Abstract
A 67-year-old man had a few month history of deteriorating visual acuity. He had originally presented to ophthalmology with right-sided visual blurring. This subsequently progressed to involve the left eye. At this point, he was empirically treated with high-dose glucocorticoids, both orally and intravenously, with the suspicion that giant cell arteritis was causing acute visual deterioration of his left eye. Unfortunately, his symptoms did not improve. During an admission to hospital for a pneumonia, he underwent further investigations for this bilateral visual loss. He was diagnosed with left neuroretinitis and right vitritis. A thorough workup revealed positive syphilis serology and cerebrospinal fluid was positive on venereal disease research laboratory testing. He was diagnosed and treated for neurosyphilis with intravenous benzylpenicillin 4 million units 4 hourly for 14 days. His left-sided vision improved but he still suffers from severe visual impairment in his right eye.
Keywords: neuroimaging, syphilis
Background
Syphilis is a sexually transmitted infection caused by the spirochete Treponema pallidum. The infection follows a course of three stages: primary, secondary and tertiary. Visual symptoms, such as blurring of vision and photopsia, occur secondary to ocular syphilis. Ocular syphilis may be diagnosed at any stage of the infection and may involve any eye structure.1 Untreated, it can lead to permanent blindness. Furthermore, ocular syphilis may be associated with central nervous system (CNS) involvement. This can lead to further complications such as muscle paresis, sensory deficits and dementia.1 Clinicians need to be aware of the varied presentations of ocular syphilis to correctly diagnose and manage these patients in a timely manner.
Case presentation
A 67-year-old man was admitted to hospital with a pneumonia. During history-taking, he mentioned that he was being investigated by the ophthalmologists for decreased bilateral visual acuity. He had initially noticed a decrease in visual acuity in his right eye while driving 8 months previously. This had gradually worsened until he was unable to count fingers from that eye.
At first, a right-sided vitreous haemorrhage or a central retinal vein occlusion had been suspected by the ophthalmologists. Subsequently, on developing acute deterioration of left-sided visual acuity, he was started on an empiric course of glucocorticoids to cover for possible giant cell arteritis (GCA). Prednisolone 50 mg daily was given for 5 weeks and was then tailed down by 10 mg per week to 30 mg daily. At this point, due to a further worsening in visual acuity, intravenous methylprednisolone 1 g daily for 3 days was administered and oral prednisolone was increased to 60 mg daily. A temporal artery biopsy had showed no evidence of inflammation. An ultrasound (US) Doppler of both carotid arteries showed no evidence of carotid artery stenosis. Unfortunately, glucocorticoid therapy did not improve his symptoms, and at the time of admission to hospital, he was taking prednisolone at a dose of 20 mg daily.
During his inpatient stay, he was, once again, reviewed by the ophthalmologists who established that he could only count fingers from the right eye, while his left visual acuity was 6/60. Both corneas and anterior chambers were normal. Lenticular opacity was increased bilaterally, being worse on the right. The right intraocular pressure was 13 mm Hg, while the left was 12 mm Hg. There was bilateral sluggish pupillary response to light. The right fundus could not be visualised on direct ophthalmoscopy. The left fundus revealed a swollen optic disc, macular pallor and superior and inferior Roth spots.
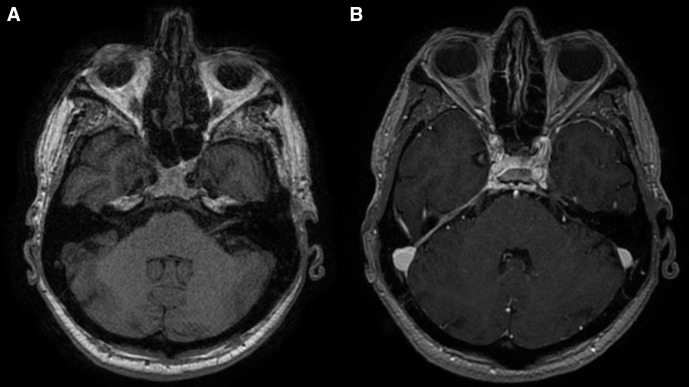
Neuroretinitis was then suspected, possibly secondary to an infection. Other differential diagnoses included haematological malignancies or anterior ischaemic optic neuropathy. GCA was thought to be unlikely since the patient’s decline in visual acuity had persisted despite adequate glucocorticoid cover and due to the lack of other symptoms typical of GCA. This was supported on review by the rheumatologists. Blood investigations for infective and autoimmune conditions were performed during the hospital admission. A transthoracic echocardiogram did not reveal any obvious vegetations. A CT trunk showed only small bibasal consolidations, but no evidence of malignancy. An MRI scan of the brain was normal, while an MRI scan of the orbits showed increased fluid-attenuated inversion recovery (FLAIR) signal intensity in the right globe (figure 1) and linear right retinal enhancement following administration of contrast (figure 2), with unremarkable findings on the left.
Figure 1.
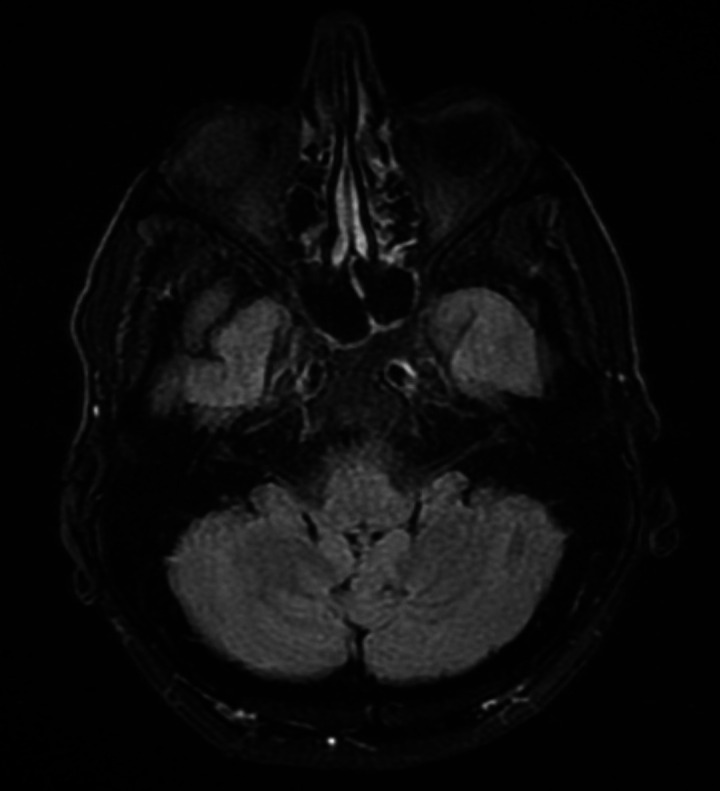
MRI showing increased fluid-attenuated inversion recovery (FLAIR) signal intensity in the right globe compared with the left counterpart.
Figure 2.
(A) T1-weighted MRI pre contrast image of the right retina compared with (B) a T1-weighted MRI postcontrast showing linear enhancement of the right retina.
Syphilis serology turned out to be positive, with a venereal disease research laboratory (VDRL) titre value of >1:256, positive syphilis IgM and T. pallidum haemagglutination (TPHA) with a titre of >1:10 240 (table 1). A lumbar puncture was subsequently performed and the cerebrospinal fluid (CSF) VDRL test was also positive at 1:128+++ with a high lymphocyte count in the CSF (table 2).
Table 1.
Blood investigations on admission to hospital with pneumonia (NB: at this point, the patient was taking prednisolone 20 mg daily)
| Parameter | Result | Range |
| White cell count | 22.02 | 4.3–11.4×109/L |
| Neutrophils | 18.6 | 2.1–7.7×109/L |
| ESR | 57 | 12–16 mm first hour |
| Blood cultures | Negative | |
| Toxoplasma IgG | Positive | |
| Cytomegalovirus IgM | Negative | |
| HIV antigen | Negative | |
| Hepatitis B surface antigen | Negative | |
| Hepatitis C antibody | Negative | |
| Antinuclear antibody | Negative | |
| Syphilis IgM | Positive | |
| Syphilis TPHA | >1:10 240 | |
| Blood for VDRL | Titre value >1: 256 |
ESR, erythrocyte sedimentation rate; TPHA, Treponema pallidum haemagglutination; VDRL, venereal disease research laboratory.
Table 2.
Cerebrospinal fluid (CSF) investigations
| CSF parameter | Result | Range |
| Colour | Colourless | |
| Turbidity | Nil | |
| Supernatant | Clear | |
| Coagulum | Absent | |
| Protein | 44.6 mg/dL | 15–45 mg/dL |
| Glucose | 7.29 | 2.8–4.4 mmol/L |
| Chloride | 117 | 120–130 mmol/L |
| Erythrocytes | 0 (×109/L) | |
| Nucleated cell count | 0.031 (×109/L) | |
| Polymorphonuclears | 0.01 (×109/L) | |
| Lymphocytes/mononuclears | 29 cells/mm3 | Up to 5 cells/mm3 |
| Other cells | 0.01 (×109/L) | |
| CSF for VDRL | 1:128 +++ | |
| CSF for cryptococcal antigen | Negative |
VDRL, venereal disease research laboratory.
The patient’s clinical presentation was diagnostic of neurosyphilis with ocular involvement. The patient was treated with a course of intravenous benzylpenicillin at a dose of 4 million units (MU) 4-hourly for 14 days. On further questioning, he admitted to having had sexual intercourse with different partners without protection. He also admitted to oral sexual intercourse with a casual sex worker. He denied a history of genital ulcers, chancres or rashes. He mentioned having suffered from a urethral discharge 9 months prior, for which he was prescribed an antibiotic but had not taken it.
He completed antibiotic treatment successfully and was discharged, with gradual tailing down of the glucocorticoids. At an outpatient review 2 months later, vision from the left eye was noted to have improved to 6/12, but unfortunately right-sided visual acuity remained poor.
Investigations
An MRI of the head was essentially normal, save for a few punctate white matter hyperintensities suggestive of small-vessel disease.
An MRI of the orbits showed increased FLAIR signal intensity in the right globe and linear right retinal enhancement following administration of contrast in keeping with right-sided vitritis. The left optic nerve appeared unremarkable.
A CT scan of the neck, thorax, abdomen, and pelvis showed no evidence of a neoplastic process.
An US Doppler of the carotid arteries showed no evidence of carotid stenosis.
A transthoracic echocardiogram showed no obvious vegetations.
Differential diagnosis
GCA was the preliminary diagnosis of the ophthalmologists when the patient re-presented with an acute loss of left-sided visual acuity, having first experienced right-sided visual loss. As a result, glucocorticoids were prescribed, with no improvement in left visual acuity. On the contrary, vision continued to deteriorate, and this diagnosis was thus questioned when the patient was incidentally admitted to hospital with a pneumonia. Vision loss in GCA may involve one or both eyes, starting either concurrently or successively. Vision loss in GCA can be due to, most commonly, anterior ischaemic optic neuropathy; but also central retinal artery occlusion, cilioretinal artery occlusion or an occipital lobe infarct.2
Neuroretinitis is an atypical type of optic neuritis which presents with visual loss, optic disc oedema and peripapillary or macular hard exudates usually arranged in a star shape around the fovea.3 The differential diagnoses is wide and includes hypertensive, infiltrative and renal retinopathies, papillitis, papilloedema secondary to raised intracranial pressure, anterior ischaemic optic neuropathy and retinal vein occlusion.3
Causes for neuroretinitis include infective, autoimmune and idiopathic. Infective causes include herpes simplex, hepatitis B, mumps, herpes zoster, HIV, toxoplasma, toxocara, histoplasmosis, syphilis, leptospirosis, cat scratch disease, Lyme’s disease and tuberculosis. Autoimmune conditions include sarcoidosis, ulcerative colitis, polyarteritis nodosa, tubulointerstitial nephritis and uveitis, systemic lupus erythematosus and the antiphospholipid syndrome.
Our patient was initially thought to have an eye haemorrhage and subsequently central retinal vein occlusion affecting the right eye. Further investigations were performed on developing left visual loss with no improvement with glucocorticoids. An autoimmune screen was negative. A workup for infective causes resulted in a positive syphilis serology.
Outcome and follow-up
At an outpatient visit 2 months after cessation of antibiotic treatment, his left-sided vision had significantly improved but he could just perceive light from the right eye. Further ophthalmic follow-up was planned.
Discussion
In recent years, there has been an upward trend in the number of new cases of syphilis worldwide. In Europe, the highest rate has been observed in Malta, with 17.9 cases per 100 000 population in 2018, according to European Centre for Disease Prevention and Control (ECDC) figures.4
Neurosyphilis is an infection of the CNS caused by the spirochete T. pallidum. It can occur at any stage of syphilitic infection, including primary and secondary syphilis. It is nowadays seen most commonly in patients with HIV, having declined in incidence with the widespread usage of antibiotics.1
Ocular syphilis, as in our case, can affect any ocular structure. It is known as the great imitator as it can present with many different clinical manifestations. Ocular syphilis can occur at any stage of syphilitic infection from primary to tertiary. It can occur as part of a CNS infection or independent of CNS involvement. Thus, a lumbar puncture must be performed in all cases of ocular syphilis. Unfortunately, untreated ocular syphilis may lead to permanent blindness.5
Patients usually present with blurring of vision, photopsia and blindness. Headache, altered mental status or hearing loss occurs in about 20% of ocular syphilis. At the ocular surface, it may cause conjunctivitis, episcleritis or scleritis. Uveitis is the most common presentation of ocular syphilis.6 Syphilitic uveitis may lead to elevated intraocular pressure, named the ocular hypertension syndrome. In the posterior segment, syphilis may cause chorioretinitis, retinitis, vasculitis, vitritis and panuveitis. Chorioretinitis is frequently multifocal and associated with vitreous inflammation. A ground glass retinitis is typical for syphilis.6 Complications of ocular syphilis include cataracts, ocular hypertension and glaucoma. Posterior segment complications included cystoid macular oedema and the development of an epiretinal membrane.7
Diagnosis of syphilis is based on clinical features and serology. A positive non-treponemal test (eg, VDRL or rapid plasma reagin (RPR) tests) should be confirmed by a treponemal test, such as the TPHA. Non-treponemal tests have a lower sensitivity, while the treponemal tests are highly sensitive.8
Peng et al compare two different algorithms to diagnose syphilis.9 These are the US Centers for Disease Control and Prevention (CDC) and the ECDC algorithms. The US CDC algorithm recommends using a non-treponemal test initially and following this a treponemal test as a confirmatory test. The ECDC algorithm prefers a treponemal test and then a confirmatory test with a non-treponemal test. They found a high consistency between both the US CDC and ECDC algorithms in populations with low and high syphilis prevalence. The US CDC algorithm had a slight advantage as it diagnosed more cases in a low prevalence population.
CSF examination is required for patients presenting with features of ophthalmic and neurosyphilis, with a lymphocyte count of >5 to 10 cells/mm3, protein concentration >40 mg/dL and a reactive CSF-VDRL being typical of neurological involvement.10 The CSF-VDRL is the gold standard for the diagnosis of neurosyphilis, being highly specific but not completely sensitive. Thus, a negative CSF-VDRL does not rule out a diagnosis of neurosyphilis, while a false positive CSF-VDRL may occur if the CSF is visibly blood stained.11 Our patient had asymptomatic neurosyphilis.
CSF treponemal tests include CSF fluorescent treponemal antibody absorption (CSF FTA-Abs) and CSF Treponema pallidum particle agglutination assay. These are extremely sensitive tests, with a sensitivity approaching 100%, and reliably exclude the diagnosis of neurosyphilis in asymptomatic patients. Unfortunately, however, they do not exclude neurosyphilis in symptomatic patients.12 CSF FTA-Abs have a high rate of false positive results and are not recommended.13 CSF PCR are reported to be 40%–70% sensitive and 60%–100% specific.13
All cases of ocular syphilis are treated as neurosyphilis regardless of CSF results. Treatment of ocular syphilis necessitates administration of intravenous penicillin G at a dose of 18–24 MU daily for 10–14 days. Alternatively, a combination of procaine penicillin G 2.4 MU once daily and probenecid 500 mg four times daily can be administered for 10–14 days. Intramuscular benzathine penicillin 2.4 MU given weekly for 3 weeks may be considered thereafter to give a comparable total duration of therapy as for latent syphilis.8
RPR or VDRL titres should be obtained at 6, 12 and 24 months after treatment and more frequently in HIV patients. In primary or secondary syphilis, a fourfold decrease in non-treponemal antibody testing must be achieved after 6 months; in latent or tertiary syphilis this should be achieved after twelve months. If not, retreatment must be considered and HIV status rechecked.8
In patients with positive CSF results suggestive of syphilis, a lumbar puncture should be done every 6 months post-treatment until it is normal. CSF lymphocyte count is the most sensitive way to assess response to treatment. CSF protein and CSF VDRL may stay elevated for longer.14 Retreatment should be considered if the CSF white blood cell count has not decreased after 6 months of treatment or if the CSF protein and VDRL persist after 2 years of treatment.7
There is lack of data in the literature describing the frequency of neurosyphilis in patients with ocular syphilis. CSF examination remains the cornerstone of diagnosis for neurosyphilis, but we still lack a perfect gold standard test. A review by Spoor et al documented that 60% of patients with ocular syphilis had lymphocytic pleocytosis, elevated protein or both abnormalities on CSF examination.15 In a case series of 13 patients with ocular syphilis by Borges et al, 33% of patients had CSF abnormalities that led to diagnosis of neurosyphilis. Out of these, none had positive CSF VDRL results and one had positive CSF FTA-Abs.13 This was a small sample of patients and does not allow for generalisation. In a review by Rodrigues et al, consisting of 21 patients with ocular syphilis, 75% had non-reactive CSF VDRL but 100% had positive CSF FTA-Abs.16
The aim of our case report is to increase awareness of the protean manifestations of syphilis, given its re-emergence worldwide. Our patient presented with ocular symptoms as the initial complaint for his newly diagnosed syphilitic infection. He is an HIV-negative heterosexual male. Roy et al report a similar case to ours in which ocular syphilis was diagnosed in an immunocompetent patient. Their case presented with ocular syphilis after secondary syphilis. This patient did not have neurological involvement.5 Boghdadi and Feldman report ocular syphilis with no neurological involvement in an HIV-negative woman.17 Sara and McAllister report a series of three cases of ocular syphilis, two of which occurred in HIV-negative individuals.18 Hong et al report a series of patients were ocular syphilis was the initial presentation of syphilis.19 Paulraj et al also report a patient with ocular syphilis as the initial manifestation for syphilis in an HIV-negative patient.20
Accurate and thorough history taking must be emphasised to pick up these potentially devastating conditions. Described as ‘the great imitator’, patients with syphilis may present to any medical specialty. Given the devastating complications that may arise with delayed treatment of ocular syphilis, namely permanent visual loss, and the availability of safe and effective antimicrobial treatment, all clinicians should be vigilant when faced with a patient with decreased visual acuity and syphilis should be considered in the differential diagnosis. Syphilis should also be considered in patients without the typical risk factors, especially considering the fact that our patient was HIV negative and heterosexual.
Learning points.
All patients diagnosed with syphilis should be tested for HIV and other sexually transmitted infections.
Syphilis should be considered in the differential diagnosis of patients presenting with unexplained ocular symptoms, as delay in treatment can lead to irreversible blindness.
Patients diagnosed with ocular syphilis need to undergo a lumbar puncture, and are invariably treated as having neurosyphilis. This remains the case even if cerebrospinal fluid results are not suggestive of central nervous system involvement.
All patients with a provisional diagnosis of giant cell arteritis should be reviewed by a rheumatologist, especially considering the morbidity and mortality associated with long-term glucocorticoid treatment.
Diagnosis of giant cell arteritis should immediately be reconsidered if there is no response to high-dose glucocorticoids.
Acknowledgments
We would like to acknowledge Dr Rueben Grech, Consultant General and Neuro Radiologist for his help in choosing the MRI images.
Footnotes
Contributors: AM and DH wrote the case with constant guidance and reviews by PJC and SV.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.STD Facts . Syphilis (detailed). Available: https://www.cdc.gov/std/syphilis/stdfact-syphilis-detailed.htm [Accessed 09 Dec 2020].
- 2.Vodopivec I, Rizzo JF. Ophthalmic manifestations of giant cell arteritis. Rheumatology 2018;57:ii63–72. 10.1093/rheumatology/kex428 [DOI] [PubMed] [Google Scholar]
- 3.Narayan SK, Kaliaperumal S, Srinivasan R. Neuroretinitis, a great mimicker. Ann Indian Acad Neurol 2008;11:109–13. 10.4103/0972-2327.41879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syphilis - annual epidemiological report for 2018. Available: https://www.ecdc.europa.eu/en/publications-data/syphilis-annual-epidemiological-report-2018 [Accessed 06 Dec 2020].
- 5.Roy M, Roy AK, Farrell JJ. Ocular syphilis in an immunocompetent host. IDCases 2020;19:e00684. 10.1016/j.idcr.2019.e00684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.How to recognize ocular syphilis. Available: https://www.reviewofophthalmology.com/article/how-to-recognize-ocular-syphilis [Accessed 07 Dec 2020].
- 7.Furtado JM, Arantes TE, Nascimento H, et al. Clinical manifestations and ophthalmic outcomes of ocular syphilis at a time of re-emergence of the systemic infection. Sci Rep 2018;8:12071. 10.1038/s41598-018-30559-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syphilis - 2015 STD treatment guidelines. Available: https://www.cdc.gov/std/tg2015/syphilis.htm [Accessed 07 Dec 2020].
- 9.Peng J, Lu Y, Yu H, et al. Analysis of 2 reverse syphilis testing algorithms in diagnosis of syphilis: a Large-Cohort prospective study. Clin Infect Dis 2018;67:947–53. 10.1093/cid/ciy198 [DOI] [PubMed] [Google Scholar]
- 10.Müller M, Ewert I, Hansmann F, et al. Detection of treponema pallidum in the vitreous by PCR. Br J Ophthalmol 2007;91:592–5. 10.1136/bjo.2006.110288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nayak S, Acharjya B. VDRL test and its interpretation. Indian J Dermatol 2012;57:3–8. 10.4103/0019-5154.92666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh AE. Ocular and neurosyphilis: epidemiology and approach to management. Curr Opin Infect Dis 2020;33:66–72. 10.1097/QCO.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 13.Borges CR, Almeida SMde, Sue K, et al. Neurosyphilis and ocular syphilis clinical and cerebrospinal fluid characteristics: a case series. Arq Neuropsiquiatr 2018;76:373–80. 10.1590/0004-282x20180054 [DOI] [PubMed] [Google Scholar]
- 14.Marra CM, Maxwell CL, Tantalo LC, et al. Normalization of serum rapid plasma reagin titer predicts normalization of cerebrospinal fluid and clinical abnormalities after treatment of neurosyphilis. Clin Infect Dis 2008;47:893–9. 10.1086/591534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spoor TC, Wynn P, Hartel WC, et al. Ocular syphilis. Acute and chronic. J Clin Neuroophthalmol 1983;3:197–203. [PubMed] [Google Scholar]
- 16.Rodrigues RAM, Nascimento HMdo, Muccioli C. Yellowish dots in the retina: a finding of ocular syphilis? Arq Bras Oftalmol 2014;77:324–6. 10.5935/0004-2749.20140081 [DOI] [PubMed] [Google Scholar]
- 17.Boghdadi G, Feldman M. An old disease with an unfamiliar face: a case report of ocular syphilis. J Med Cases 2020;11:77–8. 10.14740/jmc3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sara SA, McAllister AS. Three cases of ocular syphilis and the resurgence of the disease in queensland. Int Med Case Rep J 2016;9:279–83. 10.2147/IMCRJ.S111349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong M-C, Sheu S-J, Wu T-T, et al. Ocular uveitis as the initial presentation of syphilis. J Chin Med Assoc 2007;70:274–80. 10.1016/S1726-4901(07)70004-7 [DOI] [PubMed] [Google Scholar]
- 20.Paulraj S, Ashok Kumar P, Gambhir HS. Eyes as the window to syphilis: a rare case of ocular syphilis as the initial presentation of syphilis. Cureus 2020;12:e6998. 10.7759/cureus.6998 [DOI] [PMC free article] [PubMed] [Google Scholar]