Abstract
Dementia with Lewy bodies (DLB) research has seen a significant growth in international collaboration over the last three decades. However, researchers face a challenge in identifying large and diverse samples capable of powering longitudinal studies and clinical trials. The DLB research community has begun to focus efforts on supporting the development and harmonization of consortia, while also continuing to forge networks within which data and findings can be shared. This article describes the current state of DLB research collaborations on each continent. We discuss several established DLB cohorts, many of whom have adopted a common framework, and identify emerging collaborative initiatives that hold the potential to expand DLB networks and diversify research cohorts. Our findings identify geographical areas into which the global DLB networks should seek to expand, and we propose strategies, such as the creation of data‐sharing platforms and the harmonization of protocols, which may further potentiate international collaboration.
Keywords: collaboration, consortia, dementia with Lewy bodies, global strategy, Lewy body dementia
1. BACKGROUND
Dementia with Lewy bodies (DLB) is characterized clinically by a combination of core features, including fluctuating cognition, visual hallucinations, spontaneous parkinsonism, and rapid eye movement behavior disorder (RBD).1, 2 Although the second most common form of neurodegenerative dementia,3, 4 DLB is under‐researched (Figure 1), under‐recognized, frequently misdiagnosed, and undertreated.5, 6 It is associated with a poor prognosis7 and limited therapeutic options.8
FIGURE 1.
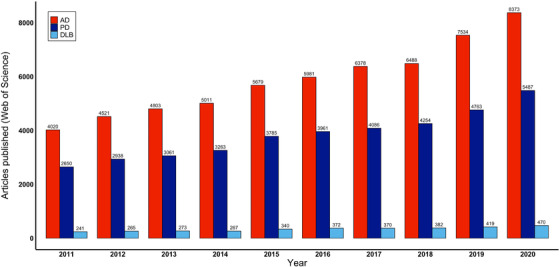
Articles published on dementia with Lewy bodies, Alzheimer's disease, and Parkinson's disease between 2011 and 2020. Articles (excluding review articles) were identified through Web of Science searches for “Alzheimer's disease” (AD), “Parkinson's disease” (PD), and “Dementia with Lewy bodies” (DLB) on March 12, 2021
Together, DLB and Parkinson's disease dementia (PDD) comprise the Lewy body dementias (LBD), a spectrum of disease with overlapping neurobiological mechanisms. Where PDD describes dementia arising in the context of established idiopathic Parkinson's disease, DLB refers to the onset of cognitive symptoms before, or within 1 year of, the onset of parkinsonism.1
The research recommendations for DLB and other dementias as part of the Alzheimer's Disease‐Related Dementias (ADRD) Summit in 2019 specifically cited the establishment of consortia as an imperative for advancing DLB research, highlighting the need for multicenter collaboration in achieving adequately powered therapeutic trials.9 The ADRD Summit 2019 also advocated merging datasets, particularly in studies using longitudinal and biomarker enriched designs, and underlined the need for genetic and environmental diversity of these research cohorts. Similarly, the fourth consensus report of the DLB Consortium1 highlighted the potential of global harmonization in DLB research, including common platforms for data and biomarker collection, and a consensus on outcome measures applied in clinical trials.
The Alzheimer's Association International Society to Advance Alzheimer's Research and Treatment (ISTAART) Lewy Body Dementias Professional Interest Area (LBD PIA), founded in 2019, is focused on advancing clinical care and research on DLB and PDD. To address many of the unmet needs outlined above, the LBD PIA established three working groups, focused on prodromal DLB, trial methods, and DLB consortia.
2. AIMS AND METHODS
The LBD PIA Consortia Working Group (CWG) aims to create a worldwide network of DLB research groups. The first goal of the CWG has been to describe the current state of global DLB research collaborations, excluding other LBD such as PDD, for this specific endeavor. During the first CWG meeting in October 2020, a writing group was established (FD, JK, JBL, DA). The main objectives were to detail factors specific to DLB that necessitate multi‐site and multinational datasets and identify existing national and international DLB research networks, as well as explore strategies to enhance global collaboration.
The authors did not explore PDD consortia for this particular endeavor, but did, over the course of the exercise, identify organizations and research cohorts that have approached DLB and PDD together under their collective term of LBD. Where necessary, we refer to LBD to remain consistent with these organizations but have taken care to ensure that LBD and DLB have not been used interchangeably.
The writing group used their pre‐existing networks to identify active DLB consortia, and invited representatives from each to participate as named co‐authors. All CWG members were then invited to critique the manuscript. The exercise focused on multicenter consortia, comprising groups from more than one geographical area. It also aimed to identify developing registries or networks that have emerged in geographical areas in which active DLB consortia are not operating.
2.1. Why are DLB consortia needed?
Two central research themes are consistently identified as strategic needs in developing DLB research:1, 9 (1) clinical and biomarker characterization of the longitudinal course of DLB, and (2) the creation of trial‐ready cohorts to facilitate publicly and privately sponsored clinical trials. While DLB represents 10% to 20% of dementia cases,10 less than one third of such cases are typically diagnosed in routine clinical care.3 Adequately powered longitudinal or therapeutic DLB research therefore necessitates specialized multi‐site consortia, as this would represent a logistic challenge for even larger research centers with access to expert clinical opinion and diagnostic investigations.5 The clinical heterogeneity of DLB, which can result in patients presenting to a range of clinical settings, can also complicate recruitment, and can contribute to the incorrect randomization of DLB patients to AD clinical trials, and variable treatment response in DLB trials.
Important subanalyses, such as those exploring the lower rate of core symptoms in women than in men with comparative DLB pathology,11 and the role this might play in DLB's clinical preponderance in men3 are also precluded by small sample sizes. Similarly, examination of the influence of geographical and ethnic factors on disease, as has been observed in PD samples,12 has not yet been possible in DLB cohorts.
RESEARCH IN CONTEXT
Review: Through both pre‐existing networks and the membership of the LBD PIA, the authors identify active multicenter DLB consortia. They also describe emerging initiatives in countries without DLB consortia.
Interpretation: International collaboration among the DLB community has increased significantly in recent decades, and common characteristics of national and continental consortia demonstrate the potential to address unmet scientific and strategic needs in the field.
Future directions: Expansion and diversification of DLB networks are critical in supporting scientific progress. Standardization of methodologies and practices, and the development of mechanisms to facilitate data sharing, are important in maximizing the potential of DLB consortia.
HIGHLIGHTS.
International collaborative research involving dementia with Lewy bodies (DLB) has grown significantly in recent decades.
The large sample sizes required for genetic studies, longitudinal studies, and therapeutic trials in DLB support collaboration between international centers.
New DLB initiatives and networks have emerged in recent years, but further expansion and diversification are critical in supporting scientific progress.
Strategies such as the creation of data‐sharing platforms and the harmonization of protocols would support further collaboration.
Prodromal DLB studies represent an even greater challenge to single‐center designs. The recently published prodromal DLB criteria13 have not yet been adopted within most clinical settings, complicating case identification and recruitment. Studies investigating prodromal DLB cohorts will require longer follow‐up than those with DLB participants, and thus require more extensive resources.
Identifying opportunities to accumulate large sample sizes is particularly important in the nascent field of DLB genetic research. The scale of this challenge, and the emerging capacity of the DLB community to rise to it, is best exemplified in a recent genome‐wide association analysis in which five independent risk loci were identified. The study included 2591 individuals with LBD and 4391 healthy controls, recruited from 17 European and 27 North American sites.14 Genetic studies also highlight the importance of increasing diversity of DLB cohorts, wherein there has been an over‐representation of European and American populations.15 The role of other epigenetic features and comorbidities further emphasizes the need for greater diversity, exemplified by the higher incidence of dementia in the African American and Caribbean Hispanic ethnic groups in the United States.16
Sharing laboratory resources and expertise could potentiate research studies outside major research centers, particularly those outside more economically developed countries, allowing analysis of more diverse and globally representative groups. This is also supported through the standardization of validated protocols, such as BIOMARKAPD17 and the recently established European Cluster for Imaging Biomarkers (ECIB) guidelines (www.ebra.eu).
Finally, the benefits of collaboration extend beyond individual studies. There is an increasing number of funding initiatives aimed specifically at multi‐site and multinational efforts. The networks created by collaboration will also provide researchers with larger platforms from which to disseminate their work, and larger audiences with whom we can engage and educate, raising the profile of DLB globally. This, in turn, encourages more research funding, study participation, and collaboration, further “widening the net” of our existing networks.
2.2. The international DLB consortium and diagnostic criteria
International DLB networks have been pivotal to the conceptual evolution of the disease. Groups from Japan, the UK, and the United States dominated DLB research prior to the first international consortium.18 Indeed, 21 of the 25 authors were from institutions in these three countries. By 1995, DLB was an emerging entity with 137 papers being published that year. However, few of these reflected any collaboration between international groups (Web of Science; Figure 2). Subsequent DLB consortium meetings have seen the growth and diversification of the DLB research community. Although researchers from Japan, the UK, and the United States comprised 69% (31/45) and 79% (50/63) of the authorship of the third19 and fourth1 consensus documents, authors from 13 other countries were also represented.
FIGURE 2.
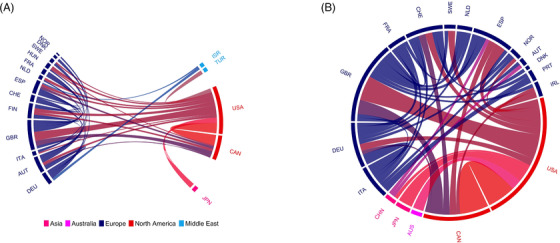
Multinational collaborations on dementia with Lewy bodies (DLB) publications (2000 and 2020). Each arc represents one of the 20 countries most frequently represented by authorship on a multinational paper published either in 2000 (n = 224; A) or 2020 (n = 470; B). Papers identified through a Web of Science search for “Dementia with Lewy bodies” on February 10, 2021. The size of each arc is proportionate to the number of multinational papers published by institutions in respective countries. Connecting lines between arcs represent co‐authorship between researchers affiliated with institutions in different countries, and the width of each line corresponds to the number of multinational articles co‐authored with researchers from connected nations. Analysis was conducted using the bibliometrix package for R.28 AUS, Australia; AUT, Austria; BEL, Belgium; CAN, Canada; CHE, Switzerland; CHN, China; DEU, Germany; DNK, Denmark; ESP, Spain; FIN, Finland; FRA, France; GBR, United Kingdom; HUN, Hungary; IRL, Ireland; ISR, Israel; ITA, Italy; JPN, Japan; KOR, South Korea; NLD, Netherlands; NOR, Norway; QAT, Qatar; SWE, Sweden; TUR, Turkey; USA, United States of America
Collaborative research both contributed to and arose from the major conceptual developments brought forward by the third19 and fourth1 consensus criteria, such as the inclusion of dopamine transporter single photon emission computed tomography (SPECT; DaTscan) imaging, which paved the way for a phase III multicenter study assessing its utility at 40 European sites.20 Work conducted by the Mayo Clinic, USA, 21 and Barcelona, Spain, groups22 led to RBD's inclusion as a core feature in the fourth criteria, later supported by a multicenter study involving 24 sites across 11 countries in North America, Europe, Asia, and Australia.23 The fourth consensus criteria also included abnormal cardiac meta‐iodobenzylguanidine (MIBG) uptake as an indicative marker after the publication of encouraging data from Japanese and Italian multicenter trials.24, 25 The publication of criteria for the diagnosis of prodromal DLB13 is further testament to the development of international collaboration in recent years (Figures 1 and 2).26, 27 The lack of established biomarkers for prodromal DLB, however, highlights the need for a coordinated global effort in developing large prospective longitudinal cohorts from a wide range of geographical regions.
2.3. DLB consortia in Europe
The following sections highlight DLB consortia across the globe, beginning with Europe. Although several European laboratories produce research arising from established DLB cohorts, a full discussion of all of these is beyond the scope of this paper; we have focused only on consortia comprising multiple geographical sites (Table 1).
TABLE 1.
Multicenter DLB consortia recruiting participants as of March 22, 2021
| Name Website Country | Year established | Enrolled patients with DLB (n) | Neuropathology/ CSF | Blood | Genetic | MRI | FDG‐PET | DaTSCAN | EEG | Funder(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| EUROPE | ||||||||||
| European DLB Consortium (E‐DLB) www.e‐dlb.com Multinational | 2016 | 1437* | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | European Joint Program—Neurodegenerative Disease (JPND) |
| PDWAVES www.pdwaves.eu Multinational | 2014 | 60* | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | n/a |
| Italian DLB consortium** Italy | 2016 | 187* | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Italian Minister of Health | |
| UK‐ENLIST** United Kingdom | 2019 | 19* | ✓ | ✓ | ✓ | x | x | x | x | Alzheimer's Research UK |
| NORTH AMERICA*** | ||||||||||
| Comprehensive Assessment of Neurodegeneration and Dementia (COMPASS‐ND) www.ccna‐ccnv.ca Canada | 2017 | 30* | ✓ | ✓ | ✓ | ✓ | x | x | x | Canadian Institutes of Health Research |
| DLB Consortium (DLBC) https://pdbp.ninds.nih.gov/index.php/Dementia‐with‐Lewy‐Bodies‐Consortium USA | 2017 | 122* | ✓ | ✓ | ✓ | ✓ | x | ✓ | x | NINDS |
| Alzheimer's Dementia Care (ADC) program https://naccdata.org/ USA | 1985 | 824a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | x | NIH‐NIA |
| Longitudinal Imaging Biomarkers of Disease Progression in DLB USA | 2018 | 69 | ✓ | ✓ | ✓ | ✓ | x | ✓ | x | NINDS |
| SOUTH AMERICAc | ||||||||||
| Multi‐partner consortium to expand dementia research in Latin America (ReDLat) Multinational | 2019 | 210 a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NIH‐NIA; Alzheimer's Association; Rainwater Charitable Foundation (Tau Consortium); Global Brain Health Institute; Takeda |
| Brazilian Biobank for Aging Studies Brazil | 2004 | 87a | ✓ | ✓ | ✓ | x | x | x | x | São Paulo Research Foundation (FAPESP) |
| DLB Consortium (COL‐DLB) Colombia | 2019 | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | n/a |
| ASIAc | ||||||||||
| Tianjin Dementia Institute China | 2016 | 512 | x | ✓ | x | ✓ | ✓ | x | ✓ | National research on the prevention and control of major chronic non‐communicable diseases |
| Dementia collaborative research network (PKU‐DCRN) | ||||||||||
| China | 2006 | 220 a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Ministry of Science and Technology |
| The Innovation Center for Neurological Disorders (CMU‐ICND) China | 2019 | 50 a | ✓ | ✓ | x | ✓ | ✓ | x | ✓ | Ministry of Science and Technology |
| Dementia with Lewy Bodies Society Japan (DLBSJ) Japan | 2007 | 200 a | ✓ | ✓ | ✓ | ✓ | x | ✓ | ✓ | Japan Foundation for Neuroscience and Mental Health |
| AUSTRALIAc | ||||||||||
| ForeFront DLB Australia | 2017 | 30 a | ✓ | ✓ | ✓ | ✓ | x | x | x | National Health and Medical Research Council (NHMRC) Dementia Research Team Grant; NHMRC‐ARC Dementia Fellowship; NHMRC Investigator Grant |
| Lewy body study Australia | 2018 | 36a | ✓ | ✓ | ✓ | ✓ | x | x | x | Yulgilbar Alzheimer's Research Program |
Abbreviations: ARC, Australian Research Council; CMU‐ICND, Capital Medical University Innovation Center for Neurological Disorders; CSF, cerebrospinal fluid; DLB, dementia with Lewy bodies; EEG, electroencephalography; FDG‐PET, fluorodeoxyglucose‐positron emission tomography; MRI, magnetic resonance imaging; NIH‐NIA, National Institutes of Health National Institute on Aging; PKU‐DCRN, Peking University Dementia Collaborative Research Network.
The initiative also recruits subjects with other disorders/controls; information contained in the table relates only to participants with DLB.
Patients in respective national initiatives are also enrolled in the European DLB Consortium (E‐DLB).
Patients in these regions are enrolled in no more than one of the listed initiatives.
2.3.1. Italy
In 2016, the Italian Neurological Society for Dementia (SINdem) led the creation of an Italian DLB study group that aimed to identify established cohorts, develop standardized methods of data collection, and optimize the diagnostic accuracy of DLB and prodromal DLB. Six working groups were subsequently created to coordinate the analysis of data collected from participating centers. These focused on neuroimaging (magnetic resonance imaging [MRI], functional MRI [fMRI], diffusion tensor imaging [DTI]), functional imaging, (DaTscan, fluorodeoxyglucose positron emission tomography [FDG‐PET], amyloid‐PET, SPECT‐MIBG), neuroelectrophysiology (resting state electroencephalography [rsEEG], visual evoked potentials, magnetoencephalography), cerebrospinal fluid (CSF) biomarkers, neuropsychological measures, and genetic analyses. The group also aimed to develop sensitive and specific tools measuring longitudinal clinical changes in DLB symptoms, and in 2018 commenced a project, funded by the Italian Minister of Health, providing two‐tiered recommendations for diagnostic and prognostic biomarker collection.
The Italian network is currently recruiting subjects with DLB, PDD, and Alzheimer's disease (AD) at its 133 participating dementia centers. The first step of the initiative was to develop a semi‐structured questionnaire among all participating centers, surveying the prevalence and incidence of DLB; perspectives on clinical assessment; the relevance and availability of diagnostic tools; pharmacological management of cognitive, motor, and behavioral disturbances; and causes of hospitalization, with specific focus on delirium and its treatment. It also led the way for the creation of a large longitudinal cohort, now available for multicenter studies.28
2.3.2. United Kingdom (UK)
Building on UK DLB research, conducted particularly in Newcastle and Cambridge, ENLIST‐UK (“A Lewy body dementia network longitudinal study in the UK''), is a three‐year observational longitudinal study started in 2019, funded by Alzheimer's Research UK and investigating predictors of cognitive decline in DLB. ENLIST‐UK is coordinated at King's College London (KCL) with Cambridge, Exeter, Newcastle, and University College London acting as participating sites. ENLIST‐UK aims to recruit subjects with probable, possible, and prodromal DLB as well as AD and Parkinson's disease (PD). Clinical and imaging data, as well as CSF and blood, will be collected from each participant and stored according to a standardized protocol.17 The ENLIST‐UK study will create a unified cohort of UK DLB patients with biological and longitudinal data.
2.3.3. European DLB consortium (www.e‐dlb.com)
E‐DLB was founded in 2015 from a grant awarded by the European Joint Programme–Neurodegenerative Disease (JPND) to standardize the design and methods for longitudinal prospective studies in DLB (https://www.neurodegenerationresearch.eu/wp‐content/uploads/2015/10/JPND‐Report‐Aarsland.pdf). Its primary aim is to establish a repository for studies investigating the longitudinal development of DLB using a standardized protocol. It currently involves 49 European centers from 20 countries, including those both the Italian SINdem and ENLIST‐UK cohorts, each recruiting and following patients with manifest and prodromal DLB. Thus, all patients prospectively recruited in the Italian SINdem and ENLIST‐UK are included in the prospective E‐DLB cohort. Ten affiliated non‐European centers have also adopted the E‐DLB protocol.
Standardized clinical assessments and biomarker collection in E‐DLB are guided by the JPND protocol. Three levels of complexity, dependent on available resources, are specified, the lowest of which describes procedures likely to be available at all specialized centers seeing DLB patients.
Prior to the prospective cohort, for which recruitment is ongoing, E‐DLB created a retrospective database of more than 1200 DLB patients.29 Data from E‐DLB have been analyzed by different study groups, focusing on neuroimaging, neurophysiology, plasma and CSF biomarkers, and genetics (https://www.e‐dlb.com). Data can be accessed via application to the E‐DLB steering committee and material transfer agreements are in place for this purpose. Biomarker hubs providing infrastructure and logistical support for biomarker collection and sample/data storage are also available.
Another central aim of E‐DLB is to collaborate with international networks, such as the Mayo Clinic DLB program.30 It has led to the development of European subinitiatives such as the Nordic and Spanish DLB networks.
2.3.4. PDWAVES (www.pdwaves.eu)
The “PDWAVES” Consortium, established in 2014, comprises investigators at 16 institutions in Italy, Switzerland, Austria, and Turkey. It aims to advance the use of rsEEG “waves” in improving the understanding of neurophysiological mechanisms underlying the regulation of brain arousal and vigilance in quiet wakefulness in patients with DLB (n = 60), PD, or AD. PDWAVES also seeks to qualify the clinical use of EEG biomarkers for diagnostic, prognostic, disease prediction, monitoring, and therapy response purposes. Core activities include the development of retrospective studies that include clinical, neuroimaging, and rsEEG data,31 as well as dissemination and outreach activities.
2.4. DLB consortia in North America
Research centers in North America have contributed significantly to the conceptual and scientific development of DLB and have laid the foundations for many consortia discussed in this article.1, 19 In the past, the North American DLB research community has benefitted from a convergence of funding from governmental and advocacy organizations focused primarily on AD and PD.32, 33
The National Alzheimer's Project Act (NAPA) established a “national plan” aimed at the prevention and treatment of AD and, among other dementias, DLB. Highlighting global collaboration through the establishment of DLB consortia as a key strategy, NAPA has opened up new funding streams for US researchers and their collaborators. For example, a recent expansion in the National Institute of Health's dementia research program has permitted additional funding focused on DLB, including the establishment of the US‐based Dementia with Lewy Bodies Consortium (US‐DLBC; see below).
2.4.1. Alzheimer's Disease Research Centers (ADRCs)
The Alzheimer's Dementia Care (ADC) program exemplifies how US multicenter DLB datasets have arisen from AD projects. Created by the National Institute on Aging (NIA), ADC initially aimed to collect and maintain a “Minimum Data Set” (demographics, diagnoses, and some neuropathological data) created through retrospective case note review and overseen by the National Alzheimer's Coordinating Center (NACC).32 The project has evolved to include 31 Alzheimer's Disease Research Centers (ADRCs). Since 2005, the ADC has been prospectively collecting longitudinal data using a standardized approach to the diagnosis and characterization of patients with dementia; this “Uniform Data Set” (UDS) is now in its third iteration.32
In 2017, an LBD module, an additional collection of scales, and checklists aimed at better characterizing patients with LBD, was added to the UDS. This included the Unified Parkinson's Disease Rating Scale (UPDRS) and Mayo Sleep Questionnaires among others (https://naccdata.org/data‐collection/forms‐documentation/lbd‐31). The module allows the collection and standardization of neuroimaging and polysomnography data from case note review. A 2018 analysis of the UDS from the NACC reported that 824 patients had been assessed with LBD (including PD), including MRI (n = 114), CSF (n = 34), genetic (n = 551), and neuropathological data (n = 353).32
The Mayo Clinic DLB program, a consortium of Mayo Clinic Facilities in Minnesota and Florida, deserve specific discussion as ADRCs contributing significantly to the DLB research landscape. Established in 1991, the program has recruited large cohorts of patients with DLB to several landmark studies.34, 35 The sleep disorders clinic has proven a rich resource for the identification and longitudinal assessment of patients with idiopathic RBD.34 Mayo Clinics’ Longitudinal Imaging Biomarkers of Disease Progression in DLB, established in June 2018, aims to recruit 90 patients with DLB, and 45 healthy controls, for longitudinal assessment. Participants will complete six visits over the 5‐year study. As well as annual FP‐CIT, MRI, and lumbar puncture, they will undergo amyloid PET and tau AV14‐51 PET.
2.4.2. The North American Prodromal Synucleinopathy (NAPS) Consortium
The North American Prodromal Synucleinopathy (NAPS) Consortium, formed in 2018, has aimed to enroll 300 participants with RBD, but without LBD, PD, or multisystem atrophy, anticipating one or more future clinical trials of neuroprotective treatments to delay the onset or prevent the overt synucleinopathies. The consortium is led by Washington University in St. Louis, and comprises 10 centers (nine US, one Canadian). Participants undergo a standardized assessment that adopts the UDS structure, as well as blood sampling and optional lumbar puncture. The consortium recently published preliminary neuropsychological data from 70 participants.36 An extension of this program—the NAPS2 Consortium—will begin in 2021 and continue with annual visits for RBD participants over the subsequent 5 years.
2.4.3. Dementia with Lewy bodies Consortium (US‐DLBC)
In 2017, the National Institute of Neurological Diseases and Stroke (NINDS) published requests for applications aimed toward developing biomarkers for DLB and PDD as part of the Parkinson's Disease Biomarkers Program (PDBP). One funded project established a consortium of 10 sites across the US with expertise in DLB (US‐DLBC; https://pdbp.ninds.nih.gov/index.php/Dementia‐with‐Lewy‐Bodies‐Consortium). The US‐DLBC aims to recruit a cohort of 216 subjects with DLB, PDD, and DLB‐mild cognitive impairment (MCI) across its 10 sites in the United States. Each participant has a comprehensive clinical assessment at baseline, 6 months, and then annually for the duration of the study. CSF is collected yearly in most participants, using PDBP protocols. Furthermore, Alzheimer's Disease Neuroimaging Initiative‐3 (ADNI‐3)–compatible MRI and DaTscan will be conducted at baseline and year two. A subset of participants will consent to autopsy, and the study cohort has been recruited with the secondary purpose of creating a sample available for additional therapeutic studies. Clinical data are entered into the PDBP (https://pdbp.ninds.nih.gov/data‐management‐resource) and clinical and neuropathologic data into the NACC database (https://naccdata.org).
To date, the US‐DLBC has recruited 122 DLB or MCI‐DLB participants, the majority with data available on imaging (MRI, DaTscan), clinical characterization, and biosamples, including CSF and blood products. A large proportion of participants (> 80%) have consented to post mortem brain donation.
2.4.4. Research Centers of Excellence
The Lewy Body Dementia Association (LBDA), the largest and most active DLB charity, developed a Research Centers of Excellence program in 2017, which comprises expert clinicians and researchers across 26 US centers with two primary goals: (1) to increase the quality of clinical care for patients with LBD and (2) to accelerate research in therapeutics for LBD.37 Similar to the LBD PIA, several working groups are focused on specific topics pertaining to LBD. In 2019, the LBDA also started the Industry Advisory Council (IAC) to provide a collaborative forum for discussion among LBD experts, pharmaceutical industries, governmental agencies, and the nonprofit LBDA to address challenges and opportunities for LBD clinical trials.37
2.5. Canada
The Canadian DLB initiative is a team within a larger consortium, the Canadian Consortium on Neurodegeneration in Aging (CCNA; www.ccna‐ccnv.ca). Established in 2014, the CCNA is currently recruiting cognitively intact older adults and people with neurodegenerative disorders across the country.38 Participants undergo a standardized imaging, clinical, and neuropsychological protocol that also includes biofluid collection and optional lumbar puncture. Follow‐up is supported at 1 and 2 years, and participants have the option to volunteer for brain donation. With more than 1100 enrolled to date, the Lewy body cohort includes 30 with DLB, and 17 with PDD. It is hoped that this cohort will grow to a target of 200 people with LBD. The consortium aims to identify diagnostic and prognostic biomarkers across the Lewy body spectrum.
2.6. DLB consortia in Australia
The ForeFront DLB initiative, based in Sydney, has been active since 2017. It recruits patients for longitudinal assessment with established DLB, as well as PD, PDD, MCI, idiopathic RBD, and other prodromal DLB subgroups. A sample of these participants has undergone detailed baseline motor, neuropsychiatric, sleep, MRI, chronobiology, genetic, clinical chemistry, and gait measure assessments. Clinical data from probable DLB cases using the Fourth consensus diagnostic criteria1 has been published, which demonstrated a clustering of the specific core and supportive DLB features as well as proposing a novel DLB‐Parkinsonism scale to help standardize diagnosis.39 Another Australian initiative, The Lewy Body Study, is based in Melbourne with collaborators in Perth. It represents a prospective observational longitudinal study focused on the impact of proteinopathies and vascular impairments on disease trajectories in patients with LBD. Participants undergo standardized clinical assessments as well as MRI, PET, and blood biomarkers with optional CSF collection.
2.7. DLB consortia in Latin America
Several multicenter dementia initiatives have been established across Latin America (Table 1). The Brazilian Biobank for Aging Studies at University of São Paolo, led by Lea Grinberg and colleagues, has collected more than 1300 brains since 2004 from patients with neurodegenerative diseases, including DLB and PD. Supported by multiple local and international stakeholders, the Biobank has produced multiple publications, and contributes DLB prevalence data to a national population‐based cohort. The Federal University of São Paulo has produced several single‐center studies assessing predictors of dementia, language impairments, or differential diagnoses between LBD and AD.40 Ongoing projects include the comparison of CSF and serum markers in DLB and AD.
The Colombian Consortium for the Study of Dementia with Lewy Bodies (COL‐DLB), a multicenter longitudinal study led by groups in Bogotá, Medellín, and Cali, was created in 2019. COL‐DLB aims to create a registry of patients with DLB with clinical, neuroimaging (MRI, FDG‐PET, DaTscan), CSF and blood biomarkers (p‐tau, neurofilament light chain, and neurogranin), and EEG data collected in accordance with the E‐DLB protocol.41
The Peruvian Institute of Neurosciences has recruited various cohorts of patients with PD and DLB since 2013. These have mainly focused on clinical and neuropsychological data. Efforts are underway to harmonize the protocol to that of E‐DLB.
The consortium to expand dementia research in Latin America (ReDLat) is a multinational initiative supported by NIH‐NIA, the Alzheimer's Association, the Rainwater Charitable Foundation (Tau Consortium), the Global Brain Health Institute, and Takeda Pharmaceutical Company. It aims to recruit participants with AD, frontotemporal dementia (FTD), and now also DLB, and controls from 10 sites in Argentina, Brazil, Chile, Colombia, Mexico, Peru, and the United States. By combining standardized genetic (whole genome) data, multimodal neuroimaging, behavioral (cognitive and socioeconomic/sociodemographic determinants of health), and EEG (in a subsample) measures and machine learning, ReDLat will compare the genetic and sociodemographic risk factors for AD and FTD in Latin American countries to those in US populations. Every DLB patient identified during ReDLat recruitment will undergo complete assessment using the study protocol. The program aims to amass more than 4200 patients, of whom an estimated 5% to 10% will have DLB, across Latin America by 2025.42, 43
2.8. DLB consortia in Asia
2.8.1. Japan
The DLB Society Japan (DLBSJ) was established by Dr. Kenji Kosaka in 2007 and has more than 400 members. It is funded by a grant from the Japan Foundation for Neuroscience and Mental Health. The DLBSJ's primary purpose is to stimulate basic and clinical research on DLB and to increase public awareness in finding better management options. Core members and their multicenter networks have contributed significantly to the advancement of diagnostic techniques. In particular, DLBSJ have led research on the use of MIBG myocardial scintigraphy25 and standardized methods for its use in discriminating probable DLB and probable AD.
Japanese groups have an impressive track record in planning and completing several clinical trials in DLB. Multicenter phase 3 randomized controlled trials of donepezil for cognition (n = 142)44 and zonisamide for parkinsonism (n = 346)45 have led to both agents being approved by the Japanese government for use in DLB.
2.8.2. India
Dementia diagnosis in India has previously been limited by the lack of standardization of assessments in movement disorders and cognitive neurology, but in recent years progress has been made toward rectifying this. The Parkinson's Research Alliance of India (PRAI) movement disorders group has developed nationwide research, including a recently formed Indian DLB consortium. PRAI coordinates the collection and annual follow‐up of a registry of patients with DLB and PDD across India. Standardized evaluation will follow the E‐DLB protocol, with one of two levels of detail of data collection (minimal and detailed) chosen by participating centers. The assessment includes the Indian Council of Medical Research neurocognitive toolbox (ICMR‐NCTB), a recent endeavor to standardize cognitive assessment scales for Indian populations.46 It is available in five languages, and it includes cultural adaptations in existing scales. Cognitive tests for the illiterate population have been implemented when possible, and new tests added by a group of multidisciplinary experts from across India.
2.8.3. China
Several multicenter DLB registries have been developed in China with the support of an established national dementia research infrastructure (Table 1). The Peking University Dementia Collaborative Research Network (PKU‐DCRN), comprising 30 participating hospitals, maintain a DLB registry comprising 220 cases. The organization has also recently begun a community‐based DLB screening program, in which older adults with RBD, visual hallucinations, or parkinsonism are invited to undergo comprehensive cognitive assessment, MRI, and DaTscan. The China Dementia with Lewy Bodies Collaboration Center (CDCC) registry, based at Tianjin Dementia Institute, has recruited 512 DLB cases.
The Innovation Center for Neurological Disorders in Xuanwu Hospital, Capital Medical University (CMU‐ICND), has established a database of 50 patients with DLB, and Zhejiang University has recruited 50 DLB cases with planned neuropathological follow‐up at China's National Brain Bank. These registries collect cognitive, neuropsychiatric data; multi‐modality MRI imaging; and in some instances, EEG, polysomnography, and DaTscan data. Blood and CSF samples are retrieved from a small proportion of participants.
Plans are in development to harmonize these registries with each other, and with other international consortia. It is also hoped that harmonization will allow identification of a large trial‐ready cohort suitable for studies investigating early diagnosis and intervention. One such trial investigating the efficacy and safety of sodium oligomannate (GV‐971) for cognitive impairment in DLB is scheduled to begin in the next year.
3. DISCUSSION
We have presented the work and future directions of DLB research initiatives from around the world (Tables 1 and 2). The growth and diversification of the global DLB community are evidenced by the authorship of diagnostic criteria, as well as by the volume of multicenter papers published per year (Figures 1 and 2). Regular communication is critical in sustaining this growth and ensures that consensus can continue to form around the growing evidence base. The feasibility of more frequent international DLB meetings should therefore be explored along with greater use of online platforms.
TABLE 2.
Selected recent publications, and planned future directions, of DLB research groups in each region
| Selected recent publications (Authors, Title, Journal, Year) | Future directions |
|---|---|
| EUROPE | |
| Abdelnour et al. The combined effect of amyloid beta and tau biomarkers on brain atrophy in dementia with Lewy bodies. Neuroimage Clin. 202050. |
|
| Oppedal et al. A signature pattern of cortical atrophy in dementia with Lewy bodies: A study on 333 patients from the European DLB consortium. Alzheimer's Dement. 201951. | |
| Roberts et al. Accuracy of cardiac innervation scintigraphy for mild cognitive impairment with Lewy bodies. Neurology. 202152. | |
| van der Zande et al. Diagnostic and prognostic value of EEG in prodromal dementia with Lewy bodies. Neurology. 202053. | |
| NORTH AMERICA | |
| Chen et al. beta‐Amyloid PET and (123)I‐FP‐CIT SPECT in mild cognitive impairment at risk for Lewy body dementia. Neurology. 202154. |
|
| Fields et al. The North American Prodromal Synucleinopathy (NAPS) Consortium: Baseline neuropsychological findings in 136 participants.Alzheimer's Dement. 202036. | |
| Naasan et al. Psychosis in neurodegenerative disease: differential patterns of hallucination and delusion symptoms. Brain. 202155. | |
| Chia et al. Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat Genet. 202114. | |
| SOUTH AMERICA | |
| Borda MG et al. Colombian consortium for the study of Lewy body dementia COL‐DLB. J Neurol Sci. 2020 41. |
|
| de Oliveira FF, et al. Neuropsychiatric feature profiles of patients with Lewy body dementia. Clin Neurol Neurosurg. 202040. | |
| Ibanez et al. The Latin America and the Caribbean Consortium on Dementia (LAC‐CD): From Networking to Research to Implementation Science. J Alzheimers Dis. 202142. | |
| ASIA | |
| Gan et al. Clinical characteristics of Lewy body dementia in Chinese memory clinics. BMC Neurol. 202156. |
|
| Murata et al. Effect of zonisamide on parkinsonism in patients with dementia with Lewy bodies: A phase 3 randomized clinical trial. Parkinsonism Relat Disord. 202045. | |
| Iyer GK, et al. Standardising dementia diagnosis across linguistic and educational diversity: Study design of the Indian Council of Medical Research‐Neurocognitive Tool Box (ICMR‐NCTB). J Int Neuropsychol Soc. 202046. | |
| AUSTRALIA | |
| Matar et al. Clinical features of Lewy body dementia: insights into diagnosis and pathophysiology. J Neurol. 202039. |
|
| Matar et al. Cognitive fluctuations in Lewy body dementia: towards a pathophysiological framework. Brain. 202057. | |
| Phillips et al. Evaluating the sustained attention response task to quantify cognitive fluctuations in dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 202058. | |
Abbreviation: DLB, dementia with Lewy bodies.
The ISTAART LBD PIA group has helped establish regular communication among members of the LBD community, filling the gap between international DLB consortia meetings. The publication of this article and the inclusion of a dedicated Featured Research session at the Alzheimer's Association International Conference 2020 demonstrate the advantages that have already arisen from the group. Virtual meetings have widened access beyond the groups that have traditionally dominated the field. While recognizing potential biologic underpinnings to risk for LBD in different cohorts, consideration should be paid to how the democratization of the community can be extended, particularly in supporting the participation of women, people of color, and researchers from low‐income countries in the DLB community.
Established consortia in Europe and the United States have recently been joined by continental initiatives in Australia and Latin America. Emerging Chinese and Indian networks hold enormous potential in accessing large populations capable of diversifying global DLB subjects. However, the absence of initiatives in Africa and the Middle East highlight that DLB cohorts are not yet reflective of a global population, and identify these regions as the next frontiers in DLB research. A strategic goal among international DLB organizations should be determining how best to support research and practice in these areas, such as clearer, more established mechanisms for accessing mentorship. The authors would welcome requests for advice or support from anyone considering establishing a DLB cohort in their respective center.
A further consideration in the development of consortia is the standardization of methodologies and practices. Research consortia, such as the ADNI47 and Parkinson's Progression Markers Initiative (PPMI)48 have achieved success both in both creating large datasets and in nurturing a global infrastructure that the DLB community should aim to replicate. Currently, this is challenged by a lack of information sharing; only a handful of the consortia discussed in this article have published protocols in peer‐reviewed journals. A single, open access, curated resource of active DLB consortia, or a bespoke journal for DLB research, could help rectify this. As novel methodologies emerge, such as the use of electronic health‐care records to enrich research cohorts with naturalistic clinical data, DLB‐specific platforms will be critical in supporting early standardization of these techniques. Inclusion of standardized operating procedures for the collection and analysis of EEG and neuroimaging data, and the adoption of data‐sharing systems like theHiveDB,49 could also allow groups to benefit from the community's shared expertise.
A more extensive and cohesive DLB research community could help support the development of single, recognizable points of contact with which engagement with pharmaceutical and biotechnology industries, regulatory industries, and political entities, could be facilitated. Global collaboration in these areas could assist the development of new therapeutic approaches by increasing the availability and size of trial‐ready cohorts as well as helping to share the expertise of groups such as DLBSJ, who have successfully worked with industry.
Involvement of patients and caregivers is crucial in guiding and supporting research. The US LBD Association and UK Lewy Body Society perform excellent work in their respective jurisdictions, and a new Lewy Body Ireland society is being established, but the creation of a single global charity could provide further funding opportunities, pooling of resources, and opportunities to engage the global public in raising awareness of DLB.
4. CONCLUSIONS
The development of global research networks should assist in addressing currently unmet needs in DLB research, facilitating better diagnostic definition, harmonization of protocols, and larger sample sizes. As national and continental consortia continue to grow, an important focus of these networks will be to ensure that levels of development at each are complementary, rather than competing. Participation of respective consortia in standardization of trial methodology, a key aim of the ISTAART LBD PIA, would support the development of a global DLB cohort with clinical, genetic, and biomarker characterization, as well as potentiate the work of individual groups.
Creation of common data‐sharing platforms, and curated repositories of resources, could enable more detailed and powerful analyses of existing cohorts and advance the understanding of clinical symptoms and prognosis. This infrastructure would facilitate the creation of a global DLB registry, and provide opportunities to develop new, more effective treatments through identification of trial‐ready DLB cohorts.
CONFLICTS OF INTEREST
Dag Aarsland has received research support and/or honoraria from Astra‐Zeneca, H. Lundbeck, Novartis Pharmaceuticals; and GE Health, and serves as paid consultant for H. Lundbeck, Eisai, and Axovant.
The authors have no other interests to declare.
ACKNOWLEDGMENTS
This manuscript was facilitated by the Alzheimer's Association International Society to Advance Alzheimer's Research and Treatment (ISTAART), through the Lewy body dementias professional interest area (PIA). The views and opinions expressed by authors in this publication represent those of the authors and do not necessarily reflect those of the greater PIA membership, ISTAART, or the Alzheimer's Association. The authors are particularly grateful for the support and diligence of Jodi Titiner and Chris Weber in supporting the LBD PIA and the production of this manuscript. Laura Bonanni, Bradley Boeve, Miguel Germán Borda, Fabrizia D'Antonio, Manabu Ikeda, Yong Ji, Rukmini Mridula Kandadai, Joseph Kane, Simon Lewis, Huali Wang, Yueyi Yu, and Jing Zhang have no funding sources to acknowledge. Dag Aarsland is a Royal Society Wolfson Research Merit Award Holder and would like to thank the Wolfson Foundation and the Royal Society for their support. Professor Aarsland is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. Claudio Babiloni is supported by the European H2020 Marie S. Curie ITN‐ETN grant. Richard Camicioli has received research funding support from the Canadian Institutes for Health Research (CIHR), the Lewy body Dementia Team of the Canadian Consortium on Neurodegeneration of Aging (CCNA), Brain Canada, and the Michael J. Fox Foundation. Agustin Ibañez is partially supported by grants from Alzheimer's Association GBHI ALZ UK‐20‐639295, Takeda CW2680521, CONICET, ANID/FONDAP/15150012, ANID/Fondecyt 1210195, Sistema General de Regalías (BPIN2018000100059), Universidad del Valle (CI 5316), and the MULTI‐PARTNER CONSORTIUM TO EXPAND DEMENTIA RESEARCH IN LATIN AMERICA (ReDLat, supported by National Institutes of Health, National Institutes of Aging [R01 AG057234], Alzheimer's Association [SG‐20‐725707], Tau Consortium, and Global Brain Health Institute). The contents of this publication are solely the responsibility of the authors and do not represent the official views of these institutions. James Leverenz is supported by the National Institute of Health (UO1 NS100610 and P30 AG062428), the Douglas Herthel DVM Memorial Research Fund, and the Jane and Lee Seidman Fund.
COLLABORATORS
1.
Carla Abdelnour
Fundació ACE. Barcelona Alzheimer Treatment and Research Center, Barcelona, Spain;
Universitat Autónoma de Barcelona, Barcelona, Spain
Matthew J. Barrett
Department of Neurology, Virginia Commonwealth University, Richmond, Virginia, USA
Ece Bayram
Parkinson and Other Movement Disorders Center, Department of Neurosciences, University of California San Diego, California, USA
Rupam Borgohain
Department of Neurology, Nizam's Institute of Medical Sciences, Hyderabad, India
Paulo Henrique Ferreira Bertolucci
Department of Neurology and Neurosurgery, Escola Paulista de Medicina, Federal University of São Paulo (UNIFESP), Sao Paulo, Brazil
Tinatin Chabrashvili
State University of New York Upstate Medical University, Syracuse, New York, USA
Kallol Ray Chaudhuri
Parkinson Foundation Centre of Excellence, Kings College London, London, UK
Shubham Dubey
Department of Neurology, Meenakshi Medical College Hospital and Research Institute, Kanchipuram, India
Daniel Ferreira
Division of Clinical Geriatrics, Center for Alzheimer Research, Department of Neurobiology, Care Sciences, and Society, Karolinska Institutet, Stockholm, Sweden; Department of Radiology, Mayo Clinic, Rochester, MN, United States
Percy Griffin
Department of Neurology, Washington University School of Medicine in St. Louis, Missouri, USA
Alzheimer's Association, Chicago, Illinois, USA
Maria Camila Gonzalez Velez
The Norwegian Centre for Movement Disorders, University of Stavanger, Stavanger, Norway
Josue D. Gonzalez Murcia
Department of Neurodegenerative Science, Van Andel Research Institute, Grand Rapids, Michigan, USA
Vinay Goyal
Department of Neurology, Medanta Hospital, Gurugram, India
Samantha K. Holden
University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA.
Jakob Hort
Memory Clinic, Department of Neurology, Charles University, 2nd Faculty of Medicine and Motol University Hospital, Prague, Czech Republic
Alberto Jaramillo‐Jimenez
Centre for Age‐Related Medicine (SESAM), Stavanger University Hospital, Stavanger, Norway
Faculty of Health Sciences, University of Stavanger, Stavanger, Norway
Grupo de Neurociencias de Antioquia, Universidad de Antioquia School of Medicine, Medellín, Colombia
Grupo Neuropsicología y Conducta, Universidad de Antioquia, School of Medicine, Medellín, Colombia
Iracena Leroi
Global Brain Health Institute, California, USA & Dublin, Ireland
Trinity College Dublin, Dublin, Ireland
Miao Qu
Neurology Department, Xuan Wu Hospital of Capital Medical University, Beijing, China
L.K. Prashanth
Parkinson's Disease and Movement Disorders Clinic, Bangalore, India
Center for Parkinson's Disease and Movement Disorders, Vikram Hospital, Bangalore, India
Parkinson Research Alliance of India (PRAI)
Ian McKeith
Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, UK
John T. O'Brien
Department of Psychiatry, University of Cambridge, UK
Cambridgeshire and Peterborough NHS Foundation Trust, UK
Mie Rizig
Institute of Neurology, University College London, London, UK
Arvid Rongve
Department of Research and Innovation, Helse Fonna, Haugesund, Norway
Institute of Clinical Medicine (K1), The University of Bergen, Norway
Sonia W. Scholz
Neurodegenerative Diseases Research Unit, National Institute of Neurological Disorders and Stroke, Bethesda, Maryland, USA
Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA
Angela S. Taylor
Lewy Body Dementia Association, Atlanta, Georgia, USA
John‐Paul Taylor
Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, UK
Alan J. Thomas
Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, UK
Jodi Titiner
Alzheimer's Association, Chicago, Illinois, USA
Jon B. Toledo
Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, Florida, USA
Prabitha Urwyler
Gerontechnology and Rehabilitation, ARTORG Center for Biomedical Engineering, University of Bern, Bern, Switzerland
University Neurorehabilitation Unit, Department of Neurology, Inselspital, Bern University Hospital, Bern, Switzerland
Marleen van de Beek
Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, the Netherlands
Rosie Watson
Population Health and Immunity Division, Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia
Department of Medicine, Royal Melbourne Hospital, The University of Melbourne, Melbourne, Australia
Chris Weber
Alzheimer's Association, Chicago, Illinois, USA
Rimona S. Weil
Dementia Research Centre, University College London, UK
Wellcome Centre for Human Neuroimaging, University College London, UK
Movement Disorders Consortium, National Hospital for Neurology and Neurosurgery, London, UK
Edward Wilson
Department of Neurology, Stanford University, Stanford, California, USA
Kathryn A. Wyman‐Chick
HealthPartners Center for Memory and Aging and Struthers Parkinson's Center, Saint Paul, Minnesota, USA
Masahito Yamada
Department of Neurology & Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Japan
Kudanzaka Hospital, Chiyoda City, Japan
D'Antonio F, Kane JPM, Ibañez A, et al., the ISTAART Lewy body dementias Consortia Working Group . Dementia with Lewy bodies research consortia: A global perspective from the ISTAART Lewy Body Dementias Professional Interest Area working group. Alzheimer's Dement. 2021;13:e12235. 10.1002/dad2.12235
Fabrizia D'Antonio and Joseph P.M. Kane are co‐first authors.
REFERENCES
- 1.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88‐100. 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attems J, Toledo JB, Walker L, et al. Neuropathological consensus criteria for the evaluation of Lewy pathology in post‐mortem brains: a multi‐centre study. Acta Neuropathol. 2021;141(2):159‐172. 10.1007/s00401-020-02255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kane JPM, Surendranathan A, Bentley A, et al. Clinical prevalence of Lewy body dementia. Alzheimers Res Ther. 2018;10(1):19. 10.1186/s13195-018-0350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens T, Livingston G, Kitchen G, Manela M, Walker Z, Katona C. Islington study of dementia subtypes in the community. Br J Psychiatry. 2002;180:270‐276. 10.1192/bjp.180.3.270. [DOI] [PubMed] [Google Scholar]
- 5.Surendranathan A, Kane JPM, Bentley A, et al. Clinical diagnosis of Lewy body dementia. BJPsych open. 2020;6(4):e61. 10.1192/bjo.2020.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galvin JE, Duda JE, Kaufer DI, Lippa CF, Taylor A, Zarit SH. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord. 2010;16(6):388‐392. 10.1016/j.parkreldis.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mueller C, Ballard C, Corbett A, Aarsland D. The prognosis of dementia with Lewy bodies. Lancet Neurol. 2017;16(5):390‐398. 10.1016/S1474-4422(17)30074-1. [DOI] [PubMed] [Google Scholar]
- 8.Taylor J‐P, McKeith IG, Burn DJ, et al. New evidence on the management of Lewy body dementia. Lancet Neurol. 2020;19(2):157‐169. 10.1016/S1474-4422(19)30153-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider Julie, Jeon Sofia GJTCRA . ADRD Summit 2019 Report to the National Advisory Neurological Disorders and Stroke Council.; 2019. [Google Scholar]
- 10.Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther. 2014;6(9):82. 10.1186/s13195-014-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayram E, Coughlin DG, Banks SJ, Litvan I. Sex differences for phenotype in pathologically defined dementia with Lewy bodies. J Neurol Neurosurg & Psychiatry. 2021:325668. 10.1136/jnnp-2020-325668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbas MM, Xu Z, Tan LCS. Epidemiology of Parkinson's Disease—East Versus West. Mov Disord Clin Pract. 2018;5(1):14‐28. 10.1002/mdc3.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKeith IG, Ferman TJ, Thomas AJ, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology. 2020;94(17):743‐755. 10.1212/WNL.0000000000009323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chia R, Sabir MS, Bandres‐Ciga S, et al. Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat Genet. 2021. 10.1038/s41588-021-00785-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orme T, Guerreiro R, Bras J. The Genetics of Dementia with Lewy Bodies: current Understanding and Future Directions. Curr Neurol Neurosci Rep. 2018;18(10):67. 10.1007/s11910-018-0874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13(1):72‐83. 10.1016/j.jalz.2016.06.2360. [DOI] [PubMed] [Google Scholar]
- 17.Reijs BLR, Teunissen CE, Goncharenko N, et al. The Central Biobank and Virtual Biobank of BIOMARKAPD: a Resource for Studies on Neurodegenerative Diseases. Front Neurol. 2015;6:216. 10.3389/fneur.2015.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113‐1124. 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 19.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863‐1872. 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 20.McKeith I, O'Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I‐FP‐CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6(4):305‐313. 10.1016/S1474-4422(07)70057-1. [DOI] [PubMed] [Google Scholar]
- 21.Boeve BF, Silber MH, Ferman TJ, et al. REM sleep behavior disorder and degenerative dementia: an association likely reflecting Lewy body disease. Neurology. 1998;51(2):363‐370. 10.1212/wnl.51.2.363. [DOI] [PubMed] [Google Scholar]
- 22.Iranzo A, Stockner H, Serradell M, et al. Five‐year follow‐up of substantia nigra echogenicity in idiopathic REM sleep behavior disorder. Mov Disord. 2014;29(14):1774‐1780. 10.1002/mds.26055. [DOI] [PubMed] [Google Scholar]
- 23.Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142(3):744‐759. 10.1093/brain/awz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiraboschi P, Corso A, Guerra UP, et al. (123) I‐2β‐carbomethoxy‐3β‐(4‐iodophenyl)‐N‐(3‐fluoropropyl) nortropane single photon emission computed tomography and (123) I‐metaiodobenzylguanidine myocardial scintigraphy in differentiating dementia with lewy bodies from other dementias: a comparativ. Ann Neurol. 2016;80(3):368‐378. 10.1002/ana.24717. [DOI] [PubMed] [Google Scholar]
- 25.Yoshita M, Arai H, Arai H, et al. Diagnostic accuracy of 123I‐meta‐iodobenzylguanidine myocardial scintigraphy in dementia with Lewy bodies: a multicenter study. PLoS One. 2015;10(3):e0120540. 10.1371/journal.pone.0120540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanc F, Colloby SJ, Philippi N, et al. Cortical Thickness in Dementia with Lewy Bodies and Alzheimer's Disease: a Comparison of Prodromal and Dementia Stages. PLoS One. 2015;10(6):e0127396. 10.1371/journal.pone.0127396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonanni L, Perfetti B, Bifolchetti S, et al. Quantitative electroencephalogram utility in predicting conversion of mild cognitive impairment to dementia with Lewy bodies. Neurobiol Aging. 2015;36(1):434‐445. 10.1016/j.neurobiolaging.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonanni L, Cagnin A, Agosta F, et al. The Italian dementia with Lewy bodies study group (DLB‐SINdem): toward a standardization of clinical procedures and multicenter cohort studies design. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2017;38(1):83‐91. 10.1007/s10072-016-2713-8. [DOI] [PubMed] [Google Scholar]
- 29.Kramberger MG, Auestad B, Garcia‐Ptacek S, et al. Long‐Term Cognitive Decline in Dementia with Lewy Bodies in a Large Multicenter, International Cohort. J Alzheimers Dis. 2017;57(3):787‐795. 10.3233/JAD-161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira D, Przybelski SA, Lesnick TG, et al. β‐Amyloid and tau biomarkers and clinical phenotype in dementia with Lewy bodies. Neurology. 2020;95(24):e3257‐e3268. 10.1212/WNL.0000000000010943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babiloni C, Pascarelli MT, Lizio R, et al. Abnormal cortical neural synchronization mechanisms in quiet wakefulness are related to motor deficits, cognitive symptoms, and visual hallucinations in Parkinson's disease patients: an electroencephalographic study. Neurobiol Aging. 2020;91:88‐111. 10.1016/j.neurobiolaging.2020.02.029. [DOI] [PubMed] [Google Scholar]
- 32.Besser L, Kukull W, Knopman DS, et al. Version 3 of the National Alzheimer's Coordinating Center's Uniform Data Set. Alzheimer Dis Assoc Disord. 2018;32(4):351‐358. 10.1097/WAD.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gwinn K, David KK, Swanson‐Fischer C, et al. Parkinson's disease biomarkers: perspective from the NINDS Parkinson's Disease Biomarkers Program. Biomark Med. 2017;11(6):451‐473. 10.2217/bmm-2016-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferman TJ, Boeve BF, Smith GE, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology. 2011;77(9):875‐882. 10.1212/WNL.0b013e31822c9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graff‐Radford J, Murray ME, Lowe VJ, et al. Dementia with Lewy bodies: basis of cingulate island sign. Neurology. 2014;83(9):801‐809. 10.1212/WNL.0000000000000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fields JA, Boeve BF, Forsberg LK, et al. The North American Prodromal Synucleinopathy (NAPS) Consortium: baseline neuropsychological findings in 136 participants. Alzheimer's Dement. 2020;16(S6):e044834. doi: 10.1002/alz.044834 [DOI] [Google Scholar]
- 37.Goldman JG, Forsberg LK, Boeve BF, et al. Challenges and opportunities for improving the landscape for Lewy body dementia clinical trials. Alzheimers Res Ther. 2020;12(1):137. 10.1186/s13195-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chertkow H, Borrie M, Whitehead V, et al. The Comprehensive Assessment of Neurodegeneration and Dementia: canadian Cohort Study. Can J Neurol Sci Le J Can des Sci Neurol. 2019;46(5):499‐511. 10.1017/cjn.2019.27. [DOI] [PubMed] [Google Scholar]
- 39.Matar E, Ehgoetz Martens KA, Halliday GM, Lewis SJG. Clinical features of Lewy body dementia: insights into diagnosis and pathophysiology. J Neurol. 2020;267(2):380‐389. 10.1007/s00415-019-09583-8. [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira FF, Machado FC, Sampaio G, de Marin S MC, Naffah‐Mazzacoratti M da G, Bertolucci PHF. Neuropsychiatric feature profiles of patients with Lewy body dementia. Clin Neurol Neurosurg. 2020;194:105832. 10.1016/j.clineuro.2020.105832. [DOI] [PubMed] [Google Scholar]
- 41.Borda MG, Lopera F, Buritica O, et al. Colombian consortium for the study of Lewy body dementia COL‐DLB. J Neurol Sci. 2020;412:116807. 10.1016/j.jns.2020.116807. [DOI] [PubMed] [Google Scholar]
- 42.Ibanez A, Parra MA, Butlerfor C. The Latin America and the Caribbean Consortium on Dementia (LAC‐CD): from Networking to Research to Implementation Science. J Alzheimers Dis. 2021. 10.3233/JAD-201384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parra MA, Baez S, Sedeño L, et al. Dementia in Latin America: paving the way toward a regional action plan. Alzheimer's Dement. 2020. 10.1002/alz.12202. n/a(n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikeda M, Mori E, Matsuo K, Nakagawa M, Kosaka K. Donepezil for dementia with Lewy bodies: a randomized, placebo‐controlled, confirmatory phase III trial. Alzheimers Res Ther. 2015;7(1):4. 10.1186/s13195-014-0083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murata M, Odawara T, Hasegawa K, et al. Effect of zonisamide on parkinsonism in patients with dementia with Lewy bodies: a phase 3 randomized clinical trial. Parkinsonism Relat Disord. 2020;76:91‐97. 10.1016/j.parkreldis.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Iyer GK, Paplikar A, Alladi S, et al. Standardising Dementia Diagnosis Across Linguistic and Educational Diversity: study Design of the Indian Council of Medical Research‐Neurocognitive Tool Box (ICMR‐NCTB). J Int Neuropsychol Soc. 2020;26(2):172‐186. 10.1017/S1355617719001127. [DOI] [PubMed] [Google Scholar]
- 47.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201‐209. 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marek K, Chowdhury S, Siderowf A, et al. The Parkinson's progression markers initiative (PPMI) ‐ establishing a PD biomarker cohort. Ann Clin Transl Neurol. 2018;5(12):1460‐1477. 10.1002/acn3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muehlboeck J‐S, Westman E, Simmons A, TheHiveDB image data management and analysis framework. Front Neuroinform. 2014;7:49. 10.3389/fninf.2013.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdelnour C, van Steenoven I, Londos E, et al. Alzheimer's disease cerebrospinal fluid biomarkers predict cognitive decline in lewy body dementia. Mov Disord. 2016;31(8):1203‐1208. 10.1002/mds.26668. [DOI] [PubMed] [Google Scholar]
- 51.Oppedal K, Ferreira D, Cavallin L, et al. A signature pattern of cortical atrophy in dementia with Lewy bodies: a study on 333 patients from the European DLB consortium. Alzheimer's Dement. 2018:1‐10. 10.1016/j.jalz.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Roberts G, Durcan R, Donaghy PC, et al. Accuracy of Cardiac Innervation Scintigraphy for Mild Cognitive Impairment With Lewy Bodies. Neurology. 2021;96(23):e2801‐e2811. 10.1212/WNL.0000000000012060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Zande JJ, Gouw AA, van Steenoven I, et al. Diagnostic and prognostic value of EEG in prodromal dementia with Lewy bodies. Neurology. 2020;95(6):e662‐e670. 10.1212/WNL.0000000000009977. [DOI] [PubMed] [Google Scholar]
- 54.Chen Q, Lowe VJ, Boeve BF, et al. β‐Amyloid PET and (123)I‐FP‐CIT SPECT in Mild Cognitive Impairment at Risk for Lewy Body Dementia. Neurology. 2021;96(8):e1180‐9. 10.1212/WNL.0000000000011454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naasan G, Shdo SM, Rodriguez EM, et al. Psychosis in neurodegenerative disease: differential patterns of hallucination and delusion symptoms. Brain. 2021;144(3):999‐1012. 10.1093/brain/awaa413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gan J, Liu S, Wang X, et al. Clinical characteristics of Lewy body dementia in Chinese memory clinics. BMC Neurol. 2021;21(1):144. 10.1186/s12883-021-02169-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matar E, Shine JM, Halliday GM, Lewis SJG. Cognitive fluctuations in Lewy body dementia: towards a pathophysiological framework. Brain. 2020;143(1):31‐46. 10.1093/brain/awz311. [DOI] [PubMed] [Google Scholar]
- 58.Phillips JR, Matar E, Martens KAE, Halliday GM, Moustafa AA, Lewis SJG. Evaluating the sustained attention response task to quantify cognitive fluctuations in dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2020;33(6):333‐339. 10.1177/0891988719882093. [DOI] [PubMed] [Google Scholar]


