Abstract
Background
Knee osteoarthritis (KOA) is the most common cause of chronic knee pain, and disability and different modalities have been used to improve pain and function. Botulinum toxin intra-articular injection is proposed to manage resistant joint pains.
Objectives
This study was carried out to compare therapeutic effects of intra-articular botulinum neurotoxin (BTX) versus physical therapy (PT) in KOA.
Methods
In this single-blind randomized clinical trial, patients with KOA attending to Imam-Reza Hospital, Tehran, Iran, from June 2018 to March 2019 were enrolled. Patients who met the inclusion criteria were randomly divided into BTX receiving a single intra-articular dose of 100 units (250 units from disport brand) and PT groups. The study was described for patients, and informed consent forms were received. For assessment of the pain and related severity, the VAS score and KOOS scales were used. Post-intervention assessment was done 1, 3, and 6 months after the intervention. The level of significance was set at α = 0.05. All data analyses were performed with SPSS version 26 for windows.
Results
In this study, 50 patients were randomly divided into BTX and PT groups. All patients completed the study, and there was no loss to follow-up. There was no significant difference between demographic data of the two groups, including age and BMI. The VAS score was similar in the two groups at the beginning. KOOS subscales were not significantly different, but the quality of life was better in the BTX than the PT group (86.2 ± 15 vs. 72.1 ± 11.5, P < 0.001). One month after the intervention, all KOOS subscales were improved in the BTX group in comparison to the PT group (P < 0.001). This difference was statistically significant in the 3rd (P < 0.001 in all comparisons except Sport/Rec subscale in which P = 0.02) and 6th months (P < 0.001) after the intervention, and the improvement in all KOOS subscales and VAS score were higher in the BTX group than the PT group. The trend of KOOS subscales and VAS score was improved over time in the BTX (P < 0.001 in all tests), but the PT group showed no improvement (P > 0.05) except for Sport/Rec and VAS score (P < 0.001).
Conclusions
Totally, it is concluded that the use of BTX can reduce pain and improve the function and quality of life in patients with KOA.
Keywords: Knee Osteoarthritis, Botulinum Neurotoxin, Physiotherapy, KOOS
1. Background
Osteoarthritis is the most common disease of synovial joints and the main cause of disability in the elderly. There is an increasing trend in osteoarthritis prevalence rate due to increased elderly population (1). Articular pain due to knee osteoarthritis (KOA) is the main cause of disability and activity restrictions and treatment-seeking, especially in elderly people (2). Pain management is the main therapeutic goal to achieve better joint function (3).
Non-invasive [e.g., physiotherapy (PT), TDCS (4), etc.] and invasive treatment options for KOA have been investigated. Platelet-rich plasma (PRP) (5, 6), prolotherapy (7, 8) have currently shown ideal outcomes. Hyaluronic acid, PRP, and prolotherapy require multiple injections that increase the risk of joint infections (9, 10). The next therapeutic step, but not in very young or aged subjects, is total knee arthroplasty, especially in recalcitrant pain (11). Pathologic assessments show osteophyte formation, subchondral changes, bone marrow edema, and articular surface destructions that decrease the joint space and stability beside soft tissue alterations such as synovial inflammation, capsular thickening, and ligament laxity (12).
Regarding peripheral sensitivity, intra-articular neurotoxins may be effective modalities (13, 14).
Botulinum toxin (BTX), produced by the bacterium Clostridium botulinum, acts on both sensory and motor neurons. Irreversible binding of BTX to presynaptic receptors of motor endplates inhibits acetylcholine release. This leads to the reduction of muscle activity and consequent muscle weakness (15). The BTX decreases the production of substance P and other pain generator substances by attaching to C fibers (16). BTX intra-articular injection is proposed to manage resistant joint pains (12). Although some primary studies showed some encouraging results for botulinum toxins, there were controversial results by other studies (17). Studies have shown better improvement in pain and function scores in short term, but there is a lack of evidence for long-term efficacy of BTX (18). According to different methods of physiotherapy interventions in KOA, modalities like local heat, TENS and pulsed ultrasound besides exercise therapy are effective in pain reduction and function of KOA patients (19-22).
2. Objectives
In this study, the long-term therapeutic effects of intra-articular were compared in the BTX and PT groups in patients with KOA.
3. Methods
3.1. Design and Setting
The original study was registered with the registration number IRCT20181217042028N2 at the Iranian Registry of Clinical Trials. We performed a single-blind randomized clinical trial from June 2018 for 9 months. The study was conducted in the Department of Physical Medicine and Rehabilitation at the Imam Reza Hospital.
3.2. Ethical Considerations
This study was approved by the Ethics Committee of Institutional Review Board of Aja University of Medical Sciences with the number of IR.AJAUMS.REC.1397.012. The researchers clarified all possible side effects, and informed consent forms were signed by the participants. The patients were free to withdraw from the study at any time.
3.3. Eligibility and Recruitment
The patients between 30 and 70 years of age who met the American College of Rheumatology criteria for KOA were recruited (23). Those with knee pain for more than 3 months, morning stiffness less than 30 min and joint crepitus were included in the study (24).
The patients with a history of diseases affecting knee joints like rheumatoid arthritis and gout and neuromuscular diseases were excluded from the study. Previous intra-articular injections, history of knee joint surgery, and trauma were the other exclusion criteria. Contraindications to intra-articular injection e.g., sepsis, intra-articular infections, intra-articular fracture, or uncontrolled coagulopathy were also considered the exclusion criteria (25).
All potential participants were evaluated based on the signs and symptoms of KOA. A standing lateral, anteroposterior and patellar view radiographs were taken. The study protocol was explained to all participants during the initial interview and after signing the written informed consent, the participants were allocated to one of the study groups.
3.4. Interventions
In the BTX group, the botulinum toxin was injected as a single intra-articular dose of 100 units (250 units from disport brand). The solution was diluted with 5 milliliters of normal saline and, after initial aspiration, was injected into the medial or lateral patellar tendon by a trained physician. In the PT group, exercise therapy besides modalities such as TENS (80 - 100 Hz, 100 - 200 milliseconds for 20 minutes), pulsed ultrasound (5: 1, 0.8 - 1.5 w/cm2 for five minutes), and superficial heat were used. Knee isometric exercises for quadriceps strengthening and calf/hamstring muscle stretching exercises were trained.
3.5. Outcome Measures
Pain as the primary outcome was evaluated with visual analog scale (VAS) in which 0 representing no pain and 10 showed the most severe pain. The Knee injury and Osteoarthritis Outcome Score (KOOS) was the secondary outcome. The KOOS is a self-report questionnaire and includes questions about pain, symptoms, activities during daily living, sport and recreational (Sport/Rec) activities, and quality of life (QOL). A normalized score from zero (extreme symptoms) to 100 (no symptoms) is given to each question. Salavati et al. validated the Persian version of KOOS showed that this version was culturally adapted, reliable, and valid (26). All measurements were performed at baseline and repeated at 1, 3, and 6 months after the intervention. All possible side effects were evaluated at each session.
3.6. Randomization and Blinding
This was a single-blind randomized clinical trial, and only outcome assessors were blinded. We used block randomization to randomly allocate 25 participants to each group. Random numbers were generated using a computer sequence generator software. Allocation concealment was done by sealed envelopes.
3.7. Statistical Analyses
The results are presented as mean (SD). Shapiro-Wilk test was used to test the normality of variables. Homogeneity of variances was investigated by Levene’s test. Independent sample t-test was used to compare means between the two groups. We used repeated measures ANOVA to compare the trend of variable means through time. The level of significance was set at α = 0.05. All data were analyzed with SPSS version 26 for windows.
4. Results
In this study, we enrolled 80 patients among whom 21 patients did not meet the inclusion criteria, and 9 patients were excluded, and overall, 50 patients randomized (Figure 1). All patients completed the study, and there was no loss to follow-up. In the BTX and PT groups, the subjects were female in 73 and 80%, respectively (P > 0.05). Table 1 compares baseline characteristics of the BTX and PT groups. The OA grade was 3 and 4 in 53 and 47% in the BTX group, respectively, and it was 3 and 4 in 60 and 40% in the PT group, respectively (P > 0.05). There was no significant difference between demographic data of the two groups, including age (77.7 ± 7.3 vs. 63.0 ± 8.0, P = 0.102) and BMI (31.3 ± 4.7 vs. 29.2 ± 3.5, P = 0.101). The VAS score was similar in the two groups at the beginning (6.67 ± 1.60 vs. 6.60 ± 1.84, P = 0.893). KOOS subscales like symptom, pain, ADL, and Sport/Rec activities did not significantly differ, but QOL was better in the BTX group than the PT group (86.2 ± 15 vs. 72.1 ± 11.5, P < 0.001). The only adverse effect in our study was severe pain in two cases in the BTX group that was improved by acetaminophen.
Figure 1. Patients’ flow diagram.
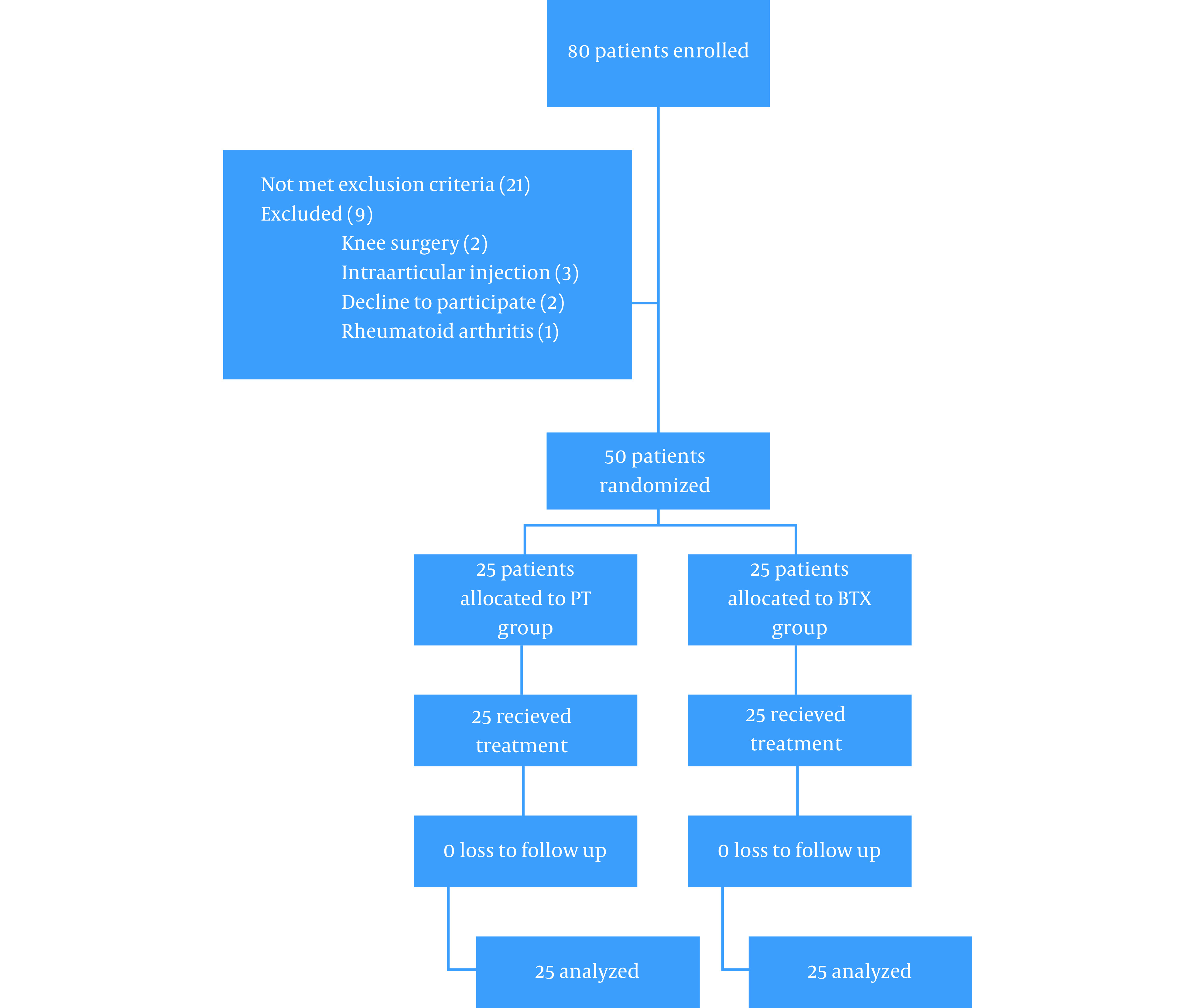
Table 1. Participants’ Baseline Characteristics a.
| Variables | BTX | PT | P-Value |
|---|---|---|---|
| Age b | 77.7 ± 7.3 | 63.0 ± 8.0 | 0.102 |
| Grade | 3.5 ± 0.5 | 3.5 ± 0.5 | 0.650 |
| Weight (kg) | 81.2 ± 12.2 | 76.4 ± 10.0 | 0.154 |
| Height (m) | 161 ± 7 | 161 ± 10 | 0.774 |
| BMI (kg/m 2 ) | 31.3 ± 4.7 | 29.2 ± 3.5 | 0.101 |
| Sex c (%) | 0.425 | ||
| Male | 27 | 20 | |
| Female | 73 | 80 | |
| VAS | 6.67 ± 1.60 | 6.60 ± 1.84 | 0.893 |
| KOOS | |||
| Symptoms | 58.21 ± 23.58 | 53.92 ± 10.86 | 0.452 |
| Pain | 38.88 ± 18.22 | 44.02 ± 12.69 | 0.279 |
| ADL | 34.36 ± 13.29 | 42.20 ± 12.53 | 0.052 |
| Sports/Rec | 9.83 ± 19.14 | 12.75 ± 13.71 | 0.560 |
| QOL | 13.75 ± 15.07 | 27.81 ± 11.55 | 0.001 d |
a Values are expressed as mean ± SD unless otherwise indicated.
b Independent sample t-test.
c Fisher’s exact test.
d Significance at the level of 0.05.
Table 2 compares the difference between post-intervention and baseline measurements between the two groups by independent sample t-test. As shown in Table 2, one month after the intervention, all KOOS subscales were improved in the BTX group in comparison to the PT group (P < 0.001). This difference was statistically significant in the 3rd (P < 0.001 in all comparisons except Sport/Rec subscale in which P = 0.02) and 6th months (P < 0.001) after the intervention, and the improvement in all KOOS subscales and VAS score were higher in the BTX group than the PT group.
Table 2. Comparison of KOOS Subscales and VAS Score Between the Two Groups in Post-intervention Assessments a.
| Variables | Pain (VAS) | KOOS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Pain | ADL | Sport/Rec | QOL | ||||||||
| 1 month - baseline | ||||||||||||
| BTX | -3.1 | 1.2 | 26.3 | 17.5 | 40.8 | 13.1 | 39.2 | 10.6 | 34.0 | 13.7 | 37.9 | 15.1 |
| PT | -1.3 | 1.4 | 5.1 | 12.3 | 2.0 | 12.5 | 3.0 | 12.7 | 10.0 | 11.3 | 4.3 | 9.5 |
| Significant b | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||||
| 3 month - baseline | ||||||||||||
| BTX | -3.7 | 1.3 | 28.8 | 19.0 | 43.7 | 13.6 | 40.8 | 10.3 | 34.0 | 13.7 | 38.1 | 14.8 |
| PT | -1.8 | 1.4 | 5.8 | 16.7 | 6.3 | 19.2 | 2.8 | 10.5 | 19.2 | 29.7 | 10.9 | 23.8 |
| Significant | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.02 | < 0.001 | ||||||
| 6 month - baseline | ||||||||||||
| BTX | -3.9 | 1.3 | 29.3 | 19.4 | 43.5 | 12.7 | 40.5 | 10.4 | 30.7 | 14.2 | 37.5 | 15.6 |
| PT | -1.5 | 1.0 | 0.5 | 11.8 | 0.1 | 13.0 | 2.8 | 10.5 | 10.2 | 11.8 | 3.8 | 9.8 |
| Significant | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||||
a All measurements are stated as mean difference ± standard deviation.
b Between-group analysis was done with independent sample t-test (significance at the level of 0.05).
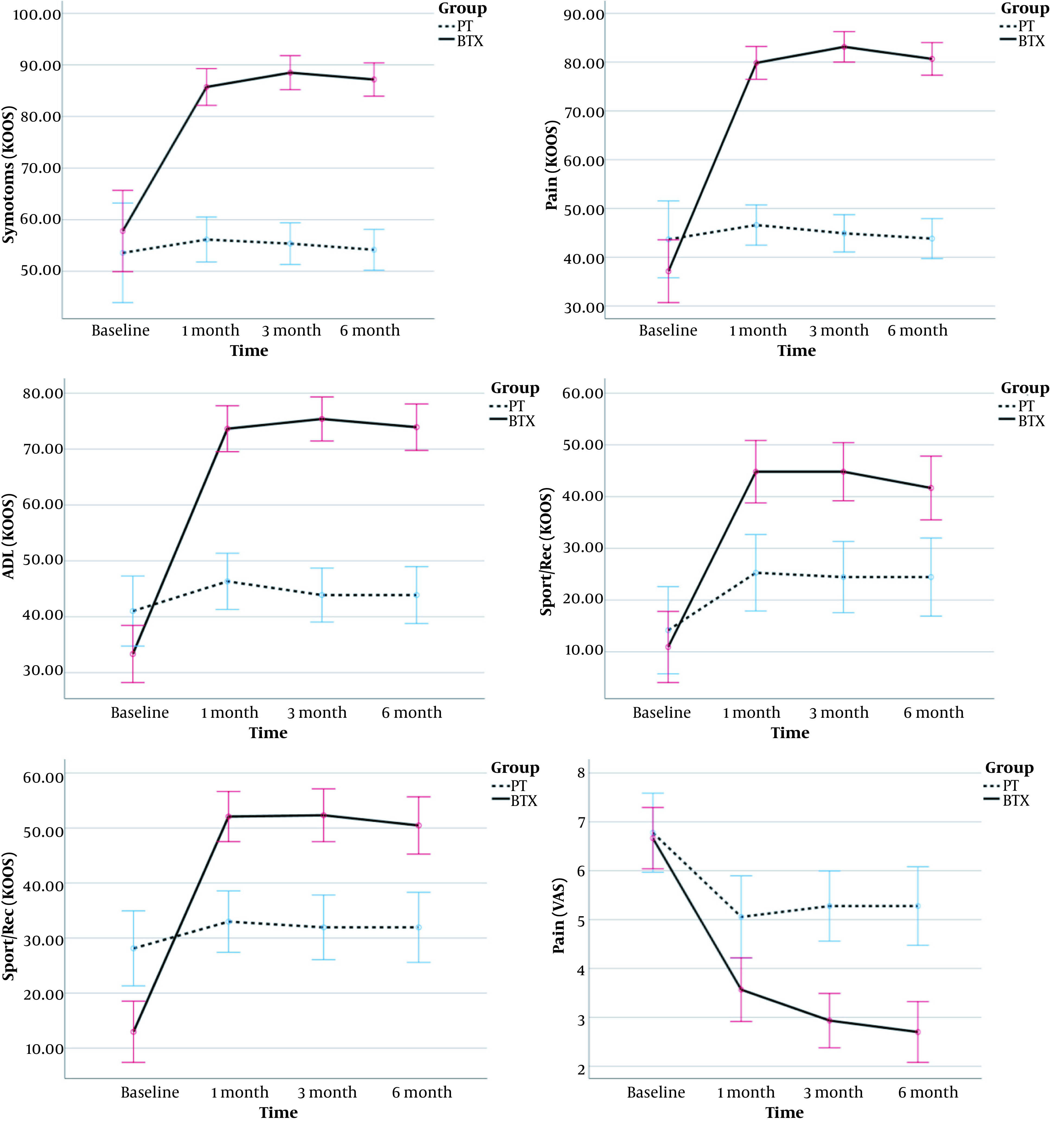
Figure 2 shows the trend of improvement in KOOS subscales and VAS score, which is calculated by within-group analysis of repeated-measurement ANOVA (RMANOVA). The trend of KOOS subscales and VAS score were improved over time in the BTX group (P < 0.001 in all tests), but the PT group showed no improvement (P > 0.05 except for Sport/Rec and VAS (P < 0.001). In addition, the difference was significant between the two groups in the 1st, 3rd, and 6th months of the intervention, and the BTX group had higher levels of improvement than the PT group. Although QOL was worse in the BTX group at the beginning, it has higher scores in the following investigations. The mixed ANOVA analysis compared the two groups in terms of pain (VAS). The assumption of sphericity was violated for the pain (Muchly's W for pain = 0.71, P = 0.009). Therefore, Greenhouse-Geisser correction was used for the degrees of freedom; epsilon pain = 0.83. The interaction effect of group × time were significant [F (2.4, 16.8) = 17.7, P < 0.001] and the effect size (partial Eta square) was 0.278.
Figure 2. Comparing change of mean outcomes throughout the study between PT and BTX groups. Error bars represent the 95% CI for the outcomes.
5. Discussion
One of the main challenges in the management of KOA is cases with high-severity disease. The intra-articular injections may impose some costs and some possible adverse effects and need multiple injections for better persistent outcomes and may be effective only in low grades (27, 28). BTX injection is a novel therapeutic method for resistant painful conditions. Regarding small studies in this era and uncertainty about appropriate dose, BTX injection is not a conventional method (29). In this study, long-term efficacy of PT versus BTX injection was assessed.
The results of our study showed higher efficacy for BTX versus PT in short and long term. In our study, the VAS score was significantly lower after treatment in both groups, but the difference was statistically significant between the two groups after the intervention. Also, these results were seen for knee function according to KOOS subscales. The study conducted by Bao et al. (30) showed better efficacy for exercise plus botulinum toxin versus hyaluronate and normal saline (control group). However, Mendes et al. showed that hyaluronic acid has higher effectiveness than botulinum toxin in short-term (4 weeks) follow-up (18). In Mendes study, both groups showed improvement in VAS score and Western Ontario and McMaster Universities Osteoarthritis Index, but this improvement was higher in hyaluronate group.
The long-term efficacy of botulinum toxin has controversial results. A study done by Sun (31) showed no significant difference in six-month follow-up between botulinum toxin and hyaluronate plus exercise in ankle osteoarthritis. Our study showed that in the long term, both pain and function in KOA were improved in the BTX group versus the PT group. The difference seen in other studies may be due to lower osteoarthritis grades. There is no evidence of botulinum toxin effects on inflammatory pain in human studies (32). The study by Singh (33) on BTX-A resulted in better function and less articular stiffness, and the WOMAC score was reduced. Better ADL and quality of life in our study showed better performance and function of the knee in patients.
The mechanism of BTX to reduce pain in KOA is not well known. It is shown that substances like serotonin, prostaglandins, bradykinin and histamine have nociception activity on free nerve endings. It has been reported in the rat models that joint damage or inflammation caused by KOA could result in the production of various substances (e.g., prostaglandins, histamine, and serotonin) and then activating C- and A-delta fibers in peripheral articular tissue (34). Sensitizations of damaged joint tissue result in increased pain, and this pain is difficult to control with conventional therapy (35). Lately, it is found that BTX-A is capable of blocking central and peripheral sensitizations by inhibiting neurotransmitter release (36). Also, other studies showed that BTX-A might have an antinociceptive effect by downregulation of the expression of voltage-gated sodium channel on rat models (37). Accordingly, a plausible explanation for pain inhibition of BTX-A is reducing neurotransmitter release such as substance P, etc., thus blocking the pain signal pathway.
Muscular weakness is the most common adverse effect due to botulinum toxin injection, especially in cervical dystopia cases. Other side effects include arrhythmia, dysphagia, anaphylactic shock, skin rashes, and flu-like syndrome. None of these were seen in our study. As mentioned, the only adverse effect in our study was severe pain in two cases that was improved by acetaminophen. This is due to high volume injection in the joint with severe OA that is destructed with high sensation status. Also, the intra-articular versus systemic injection may be used as a safe method. Also, there were no adverse effects in the PT group.
Totally, it is concluded that the use of BTX can reduce pain and improve the function and quality of life in patients with high-severity KOA. However, further dose-finding and safety studies with larger sample size are required to get more definite applicable results.
5.1. Conclusion
It is hypothesized that botulinum toxin can reduce neurotransmitter release; thus blocking the pain signal pathway. In this study, we concluded that the use of BTX can reduce pain and improve the function and quality of life in patients with KOA. This improvement was significant in long-term follow-up in the BTX group even in 6 months after the intervention. We can conclude that BTX can be a suitable long-term treatment option for KOA, even in high grades arthritis.
Footnotes
Authors' Contribution: Study concept and design, A.D., Z.R., and M.K.M.; Analysis and interpretation of data, S.M.T. and F. A.; Drafting of the manuscript, R.K.M., F. A.; Critical revision of the manuscript for important intellectual content, Z.R., A.D., and M.K.M.; Statistical analysis, S.M.T.
Clinical Trial Registration Code: IRCT20181217042028N2.
Conflict of Interests: The authors declare that they have no competing interests.
Ethical Approval: This study was approved by the Ethics Committee of Institutional Review Board of Aja University of Medical Sciences with the number of IR.AJAUMS.REC.1397.012.
Funding/Support: The authors received no financial support from any public or private sources.
Informed Consent: Informed consent forms were signed by the participants.
Contributor Information
Zahra Rezasoltani, Email: z.rezasoltani@ajaums.ac.ir.
Afsaneh Dadarkhah, Email: a.dadarkhah@ajaums.ac.ir.
Seyed Morteza Tabatabaee, Email: smt.1368@gmail.com.
Fateme Abdorrazaghi, Email: f.m.abdorrazaghi@gmail.com.
Morteza Kazempour Mofrad, Email: mkmmofrad@yahoo.com.
Reza Kazempour Mofrad, Email: reazakazempoor@sbmu.ac.ir.
References
- 1.Hatefi M, Parvizi R, Borji M, Tarjoman A. Effect of self-management program on Pain and Disability Index in elderly men with osteoarthritis. Anesth Pain Med. 2019;9(4):e92672. doi: 10.5812/aapm.92672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imani F, Patel VB. Therapeutic challenges for knee osteoarthritis. Anesth Pain Med. 2019;9(3):e95377. doi: 10.5812/aapm.95377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enteshari-Moghaddam A, Isazadehfar K, Habibzadeh A, Hemmati M. Efficacy of methotrexate on pain severity reduction and improvement of quality of life in patients with moderate to severe knee osteoarthritis. Anesth Pain Med. 2019;9(3):e89990. doi: 10.5812/aapm.89990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azizi S, Rezasoltani Z, Najafi S, Mohebi B, Tabatabaee SM, Dadarkhah A. Transcranial direct current stimulation for knee osteoarthritis: a single-blind randomized sham-controlled trial. Neurophysiol Clin. 2020 doi: 10.1016/j.neucli.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Rahimzadeh P, Imani F, Faiz SHR, Entezary SR, Zamanabadi MN, Alebouyeh MR. The effects of injecting intra-articular platelet-rich plasma or prolotherapy on pain score and function in knee osteoarthritis. Clin Interv Aging. 2018;13:73–9. doi: 10.2147/CIA.S147757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahimzadeh P, Imani F, Faiz SH, Alebouyeh MR, Azad-Ehyaei D, Bahari L, et al. Adding intra-articular growth hormone to platelet rich plasma under ultrasound guidance in knee osteoarthritis: A comparative double-blind clinical trial. Anesth Pain Med. 2016;6(6):e41719. doi: 10.5812/aapm.41719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rezasoltani Z, Azizi S, Najafi S, Sanati E, Dadarkhah A, Abdorrazaghi F. Physical therapy, intra-articular dextrose prolotherapy, botulinum neurotoxin, and hyaluronic acid for knee osteoarthritis: randomized clinical trial. Int J Rehabil Res. 2020;43(3):219–27. doi: 10.1097/MRR.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 8.Imani F, Hejazian K, Kazemi MR, Narimani-Zamanabadi M, Malik KM. Adding ozone to dextrose and somatropin for intra-articular knee prolotherapy: A randomized single-blinded controlled trial. Anesth Pain Med. 2020;10(5):e110277. doi: 10.5812/aapm.110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rezasoltani Z, Taheri M, Mofrad MK, Mohajerani SA. Periarticular dextrose prolotherapy instead of intra-articular injection for pain and functional improvement in knee osteoarthritis. J Pain Res. 2017;10:1179–87. doi: 10.2147/JPR.S127633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahimzadeh P, Imani F, Faiz SH, Entezary SR, Nasiri AA, Ziaeefard M. Investigation the efficacy of intra-articular prolotherapy with erythropoietin and dextrose and intra-articular pulsed radiofrequency on pain level reduction and range of motion improvement in primary osteoarthritis of knee. J Res Med Sci. 2014;19(8):696–702. [PMC free article] [PubMed] [Google Scholar]
- 11.Curatolo M, Bogduk N. Pharmacologic pain treatment of musculoskeletal disorders: current perspectives and future prospects. Clin J Pain. 2001;17(1):25–32. doi: 10.1097/00002508-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Kidd BL. Osteoarthritis and joint pain. Pain. 2006;123(1-2):6–9. doi: 10.1016/j.pain.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Schaible HG, Schmelz M, Tegeder I. Pathophysiology and treatment of pain in joint disease. Adv Drug Deliv Rev. 2006;58(2):323–42. doi: 10.1016/j.addr.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Najafi S, Sanati E, Khademi M, Abdorrazaghi F, Mofrad RK, Rezasoltani Z. Intra-articular botulinum toxin type A for treatment of knee osteoarthritis: Clinical trial. Toxicon. 2019;165:69–77. doi: 10.1016/j.toxicon.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Dhaliwal HP, Kukreja RV, Singh BR. The botulinum toxin as a therapeutic agent: Molecular structure and mechanism of action in motor and sensory systems. Semin Neurol. 2016;36(1):10–9. doi: 10.1055/s-0035-1571215. [DOI] [PubMed] [Google Scholar]
- 16.Maren LM, Krug HE, Singh Intra-articular botulinum toxin A for osteoarticular pain. Toxicon. 2008;51:49. doi: 10.1016/j.toxicon.2008.04.148. [DOI] [Google Scholar]
- 17.Safarpour Y, Jabbari B. Botulinum toxin treatment of pain syndromes -an evidence based review. Toxicon. 2018;147:120–8. doi: 10.1016/j.toxicon.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Mendes JG, Natour J, Nunes-Tamashiro JC, Toffolo SR, Rosenfeld A, Furtado RNV. Comparison between intra-articular Botulinum toxin type A, corticosteroid, and saline in knee osteoarthritis: a randomized controlled trial. Clin Rehabil. 2019;33(6):1015–26. doi: 10.1177/0269215519827996. [DOI] [PubMed] [Google Scholar]
- 19.Zhou XY, Zhang XX, Yu GY, Zhang ZC, Wang F, Yang YL, et al. Effects of low-intensity pulsed ultrasound on knee osteoarthritis: A meta-analysis of randomized clinical trials. Biomed Res Int. 2018;2018:7469197. doi: 10.1155/2018/7469197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page CJ, Hinman RS, Bennell KL. Physiotherapy management of knee osteoarthritis. Int J Rheum Dis. 2011;14(2):145–51. doi: 10.1111/j.1756-185X.2011.01612.x. [DOI] [PubMed] [Google Scholar]
- 21.Mohamed HG, Mohamed MAF. Effect of local heat application on complaints of patients with moderate knee osteoarthritis. Am J Nurs Res. 2019;7:148–59. [Google Scholar]
- 22.Vance CG, Rakel BA, Blodgett NP, DeSantana JM, Amendola A, Zimmerman MB, et al. Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: a randomized controlled trial. Phys Ther. 2012;92(7):898–910. doi: 10.2522/ptj.20110183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skou ST, Koes BW, Gronne DT, Young J, Roos EM. Comparison of three sets of clinical classification criteria for knee osteoarthritis: a cross-sectional study of 13,459 patients treated in primary care. Osteoarthritis Cartilage. 2020;28(2):167–72. doi: 10.1016/j.joca.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886–93. doi: 10.1007/s11999-016-4732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephens MB, Beutler AI, O'Connor FG. Musculoskeletal injections: A review of the evidence. Am Fam Physician. 2008;78(8):971–6. [PubMed] [Google Scholar]
- 26.Salavati M, Mazaheri M, Negahban H, Sohani SM, Ebrahimian MR, Ebrahimi I, et al. Validation of a Persian-version of Knee injury and Osteoarthritis Outcome Score (KOOS) in Iranians with knee injuries. Osteoarthritis Cartilage. 2008;16(10):1178–82. doi: 10.1016/j.joca.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Rutjes AW, Juni P, da Costa BR, Trelle S, Nuesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta-analysis. Ann Intern Med. 2012;157(3):180–91. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 28.Imani F, Rahimzadeh P, Abolhasan Gharehdag F, Faiz SH. Sonoanatomic variation of pes anserine bursa. Korean J Pain. 2013;26(3):249–54. doi: 10.3344/kjp.2013.26.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puett DW, Griffin MR. Published trials of nonmedicinal and noninvasive therapies for hip and knee osteoarthritis. Ann Intern Med. 1994;121(2):133–40. doi: 10.7326/0003-4819-121-2-199407150-00010. [DOI] [PubMed] [Google Scholar]
- 30.Bao X, Tan JW, Flyzik M, Ma XC, Liu H, Liu HY. Effect of therapeutic exercise on knee osteoarthritis after intra-articular injection of botulinum toxin type A, hyaluronate or saline: A randomized controlled trial. J Rehabil Med. 2018;50(6):534–41. doi: 10.2340/16501977-2340. [DOI] [PubMed] [Google Scholar]
- 31.Sun SF, Hsu CW, Lin HS, Chou YJ, Chen JY, Wang JL. Efficacy of intraarticular botulinum toxin A and intraarticular hyaluronate plus rehabilitation exercise in patients with unilateral ankle osteoarthritis: A randomized controlled trial. J Foot Ankle Res. 2014;7(1):9. doi: 10.1186/1757-1146-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sycha T, Samal D, Chizh B, Lehr S, Gustorff B, Schnider P, et al. A lack of antinociceptive or antiinflammatory effect of botulinum toxin A in an inflammatory human pain model. Anesth Analg. 2006;102(2):509–16. doi: 10.1213/01.ane.0000194447.46763.73. [DOI] [PubMed] [Google Scholar]
- 33.Singh JA. Stem cells and other innovative intra-articular therapies for osteoarthritis: what does the future hold? BMC Med. 2012;10:44. doi: 10.1186/1741-7015-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain. 1993;55(1):5–54. doi: 10.1016/0304-3959(93)90183-P. [DOI] [PubMed] [Google Scholar]
- 35.O'Leary H, Smart KM, Moloney NA, Doody CM. Nervous system sensitization as a predictor of outcome in the treatment of peripheral musculoskeletal conditions: A systematic review. Pain Pract. 2017;17(2):249–66. doi: 10.1111/papr.12484. [DOI] [PubMed] [Google Scholar]
- 36.Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26(5):785–93. doi: 10.1016/j.neuro.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Yang KY, Kim MJ, Ju JS, Park SK, Lee CG, Kim ST, et al. Antinociceptive effects of botulinum toxin type A on trigeminal neuropathic pain. J Dent Res. 2016;95(10):1183–90. doi: 10.1177/0022034516659278. [DOI] [PubMed] [Google Scholar]


