 | DD | 0.01 M NaCl and 0.1 M Na2SO4 | Cl–: 1.55 ± 0.06 | FSO4Cl = 27.9 ± 5.0 | (193) |
| SO42–: 0.57 ± 0.09 | |||||||
| 26 | PSS/PAH on AMX | 11 | DD | 0.1 M NaCl and 0.01 M Na2SO4 | Cl–: 7.40 ± 0.53 | FSO4Cl > 200 | (193) |
| SO42–: not detected | |||||||
| 27 | AMX | bare | DD | 0.01 M NaCl and 0.01 M Na2SO4 | Cl–: 6.12 ± 0.12 | FSO4Cl = 1.66 ± 0.08 | (193) |
| SO42–: 3.70 ± 0.22 | |||||||
| 28 | AMX | bare | DD | 0.1 M NaCl and 0.1 M Na2SO4 | Cl–: 30.0 ± 1.9 | FSO4Cl = 13.0 ± 0.4 | (193) |
| SO42–: 2.30 ± 0.19 | |||||||
| 29 | AMX | bare | DD | 0.01 M NaCl and 0.1 M Na2SO4 | Cl–: 2.85 ± 0.07 | FSO4Cl = 4.3 ± 0.1 | (193) |
| SO42–: 6.61 ± 0.12 | |||||||
| 30 | AMX | bare | DD | 0.1 M NaCl and 0.01 M Na2SO4 | Cl–: 35.16 ± 3.17 | FSO4Cl = 9.9 ± 1.0 | (193) |
| SO42–: 0.361 ± 0.064 | |||||||
| 31 | PSS/PAH on AMX | 11 | ED | CC, 1.13 mA/cm2, 0.01 M NaCl and 0.01 M Na2SO4 | Cl–: 6.72 ± 0.13 | FSO4Cl = 7.4 ± 0.6 | (193) |
| SO42–: 0.91 ± 0.09 | |||||||
| 32 | PSS/PAH on AMX | 11 | ED | CC, 1.13 mA/cm2, 0.1 M NaCl and 0.1 M Na2SO4 | Cl–: 19.37 ± 0.37 | FSO4Cl = 69.3 ± 5.2 | (193) |
| SO42–: 0.28 ± 0.02 | |||||||
| 33 | PSS/PAH on AMX | 11 | ED | CC, 1.13 mA/cm2, 0.01 M NaCl and 0.1 M Na2SO4 | Cl–: 4.54 ± 0.21 | FSO4Cl = 17.3 ± 2.4 | (193) |
| SO42–: 2.65 ± 0.28 | |||||||
| 34 | PSS/PAH on AMX | 11 | ED | CC, 1.13 mA/cm2, 0.1 M NaCl and 0.01 M Na2SO4 | Cl–: 18.38 ± 0.77 | FSO4Cl > 81 | (193) |
| SO42–: 0.018 ± 0.008 | |||||||
| 35 | AMX | bare | ED | CC, 1.13 mA/cm2, 0.01 M NaCl and 0.01 M Na2SO4 | Cl–: 7.38 ± 0.31 | FSO4Cl = 1.32 ± 0.01 | (193) |
| SO42–: 5.57 ± 0.26 | |||||||
| 36 | AMX | bare | ED | CC, 1.13 mA/cm2, 0.1 M NaCl and 0.1 M Na2SO4 | Cl–: 34.11 ± 1.63 | FSO4Cl = 10.9 ± 0.2 | (193) |
| SO42–: 3.13 ± 0.12 | |||||||
| 37 | AMX | bare | ED | CC, 1.13 mA/cm2, 0.01 M NaCl and 0.1 M Na2SO4 | Cl–: 3.62 ± 0.29 | FSO4Cl = 3.2 ± 0.1 | (193) |
| SO42–: 11.34 ± 0.57 | |||||||
| 38 | AMX | bare | ED | CC, 1.13 mA/cm2, 0.1 M NaCl and 0.01 M Na2SO4 | Cl–: 57.76 ± 5.04 | FSO4Cl = 8.4 ± 1.1 | (193) |
| SO42–: 0.695 ± 0.11 | |||||||
| 39 | PSS/PDADMAC | 15 | CDI | CV, (±)1 V, 10 mM NaCl, 10 mM Na2SO4 | ND | 7 < βSO4Cl < 14 | (194) |
| 40 | PSS/PDADMAC | 14 | CDI | CV, (±)1 V, 10 mM NaCl, 10 mM Na2SO4 | ND | βSO4Cl ≈ 2 | (194) |
| 41 | PSS/PDADMAC | 5 | CDI | CV, (±)1 V, 10 mM NaCl, 10 mM Na2SO4 | ND | 3 < βSO4Cl < 6 | (194) |
| 42 | PSS/PDADMAC | 23 | CDI | CV, (±)1 V, 10 mM NaCl, 10 mM Na2SO4 | ND | βSO4Cl ≈ 1b | (194) |
| 43 | PSS/PDADMAC | 14 | CDI | CV, (±)1 V, 10 mM NaCl, 10 mM Na2SO4 | ND | βSO4Cl ≈ 1.5b | (194) |
| 44 | AMX | bare | CDI | CV, (±)1 V, 10 mM NaCl, 10 mM Na2SO4 | ND | βSO4Cl ≈ 2 | (194) |
| 45 | ACS | bare | CDI | CV, (±)1 V, 10 mM NaCl, 10 mM Na2SO4 | ND | βSO4Cl ≈ 7 | (194) |
| 46 | PSS/PDADMAC | 15 | CDI | CV, (±)1 V, 10 mM NaCl, 10 mM NaH2PO4 | ND | 1.5 < βH2PO4Cl < 5.5b | (194) |
| 47 | PSS/PDADMAC | 15 | CDI | CV, (±)1 V, 10 mM NaCl, 10 mM NaNO3 | ND | βClNO3 ≈ 1.5b | (194) |
ED: Electrodialysis. RED: Reverse electrodialysis. DD: Diffusion dialysis. CDI: Capacitive deionization. CC and CV represent a desalination process with constant current or constant voltage, respectively. PSS-MA stands for poly(styrenesulfonic acid-co-maleic acid) sodium salt.
Estimated from the graphs reported in the cited references.
As stated above, building PEMs on dense membranes improves their mono-/divalent ion selectivities. The terminating layer has a major effect on monovalent ion selectivity since the main mechanism of selectivity is the charge exclusion of divalent ions. As can be seen in multiple entries of Table 3 (i.e., 1, 5–9, 11–13), the terminating layer needs to be the polycation in order to achieve monovalent cation selectivity. Similarly, the terminating layer should be the polyanion to achieve monovalent anion selectivity as shown in multiple entries (i.e., 1, 3, 5–7, 9–12, 14) in Table 4. In entry 10 of Table 3, bare Nafion 115 shows FMgK ≈ 2 selectivity and by using the same conditions, FMg > 1000 was achieved with PEM-coated Nafion 115. In an anion selectivity study (entries 1 and 2 of Table 4), a similar switch can be observed. Since the bare CMX and AMX have fixed negative and positive charges, respectively, divalent cations interact more with CMX, while AMX shows affinity toward divalent anions. Another important parameter for tuning ion selectivity is the number of layers in the PEM, as the increased charge density and thickness of the PEM can further increase the rejection of divalent ions. For instance, by increasing the number of PE layers from 3 to 11, FCoLi increased from >23 to >6500 (in entries 13–15 of Table 3). The effect of the number of layers on anion selectivity can be seen, for example, in entries 39–42 of Table 4, where the rejection of SO42– with 5 layers was reduced by a factor of ∼2 with 15 layers of PEs. However, increasing the number of layers to 23 did not improve the Cl– selectivity further. As explained in detail36,198 and demonstrated in numerous studies,183,185,190,194 the overcompensation of charge by the polycation can result in an excess of positive charges in the PEM. While, in case of monovalent cation selectivity, this is a desired effect, an overall positive charge can reduce the rejection of divalent anions and therefore result in a lower mono/divalent anion selectivity. The examples show that the type and the amount of charge of the PEM and the valence of ions can determine the affinity of the PEM toward ions. Next to the effect of charge (type and valency), also the hydration energy of ions is a key factor in selectivity. For instance, K+/Li+ (entry 21, Table 3), NO3–/Cl– and H2PO4– (entries 46 and 47, Table 4) selectivities can be explained by the differences in the hydration energy values. To be specific, ions with a smaller hydration energy will pass through the PEM-coated membranes more easily.
Moreover, the coating procedure of the PEMs can have a significant effect on the selectivity value. For instance, in entries 28 and 30 of Table 3, the only difference in between the experiments was the recipe for preparing the coating. In recipe 1 (entry 28) has a 15 min rinsing step with 0.5 M NaCl, while in recipe 2 (entry 30), the rinsing step (1 min) is with demineralized water. Faster rinsing steps with demineralized water causes a higher intrinsic charge compensation between the PEs, resulting in a denser and less hydrated PEM. Therefore, with recipe 2, a less hydrated PEM can be established and a higher selectivity value (RMgNa = 7.8) was achieved compared to the PEM prepared with recipe 1 (RMg = 5.7). The degree of hydration in PEMs can also be tuned by cross-linking as shown in an anion separation study, which can be explained by two factors. First, cross-linking causes more compact PEMs that increases the rejection larger ions. Second, the sulfonate groups of the cross-linking agent increase the amount of negative charge within the PEM, resulting in a higher rejection of divalent anions. In Table 4, the cross-linked PEM (entry 10, Table 4) shows a ∼2 times higher PSO4Cl value compared to the PEM without cross-linking (entry 12, Table 4).
Not only the characteristics of the PEM and ions, but also the experimental conditions of desalination process are crucial while optimizing the ion selectivity. We first highlight an example that includes an ED process performed at different salt concentrations. With a source phase concentration of 0.01 M for both KNO3 and Mg(NO3)2, FMgK was found to be 22.1 ± 3.5 (Table 3, entry 6). For the same system, when the concentration of both salts was increased to 0.02 M, FMg increased to 96 ± 26 (entry 8, Table 3). Similarly, when the salt concentrations were 0.1 M, FMgK was reported to >20 000, further indicating the importance of the salt concentration for the system. It was hypothesized that the relatively lower source-phase concentrations caused more water splitting and therefore a higher Mg2+ flux. In entries 11 and 12 of Table 3, the same effect can be observed, where the higher concentration resulted in increased FCo (>360 vs >1600). Also, in another ED study, a 10 times higher source-phase concentration resulted in ∼10 times higher FSO4Cl (entries 31 and 32, Table 4). Although the higher FSO4 could be sourced from the charge screening or ion adsorption in the PEM, a ∼10 times increase in FSO4Cl even with the bare AMX (entries 35 and 36, Table 4) showed that this increase is not due to the PEM. Instead, the increase in Cl– flux in a higher source concentration is the main reason for the improved selectivity. Moreover, in both ED and diffusion dialysis (DD) studies, FSO4 are higher when the source-phase contains an excess of Cl– compared to SO42– (entries 26 and 34, Table 4). Therefore, both total salt concentration and the ion ratio affect the ion selectivity.
Another experimental condition is that the amount of driving force during the desalination process. In a constant current (CC) operation, when the current density increases, less rejections of divalent ions are observed. For instance, increasing the current density from 1.27 to 2.54 mA/cm2, FMgK decreased from >1000 to 22.1 (entries 5 and 6, respectively, in Table 3). Therefore, while comparing two FMg values from two different studies, the amount of current/voltage as well as the type of method should be considered to achieve a fair comparison. One indication is the flux values of the ions to compare the selectivity values. For instance, in an ED study, FMgNa is 1.7 when the flux of Na+ is ∼3.5 × 104 nmol·cm–2·s–1 (entry 33, Table 3). However, in another ED study, FMg > 1000 can be achieved since the driving force, and therefore the flux of the monovalent cation (K+, in this case) is much (×104) smaller (entry 5, Table 3).
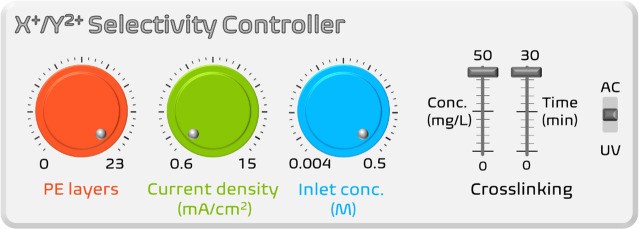
In conclusion, for an optimized system, the coating conditions of the PEM build-up (i.e., rinsing step, number of layers, degree of cross-linking), operational parameters (i.e., the composition of the salt solution), as well as the current density/voltage values should be considered carefully. To sum up, the combination of higher number of layers, therefore the charge density of the PEM, higher inlet concentration, and the smaller driving force (current/voltage) lead to higher X+/Y2+ selectivity values (Figure 6). Also, the stability of the PEM depends on the duration of the process, as well as the operating conditions. For instance, overlimiting current values (depending on the operation) can cause water splitting and even electromigration of the PEs.188 As a result, reduction in current efficiency, fouling of membrane with insoluble metal hydroxides, and even lack of stability in long-term operations can occur. Also, film stability in ED can be affected by the chlorine generation during the operation.199
Figure 6.
Schematic representation of the main parameters that affect the mono/divalent cation selectivity in PEM-coated dense membranes. The values are taken from the citations that are listed in Tables 3 and 4 and, as such, do not represent optimized values. AC and UV stand for alternating current electrical field and ultraviolet approaches to establish cross-linking between PEs with agents like 1,4-bis(2′,3′-epoxypropyl) perfluoro-1-butane and (4,4-diazos-tilbene-2,2-disulfonic acid disodium salt, respectively.
4.2. Fouling Control
PEM-coated AEMs were also used in antifouling studies in ED185,186 and reverse ED.192 When the terminating layer is PSS, the negatively charged hydrophilic outermost layer improved the antifouling properties of the AEM against various foulants including sodium dodecylbenzenesulfonate,185 sodium dodecyl sulfate (SDS),186 and humic acid.192 For example, Zhao et al. demonstrated that deposition of (PSS/PDADMAC)5.5 on AEM prevented the SDS formation on the membrane, and therefore, the electrical resistance reduced and ion transport through the membrane was unaffected in the presence of SDS (Figure 5e).186 Moreover, a PEM coating can simultaneously enhance the energy conversion efficiency by 3-fold compared to the pristine AEM, while still perform as an antifouling layer.192 Likewise, ROMs have been combined with PEMs to reduce membrane fouling.201−203 For instance, Ishigami et al. coated ROMs with (PAH/PSS)3 and (PAH/PSS)6 and concluded that the surface roughness decreased, and hydrophilicity increased with higher number of layers.201 In filtration experiments, the modified membranes were tested against hydrophobic foulants, humic acid and dodecyl trimethylammonium bromide (DTAB). The polyanion-terminated PEM coating reduced the amount of fouling in all cases, even for a cationic surfactant (DTAB). Moreover, a real-time surface technique called quartz crystal microbalance with dissipation (QCM-D) was used to determine bovine serum albumin (BSA) fouling. The QCM-D fouling study showed that the PEM coating resulted in ∼2 times less protein fouling. The surface mass densities of adsorbed BSA were calculated as 3.0 and 1.5 mg·m–2 for pristine gold sensors and gold sensors coated with (PAH/PSS)3, respectively, further proving the antifouling character of the PEM. More recently, PEMs were used as sacrificial coatings for fouling control for ROMs.131,204 When the membrane was fouled with organic foulants, both the biofilm and the PEM were flushed with concentrated brine solution and a fresh PEM was coated in situ (Figure 5e).204
4.3. Catalytic Effect in Dissociation Water
Another application of PEM-coated IEMs is to improve the water splitting capability of the membrane.184,205 In 2013, Abdu et al. deposited a PEM in between the anion-exchange and cation-exchange layers of a BPM.205 The PEM enhanced the rate of water dissociation because of the fixed charge groups of the PEM that behave as catalysts. In 2014, the same research group reported this also for a PEM-coated CEM.184 In that work, water dissociation occurred with PEI-terminated multilayers, while PSS-terminated multilayers showed no significant catalytic effect, allowing switchable water splitting at the membrane–PEM interface.
4.4. Solvent Transport and Separation
Furthermore, PEMs can improve the performance of direct methanol fuel cells when applied on Nafion membranes.206,207 Jiang and Tang showed that (PDADMAC/PAA)n multilayers reduced the methanol transport of Nafion membranes significantly as well as lowered the proton conductivity of the membrane.206 LbL assembly of different polyelectrolyte combinations have also been employed in RO for the separation of isopropanol–water mixtures yielding promising separation factors up to 1075.208
4.5. Stability
Besides their various applications, PEMs also have been proven to be highly stable coatings on dense membranes. They can be built at different pH values ranging from 2.3 to 9.3,78 remain intact in salt solutions up to 0.5 M,190 and under electric fields.183,194 To improve the chemical and physical stability of PEMs further, covalent bonds within the loose multilayers can be formed via UV irradiation.195,209
5. Polyelectrolytes and Polyelectrolyte Complexes as Free-Standing Membranes
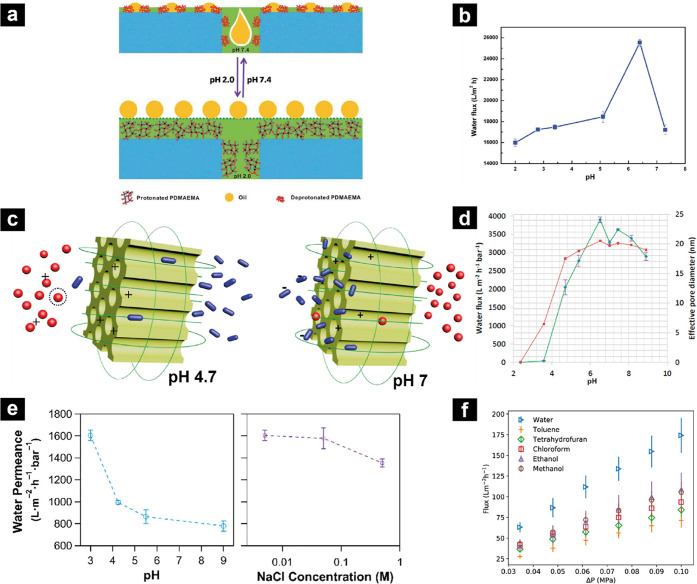
So far, PE or PEM coatings on membranes have been discussed, and we gave numerous reasons and examples why PEs are advantageous for many membrane applications by their nature. In this part, we will discuss membranes that are entirely composed of PEs either in homopolymer form or in copolymer form. To distinguish from the membranes with PE or PEM coatings, in this section, membranes will be referred to as “free-standing membranes”. Most of the membranes discussed, here, are indeed free-standing; however, the ones on macroporous substrates (for mechanical support during filtration) are also included. Blends or cases where PEs are incorporated to the membrane after the membrane formation are out of the scope of this part. First, material properties of PEs and PECs for membrane applications will be discussed. Next an oversight is given of free-standing membrane preparation, and finally, the advanced functions of these kinds of membranes will be discussed in detail.
5.1. General Properties and Functionalities
As explained earlier, PEs are water-soluble because of the charges along their polymer chains. However, when oppositely charged PEs form a complex via electrostatic interactions, strong ionic cross-linking makes the material resistant to many solvents.30 While such stability can be considered as a real advantage, it also means that it is not possible to process PECs in a conventional manner. Until the end of the 2000s, the lack of processability of PECs was still an issue. Some dense films from PECs could be obtained and were studied mostly for pervaporation applications.210 However, obtaining a porous film with good control over structure was not possible. In 2009, Schlenoff and co-workers showed that these materials are plasticized by salt and introduced the term “saloplastic”.211 Using salt as a plasticizer allowed the PECs to be formed in different shapes and sizes, proving that PECs are actually processable just like thermoplastics.33,212−214 This accelerated the research on saloplastic materials, and many papers have been published on advanced functional saloplastics with self-healing,215−217 shape recovery,218 patternability,219 antifouling,18 gas barrier,220 and chemical stability212 features. Additionally, incorporation of PECs with other kinds of materials, such as nanoparticles, is also demonstrated indicating it is possible to have materials with even more advanced functions.221−224 Moreover, PECs are plasticized and then processed after being exposed to saline aqueous solutions without using toxic organic solvents suggesting saloplastic materials can be prepared in a sustainable manner. The introduction of saloplastics to the literature signaled that PEC films can be obtained in many ways and these films can have numerous functions, which will be particularly useful for membrane applications.
5.2. Free-Standing Membrane Formation
Investigations on PEC film225 and membrane30,226 formation already started decades ago. However, it hit the significant obstacle of lack of processability of PECs as discussed above. When dry, PECs are infusible, brittle, and resistive to most of the solvents.30,227 Therefore, shaping PECs in desired forms, including membrane formation, was not possible with conventional methods like phase separation. On the other hand, PE bulk films were soft, sticky, highly swollen in the presence of water, and very sensitive to humidity. Although these are desired features for some applications, they are not ideal for filtration-based separations, and a complex formation or a kind of cross-linking is needed for most of the cases to suppress these effects.228
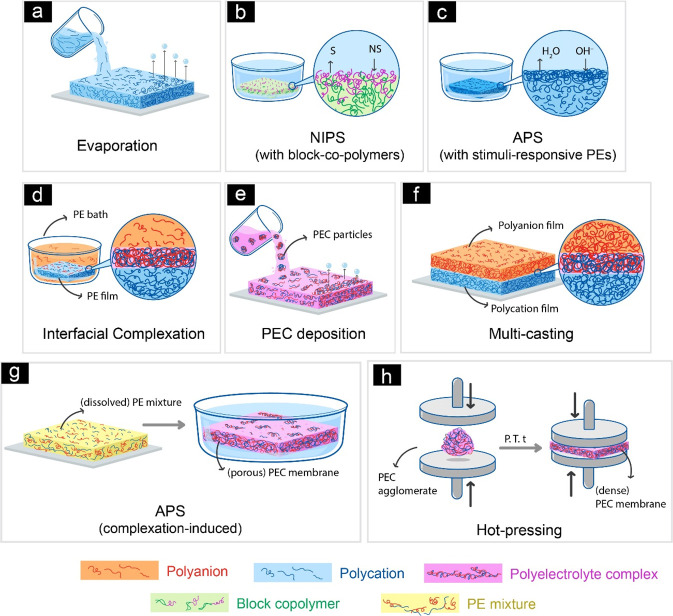
Preparation of the free-standing membranes from only one PE was possible with conventional methods, such as solvent evaporation229 (Figure 7a) or nonsolvent induced phase separation (NIPS)230 (Figure 7b). On the other hand, to prepare free-standing PEC membranes, researchers had to develop new methods. Therefore, most of the research is focused on preparing and evaluating the performance of the membranes rather than developing them further to have advanced functions. Interfacial complexation, multicasting, and PEC deposition are among these methods which are illustrated in Figure 7. In interfacial complexation (Figure 7d), a PE film is immersed in a bath containing oppositely charged PEs.19,231 In multicasting methods (Figure 7f), either PEC dispersion is cast, dried, and cast again on top of the previous one,22 or a PE solution is cast followed by casting of oppositely charged PE solution on top of it.226,232 In the PEC deposition method (Figure 7e), dilute solutions of oppositely charged PEs mixed to obtain a dispersion. Then, this PEC dispersion is cast followed by evaporation of the solvent (usually water) to form the film228,233−236 or the dispersion is filtered where the membrane is obtained on that filter.237,238 Mostly for these methods, membrane formation occurs completely in aqueous media, which is a very desired feature, especially in terms of sustainability and clean production. However, these methods suffer from several problems including nonuniform ionic cross-linking, very long evaporation times, lack of scalability, and low control over membrane structure (i.e., mostly dense films are obtained). Recently, Krishna B et al. demonstrated a new method for the formation of dense PEC membranes, which is obtained by pressing a PEC agglomerate at a certain temperature33 (Figure 7h), and many of the drawbacks mentioned before are not seen for hot-pressed membranes. It is also possible to have free-standing membranes with conventional phase separation methods. For this, either poly(ionic liquids) (PILs)28,239−242 or copolymers of regular membrane polymers with PEs20,243−248 are used. Although the organic solvent used in the phase separation methods becomes a big issue,9 membrane preparation is very well established with great control over the structure.
Figure 7.
Schematic drawings of PE and PEC free-standing membrane preparation methods. (a) Solvent evaporation method (solvents including water), (b) membrane preparation from block copolymers via NIPS technique, (c) membrane preparation with stimuli-responsive PEs via APS, (d) interfacial complexation technique, while the type of PE is not important, here, the viscous film is the polycation which is immersed in polyanion bath, (e) PEC deposition membranes prepared through removing water from PEC dispersion, (f) multicasting technique, each layer can be with any type of PE solution, as well as with PEC solutions, (g) membrane preparation via complexation induced APS, (h) dense membrane preparation by hot-pressing.
If it is possible to process PECs just like regular thermoplastics, it means that it is also possible to prepare free-standing PEC membranes with a phase separation approach just like NIPS membranes. Indeed, de Vos introduced the aqueous phase separation (APS) approach for the preparation of membranes made of PEs and PECs in totally water-based processes.249 To make membranes from a single polymer, weak PEs are used. Casting solutions are prepared at a pH where the weak PE is charged, and soluble in water, and after casting, the film is immersed in a bath at a pH that the PE is insoluble in water250,251 (Figure 7c). On the other hand, PEC membranes are obtained either via the pH- or salinity-switch method (Figure 7g). In the former, a homogeneous mixture of oppositely charged PEs is obtained by keeping the pH at a point where weak PE is uncharged and not interacting with the other one. Casting is followed by immersion of the mixture in a bath where both of the PEs are charged, interacting with each other, and forming the PEC membrane.252 In the salinity-switch method, a casting solution is prepared at high salinity to screen the charges of the PEs and, therefore, preventing the complex formation. When this solution is in the coagulation bath of low salinity, then the charge screening disappears, and PEs form the complex.253 The last method is also investigated by others with the emphasis of how sustainable and scalable the method is.254,255 It is clear that with the APS approach, it is possible to combine the know-how and versatility of the NIPS method with the desired features of PE membranes.
5.3. Responsiveness
Most of the recent research on advanced functional, free-standing PE-based membranes is on stimuli-responsive behavior. PEs change the chain conformation depending on several factors, including ionic strength, solvent type, temperature, and pH. Therefore, membranes made from a PE that responds to these external stimuli would be expected to be stimuli-responsive as well. There are many papers on pH-responsive membranes made with both homopolymers251,256−258 and copolymers.20,21,238,243,245−247,259−264 The responsive behavior is observed as either a change in surface properties, a gating mechanism where pore size changes with the external stimuli, or swelling of the whole material. Weak polyelectrolytes such as poly(2-dimethylamino ethyl methacrylate) (PDMAEMA), poly(4-vinylpyridine) (P4VP), and PAA are frequently used. Mostly, at a pH where the PE is charged, the polymer chains get an extended conformation due to repulsion of the monomers leading to swelling of the polymer. Xiang et al. showed that it is possible to control hydrophilic properties of the membranes composed of PDMAEMA and poly(vinylidene fluoride) (PVDF) copolymer.20 At pH ∼7, PDMAEMA chains are collapsed and PVDF is more exposed; therefore, the membrane is more hydrophobic letting oil permeate. On the other hand, at low pH (∼2), PDMAEMA chains are extended and cover the PVDF, resulting in more hydrophilic membranes where oil/water separations are possible (Figure 8a). Figure 8c shows the pH-dependent flux of PS-b-P4VP membranes, where a small pH change enables one to alter the water flux by orders of magnitude.21 Here, the flux is higher when P4VP chains are collapsed (i.e., when the gates are open). On the other hand, Figure 8e also shows the pH-dependent flux behavior of a membrane composed completely of P4VP homopolymer.251 Unlike the previous ones, this membrane has a lower resistance for water permeation at low pH where P4VP is charged and extended since the swelling of the whole material leads to more open structures.
Figure 8.
(a) Sketch showing the pH-responsive oil/water separation of PVDF-co-PDMAEMA membranes and (b) pH-responsive pure water flux of PVDF-co-PDMAEMA membranes. (a and b) Reproduced with permission from ref (20). Copyright 2015 The Royal Society of Chemistry. (c) A sketch showing the separation of similarly sized proteins with positively charged polystyrene-b-poly-4-vinylpyridine block copolymer (PS-co-P4VP) membranes, which protein to be retained is depending on the net charge of the protein which depends on the pH of the medium and (d) pure water flux (green line) vs pH plot for PS-co-P4VP membranes). (c and d) Reproduced with permission from ref (21). Copyright 2012 American Chemical Society. (e) pH- and ionic strength-dependent behavior of the flux of membranes completely composed of P4VP homopolymer. Reproduced with permission from ref (251). Copyright 2019 American Chemical Society. (f) Flux over transmembrane pressure plot showing the linear relationship for PSS-poly(N-ethyl-4-vinylpyridinium) (QVPC2) PEC membranes. Reproduced with permission from ref (254). Copyright 2019 American Chemical Society.
Since the transition between a coiled and extended form of the PE chains is not sharp, membrane permeability can easily be fine-tuned with pH. Moreover, especially for the membranes from copolymers, permeability also depends on the surface properties. When the hydrophilic PE is in a coiled form, the hydrophobic part of the copolymer is more in contact with the filtration medium, a decrease in membrane flux can be observed with the increase of the hydrophobicity (Figure 8b, d).20,21 Finally, the pH affects the charge density of the weak PEs and consequently the charge exclusion mechanism of the separation, which is a key factor for the separation of charged compounds like proteins, dyes, salts, and micropollutants. For example, Qiu et al. studied the separation of similarly sized proteins where the membrane charge is one of the factors to control this separation as sketched in Figure 8d.21
Most of the papers on stimuli-responsive membranes investigated the effect on flux. However, only a few investigated the membranes with a rejection test of a compound,245,246,248,251,265,266 and to our knowledge, there are very few investigating the separation of similar species.21,258 The reported rejection tests are very frequently done with BSA to examine the fouling behavior of the membranes, which will be discussed later.
Other than pH, also ionic strength,230,251,263,266 temperature,243,247,259,267 and redox-responsive268 behavior of PE bulk membranes is studied. The mechanism is again completely dominated by the conformation of the PE. The gate mechanism studied for drug-release and dialysis membranes as well that are excluded from this Review.
5.4. Fouling Control
As stated earlier, fouling is a major problem for membrane applications. Hydrophobic membranes tend to foul easily, but as membranes get more hydrophilic, there will be more water molecules between the membrane and the foulant and therefore it will be less favorable for the foulant to be adsorbed on the surface.246,248 Therefore, many membrane studies have been dedicated to increasing the hydrophilicity of the membranes using PEs. PEs due to their charged nature are hydrophilic, a feature that can even be tuned by their charge density. Many studies on stimuli-responsive behavior of the membranes also investigated the fouling behavior of the PE free-standing membranes.245,246,248,251,265,266 It is reported that as the membranes get hydrophilic, it becomes more difficult to foul the membrane.20,246,269 Moreover, when fouled it is easier to clean the membrane by adjusting the pH where the PE is charged. Willott et al. showed that cross-linking P4VP free-standing membranes introduces fixed charges and by controlling the cross-linking degree, it is possible to control the self-cleaning degree of the membranes.251 Very frequently, the flux recovery data (pure water flux after cleaning with respect to the one before fouling) of PE-based free-standing membranes is over 90%. This means that although it is not possible to do the filtrations at a pH where the membrane shows high resistance to fouling, it can be easily cleaned, and cleaned membranes can perform as well as new membranes. The literature on this is predominantly on copolymer membranes which are prepared with NIPS. There are a few studies on antifouling saloplastic material,18 and to our knowledge, the work of Willott et al. is the only study that investigates stimuli-responsive and self-cleaning functions of homopolymer free-standing membranes251 prepared with APS.
5.5. Stability
PECs and many PEs are known with being chemically resistive toward organic solvents. As in other polymers, the chemical stability of PEs and PECs is highly dependent on solvent–polymer interactions. Moreover, the chemical stability of a membrane is determined by the stability of the polymer, the extent of cross-linking, and the extent of the entanglements. For PECs, electrostatic interactions between oppositely charged PEs, which are also called ionic cross-links, contribute to the stability of the membrane. Free-standing pervaporation membranes and PEMs used for organic solvent nanofiltration (OSN) already indicate the great compatibility of PE-based membranes. For free-standing PEC membranes, Sadman et al. reported stable fluxes over varying transmembrane pressure for several organic solvents Figure 8f254 and Fares showed swelling behavior of PSS–PDADMAC-based saloplastics,270 pointing out that PEC membranes will be particularly useful for filtrations with organic solvents. Other than stability against organic solvents, pH stability of these membranes is also important. Here, the extent of ionic cross-linking is more important which is mostly dependent on PE type. For example, Baig et al. reported the pH stability of PSS–PEI free-standing membranes is only up to pH 10;271 this is because of the pH-dependent behavior of the weak polycation PEI. It is speculated that, at conditions higher than pH 10, the membrane starts to decomplex because of PEI becomes less charged because of the excessive deprotonation. On the other hand, it is reported that PSS–PDADMAC membranes are stable at pH 0–14.33,253 Pham et al. showed that anion exchange membranes from ionenes, a class of PEs, are stable in alkaline solutions and organic solvents like dimethyl sulfoxide, N-methyl-2-pyrrolidone and dimethylacetamid.272 Krishna B et al. exposed the hot-pressed PSS–PDADMAC membranes to 1 M HCl and 1 M NaOH solutions for 60 days and the membranes exhibit great stability against this extreme pH without sacrificing from the permselectivities.33 The difference in the stability of these PEC membranes does not only result from different types of PEs, as also the polyanion to polycation ratio in the complex, the strength of ion–ion interactions, and the extent of cross-linking and entanglements are affecting factors. This implies that it is possible to tune the stability of these membranes further with an informed selection of PEs and membrane preparation conditions.
6. Conclusion and Outlook
In the past decade, the use of polyelectrolytes to modify or produce membranes has received much attention and has led to many examples of highly promising functionalities. Attaching just a single layer of polyelectrolyte, either as a brush or a physiosorbed layer, is already enough to produce membranes with excellent antifouling properties, responsive membranes through a gating mechanism and even antiviral and/or antibacterial properties. Moreover, when applying polyelectrolytes in a complex form, through the formation of polyelectrolyte multilayers, more advanced functionalities become possible. Next to antifouling and pH- and salt-responsive coatings, it has now also become possible to create membranes with sacrificial coatings, for easy cleaning, and membranes with very specific selectivities. Polyelectrolyte multilayers, both on porous and dense membranes can allow very high selectivities between multivalent and monovalent ions, going beyond what is possible with more traditional membrane coatings and materials. Other specific selectivities, including selectivity between monovalent ions and membranes with a high retention to small organics molecules and a low salt retention also become possible. In the past decade, it has also become clear that porous free-standing membranes can be completely made out from polyelectrolyte complexes. Some approaches are especially promising as they allow the formation of membranes without use of organic solvents, providing a platform to make membrane production much more sustainable. The produced free-standing membranes can be as diverse as membranes produced with organic solvents, besides they can have the same advanced functionalities, ranging from stimuli-responsive properties to separation behavior. Some of the PEM- and PEC-based free-standing membranes also demonstrate very high stabilities under problematic conditions, for example in organic solvents or when exposed to extreme pH conditions.
Overall, it can thus certainly be concluded that polyelectrolytes are highly promising materials to allow the formation of next-generation membranes with advanced functionalities. However, in many ways the field is still developing, and much more exciting work on these versatile materials are expected in the near future. For example, by focusing not on just one single functionality but rather using polyelectrolytes in smart ways to achieve multifunctional membranes, where for example, low fouling, easy to clean, and specific selectivities are combined.169
A natural advantage of polyelectrolytes is their solubility in water, allowing the fabrication of polyelectrolyte coatings and even complete membranes without the need for organic solvents. While a clear advantage from a sustainability point of view, this can certainly be pushed further. One idea is to use biobased PEs in membrane separations. Although, there are few recent studies that have focused on the monovalent anion selectivity and pervaporation with biodegradable PEs (mostly chitosan derivatives),192,196,197,228 there is much room to explore the use of PEs such as pectin, alginic acid, and cellulose derivatives in the context of membrane-based separations.
Moreover, it has been well established that polyelectrolyte complexes have self-healing properties, allowing them to heal from small and larger damage in sufficiently salt water. For PEM coatings and for the free-standing membranes such self-healing properties would be a huge advantage, one that has the potential to lengthen the product lifetime, further improving sustainability. Still, up until now the self-healing properties of PEC membranes and PEM membrane coatings has received no real attention. In addition, polyelectrolyte complexes tend to lose their stability under extremely saline conditions, or in the presence of surfactants. For those conditions good cross-linking approaches will become necessary. For example, microfiltration membranes made from PILs by Dani et al. are stable against high ionic strength solutions since the films are covalently cross-linked by UV-light.273 On the other hand, this feature can be useful for membrane reuse. Wu et al. showed it is possible to reprocesses PIL–PAA membranes in high concentration salt solutions.274
One other great advantage of PE- and PEC-based materials is that additives can easily be incorporated/intercalated to allow additional functionalities and further improvement of membrane properties. Indeed, based on the successes of the early examples of PEMs including ion-selective receptors,275−278 the recovery and harvest of specific ions from aqueous solutions via PEM-coated dense membranes can be further tuned in the future. The incorporation of tailored ion-selective groups184 may allow selectivity between monovalent ions for PEM-based membranes. Recently, the intercalation of SDS bilayers in a (PDADMAC/PSS)n multilayer allowed for thinner multilayers, with increased pore size, and a higher hydrophilicity, which resulted in a 100% increase in water permeability without compromising the SO42– rejection.279 These results highlight how small molecular additives may become a novel approach to enhance and fine-tune the performance of PEM-coated membranes. Additionally, nanoparticles280−282 and metallo-polyelectrolytes283 can be incorporated into the multilayer to increase PEM-based membrane performance (e.g., permeability, selectivity, strength, and hydrophilicity).280,284 Here, future research is expected to focus on membranes with incorporated ion-selective nanoparticles for selective adsorption and recovery (via pH regeneration) of specific resources from wastewater, thereby combining filtration and adsorption in a single step process. Finally, polyelectrolyte multilayers could be combined with functional biological moieties, such as enzymes. Current PEM-based membranes can stop already stop MPs with a high efficiency. However, recent work on PEM-based biocatalytic membranes, with incorporated, show that MP rejection and degradation could be combined in a single process.285−287
We do stress that, in future research on improved membrane selectivities, it is very important to work with standardized process conditions, and standardized selectivity definitions and rejection/permeation performances that allow direct comparison of different studies. Moreover, these separation performance values should be reported as an average of multiple (minimum 3) experiments and standard deviations should be reported to facilitate a better understanding of the reproducibility of the production process of modified and new membranes.
Finally, the natural stability of polyelectrolytes and their complex in organic solvents, coupled to their high cross-link densities make PE- and PEC-based membranes relevant for many applications that go beyond water treatment. It has already been shown that PEM-based membranes can be very relevant for solvent filtration,36 while PEC-based membranes have also been shown to dramatically reduce the permeability of oxygen in packaging materials.220,288 Such oxygen barrier properties, also point to the relevance of these materials for the fabrication of advanced gas separation membranes, which are relevant for, for example, CO separation and storage.289 We, thus, foresee a very bright future for polyelectrolyte-based advanced functional membranes, for applications in water treatment, but also in industrial processes that require the separation of organic solvents and gases.
Acknowledgments
This work was supported by the European Union Horizon 2020 research and innovation program (ERC CoG 682444 E-motion to LdS and ERC StG 714744 SAMBA to W.M.d.V. and E.N.D.) and was performed in the cooperation framework of Wetsus, European Centre of Excellence for Sustainable Water Technology. Wetsus is cofunded by the Dutch Ministry of Economic Affairs and Ministry of Infrastructure and Environment, the European Union Regional Development Fund, the Province of Fryslân, and the Northern Netherlands Provinces. Xiaoliu Wen is credited for the creation of the artwork in Figures 1, 2, 3, and 7.
Glossary
Abbreviations
- AEM
anion-exchange membrane
- AMX
standard-grade anion exchange membrane
- APS
aqueous phase separation
- BCM
block copolymer micelles
- BPM
bipolar membrane
- BSA
bovine serum albumin
- CC
constant current
- CDI
capacitive deionization
- CEM
cation-exchange membrane
- CMX
standard-grade cation exchange membrane
- CV
constant voltage
- DD
diffusion dialysis
- DMF
dimethylformamide
- DTAB
dodecyl trimethylammonium bromide
- ED
electrodialysis
- HA
hyaluronic acid
- HF
hollow fiber
- IEM
ion-exchange membrane
- LbL
layer-by-layer
- MF
microfiltration
- MP
micropollutant
- NF
nanofiltration
- NIPS
nonsolvent induced phase separation
- OSN
organic solvent nanofiltration
- PE
polyelectrolyte
- PEB
polyelectrolyte brush
- PEC
polyelectrolyte complex
- PEM
polyelectrolyte multilayer
- PIL
poly(ionic liquid)
- QCM-D
quartz crystal microbalance with dissipation
- RED
reverse electrodialysis
- RM
resistance measurement
- RO
reverse osmosis
- ROM
reverse osmosis membrane
- RP
receiving phase
- SDS
sodium dodecyl sulfate
- TFC
thin-film composite
- THF
tetrahydrofuran
- UF
ultrafiltration
Polymers
- alkynyl-PMETA
poly(2-(methacryloyloxy)ethyl trimethylammonium chloride)
- HACC
2-hydroxypropyltrimethylammonium chloride chitosan
- NSBC
N–O-sulfonic acid benzyl chitosan
- P4VP
poly(4-vinylpyridine)
- PAA
poly(acrylic acid)
- PAH
poly(allylamine hydrochloride)
- PAMA
poly(alkyl methacrylate)
- PAN
poly(acrylonitrile)
- PAS
poly(phenylene sulfone)
- PDADMAC
poly(diallyldimethylammonium chloride)
- PDMAEMA
poly(2-dimethylamino ethyl methacrylate)
- PEI
poly(ethylenimine)
- PES
poly(ethersulfone)
- PET
poly(ethylene terephthalate)
- PMAA
poly(methacrylic acid)
- PMPC
poly(2-methacryloyloxyethyl phosphorylcholine)
- PMPC-co-AA
poly(2-methacryloyloxyethyl phosphorylcholine-co-acrylic acid)
- poly(OEGMA-r-HEMA)
poly(oligo(ethylene glycol) methacrylate-random-2-hydroxyethyl methacrylate)
- PSf
poly(sulfone)
- PSBMA
poly(sulfobetaine methacrylate)
- PS-co-P4VP
polystyrene-b-poly-4-vinylpyridine block copolymer
- PSPMA
poly(3-sulfopropyl methacrylate)
- PSS
poly(sodium 4-styrenesulfonate)
- PSS-MA
poly(styrenesulfonic acid-co-maleic acid) sodium salt
- PVA
poly(vinyl alcohol)
- PVDF
poly(vinylidene fluoride)
- PVS
poly(vinyl sulfonate)
- PVSA
poly(vinylsulfonic acid) sodium salt
- QVPC2
poly(N-ethyl-4-vinylpyridinium)
- SPEEK
sulfonated poly(ether ether ketone)
- SPES
sulfonated poly(ether sulfone)
Author Contributions
§ E.N.D., S.S., and E.V. contributed equally to this paper.
The authors declare no competing financial interest.
References
- Baker R. W.Membrane Technology and Applications, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp 1–450. [Google Scholar]
- Strathmann H.Introduction to Membrane Science and Technology, 1st ed.; Wiley-VCH Verlag & Co. KGaA: Weinheim, 2011; pp 1–323. [Google Scholar]
- Global Membrane Separation Technology Markets Report 2021–2027—Expansion and New Equipment Drive Demand for Membranes in Industry. Markets Insider https://markets.businessinsider.com/news/stocks/global-membrane-separation-technology-markets-report-2021-2027-expansion-and-new-equipment-drive-demand-for-membranes-in-industry-1030749538 (accessed 2021-08-23).
- Smitha B.; Suhanya D.; Sridhar S.; Ramakrishna M. Separation of Organic–Organic Mixtures by Pervaporation—A Review. J. Membr. Sci. 2004, 1–21. 10.1016/j.memsci.2004.03.042. [DOI] [Google Scholar]
- Khanzada N. K.; Farid M. U.; Kharraz J. A.; Choi J.; Tang C. Y.; Nghiem L. D.; Jang A.; An A. K. Removal of Organic Micropollutants Using Advanced Membrane-Based Water and Wastewater Treatment: A Review. J. Membr. Sci. 2020, 117672. 10.1016/j.memsci.2019.117672. [DOI] [Google Scholar]
- Zhang Y.; Sunarso J.; Liu S.; Wang R. Current Status and Development of Membranes for CO2/CH4 Separation: A Review. Int. J. Greenhouse Gas Control 2013, 12, 84–107. 10.1016/j.ijggc.2012.10.009. [DOI] [Google Scholar]
- Zhang R.; Liu Y.; He M.; Su Y.; Zhao X.; Elimelech M.; Jiang Z. Antifouling Membranes for Sustainable Water Purification: Strategies and Mechanisms. Chemical Society Reviews 2016, 5888–5924. 10.1039/c5cs00579e. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Srivastava A.; Galaev I. Y.; Mattiasson B. Smart Polymers: Physical Forms and Bioengineering Applications. Progress in Polymer Science (Oxford) 2007, 1205–1237. 10.1016/j.progpolymsci.2007.05.003. [DOI] [Google Scholar]
- Figoli A.; Marino T.; Simone S.; Di Nicolò E.; Li X.-M.; He T.; Tornaghi S.; Drioli E. Towards Non-Toxic Solvents for Membrane Preparation: A Review. Green Chem. 2014, 16 (9), 4034–4059. 10.1039/C4GC00613E. [DOI] [Google Scholar]
- Li X.; Shen S.; Xu Y.; Guo T.; Dai H.; Lu X. Application of Membrane Separation Processes in Phosphorus Recovery: A Review. Sci. Total Environ. 2021, 144346. 10.1016/j.scitotenv.2020.144346. [DOI] [PubMed] [Google Scholar]
- Remmen K.; Schäfer R.; Hedwig S.; Wintgens T.; Wessling M.; Lenz M. Layer-by-Layer Membrane Modification Allows Scandium Recovery by Nanofiltration. Environ. Sci. Water Res. Technol. 2019, 5 (10), 1683–1688. 10.1039/C9EW00509A. [DOI] [Google Scholar]
- Li X.; Mo Y.; Qing W.; Shao S.; Tang C. Y.; Li J. Membrane-Based Technologies for Lithium Recovery from Water Lithium Resources: A Review. J. Membr. Sci. 2019, 117317. 10.1016/j.memsci.2019.117317. [DOI] [Google Scholar]
- López J.; Gibert O.; Cortina J. L. Integration of Membrane Technologies to Enhance the Sustainability in the Treatment of Metal-Containing Acidic Liquid Wastes. An Overview. Separation and Purification Technology 2021, 118485. 10.1016/j.seppur.2021.118485. [DOI] [Google Scholar]
- Platt S.; Nyström M.; Bottino A.; Capannelli G. Stability of NF Membranes under Extreme Acidic Conditions. J. Membr. Sci. 2004, 239 (1), 91–103. 10.1016/j.memsci.2003.09.030. [DOI] [Google Scholar]
- Ricci B. C.; Ferreira C. D.; Aguiar A. O.; Amaral M. C. S. Integration of Nanofiltration and Reverse Osmosis for Metal Separation and Sulfuric Acid Recovery from Gold Mining Effluent. Sep. Purif. Technol. 2015, 154, 11–21. 10.1016/j.seppur.2015.08.040. [DOI] [Google Scholar]
- Fernández P.; Riera F. A.; Álvarez R.; Álvarez S. Nanofiltration Regeneration of Contaminated Single-Phase Detergents Used in the Dairy Industry. J. Food Eng. 2010, 97 (3), 319–328. 10.1016/j.jfoodeng.2009.10.023. [DOI] [Google Scholar]
- Bes-Piá A.; Iborra-Clar M. I.; Iborra-Clar A.; Mendoza-Roca J. A.; Cuartas-Uribe B.; Alcaina-Miranda M. I. Nanofiltration of Textile Industry Wastewater Using a Physicochemical Process as a Pre-Treatment. Desalination 2005, 178 (1–3), 343–349. 10.1016/j.desal.2004.11.044. [DOI] [Google Scholar]
- Kurtz I. S.; Sui S.; Hao X.; Huang M.; Perry S. L.; Schiffman J. D. Bacteria-Resistant, Transparent, Free-Standing Films Prepared from Complex Coacervates. ACS Appl. Bio Mater. 2019, 2 (9), 3926–3933. 10.1021/acsabm.9b00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop S.; Thielking H.; Kulicke W.-M. Simplex Membranes of Sulphoethylcellulose and Poly(Diallydimethylammonium Chloride) for the Pervaporation of Water-Alcohol Mixtures. J. Appl. Polym. Sci. 2000, 77 (14), 3169–3177. . [DOI] [Google Scholar]
- Xiang Y.; Shen J.; Wang Y.; Liu F.; Xue L. A pH-Responsive PVDF Membrane with Superwetting Properties for the Separation of Oil and Water. RSC Adv. 2015, 5 (30), 23530–23539. 10.1039/C5RA00739A. [DOI] [Google Scholar]
- Qiu X.; Yu H.; Karunakaran M.; Pradeep N.; Nunes S. P.; Peinemann K.-V. Selective Separation of Similarly Sized Proteins with Tunable Nanoporous Block Copolymer Membranes. ACS Nano 2013, 7 (1), 768–776. 10.1021/nn305073e. [DOI] [PubMed] [Google Scholar]
- Long Q.; Zhang Z.; Qi G.; Wang Z.; Chen Y.; Liu Z.-Q. Fabrication of Chitosan Nanofiltration Membranes by the Film Casting Strategy for Effective Removal of Dyes/Salts in Textile Wastewater. ACS Sustainable Chem. Eng. 2020, 8 (6), 2512–2522. 10.1021/acssuschemeng.9b07026. [DOI] [Google Scholar]
- Krasemann L.; Tieke B. Selective Ion Transport across Self-Assembled Alternating Multilayers of Cationic and Anionic Polyelectrolytes. Langmuir 2000, 16 (2), 287–290. 10.1021/la991240z. [DOI] [Google Scholar]
- Adusumilli M.; Bruening M. L. Variation of Ion-Exchange Capacity, ζ Potential, and Ion-Transport Selectivities with the Number of Layers in a Multilayer Polyelectrolyte Film. Langmuir 2009, 25 (13), 7478–7485. 10.1021/la900391q. [DOI] [PubMed] [Google Scholar]
- Hong S. U.; Malaisamy R.; Bruening M. L. Separation of Fluoride from Other Monovalent Anions Using Multilayer Polyelectrolyte Nanofiltration Membranes. Langmuir 2007, 23 (4), 1716–1722. 10.1021/la061701y. [DOI] [PubMed] [Google Scholar]
- Hong S. U.; Malaisamy R.; Bruening M. L. Optimization of Flux and Selectivity in Cl-/SO42- Separations with Multilayer Polyelectrolyte Membranes. J. Membr. Sci. 2006, 283 (1–2), 366–372. 10.1016/j.memsci.2006.07.007. [DOI] [Google Scholar]
- Hong S. U.; Ouyang L.; Bruening M. L. Recovery of Phosphate Using Multilayer Polyelectrolyte Nanofiltration Membranes. J. Membr. Sci. 2009, 327 (1–2), 2–5. 10.1016/j.memsci.2008.11.035. [DOI] [Google Scholar]
- Kammakakam I.; Bara J. E.; Jackson E. M. Dual Anion–Cation Crosslinked Poly(Ionic Liquid) Composite Membranes for Enhanced CO 2 Separation. ACS Appl. Polym. Mater. 2020, 2 (11), 5067–5076. 10.1021/acsapm.0c00877. [DOI] [Google Scholar]
- Li P.; Pramoda K. P.; Chung T.-S. CO 2 Separation from Flue Gas Using Polyvinyl-(Room Temperature Ionic Liquid)–Room Temperature Ionic Liquid Composite Membranes. Ind. Eng. Chem. Res. 2011, 50 (15), 9344–9353. 10.1021/ie2005884. [DOI] [Google Scholar]
- Michaels A. S. Polyelectrolyte Complexes. Ind. Eng. Chem. 1965, 57 (10), 32–40. 10.1021/ie50670a007. [DOI] [Google Scholar]
- Li X.; Goyens W.; Ahmadiannamini P.; Vanderlinden W.; De Feyter S.; Vankelecom I. Morphology and Performance of Solvent-Resistant Nanofiltration Membranes Based on Multilayered Polyelectrolytes: Study of Preparation Conditions. J. Membr. Sci. 2010, 358 (1–2), 150–157. 10.1016/j.memsci.2010.04.039. [DOI] [Google Scholar]
- de Grooth J.; Haakmeester B.; Wever C.; Potreck J.; de Vos W. M.; Nijmeijer K. Long Term Physical and Chemical Stability of Polyelectrolyte Multilayer Membranes. J. Membr. Sci. 2015, 489, 153–159. 10.1016/j.memsci.2015.04.031. [DOI] [Google Scholar]
- Krishna B A.; Lindhoud S.; de Vos W. M. Hot-Pressed Polyelectrolyte Complexes as Novel Alkaline Stable Monovalent-Ion Selective Anion Exchange Membranes. J. Colloid Interface Sci. 2021, 593, 11–20. 10.1016/j.jcis.2021.02.077. [DOI] [PubMed] [Google Scholar]
- Elshof M.G.; de Vos W.M.; de Grooth J.; Benes N.E. On the Long-Term PH Stability of Polyelectrolyte Multilayer Nanofiltration Membranes. J. Membr. Sci. 2020, 615 (July), 118532. 10.1016/j.memsci.2020.118532. [DOI] [Google Scholar]
- Li X.; Liu C.; Van der Bruggen B. Polyelectrolytes Self-Assembly: Versatile Membrane Fabrication Strategy. J. Mater. Chem. A 2020, 8 (40), 20870–20896. 10.1039/D0TA07154D. [DOI] [Google Scholar]
- Joseph N.; Ahmadiannamini P.; Hoogenboom R.; Vankelecom I. F. J. Layer-by-Layer Preparation of Polyelectrolyte Multilayer Membranes for Separation. Polym. Chem. 2014, 5 (6), 1817–1831. 10.1039/C3PY01262J. [DOI] [Google Scholar]
- Zhao Q.; An Q. F.; Ji Y.; Qian J.; Gao C. Polyelectrolyte Complex Membranes for Pervaporation, Nanofiltration and Fuel Cell Applications. J. Membr. Sci. 2011, 379 (1–2), 19–45. 10.1016/j.memsci.2011.06.016. [DOI] [Google Scholar]
- Zhang W.; Zhao Q.; Yuan J. Porous Polyelectrolytes: The Interplay of Charge and Pores for New Functionalities. Angew. Chem., Int. Ed. 2018, 57 (23), 6754–6773. 10.1002/anie.201710272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.; Dellatore S. M.; Miller W. M.; Messersmith P. B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science (Washington, DC, U. S.) 2007, 318 (5849), 426–430. 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danglad-Flores J.; Eickelmann S.; Riegler H. Deposition of Polymer Films by Spin Casting: A Quantitative Analysis. Chem. Eng. Sci. 2018, 179, 257–264. 10.1016/j.ces.2018.01.012. [DOI] [Google Scholar]
- Khlyustova A.; Cheng Y.; Yang R. Vapor-Deposited Functional Polymer Thin Films in Biological Applications. Journal of Materials Chemistry B 2020, 6588–6609. 10.1039/d0tb00681e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballauff M.; Borisov O. Polyelectrolyte Brushes. Curr. Opin. Colloid Interface Sci. 2006, 11 (6), 316–323. 10.1016/j.cocis.2006.12.002. [DOI] [Google Scholar]
- Xu X.; Billing M.; Ruths M.; Klok H. A.; Yu J. Structure and Functionality of Polyelectrolyte Brushes: A Surface Force Perspective. Chem. - Asian J. 2018, 13 (22), 3411–3436. 10.1002/asia.201800920. [DOI] [PubMed] [Google Scholar]
- Das S.; Banik M.; Chen G.; Sinha S.; Mukherjee R. Polyelectrolyte Brushes: Theory, Modelling, Synthesis and Applications. Soft Matter 2015, 11 (44), 8550–8583. 10.1039/C5SM01962A. [DOI] [PubMed] [Google Scholar]
- Zdyrko B.; Luzinov I. Polymer Brushes by the “Grafting to” Method. Macromol. Rapid Commun. 2011, 32 (12), 859–869. 10.1002/marc.201100162. [DOI] [PubMed] [Google Scholar]
- Zoppe J. O.; Ataman N. C.; Mocny P.; Wang J.; Moraes J.; Klok H. A. Surface-Initiated Controlled Radical Polymerization: State-of-the-Art, Opportunities, and Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117 (3), 1105–1318. 10.1021/acs.chemrev.6b00314. [DOI] [PubMed] [Google Scholar]
- Alexander S. Adsorption of Chain Molecules With a Polar Head. a Scaling Description. J. Phys. (Paris) 1977, 38 (8), 983–987. 10.1051/jphys:01977003808098300. [DOI] [Google Scholar]
- de Gennes P. G. Conformations of Polymers Attached to an Interface. Macromolecules 1980, 13 (5), 1069–1075. 10.1021/ma60077a009. [DOI] [Google Scholar]
- Pincus P. Colloid Stabilization with Grafted Polyelectrolytes. Macromolecules 1991, 24 (10), 2912–2919. 10.1021/ma00010a043. [DOI] [Google Scholar]
- Borisov O.; Birshtein T.; Zhulina E. Collapse of Grafted Polyelectrolyte Layer. J. Phys. II 1991, 1 (5), 521–526. 10.1051/jp2:1991186. [DOI] [Google Scholar]
- Raviv U.; Giasson S.; Kampf N.; Gohy J. F.; Jéröme R.; Klein J. Lubrication by Charged Polymers. Nature 2003, 425 (6954), 163–165. 10.1038/nature01970. [DOI] [PubMed] [Google Scholar]
- Zhulina E. B.; Rubinstein M. Lubrication by Polyelectrolyte Brushes. Macromolecules 2014, 47 (16), 5825–5838. 10.1021/ma500772a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki Y.; Kobayashi M.; Murakami D.; Takahara A. Anti-Fouling Behavior of Polymer Brush Immobilized Surfaces. Polym. J. 2016, 48 (4), 325–331. 10.1038/pj.2015.137. [DOI] [Google Scholar]
- Stuart M. A. C.; Huck W. T. S.; Genzer J.; Müller M.; Ober C.; Stamm M.; Sukhorukov G. B.; Szleifer I.; Tsukruk V. V.; Urban M.; Winnik F.; Zauscher S.; Luzinov I.; Minko S. Emerging Applications of Stimuli-Responsive Polymer Materials. Nat. Mater. 2010, 9 (2), 101–113. 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Brió Pérez M.; Cao C.; de Beer S. Switching (Bio-) Adhesion and Friction in Liquid by Stimulus Responsive Polymer Coatings. Eur. Polym. J. 2021, 147, 110298. 10.1016/j.eurpolymj.2021.110298. [DOI] [Google Scholar]
- Keating J. J.; Imbrogno J.; Belfort G. Polymer Brushes for Membrane Separations: A Review. ACS Appl. Mater. Interfaces 2016, 8 (42), 28383–28399. 10.1021/acsami.6b09068. [DOI] [PubMed] [Google Scholar]
- Chen S.; Li L.; Zhao C.; Zheng J. Surface Hydration: Principles and Applications toward Low-Fouling/Nonfouling Biomaterials. Polymer 2010, 51 (23), 5283–5293. 10.1016/j.polymer.2010.08.022. [DOI] [Google Scholar]
- Maan A. M. C.; Hofman A. H.; de Vos W. M.; Kamperman M. Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Adv. Funct. Mater. 2020, 30 (32), 2000936. 10.1002/adfm.202000936. [DOI] [Google Scholar]
- Yang Z.; Tarabara V. V.; Bruening M. L. Adsorption of Anionic or Cationic Surfactants in Polyanionic Brushes and Its Effect on Brush Swelling and Fouling Resistance during Emulsion Filtration. Langmuir 2015, 31 (43), 11790–11799. 10.1021/acs.langmuir.5b01938. [DOI] [PubMed] [Google Scholar]
- Li P.; Ding Z.; Yin Y.; Yu X.; Yuan Y.; Brió Pérez M.; de Beer S.; Vancso G. J.; Yu Y.; Zhang S. Cu2+-Doping of Polyanionic Brushes: A Facile Route to Prepare Implant Coatings with Both Antifouling and Antibacterial Properties. Eur. Polym. J. 2020, 134, 109845. 10.1016/j.eurpolymj.2020.109845. [DOI] [Google Scholar]
- Higaki Y.; Nishida J.; Takenaka A.; Yoshimatsu R.; Kobayashi M.; Takahara A. Versatile Inhibition of Marine Organism Settlement by Zwitterionic Polymer Brushes. Polym. J. 2015, 47 (12), 811–818. 10.1038/pj.2015.77. [DOI] [Google Scholar]
- Schönemann E.; Koc J.; Aldred N.; Clare A. S.; Laschewsky A.; Rosenhahn A.; Wischerhoff E. Synthesis of Novel Sulfobetaine Polymers with Differing Dipole Orientations in Their Side Chains, and Their Effects on the Antifouling Properties. Macromol. Rapid Commun. 2020, 41 (1), 1900447. 10.1002/marc.201900447. [DOI] [PubMed] [Google Scholar]
- Lin X.; Jain P.; Wu K.; Hong D.; Hung H. C.; O’Kelly M. B.; Li B.; Zhang P.; Yuan Z.; Jiang S. Ultralow Fouling and Functionalizable Surface Chemistry Based on Zwitterionic Carboxybetaine Random Copolymers. Langmuir 2019, 35 (5), 1544–1551. 10.1021/acs.langmuir.8b02540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisocherová H.; Ševců V.; Adam P.; Špačková B.; Hegnerová K.; de los Santos Pereira A.; Rodriguez-Emmenegger C.; Riedel T.; Houska M.; Brynda E.; Homola J. Functionalized Ultra-Low Fouling Carboxy- and Hydroxy-Functional Surface Platforms: Functionalization Capacity, Biorecognition Capability and Resistance to Fouling from Undiluted Biological Media. Biosens. Bioelectron. 2014, 51, 150–157. 10.1016/j.bios.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Van Andel E.; Lange S. C.; Pujari S. P.; Tijhaar E. J.; Smulders M. M. J.; Savelkoul H. F. J.; Zuilhof H. Systematic Comparison of Zwitterionic and Non-Zwitterionic Antifouling Polymer Brushes on a Bead-Based Platform. Langmuir 2019, 35 (5), 1181–1191. 10.1021/acs.langmuir.8b01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong C. S.; Al-anzi B.; Lau W. J.; Goh P. S.; Lai G. S.; Ismail A. F.; Ong Y. S. Anti-Fouling Double-Skinned Forward Osmosis Membrane with Zwitterionic Brush for Oily Wastewater Treatment. Sci. Rep. 2017, 7 (1), 1–11. 10.1038/s41598-017-07369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K.; Duan H.; Chen G. Y.; Liu X.; Yang W.; Wang D. Cleaning of Oil Fouling with Water Enabled by Zwitterionic Polyelectrolyte Coatings: Overcoming the Imperative Challenge of Oil–Water Separation Membranes. ACS Nano 2015, 9 (9), 9188–9198. 10.1021/acsnano.5b03791. [DOI] [PubMed] [Google Scholar]
- Carroll T.; Booker N. A.; Meier-Haack J. Polyelectrolyte-Grafted Microfiltration Membranes to Control Fouling by Natural Organic Matter in Drinking Water. J. Membr. Sci. 2002, 203 (1–2), 3–13. 10.1016/S0376-7388(01)00701-3. [DOI] [Google Scholar]
- Gong L.; Zhang J.; Wang W.; Xiang L.; Pan M.; Yang W.; Han L.; Wang J.; Yan B.; Zeng H. Ion-Specific Effect on Self-Cleaning Performances of Polyelectrolyte-Functionalized Membranes and the Underlying Nanomechanical Mechanism. J. Membr. Sci. 2021, 634, 119408. 10.1016/j.memsci.2021.119408. [DOI] [Google Scholar]
- Francolini I.; Vuotto C.; Piozzi A.; Donelli G. Antifouling and Antimicrobial Biomaterials: An Overview. APMIS 2017, 392–417. 10.1111/apm.12675. [DOI] [PubMed] [Google Scholar]
- Xu G.; Liu P.; Pranantyo D.; Xu L.; Neoh K. G.; Kang E. T. Antifouling and Antimicrobial Coatings from Zwitterionic and Cationic Binary Polymer Brushes Assembled via “Click” Reactions. Ind. Eng. Chem. Res. 2017, 56 (49), 14479–14488. 10.1021/acs.iecr.7b03132. [DOI] [Google Scholar]
- Xu G.; Liu P.; Pranantyo D.; Neoh K. G.; Kang E. T.; Lay-Ming Teo S. One-Step Anchoring of Tannic Acid-Scaffolded Bifunctional Coatings of Antifouling and Antimicrobial Polymer Brushes. ACS Sustainable Chem. Eng. 2019, 7 (1), 1786–1795. 10.1021/acssuschemeng.8b05789. [DOI] [Google Scholar]
- Asha A. B.; Chen Y.; Zhang H.; Ghaemi S.; Ishihara K.; Liu Y.; Narain R. Rapid Mussel-Inspired Surface Zwitteration for Enhanced Antifouling and Antibacterial Properties. Langmuir 2019, 35 (5), 1621–1630. 10.1021/acs.langmuir.8b03810. [DOI] [PubMed] [Google Scholar]
- Sinclair T. R.; Robles D.; Raza B.; van den Hengel S.; Rutjes S. A.; de Roda Husman A. M.; de Grooth J.; de Vos W. M.; Roesink H. D. W. Virus Reduction through Microfiltration Membranes Modified with a Cationic Polymer for Drinking Water Applications. Colloids Surf., A 2018, 551, 33–41. 10.1016/j.colsurfa.2018.04.056. [DOI] [Google Scholar]
- Sinclair T. R.; Patil A.; Raza B. G.; Reurink D.; van den Hengel S. K.; Rutjes S. A.; de Roda Husman A. M.; Roesink H. D. W.; de Vos W. M. Cationically Modified Membranes Using Covalent Layer-by-Layer Assembly for Antiviral Applications in Drinking Water. J. Membr. Sci. 2019, 570–571, 494–503. 10.1016/j.memsci.2018.10.081. [DOI] [Google Scholar]
- Yang Z.; Takagi R.; Zhang X.; Yasui T.; Zhang L.; Matsuyama H. Engineering a Dual-Functional Sulfonated Polyelectrolyte-Silver Nanoparticle Complex on a Polyamide Reverse Osmosis Membrane for Robust Biofouling Mitigation. J. Membr. Sci. 2021, 618, 118757. 10.1016/j.memsci.2020.118757. [DOI] [Google Scholar]
- Yaneva J.; Dimitrov D. I.; Milchev A.; Binder K. Nanoinclusions in Polymer Brushes with Explicit Solvent - A Molecular Dynamics Investigation. J. Colloid Interface Sci. 2009, 336 (1), 51–58. 10.1016/j.jcis.2009.03.062. [DOI] [PubMed] [Google Scholar]
- Yang L.; Tang C.; Ahmad M.; Yaroshchuk A.; Bruening M. L. High Selectivities among Monovalent Cations in Dialysis through Cation-Exchange Membranes Coated with Polyelectrolyte Multilayers. ACS Appl. Mater. Interfaces 2018, 10 (50), 44134–44143. 10.1021/acsami.8b16434. [DOI] [PubMed] [Google Scholar]
- Milchev A.; Dimitrov D. I.; Binder K. Excess Free Energy of Nanoparticles in a Polymer Brush. Polymer 2008, 49 (17), 3611–3618. 10.1016/j.polymer.2008.04.032. [DOI] [Google Scholar]
- Merlitz H.; Wu C. X.; Sommer J. U. Inclusion Free Energy of Nanoparticles in Polymer Brushes. Macromolecules 2012, 45 (20), 8494–8501. 10.1021/ma301781b. [DOI] [Google Scholar]
- De Beer S.; Mensink L. I. S.; Kieviet B. D. Geometry-Dependent Insertion Forces on Particles in Swollen Polymer Brushes. Macromolecules 2016, 49 (3), 1070–1078. 10.1021/acs.macromol.5b01960. [DOI] [Google Scholar]
- De Vos W. M.; Biesheuvel M.; De Keizer A.; Kleijn M.; Cohen Stuart M. A. Adsorption of Anionic Surfactants in a Nonionic Polymer Brush Experiments, Comparison with Mean-Field Theory, and Implications for Brush-Particle Interaction. Langmuir 2009, 25 (16), 9252–9261. 10.1021/la900791b. [DOI] [PubMed] [Google Scholar]
- De Vos W. M.; Leermakers F. A. M.; Lindhoud S.; Prescott S. W. Modeling the Structure and Antifouling Properties of a Polymer Brush of Grafted Comb-Polymers. Macromolecules 2011, 44 (7), 2334–2342. 10.1021/ma1028642. [DOI] [Google Scholar]
- Milner S. T.; Witten T. A.; Cates M. E. Effects of Polydispersity in the End-Grafted Polymer Brush. Macromolecules 1989, 22 (2), 853–861. 10.1021/ma00192a057. [DOI] [Google Scholar]
- de Vos W. M.; Leermakers F. A. M. Modeling the Structure of a Polydisperse Polymer Brush. Polymer 2009, 50 (1), 305–316. 10.1016/j.polymer.2008.10.025. [DOI] [Google Scholar]
- Qi S.; Klushin L. I.; Skvortsov A. M.; Schmid F. Polydisperse Polymer Brushes: Internal Structure, Critical Behavior, and Interaction with Flow. Macromolecules 2016, 49 (24), 9665–9683. 10.1021/acs.macromol.6b02026. [DOI] [Google Scholar]
- de Vos W. M.; Leermakers F. A. M.; de Keizer A.; Kleijn J. M.; Cohen Stuart M. A. Interaction of Particles with a Polydisperse Brush: A Self-Consistent-Field Analysis. Macromolecules 2009, 42 (15), 5881–5891. 10.1021/ma900819b. [DOI] [Google Scholar]
- Qi S. Particle Penetration into Polydisperse Polymer Brushes: A Theoretical Analysis. Macromol. Theory Simul. 2017, 26 (5), 1–9. 10.1002/mats.201700029. [DOI] [Google Scholar]
- Swift T.; Swanson L.; Geoghegan M.; Rimmer S. The pH-Responsive Behaviour of Poly(Acrylic Acid) in Aqueous Solution Is Dependent on Molar Mass. Soft Matter 2016, 12 (9), 2542–2549. 10.1039/C5SM02693H. [DOI] [PubMed] [Google Scholar]
- Farina R.; Laugel N.; Yu J.; Tirrell M. Reversible Adhesion with Polyelectrolyte Brushes Tailored via the Uptake and Release of Trivalent Lanthanum Ions. J. Phys. Chem. C 2015, 119 (26), 14805–14814. 10.1021/acs.jpcc.5b02121. [DOI] [Google Scholar]
- Hollingsworth N. R.; Wilkanowicz S. I.; Larson R. G. Salt- And PH-Induced Swelling of a Poly(Acrylic Acid) Brush: Via Quartz Crystal Microbalance w/Dissipation (QCM-D). Soft Matter 2019, 15 (39), 7838–7851. 10.1039/C9SM01289C. [DOI] [PubMed] [Google Scholar]
- Weir M. P.; Heriot S. Y.; Martin S. J.; Parnell A. J.; Holt S. A.; Webster J. R. P.; Jones R. A. L. Voltage-Induced Swelling and Deswelling of Weak Polybase Brushes. Langmuir 2011, 27 (17), 11000–11007. 10.1021/la201343w. [DOI] [PubMed] [Google Scholar]
- Hoy O.; Zdyrko B.; Lupitskyy R.; Sheparovych R.; Aulich D.; Wang J.; Bittrich E.; Eichhorn K.-J.; Uhlmann P.; Hinrichs K.; Müller M.; Stamm M.; Minko S.; Luzinov I. Synthetic Hydrophilic Materials with Tunable Strength and a Range of Hydrophobic Interactions. Adv. Funct. Mater. 2010, 20 (14), 2240–2247. 10.1002/adfm.201000170. [DOI] [Google Scholar]
- Raftari M.; Zhang Z.; Carter S. R.; Leggett G. J.; Geoghegan M. Frictional Properties of a Polycationic Brush. Soft Matter 2014, 10 (16), 2759–2766. 10.1039/c3sm53201a. [DOI] [PubMed] [Google Scholar]
- Xu G.; Neoh K. G.; Kang E. T.; Teo S. L. M. Switchable Antimicrobial and Antifouling Coatings from Tannic Acid-Scaffolded Binary Polymer Brushes. ACS Sustainable Chem. Eng. 2020, 8 (6), 2586–2595. 10.1021/acssuschemeng.9b07836. [DOI] [Google Scholar]
- Zeng H.; Zhang Y.; Mao S.; Nakajima H.; Uchiyama K. A Reversibly Electro-Controllable Polymer Brush for Electro-Switchable Friction. J. Mater. Chem. C 2017, 5 (24), 5877–5881. 10.1039/C7TC01624G. [DOI] [Google Scholar]
- Nordgren N.; Rutland M. W. Tunable Nanolubrication between Dual-Responsive Polyionic Grafts. Nano Lett. 2009, 9 (8), 2984–2990. 10.1021/nl901411e. [DOI] [PubMed] [Google Scholar]
- Zhou F.; Huck W. T. S. Three-Stage Switching of Surface Wetting Using Phosphate-Bearing Polymer Brushes. Chem. Commun. 2005, (48), 5999–6001. 10.1039/b512106j. [DOI] [PubMed] [Google Scholar]
- Azzaroni O.; Brown A. A.; Huck W. T. S. UCST Wetting Transitions of Polyzwitterionic Brushes Driven by Self-Association. Angew. Chem. 2006, 118 (11), 1802–1806. 10.1002/ange.200503264. [DOI] [PubMed] [Google Scholar]
- Yang J.; Chen H.; Xiao S.; Shen M.; Chen F.; Fan P.; Zhong M.; Zheng J. Salt-Responsive Zwitterionic Polymer Brushes with Tunable Friction and Antifouling Properties. Langmuir 2015, 31 (33), 9125–9133. 10.1021/acs.langmuir.5b02119. [DOI] [PubMed] [Google Scholar]
- Du T.; Ma S.; Pei X.; Wang S.; Zhou F. Bio-Inspired Design and Fabrication of Micro/Nano-Brush Dual Structural Surfaces for Switchable Oil Adhesion and Antifouling. Small 2017, 13 (4), 1602020. 10.1002/smll.201602020. [DOI] [PubMed] [Google Scholar]
- Sheiko S. S.; Panyukov S.; Rubinstein M. Bond Tension in Tethered Macromolecules. Macromolecules 2011, 44 (11), 4520–4529. 10.1021/ma200328h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok H. A.; Genzer J. Expanding the Polymer Mechanochemistry Toolbox through Surface-Initiated Polymerization. ACS Macro Lett. 2015, 4 (6), 636–639. 10.1021/acsmacrolett.5b00295. [DOI] [PubMed] [Google Scholar]
- Wang J.; Klok H. A. Swelling-Induced Chain Stretching Enhances Hydrolytic Degrafting of Hydrophobic Polymer Brushes in Organic Media. Angew. Chem., Int. Ed. 2019, 58 (29), 9989–9993. 10.1002/anie.201904436. [DOI] [PubMed] [Google Scholar]
- Li Y.; Lin Y.; Ko Y.; Kiserow D.; Genzer J. Visualization of Mechanochemically-Assisted Degrafting of Surface-Tethered Poly(Acrylic Acid) Brushes. ACS Macro Lett. 2018, 7 (6), 609–613. 10.1021/acsmacrolett.8b00241. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Lv B.; Lu Z.; He J.; Zhang S.; Chen H.; Ma H. Predicting Au-S Bond Breakage from the Swelling Behavior of Surface Tethered Polyelectrolytes. Soft Matter 2011, 7 (24), 11496–11500. 10.1039/c1sm05895a. [DOI] [Google Scholar]
- Brió Pérez M.; Cirelli M.; de Beer S. Degrafting of Polymer Brushes by Exposure to Humid Air. ACS Appl. Polym. Mater. 2020, 2 (8), 3039–3043. 10.1021/acsapm.0c00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paripovic D.; Klok H. A. Improving the Stability in Aqueous Media of Polymer Brushes Grafted from Silicon Oxide Substrates by Surface-Initiated Atom Transfer Radical Polymerization. Macromol. Chem. Phys. 2011, 212 (9), 950–958. 10.1002/macp.201000729. [DOI] [Google Scholar]
- Li Y.; Ko Y.; Lin Y.; Kiserow D.; Genzer J. Enhanced Stability of Surface-Tethered Diblock Copolymer Brushes with a Neutral Polymer Block and a Weak Polyelectrolyte Block: Effects of Molecular Weight and Hydrophobicity of the Neutral Block. Macromolecules 2017, 50 (21), 8580–8587. 10.1021/acs.macromol.7b01825. [DOI] [Google Scholar]
- Quintana R.; Gosa M.; Jańczewski D.; Kutnyanszky E.; Vancso G. J. Enhanced Stability of Low Fouling Zwitterionic Polymer Brushes in Seawater with Diblock Architecture. Langmuir 2013, 29 (34), 10859–10867. 10.1021/la402287a. [DOI] [PubMed] [Google Scholar]
- Divandari M.; Dehghani E. S.; Spencer N. D.; Ramakrishna S. N.; Benetti E. M. Understanding the Effect of Hydrophobic Protecting Blocks on the Stability and Biopassivity of Polymer Brushes in Aqueous Environments: A Tiramisù for Cell-Culture Applications. Polymer 2016, 98, 470–480. 10.1016/j.polymer.2016.04.042. [DOI] [Google Scholar]
- Yu Y.; Vancso G. J.; de Beer S. Substantially Enhanced Stability against Degrafting of Zwitterionic PMPC Brushes by Utilizing PGMA-Linked Initiators. Eur. Polym. J. 2017, 89, 221–229. 10.1016/j.eurpolymj.2017.02.033. [DOI] [Google Scholar]
- Yu Y.; Cirelli M.; Li P.; Ding Z.; Yin Y.; Yuan Y.; De Beer S.; Vancso G. J.; Zhang S. Enhanced Stability of Poly(3-Sulfopropyl Methacrylate Potassium) Brushes Coated on Artificial Implants in Combatting Bacterial Infections. Ind. Eng. Chem. Res. 2019, 58 (47), 21459–21465. 10.1021/acs.iecr.9b03980. [DOI] [Google Scholar]
- Rodriguez-Emmenegger C.; Preuss C. M.; Yameen B.; Pop-Georgievski O.; Bachmann M.; Mueller J. O.; Bruns M.; Goldmann A. S.; Bastmeyer M.; Barner-Kowollik C. Controlled Cell Adhesion on Poly(Dopamine) Interfaces Photopatterned with Non-Fouling Brushes. Adv. Mater. 2013, 25 (42), 6123–6127. 10.1002/adma.201302492. [DOI] [PubMed] [Google Scholar]
- Decher G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science (Washington, DC, U. S.) 1997, 277 (5330), 1232–1237. 10.1126/science.277.5330.1232. [DOI] [Google Scholar]
- Glinel K.; Déjugnat C.; Prevot M.; Schöler B.; Schönhoff M.; Klitzing R. v. Responsive Polyelectrolyte Multilayers. Colloids Surf., A 2007, 303 (1–2), 3–13. 10.1016/j.colsurfa.2007.02.052. [DOI] [Google Scholar]
- Izumrudov V. A.; Mussabayeva B. K.; Murzagulova K. B. Polyelectrolyte Multilayers: Preparation and Applications. Russ. Chem. Rev. 2018, 87 (2), 192–200. 10.1070/RCR4767. [DOI] [Google Scholar]
- Liu C.; Shi L.; Wang R. Crosslinked Layer-by-Layer Polyelectrolyte Nanofiltration Hollow Fiber Membrane for Low-Pressure Water Softening with the Presence of SO42- in Feed Water. J. Membr. Sci. 2015, 486, 169–176. 10.1016/j.memsci.2015.03.050. [DOI] [Google Scholar]
- Cheng W.; Liu C.; Tong T.; Epsztein R.; Sun M.; Verduzco R.; Ma J.; Elimelech M. Selective Removal of Divalent Cations by Polyelectrolyte Multilayer Nanofiltration Membrane: Role of Polyelectrolyte Charge, Ion Size, and Ionic Strength. J. Membr. Sci. 2018, 559, 98–106. 10.1016/j.memsci.2018.04.052. [DOI] [Google Scholar]
- Ng L. Y.; Mohammad A. W.; Ng C. Y.; Leo C. P.; Rohani R. Development of Nanofiltration Membrane with High Salt Selectivity and Performance Stability Using Polyelectrolyte Multilayers. Desalination 2014, 351, 19–26. 10.1016/j.desal.2014.07.020. [DOI] [Google Scholar]
- Ouyang L.; Malaisamy R.; Bruening M. L. Multilayer Polyelectrolyte Films as Nanofiltration Membranes for Separating Monovalent and Divalent Cations. J. Membr. Sci. 2008, 310 (1–2), 76–84. 10.1016/j.memsci.2007.10.031. [DOI] [Google Scholar]
- Wang J.; Wang Z.; Liu Y.; Wang J.; Wang S. Surface Modification of NF Membrane with Zwitterionic Polymer to Improve Anti-Biofouling Property. J. Membr. Sci. 2016, 514, 407–417. 10.1016/j.memsci.2016.05.014. [DOI] [Google Scholar]
- Chaudhury S.; Wormser E.; Harari Y.; Edri E.; Nir O. Tuning the Ion-Selectivity of Thin-Film Composite Nanofiltration Membranes by Molecular Layer Deposition of Alucone. ACS Appl. Mater. Interfaces 2020, 12 (47), 53356–53364. 10.1021/acsami.0c16569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z.; Fathizadeh M.; Huang Y.; Chu K. H.; Yoon Y.; Wang L.; Xu W. L.; Yu M. TiO2 Nanofiltration Membranes Prepared by Molecular Layer Deposition for Water Purification. J. Membr. Sci. 2016, 510, 72–78. 10.1016/j.memsci.2016.03.011. [DOI] [Google Scholar]
- Zhou X.; Zhao Y. Y.; Kim S. R.; Elimelech M.; Hu S.; Kim J. H. Controlled TiO2 Growth on Reverse Osmosis and Nanofiltration Membranes by Atomic Layer Deposition: Mechanisms and Potential Applications. Environ. Sci. Technol. 2018, 52 (24), 14311–14320. 10.1021/acs.est.8b03967. [DOI] [PubMed] [Google Scholar]
- Gao S.; Zhu Y.; Gong Y.; Wang Z.; Fang W.; Jin J. Ultrathin Polyamide Nanofiltration Membrane Fabricated on Brush-Painted Single-Walled Carbon Nanotube Network Support for Ion Sieving. ACS Nano 2019, 13 (5), 5278–5290. 10.1021/acsnano.8b09761. [DOI] [PubMed] [Google Scholar]
- Hu R.; He Y.; Zhang C.; Zhang R.; Li J.; Zhu H. Graphene Oxide-Embedded Polyamide Nanofiltration Membranes for Selective Ion Separation. J. Mater. Chem. A 2017, 5, 25632–25640. 10.1039/C7TA08635K. [DOI] [Google Scholar]
- Homayoonfal M.; Akbari A.; Mehrnia M. R. Preparation of Polysulfone Nanofiltration Membranes by UV-Assisted Grafting Polymerization for Water Softening. Desalination 2010, 263 (1–3), 217–225. 10.1016/j.desal.2010.06.062. [DOI] [Google Scholar]
- Yuan B.; Li P.; Wang P.; Yang H.; Sun H.; Li P.; Sun H.; Niu Q. J. Novel Aliphatic Polyamide Membrane with High Mono-/Divalent Ion Selectivity, Excellent Ca2+, Mg2+ Rejection, and Improved Antifouling Properties. Sep. Purif. Technol. 2019, 224, 443–455. 10.1016/j.seppur.2019.05.021. [DOI] [Google Scholar]
- te Brinke E.; Reurink D. M.; Achterhuis I.; de Grooth J.; de Vos W. M. Asymmetric Polyelectrolyte Multilayer Membranes with Ultrathin Separation Layers for Highly Efficient Micropollutant Removal. Appl. Mater. Today 2020, 18, 100471. 10.1016/j.apmt.2019.100471. [DOI] [Google Scholar]
- Nava-Ocampo M. F.; Bucs S. S.; Farinha A. S. F.; Son M.; Logan B. E.; Vrouwenvelder J. S. Sacrificial Coating Development for Biofouling Control in Membrane Systems. Desalination 2020, 496, 114650. 10.1016/j.desal.2020.114650. [DOI] [Google Scholar]
- Esker A. R.; Mengel C.; Wegner G. Ultrathin Films of a Polyelectrolyte with Layered Architecture. Science (Washington, DC, U. S.) 1998, 280 (5365), 892–895. 10.1126/science.280.5365.892. [DOI] [PubMed] [Google Scholar]
- Richardson J. J.; Björnmalm M.; Caruso F. Technology-Driven Layer-by-Layer Assembly of Nanofilms. Science (Washington, DC, U. S.) 2015, 348 (6233), aaa2491. 10.1126/science.aaa2491. [DOI] [PubMed] [Google Scholar]
- Sanyal O.; Lee I. Recent Progress in the Applications of Layer-by-Layer Assembly to the Preparation of Nanostructured Ion-Rejecting Water Purification Membranes. J. Nanosci. Nanotechnol. 2014, 14 (3), 2178–2189. 10.1166/jnn.2014.8541. [DOI] [PubMed] [Google Scholar]
- de Grooth J.; Oborný R.; Potreck J.; Nijmeijer K.; de Vos W. M. The Role of Ionic Strength and Odd – Even Effects on the Properties of Polyelectrolyte Multilayer Nano Fi Ltration Membranes. J. Membr. Sci. 2015, 475, 311–319. 10.1016/j.memsci.2014.10.044. [DOI] [Google Scholar]
- DuChanois R. M.; Epsztein R.; Trivedi J. A.; Elimelech M. Controlling Pore Structure of Polyelectrolyte Multilayer Nanofiltration Membranes by Tuning Polyelectrolyte-Salt Interactions. J. Membr. Sci. 2019, 581, 413–420. 10.1016/j.memsci.2019.03.077. [DOI] [Google Scholar]
- Ilyas S.; Abtahi S. M.; Akkilic N.; Roesink H. D. W.; de Vos W. M. Weak Polyelectrolyte Multilayers as Tunable Separation Layers for Micro-Pollutant Removal by Hollow Fiber Nanofiltration Membranes. J. Membr. Sci. 2017, 537, 220–228. 10.1016/j.memsci.2017.05.027. [DOI] [Google Scholar]
- Ng L. Y.; Mohammad A. W.; Ng C. Y. A Review on Nanofiltration Membrane Fabrication and Modification Using Polyelectrolytes: Effective Ways to Develop Membrane Selective Barriers and Rejection Capability. Adv. Colloid Interface Sci. 2013, 197–198, 85–107. 10.1016/j.cis.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Cheng C.; Yaroshchuk A.; Bruening M. L. Fundamentals of Selective Ion Transport through Multilayer Polyelectrolyte Membranes. Langmuir 2013, 29 (6), 1885–1892. 10.1021/la304574e. [DOI] [PubMed] [Google Scholar]
- Ahmadiannamini P.; Bruening M. L.; Tarabara V. V. Sacrificial Polyelectrolyte Multilayer Coatings as an Approach to Membrane Fouling Control: Disassembly and Regeneration Mechanisms. J. Membr. Sci. 2015, 491, 149–158. 10.1016/j.memsci.2015.04.041. [DOI] [Google Scholar]
- Virga E.; de Grooth J.; Žvab K.; de Vos W. M. Stable Polyelectrolyte Multilayer-Based Hollow Fiber Nanofiltration Membranes for Produced Water Treatment. ACS Appl. Polym. Mater. 2019, 1 (8), 2230–2239. 10.1021/acsapm.9b00503. [DOI] [Google Scholar]
- Chandra P. N.; Usha K. Materials Today : Proceedings Removal of Atrazine Herbicide from Water by Polyelectrolyte Multilayer Membranes. Mater. Today Proc. 2021, 41 (3), 622–627. 10.1016/j.matpr.2020.05.263. [DOI] [Google Scholar]
- Wang Y.; Zucker I.; Boo C.; Elimelech M. Removal of Emerging Wastewater Organic Contaminants by Polyelectrolyte Multilayer Nanofiltration Membranes with Tailored Selectivity. ACS EST Engg. 2021, 1 (3), 404–414. 10.1021/acsestengg.0c00160. [DOI] [Google Scholar]
- Yuan W.; Weng G. M.; Lipton J.; Li C. M.; Van Tassel P. R.; Taylor A. D. Weak Polyelectrolyte-Based Multilayers via Layer-by-Layer Assembly: Approaches, Properties, and Applications. Adv. Colloid Interface Sci. 2020, 282, 102200. 10.1016/j.cis.2020.102200. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Wang Z.; Epsztein R.; Zhan C.; Li W.; Fortner J. D.; Pham T. A.; Kim J. H.; Elimelech M. Intrapore Energy Barriers Govern Ion Transport and Selectivity of Desalination Membranes. Sci. Adv. 2020, 6 (48), eabd9045. 10.1126/sciadv.abd9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirir Y. I.; Hanafi Y.; Ghoufi A.; Szymczyk A. Theoretical Investigation of the Ionic Selectivity of Polyelectrolyte Multilayer Membranes in Nanofiltration. Langmuir 2015, 31 (1), 451–457. 10.1021/la5044188. [DOI] [PubMed] [Google Scholar]
- Tosun J.; Schaub S.; Fleig A. What Determines Regulatory Preferences? Insights from Micropollutants in Surface Waters. Environ. Sci. Policy 2020, 106, 136–144. 10.1016/j.envsci.2020.02.001. [DOI] [Google Scholar]
- Al-Rifai J. H.; Khabbaz H.; Schäfer A. I. Removal of Pharmaceuticals and Endocrine Disrupting Compounds in a Water Recycling Process Using Reverse Osmosis Systems. Sep. Purif. Technol. 2011, 77 (1), 60–67. 10.1016/j.seppur.2010.11.020. [DOI] [Google Scholar]
- Malaisamy R.; Bruening M. L. High-Flux Nanofiltration Membranes Prepared by Adsorption of Multilayer Polyelectrolyte Membranes on Polymeric Supports. Langmuir 2005, 21 (23), 10587–10592. 10.1021/la051669s. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zucker I.; Boo C.; Elimelech M. Removal of Emerging Wastewater Organic Contaminants by Polyelectrolyte Multilayer Nanofiltration Membranes with Tailored Selectivity. ACS ES&T Eng. 2021, 1 (3), 404–414. 10.1021/acsestengg.0c00160. [DOI] [Google Scholar]
- Kamp J.; Emonds S.; Wessling M. Designing Tubular Composite Membranes of Polyelectrolyte Multilayer on Ceramic Supports with Nanofiltration and Reverse Osmosis Transport Properties. J. Membr. Sci. 2021, 620, 118851. 10.1016/j.memsci.2020.118851. [DOI] [Google Scholar]
- Hong S. U.; Bruening M. L. Separation of Amino Acid Mixtures Using Multilayer Polyelectrolyte Nanofiltration Membranes. J. Membr. Sci. 2006, 280 (1–2), 1–5. 10.1016/j.memsci.2006.04.028. [DOI] [Google Scholar]
- Hong S. U.; Miller M. D.; Bruening M. L. Removal of Dyes, Sugars, and Amino Acids from NaCl Solutions Using Multilayer Polyelectrolyte Nanofiltration Membranes. Ind. Eng. Chem. Res. 2006, 45 (18), 6284–6288. 10.1021/ie060239+. [DOI] [Google Scholar]
- Reurink D. M.; Willott J. D.; Roesink H. D. W.; De Vos W. M. Role of Polycation and Cross-Linking in Polyelectrolyte Multilayer Membranes. ACS Appl. Polym. Mater. 2020, 2 (11), 5278–5289. 10.1021/acsapm.0c00992. [DOI] [Google Scholar]
- Avram A. M.; Ahmadiannamini P.; Vu A.; Qian X.; Sengupta A.; Wickramasinghe S. R. Polyelectrolyte Multilayer Modified Nanofiltration Membranes for the Recovery of Ionic Liquid from Dilute Aqueous Solutions. J. Appl. Polym. Sci. 2017, 134 (39), 45349. 10.1002/app.45349. [DOI] [Google Scholar]
- Abtahi S. M.; Ilyas S.; Joannis Cassan C.; Albasi C.; de Vos W. M. Micropollutants Removal from Secondary-Treated Municipal Wastewater Using Weak Polyelectrolyte Multilayer Based Nanofiltration Membranes. J. Membr. Sci. 2018, 548, 654–666. 10.1016/j.memsci.2017.10.045. [DOI] [Google Scholar]
- Abtahi S. M.; Marbelia L.; Gebreyohannes A. Y.; Ahmadiannamini P.; Joannis-Cassan C.; Albasi C.; de Vos W. M.; Vankelecom I. F. J. Micropollutant Rejection of Annealed Polyelectrolyte Multilayer Based Nanofiltration Membranes for Treatment of Conventionally-Treated Municipal Wastewater. Sep. Purif. Technol. 2019, 209, 470–481. 10.1016/j.seppur.2018.07.071. [DOI] [Google Scholar]
- Magnenet C.; Jurin F. E.; Lakard S.; Buron C. C.; Lakard B. Polyelectrolyte Modification of Ultrafiltration Membrane for Removal of Copper Ions. Colloids Surf., A 2013, 435, 170–177. 10.1016/j.colsurfa.2012.12.028. [DOI] [Google Scholar]
- Alghamdi A. M. Fast and Versatile Pathway in Fabrication of Polyelectrolyte Multilayer Nanofiltration Membrane with Tunable Properties. J. Chem. 2021, 9978596. 10.1155/2021/9978596. [DOI] [Google Scholar]
- Jin W.; Toutianoush A.; Tieke B. Use of Polyelectrolyte Layer-by-Layer Assemblies as Nanofiltration and Reverse Osmosis Membranes. Langmuir 2003, 19 (7), 2550–2553. 10.1021/la020926f. [DOI] [Google Scholar]
- Liu Y.; Chen G. Q.; Yang X.; Deng H. Preparation of Layer-by-Layer Nanofiltration Membranes by Dynamic Deposition and Crosslinking. Membranes (Basel, Switz.) 2019, 9 (2), 20. 10.3390/membranes9020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W.; Ngo H. H.; Li J. A Mini-Review on Membrane Fouling. Bioresour. Technol. 2012, 122, 27–34. 10.1016/j.biortech.2012.04.089. [DOI] [PubMed] [Google Scholar]
- Porcelli N.; Judd S. Chemical Cleaning of Potable Water Membranes: A Review. Sep. Purif. Technol. 2010, 71 (2), 137–143. 10.1016/j.seppur.2009.12.007. [DOI] [Google Scholar]
- Shi X.; Tal G.; Hankins N. P.; Gitis V. Fouling and Cleaning of Ultrafiltration Membranes: A Review. Journal of Water Process Engineering 2014, 1, 121–138. 10.1016/j.jwpe.2014.04.003. [DOI] [Google Scholar]
- Goosen M. F. A.; Sablani S. S.; Al-Hinai H.; Al-Obeidani S.; Al-Belushi R.; Jackson D. Fouling of Reverse Osmosis and Ultrafiltration Membranes: A Critical Review. Sep. Sci. Technol. 2005, 39 (10), 2261–2297. 10.1081/SS-120039343. [DOI] [Google Scholar]
- Nunes S. P. Can Fouling in Membranes Be Ever Defeated?. Curr. Opin. Chem. Eng. 2020, 28, 90–95. 10.1016/j.coche.2020.03.006. [DOI] [Google Scholar]
- Fadhillah F.; Alghamdi A. M.; Alsubei M. D.; Aljlil S. A. Synthesis of Protein-Fouling-Resistance Polyelectrolyte Multilayered Nanofiltration Membranes through Spin-Assisted Layer-by-Layer Assembly. J. King Saud Univ., Eng. Sci. 2021, 33 (2), 81–87. 10.1016/j.jksues.2020.04.003. [DOI] [Google Scholar]
- Virga E.; Žvab K.; de Vos W. M. Fouling of Nanofiltration Membranes Based on Polyelectrolyte Multilayers: The Effect of a Zwitterionic Final Layer. J. Membr. Sci. 2021, 620, 118793. 10.1016/j.memsci.2020.118793. [DOI] [Google Scholar]
- Ilyas S.; de Grooth J.; Nijmeijer K.; De Vos W. M. Multifunctional Polyelectrolyte Multilayers as Nanofiltration Membranes and as Sacrificial Layers for Easy Membrane Cleaning. J. Colloid Interface Sci. 2015, 446, 386–393. 10.1016/j.jcis.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Syed J. A.; Meng X. Intelligent Anticorrosion Saline-Enabled Self-Healing Polyelectrolyte Multilayer Coatings. Advances in Smart Coatings and Thin Films for Future Industrial and Biomedical Engineering Applications 2020, 207–243. 10.1016/B978-0-12-849870-5.00006-9. [DOI] [Google Scholar]
- Jiang T.; Moghaddam S. Z.; Thormann E. A pH-Responsive Polyelectrolyte Multilayer Film with Tunable Interfacial Properties. Polymer 2021, 214, 123367. 10.1016/j.polymer.2020.123367. [DOI] [Google Scholar]
- Xu L.; Wang H.; Chu Z.; Cai L.; Shi H.; Zhu C.; Pan D.; Pan J.; Fei X.; Lei Y. Temperature-Responsive Multilayer Films of Micelle-Based Composites for Controlled Release of a Third-Generation EGFR Inhibitor. ACS Appl. Polym. Mater. 2020, 2 (2), 741–750. 10.1021/acsapm.9b01051. [DOI] [Google Scholar]
- Irigoyen J.; Han L.; Llarena I.; Mao Z.; Gao C.; Moya S. E. Responsive Polyelectrolyte Multilayers Assembled at High Ionic Strength with an Unusual Collapse at Low Ionic Strength. Macromol. Rapid Commun. 2012, 33 (22), 1964–1969. 10.1002/marc.201200471. [DOI] [PubMed] [Google Scholar]
- De Grooth J.; Dong M.; De Vos W. M.; Nijmeijer K. Building Polyzwitterion-Based Multilayers for Responsive Membranes. Langmuir 2014, 30 (18), 5152–5161. 10.1021/la500857b. [DOI] [PubMed] [Google Scholar]
- Cho K. L.; Hill A. J.; Caruso F.; Kentish S. E. Chlorine Resistant Glutaraldehyde Crosslinked Polyelectrolyte Multilayer Membranes for Desalination. Adv. Mater. 2015, 27 (17), 2791–2796. 10.1002/adma.201405783. [DOI] [PubMed] [Google Scholar]
- Li X.; De Feyter S.; Chen D.; Aldea S.; Vandezande P.; Du Prez F.; Vankelecom I. F. J. Solvent-Resistant Nanofiltration Membranes Based on Multilayered Polyelectrolyte Complexes. Chem. Mater. 2008, 20 (12), 3876–3883. 10.1021/cm703072k. [DOI] [Google Scholar]
- Menne D.; Kamp J.; Erik Wong J.; Wessling M. Precise Tuning of Salt Retention of Backwashable Polyelectrolyte Multilayer Hollow Fiber Nanofiltration Membranes. J. Membr. Sci. 2016, 499, 396–405. 10.1016/j.memsci.2015.10.058. [DOI] [Google Scholar]
- Kang J.; Dähne L. Strong Response of Multilayer Polyelectrolyte Films to Cationic Surfactants. Langmuir 2011, 27 (8), 4627–2634. 10.1021/la104610a. [DOI] [PubMed] [Google Scholar]
- Duong P. H. H.; Zuo J.; Chung T. S. Highly Crosslinked Layer-by-Layer Polyelectrolyte FO Membranes: Understanding Effects of Salt Concentration and Deposition Time on FO Performance. J. Membr. Sci. 2013, 427, 411–421. 10.1016/j.memsci.2012.10.014. [DOI] [Google Scholar]
- An Q.; Huang T.; Shi F. Covalent Layer-by-Layer Films: Chemistry, Design, and Multidisciplinary Applications. Chem. Soc. Rev. 2018, 47, 5061–5098. 10.1039/C7CS00406K. [DOI] [PubMed] [Google Scholar]
- Virga E.; de Grooth J.; Zvab K.; de Vos W. M. Stable Polyelectrolyte Multilayer-Based Hollow Fiber Nanofiltration Membranes for Produced Water Treatment. ACS Appl. Polym. Mater. 2019, 1 (8), 2230–2239. 10.1021/acsapm.9b00503. [DOI] [Google Scholar]
- Luo T.; Abdu S.; Wessling M. Selectivity of Ion Exchange Membranes: A Review. J. Membr. Sci. 2018, 555, 429–454. 10.1016/j.memsci.2018.03.051. [DOI] [Google Scholar]
- Sahin S.; Dykstra J. E.; Zuilhof H.; Zornitta R. L.; de Smet L. C. P. M. Modification of Cation-Exchange Membranes with Polyelectrolyte Multilayers to Tune Ion Selectivity in Capacitive Deionization. ACS Appl. Mater. Interfaces 2020, 12 (31), 34746–34754. 10.1021/acsami.0c05664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdu S.; Martí-Calatayud M.-C.; Wong J. E.; García-Gabaldón M.; Wessling M. Layer-by-Layer Modification of Cation Exchange Membranes Controls Ion Selectivity and Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 1843–1854. 10.1021/am4048317. [DOI] [PubMed] [Google Scholar]
- Mulyati S.; Takagi R.; Fujii A.; Ohmukai Y.; Matsuyama H. Simultaneous Improvement of the Monovalent Anion Selectivity and Antifouling Properties of an Anion Exchange Membrane in an Electrodialysis Process, Using Polyelectrolyte Multilayer Deposition. J. Membr. Sci. 2013, 431, 113–120. 10.1016/j.memsci.2012.12.022. [DOI] [Google Scholar]
- Zhao Z.; Shi S.; Cao H.; Li Y.; Van der Bruggen B. Layer-by-Layer Assembly of Anion Exchange Membrane by Electrodeposition of Polyelectrolytes for Improved Antifouling Performance. J. Membr. Sci. 2018, 558 (1), 1–8. 10.1016/j.memsci.2018.04.035. [DOI] [Google Scholar]
- White N.; Misovich M.; Yaroshchuk A.; Bruening M. L. Coating of Nafion Membranes with Polyelectrolyte Multilayers to Achieve High Monovalent/Divalent Cation Electrodialysis Selectivities. ACS Appl. Mater. Interfaces 2015, 7, 6620–6628. 10.1021/am508945p. [DOI] [PubMed] [Google Scholar]
- White N.; Misovich M.; Alemayehu E.; Yaroshchuk A.; Bruening M. L. Highly Selective Separations of Multivalent and Monovalent Cations in Electrodialysis through Nafion Membranes Coated with Polyelectrolyte Multilayers. Polymer 2016, 103, 478–485. 10.1016/j.polymer.2015.12.019. [DOI] [Google Scholar]
- Yang L.; Tang C.; Ahmad M.; Yaroshchuk A.; Bruening M. Correction to “High Selectivities among Monovalent Cations in Dialysis through Cation-Exchange Membranes Coated with Polyelectrolyte Multilayers.. ACS Appl. Mater. Interfaces 2021, 13 (18), 22073–22073. 10.1021/acsami.1c06321. [DOI] [PubMed] [Google Scholar]
- Rijnaarts T.; Reurink D. M.; Radmanesh F.; de Vos W. M.; Nijmeijer K. Layer-by-Layer Coatings on Ion Exchange Membranes: Effect of Multilayer Charge and Hydration on Monovalent Ion Selectivities. J. Membr. Sci. 2019, 570–571, 513–521. 10.1016/j.memsci.2018.10.074. [DOI] [Google Scholar]
- Zhao Y.; Gao C.; Van Der Bruggen B. Technology-Driven Layer-by-Layer Assembly of a Membrane for Selective Separation of Monovalent Anions and Antifouling. Nanoscale 2019, 11 (5), 2264–2274. 10.1039/C8NR09086F. [DOI] [PubMed] [Google Scholar]
- Gao H.; Zhang B.; Tong X.; Chen Y. Monovalent-Anion Selective and Antifouling Polyelectrolytes Multilayer Anion Exchange Membrane for Reverse Electrodialysis. J. Membr. Sci. 2018, 567, 68–75. 10.1016/j.memsci.2018.09.035. [DOI] [Google Scholar]
- Ahmad M.; Tang C.; Yang L.; Yaroshchuk A.; Bruening M. L. Layer-by-Layer Modification of Aliphatic Polyamide Anion-Exchange Membranes to Increase Cl–/SO42– Selectivity. J. Membr. Sci. 2019, 578, 209–219. 10.1016/j.memsci.2019.02.018. [DOI] [Google Scholar]
- Singh K.; Sahin S.; Gamaethiralalage J. G.; Zornitta R. L.; de Smet L. C. P. M. Simultaneous, Monovalent Ion Selectivity with Polyelectrolyte Multilayers and Intercalation Electrodes in Capacitive Deionization. Chem. Eng. J. 2021, 128329. 10.1016/j.cej.2020.128329. [DOI] [Google Scholar]
- Liu H.; Ruan H.; Zhao Y.; Pan J.; Sotto A.; Gao C.; van der Bruggen B.; Shen J. A Facile Avenue to Modify Polyelectrolyte Multilayers on Anion Exchange Membranes to Enhance Monovalent Selectivity and Durability Simultaneously. J. Membr. Sci. 2017, 543 (June), 310–318. 10.1016/j.memsci.2017.08.072. [DOI] [Google Scholar]
- Zhao Y.; Zhu J.; Ding J.; Van der Bruggen B.; Shen J.; Gao C. Electric-Pulse Layer-by-Layer Assembled of Anion Exchange Membrane with Enhanced Monovalent Selectivity. J. Membr. Sci. 2018, 548, 81–90. 10.1016/j.memsci.2017.11.007. [DOI] [Google Scholar]
- Gamaethiralalage J. G.; Singh K.; Sahin S.; Yoon J.; Elimelech M.; Suss M. E.; Liang P.; Biesheuvel P. M.; Zornitta R. L.; de Smet L. C. P. M. Recent Advances in Ion Selectivity with Capacitive Deionization. Energy Environ. Sci. 2021, 14, 1095–1120. 10.1039/D0EE03145C. [DOI] [Google Scholar]
- Riegler H.; Essler F. Polyelectrolytes. 2: Intrinsic or Extrinsic Charge Compensation? Quantitative Charge Analysis of PAH/PSS Multilayers. Langmuir 2002, 18 (17), 6694–6698. 10.1021/la020108n. [DOI] [Google Scholar]
- Cheng C.; White N.; Shi H.; Robson M.; Bruening M. L. Cation Separations in Electrodialysis through Membranes Coated with Polyelectrolyte Multilayers. Polymer 2014, 55, 1397–1403. 10.1016/j.polymer.2013.12.002. [DOI] [Google Scholar]
- Zhao Y.; Tang K.; Liu H.; Van der Bruggen B.; Sotto Díaz A.; Shen J.; Gao C. An Anion Exchange Membrane Modified by Alternate Electro-Deposition Layers with Enhanced Monovalent Selectivity. J. Membr. Sci. 2016, 520, 262–271. 10.1016/j.memsci.2016.07.026. [DOI] [Google Scholar]
- Ishigami T.; Amano K.; Fujii A.; Ohmukai Y.; Kamio E.; Maruyama T.; Matsuyama H. Fouling Reduction of Reverse Osmosis Membrane by Surface Modification via Layer-by-Layer Assembly. Sep. Purif. Technol. 2012, 99, 1–7. 10.1016/j.seppur.2012.08.002. [DOI] [Google Scholar]
- Karkhanechi H.; Razi F.; Sawada I.; Takagi R.; Ohmukai Y.; Matsuyama H. Improvement of Antibiofouling Performance of a Reverse Osmosis Membrane through Biocide Release and Adhesion Resistance. Sep. Purif. Technol. 2013, 105, 106–113. 10.1016/j.seppur.2012.12.016. [DOI] [Google Scholar]
- Ma W.; Soroush A.; Van Anh Luong T.; Brennan G.; Rahaman M. S.; Asadishad B.; Tufenkji N. Spray- and Spin-Assisted Layer-by-Layer Assembly of Copper Nanoparticles on Thin-Film Composite Reverse Osmosis Membrane for Biofouling Mitigation. Water Res. 2016, 99, 188–199. 10.1016/j.watres.2016.04.042. [DOI] [PubMed] [Google Scholar]
- Son M.; Yang W.; Bucs S. S.; Nava-Ocampo M. F.; Vrouwenvelder J. S.; Logan B. E. Polyelectrolyte-Based Sacrificial Protective Layer for Fouling Control in Reverse Osmosis Desalination. Environ. Sci. Technol. Lett. 2018, 5, 584–90. 10.1021/acs.estlett.8b00400. [DOI] [Google Scholar]
- Abdu S.; Sricharoen K.; Wong J. E.; Muljadi E. S.; Melin T.; Wessling M. Catalytic Polyelectrolyte Multilayers at the Bipolar Membrane Interface. ACS Appl. Mater. Interfaces 2013, 5, 10445–10455. 10.1021/am403019y. [DOI] [PubMed] [Google Scholar]
- Jiang S. P.; Tang H. Methanol Crossover Reduction by Nafion Modification via Layer-by-Layer Self-Assembly Techniques. Colloids Surf., A 2012, 407, 49–57. 10.1016/j.colsurfa.2012.05.006. [DOI] [Google Scholar]
- Xiang Y.; Lu S.; Jiang S. P. Layer-by-Layer Self-Assembly in the Development of Electrochemical Energy Conversion and Storage Devices from Fuel Cells to Supercapacitors. Chem. Soc. Rev. 2012, 41 (21), 7291–7321. 10.1039/c2cs35048c. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Qian J.; Yang Y.; An Q.; Liu X.; Gui Z. Polyelectrolyte Layer-by-Layer Self-Assembly Enhanced by Electric Field and Their Multilayer Membranes for Separating Isopropanol-Water Mixtures. J. Membr. Sci. 2008, 320 (1–2), 73–77. 10.1016/j.memsci.2008.03.055. [DOI] [Google Scholar]
- Nguyen T. T. T.; Belbekhouche S.; Dubot P.; Carbonnier B.; Grande D. From the Functionalization of Polyelectrolytes to the Development of a Versatile Approach to the Synthesis of Polyelectrolyte Multilayer Films with Enhanced Stability. J. Mater. Chem. A 2017, 5 (46), 24472–24483. 10.1039/C7TA06855G. [DOI] [Google Scholar]
- Nam S. Y.; Lee M. L. Pervaporation and Properties of Chitosan-Poly(Acrylic Acid) Complex Membranes. J. Membr. Sci. 1997, 135 (2), 161–171. 10.1016/S0376-7388(97)00144-0. [DOI] [Google Scholar]
- Porcel C. H.; Schlenoff J. B. Compact Polyelectrolyte Complexes: “Saloplastic” Candidates for Biomaterials. Biomacromolecules 2009, 10 (11), 2968–2975. 10.1021/bm900373c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.; Perry S. L.; Schiffman J. D. Complex Coacervation: Chemically Stable Fibers Electrospun from Aqueous Polyelectrolyte Solutions. ACS Macro Lett. 2017, 6 (5), 505–511. 10.1021/acsmacrolett.7b00173. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Schlenoff J. B. Tough Strained Fibers of a Polyelectrolyte Complex: Pretensioned Polymers. RSC Adv. 2014, 4 (87), 46675–46679. 10.1039/C4RA08733J. [DOI] [Google Scholar]
- Kelly K. D.; Schlenoff J. B. Spin-Coated Polyelectrolyte Coacervate Films. ACS Appl. Mater. Interfaces 2015, 7 (25), 13980–13986. 10.1021/acsami.5b02988. [DOI] [PubMed] [Google Scholar]
- Reisch A.; Roger E.; Phoeung T.; Antheaume C.; Orthlieb C.; Boulmedais F.; Lavalle P.; Schlenoff J. B.; Frisch B.; Schaaf P. On the Benefits of Rubbing Salt in the Cut: Self-Healing of Saloplastic PAA/PAH Compact Polyelectrolyte Complexes. Adv. Mater. 2014, 26 (16), 2547–2551. 10.1002/adma.201304991. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Wang C.; Zhu G.; Zacharia N. S. Self-Healing of Bulk Polyelectrolyte Complex Material as a Function of PH and Salt. ACS Appl. Mater. Interfaces 2016, 8 (39), 26258–26265. 10.1021/acsami.6b06776. [DOI] [PubMed] [Google Scholar]
- Luo F.; Sun T. L.; Nakajima T.; Kurokawa T.; Zhao Y.; Sato K.; Ihsan A. B.; Li X.; Guo H.; Gong J. P. Oppositely Charged Polyelectrolytes Form Tough, Self-Healing, and Rebuildable Hydrogels. Adv. Mater. 2015, 27 (17), 2722–2727. 10.1002/adma.201500140. [DOI] [PubMed] [Google Scholar]
- Murakawa K.; King D. R.; Sun T.; Guo H.; Kurokawa T.; Gong J. P. Polyelectrolyte Complexation via Viscoelastic Phase Separation Results in Tough and Self-Recovering Porous Hydrogels. J. Mater. Chem. B 2019, 7 (35), 5296–5305. 10.1039/C9TB01376H. [DOI] [PubMed] [Google Scholar]
- Gai M.; Frueh J.; Kudryavtseva V. L.; Mao R.; Kiryukhin M. V.; Sukhorukov G. B. Patterned Microstructure Fabrication: Polyelectrolyte Complexes vs Polyelectrolyte Multilayers. Sci. Rep. 2016, 6 (1), 37000. 10.1038/srep37000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J.; Long C. T.; Grunlan J. C. Transparent Polyelectrolyte Complex Thin Films with Ultralow Oxygen Transmission Rate. Langmuir 2018, 34 (37), 11086–11091. 10.1021/acs.langmuir.8b02391. [DOI] [PubMed] [Google Scholar]
- Fu J.; Wang Q.; Schlenoff J. B. Extruded Superparamagnetic Saloplastic Polyelectrolyte Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7 (1), 895–901. 10.1021/am5074694. [DOI] [PubMed] [Google Scholar]
- Meng X.; Schiffman J. D.; Perry S. L. Electrospinning Cargo-Containing Polyelectrolyte Complex Fibers: Correlating Molecular Interactions to Complex Coacervate Phase Behavior and Fiber Formation. Macromolecules 2018, 51 (21), 8821–8832. 10.1021/acs.macromol.8b01709. [DOI] [Google Scholar]
- Chiang H.; Kolibaba T. J.; Eberle B.; Grunlan J. C. Super Gas Barrier of a Polyelectrolyte/Clay Coacervate Thin Film. Macromol. Rapid Commun. 2021, 42, 2000540. 10.1002/marc.202000540. [DOI] [PubMed] [Google Scholar]
- Wu J. K.; Ye C. C.; Liu T.; An Q. F.; Song Y. H.; Lee K. R.; Hung W. S.; Gao C. J. Synergistic Effects of CNT and GO on Enhancing Mechanical Properties and Separation Performance of Polyelectrolyte Complex Membranes. Mater. Des. 2017, 119, 38–46. 10.1016/j.matdes.2017.01.056. [DOI] [Google Scholar]
- Michaels A. S.; Miekka R. G. Polycation-Polyanion Complexes: Preparation and Properties of Poly-(Vinylbenzyltrimethylammonium) Poly-(Styrenesulfonate). J. Phys. Chem. 1961, 65 (10), 1765–1773. 10.1021/j100827a020. [DOI] [Google Scholar]
- Schwarz H.-H.; Richau K.; Paul D. Membranes from Polyelectrolyte Complexes. Polym. Bull. 1991, 25 (1), 95–100. [Google Scholar]
- Schaaf P.; Schlenoff J. B. Saloplastics: Processing Compact Polyelectrolyte Complexes. Adv. Mater. 2015, 27 (15), 2420–2432. 10.1002/adma.201500176. [DOI] [PubMed] [Google Scholar]
- Choudhari S. K.; Premakshi H. G.; Kariduraganavar M. Y. Preparation and Pervaporation Performance of Chitosan-Poly(Methacrylic Acid) Polyelectrolyte Complex Membranes for Dehydration of 1,4-Dioxane. Polym. Eng. Sci. 2016, 56 (6), 715–724. 10.1002/pen.24298. [DOI] [Google Scholar]
- Uragami T.; Shinomiya H. Concentration of Aqueous Dimethyl Sulfoxide Solutions through a Chitosan Membrane by Permeation with a Temperature Difference. J. Membr. Sci. 1992, 74 (1–2), 183–191. 10.1016/0376-7388(92)87082-9. [DOI] [Google Scholar]
- Su Y.; Li C. Tunable Water Flux of a Weak Polyelectrolyte Ultrafiltration Membrane. J. Membr. Sci. 2007, 305 (1–2), 271–278. 10.1016/j.memsci.2007.08.029. [DOI] [Google Scholar]
- Achari D.; Rachipudi P.; Naik S.; Karuppannan R.; Kariduraganavar M. Polyelectrolyte Complex Membranes Made of Chitosan—PSSAMA for Pervaporation Separation of Industrially Important Azeotropic Mixtures. J. Ind. Eng. Chem. 2019, 78, 383–395. 10.1016/j.jiec.2019.05.031. [DOI] [Google Scholar]
- Schwarz H.-H.; Apostel R.; Paul D. Membranes Based on Polyelectrolyte–Surfactant Complexes for Methanol Separation. J. Membr. Sci. 2001, 194 (1), 91–102. 10.1016/S0376-7388(01)00520-8. [DOI] [Google Scholar]
- Ball V.; Michel M.; Toniazzo V.; Ruch D. The Possibility of Obtaining Films by Single Sedimentation of Polyelectrolyte Complexes. Ind. Eng. Chem. Res. 2013, 52 (16), 5691–5699. 10.1021/ie303535s. [DOI] [Google Scholar]
- Hu C.; Li B.; Guo R.; Wu H.; Jiang Z. Pervaporation Performance of Chitosan–Poly(Acrylic Acid) Polyelectrolyte Complex Membranes for Dehydration of Ethylene Glycol Aqueous Solution. Sep. Purif. Technol. 2007, 55 (3), 327–334. 10.1016/j.seppur.2007.01.005. [DOI] [Google Scholar]
- Han G. L.; Gong Y.; Zhang Q. G.; Zhu A. M.; Ye M. L.; Liu Q. L. Facile Preparation of Homogeneous Polyelectrolyte Complex Membranes for Separation of Methanol/Methyl Tert-Butyl Ether Mixtures. J. Membr. Sci. 2013, 447, 246–252. 10.1016/j.memsci.2013.07.038. [DOI] [Google Scholar]
- Jiang H.; Zuo Y.; Cheng L.; Wang H.; Gu A.; Li Y. A Homogenous CS/NaCMC/n-HA Polyelectrolyte Complex Membrane Prepared by Gradual Electrostatic Assembling. J. Mater. Sci.: Mater. Med. 2011, 22 (2), 289–297. 10.1007/s10856-010-4195-1. [DOI] [PubMed] [Google Scholar]
- Bernabé P.; Peniche C.; Argüelles-Monal W. Swelling Behavior of Chitosan/Pectin Polyelectrolyte Complex Membranes. Effect of Thermal Cross-Linking. Polym. Bull. 2005, 55 (5), 367–375. 10.1007/s00289-005-0439-5. [DOI] [Google Scholar]
- Recillas M.; Silva L. L.; Peniche C.; Goycoolea F. M.; Rinaudo M.; Román J. S.; Argüelles-Monal W. M. Thermo- and PH-Responsive Polyelectrolyte Complex Membranes from Chitosan-g-N-Isopropylacrylamide and Pectin. Carbohydr. Polym. 2011, 86 (3), 1336–1343. 10.1016/j.carbpol.2011.06.047. [DOI] [Google Scholar]
- Yang Y.; Zhang Q.; Li S.; Zhang S. Preparation and Characterization of Porous Polyelectrolyte Complex Membranes for Nanofiltration. RSC Adv. 2015, 5 (5), 3567–3573. 10.1039/C4RA13699C. [DOI] [Google Scholar]
- Shaplov A. S.; Morozova S. M.; Lozinskaya E. I.; Vlasov P. S.; Gouveia A. S. L.; Tomé L. C.; Marrucho I. M.; Vygodskii Y. S. Turning into Poly(Ionic Liquid)s as a Tool for Polyimide Modification: Synthesis, Characterization and CO 2 Separation Properties. Polym. Chem. 2016, 7 (3), 580–591. 10.1039/C5PY01553G. [DOI] [Google Scholar]
- Shao Y.; Wang Y.; Li X.; Kheirabad A. K.; Zhao Q.; Yuan J.; Wang H. Crosslinking of a Single Poly(Ionic Liquid) by Water into Porous Supramolecular Membranes. Angew. Chem., Int. Ed. 2020, 59 (39), 17187–17191. 10.1002/anie.202002679. [DOI] [PubMed] [Google Scholar]
- Täuber K.; Zhao Q.; Antonietti M.; Yuan J. Tuning the Pore Size in Gradient Poly(Ionic Liquid) Membranes by Small Organic Acids. ACS Macro Lett. 2015, 4 (1), 39–42. 10.1021/mz500674d. [DOI] [PubMed] [Google Scholar]
- Schacher F.; Ulbricht M.; Müller A. H. E. Self-Supporting, Double Stimuli-Responsive Porous Membranes From Polystyrene-Block-Poly(N,N-Dimethylaminoethyl Methacrylate) Diblock Copolymers. Adv. Funct. Mater. 2009, 19 (7), 1040–1045. 10.1002/adfm.200801457. [DOI] [PubMed] [Google Scholar]
- Liu C.; Bi W.; Chen D.; Zhang S.; Mao H. Positively Charged Nanofiltration Membrane Fabricated by Poly(Acid–Base) Complexing Effect Induced Phase Inversion Method for Heavy Metal Removal. Chin. J. Chem. Eng. 2017, 25 (11), 1685–1694. 10.1016/j.cjche.2017.06.001. [DOI] [Google Scholar]
- Wang N.; Wang J.; Zhang P.; Wang W.; Sun C.; Xiao L.; Chen C.; Zhao B.; Kong Q.; Zhu B. Metal Cation Removal by P(VC-r-AA) Copolymer Ultrafiltration Membranes. Front. Chem. Sci. Eng. 2018, 12 (2), 262–272. 10.1007/s11705-017-1682-7. [DOI] [Google Scholar]
- Wang L.; Pan K.; Li L.; Cao B. Surface Hydrophilicity and Structure of Hydrophilic Modified PVDF Membrane by Nonsolvent Induced Phase Separation and Their Effect on Oil/Water Separation Performance. Ind. Eng. Chem. Res. 2014, 53, 6401–6408. 10.1021/ie4042388. [DOI] [Google Scholar]
- Jiang P.; Zhang J.; Jia S. S.; Wu Y.; Wu Q. Self-Supporting PH- and Temperature-Responsive Ethyl Cellulose-g-PDMAEMA Microporous Membranes through Non–Solvent-Induced Phase Separation Process. Int. J. Polym. Mater. 2017, 66 (12), 609–617. 10.1080/00914037.2016.1252355. [DOI] [Google Scholar]
- Liu Z.; Mi Z.; Chen C.; Zhou H.; Zhao X.; Wang D. Preparation of Hydrophilic and Antifouling Polysulfone Ultrafiltration Membrane Derived from Phenolphthalin by Copolymerization Method. Appl. Surf. Sci. 2017, 401, 69–78. 10.1016/j.apsusc.2016.12.228. [DOI] [Google Scholar]
- de Vos W. M. (Universiteit Twente ) Aqueous phase separation method. U.S. Patent US20180318775A1, 2018.
- Nielen W. M.; Willott J. D.; de Vos W. M. Aqueous Phase Separation of Responsive Copolymers for Sustainable and Mechanically Stable Membranes. ACS Appl. Polym. Mater. 2020, 2 (4), 1702–1710. 10.1021/acsapm.0c00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott J. D.; Nielen W. M.; de Vos W. M. Stimuli-Responsive Membranes through Sustainable Aqueous Phase Separation. ACS Appl. Polym. Mater. 2020, 2 (2), 659–667. 10.1021/acsapm.9b01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig M. I.; Durmaz E. N.; Willott J. D.; Vos W. M. Sustainable Membrane Production through Polyelectrolyte Complexation Induced Aqueous Phase Separation. Adv. Funct. Mater. 2020, 30 (5), 1907344. 10.1002/adfm.201907344. [DOI] [Google Scholar]
- Durmaz E. N.; Baig M. I.; Willott J. D.; de Vos W. M. Polyelectrolyte Complex Membranes via Salinity Change Induced Aqueous Phase Separation. ACS Appl. Polym. Mater. 2020, 2 (7), 2612–2621. 10.1021/acsapm.0c00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadman K.; Delgado D. E.; Won Y.; Wang Q.; Gray K. A.; Shull K. R. Versatile and High-Throughput Polyelectrolyte Complex Membranes via Phase Inversion. ACS Appl. Mater. Interfaces 2019, 11 (17), 16018–16026. 10.1021/acsami.9b02115. [DOI] [PubMed] [Google Scholar]
- Kamp J.; Emonds S.; Borowec J.; Restrepo Toro M. A.; Wessling M. On the Organic Solvent Free Preparation of Ultrafiltration and Nanofiltration Membranes Using Polyelectrolyte Complexation in an All Aqueous Phase Inversion Process. J. Membr. Sci. 2021, 618, 118632. 10.1016/j.memsci.2020.118632. [DOI] [Google Scholar]
- Tokarev I.; Orlov M.; Minko S. Responsive Polyelectrolyte Gel Membranes. Adv. Mater. 2006, 18 (18), 2458–2460. 10.1002/adma.200601288. [DOI] [Google Scholar]
- Orlov M.; Tokarev I.; Scholl A.; Doran A.; Minko S. pH-Responsive Thin Film Membranes from Poly (2-Vinylpyridine): Water Vapor-Induced Formation of a Microporous Structure. Macromolecules 2007, 40, 2086–2091. 10.1021/ma062821f. [DOI] [Google Scholar]
- Chakrabarty T.; Shahi V. K. Modified Chitosan-Based, PH-Responsive Membrane for Protein Separation. RSC Adv. 2014, 4 (95), 53245–53252. 10.1039/C4RA05314A. [DOI] [Google Scholar]
- Xue J.; Chen L.; Wang H. L.; Zhang Z. B.; Zhu X. L.; Kang E. T.; Neoh K. G. Stimuli-Responsive Multifunctional Membranes of Controllable Morphology from Poly(Vinylidene Fluoride)- Graft -Poly[2-(N,N-Dimethylamino)Ethyl Methacrylate] Prepared via Atom Transfer Radical Polymerization. Langmuir 2008, 24 (24), 14151–14158. 10.1021/la801402u. [DOI] [PubMed] [Google Scholar]
- Nunes S. P.; Behzad A. R.; Hooghan B.; Sougrat R.; Karunakaran M.; Pradeep N.; Vainio U.; Peinemann K.-V. Switchable PH-Responsive Polymeric Membranes Prepared via Block Copolymer Micelle Assembly. ACS Nano 2011, 5 (5), 3516–3522. 10.1021/nn200484v. [DOI] [PubMed] [Google Scholar]
- Jiang P.; Li D.; Liu Y.; Hao X.; Zhang X.; Gao J.; Deng K. Remarkable PH-Responsive Microfiltration Membrane from Well-Defined PS-b-PDEAEMA Copolymers by ATRP Method. J. Macromol. Sci., Part A: Pure Appl.Chem. 2013, 50 (9), 991–1001. 10.1080/10601325.2013.814326. [DOI] [Google Scholar]
- Su Y.; Sun M.; Wang L.; Jiang Z. Ion-Pair Formation and Ion-Specific Flux of a Weak Polyelectrolyte Membrane. J. Phys. Chem. B 2009, 113 (28), 9454–9460. 10.1021/jp901618k. [DOI] [PubMed] [Google Scholar]
- Zhai G.; Toh S. C.; Tan W. L.; Kang E. T.; Neoh K. G.; Huang C. C.; Liaw D. J. Poly(Vinylidene Fluoride) with Grafted Zwitterionic Polymer Side Chains for Electrolyte-Responsive Microfiltration Membranes. Langmuir 2003, 19 (17), 7030–7037. 10.1021/la034440q. [DOI] [Google Scholar]
- Su Y.; Zheng L.; Li C.; Jiang Z. Smart Zwitterionic Membranes with On/Off Behavior for Protein Transport. J. Phys. Chem. B 2008, 112 (38), 11923–11928. 10.1021/jp804422t. [DOI] [PubMed] [Google Scholar]
- Sun Q.; Su Y.; Ma X.; Wang Y.; Jiang Z. Improved Antifouling Property of Zwitterionic Ultrafiltration Membrane Composed of Acrylonitrile and Sulfobetaine Copolymer. J. Membr. Sci. 2006, 285 (1–2), 299–305. 10.1016/j.memsci.2006.08.035. [DOI] [Google Scholar]
- Su Y.; Mu C.; Li C.; Jiang Z. Antifouling Property of a Weak Polyelectrolyte Membrane Based on Poly(Acrylonitrile) during Protein Ultrafiltration. Ind. Eng. Chem. Res. 2009, 48 (6), 3136–3141. 10.1021/ie801393z. [DOI] [Google Scholar]
- Chen F.; Guo J.; Xu D.; Yan F. Thermo- and PH-Responsive Poly(Ionic Liquid) Membranes. Polym. Chem. 2016, 7 (6), 1330–1336. 10.1039/C5PY01927C. [DOI] [Google Scholar]
- Zhang K.; Feng X.; Sui X.; Hempenius M. A.; Vancso G. J. Breathing Pores on Command: Redox-Responsive Spongy Membranes from Poly(Ferrocenylsilane)S. Angew. Chem., Int. Ed. 2014, 53 (50), 13789–13793. 10.1002/anie.201408010. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Chen P.; Liu Y.; Guo H.; Sun N.; Cai Q.; Yu Y.; Zhao F. Exploration of Antifouling Zwitterionic Polyimide Ultrafiltration Membrane Based on Novel Aromatic Diamine Monomer. Sep. Purif. Technol. 2021, 255, 117738. 10.1016/j.seppur.2020.117738. [DOI] [Google Scholar]
- Fares H. M.; Wang Q.; Yang M.; Schlenoff J. B. Swelling and Inflation in Polyelectrolyte Complexes. Macromolecules 2019, 52 (2), 610–619. 10.1021/acs.macromol.8b01838. [DOI] [Google Scholar]
- Baig M. I.; Sari P. P. I.; Li J.; Willott J. D.; de Vos W. M. Sustainable Aqueous Phase Separation Membranes Prepared through Mild PH Shift Induced Polyelectrolyte Complexation of PSS and PEI. J. Membr. Sci. 2021, 625, 119114. 10.1016/j.memsci.2021.119114. [DOI] [Google Scholar]
- Pham T. H.; Olsson J. S.; Jannasch P. N-Spirocyclic Quaternary Ammonium Ionenes for Anion-Exchange Membranes. J. Am. Chem. Soc. 2017, 139 (8), 2888–2891. 10.1021/jacs.6b12944. [DOI] [PubMed] [Google Scholar]
- Dani A.; Täuber K.; Zhang W.; Schlaad H.; Yuan J. Stable Covalently Photo-Crosslinked Poly(Ionic Liquid) Membrane with Gradient Pore Size. Macromol. Rapid Commun. 2017, 38 (16), 1700167. 10.1002/marc.201700167. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Regan M.; Zhang W.; Yuan J. Reprocessable Porous Poly(Ionic Liquid) Membranes Derived from Main-Chain Polyimidazolium. Eur. Polym. J. 2018, 103, 214–219. 10.1016/j.eurpolymj.2018.03.035. [DOI] [Google Scholar]
- Paltrinieri L.; Remmen K.; Müller B.; Chu L.; Köser J.; Wintgens T.; Wessling M.; de Smet L. C. P. M.; Sudhölter E. J. R. Improved Phosphoric Acid Recovery from Sewage Sludge Ash Using Layer-by-Layer Modified Membranes. J. Membr. Sci. 2019, 587, 117162. 10.1016/j.memsci.2019.06.002. [DOI] [Google Scholar]
- Kazemabad M.; Verliefde A.; Cornelissen E. R.; D'Haese A. Crown Ether Containing Polyelectrolyte Multilayer Membranes for Lithium Recovery. J. Membr. Sci. 2020, 595, 117432. 10.1016/j.memsci.2019.117432. [DOI] [Google Scholar]
- Liang Y.; Lin S. Mechanism of Permselectivity Enhancement in Polyelectrolyte-Dense Nanofiltration Membranes via Surfactant-Assembly Intercalation. Environ. Sci. Technol. 2021, 55 (1), 738–748. 10.1021/acs.est.0c06866. [DOI] [PubMed] [Google Scholar]
- Paltrinieri L.; Poltorak L.; Chu L.; Puts T.; van Baak W.; Sudhölter E. J. R.; de Smet L. C. P. M. Hybrid Polyelectrolyte-Anion Exchange Membrane and Its Interaction with Phosphate. React. Funct. Polym. 2018, 133, 126–135. 10.1016/j.reactfunctpolym.2018.10.005. [DOI] [Google Scholar]
- Sarma R.; Islam M. S.; Miller A. F.; Bhattacharyya D. Layer-by-Layer-Assembled Laccase Enzyme on Stimuli-Responsive Membranes for Chloro-Organics Degradation. ACS Appl. Mater. Interfaces 2017, 9 (17), 14858–14867. 10.1021/acsami.7b01999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Yan Z.; Bai L. Layer-by-Layer Assembly of Polyelectrolyte and Gold Nanoparticle for Highly Reproducible and Stable SERS Substrate. Appl. Surf. Sci. 2016, 360 (B), 437–441. 10.1016/j.apsusc.2015.09.151. [DOI] [Google Scholar]
- Ng L. Y.; Mohammad A. W.; Leo C. P.; Hilal N. Polymeric Membranes Incorporated with Metal/Metal Oxide Nanoparticles: A Comprehensive Review. Desalination 2013, 308, 15–33. 10.1016/j.desal.2010.11.033. [DOI] [Google Scholar]
- Kotov N. A.; Magonov S.; Tropsha E. Layer-by-Layer Self-Assembly of Alumosilicate-Polyelectrolyte Composites: Mechanism of Deposition, Crack Resistance, and Perspectives for Novel Membrane Materials. Chem. Mater. 1998, 10 (3), 886–895. 10.1021/cm970649b. [DOI] [Google Scholar]
- Zhu T.; Sha Y.; Yan J.; Pageni P.; Rahman M. A.; Yan Y.; Tang C. Metallo-Polyelectrolytes as a Class of Ionic Macromolecules for Functional Materials. Nature Communications 2018, 9, 4329. 10.1038/s41467-018-06475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabihzadeh Khajavi M.; Ebrahimi A.; Yousefi M.; Ahmadi S.; Farhoodi M.; Mirza Alizadeh A.; Taslikh M. Strategies for Producing Improved Oxygen Barrier Materials Appropriate for the Food Packaging Sector. Food Eng. Rev. 2020, 12 (3), 346–363. 10.1007/s12393-020-09235-y. [DOI] [Google Scholar]
- Dizge N.; Epsztein R.; Cheng W.; Porter C. J.; Elimelech M. Biocatalytic and Salt Selective Multilayer Polyelectrolyte Nanofiltration Membrane. J. Membr. Sci. 2018, 549, 357–365. 10.1016/j.memsci.2017.12.026. [DOI] [Google Scholar]
- Li X.; Xu Y.; Goh K.; Chong T. H.; Wang R. Layer-by-Layer Assembly Based Low Pressure Biocatalytic Nanofiltration Membranes for Micropollutants Removal. J. Membr. Sci. 2020, 615, 118514. 10.1016/j.memsci.2020.118514. [DOI] [Google Scholar]
- Srivastava S.; Kotov N. A. Composite Layer-by-Layer (LBL) Assembly with Inorganic Nanoparticles and Nanowires. Acc. Chem. Res. 2008, 41 (12), 1831–1841. 10.1021/ar8001377. [DOI] [PubMed] [Google Scholar]
- Kim S.; Lee K.-S.; Lee K. Polyelectrolyte Complex Membranes Based on Two Anionic Polysaccharides Composed of Sodium Alginate and Carrageenan: The Effect of Annealing on the Separation of Methanol/Water Mixtures. J. Appl. Polym. Sci. 2006, 102 (6), 5781–5788. 10.1002/app.23903. [DOI] [Google Scholar]
- Nikolaeva D.; Luis P. Top-down Polyelectrolytes for Membrane-Based Post-Combustion CO2 Capture. Molecules 2020, 25 (2), 323–343. 10.3390/molecules25020323. [DOI] [PMC free article] [PubMed] [Google Scholar]