Abstract
Introduction
Low surface contamination levels of hazardous drugs in compounding areas can be used as indicators of exposure and efficacy of cleaning procedures. We report the efficacy results of the KIRO® Oncology self-cleaning automated compounding system for decontamination of cytotoxic drugs, assessed in an oncology health center using a sanitizing method and an alkaline method.
Methods
The study was conducted for six-days over a three-week period. A mixture with known levels of 5-fluorouracil, ifosfamide, cyclophosphamide, gemcitabine, etoposide, methotrexate, paclitaxel, docetaxel and carboplatin was added to the KIRO® Oncology’s compounding area surface before each self-cleaning method was used. Contamination levels were determined, with a surface wipe sampling kit, at the end of the self-cleaning process.
Results
Background surface contamination for quantified levels of cytotoxic drugs during routine use of KIRO® Oncology was below limit of quantification (<LOQ) for all drugs, except for carboplatin, which has a very low LOQ (0.2 ng/sample). The quantified drug levels detected on surface wipe samples after self-cleaning using both methods in the KIRO® Oncology’s compounding area surface sections were all <LOQ when spiking with 1 ng/cm2 (ten times the ‘safe’ reference value), except for carboplatin (alkaline method only), although its levels were still below the ‘safe’ reference value (0.1 ng/cm2). For surface contamination levels when spiking with 100 ng/cm2, both self-cleaning methods had decontamination efficacies >99.8% for all cytotoxic drugs analyzed.
Conclusion
This study provides evidence on the efficacy of the KIRO® Oncology automatic self-cleaning system for surface area decontamination during the preparation of cytotoxic drugs.
Keywords: Self-cleaning, automated, compounding, decontamination, cytotoxic drug
Introduction
Continuous exposure to cytotoxic drugs increases the risk of mutagenic, carcinogenic or teratogenic effects in healthcare workers. Although many countries have adopted guidelines and recommendations for the safe handling of cytotoxic drugs, no standards exist for acceptable cytotoxic drug levels in compounding areas.1 Because little is known about the potential health risks associated with low-level multidrug environmental surface contamination, contamination levels should be kept as low as reasonably achievable (“ALARA”).2
Some studies have recommended using threshold guidance values (“TGVs”) based on contamination levels of 100 hospitals in Germany, considering the 75th percentile level (0.03 ng/cm2) the achievable TGV.3 Other studies have recommended the use of the 90th percentile and suggested a substance-independent guidance value of 0.1 ng/cm2 based on their findings for 5-fluorouracil contamination in 130 hospitals in Germany.4
A Dutch study established 0.1 ng/cm2 to be the surface contamination level below which cyclophosphamide was not detectable in the urine of healthcare workers, and recommended a general target surface contamination level of 0.1 ng/cm2 (percentile 90th) and a prohibitory level of 10 ng/cm2 (percentile 99th).5 These values have been widely used as the reference for cytotoxic drug levels on surfaces of compounding facilities for most of the drugs, although some studies have described more detailed drug-specific threshold values.6
However, routine monitoring of chemical contamination levels in compounding facilities is still not a standard practice for process or cleaning qualification, neither for quality control nor for worker healthcare evaluation. This lack of routine monitoring may be due to the limited number of validated tests for the quantification of cytotoxic drugs.7 Additionally, the high cost of these tests limits the availability of data to assess the impact of implementing quality systems or corrective actions.2 As a result, chemical contamination with cytotoxic drugs is found inside and outside the direct compounding areas in pharmacy facilities, in the final products, and on the operator garments, regardless of whether isolators or laminar airflow cabinets are being used. These results are particularly pronounced in those pharmacies where there is not a consistent cleaning protocol in operation.8–13
Advanced containment technologies, such as closed-system transfer devices (CSTDs) or robotic systems highly reduce, but do not completely eliminate, chemical contamination levels in compounding areas and final preparations.14–23 Therefore, effective cleaning methods capable of inactivating all cytotoxic drugs currently used for the treatment of cancer patients are still warranted.
KIRO® Oncology (Kiro Grifols, a Grifols company, Gipuzkoa, Spain) is an automated pharmacy compounding device for the preparation of compounded sterile products that includes a self-cleaning system for the chemical decontamination of the compounding area. Here we report the results of a study conducted in an oncology health center in which the efficacy of the KIRO® Oncology self-cleaning system for the decontamination of common cytotoxic drugs was assessed.
Materials and methods
Objectives
The goal of this study was to determine the efficacy of the KIRO® Oncology self-cleaning system for the decontamination of common cytotoxic drugs. To this end, a mixture with known levels of nine commercially available cytotoxic drugs was added to the system’s compounding area, and contamination levels were determined after spiking followed by the sanitizing cleaning method and after spiking followed by the alkaline cleaning method.
The study was conducted during three weeks (two working days per week) after routine use of the system for compounding at Fundación Onkologikoa (Gipuzkoa, Spain), a reference health center committed to the prevention, diagnosis, research and treatment of cancer.
Automatic compounding system description
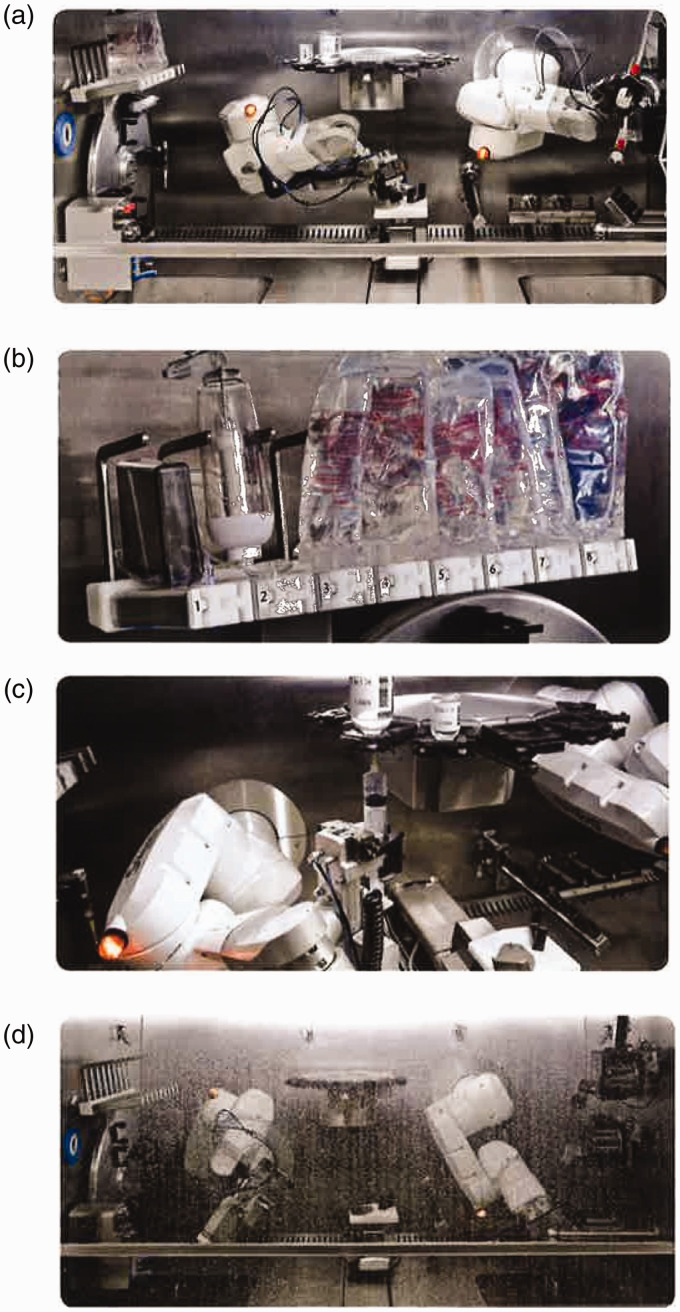
KIRO® Oncology is an automated system designed for the compounding of intravenous (IV) treatments, including cytotoxics and biologicals.24,25 The compounding area comprises two robotic arms able to perform separate tasks simultaneously (Figure 1(a)) and cameras for the identification of vials, IV bags, and supplies by comparing images and barcodes with those stored in the system database. Gravimetric controls are performed at each step of the compounding process and final products are released based on their accuracy to the prescribed dose (final containers: infusion bags, capped syringes, cassettes or elastomeric pumps, Figure 1(b)). Additionally, partially used vials can be stored in the system and their use in subsequent doses tracked.
Figure 1.
Some features of the Kiro® Oncology system: (a) two robotic arms to perform separate tasks simultaneously; (b) final containers: infusion bags, syringes, cassettes, or elastomeric pumps; (c) Two peristaltic pumps for diluent filling of empty containers and reconstitution of lyophilized drug vials; (d) Self-cleaning for decontamination of cytotoxic drugs.
Furthermore, two peristaltic pumps complete the diluent filling of empty containers and lyophilized drug vials (Figure 1(c)).
KIRO® Oncology’s self-cleaning system (Figure 1(d)) consists of spraying sequentially 15 L of cleaning solution on the surfaces of the compounding area and on the adaptors. Drying of the machine is carried out by means of the airflow system, with the possibility of adding germicidal UVC light. The cleaning solutions are drained through the system entirely and collected in a waste container for disposal according to institution´s policies and local regulations.
Self-cleaning procedure description
The cleaning solution to be used can combine different cleaning agents to attain chemical decontamination, sanitizing and/or disinfection effects. In this study, to assess the decontamination efficacy of two cleaning methods, the following cleaning procedures were used: i) a sanitizing cleaning method spraying 5 L of sanitizing solution, 2% Sporklenz Ready To Use (Steris Corporation, Mentor OH, USA) after having sprayed 10 L of sterile water, and ii) an alkaline cleaning method consisting of spraying 5 L of an alkaline solution, 1% Proklenz (Steris Corporation, Mentor OH, USA) followed by the spraying of 10 L of sanitizing solution.
Cytotoxic drug solutions preparation and spiking
Nine cytotoxic drugs commonly used in compounding facilities were chosen for this study: 5-fluorouracil (50 mg/mL, Accord Healthcare S.L.U., Barcelona, Spain), ifosfamide (50 mg/mL Baxter Healthcare Ltd, Thetford, UK), cyclophosphamide (20 mg/mL after reconstitution with 50 mL sterile water for injection, Baxter Oncology GmbH, Halle, Germany), gemcitabine (38 mg/mL, Pfizer, S.L., Alcobendas, Spain), etoposide (20 mg/mL, Sandoz Farmacéutica S.A., Madrid, Spain), methotrexate (25 mg/mL, Pfizer S.L., Alcobendas, Spain), paclitaxel (6 mg/mL, Pfizer, Alcobendas, Spain), docetaxel (20 mg/mL, Aurovitas Spain, S.A.U., Madrid, Spain) and carboplatin (10 mg/mL, Accord Healthcare, S.L.U., Barcelona, Spain).
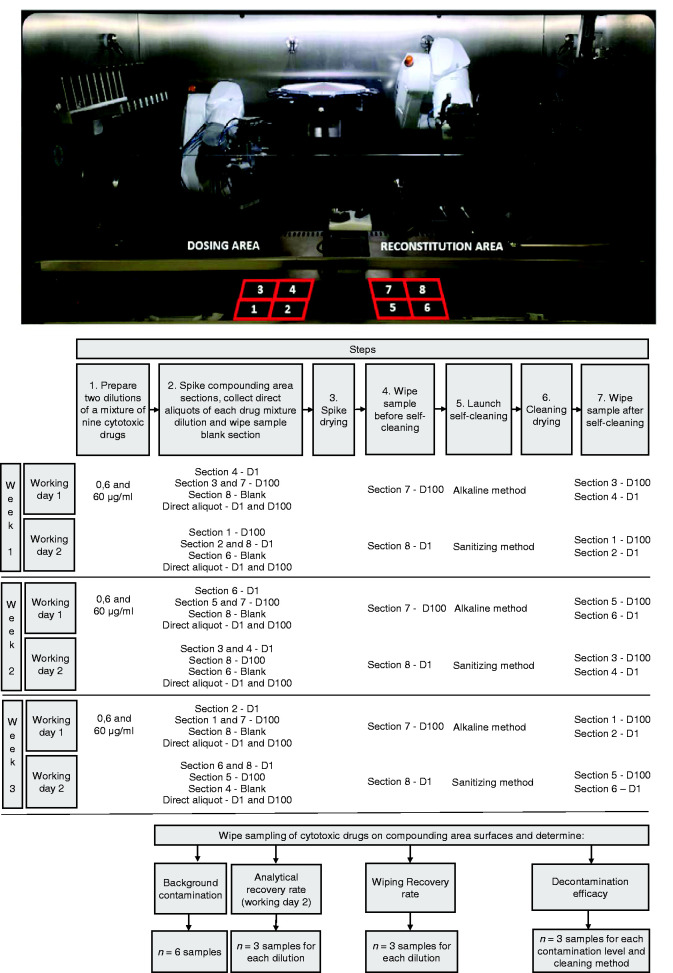
With the aim of covering a wide range of drug contamination levels that may potentially arise in the compounding area of the system, two levels of surface contamination were defined for the study: D1 (1 ng/cm2) and D100 (100 ng/cm2). To this end, two dilutions containing 0.6 µg/mL or 60 µg/mL of each drug were prepared weekly with the aid of a variable volume micropipette (Transferpette® 100–1000 µL) and a dropping funnel. Dilutions were stored at room temperature for the first and second spiking days. At each of the three study weeks, on two consecutive days the lower stainless steel surface of the compounding area was divided into eight sections of 150 cm2 as indicated in Figure 2. On each study day, ten 25 µL drops of a drug mixture dilution were spiked with a micropipette on three surface sections and were left to dry. Of the five remaining sections, one of them served as the blank, while the other four sections were sampled to test the other self-cleaning method. The surfaces were spiked prior to using each of the two self-cleaning methods studied.
Figure 2.
Eight sections of the KIRO® Oncology compounding area on which different drug mixtures were added for the study (upper panel: picture) following the rotation plan for the six working days of the study (lower panel: flowchart of sampling methodology to determine decontamination efficacy, and analytical and wiping recovery rates) (D1 = 1 ng/cm2 and D100 = 100 ng/cm2).
Sampling of cytotoxic drugs on compounding surface
Once the surfaces were dry, a surface wipe sampling kit (PharmaMonitor, Berner International GmbH, Elmshorn, Germany) was used to collect the wipe samples from the different compounding area sections as indicated in Figure 2 on each study day, before and after the automated self-cleaning of the KIRO® Oncology was launched. The alkaline cleaning method was used the first day and the sanitizing cleaning method was used the second day of each study week. For both cleaning methods, the system was allowed to dry for one hour before collecting the wipe samples. Samples before self-cleaning were used to determine the wiping recovery rate of the wipe sampling and analytical method combined. Samples after self-cleaning were used to determine decontamination efficacy of the self-cleaning process.
Since the system was routinely used for the preparation of antineoplastic drugs during the study period, in order to determine the background contamination level of the compounding area, at each of the six study days a blank wipe sample was collected from the section in which no drug mixture was added.
In addition to the wipe samples, on the second day of each study week, two direct aliquots containing 180 ng and 18 µg (18,000 ng) of each of the nine cytotoxic drugs were also added to two wipes, in order to evaluate the analytical recovery rate of Multimethod 2 in the absence of a wiping process. The analytical recovery rates of drug mixture dilutions were calculated for the direct aliquots collected on the second day of each of the three study weeks.
Overall, 30 samples were evaluated at the end of the study: Three samples for each surface contamination level (D1 and D100) and cleaning method (Sanitizing and Alkaline) for self-cleaning decontamination efficacy calculation (12 samples, [4 samples per week]); three samples for each dilution for analytical recovery rate calculation (6 samples, [2 dilutions per week]); three samples for each dilution for wiping recovery rate calculation (6 samples, [2 surface contamination levels per week]); and six samples for determination of background surface contamination level, one per test day (6 samples, [2 blank wipe samples per week]).
Quantification of cytotoxic drug levels
Wipe samples were shipped to the test laboratory (Institute for Energy and Environmental Technology, Duisburg, Germany) for simultaneous analysis and quantification of the nine drugs in a single sample using Multimethod 2 (Berner International GmbH, Elmshorn, Germany), which included liquid chromatography-mass spectrometry, and inductively coupled plasma mass spectrometry for the quantification of total platinum.26 Sensitivity and recovery variability of Multimethod 2 were expressed as the limit of quantification (LOQ) and uncertainty (%) reported by the test laboratory, respectively.
Reported drug quantities were summarized descriptively using mean ± standard deviation (SD). When drug levels were reported by the analytical test method to be below the LOQ, the results were expressed as <LOQ.
Calculation of analytical and wiping recovery rates
The analytical recovery rate was calculated as the percentage of drug quantified and referred to the amount of each drug added directly to the wipe.
The % Analytical recovery rate = (drug [ng/sample]/drug added to the wipe [ng])*100.
The wiping recovery rate was calculated as the percentage of drug quantified in sections wipe sampled before self-cleaning referred to the amount of each drug added to that compounding area section.
The % wiping recovery rate = drug before self-cleaning (ng/cm2)*(surface [cm2]/drug added to the compounding area section [ng])*100.
Calculation of self-cleaning efficacy
Decontamination efficacy of the self-cleaning system was calculated as the percentage of the difference between the mean cytotoxic drug quantified in wipe samples before and after self-cleaning.
The % decontamination efficacy = (drug before self-cleaning [ng/cm2] − drug after self-cleaning [ng/cm2]) × 100/drug before self-cleaning [ng/cm2]
When levels <LOQ were reported, the LOQ was used as the quantified drug level after self-cleaning to determine the minimum decontamination efficacy that could be calculated based on the study method used.
For the designed study conditions, the maximum detectable self-cleaning efficacy for 100 ng/cm2 and 1 ng/cm2 contamination levels was calculated taking into account the LOQ for each drug and the mean wiping recovery rate of three days for each drug and contamination level.
Results
Analytical recovery rate
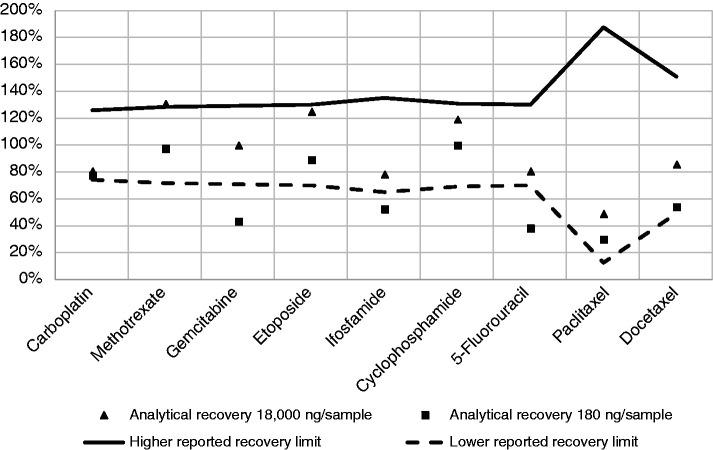
Calculated analytical recovery rates for 180 ng and 18,000 ng direct samples added to a wipe are presented in Figure 3. For 18,000 ng samples, analytical recovery rates for all drugs matched the expected values according to uncertainty levels reported by the test laboratory for Multimethod 2 for the quantification of multiple cytotoxic drugs in a single surface wipe sample (see Supplementary Table 1). However, for paclitaxel, the uncertainty reported was as high as 88%, which corresponds to expected recoveries (uncertainty limits) ranging from 12% to 188% of the real contamination level. The analytical recovery rates determined for paclitaxel were 29% and 49% for wipe samples expecting to have 180 ng/sample and 18,000 ng/sample, respectively, in agreement with the reported uncertainty.
Figure 3.
Mean analytical recovery (%) of nine cytotoxic drugs determined in duplicated analytical measurements in wipes with 18,000 ng and 180 ng of each drug, indicating the wipe method uncertainty limits (100% analytical recovery ± uncertainty reported by the test laboratory for Multimethod 2). See Supplementary Table 1 for mean and standard deviation of the cytotoxic drug levels quantified in wipes, and for LOQ and uncertainty reported for each drug for Multimethod 2.
Furthermore, for 180 ng samples, well above the reported LOQ presented in Supplementary Table 1, analytical recovery rates calculated for gemcitabine (43%), ifosfamide (52%) and 5-fluorouracil (38%) were below the lower uncertainty limit according to the uncertainties reported by the test laboratory for Multimethod 2 for these drugs (71%, 65%, and 70%, respectively) (see Figure 3 and Supplementary Table 1). The analytical recovery rates of drug mixture dilutions indicated that they were stable after being collected on the second day of each of the three study weeks.
Background contamination
Background contamination levels that may exist on KIRO® Oncology compounding area surfaces during routine use of the system were determined by wiping sample sections in which no drug was added. The quantified levels of cytotoxic drugs during routine use of KIRO® Oncology were <LOQ for all drugs except for carboplatin levels of 0.03 ng/cm2 in two of the three sampling days and 0.06 ng/cm2 of 5-fluorouracil in one of the three sampling days.
Wiping recovery rate
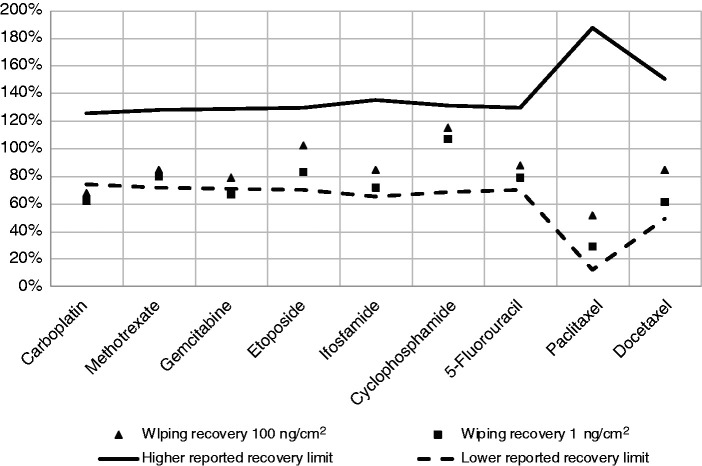
The amount of drug quantified on surface wipe samples collected on three days from 150 cm2 KIRO® Oncology compounding area sections after having been contaminated with 150 ng and 15,000 ng of a mixture of the nine cytotoxic drugs, and before self-cleaning, is presented in Supplementary Table 2. Recovery rates were slightly lower than those expected based on the reported uncertainties only for carboplatin (62%) and gemcitabine (67%) for contamination levels of 1 ng/cm2 (D1), and only for carboplatin (68%) for contamination levels of 100 ng/cm2 (D100) (see Figure 4 and Supplementary Table 2).
Figure 4.
Mean wiping recovery (%) of nine cytotoxic drugs calculated based on cytotoxic drug levels determined on surface wipe samples before self-cleaning on three independent days for surface contamination levels of 100 ng/cm2 and 1 ng/cm2, indicating the wipe method uncertainty limits (100% wiping recovery ± uncertainty reported by the test laboratory for Multimethod 2). See Supplementary Table 2 for mean and standard deviation of the cytotoxic drug levels quantified in surface wipe samples.
Cytotoxic drug levels after self-cleaning
Table 1 shows the quantified drug levels found on surface wipe samples after self-cleaning using the sanitizing method in the KIRO® Oncology’s compounding area sections which were experimentally contaminated with commonly used cytotoxic drugs. For the sanitizing cleaning method, levels below the LOQ were reported for all drugs after self-cleaning when spiking with 1 ng/cm2 (D1). When the added amount was 100 ng/cm2 (D100), levels below the LOQ were reported for all drugs except for carboplatin and methotrexate, for which mean reported contamination levels of the three study weeks were 0.011 ng/cm2 and 0.100 ng/cm2, respectively.
Table 1.
Cytotoxic drug levels determined on surface wipe samples after self-cleaning with sanitizing solution on three independent days (mean ± standard deviation; n = 3) for two drug contamination levels (D1 = 1 ng/cm2 and D100 = 100 ng/cm2).
| Cytotoxic drug |
D1 |
D100 |
||
|---|---|---|---|---|
| ng/150 cm2 | ng/cm2 | ng/150 cm2 | ng/cm2 | |
| Carboplatin | <0.2 | <0.0013 | 1.575 ± 0.745 | 0.011 ± 0.005 |
| Methotrexate | <3 | <0.02 | 15.025 ± 17.025 | 0.100 ± 0.113 |
| Gemcitabine | <3 | <0.02 | <3 | <0.02 |
| Etoposide | <3 | <0.02 | <3 | <0.02 |
| Ifosfamide | <3 | <0.02 | <3 | <0.02 |
| Cyclophosphamide | <3 | <0.02 | <3 | <0.02 |
| 5-Fluorouracil | <10 | <0.07 | <10 | <0.07 |
| Paclitaxel | <10 | <0.07 | <10 | <0.07 |
| Docetaxel | <20 | <0.13 | <20 | <0.13 |
Non quantifiable drug levels reported are expressed as below (<) the limit of quantification for each drug.
For the alkaline self-cleaning method, Table 2 shows levels below the LOQ being reported for all drugs except for carboplatin when spiking with 1 ng/cm2 (D1) and 100 ng/cm2 (D100), resulting in a detection of 0.0019 ng/cm2 and 0.0072 ng/cm2, respectively.
Table 2.
Cytotoxic drug levels determined on surface wipe samples after self-cleaning with the alkaline cleaning method on three independent days (mean ± standard deviation; n = 3) for two drug contamination levels (D1 = 1 ng/cm2 and D100 = 100 ng/cm2).
| Cytotoxic drug |
D1 |
D100 |
||
|---|---|---|---|---|
| ng/150 cm2 | ng/cm2 | ng/150 cm2 | ng/cm2 | |
| Carboplatin | 0.278 ± 0.221 | 0.002 ± 0.001 | 1.075 ± 0.240 | 0.007 ± 0.002 |
| Methotrexate | <3 | <0.02 | <3 | <0.02 |
| Gemcitabine | <3 | <0.02 | <3 | <0.02 |
| Etoposide | <3 | <0.02 | <3 | <0.02 |
| Ifosfamide | <3 | <0.02 | <3 | <0.02 |
| Cyclophosphamide | <3 | <0.02 | <3 | <0.02 |
| 5-Fluorouracil | <1 | <0.07 | <10 | <0.07 |
| Paclitaxel | <10 | <0.07 | <10 | <0.07 |
| Docetaxel | <20 | <0.13 | <20 | <0.13 |
Non-quantifiable drug levels reported are expressed as below (<) the limit of quantification for each drug.
Self-cleaning efficacy
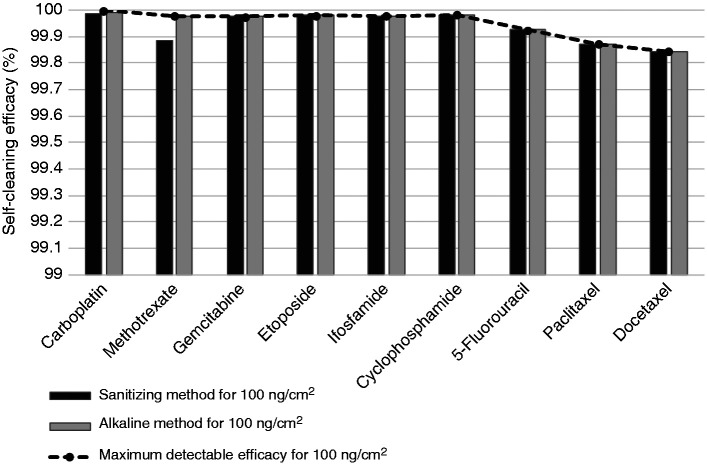
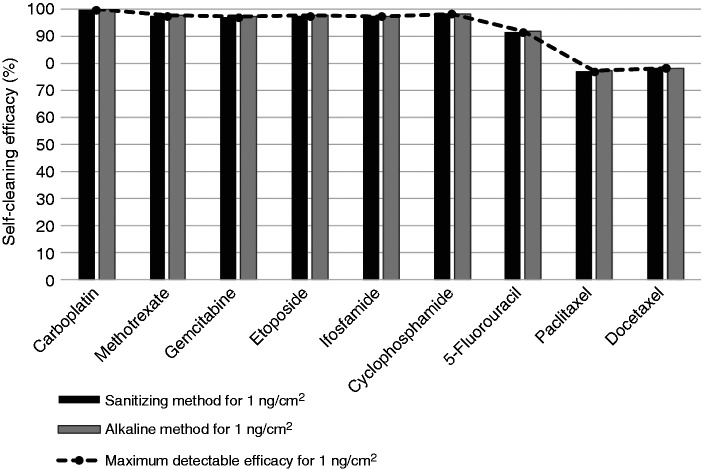
The calculated self-cleaning efficacy for each cleaning method (sanitizing method and alkaline method), drug, and contamination level are presented in Figures 5 and 6 for 100 ng/cm2 (D100) and 1 ng/cm2 (D1) contamination levels respectively, along with the maximum detectable self-cleaning efficacy for each contamination level and drug.
Figure 5.
Calculated self-cleaning efficacy with sanitizing and alkaline methods for the decontamination of cytotoxic drug level D100 (100 ng/cm2), and maximum detectable self-cleaning efficacy for each drug for this contamination level.
Figure 6.
Calculated self-cleaning efficacy with sanitizing and alkaline methods for the decontamination of cytotoxic drug level D1 (1 ng/cm2), and maximum detectable self-cleaning efficacy for each drug for this contamination level.
For surface contamination levels of 100 ng/cm2 (D100), both self-cleaning methods had minimum decontamination efficacies of 99.8% for all nine cytotoxic drugs analyzed. For surface contamination levels of 1 ng/cm2 (D1), calculated decontamination efficacy for both self-cleaning methods was above 99% for carboplatin, above 98% for cyclophosphamide, above 97% for methotrexate, gemcitabine, etoposide and ifosfamide, above 91% for 5-fluorouracil, 78% for docetaxel and 77% for paclitaxel.
Discussion
This study, perfomed during the use of KIRO® Oncology for the preparation of cytotoxic drugs in a hospital pharmacy, provides evidence of the efficacy of KIRO® Oncology’s automatic self-cleaning system for the decontamination of nine commonly used cytotoxic drugs from the system’s compounding area surfaces.
Analytical recovery rates for all drugs matched the expected values according to reported uncertainty levels for 18,000 ng samples. However, for 180 ng samples well above the reported LOQ, analytical recovery rates determined for gemcitabine, ifosfamide and 5-fluorouracil were slightly below the expected according to the uncertainties reported for these drugs.
It is to be noted that when using quantification methods for detecting trace amounts of multiple cytotoxic drugs simultaneously, the quantification uncertainty was high for some of the drugs included in the analytical method used in the study. For example, in the extreme case of paclitaxel (reported uncertainty of 88%), this study determined a mean wiping recovery level of 51% for contamination levels of 100 ng/cm2 and a mean wiping recovery level of 29% for contamination levels of 1 ng/cm2.
For carboplatin and gemcitabine mean wiping recovery rates were slightly lower than expected based on the reported uncertainties. Additionally, considerable variability for the recovery rates on each test day for all test drugs was observed.
Variability of multidrug wipe sampling methods for the quantification of trace levels of cytotoxic drugs may limit their applicability for accurate determination of surface contamination levels.2,7 However, being able to determine multiple drugs in a single sample provides the opportunity to use these methods to easily monitor workplace surfaces and improve practices and cleaning procedures through the interpretation of contamination level trends.
In 2011, Sessink measured the environmental contamination with cyclophosphamide in hospital pharmacies and established the following reference values: 0.1 ng/cm2 (“safe”) and 10 ng/cm2 (“prohibitory”).5,16 Our results showed that during routine use of KIRO® Oncology for the production of cytotoxic drugs in a hospital pharmacy, surface contamination levels after self-cleaning were <LOQ for all drugs, except for carboplatin which has a lower LOQ (0.2 ng/sample). The contamination level of carboplatin was 0.01 ng/cm2, being 10 times lower than the “safe” threshold value of 0.1 ng/cm2. Therefore, background contamination of KIRO® Oncology under routine production at Fundación Onkologikoa was determined not to interfere with the purpose of the study.
The quantified drug levels found on surface wipe samples after self-cleaning with sanitizing solution in the areas experimentally contaminated with cytotoxic drugs were <LOQ for all drugs when spiking with 1 ng/cm2 (ten times the “safe” reference value), and 100 ng/cm2 (ten times the “prohibitory” level5) except for carboplatin and methotrexate, which presented levels below the “safe” reference value of 0.1 ng/cm2.
For the alkaline self-cleaning method, levels <LOQ were reported for all drugs except for carboplatin, for which levels below the “safe” reference value of 0.1 ng/cm2 were detected after self-cleaning when spiking with 1 ng/cm2 (ten times the “safe” reference value), and 100 ng/cm2 (ten times the “prohibitory” level5).
Remarkably, contamination levels for all cytotoxic drugs except for docetaxel were determined to meet the “safe” reference value of 0.1 ng/cm2 after self-cleaning with both cleaning methods for quantities of 1 ng/cm2 and 100 ng/cm2. For docetaxel, contamination levels were reported to be below the analytical LOQ after self-cleaning with both methods for both quantities. However, since the LOQ for docetaxel was higher that the “safe” reference value of 0.1 ng/cm2, it was determined that docetaxel levels after self-cleaning were close to but not below the “safe” reference value.
These results are relevant because even when using containment systems to reduce surface contamination during compounding, some contamination may persist18 and cleaning methods of demonstrated efficacy are needed for automated pharmacy compounding devices.
For surface contamination levels of 100 ng/cm2, both self-cleaning methods (sanitizing and alkaline) had efficacies greater than 99.8% for all nine cytotoxic drugs analyzed. Surface contamination levels were close to or below the LOQ after self-cleaning for all samples representing surface contamination levels of 1 ng/cm2. Additionally, wiping recovery rates for these contamination levels presented considerable variability and high uncertainties for drugs such as paclitaxel. Therefore, the calculated self-cleaning efficacy for surface contamination levels of 1 ng/cm2 was limited to the maximum detectable efficacy.
Previous studies have described routine cleaning methods in biosafety cabinets over time to have decontamination efficacies that may vary from 49% to 82% for commonly used cytostatic drugs.24 Other studies showed that more intensive cleaning procedures can yield a decontamination efficacy greater than 99%.24,27,28 These findings underline the importance of setting up effective, consistent and reliable cleaning methods, as a way of holding contamination levels ALARA to protect healthcare workers.
This study demonstrated that the KIRO® Oncology’s self-cleaning system has a minimum decontamination efficacy of 99.8% for nine commonly used cytotoxic drugs (5-fluorouracil, cyclophosphamide, ifosfamide, gemcitabine, etoposide, methotrexate, docetaxel and carboplatin) when contamination levels were up to 100 ng/cm2, equivalent to ten times the “prohibitory” reference value. Under these conditions, mean cytotoxic drug contamination levels remaining after self-cleaning were below the target level of 0.1 ng/cm2. However, it should be noted that decontamination efficacy results are subject to considerable variability due to the LOQ and uncertainties reported for multidrug analytical methods used for the detection of low cytotoxic drug levels in surfaces of compounding facilities. Therefore, results obtained by means of multidrug analytical methods may not fully extrapolate to those obtained if each drug had been analyzed individually. In addition, analyses were performed on a single and limited area in the working surface, and decontamination efficacy may be different for other areas which are less accessible to the self-cleaning system.
In summary, this study provides evidence of automatic cleaning methods being an effective novel alternative for avoiding daily manual cleaning tasks when efficacy is influenced by inter-individual variability and involves additional health risks to compounding personnel. Chemical contamination monitoring of the direct compounding area, surrounding areas, final preparations, user’s garments and exposure level determinations during routine production will be required to conclude the benefit of this self-cleaning system in comparison to containment and manual cleaning methods.
Supplemental Material
Supplemental material, sj-pdf-1-opp-10.1177_1078155220951866 for Evaluation of the efficacy of a self-cleaning automated compounding system for the decontamination of cytotoxic drugs by Naiara Telleria, Nerea García, Jaione Grisaleña, Naiara Algaba, Eider Bergareche, María José Tamés and Gerardo Cajaraville in Journal of Oncology Pharmacy Practice
Acknowledgements
Writing assistance for the preparation of this manuscript was provided under the direction of the authors by Carme Romera PhD, Eugenio Rosado PhD, and Jordi Bozzo PhD, CMPP (Grifols). Editorial assistance was provided by MaryJane Silvey (WriteMonitor, LLC, Durham, NC, USA).
Footnotes
Data availability: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This study is the result of a collaboration agreement between the Fundación Onkologikoa (Donostia, Gipuzkoa, Spain) and KIRO Grifols S.L., the manufacturer of KIRO® Oncology. NT, NG, JG, NA, and EB are employees of KIRO Grifols S.L. GC has been a consultant for KIRO Grifols S.L. and has received support from Grifols for presentations at symposia.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Mathias PI, MacKenzie BA, Toennis CA, et al. Survey of guidelines and current practices for safe handling of antineoplastic and other hazardous drugs used in 24 countries. J Oncol Pharm Pract 2019; 25: 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connor TH, Zock MD, Snow AH.Surface wipe sampling for antineoplastic (chemotherapy) and other hazardous drug residue in healthcare settings: methodology and recommendations. J Occup Environ Hyg 2016; 13: 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schierl R, Bohlandt A, Nowak D.Guidance values for surface monitoring of antineoplastic drugs in German pharmacies. Ann Occup Hyg 2009; 53: 703–711. [DOI] [PubMed] [Google Scholar]
- 4.Kiffmeyer TK, Tuerk J, Hahn M, et al. Application and assessment of a regular environmental monitoring of the antineoplastic drug contamination level in pharmacies – the MEWIP project. Ann Occup Hyg 2013; 57: 444–455. [DOI] [PubMed] [Google Scholar]
- 5.Sessink PJM.Environmental contamination with cytostatic drugs: past, present, future. Saf Consid Oncol Pharm Special Edition. Fall 2011: 3–5. [Google Scholar]
- 6.Sottani C, Grignani E, Oddone E, et al. Monitoring surface contamination by antineoplastic drugs in Italian hospitals: performance-based hygienic guidance values (HGVs) project. Ann Work Expo Health 2017; 61: 994–1002. [DOI] [PubMed] [Google Scholar]
- 7.Marie P, Christophe C, Manon R, et al. Environmental monitoring by surface sampling for cytotoxics: a review. Environ Monit Assess 2017; 189: 5762–5769. [DOI] [PubMed] [Google Scholar]
- 8.Connor TH, Anderson RW, Sessink PJ, et al. Surface contamination with antineoplastic agents in six cancer treatment centers in Canada and the United States. Am J Health Syst Pharm 1999; 56: 1427–1432. [DOI] [PubMed] [Google Scholar]
- 9.Schulz H, Bigelow S, Dobish R, et al. Antineoplastic agent workplace contamination study: the Alberta cancer board pharmacy perspective. J Oncol Pharm Pract 2005; 11: 101–109. [DOI] [PubMed] [Google Scholar]
- 10.Mason HJ, Blair S, Sams C, et al. Exposure to antineoplastic drugs in two UK hospital pharmacy units. Ann Occup Hyg 2005; 49: 603–610. [DOI] [PubMed] [Google Scholar]
- 11.Crauste-Manciet S, Sessink PJ, Ferrari S, et al. Environmental contamination with cytotoxic drugs in healthcare using positive air pressure isolators. Ann Occup Hyg 2005; 49: 619–628. [DOI] [PubMed] [Google Scholar]
- 12.Acampora A, Castiglia L, Miraglia N, et al. A case study: surface contamination of cyclophosphamide due to working practices and cleaning procedures in two Italian hospitals. Ann Occup Hyg 2005; 49: 611–618. [DOI] [PubMed] [Google Scholar]
- 13.Kopp B, Crauste-Manciet S, Guibert A, et al. Environmental and biological monitoring of platinum-containing drugs in two hospital pharmacies using positive air pressure isolators. Ann Occup Hyg 2013; 57: 374–383. [DOI] [PubMed] [Google Scholar]
- 14.Connor TH, Anderson RW, Sessink PJ, et al. Effectiveness of a closed-system device in containing surface contamination with cyclophosphamide and ifosfamide in an i.v. admixture area. Am J Health Syst Pharm 2002; 59: 68–72. [DOI] [PubMed] [Google Scholar]
- 15.Harrison BR, Peters BG, Bing MR.Comparison of surface contamination with cyclophosphamide and fluorouracil using a closed-system drug transfer device versus standard preparation techniques. Am J Health Syst Pharm 2006; 63: 1736–1744. [DOI] [PubMed] [Google Scholar]
- 16.Sessink PJ, Connor TH, Jorgenson JA, et al. Reduction in surface contamination with antineoplastic drugs in 22 hospital pharmacies in the US following implementation of a closed-system drug transfer device. J Oncol Pharm Pract 2011; 17: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark BA, Sessink PJ.Use of a closed system drug-transfer device eliminates surface contamination with antineoplastic agents. J Oncol Pharm Pract 2013; 19: 99–104. [DOI] [PubMed] [Google Scholar]
- 18.Simon N, Vasseur M, Pinturaud M, et al. Effectiveness of a closed-system transfer device in reducing surface contamination in a new antineoplastic drug-compounding unit: a prospective, controlled, parallel study. PLoS One 2016; 11: e0159052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartel SB, Tyler TG, Power LA.Multicenter evaluation of a new closed system drug-transfer device in reducing surface contamination by antineoplastic hazardous drugs. Am J Health Syst Pharm 2018; 75: 199–211. [DOI] [PubMed] [Google Scholar]
- 20.Schierl R, Masini C, Groeneveld S, et al. Environmental contamination by cyclophosphamide preparation: comparison of conventional manual production in biological safety cabinet and robot-assisted production by APOTECAchemo. J Oncol Pharm Pract 2016; 22: 37–45. [DOI] [PubMed] [Google Scholar]
- 21.Gandré B, Krämer I.Cytotoxic surface contamination in a robotic system in comparison to manual compounding. Eur J Hosp Pharm 2012; 19: 151–151. [Google Scholar]
- 22.Sessink PJ, Leclercq GM, Wouters DM, et al. Environmental contamination, product contamination and workers exposure using a robotic system for antineoplastic drug preparation. J Oncol Pharm Pract 2015; 21: 118–127. [DOI] [PubMed] [Google Scholar]
- 23.Krämer I, Federici M, Schierl R.Environmental and product contamination during the preparation of antineoplastic drugs with robotic systems. Pharm Technol Hosp Pharm 2018; 3: 153–164. [Google Scholar]
- 24.Anastasi M, Rudaz S, Queruau Lamerie T, et al. Efficacy of two cleaning solutions for the decontamination of 10 antineoplastic agents in the biosafety cabinets of a hospital pharmacy. Ann Occup Hyg 2015; 59: 895–908. [DOI] [PubMed] [Google Scholar]
- 25.Jobard M, Brandely-Piat ML, Chast F, et al. Qualification of a chemotherapy-compounding robot. J Oncol Pharm Pract 2020; 26: 312–324. [DOI] [PubMed] [Google Scholar]
- 26.Holland JF, Enke CG, Allison J, et al. Mass spectrometry on the chromatographic time scale: realistic expectations. Anal Chem 1983; 55: 997A–1012A. [Google Scholar]
- 27.Queruau Lamerie T, Nussbaumer S, Decaudin B, et al. Evaluation of decontamination efficacy of cleaning solutions on stainless steel and glass surfaces contaminated by 10 antineoplastic agents. Ann Occup Hyg 2013; 57: 456–469. [DOI] [PubMed] [Google Scholar]
- 28.Ade A, Chauchat L, Freve JO, et al. Comparison of decontamination efficacy of cleaning solutions on a biological safety cabinet workbench contaminated by cyclophosphamide. Can J Hosp Pharm 2017; 70: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-opp-10.1177_1078155220951866 for Evaluation of the efficacy of a self-cleaning automated compounding system for the decontamination of cytotoxic drugs by Naiara Telleria, Nerea García, Jaione Grisaleña, Naiara Algaba, Eider Bergareche, María José Tamés and Gerardo Cajaraville in Journal of Oncology Pharmacy Practice